Abstract
Inter-α-trypsin inhibitor (IαI) family members are ancient and unique molecules that have evolved over several hundred million years of vertebrate evolution. IαI is a complex containing the proteoglycan bikunin to which heavy chain proteins are covalently attached to the chondroitin sulfate chain. Besides its matrix protective activity through protease inhibitory action, IαI family members interact with extracellular matrix molecules and most notably hyaluronan, inhibit complement, and provide cell regulatory functions. Recent evidence for the diverse roles of the IαI family in both biology and pathology is reviewed and gives insight into their pivotal roles in tissue homeostasis. In addition, the clinical uses of these molecules are explored, such as in the treatment of inflammatory conditions including sepsis and Kawasaki disease, which has recently been associated with severe acute respiratory syndrome coronavirus 2 infection in children:
Keywords: bikunin, chondroitin sulfate, extracellular matrix, glycosaminoglycan, heavy chain, inter-α inhibitor proteins, inter-α-trypsin inhibitor, proteoglycan, TSG-6
Introduction
Since its discovery in the 1960s, the precise functional role of inter-α-trypsin inhibitor (IαI) has been a bit of a mystery as there are no known IαI deficiency syndromes and probably due to its critical role in female reproductive biology.1–3 Despite displaying serine protease inhibitory activity,4 the need for this activity in serum was unclear given that serum contains an excess of serine protease inhibitory capacity, suggesting that IαI was a redundant serum protease inhibitor. More recent studies on IαI have revealed its localization in many tissues with diverse roles in cell regulation and matrix integrity (Table 1) in both health and disease.5,6 Although the liver is recognized as the main source of IαI,7–12 many other tissues synthesize IαI family components, including the kidney,13 reproductive tissues,14 lung,15 connective tissues,16–18 and central nervous system19,20 (Table 2). Much research focus has been placed on the roles of IαI in pathology, and notably in inflammation, whereas its physiological roles, apart from those in ovulation, are less explored. Recent evidence for the diverse roles of IαI in both biology and pathology is reviewed and gives insight into the pivotal roles this unusual molecule plays in tissue homeostasis and the emerging roles in diagnostics and therapeutics.
Table 1.
Roles of Bikunin, HCs, and the IαI Family.
| IαI Family | Bikunin | HCs |
|---|---|---|
| ■ Stabilizes HA via crosslinking with HCs ■ Controls neutrophil activation ■ Plasmin inhibitor ■ Inhibits complement ■ Inhibits hyaluronidase |
■ Serine protease inhibitor ■ Inhibits cell migration/invasion ■ Decreases cell proliferation ■ Disrupts growth factor signaling ■ Inhibits cytokine release ■ Inhibits calcium channel–dependent signaling ■ Inhibits calcium oxalate crystallization |
■ Bind extracellular matrix components such as
vitronectin ■ Inhibit complement |
Abbreviations: HA, hyaluronan; HCs, heavy chains; IαI, inter-α-trypsin inhibitor.
Table 2.
Summary of Expression and Localization of IαI Family Components.
| Tissue | IαI Family Localization | IαI Family Component Localization | IαI Family Gene Expression |
|---|---|---|---|
| Liver | IαI: human hepatocytes10 | HCs: mouse hepatocytes and Kupffer cells21 | Bikunin: mouse22 and human tissue23,24 |
| Pancreas | Bikunin: human tissue23 | Bikunin: human tissue23–25 | |
| Kidney | PαI: human proximal tubular epithelial cells11 | HCs: mouse proximal tubule epithelial cells21 | Bikunin and HC3: human proximal tubular epithelial cells11 |
| Reproductive tissues | IαI and PαI: human amniotic membrane12 | HCs: mouse theca and stromal cells surrounding mature follicles,
follicular fluid, and the cumulus cell surface during ovulation21
Bikunin and HC1-3: human amniotic membrane12 |
Bikunin and HC1-3: human amniotic membrane12 |
| Lung | Bikunin·and HC5: human lung fibroblasts13 | HCs: human bronchiolar epithelial cells6,21,26
Bikunin and HC1: human lung-resident mast and polymorphonuclear cells6,26 |
Bikunin and HC1-3: human lung tissue6
HC3-5: human lung fibroblasts13 |
| Connective tissues | IαI: human cartilage14
Bikunin/HCs: ovine stifle joint articular cartilage15 IαI: canine intervertebral disk16 |
Bikunin/HCs: human cartilage14 and intervertebral disk27
HCs: mouse growth plate21 |
HC1-2: human cartilage tissue14 |
| Skin | Bikunin: human keratinocyte cells28
Bikunin, HC1, and HC2: human tissue keratinocytes28,29 HCs: mouse tissue keratinocytes21 |
Bikunin: human keratinocyte cells28,30 | |
| Central nervous system | IαI and PαI: human adult brain,17 ovine cerebrospinal fluid, and cerebral cortex during development18 | Bikunin/HCs: human fetal and adult cerebral cortex localized to
neurons and astrocytes18
HCs: mouse nerves21 Bikunin/HCs: immunolocalized intracellularly to neurons, microglial cells, and astrocytes isolated from embryonic mouse cerebral cortex31 |
Bikunin and HC1-5: cultured neurons from embryonic mouse
cerebral cortex31
Bikunin: rat hippocampus, cerebral cortex, and pitutiary32 HC2-3: adult mouse brain33 |
Abbreviations: HA, hyaluronan; HCs, heavy chains; IαI, inter-α-trypsin inhibitor; PαI, pre-α-inhibitor.
Overview of IαI
Structure of IαI
IαI is an approximately 225-kDa complex containing bikunin and two heavy chain (HC) proteins, designated HC1 and HC2 (Fig. 1).34 IαI is present in many fluid compartments including blood, peritoneal, amniotic, cerebrospinal, and synovial fluids, whereas bikunin is also excreted in the urine.23,28,35 IαI is also present in many tissues including the liver, pancreas, kidneys, ovary, amniotic membrane, lung, connective tissues, skin, and brain, suggesting broad physiological roles.22,24,26,30,31,36–38
Figure 1.
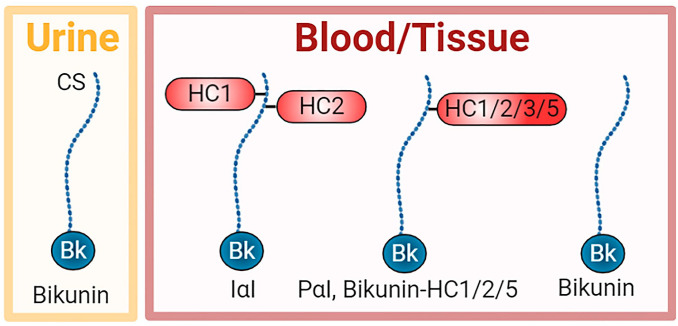
Schematic of members of the IαI family in urine, blood, and tissues. Bikunin is found alone in the urine, whereas it is also found in the blood and tissues either alone or complexed with one HC, including HC1, HC2, HC3, or HC5. HC3 covalently bound to bikunin is called pre-α-inhibitor (PαI). In addition, the predominant form is IαI, a complex of HC1 and HC2 covalently bound to the CS chain of bikunin. Abbreviations: CS, chondroitin sulfate; HC, heavy chain; IαI, inter-α-trypsin inhibitor.
Bikunin is a proteoglycan with a chondroitin sulfate (CS) chain attached to the protein core of approximately 20 kDa. HC1 and HC2 are similar in size at approximately 85 kDa. Unusually, these HCs are covalently attached to the CS chain. HC1 and HC2 are located close together on the CS chain with HC2 positioned closer to the bikunin core protein.39 Bikunin·HC1 and bikunin·HC2 conjugates have also been detected in the circulation and tissues, whereas bikunin alone has been detected in both the circulation and urine.40,41 A related structure is pre-α-inhibitor (PαI) which is a complex of bikunin with HC3 (~90 kDa) covalently attached to the CS chain in a similar way as HC1 and HC2. More recently, bikunin complexed with HC5 has also been reported (Fig. 1).15
The biosynthesis of IαI is unusual with regulation at many levels, including protein synthesis of three discrete proproteins, CS chain biosynthesis, proprotein cleavage, and assembly of the complex. The AMBP gene encodes both α1-microglobulin and bikunin. The α1-microglobulin/bikunin proprotein is posttranslationally modified on serine-10 of the bikunin protein core with a CS chain via well-established biosynthetic pathways.42,43 This is followed by the covalent attachment of HC1 and HC2. These HCs are transcribed by the ITIH1 and ITIH2 genes and their C-terminal prodomains cleaved by furin before covalent attachment to the bikunin CS chain via ester bonds between the C-terminal aspartic acid residues in the HCs and C-6 hydroxyls of internal N-acetyl galactosamine (GalNAc) residues in the CS chain in the trans Golgi.42,44–49 However, the mechanisms controlling the covalent attachment of both the number and type of HCs to the CS chain remain to be elucidated. The last step before secretion involves cleavage between α1-microglobulin and bikunin by an unknown enzyme, but hypothesized to be a furin-like protease.50,51 Although the transcription of the α1-microglobulin/bikunin proprotein is unusual, there is evidence that the coexpression of α1-microglobulin provides a chaperone role in the correct folding of bikunin.52
Little is known about the factors that promote the expression of IαI components; however, they are thought to possess tissue-specific regulation pathways.53 Regulation of ambp gene expression in the liver involves the transcription factor binding to the 5′ flanking region of the gene54; however, such regulation has not been explored in other tissues. In addition, ambp gene expression in the liver can be regulated by glutamine and cell swelling,55 as well as interleukin (IL)-6, leukemia inhibitory factor, and retinoic acid, and potentiated by dexamethasone in rat hepatocytes.56 Furthermore, IL-6 can increase bikunin expression in macrophages.57 The gene expression of IαI components in acute inflammatory states is not regulated in a coordinated way, as only the HC3 gene is upregulated, whereas the bikunin and HC2 genes are downregulated and the HC1 gene remains unaffected.58
Early structural analysis of the CS chain indicated that it contained both unsulfated and 4-sulfated CS disaccharides, with the sulfated disaccharides concentrated at the reducing end.39,59 More recently, with the advent of different purification protocols and sources of bikunin, it was discovered that the CS chain is variable in both chain length and degree of sulfation and can contain unsulfated, monosulfated, and disulfated disaccharides.39,60–62 In addition, the linkage region can be variably sulfated.59,62–69
Roles of IαI
The most widely acknowledged role of the IαI family members is the transesterification of HCs from IαI to hyaluronan (HA) in the presence of tumor necrosis factor–stimulated gene-6 (TSG-6), a process that results in the covalent modification of HA with HCs (HA·HC)70–72 and recently reviewed in detail.73 This process is conserved across mammals, birds, and reptiles.74 It requires that HCs are present as part of the IαI family members for the transfer to HA, because HCs in the bikunin knockout mouse are present in their precursor form, but are unable to complex with HA.2 It has been suggested that the energy for this reaction is contained within the ester bond between the HC and CS formed during biosynthesis.71 During the transesterification process, the HCs become covalently attached to HA via an ester bond from their C-termini to the C-6 hydroxylated GlcNAc residues in HA, and thus analogous to the linkage to CS in IαI.41,75,76 The transesterification process requires the presence of TSG-6 and divalent cations.71,73,77–80 Self-association of HCs bound to HA likely enables crosslinking via a cation-dependent mechanism.81,82 This crosslinking is reported to be dynamic with TSG-6 able to reversibly transfer HCs to bikunin and between HA chains when present in serum whereby high-molecular-weight HA can act as both an acceptor and a donor of HCs.83–85 In addition, HA oligosaccharides can accept HCs,86,87 and there is also evidence that they do not donate them.85 Although the function of HA oligosaccharide·HC complexes has not been reported, high-molecular-weight HA·HC complexes provide anti-inflammatory properties, including polarization of macrophages to the anti-inflammatory M2 phenotype.88 When the HA·HC complexes were synthesized either in vivo or in vitro by cells in culture, they could support leucocyte adhesion89,90; however, when these complexes were synthesized in vitro using isolated components, they did not support the adhesion of CD44+ cells,91 suggesting that the formation of these HA·HC complexes in more complex biological fluids involved additional components that together mediated cell adhesion. In addition, pentraxin-3 (PTX3) is essential for the formation of a crosslinked HA network in the cumulus cell oocyte complex92–94 where it crosslinks HA·HC complexes through its interactions with the HCs.95 This suggests that distinct tissue environments may contain HA·HC complexes with different associated molecular components and possibly even distinct functions.
