Abstract
The extracellular matrix (ECM) offers a structural basis for regulating cell functions while also acting as a collection point for bioactive molecules and connective tissue cells. To perform pathological functions under a pathological condition, the involved cells need to regulate the ECM to support their altered functions. This is particularly common in the development of cancer. The ECM has been recognized as a key driver of cancer development and progression, and ECM remodeling occurs at all stages of cancer progression. Thus, cancer cells need to change the ECM to support relevant cell surface adhesion receptor–mediated cell functions. In this context, it is interesting to examine how cancer cells regulate ECM remodeling, which is critical to tumor malignancy and metastatic progression. Here, we review how the cell surface adhesion receptor, syndecan, regulates ECM remodeling as cancer progresses, and explore how this can help us better understand ECM remodeling under these pathological conditions
Keywords: cancer, ECM remodeling, extracellular matrix, glycosaminoglycan, matrix metalloprotease, syndecan
Introduction
The extracellular matrix (ECM) is a key material of the microenvironment that provides essential structural and biological signals to regulate cellular functions.1,2 The ECM is the primary structural basis for living cells to undertake various cell behaviors, including adhesion, migration, growth, and differentiation. It also acts as a functional platform, as it comprises bioactive molecules and is home for connective tissue cells. Therefore, many cell functions are closely related to the composition and/or physical properties of the ECM, and its remodeling inevitably alters cell function. ECM remodeling is highly common during the development and progression of cancer.3,4 For instance, cancer cells must degrade the basement membrane, and this remodeling of ECM eventually leads to metastasis.2 In cancer, ECM remodeling must be orchestrated by cancer cells, and this process is required to support the varied functions of cancer cells, the adhesion receptors of which sense the changed ECM and trigger responses via transduced signals.
Syndecans are type I transmembrane proteins that comprise a major family of heparan sulfate proteoglycans on the cell surface of both stromal and tumor cells.5 The core protein and glycosaminoglycan chains (GAGs) of syndecan interact with various soluble and insoluble factors in the ECM and relay signals inside of cells in response to a change in the ECM.6,7 Similar to integrin, one of the major families of cell adhesion receptors, syndecans mediate both outside-in and inside-out signaling to directly regulate the expression and release of ECM components and modulate the organization of the ECM.8–10 Therefore, cancer cells can actively reshape the ECM through cell surface adhesion receptors, such as syndecans. Several structural features of syndecan are well suited for reshaping the cancer ECM; these include having a strong potential to interact with diverse extracellular ligands in the ECM; playing regulatory roles in both the ECM and cytoskeletal organization; and exhibiting cooperativity between the core protein and GAG chains.11–14 In this review, we will summarize how syndecans regulate the remodeling of ECM in the context of cancer (Fig. 1).
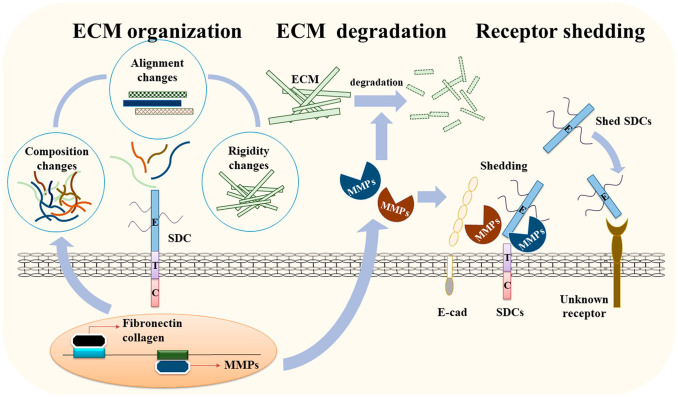
Figure 1.
The regulatory functions of SDCs in ECM remodeling during cancer progression. SDC can activate the expression of structural components of the ECM to alter the organization and physical properties of the ECM, including its stiffness, which is crucial for various cancer functions. SDC also regulates the expression of regulatory enzymes, such as MMPs, to modulate turnover of the ECM. In addition, SDC anchors MMPs on the cell surface and induces their processing into active MMP to induce the breakdown of the ECM and cell surface receptors, including SDCs. Shed SDC further regulates cancer cell activity and other cancer progression activities, such as angiogenesis. Abbreviations: SDC, syndecan; ECM, extracellular matrix; MMP, matrix metalloproteinase.
Syndecans Regulate the Composition of the ECM as Adhesion Receptors
During the progression of various types of cancer, the major structural ECM components, including fibronectin and collagen, show constant expressional changes. The expression levels of type I and IV collagens are well known to be associated with the development of a number of human cancers, including ovarian, pancreatic, melanoma, prostate, and breast cancer,15–19 and fibronectin is reportedly overexpressed in gastric, ovarian, breast, and colon cancers.20–23 Moreover, the content and distribution of collagen is modified to coordinate the functions of colon and breast cancer cells17,24,25 and alter the fibronectin distribution pattern as seen in human solid tumors.26 Together with the altered expression of ECM components, alterations in the organization of the ECM change its rigidity in tumor tissues.27–29 Increased ECM rigidity induces Rho-generated cytoskeletal tension and activates extracellular signal-regulated kinase (ERK)-dependent growth, and subsequent increases in cytoskeletal tension promote growth and the formation of focal adhesions,30 which are the sites at which cancer cells connect to the ECM. Given these cancer-related changes in the ECM, it becomes clear that cancer cells must properly modulate the ECM components.
As an adhesion receptor, syndecans regulate the expression and secretion of ECM components that have different functions in modulating cancer progression. Syndecan-1 plays antimigratory and pro-adhesion roles in several types of cancer cells. For example, syndecan-1 interacts with the ECM and transduces signals into cells to promote the adhesion of human colon cancer cells through blocking the JAK (Janus kinase)/STAT3 (signal transducer and activator of transcription-3) and Ras/Raf/MEK/ERK pathway31,32 to subsequently suppress cell migration.33 Syndecan-1 also modulates fibronectin fibrillogenesis during ECM production through integrin, thereby mediating ECM fiber alignment.34 Conversely, syndecan-2, which plays pro-migratory roles in several cancer cell types, including colon cancer cells,35,36 appears to be required to assemble fibronectin into a fibrillar matrix.37 Moreover, the interaction of syndecan-2 and fibronectin contributes to integrin α2β1 expression, focal adhesion kinase (FAK) activation, and increasing colon cancer cell migration.38,39 Syndecan-4 has been implicated in the initiation of fibronectin matrix assembly and cancer cell motility,40–42 and recent findings showed that the ability of cells to recognize patterned fibronectin substrates is dependent on syndecan-4.43 Syndecan-4 further acts as a receptor that transmits mechanotransduction signals via the activation of protein kinase Cα (PKCα) under mechanical stimulation of elastomeric substrates, and thereby regulates tumor cell spreading, actin cytoskeleton assembly, and cell contractility.44 Thus, syndecan-4 is essential for determining the ECM architecture and the mechanical properties of ECM. ECM surrounding cancer cells may also be produced from cancer-associated fibroblasts,45 and expression of syndecan-1 in stromal fibroblasts in the tumor microenvironment engages a reciprocal, cancer-promoting feedback regulation,46–48 which is consistent with invasiveness of breast cancer46 supporting that syndecan-1 in carcinoma-associated fibroblasts regulates changes in collagen architecture associated with tumor progression.
Syndecan may also regulate the ECM by altering the expression levels of the matrix metalloproteinases (MMPs). For instance, in medulloblastoma and endometrial cancer cells,49,50 the overexpression of syndecan-1 upregulates MMP-9; this degrades type IV collagen, which is the major structural component of the basement membrane. In myeloma and colon cancer cells, in contrast, syndecan-1 has been found to downregulate MMP-9,31,51 indicating different mechanism of MMP expression regulation depending on the cancer cells. Engagement of syndecan-2 and the ECM transduces signals to activate PKCγ/FAK/ERK signaling in parallel with the expression of MMP-7, which is associated with increased colon cancer activities.52 Therefore, syndecan expression regulates the expression levels of both structural and regulatory components of the ECM, and thereby contributes to the organization of the ECM.