The CS chain attached to bikunin is essential for the transesterification of its pendant HCs to HA in the formation of HA·HC.46,80 In addition, the structure of the CS chain modulates the extent of HA·HC formation, with more highly sulfated CS chains supporting a greater extent of HA·HC formation than lower sulfated CS,61 suggesting a mechanism for control of this process.
Other lesser explored roles of IαI include its ability to bind multiple extracellular matrix components, support stem cell expansion, control neutrophil activation, inhibit complement, and impart protease and hyaluronidase inhibitory activity. For example, transglutaminases can catalyze the covalent attachment of IαI onto fibrinogen and also crosslink proteins in a plasma clot.96 IαI interacts with molecules that act as integrin ligands such as fibronectin and vitronectin, and the recent crystal structure of HC1 has revealed that HCs resemble integrin β-chains containing a von Willebrand Factor A domain with a metal ion–dependent adhesion site (MIDAS) motif and an associated hybrid domain.81 However, in the case of HC1, surprisingly the interaction with fibronectin and vitronectin is not mediated by the von Willebrand Factor A domain, is not metal-dependent, and does not involve the Arg-Gly-Asp (RGD) motifs of these ligands.81 IαI binding to vitronectin promotes epithelial adhesion, migration, and proliferation.97 In addition, IαI supplementation of the defined medium supports the expansion and long-term maintenance of pluripotent stem cells, circumventing the need for the use of ill-defined preparations such as Matrigel or immobilization of cell adhesive proteins/peptides such as vitronectin.98 IαI also controls neutrophil activation via a reduction in both reactive oxygen species production and adhesion to vascular endothelial cells.99 IαI inhibits activation of the complement system, including the classical, lectin, and alternative complement pathways and specifically the processing of factor B that forms part of the alternative pathway C3 convertase.100,101 Whereas bikunin is known to possess protease inhibitory activity, IαI can inhibit plasmin and this effect can be potentiated by binding to TSG-6, which is further enhanced by the interaction of TSG-6 with heparin and heparan sulfate.102,103 In addition, IαI family members, in particular PαI, may have hyaluronidase inhibitory activity.104
Roles of Bikunin
Bikunin is involved in a wide variety of processes as demonstrated by gene analysis of the bikunin knockout mice which revealed that in the absence of bikunin there was a dysregulation of genes associated with stress, apoptosis, proteases, signaling molecules, aging, cytokines, HA metabolism, and female fertility.2,3,105 Mice deficient in bikunin exhibit reduced female fertility,2,3 although the applicability of these findings to humans has not been established. In addition, these mice exhibit a higher frequency of spontaneous lung metastasis,106 which correlates with the low level of bikunin in the urine of patients with some cancers.107,108 Mice deficient in bikunin also display an increased anxiety-like behavior.109 Interestingly, overexpression of bikunin has not been reported or associated with human pathology.
The earliest role of bikunin was ascribed to its two Künitz domains that possess protease inhibitory properties against several serine proteases, including leucocyte elastase and cathepsin G, pancreatic trypsin and chymotrypsin, plasmin, plasma and tissue kallikrein, and some of the coagulation cascade proteinases.110–113 This antiprotease activity is thought to support the growth of endothelial cells and fibroblasts.7,114 Interestingly, bikunin itself can be cleaved within the Künitz domains by mesotrypsin.115
Bikunin is also recognized to exhibit a range of cell signaling functions mediated at the cell surface via interactions with cartilage link protein (HAPLN1), an HA binding protein, and an as-yet-unidentified protein named the bikunin receptor.5,116 Notably, bikunin inhibits cell invasion via inhibition of transforming growth factor (TGF)-β1 expression, which in turn inhibits selected mitogen-activated protein (MAP) kinase signaling pathways including Src-, MEK-, and ERK-dependent urokinase-type plasminogen activator (uPA) and uPA receptor expression.117–121 Bikunin can reduce cell proliferation via disrupting the heterodimerization of CD44 with growth factors, resulting in the suppression of receptor-mediated MAP kinase signaling.122 In addition, bikunin can inhibit the dimerization of CD44 v9-containing isoforms, which in turn inhibits cell interactions with HA and consequently CD44/HA-mediated activation of MAP kinase signaling.123 Bikunin can also inhibit cytokine release and nuclear translocation of nuclear factor-κB to inhibit apoptosis.124–127 Bikunin blocks calcium channels, inhibiting both the contraction of vascular smooth muscle cells and the calcium-dependent TGF-β1 signaling cascade.121,128,129 Although bikunin itself can be upregulated via IL-6 such as macrophages, it can inhibit the production of proinflammatory cytokines.48,57,130 In addition, bikunin can inhibit the formation of calcium oxalate crystals.131
Roles of the Heavy Chains
Interestingly, six HCs have been reported with differential expression in tissue development and pathology.132,133 The roles of these HCs have not been established by mouse knockout studies; however, HC1-5 gene expression is reduced in multiple human solid tumors including breast, lung, and kidney,25 suggesting a role for these proteins in tissue homeostasis.
HC1-3 is synthesized with C-terminal prodomains that are cleaved before their covalent attachment to the bikunin CS chain.34,44,46,47 HC4 does not contain the conserved cleavage site present in HC1-3, suggesting that HC4 attachment to either the bikunin CS chain or HA is unlikely.15,34 HC5 can covalently bind to the CS chain on bikunin, whereas HC6 has only been identified at the gene level.15,134
HCs can interact with molecules such as vitronectin and fibronectin, suggesting a more diverse role in extracellular matrix interactions beyond HA.81,97 HC1 binds to vitronectin in vitro,81 and IaI potentiates cell adhesion to immobilized vitronectin.97,135 In addition, IaI supports vitronectin-mediated epithelial cell repair in an injury model involving cell adhesion, migration, and proliferation.97 These findings suggest that HCs interact with proteins such as vitronectin and fibronectin in the extracellular space and may act as a linker to HA for broader interactions in the extracellular matrix to influence the biological activity of these molecules. However, whether these interactions functionally involve HCs in IαI family complexes, bound to HA or alone, remains to be elucidated. Recently, HC1 has been shown to bind directly to complement C3 via a MIDAS and inhibit the activity of the alternative pathway C3 convertase, which has a central role in the amplification of this component of innate immunity.81
Roles of IαI in Biology
Components of the IαI family have been localized in many tissues such as the liver, kidney, pancreas, skin, lung, ovary, amniotic membrane, central nervous system, and connective tissues (Table 2), as well as multiple fluid compartments—most notably, blood.22–24,26,28,30,31,35–38 However, the presence of these components in IαI family complexes remains to be explored in some tissues as do their tissue-specific roles during homeostasis. HA matrix crosslinking roles for IαI family members have been explored during ovulation, in the amniotic membrane and gut asymmetry during intestinal morphogenesis.14,92,93,136 However, many other potential roles of these molecules in more broad extracellular matrix interactions, control of cell signaling, and matrix degradation (Table 1) remain to be explored and will likely reveal a myriad of roles of these ubiquitous molecules. This is particularly the case for tissues such as the pancreas, kidney, lung, connective tissues, skin, and central nervous system described below where IαI family member complexes and/or components have been reported without further exploration of their tissue-specific functions during homeostasis.
Liver, Pancreas, and Kidney
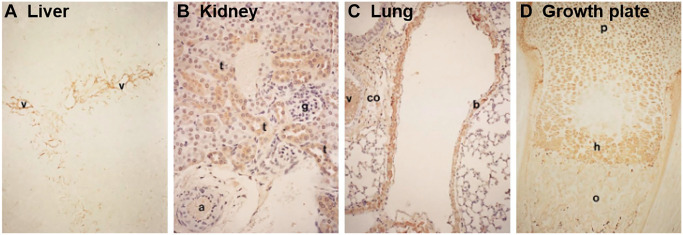
The liver constitutively produces IαI where it is passed into the circulation ready to infiltrate into tissues to exert its functions.7–11 HCs have been immunolocalized to both hepatocytes and Kupffer cells21 (Fig. 2A), and the liver is found to predominantly secrete IαI at the protein level.12 The high expression of the ambp gene in the liver during mouse development also suggests that the liver is the predominant source of IαI.13 Both the human liver and pancreas express the bikunin gene, whereas bikunin at the protein level is also present in the human pancreas.25,30,31 In addition, isolated mouse β-islet cells express genes for bikunin as well as HC1 and HC2, and these IαI components have been colocalized with both HA and TSG-6.137 TSG-6 is constitutively expressed by β-islet cells, suggesting it is possible that HA·HC forms a part of the normal islet matrix. The kidney, and specifically the proximal tubule epithelial cells, also constitutively expresses bikunin at both the gene and protein levels with predominantly bikunin·HC formed, and although the type of HC in this complex has not been determined, there is evidence that these cells express HC3 at the gene level.13,138,139 In addition, HCs have been immunolocalized to these cells (Fig. 2B).21
Figure 2.