Syndecan Regulates ECM Degradation as a Docking Receptor
Syndecan also contributes to regulating the ECM as a docking receptor that regulates extracellular events rather than transducing signals to the interior of the cell.11,53 Several reports have shown that cell surface heparan sulfate proteoglycans provide binding sites for MMP-2, -7, -9, and 13.54 Syndecan-1 is known to dock MMP-7 at the cell surface in breast, colon, and pancreatic cancer.55–57 In colon cancer cells, syndecan-2 induces MMP-7 expression, binds pro-MMP-7, and activates pro-MMP-7 into active MMP-7;58,59 this suggests that syndecan-2 acts as a docking receptor for pro-MMP-7 in colon cancer. In a Lewis lung carcinoma cell line, in contrast, syndecan-2 was found to suppress MMP-2 activation and decrease metastasis.60 Thus, syndecan can bind extracellular proteases to the cell surface, and this interaction may positively or negatively regulate the activity of MMPs. Syndecan-4 controls the activation of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 5 (ADAMTS-5) through a direct interaction,61 and the direct interaction of syndecan-4 with ADAM17 regulates the transient receptor potential channel 6 (TRPC6) protein and may contribute to glomerular pathology.62 Interestingly, the ability of syndecans to sense and regulate MMP activity depends on the ECM stiffness.44 Therefore, the altered expression of syndecan in cancer cells further regulates MMP expression (with syndecan acting as an adhesion receptor) and MMP activation (with syndecan acting as a docking receptor).
Syndecan Shedding and ECM Remodeling in Cancer Cells
The remodeling of the ECM by MMPs releases a number of bioactive fragments that further regulate numerous biological processes, including tumor growth and metastasis. All syndecan family members have been found to be cleaved by MMPs in various cancers: syndecan-1 by MMP-2, -7, and -9, MT1-MMP, and MT3-MMP;63–65 syndecan-2 by MMP-2 and -7 and MT1-MMP;60,66,67 and syndedcan-4 by MMP-2 and -9 and ADAM-17.68,69 These shedding events and the shed syndecan further regulate cancer progression. For instance, the MT-1 MMP-mediated shedding of syndecan-1 stimulates breast cancer cell proliferation.63,70 The MMP-9-mediated shedding of syndecan-1 and -4 by HeLa cells involves ECM degradation during cancer cell metastasis and invasion, and shed syndecan-1 is involved in tumor cell proliferation.68 A positive correlation between shed syndecan-1 levels and tumor size was reported in breast tumor progression,71 and shed syndecan-1 was shown to bind activated vascular endothelial growth factor receptor 2 (VEGFR2) and thereby stimulate tumor cell invasion,72 suggesting that it is involved in tumor angiogenesis. Heparanase-1, which is upregulated in many human tumors in association with increases in the metastatic potential of tumor cells, regulates syndecan-1 expression and shedding to influence breast cancer progression.73,74 This heparanase-mediated syndecan-1 shedding occurs through upregulation of ERK phosphorylation, which leads to enhanced expression of MMP-9.75 Moreover, the heparanase-induced shedding of syndecan-1 in myeloma activates VEGFR2 on adjacent endothelial cells to stimulate tumor angiogenesis.76
Although MMP expression and syndecan shedding appear to be closely correlated, the underlying mechanism of this shedding is not yet fully clear. One of the best examples is seen in the MMP7-mediated shedding of syndecan-2. As mentioned above, syndecan-2 activates MMP-7 at the surface of colon cancer cells,58 and activated MMP-7 mediates shedding of syndecan-2.66 This interplay between syndecan-2 and MMP-7 may regulate colon cancer progression. We speculate that during colon carcinogenesis, syndecan-2 is upregulated, resulting in enhanced expression and extracellular secretion of pro-MMP-7, which interacts with syndecan-2 and is activated to MMP-7. Activated MMP-7 may then enhance the extracellular domain shedding of syndecan-2 to produce the soluble form in cancer cells (Fig. 2). Meanwhile, syndecan-2-activated MMP-7 directly shed E-cadherin,77 which regulates the synthesis of ECMs, including collagen and elastin by forming adherence junction.78 The functional loss of E-cadherin in early-stage E-cadherin-expressing colon cancer cells has been shown to induce epithelial-mesenchymal transition (EMT) and subsequently enhance cell migration.79 This is consistent with the finding that MMP-7 induces syndecan-2 shedding under conditions whereby cancer cell migration and invasion are increased.80 Interestingly, shed syndecan-2 may also regulate the tumorigenic characteristics of colon cancer cells in a paracrine manner. Treatment with shed syndecan-2 has been shown to enhance the migration of HCT116 cells,36,38 promote angiogenic processes in brain microvascular endothelial cells,81 and regulate angiogenesis by inhibiting endothelial cell migration in human and rodent models to reduce tumor growth.82 Therefore, shedding of syndecan-2 may enhance angiogenic processes, thereby facilitating cancer cell growth and metastasis, and promoting the tumorigenic activities.