Immunolocalization of heavy chains (HCs) in adult mouse tissues: (A) liver, (B) kidney, (C) lung, and (D) growth plate. HCs were detected via immunohistochemistry using a polyclonal antibody raised against human serum inter-α-trypsin inhibitor and reactivity to mouse HCs, and specific staining is shown in brown. Annotations for tissue features include the following: a, artery; b, bronchiolar epithelial cells; co, connective tissue; g, glomerulus; h, hypertrophic zone; o, ossification; p, proliferative zone; t, proximal convoluted tubular cells; v, vessel. Images shown at 100× magnification. Reprinted with permission from Kobayashi et al.21
Reproductive Tissues
IαI components are localized in the ovary, including in the theca and stromal cells surrounding mature follicles as well as the follicular fluid, and on the cumulus cell surface during ovulation, where IαI required for ovulation is derived from the circulation.3,21,140 Just before ovulation an HA-rich matrix forms around the oocyte, driving expansion of the cumulus cell oocyte complex; this “cumulus” matrix is stabilized by HA·HC complexes involving the transesterification of HCs from IαI and PαI onto HA catalyzed via TSG-6, where PTX3 is essential for the formation of a crosslinked HA network.92–94 Here, PTX3 directly crosslinks HA·HC complexes through its interactions with the HCs; however, the molecular details of how the formation of these higher order HA·HC/PTX3 complexes is regulated are not yet fully understood.95 Weaker interactions between HCs are also likely to contribute to the stabilization of the cumulus matrix.81 The deposition and stabilization of HA around oocytes are necessary for fertilization. This has been demonstrated by reduced fertility in female mice deficient in bikunin, PTX3, or TSG-6 and associated with defects in the formation of HA·HC complexes and crosslinking by PTX3.2,3,70,93,141 Furthermore, these defects were rescued by the exogenous addition of each of the molecules that were knocked out.2,70,93 In addition, this HA matrix is soft, but elastic, and thought to assist in both oocyte transport in the oviduct and sperm capture.142
Bikunin and HC1-3 have been immunolocalized to the amniotic membrane epithelium and stromal cells/matrix and colocalized with both HA and TSG-6.14 In addition, analysis of the tissue revealed the formation of IαI and PαI complexes, whereas gene-level analysis of isolated amniotic membrane epithelial and stromal cells revealed that these cells expressed the genes for bikunin and HC1-3, consistent with the local expression of IαI and PαI.14 TSG-6 is also constitutively expressed in the amniotic membrane, suggesting that the HA·HC/PTX3 complexes found in this tissue28 may be synthesized from endogenous components. Interestingly, only HC1 is reported to be present in the complexes, which is surprising given our current understanding of the biochemistry of HA·HC formation.14 This matrix is reported to exert anti-inflammatory and anti-scarring properties via control of cell signaling processes.143 Bikunin is also involved in the early phase of pregnancy in the conceptus attachment to the uterine luminal surface.144
Lung
HCs have been immunolocalized to the bronchiolar epithelium (Fig. 2C).8,38,21 In addition, HC2, HC3, and bikunin have been localized to lung tissue–resident polymorphonuclear cells, whereas both HC1 and bikunin have been localized to lung tissue–resident mast cells.8,38 In addition, the human mast cell line, HMC-1, expresses the bikunin gene, suggesting that mast cells have the potential to secrete bikunin.26 Recently, human primary lung fibroblasts were reported to express the genes for HC3-5 as well as both bikunin and HC5 at the protein level in a complex that was sensitive to chondroitinase ABC digestion consistent with HC5 covalently bound to the bikunin CS chain.15 HC5·HA complexes were also found to be synthesized by these cells in the presence of TSG-6 following stimulation with TGFβ1 and were linked to the phenotypic change of fibroblasts to myofibroblasts.15 These data suggest a role for IαI family members, and HA·HC complexes, in normal wound healing.
Connective Tissues
HCs have been localized to the lacunae of chondrocytes in the hypertrophic zone of the mouse growth plate with an identical localization pattern as HA (Fig. 2D).21 This might suggest a matrix crosslinking role during endochondral ossification.126,145 In addition, HCs have also been immunolocalized to chondrocytes located between the superficial and middle zones in the mouse, whereas HC1 and HC2 were present in adult human cartilage and immunolocalized to the lacunae of chondrocytes in the superficial zone as well as the cartilage surface.16,21,27 HC1 and HC2 genes are expressed in human cartilage, whereas the expression of bikunin gene was absent.16 IαI was detected at the protein level in these extracts, and it was suggested that bikunin and IαI present in the tissues were derived from the circulation and enter the tissue via the synovial fluid.16 HCs/bikunin has been immunolocalized throughout the matrix of human intravertebral disk,27 whereas canine intravertebral disk tissue extracts contain IαI.18 More recently, bikunin has been identified in ovine stifle joint articular cartilage extracts in a complex of ~120 kDa consistent with the presence of bikunin·HC complexes, although the type of HC present was not reported.17
Skin
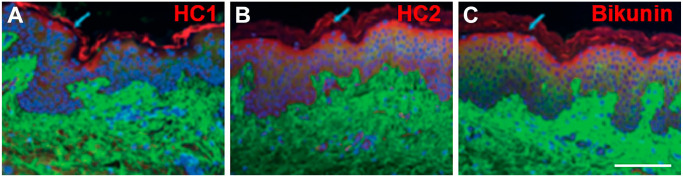
Components of IαI have been localized to keratinocytes in the epidermis,21,24 with HC1, HC2, and bikunin shown to colocalize in this region (Fig. 3).29 HC1, HC2, and bikunin have also been localized to the melanocytes and Langerhans cells in the epidermis, as well as fibroblasts, surrounding blood vessels, and perivascular lymphocytic cells in the dermis.29 In addition, bikunin is expressed on the cell membrane of outer root sheath cells and hair bulb cells.24,29 Analysis of human keratinocytes also confirmed that these cells express bikunin at both the gene and protein levels.24,26 In addition, gene expression of HC5 has been identified in human dermal fibroblasts and the dermis.133 The constitutive expression of TSG-6 in skin may suggest a role for these molecules in HA matrix crosslinking; however, the presence of IαI family complexes required to take part in the process remains to be established.29,146 HA·HC complexes are, however, likely present in normal skin,146 supporting the presence of IαI family complexes.
Figure 3.
Immunofluorescent staining of normal human skin. Combined images are shown for hyaluronan (green; A-C), HC1 (red, A), HC2 (red, B), bikunin (red; D), and cell nuclei (blue; A-C). Scale bar, 100 µm. Reprinted with permission from Tan et al.29 Abbreviation: HC, heavy chain.
Central Nervous System
IαI components have been identified in the human fetal and adult brain and localized to neurons and astrocytes, whereas this brain tissue was also found to contain IαI and PαI complexes.19 In support of these findings, developing and adult ovine cerebral cortex and cerebrospinal fluid during development contain IαI and PαI complexes.20 HCs have been immunolocalized to nerve fibers in the mouse brain along with the gene expression of HC2 and HC3.21,33 In addition, HC3 has been identified in the rat brain, as well as gene expression of bikunin has been identified in the hippocampus, cerebral cortex, and pituitary.32,147 Cultured neurons from embryonic mouse cerebral cortex express genes for bikunin, HC1-5 with bikunin/HCs immunolocalized intracellularly to isolated neurons, microglial cells, and astrocytes in culture.22 These cultured neurons were found to contain IαI and PαI; however, their culture in the presence of serum cannot exclude this as the source of these complexes. Thus, the presence of IαI family members and their local expression indicates physiological roles such as brain development. There is evidence that neuroinflammation contributes to normal brain development, which may involve the formation of HA·HC complexes as has been reported for HC3 in the central nervous system and associated with an organized HA-rich pericellular matrix.148,149 In addition to its HA-interactive properties in the brain, IαI is an inhibitor of serine proteases which plays an important role in neuronal development and plasticity, suggesting that IαI modulates neuronal cell activities.150–152
Roles of IαI in Pathology
IαI is most often associated with inflammation where elevated levels of HA·HC complexes are detected in tissues and fluids.113,153,154 Indeed, the reported pathologies detailed below involving IαI family members exhibit inflammation, whether sterile or pathogenic forms, at some stage during the disease process. For example, islets in type 1 diabetic patients early in the disease exhibit increased amounts of IαI components associated with the peri-islet matrix containing increased levels of HA, whereas the expression of IαI components is decreased as the disease progresses and correlates with reduced HC1/HC2 expression in a mouse model of type 1 diabetes.155,156 The increased formation of HA·HC in many pathologies is correlated with increased expression of TSG-6, whereas the excretion of bikunin in the urine, in part as a by-product of HA·HC formation, is elevated in many inflammatory conditions.113,153 IαI itself is modified during inflammation where the CS chain that is biosynthesized is longer and less sulfated.63,157,158 This is hypothesized to facilitate the transfer of HCs from IαI to HA.63 Lesser explored, however, are the roles of the IαI family in cell signaling, extracellular matrix interactions, and protease inhibition in the context of pathology.
Arthritis
HA·HC complexes are found in the synovial fluid and serum of patients with rheumatoid arthritis along with the expression of TSG-6 that is absent in normal synovial fluid.154,159,160 Osteoarthritis, although classically characterized as a non-inflammatory disease, is now recognized to involve inflammation with increased expression of TSG-6, but lower levels of HA·HC complexes in the synovial fluid than in rheumatoid arthritis.154,161–163 These complexes may have positive effects in the synovial fluid by preserving the hydrodynamic properties of the tissue that are compromised via the fragmentation of HA. In addition, the HA·HC complexes likely support infiltration of leucocytes into the synovial fluid90; however it remains to be established whether this either contributes to the pathology or takes part in its resolution. In some contexts, HA·HC complexes are adhesive for naïve leucocytes,90,164–166 but not in others.95 Recently, thrombin has been shown to ablate leucocyte adhesion to HA·HC matrices via cleavage of HC1 and is also likely to act in the same way on HC3-5.167 Thrombin is elevated in the synovial fluid of patients with rheumatoid arthritis,168 suggesting that thrombin-mediated modification of HA·HC complexes may modulate leucocyte adhesion. These cleaved HC fragments have been proposed to act as competitive inhibitors to HC–HC interactions between HA·HC complexes,81 and thus thrombin could alter both the physical and biological properties of these matrices.