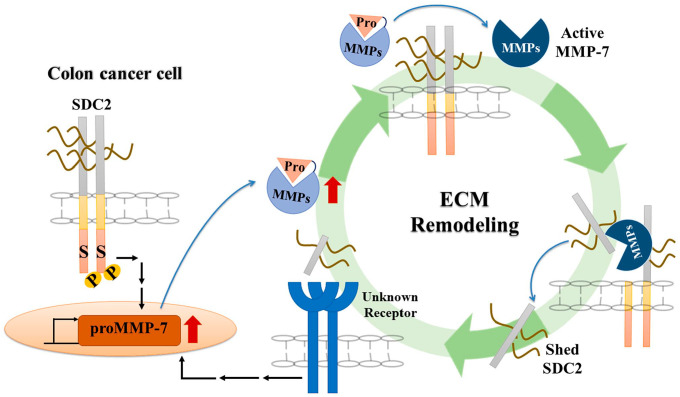
Figure 2.
An interplay of SDC2 and MMP-7 regulates cancer progression. During the development of colon cancer, increased SDC2 expression induces the expression and secretion of pro-form of MMP-7. The secreted pro-MMP-7 anchors to the cell surface through an interaction with SDC2; this induces processing of pro-MMP-7 into active MMP-7 by a yet-unknown mechanism. Active MMP-7 can enhance the extracellular domain shedding of SDC2 to produce the soluble ligand. This shed SDC2 increases cancer activity and MMP-7 expression in a positive feedback regulation. This interplay between SDC2 and MMP-7 can thus regulate colon cancer progression. Abbreviations: SDC2, syndecan-2; ECM, extracellular matrix; MMP, matrix metalloproteinase.
Since syndecan is a member of the ECM as well as a cell surface receptor, syndecan interacts all the time with ECM surrounding cancer cells. Thus, syndecan expression is influenced by cancer type and cancer stages. At the same time, syndecan need to properly regulate the remodeling of the ECM in various ways (Fig. 1). When cancer cells are needed, syndecan induces degradation of existing ECM by regulating MMP expression and/or activating MMPs. Syndecan expresses and secretes new structural ECM components and regulates their organization suitable for cancer function. If necessary, syndecan changes the rigidity of the ECM and others. Syndecan extracellular domain can be released by MMPs and produce new bioactive molecules in a shedding process. Shed syndecan-2 binds the cell surface receptor and then regulates new functions, including angiogenesis. Besides, shed syndecan-2 induces the expression of MMP-7 to further regulate ECM remodeling. ECM rigidity regulated by syndecan can also regulate the expression of syndecan itself, which suggests a positive feedback regulation of syndecan. Although an impressive number of different regulatory functions in ECM remodeling has already been attributed to the structural features of syndecans, additional properties that allow syndecans to modulate signal transmission cannot be excluded. For instance, the syndecan-associated regulations of growth factor receptor and integrins can alter the interactions of the latter proteins with ECM molecules and facilitate cancer progression.9,37,83,84 Shed syndecan can act as ligand for them during tumor progression.9 Although many parts of syndecan’s functions are related to GAGs and many soluble factors, including growth factors, interact through interaction with GAG chains and regulate the functions of cancer cells, we have not dealt in this review. All of the functions in this review mainly focused on the role(s) of the core proteins, but it could also be the collaboration with GAG chains. In any case, syndecan can regulate ECM remodeling as an adhesion receptor, a docking receptor, and soluble ligands as cancer progresses. All these features of syndecan can provide clues for a better understanding of ECM remodeling and a possible application for diagnosis and treatment under these pathological conditions.
Footnotes
Author Contributions: BJ, AK and ESO drafted the manuscript and arranged figures. All authors have read and approved the final manuscript.
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2019R1A2C2009011) (NRF-2018R1D1A1B07049726).
Contributor Information
Bohee Jang, Department of Life Sciences, Ewha Womans University, Seoul, Republic of Korea.
Ayoung Kim, Department of Life Sciences, Ewha Womans University, Seoul, Republic of Korea.
Jisun Hwang, Department of Life Sciences, Ewha Womans University, Seoul, Republic of Korea.
Hyun-Kuk Song, Department of Life Sciences, Ewha Womans University, Seoul, Republic of Korea.
Yunjeon Kim, Department of Life Sciences, Ewha Womans University, Seoul, Republic of Korea.
Eok-Soo Oh, Department of Life Sciences, Ewha Womans University, Seoul, Republic of Korea.
Literature Cited
- 1. Jean C, Gravelle P, Fournie JJ, Laurent G. Influence of stress on extracellular matrix and integrin biology. Oncogene. 2011;30(24):2697–706. [DOI] [PubMed] [Google Scholar]
- 2. Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18(5):463–71. [DOI] [PubMed] [Google Scholar]
- 3. Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol. 2015;5:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121(3):255–64. [DOI] [PubMed] [Google Scholar]
- 5. Xian X, Gopal S, Couchman JR. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2010;339(1):31–46. [DOI] [PubMed] [Google Scholar]
- 6. Walker C, Mojares E, del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int J Mol. 2018;19(10):3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates αvβ3 integrin activity in human mammary carcinoma cells. J Cell Biol. 2004;167(1):171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8(12):957–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beauvais DM, Rapraeger AC. Syndecans in tumor cell adhesion and signaling. Reprod Biol Endocrinol. 2004;2(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen B, Delaney MK, Du X. Inside-out, outside-in, and inside–outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr Opin Cell Biol. 2012;24(5):600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi Y, Chung H, Jung H, Couchman JR, Oh ES. Syndecans as cell surface receptors: unique structure equates with functional diversity. Matrix Biol. 2011;30(2):93–9. [DOI] [PubMed] [Google Scholar]
- 12. Okina E, Manon-Jensen T, Whiteford JR, Couchman JR. Syndecan proteoglycan contributions to cytoskeletal organization and contractility. Scand J Med Sci Sports. 2009;19(4):479–89. [DOI] [PubMed] [Google Scholar]
- 13. Barbouri D, Afratis N, Gialeli C, Vynios DH, Theocharis AD, Karamanos N. Syndecans as modulators and potential pharmacological targets in cancer progression. Front Oncol. 2014;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fears CY, Woods A. The role of syndecans in disease and wound healing. Matrix Biol. 2006;25(7):443–56. [DOI] [PubMed] [Google Scholar]
- 15. Januchowski R, Świerczewska M, Sterzyńska K, Wojtowicz K, Nowicki M, Zabel M. Increased expression of several collagen genes is associated with drug resistance in ovarian cancer cell lines. J Cancer. 2016;7(10):1295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shields MA, Dangi-Garimella S, Redig AJ, Munshi HG. Biochemical role of the collagen-rich tumour microenvironment in pancreatic cancer progression. Biochem J. 2012;441(2):541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Kempen LC, Rijntjes J, Mamor-Cornelissen I, Vincent-Naulleau S, Gerritsen MJP, Ruiter DJ, van Muijen GN. Type I collagen expression contributes to angiogenesis and the development of deeply invasive cutaneous melanoma. Int J Cancer. 2008;122(5):1019–29. [DOI] [PubMed] [Google Scholar]
- 18. Hall CL, Dubyk CW, Riesenberger TA, Shein D, Keller ET, van Golen KL. Type I collagen receptor (α2β1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 2008;10(8):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karousou E, D’Angelo ML, Kouvidi K, Vigetti D, Viola M, Nikitovic D, Passi A. Collagen VI and hyaluronan: the common role in breast cancer. Biomed Res Int. 2014;2014:606458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun Y, Zhao C, Ye Y, Wang Z, He Y, Li Y, Mao H. High expression of fibronectin 1 indicates poor prognosis in gastric cancer. Oncol Lett. 2020;19(1):93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kujawa KA, Zembala-Nożyńska E, Cortez AJ, Kujawa T, Kupryjańczyk J, Lisowska KM. Fibronectin and periostin as prognostic markers in ovarian cancer. Cells. 2020;9(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. You D, Jung SP, Jeong Y, Bae SY, Lee JE, Kim S. Fibronectin expression is upregulated by PI-3K/Akt activation in tamoxifen-resistant breast cancer cells. BMB Rep. 2017;50(12):615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yi W, Xiao E, Ding R, Luo P, Yang Y. High expression of fibronectin is associated with poor prognosis, cell proliferation and malignancy via the NF-κB/p53-apoptosis signaling pathway in colon cancer. Oncol Rep. 2016;36(6):3145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12):a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22(5):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Labat-Robert J, Birembaut P, Robert L, Adnet JJ. Modification of fibronectin distribution pattern in solid human tumours. Diagn Histopathol. 1981;4(4):299–306. [PubMed] [Google Scholar]