Although at low levels, HC1 was localized to the surface region of damaged cartilage in osteoarthritis as well as bikunin and HC2 with both interterritorial matrix and chondrocyte lacunae staining.16,163 It was recently shown that ADAMTS-5 and matrix metalloproteases can release HC2 from both IαI and HA·HC complexes, supporting the presence of truncated forms of HCs in osteoarthritic articular cartilage and synovial fluid.16,169
Fibrosis
Fibrosis is associated with myofibroblasts that fail to undergo apoptosis.170 Although HA·HC complexes can support phenotypic modulation of fibroblasts to myofibroblasts in normal wound healing,15 they may also support this phenotypic change during fibrosis. HA·HC complexes may also indirectly support fibrosis such as cystic fibrosis where these complexes support leucocyte attachment and are localized to the pulmonary vasculature and airway submucosa.171 This tissue localization of HA·HC complexes results in a decrease in HA levels in sputum, likely affecting mucus hydration, viscoelasticity, and the clearance of pathogens.171
Renal proximal tubular epithelial cells contribute to renal interstitial fibrosis via upregulating the secretion of the latent form of TGF-β1 with plasmin involved in its activation. IαI non-covalently binds TSG-6, and this complex exerts antiplasmin activity that controls TGF-β1 activation, which in turn modulates its expression.13 These suggest a role for IαI family members in limiting inflammation during fibrosis, and recent work has shown that HC1 binds to both the small and large latent complexes of TFG-β1-3, thus perhaps having a role in controlling the activation and bioavailability of the mature growth factor.81 Indeed, amniotic membranes composed of constitutively expressed HA·HC1/PTX3 complexes exert anti-inflammatory and antifibrotic effects via suppressing the proinflammatory activities of neutrophils and macrophages,88,172 as well as downregulating TFG-β1 expression and upregulating TGF-β3 (which counteracts TGF-β1 signaling) by fibroblasts and α-smooth muscle actin expression by myofibroblasts.28,143,173 In addition, in a mouse model of fibrotic lung injury, IαI was found to support angiogenesis and contribute to tissue repair,174 whereas HA·HC complexes derived from the amniotic membrane are antiangiogenic.175 These contrasting activities suggest that the composition of HA·HC complexes and the tissue context are important in determining the roles of IαI family members in fibrosis.
Asthma and Allergen-induced Lung Disease
Asthma is characterized by an increase in airway remodeling involving the deposition of HA in the bronchial mucosa. In a mouse model of asthma prepared via ovalbumin sensitization and challenge, the formation of HA·HC complexes stabilized the HA-rich matrix and suppressed inflammation by inhibiting tumor necrosis factor-α (TNF-α) activity through interaction with TNF receptor 1 in wild-type mice, whereas bikunin knockout mice exhibited high numbers of infiltrating neutrophils.176 Furthermore, TSG-6·HC complexes were present in bronchoalveolar lavage fluid from patients with asthma, indicating that HA·HC complexes are prevalent in asthma.113 This formation of HA·HC complexes is facilitated by the increased expression of TSG-6 where it is induced by TNF-α and IL-1β.113,177 TSG-6 also potentiates the antiplasmin activity of bikunin and inhibits tissue kallikrein, a serine protease, that acts to resolve inflammation when released from IαI.102,103,113,178 Although HA·HC complexes may provide protective effects, prevention of the formation of these complexes in the TSG-6 knockout mouse was shown to prevent airway hyperresponsiveness, suggesting that they may contribute to the pathogenesis in some lung diseases.179,180
Sepsis
Sepsis involves a systemic inflammatory response mediated by the host immune system and is associated with the release of neutrophil-derived proteases including elastase. IαI is susceptible to cleavage by elastase, releasing bikunin to exert its inhibitory activity on serine proteases.181 There is a reduction in circulating IαI in patients with sepsis which correlates with increased mortality rates, whereas administration of exogenous IαI reduced mortality.182–185 These findings correlated with bikunin knockout mice being more sensitive to lipopolysaccharide (LPS)-induced death and suggest the protective role of IαI family members.127,186 Systemic endotoxemia in a mouse model has recently been shown to induce the deposition of HCs that colocalized with intravascular HA and is thought to contribute to improved outcomes via retention of neutrophils in the liver sinusoids.187,188
Cutaneous Wound Healing and Lichen Sclerosus
The presence of HA·HC complexes in skin has been reported to be elevated after wounding and to correlate with increased TSG-6 expression, suggesting a role for IαI family members in wound healing.146 Lichen sclerosus is a chronic inflammatory skin disorder characterized by the presence of a broad hyalinized zone in the upper dermis where an increase in HA is observed.189 IαI is associated with lichen sclerosus accumulating in the superficial dermis compared with normal skin and colocalized with HA.190
Central Nervous System Injury
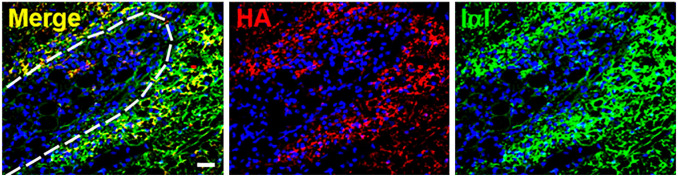
Reduced expression of IαI and PαI was observed in the brain following ischemia–reperfusion injury in sheep fetuses.191 IαI has been suggested to possess neuroprotective effects as its systemic administration immediately following neonatal cerebral hypoxic-ischemic injury in rats was found to attenuate infarct volume and reduce cell death in the cortex.192 Administration of IαI in this model has also been able to show improved working memory in adulthood.193 Bikunin is also reported to possess neuroprotective effects as administration reduced infarct volume and neutrophil infiltration in rat occlusion–reperfusion brain injury model.194 These studies suggest that IαI family members may have roles in neuroplasticity. TSG-6 is constitutively expressed by astrocytes and upregulated following injury, providing a means to form HA·HC complexes in line with the colocalization of HCs and HA in the pericellular matrix of glial scars (Fig. 4).147 The HCs themselves have been identified in disease, including HC3 being reported to be a Tau-interactive protein in human Alzheimer’s brain and the gene for HC6 identified in autism.195 Although the implication of these findings has not been explored,196 both HA and TSG-6 are increased in the brain during Alzheimer’s disease.197 In addition, genetic variants of the HCs are related to psychiatric disorders including schizophrenia and bipolar disorders, suggesting roles for the HCs themselves.198
Figure 4.
HA and IαI colocalize in the rat glial scar after injury. A spinal cord injury was created in 8- to 10-week-old female Sprague-Dawley rat using D-lysophosphatidylcholine administered to the dorsal and ventral funiculi which causes focal demyelination at the injection site. The glial scar was analyzed 15 days after injury via immunohistochemistry for the presence of HA (biotinylated HA binding protein; red), IαI (anti-IαI antibody [Dako, A0301]; green with known reactivity to HC1-3 and bikunin), and cell nuclei (DAPI; blue). Colocalization of HA and IαI immunoreactivity is shown surrounding the wound site. The dashed line indicates the wound boundary. Scale bar, 20 µm. This research was originally published in the Journal of Biological Chemistry. © The American Society for Biochemistry and Molecular Biology. Reprinted with permission from Coulson-Thomas et al.147 Abbreviations: HA, hyaluronan; IαI, inter-α-trypsin inhibitor.
Calcium Oxalate Nephrolithiasis
The formation of calcium oxalate kidney stones is triggered by reactive oxygen species which, when dysregulated, cause tissue injury and inflammation.199 IαI family components are found in kidney stones including HC1-3, along with HA, and correlated with increased gene expression for bikunin with hyperoxaluria.138,200–202 Interestingly, HA inhibits calcium oxalate crystallization in vitro.202 Renal epithelial cells increase the expression of bikunin upon exposure to oxalate and may be protective in response to oxalate-mediated nephrotoxicity via inhibiting stone formation and attachment to the epithelium.138,199,203
Cancer
IαI family members are downregulated at the gene level in multiple tumors, including breast, colon, and lung cancer, and renal cell and oral squamous cell carcinoma.25,204,205 HC1-5 gene expression is reduced in multiple solid tumors, including breast, lung, and kidney.25 Specifically, the HC5 gene has been identified as a tumor suppressor gene in multiple types of cancer, including breast, bladder, pancreatic, and colon cancer and acute myeloid leukemia.206–210 It can suppress the proliferation and migration of breast, bladder, and colon cancer cells in vitro.206,207,210 HC5 gene expression is reduced in cancer due to the aberrant hypermethylation of the promoter.208,211 Expression of the HC5 gene predicts longer overall survival in gastric and breast cancer and lung adenocarcinoma.210,212,213 Bikunin has known roles in tumor suppression by inhibiting cell–cell interactions, cell invasion, and metastasis, as well as providing serine proteinase inhibitory activity.107,117,118,214–219 In support of these findings, bikunin knockout mice have an increased prevalence of lung metastasis.106 These animal studies support human studies reporting low levels of bikunin in the urine of patients with bladder cancer and in high-grade glioma tissue.107,108 In addition, low bikunin gene expression is an independent predictive factor of death in cancer patients due to the metastatic advantage conferred by low bikunin levels and is correlated with lower 5-year survival rates.57,116
Diagnostic and Therapeutic Uses of IαI Family Members
The diagnostic value of IαI family members has been explored in cancer, with low gene and/or protein levels correlating with poorer health outcomes. 57,116 More recent proteomics approaches have reported increased levels of IαI family members in diseases, including ovarian, gastric, and non–small cell lung cancer.220–222 However, such a biomarker approach will likely miss the patients with poorer prognostic outcomes as low levels of bikunin have correlated with lower 5-year survival rates.57,116 Thus, interpretation of proteomic screens can be refined to provide prognostic markers of better health outcomes.
The diagnostic value of IαI is being explored in the context of pregnancy, with preterm delivery involving cervical ripening that can be inhibited by bikunin via inhibition of calcium channel signaling that prevents myometrial contraction.223 The level of bikunin in amniotic fluid is positively correlated with preterm delivery and thus may be a useful diagnostic biomarker.224 Preeclampsia is characterized by systemic inflammation in response to ischemic hypoxia and oxidative stress, and patients with preeclampsia display higher cerebrospinal fluid and serum levels of bikunin than normotensive pregnant patients.225–227 These differences may be of diagnostic value for early identification of patients at risk of preeclampsia.
Bikunin, also known as ulinastatin, is used clinically in the treatment of conditions including acute respiratory distress syndrome, sepsis, and pancreatitis.228–230 It is marketed for clinical use as Miraclid, Ulinase, U-Tryp, Ulistin, Ustatin, or Techpool Roan and sourced either from human urine or from recombinant expression technologies. Meta-analyses of randomized controlled trials showed that the administration of bikunin reduced mortality, reduced serum inflammatory markers, and reduced hospitalization time, among other parameters, in patients with acute respiratory distress syndrome or sepsis.228,229 Although the exact mechanisms by which bikunin exerts its therapeutic functions in these conditions remain to be elucidated, it is tempting to speculate that its protease inhibitory functions may limit damage caused by the excessive release of proteolytic enzymes, and particularly elastase, from neutrophils.231,232
Topically, autopsies of patients who died from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) revealed that their lungs were filled with a clear jelly, and while not positively identified is most likely HA-associated with acute respiratory distress syndrome, suggesting that bikunin is a potential treatment option for these patients.233,234 Similarly, HA is greatly increased in the airway lavage fluid of mice infected with the H1N1 influenza A virus, with elevated levels persisting 10 weeks after infection along with the presence of HA·HC complexes; here, removal of HA with hyaluronidase restored lung function to normal, proving another potential option for treating the lung symptoms associated with COVID-19.235 A retrospective analysis of patients diagnosed with pancreatitis and treated with bikunin had a lower mortality rate.230 Patients with Kawasaki disease, a condition characterized by vasculitis in infants and recently linked to SARS-CoV-2 infection in children,236 is usually treated intravenously with immunoglobulin together with aspirin to resolve inflammation; however, coronary artery lesions develop in some patients. A retrospective analysis of concurrent administration of bikunin was found to reduce the occurrence of coronary artery lesions, suggesting that it may be of clinical value.237
IαI itself may also provide therapeutic benefit. Animal models suggest that IαI administration during sepsis reduced the mortality rate,185,238 administration after hypoxic-ischemic brain injury provided neuroprotective effects,192 and coadministration with antimicrobial agents protected mice challenged with Bacillus anthracis from death.239
To conclude, the literature provides a wealth of evidence for the roles of IαI family in both biology and pathology. IαI family members have roles in matrix organization, cell signaling, protease inhibition, and regulation of complement activation and are found in many tissues. IαI family members have a role in inflammation where they can either act to limit and resolve this process to restore tissue homeostasis or perpetuate pathology. These contrasting activities are likely due to the composition of IαI family members in their temporal tissue context. As such, IαI family members have been explored for both diagnostic and therapeutic applications and used clinically in some countries. Although our understanding of the roles of IαI family members is largely associated with its HA matrix organization properties, future research is needed to explore their diverse roles in both physiological and pathological processes for both fundamental knowledge and to progress treatments for improved health outcomes.