- 27. Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;90(6):H2196–203. [DOI] [PubMed] [Google Scholar]
- 28. Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8(3):175–6. [DOI] [PubMed] [Google Scholar]
- 29. Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Hammer DA. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–54. [DOI] [PubMed] [Google Scholar]
- 30. Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci. 2011;124(8):1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang S, Zhang X, Wang G, Cao B, Yang H, Jin L, Mao Y. Syndecan-1 suppresses cell growth and migration via blocking JAK1/STAT3 and Ras/Raf/MEK/ERK pathways in human colon carcinoma cells. BMC Cancer. 2019;19(1):1160. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Teng Y, Ross JL, Cowell JK. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. JAKSTAT. 2014;6:e28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ogawa T, Tsubota Y, Hashimoto J, Kariya Y, Miyazaki K. The short arm of laminin γ2 chain of laminin-5 (laminin-332) binds syndecan-1 and regulates cellular adhesion and migration by suppressing phosphorylation of integrin β4 chain. Mol Biol Cell. 2007;18(5):1621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang N, Friedl A. Syndecan-1-induced ECM fiber alignment requires integrin αvβ3 and syndecan-1 ectodomain and heparan sulfate chains. PLoS ONE. 2016;11(2):e0150132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee H, Kim Y, Choi Y, Choi S, Hong E, Oh ES. Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1. Biochem Biophys Res Commun. 2011;409(1):148–53. [DOI] [PubMed] [Google Scholar]
- 36. Choi Y, Kim H, Chung H, Hwang JS, Shin JA, Han IO, Oh ES. Syndecan-2 regulates cell migration in colon cancer cells through Tiam1-mediated Rac activation. Biochem Biophys Res Commun. 2010;391(1):921–5. [DOI] [PubMed] [Google Scholar]
- 37. Klass CM, Couchman JR, Woods A. Control of extracellular matrix assembly by syndecan-2 proteoglycan. J Cell. 2000;113(3):493–506. [DOI] [PubMed] [Google Scholar]
- 38. Choi S, Kim Y, Park H, Han IO, Chung E, Lee SY, Yi JY. Syndecan-2 overexpression regulates adhesion and migration through cooperation with integrin α2. Biochem Biophys Res Commun. 2009;384(2):231–5. [DOI] [PubMed] [Google Scholar]
- 39. Kwon MJ, Kim Y, Choi Y, Kim SH, Park S, Han I, Oh ES. The extracellular domain of syndecan-2 regulates the interaction of HCT116 human colon carcinoma cells with fibronectin. Biochem Biophys Res Commun. 2013;31(3):415–20. [DOI] [PubMed] [Google Scholar]
- 40. Ilić D, Kovačič B, Johkura K, Schlaepfer DD, Tomašević N, Han Q, Streblow DN. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci. 2004;117(2):177–87. [DOI] [PubMed] [Google Scholar]
- 41. Wierzbicka-Patynowski I, Schwarzbauer JE. Regulatory role for SRC and phosphatidylinositol 3-kinase in initiation of fibronectin matrix assembly. J Biol Chem. 2002;277(22):19703–8. [DOI] [PubMed] [Google Scholar]
- 42. Midwood KS, Mao Y, Hsia HC, Valenick LV, Schwarzbauer JE. Modulation of cell–fibronectin matrix interactions during tissue repair. J Investig Dermatol Symp Proc. 2006;11(1):73–8. [DOI] [PubMed] [Google Scholar]
- 43. Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, Humphries MJ. Syndecan-4–dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol. 2007;177(3):527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang CP, Cheng CM, Su HL, Lin YW. Syndecan-4 promotes epithelial tumor cells spreading and regulates the turnover of PKCα Activity under mechanical stimulation on the elastomeric substrates. Cell Physiol Biochem. 2015;36(4):1291–304. [DOI] [PubMed] [Google Scholar]
- 45. Hu W, Wei X, Zhu L, Yin D, Wei A, Bi X, Wen Z. Enhancing proliferation and migration of fibroblast cells by electric stimulation based on triboelectric nanogenerator. Nano Energy. 2019;57:600–7. [Google Scholar]
- 46. Maeda T, Alexander CM, Friedl A. Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Res. 2004;64(2):612–21. [DOI] [PubMed] [Google Scholar]
- 47. Mundhenke C, Meyer K, Drew S, Friedl A. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 receptor binding in breast carcinomas. Am J Pathol. 2002;160(1):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Máthé M, Suba Z, Németh Z, Tátrai P, Füle T, Borgulya G, Kovalszky I. Stromal syndecan-1 expression is an adverse prognostic factor in oral carcinomas. Oral Oncol. 2006;42(5):493–500. [DOI] [PubMed] [Google Scholar]
- 49. Oh JH, Kim JH, Ahn HJ, Yoon JH, Yoo SC, Choi DS, Min CK. Syndecan-1 enhances the endometrial cancer invasion by modulating matrix metalloproteinase-9 expression through nuclear factor κB. Gynecol Oncol. 2009;114(3):509–15. [DOI] [PubMed] [Google Scholar]
- 50. Asuthkar S, Velpula KK, Nalla AK, Gogineni VR, Gondi CS, Rao JS. Irradiation-induced angiogenesis is associated with an MMP-9-miR-494-syndecan-1 regulatory loop in medulloblastoma cells. Oncogene. 2014;33(15):1922–33. [DOI] [PubMed] [Google Scholar]
- 51. Kaushal GP, Xiong X, Athota AB, Rozypal TL, Sanderson RD, Kelly T. Syndecan-1 expression suppresses the level of myeloma matrix metalloproteinase-9. Br J Haematol. 1999;104(2):365–73. [DOI] [PubMed] [Google Scholar]
- 52. Jang B, Jung H, Choi S, Lee YH, Lee ST, Oh ES. Syndecan-2 cytoplasmic domain up-regulates matrix metalloproteinase-7 expression via the protein kinase Cγ–mediated FAK/ERK signaling pathway in colon cancer. J Biol Chem. 2017;292(39):16321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Afratis NA, Nikitovic D, Multhaupt HA, Theocharis AD, Couchman JR, Karamanos NK. Syndecans–key regulators of cell signaling and biological functions. FEBS J. 2017;284(1):27–41. [DOI] [PubMed] [Google Scholar]
- 54. Yu WH, Woessner JF. Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7). J Biol Chem. 2000;275(6):4183–91. [DOI] [PubMed] [Google Scholar]
- 55. Su G, Blaine SA, Qiao D, Friedl A. Shedding of syndecan-1 by stromal fibroblasts stimulates human breast cancer cell proliferation via FGF2 activation. J Biol Chem. 2007;282(20):14906–15. [DOI] [PubMed] [Google Scholar]
- 56. Wang X, Zuo D, Chen Y, Li W, Liu R, He Y, Ba Y. Shed syndecan-1 is involved in chemotherapy resistance via the EGFR pathway in colon cancer. Br J Cancer. 2014;111(10):1965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sanderson RD, Yang Y. Syndecan-1: a dynamic regulator of the myeloma microenvironment. Clin Exp Metastasis. 2008;25(2):149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ryu HY, Lee J, Yang S, Park H, Choi S, Jung KC, Oh ES. Syndecan-2 functions as a docking receptor for pro-matrix metalloproteinase-7 in human colon cancer cells. J Biol Chem. 2009;284(51):35692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jang B, Yun JH, Choi S, Park J, Shin DH, Lee ST, Oh ES. Tyrosine 51 residue of the syndecan-2 extracellular domain is involved in the interaction with and activation of pro-matrix metalloproteinase-7. Sci Rep. 2019;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Munesue S, Yoshitomi Y, Koyama Y, Kiyono S, Oguri K, Nakanishi H, Okayama M. A novel function of syndecan-2, suppression of matrix metalloproteinase-2 activation, which causes suppression of metastasis. J Biol Chem. 2007;282(38):28164–74. [DOI] [PubMed] [Google Scholar]
- 61. Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, Pap T. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15(9):1072–6. [DOI] [PubMed] [Google Scholar]
- 62. Kim EY, Roshanravan H, Dryer SE. Syndecan-4 ectodomain evokes mobilization of podocyte TRPC6 channels and their associated pathways: an essential role for integrin signaling. Biochim Biophys Acta. 2015;1853(10):2610–20. [DOI] [PubMed] [Google Scholar]
- 63. Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278(42):40764–70. [DOI] [PubMed] [Google Scholar]
- 64. Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111(5):635–46. [DOI] [PubMed] [Google Scholar]