Footnotes
Competing Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MSL and JMW are Directors of Glycos Pty Ltd, which is focused on the generation of bioengineered glycosaminoglycans as therapeutics. AJD is a Director of Link Biologics, which is focused on the use of a TSG-6-based biological drug for inflammatory conditions. The other author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed to the literature review and manuscript preparation.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.S.L. and J.M.W. were funded by the Australian Research Council under the Linkage Project (LP140101056) and Discovery Project (DP150104242) schemes, and A.J.D. was funded by Versus Arthritis (19489, 20895, 21946, and 22277).
Contributor Information
Megan S. Lord, Graduate School of Biomedical Engineering, UNSW Sydney, Sydney, NSW, Australia.
James Melrose, Graduate School of Biomedical Engineering, UNSW Sydney, Sydney, NSW, Australia; Raymond Purves Bone and Joint Research Laboratories, Kolling Institute of Medical Research, Royal North Shore Hospital and University of Sydney, St. Leonards, NSW, Australia; Sydney Medical School, Northern, Sydney University, Royal North Shore Hospital, St. Leonards, NSW, Australia.
Anthony J. Day, Wellcome Trust Centre for Cell-Matrix Research and Lydia Becker Institute of Immunology and Inflammation, Division of Cell-Matrix Biology and Regenerative Medicine, School of Biological Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester Academic Health Science Centre, Manchester, UK
John M. Whitelock, Graduate School of Biomedical Engineering, UNSW Sydney, Sydney, NSW, Australia Stem Cell Extracellular Matrix & Glycobiology, Wolfson Centre for Stem Cells, Tissue Engineering and Modelling, Faculty of Medicine, University of Nottingham, Nottingham, UK.
Literature Cited
- 1. Steinbuch M, Loeb J. Isolation of an alpha2-globulin from human plasma. Nature. 1961;192:1196. [DOI] [PubMed] [Google Scholar]
- 2. Zhuo L, Yoneda M, Zhao M, Yingsung W, Yoshida N, Kitagawa Y, Kawamura K, Suzuki T, Kimata K. Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem. 2001;276(11):7693–6. [DOI] [PubMed] [Google Scholar]
- 3. Sato H, Kajikawa S, Kuroda S, Horisawa Y, Nakamura N, Kaga N, Kakinuma C, Kato K, Morishita H, Niwa H, Miyazaki J. Impaired fertility in female mice lacking urinary trypsin inhibitor. Biochem Biophys Res Commun. 2001;281(5):1154–60. [DOI] [PubMed] [Google Scholar]
- 4. Schulman NR. A proteolytic inhibitor with anticoagulant activity separated from human urine and plasma. J Biol Chem. 1955;213:655–71. [PubMed] [Google Scholar]
- 5. Basu M, Basu S, Pugia M. Uristatin anti-inflammatory cellular signaling. In: Pugia M, editor. Inflammatory pathways in diabetes. Cham: Springer;2015, p. 171–90. [Google Scholar]
- 6. Zhuo L, Hascall VC, Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem. 2004;279(37):38079–82. [DOI] [PubMed] [Google Scholar]
- 7. McKeehan WL, Sakagami Y, Hoshi H, McKeehan KA. Two apparent human endothelial cell growth factors from human hepatoma cells are tumor-associated proteinase inhibitors. J Biol Chem. 1986;261(12):5378–83. [PubMed] [Google Scholar]
- 8. Bourguignon J, Borghi H, Sesboue R, Diarra-Mehrpour M, Bernaudin JF, Metayer J, Martin JP, Thiberville L. Immunohistochemical distribution of inter-alpha-trypsin inhibitor chains in normal and malignant human lung tissue. J Histochem Cytochem. 1999;47(12):1625–32. [DOI] [PubMed] [Google Scholar]
- 9. Salier JP, Diarra-Mehrpour M, Sesboue R, Bourguignon J, Benarous R, Ohkubo I, Kurachi S, Kurachi K, Martin JP. Isolation and characterization of cDNAs encoding the heavy chain of human inter-alpha-trypsin inhibitor (I alpha TI): unambiguous evidence for multipolypeptide chain structure of I alpha TI. Proc Natl Acad Sci U S A. 1987;84(23):8272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bourguignon J, Diarra-Mehrpour M, Sesboüé R, Frain M, Sala-Trepat JM, Martin JP, Salier JP. Human inter-alpha-trypsin-inhibitor: characterization and partial nucleotide sequencing of a light chain-encoding cDNA. Biochem Biophys Res Commun. 1985;131(3):1146–53. [DOI] [PubMed] [Google Scholar]
- 11. Bourguignon J, Vercaigne D, Sesboüé R, Martin JP, Salier JP. Inter-alpha-trypsin-inhibitor (ITI): two distinct mRNAs in baboon liver argue for a discrete synthesis of ITI and ITI derivatives. FEBS Lett. 1983;162(2):379–83. [DOI] [PubMed] [Google Scholar]
- 12. Thøgersen IB, Enghild JJ. Biosynthesis of bikunin proteins in the human carcinoma cell line HepG2 and in primary human hepatocytes. Polypeptide assembly by glycosaminoglycan. J Biol Chem. 1995;270(31):18700–9. [DOI] [PubMed] [Google Scholar]
- 13. Janssen U, Thomas G, Glant T, Phillips A. Expression of inter-α-trypsin inhibitor and tumor necrosis factor-stimulated gene 6 in renal proximal tubular epithelial cells. Kidney Int. 2001;60(1):126–36. [DOI] [PubMed] [Google Scholar]
- 14. Zhang S, He H, Day AJ, Tseng SC. Constitutive expression of inter-alpha-inhibitor (IalphaI) family proteins and tumor necrosis factor-stimulated gene-6 (TSG-6) by human amniotic membrane epithelial and stromal cells supporting formation of the heavy chain-hyaluronan (HC-HA) complex. J Biol Chem. 2012;287(15):12433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin J, Midgley A, Meran S, Woods E, Bowen T, Phillips AO, Steadman R. Tumor necrosis factor-stimulated gene 6 (TSG-6)-mediated interactions with the inter-α-inhibitor heavy chain 5 facilitate tumor growth factor β1 (TGFβ1)-dependent fibroblast to myofibroblast differentiation. J Biol Chem. 2016;291(26):13789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshihara Y, Plaas A, Osborn B, Margulis A, Nelson F, Stewart M, Rugg MS, Milner CM, Day AJ, Nemoto K, Sandy JD. Superficial zone chondrocytes in normal and osteoarthritic human articular cartilages synthesize novel truncated forms of inter-alpha-trypsin inhibitor heavy chains which are attached to a chondroitin sulfate proteoglycan other than bikunin. Osteoarthritis Cartilage. 2008;16(11):1343–55. [DOI] [PubMed] [Google Scholar]
- 17. Smith SM, Melrose J. A retrospective analysis of the cartilage Kunitz protease inhibitory proteins identifies these as members of the inter-α-trypsin inhibitor superfamily with potential roles in the protection of the articulatory surface. Int J Mol Sci. 2019;20(3):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melrose J, Taylor TK, Ghosh P. The serine proteinase inhibitory proteins of the chondrodystrophoid (beagle) and non-chondrodystrophoid (greyhound) canine intervertebral disc. Electrophoresis. 1997;18(7):1059–63. [DOI] [PubMed] [Google Scholar]
- 19. Kim B, De La Monte S, Hovanesian V, Patra A, Chen X, Chen RH, Miller MC, Pinar MH, Lim Y-P, Stopa EG, Stonestreet BS. Ontogeny of inter-alpha inhibitor protein (IAIP) expression in human brain. J Neurosci Res. 2020;98(5):869–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spasova MS, Sadowska GB, Threlkeld SW, Lim YP, Stonestreet BS. Ontogeny of inter-alpha inhibitor proteins in ovine brain and somatic tissues. Exp Biol Med. 2014;239(6):724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi H, Sun GW, Terao T. Immunolocalization of hyaluronic acid and inter-alpha-trypsin inhibitor in mice. Cell Tissue Res. 1999;296(3):587–97. [DOI] [PubMed] [Google Scholar]
- 22. Chen X, Rivard L, Naqvi S, Nakada S, Padbury JF, Sanchez-Esteban J, Stopa EG, Lim YP, Stonestreet BS. Expression and localization of inter-alpha inhibitors in rodent brain. Neuroscience. 2016;324:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutadilok N, Ghosh P, Brooks PM. Binding of haptoglobin, inter-alpha-trypsin inhibitor, and alpha 1 proteinase inhibitor to synovial fluid hyaluronate and the influence of these proteins on its degradation by oxygen derived free radicals. Ann Rheum Dis. 1988;47(5):377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang-Yi C, Aragane Y, Maeda A, Yu-Lan P, Takahashi M, Lee-Hwa K, Tezuka T. Bikunin, a serine protease inhibitor, is present on the cell boundary of epidermis. J Invest Dermatol. 1999;113(2):182–8. [DOI] [PubMed] [Google Scholar]
- 25. Hamm A, Veeck J, Bektas N, Wild PJ, Hartmann A, Heindrichs U, Kristiansen G, Werbowetski-Ogilvie T, Del Maestro R, Knuechel R, Dahl E. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: a systematic expression analysis. BMC Cancer. 2008;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ashenagar MS, Sugihara K, Maeda A, Isogai R, Takahashi M, Aisu K, Horiuchi A, Aragane Y, Kawada A, Tezuka T. The presence of tryptase-positive and bikunin-negative mast cells in psoriatic skin lesions. Arch Dermatol Res. 2007;298(9):421–6. [DOI] [PubMed] [Google Scholar]
- 27. Roberts S, Evans H, Menage J, Urban JP, Bayliss MT, Eisenstein SM, Rugg MS, Milner CM, Griffin S, Day AJ. TNFalpha-stimulated gene product (TSG-6) and its binding protein, IalphaI, in the human intervertebral disc: new molecules for the disc. Eur Spine J. 2005;14(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He H, Li W, Tseng DY, Zhang S, Chen SY, Day AJ, Tseng SC. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem. 2009;284(30):20136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan KT, McGrouther DA, Day AJ, Milner CM, Bayat A. Characterization of hyaluronan and TSG-6 in skin scarring: differential distribution in keloid scars, normal scars and unscarred skin. J Eur Acad Dermatol Venereol. 2011;25(3):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berggård T, Oury TD, Thøgersen IB, Åkerström B, Enghild JJ. α1-microglobulin is found both in blood and in most tissues. J Histochem Cytochem. 1998;46(8):887–93. [DOI] [PubMed] [Google Scholar]