- 65. Ramani VC, Pruett PS, Thompson CA, DeLucas LD, Sanderson RD. Heparan sulfate chains of syndecan-1 regulate ectodomain shedding. J Biol Chem. 2012;287(13):9952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Choi S, Kim JY, Park JH, Lee ST, Han IO, Oh ES. The matrix metalloproteinase-7 regulates the extracellular shedding of syndecan-2 from colon cancer cells. Biochem Biophys Res Commun. 2012;417(4):1260–4. [DOI] [PubMed] [Google Scholar]
- 67. Lee YH, Park JH, Cheon DH, Kim T, Park YE, Oh ES, Lee ST. Processing of syndecan-2 by matrix metalloproteinase-14 and effect of its cleavage on VEGF-induced tube formation of HUVECs. Biochem J. 2017;474(22):3719–32. [DOI] [PubMed] [Google Scholar]
- 68. Brule S, Charnaux N, Sutton A, Ledoux D, Chaigneau T, Saffar L, Gattegno L. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology. 2006;16(6):488–501. [DOI] [PubMed] [Google Scholar]
- 69. Strand ME, Aronsen JM, Braathen B, Sjaastad I, Kvaløy H, Tønnessen T, Lunde IG. Shedding of syndecan-4 promotes immune cell recruitment and mitigates cardiac dysfunction after lipopolysaccharide challenge in mice. J Mol Cell Cardiol. 2015;88:133–44. [DOI] [PubMed] [Google Scholar]
- 70. Su G, Blaine SA, Qiao D, Friedl A. Membrane type 1 matrix metalloproteinase–mediated stromal syndecan-1 shedding stimulates breast carcinoma cell proliferation. Cancer Res. 2008;68(22):9558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Malek-Hosseini Z, Jelodar S, Talei A, Ghaderi A, Doroudchi M. Elevated syndecan-1 levels in the sera of patients with breast cancer correlate with tumor size. Breast Cancer. 2017;24(6):742–7. [DOI] [PubMed] [Google Scholar]
- 72. Jung O, Trapp-Stamborski V, Purushothaman A, Jin H, Wang H, Sanderson RD, Rapraeger AC. Heparanase-induced shedding of syndecan-1/CD138 in myeloma and endothelial cells activates VEGFR2 and an invasive phenotype: prevention by novel synstatins. Oncogenesis. 2016;5(2):e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ramani VC, Purushothaman A, Stewart MD, Thompson CA, Vlodavsky I, Au JLS, Sanderson RD. The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. FEBS J. 2013;280(10):2294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gomes AM, Stelling MP, Pavão MS. Heparan sulfate and heparanase as modulators of breast cancer progression. Biomed Res Int. 2013;2013:852093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Purushothaman A, Chen L, Yang Y, Sanderson RD. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J Cell Biol. 2008;283(47):32628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. 2010;115(12):2449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jang B, Jung H, Chung H, Moon BI, Oh ES. Syndecan-2 enhances E-cadherin shedding and fibroblast-like morphological changes by inducing MMP-7 expression in colon cancer cells. Biochem Biophys Res Commun. 2016;477(1):47–53. [DOI] [PubMed] [Google Scholar]
- 78. Row S, Liu Y, Alimperti S, Agarwal SK, Andreadis ST. Cadherin-11 is a novel regulator of extracellular matrix synthesis and tissue mechanics. J Cell Sci. 2016;129(15):2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bates RC, Mercurio A. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005;4(4):371–6. [DOI] [PubMed] [Google Scholar]
- 80. Choi S, Choi Y, Jun E, Kim IS, Kim SE, Jung SA, Oh ES. Shed syndecan-2 enhances tumorigenic activities of colon cancer cells. Oncotarget. 2015;6(6):3874–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fears CY, Gladson CL, Woods A. Syndecan-2 is expressed in the microvasculature of gliomas and regulates angiogenic processes in microvascular endothelial cells. J Cell Biol. 2006;281(21):14533–6. [DOI] [PubMed] [Google Scholar]
- 82. De Rossi G, Evans AR, Kay E, Woodfin A, McKay TR, Nourshargh S, Whiteford JR. Shed syndecan-2 inhibits angiogenesis. J Cell Sci. 2014;127(21):4788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Essner JJ, Chen E, Ekker SC. Syndecan-2. Int J Biochem Cell Biol. 2006;38(2):152–6. [DOI] [PubMed] [Google Scholar]
- 84. Vicente CM, Ricci R, Nader HB, Toma L. Syndecan-2 is upregulated in colon cancer cells through interactions with extracellular matrix produced by stromal fibroblasts. BMC Cell Biol. 2013;14(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]