- 31. Itoh H, Tomita M, Kobayashi T, Uchino H, Maruyama H, Nawa Y. Expression of inter-alpha-trypsin inhibitor light chain (bikunin) in human pancreas. J Biochem. 1996;120(2):271–5. [DOI] [PubMed] [Google Scholar]
- 32. Takano M, Mori Y, Shiraki H, Horie M, Okamoto H, Narahara M, Miyake M, Shikimi T. Detection of bikunin mRNA in limited portions of rat brain. Life Sci. 1999;65(8):757–62. [DOI] [PubMed] [Google Scholar]
- 33. Chan P, Risler JL, Raguenez G, Salier JP. The three heavy-chain precursors for the inter-α-inhibitor family in mouse: new members of the multicopper oxidase protein group with differential transcription in liver and brain. Biochem J. 1995;306(2):505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhuo L, Kimata K. Structure and function of inter-α-trypsin inhibitor heavy chains. Connect Tissue Res. 2008;49(5):311–20. [DOI] [PubMed] [Google Scholar]
- 35. Thomas GJ, Yung S, Davies M. Bikunin present in human peritoneal fluid is in part derived from the interaction of serum with peritoneal mesothelial cells. Am J Pathol. 1998;153(4):1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sánchez D, Martínez S, Lindqvist A, Åkerström B, Falkenberg C. Expression of the AMBP gene transcript and its two protein products, α1-microglobulin and bikunin, in mouse embryogenesis. Mech Dev. 2002;117(1):293–8. [DOI] [PubMed] [Google Scholar]
- 37. Isogai R, Matsukura A, Aragane Y, Maeda A, Matsukura M, Yudate T, Sugihara K, Takahashi M, Aisu K, Tezuka T. Quantitative analysis of bikunin-laden mast cells in follicular eruptions and chronic skin lesions of atopic dermatitis. Arch Dermatol Res. 2002;294(9):387–92. [DOI] [PubMed] [Google Scholar]
- 38. Odum L, Nielsen HW. Human protein HC (alpha 1-microglobulin) and inter-alpha-trypsin inhibitor in connective tissue. Histochem J. 1994;26(10):799–803. [PubMed] [Google Scholar]
- 39. Enghild JJ, Thøgersen IB, Cheng F, Fransson L-Å, Roepstorff P, Rahbek-Nielsen H. Organization of the inter-α-inhibitor heavy chains on the chondroitin sulfate originating from Ser10 of bikunin: Posttranslational modification of IαI-derived bikunin. Biochemistry. 1999;38(36):11804–13. [DOI] [PubMed] [Google Scholar]
- 40. Enghild JJ, Thogersen IB, Pizzo SV, Salvesen G. Analysis of inter-alpha-trypsin inhibitor and a novel trypsin inhibitor, pre-alpha-trypsin inhibitor, from human plasma. Polypeptide chain stoichiometry and assembly by glycan. J Biol Chem. 1989;264(27):15975–81. [PubMed] [Google Scholar]
- 41. Enghild JJ, Salvesen G, Thogersen IB, Valnickova Z, Pizzo SV, Hefta SA. Presence of the protein-glycosaminoglycan-protein covalent cross-link in the inter-alpha-inhibitor-related proteinase inhibitor heavy chain 2/bikunin. J Biol Chem. 1993;268(12):8711–6. [PubMed] [Google Scholar]
- 42. Enghild JJ, Salvesen G, Hefta SA, Thogersen IB, Rutherfurd S, Pizzo SV. Chondroitin 4-sulfate covalently cross-links the chains of the human blood protein pre-alpha-inhibitor. J Biol Chem. 1991;266(2):747–51. [PubMed] [Google Scholar]
- 43. Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6(7):530–41. [DOI] [PubMed] [Google Scholar]
- 44. Kaczmarczyk A, Thuveson M, Fries E. Intracellular coupling of the heavy chain of pre-alpha-inhibitor to chondroitin sulfate. J Biol Chem. 2002;277(16):13578–82. [DOI] [PubMed] [Google Scholar]
- 45. Morelle W, Capon C, Balduyck M, Sautiere P, Kouach M, Michalski C, Fournet B, Mizon J. Chondroitin sulphate covalently cross-links the three polypeptide chains of inter-alpha-trypsin inhibitor. Eur J Biochem. 1994;221(2):881–8. [DOI] [PubMed] [Google Scholar]
- 46. Zhuo L, Salustri A, Kimata K. A physiological function of serum proteoglycan bikunin: the chondroitin sulfate moiety plays a central role. Glycoconj J. 2002;19(4–5):241–7. [DOI] [PubMed] [Google Scholar]
- 47. Thuveson M, Fries E. Intracellular proteolytic processing of the heavy chain of rat pre-α-inhibitor: the COOH-terminal propeptide is required for coupling to bikunin. J Biol Chem. 1999;274(10):6741–6. [DOI] [PubMed] [Google Scholar]
- 48. Fries E, Blom AM. Bikunin—not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000;32(2):125–37. [DOI] [PubMed] [Google Scholar]
- 49. Bratt T, Olsson H, Sjöberg EM, Jergil B, Akerström B. Cleavage of the alpha 1-microglobulin-bikunin precursor is localized to the Golgi apparatus of rat liver cells. Biochim Biophys Acta. 1993;1157(2):147–54. [DOI] [PubMed] [Google Scholar]
- 50. Tyagi S, Salier JP, Lal SK. The liver-specific human alpha(1)-microglobulin/bikunin precursor (AMBP) is capable of self-association. Arch Biochem Biophys. 2002;399(1):66–72. [DOI] [PubMed] [Google Scholar]
- 51. Bratt T, Cedervall T, Akerstrom B. Processing and secretion of rat α1-microglobulin-bikunin expressed in eukaryotic cell lines. FEBS Lett. 1994;354:57–61. [DOI] [PubMed] [Google Scholar]
- 52. Bergwik J, Kristiansson A, Welinder C, Goransson O, Hansson SR, Gram M, Erlandsson L, Akerstrom B. Knockout of the radical scavenger alpha1-microglobulin in mice results in defective bikunin synthesis, endoplasmic reticulum stress and increased body weight [published online ahead of print February 21, 2020]. Free Radic Biol Med. doi: 10.1016/j.freeradbiomed.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 53. Daveau M, Jean L, Soury E, Olivier E, Masson S, Lyoumi S, Chan P, Hiron M, Lebreton J-P, Husson A, Jegou S, Vaudry H, Salier JP. Hepatic and extra-hepatic transcription of inter-α-inhibitor family genes under normal or acute inflammatory conditions in rat. Arch Biochem Biophys. 1998;350(2):315–23. [DOI] [PubMed] [Google Scholar]
- 54. Rouet P, Raguenez G, Tronche F, Yaniv M, N’Guyen C, Salier JP. A potent enhancer made of clustered liver-specific elements in the transcription control sequences of human α1-microglobulin/bikunin gene. J Biol Chem. 1992;267:20765–73. [PubMed] [Google Scholar]
- 55. Lavoinne A, Meisse D, Quillard M, Husson A, Renouf S, Yassad A. Glutamine and regulation of gene expression in rat hepatocytes: the role of cell swelling. Biochimie. 1998;80(10):807–11. [DOI] [PubMed] [Google Scholar]
- 56. Pierzchalski P, Rokita H, Koj A, Fries E, Akerstrom B. Synthesis of a1-microglobulin in cultured rat hepatocytes is stimulated by interleukin-6, leukemia inhibitory factor, dexamethasone and retinoic acid. FEBS Lett. 1992;298:165–8. [DOI] [PubMed] [Google Scholar]
- 57. Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Suzuki M, Terao T. Upregulation of bikunin in tumor-infiltrating macrophages as a factor of favorable prognosis in ovarian cancer. Gynecol Oncol. 2004;94(3):725–34. [DOI] [PubMed] [Google Scholar]
- 58. Daveau M, Rouet P, Scotte M, Faye L, Hiron M, Libreton JP, Salier JP. Human inter-α-inhibitor family in inflammation: simultaneous synthesis of positive and negative acute-phase proteins. Biochem J. 1993;292:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Toyoda H, Kobayashi S, Sakamoto S, Toida T, Imanari T. Structural analysis of a low-sulfated chondroitin sulfate chain in human urinary trypsin inhibitor. Biol Pharm Bull. 1993;16(9):945–7. [DOI] [PubMed] [Google Scholar]
- 60. Kakizaki I, Takahashi R, Ibori N, Kojima K, Takahashi T, Yamaguchi M, Kon A, Takagaki K. Diversity in the degree of sulfation and chain length of the glycosaminoglycan moiety of urinary trypsin inhibitor isomers. Biochim Biophys Acta. 2007;1770(2):171–7. [DOI] [PubMed] [Google Scholar]
- 61. Lord MS, Day AJ, Youssef P, Zhuo L, Watanabe H, Caterson B, Whitelock JM. Sulfation of the bikunin chondroitin sulfate chain determines heavy chain.hyaluronan complex formation. J Biol Chem. 2013;288(32):22930–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ly M, Leach FE, Laremore TN, Toida T, Amster IJ, Linhardt RJ. The proteoglycan bikunin has a defined sequence. Nat Chem Biol. 2011;7(11):827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Capon C, Mizon C, Lemoine J, Rodie-Talbere P, Mizon J. In acute inflammation, the chondroitin-4 sulphate carried by bikunin is not only longer, it is also undersulphated. Biochimie. 2003;85(1–2):101–7. [DOI] [PubMed] [Google Scholar]
- 64. Chi L, Wolff JJ, Laremore TN, Restaino OF, Xie J, Schiraldi C, Toida T, Amster IJ, Linhardt RJ. Structural analysis of bikunin glycosaminoglycan. J Am Chem Soc. 2008;130(8):2617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamada S, Oyama M, Kinugasa H, Nakagawa T, Kawasaki T, Nagasawa S, Khoo KH, Morris HR, Dell A, Sugahara K. The sulphated carbohydrate-protein linkage region isolated from chondroitin 4-sulphate chains of inter-α-trypsin inhibitor in human plasma. Glycobiology. 1995;5(3):335–41. [DOI] [PubMed] [Google Scholar]
- 66. Nilsson J, Noborn F, Gomez Toledo A, Nasir W, Sihlbom C, Larson G. Characterization of glycan structures of chondroitin sulfate-glycopeptides facilitated by sodium ion-pairing and positive mode LC-MS/MS. J Am Soc Mass Spectrom. 2017;28(2):229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yuki Y, Nomura K, Kirihara M, Shimomura M, Hiratani H, Nishimura R, Kato K. Charge isomers of urinary bikunin (trypsin inhibitor). Biochim Biophys Acta. 1993;1203(2):298–303. [DOI] [PubMed] [Google Scholar]
- 68. Persson A, Nilsson J, Vorontsov E, Noborn F, Larson G. Identification of a non-canonical chondroitin sulfate linkage region trisaccharide. Glycobiology. 2019;29(5):366–71. [DOI] [PubMed] [Google Scholar]
- 69. Gomez Toledo A, Nilsson J, Noborn F, Sihlbom C, Larson G. Positive mode LC-MS/MS analysis of chondroitin sulfate modified glycopeptides derived from light and heavy chains of the human inter-α-trypsin inhibitor complex. Mol Cell Proteomics. 2015;14(12):3118–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fulop C, Szanto S, Mukhopadhyay D, Bardos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–61. [DOI] [PubMed] [Google Scholar]
- 71. Rugg MS, Willis AC, Mukhopadhyay D, Hascall VC, Fries E, Fulop C, Milner CM, Day AJ. Characterization of complexes formed between TSG-6 and inter-alpha-inhibitor that act as intermediates in the covalent transfer of heavy chains onto hyaluronan. J Biol Chem. 2005;280(27):25674–86. [DOI] [PubMed] [Google Scholar]
- 72. Hascall VC. The journey of hyaluronan research in the Journal of Biological Chemistry. J Biol Chem. 2019;294(5):1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Day AJ, Milner CM. TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019;78–79:60–83. [DOI] [PubMed] [Google Scholar]
- 74. Sanggaard KW, Hansen L, Scavenius C, Wisniewski HG, Kristensen T, Thogersen IB, Enghild JJ. Evo-lutionary conservation of heavy chain protein transfer between glycosaminoglycans. Biochim Biophys Acta. 2010;1804(4):1011–9. [DOI] [PubMed] [Google Scholar]
- 75. Zhao M, Yoneda M, Ohashi Y, Kurono S, Iwata H, Ohnuki Y, Kimata K. Evidence for the covalent binding of SHAP, heavy chains of inter-α-trypsin inhibitor, to hyaluronan. J Biol Chem. 1995;270:26657–63. [DOI] [PubMed] [Google Scholar]
- 76. Huang L, Yoneda M, Kimata K. A serum-derived hyaluronan-associated protein (SHAP) is the heavy chain of the inter alpha-trypsin inhibitor. J Biol Chem. 1993;268(35):26725–30. [PubMed] [Google Scholar]
- 77. Briggs DC, Birchenough HL, Ali T, Rugg MS, Waltho JP, Ievoli E, Jowitt TA, Enghild JJ, Richter RP, Salustri A, Milner CM, Day AJ. Metal ion-dependent heavy chain transfer activity of TSG-6 mediates assembly of the cumulus-oocyte matrix. J Biol Chem. 2015;290(48):28708–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scavenius C, Nikolajsen CL, Stenvang M, Thogersen IB, Wyrozemski L, Wisniewski HG, Otzen DE, Sanggaard KW, Enghild JJ. The compact and biologically relevant structure of inter-alpha-inhibitor is maintained by the chondroitin sulfate chain and divalent cations. J Biol Chem. 2016;291(9):4658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sanggaard KW, Sonne-Schmidt CS, Krogager TP, Lorentzen KA, Wisniewski HG, Thogersen IB, Enghild JJ. The transfer of heavy chains from bikunin proteins to hyaluronan requires both TSG-6 and HC2. J Biol Chem. 2008;283(27):18530–7. [DOI] [PubMed] [Google Scholar]
- 80. Sanggaard KW, Sonne-Schmidt CS, Jacobsen C, Thøgersen IB, Valnickova Z, Wisniewski HG, Enghild JJ. Evidence for a two-step mechanism involved in the formation of covalent HC x TSG-6 complexes. Biochemistry. 2006;45(24):7661–8. [DOI] [PubMed] [Google Scholar]
- 81. Briggs DC, Langford-Smith AWW, Birchenough HL, Jowitt TA, Kielty CM, Enghild JJ, Baldock C, Milner CM, Day AJ. Inter-α-inhibitor heavy chain-1 has an integrin-like 3D structure mediating immune regulatory activities and matrix stabilization during ovulation. J Biol Chem. 2020;295(16):5278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yingsung W, Zhuo L, Morgelin M, Yoneda M, Kida D, Watanabe H, Ishiguro N, Iwata H, Kimata K. Molecular heterogeneity of the SHAP-hyaluronan complex. Isolation and characterization of the complex in synovial fluid from patients with rheumatoid arthritis. J Biol Chem. 2003;278(35):32710–8. [DOI] [PubMed] [Google Scholar]
- 83. Lamkin E, Cheng G, Calabro A, Hascall VC, Joo EJ, Li L, Linhardt RJ, Lauer ME. Heavy chain transfer by tumor necrosis factor-stimulated gene 6 to the bikunin proteoglycan. J Biol Chem. 2015;290(8):5156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sanggaard KW, Scavenius C, Rasmussen AJ, Wisniewski HG, Thogersen IB, Enghild JJ. The TSG-6/HC2-mediated transfer is a dynamic process shuffling heavy chains between glycosaminoglycans. J Biol Chem. 2010;285(29):21988–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lauer ME, Glant TT, Mikecz K, DeAngelis PL, Haller FM, Husni ME, Hascall VC, Calabro A. Irreversible heavy chain transfer to hyaluronan oligosaccharides by tumor necrosis factor-stimulated gene-6. J Biol Chem. 2013;288(1):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Higman VA, Briggs DC, Mahoney DJ, Blundell CD, Sattelle BM, Dyer DP, Green DE, DeAngelis PL, Almond A, Milner CM, Day AJ. A refined model for the TSG-6 link module in complex with hyaluronan: use of defined oligosaccharides to probe structure and function. J Biol Chem. 2014;289(9):5619–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mukhopadhyay D, Asari A, Rugg MS, Day AJ, Fülöp C. Specificity of the tumor necrosis factor-induced protein 6-mediated heavy chain transfer from inter-α-trypsin inhibitor to hyaluronan: implications for the assembly of the cumulus extracellular matrix. J Biol Chem. 2004;279(12):11119–28. [DOI] [PubMed] [Google Scholar]
- 88. He H, Zhang S, Tighe S, Son J, Tseng SCG. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem. 2013;288(36):25792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lauer ME, Mukhopadhyay D, Fulop C, de la Motte CA, Majors AK, Hascall VC. Primary murine airway smooth muscle cells exposed to poly(I, C) or tunicamycin synthesize a leukocyte-adhesive hyaluronan matrix. J Biol Chem. 2009;284(8):5299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhuo L, Kanamori A, Kannagi R, Itano N, Wu J, Hamaguchi M, Ishiguro N, Kimata K. SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J Biol Chem. 2006;281(29):20303–14. [DOI] [PubMed] [Google Scholar]
- 91. Baranova NS, Foulcer SJ, Briggs DC, Tilakaratna V, Enghild JJ, Milner CM, Day AJ, Richter RP. Inter-α-inhibitor impairs TSG-6-induced hyaluronan cross-linking. J Biol Chem. 2013;288(41):29642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen L, Mao SJ, McLean LR, Powers RW, Larsen WJ. Proteins of the inter-alpha-trypsin inhibitor family stabilize the cumulus extracellular matrix through their direct binding with hyaluronic acid. J Biol Chem. 1994;269(45):28282–7. [PubMed] [Google Scholar]
- 93. Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P, Salvatori G, Mahoney DJ, Day AJ, Siracusa G, Romani L, Mantovani A. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131(7):1577–86. [DOI] [PubMed] [Google Scholar]
- 94. Mukhopadhyay D, Hascall VC, Day AJ, Salustri A, Fülöp C. Two distinct populations of tumor necrosis factor-stimulated gene-6 protein in the extracellular matrix of expanded mouse cumulus cell-oocyte complexes. Arch Biochem Biophys. 2001;394(2):173–81. [DOI] [PubMed] [Google Scholar]
- 95. Baranova NS, Inforzato A, Briggs DC, Tilakaratna V, Enghild JJ, Thakar D, Milner CM, Day AJ, Richter RP. Incorporation of pentraxin 3 into hyaluronan matrices is tightly regulated and promotes matrix cross-linking. J Biol Chem. 2014;289(44):30481–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Scavenius C, Sanggaard KW, Nikolajsen CL, Bak S, Valnickova Z, Thøgersen IB, Jensen ON, Højrup P, Enghild JJ. Human inter-α-inhibitor is a substrate for factor XIIIa and tissue transglutaminase. Biochim Biophys Acta. 2011;1814(12):1624–30. [DOI] [PubMed] [Google Scholar]
- 97. Adair JE, Stober V, Sobhany M, Zhuo L, Roberts JD, Negishi M, Kimata K, Garantziotis S. Inter-alpha-trypsin inhibitor promotes bronchial epithelial repair after injury through vitronectin binding. J Biol Chem. 2009;284(25):16922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pijuan-Galitó S, Tamm C, Schuster J, Sobol M, Forsberg L, Merry CLR, Annerén C. Human serum-derived protein removes the need for coating in defined human pluripotent stem cell culture. Nat Commun. 2016;7(1):12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Htwe SS, Wake H, Liu K, Teshigawara K, Stonestreet BS, Lim Y-P, Nishibori M. Inter-α inhibitor proteins maintain neutrophils in a resting state by regulating shape and reducing ROS production. Blood Adv. 2018;2(15):1923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Okroj M, Holmquist E, Sjolander J, Crrales L, Saxne T, Wisniewski HG. Heavy chains of inter alpha trypsin inhibitor (IaI) inhibit the human complement system at early stages of the cascade. J Biol Chem. 2012;287(24):20100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Garantziotis S, Hollingsworth JW, Ghanayem RB, Timberlake S, Zhuo L, Kimata K, Schwartz DA. Inter-alpha-trypsin inhibitor attenuates complement activation and complement-induced lung injury. J Immunol. 2007;179(6):4187–92. [DOI] [PubMed] [Google Scholar]
- 102. Wisniewski HG, Hua JC, Poppers DM, Naime D, Vilcek J, Cronstein BN. TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-alpha-inhibitor and exerts a strong anti-inflammatory effect in vivo. J Immunol. 1996;156(4):1609–15. [PubMed] [Google Scholar]
- 103. Mahoney DJ, Mulloy B, Forster MJ, Blundell CD, Fries E, Milner CM, Day AJ. Characterization of the interaction between tumor necrosis factor-stimulated gene-6 and heparin: implications for the inhibition of plasmin in extracellular matrix microenvironments. J Biol Chem. 2005;280(29):27044–55. [DOI] [PubMed] [Google Scholar]
- 104. Mio K, Carrette O, Maibach HI, Stern R. Evidence that the serum inhibitor of hyaluronidase may be a member of the inter-alpha-inhibitor family. J Biol Chem. 2000;275(42):32413–21. [DOI] [PubMed] [Google Scholar]
- 105. Suzuki M, Kobayashi H, Tanaka Y, Kanayama N, Terao T. Reproductive failure in mice lacking inter—alpha—trypsin inhibitor (ITI)—ITI target genes in mouse ovary identified by microarray analysis. J Endocrinol. 2004;183(1):29–38. [DOI] [PubMed] [Google Scholar]
- 106. Yagyu T, Kobayashi H, Matsuzaki H, Wakahara K, Kondo T, Kurita N, Sekino H, Inagaki K. Enhanced spontaneous metastasis in bikunin-deficient mice. Int J Cancer. 2006;118(9):2322–8. [DOI] [PubMed] [Google Scholar]
- 107. Werbowetski-Ogilvie TE, Agar NY, Waldkircher de Oliveira RM, Faury D, Antel JP, Jabado N, Del Maestro RF. Isolation of a natural inhibitor of human malignant glial cell invasion: inter alpha-trypsin inhibitor heavy chain 2. Cancer Res. 2006;66(3):1464–72. [DOI] [PubMed] [Google Scholar]
- 108. Tsui KH, Tang P, Lin CY, Chang PL, Chang CH, Yung BY. Bikunin loss in urine as useful marker for bladder carcinoma. J Urol. 2010;183(1):339–44. [DOI] [PubMed] [Google Scholar]
- 109. Goulding DR, Nikolova VD, Mishra L, Zhuo L, Kimata K, McBride SJ, Moy SS, Harry GJ, Garantziotis S. Inter-α-inhibitor deficiency in the mouse is associated with alterations in anxiety-like behavior, exploration and social approach. Genes Brain Behav. 2019;18(1):e12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Salier JP. Inter-alpha-trypsin inhibitor: emergence of a family within the Kunitz-type protease inhibitor superfamily. Trends Biochem Sci. 1990;15(11):435–9. [DOI] [PubMed] [Google Scholar]
- 111. Itoh H, Ide H, Ishikawa N, Nawa Y. Mast cell protease inhibitor, trypstatin, is a fragment of inter-alpha-trypsin inhibitor light chain. J Biol Chem. 1994;269(5):3818–22. [PubMed] [Google Scholar]
- 112. Wilharm E, Parry MA, Friebel R, Tschesche H, Matschiner G, Sommerhoff CP, Jenne DE. Generation of catalytically active granzyme K from Escherichia coli inclusion bodies and identification of efficient granzyme K inhibitors in human plasma. J Biol Chem. 1999;274(38):27331–7. [DOI] [PubMed] [Google Scholar]
- 113. Forteza R, Casalino-Matsuda SM, Monzon ME, Fries E, Rugg MS, Milner CM, Day AJ. TSG-6 potentiates the antitissue kallikrein activity of inter-alpha-inhibitor through bikunin release. Am J Respir Cell Mol Biol. 2007;36(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Manilal SB, Scott GK. Further evidence for the mitogenic action of urinary trypsin inhibitor. Biochem Mol Biol Int. 1996;39(4):711–20. [DOI] [PubMed] [Google Scholar]
- 115. Pendlebury D, Wang R, Henin RD, Hockla A, Soares AS, Madden BJ, Kazanov MD, Radisky ES. Sequence and conformational specificity in substrate recognition: several human Kunitz protease inhibitor domains are specific substrates of mesotrypsin. J Biol Chem. 2014;289(47):32783–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Suzuki M, Yamakawa T, Morishita H, Terao T. Reduced bikunin gene expression as a factor of poor prognosis in ovarian carcinoma. Cancer. 2003;98(2):424–30. [DOI] [PubMed] [Google Scholar]
- 117. Kobayashi H, Suzuki M, Hirashima Y, Terao T. The protease inhibitor bikunin, a novel anti-metastatic agent. Biol Chem. 2003;384(5):749–54. [DOI] [PubMed] [Google Scholar]
- 118. Kobayashi H, Suzuki M, Tanaka Y, Hirashima Y, Terao T. Suppression of urokinase expression and invasiveness by urinary trypsin inhibitor is mediated through inhibition of protein kinase C- and MEK/ERK/c-Jun-dependent signaling pathways. J Biol Chem. 2001;276(3):2015–22. [DOI] [PubMed] [Google Scholar]
- 119. Hamasuna R, Kataoka H, Meng JY, Itoh H, Moriyama T, Wakisaka S, Koono M. Reduced expression of hepatocyte growth factor activator inhibitor type-2/placental bikunin (HAI-2/PB) in human glioblastomas: implication for anti-invasive role of HAI-2/PB in glioblastoma cells. Int J Cancer. 2001;93(3):339–45. [DOI] [PubMed] [Google Scholar]
- 120. Kobayashi H, Suzuki M, Kanayama N, Nishida T, Takigawa M, Terao T. Suppression of urokinase receptor expression by bikunin is associated with inhibition of upstream targets of extracellular signal-regulated kinase-dependent cascade. Eur J Biochem. 2002;269(16):3945–57. [DOI] [PubMed] [Google Scholar]
- 121. Kobayashi H, Suzuki M, Tanaka Y, Kanayama N, Terao T. A Kunitz-type protease inhibitor, bikunin, inhibits ovarian cancer cell invasion by blocking the calcium-dependent transforming growth factor-beta 1 signaling cascade. J Biol Chem. 2003;278(10):7790–9. [DOI] [PubMed] [Google Scholar]
- 122. Wakahara K, Kobayashi H, Yagyu T, Matsuzaki H, Kondo T, Kurita N, Sekino H, Inagaki K, Suzuki M, Kanayama N, Terao T. Bikunin down-regulates heterodimerization between CD44 and growth factor receptors and subsequently suppresses agonist-mediated signaling. J Cell Biochem. 2005;94(5):995–1009. [DOI] [PubMed] [Google Scholar]
- 123. Suzuki M, Kobayashi H, Fujie M, Nishida T, Takigawa M, Kanayama N, Terao T. Kunitz-type protease inhibitor bikunin disrupts phorbol ester-induced oligomerization of CD44 variant isoforms containing epitope v9 and subsequently suppresses expression of urokinase-type plasminogen activator in human chondrosarcoma cells. J Biol Chem. 2002;277(10):8022–32. [DOI] [PubMed] [Google Scholar]
- 124. Kanayama S, Yamada Y, Onogi A, Shigetomi H, Ueda S, Tsuji Y, Haruta S, Kawaguchi R, Yoshida S, Sakata M, Sado T, Kitanaka T, Oi H, Yagyu T, Kobayashi H. Molecular structure and function analysis of bikunin on down-regulation of tumor necrosis factor-alpha expression in activated neutrophils. Cytokine. 2008;42(2):191–7. [DOI] [PubMed] [Google Scholar]
- 125. Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Inagaki K, Kondo T, Kurita N, Suzuki M, Kanayama N, Terao T. Suppression of lipopolysaccharide-induced cytokine production of gingival fibroblasts by a soybean, Kunitz trypsin inhibitor. J Periodontal Res. 2005;40(6):461–8. [DOI] [PubMed] [Google Scholar]
- 126. Matsuzaki H, Kobayashi H, Yagyu T, Wakahara K, Kondo T, Kurita N, Sekino H, Inagaki K, Suzuki M, Kanayama N, Terao T. Bikunin inhibits lipopolysaccharide-induced tumor necrosis factor alpha induction in macrophages. Clin Diagn Lab Immunol. 2004;11(6):1140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wakahara K, Kobayashi H, Yagyu T, Matsuzaki H, Kondo T, Kurita N, Sekino H, Inagaki K, Suzuki M, Kanayama N, Terao T. Bikunin suppresses lipopolysaccharide-induced lethality through down-regulation of tumor necrosis factor- alpha and interleukin-1 beta in macrophages. J Infect Dis. 2005;191(6):930–8. [DOI] [PubMed] [Google Scholar]
- 128. Kanayama N, Halim A, Maehara K, Kajiwara Y, Fujie M, Terao T. Kunitz-type trypsin inhibitor prevents LPS-induced increase of cytosolic free Ca2+ in human neutrophils and HUVEC cells. Biochem Biophys Res Commun. 1995;207(1):324–30. [DOI] [PubMed] [Google Scholar]
- 129. Kanayama N, Maehara K, She L, Belayet HM, Khatun S, Tokunaga N, Terao T. Urinary trypsin inhibitor suppresses vascular smooth muscle contraction by inhibition of Ca2+ influx. Biochim Biophys Acta. 1998;1381(2):139–46. [DOI] [PubMed] [Google Scholar]
- 130. Nakamura H, Abe S, Shibata Y, Sata M, Kato S, Saito H, Hino T, Takahashi H, Tomoike H. Inhibition of neutrophil elastase-induced interleukin-8 gene expression by urinary trypsin inhibitor in human bronchial epithelial cells. Int Arch Allergy Immunol. 1997;112(2):157–62. [DOI] [PubMed] [Google Scholar]
- 131. Atmani F, Lacour B, Drüeke T, Daudon M. Isolation and purification of a new glycoprotein from human urine inhibiting calcium oxalate crystallization. Urol Res. 1993;21(1):61–6. [DOI] [PubMed] [Google Scholar]
- 132. Hennies HC. All is balanced: inter-alpha-trypsin inhibitors as unseen extracellular matrix proteins in epidermal morphology and differentiation. Exp Dermatol. 2015;24(9):661–2. [DOI] [PubMed] [Google Scholar]
- 133. Huth S, Heise R, Vetter-Kauczok CS, Skazik C, Marquardt Y, Czaja K, Knuchel R, Merk HF, Dahl E, Baron JM. Inter-alpha-trypsin inhibitor heavy chain 5 (ITIH5) is overexpressed in inflammatory skin diseases and affects epidermal morphology in constitutive knockout mice and murine 3D skin models. Exp Dermatol. 2015;24(9):663–8. [DOI] [PubMed] [Google Scholar]
- 134. Clark HF, Gurney AL, Abaya E, Baker K, Baldwin D, Brush J, Chen J, Chow B, Chui C, Crowley C, Currell B, Deuel B, Dowd P, Eaton D, Foster J, Grimaldi C, Gu Q, Hass PE, Heldens S, Huang A, Kim HS, Klimowski L, Jin Y, Johnson S, Lee J, Lewis L, Liao D, Mark M, Robbie E, Sanchez C, Schoenfeld J, Seshagiri S, Simmons L, Singh J, Smith V, Stinson J, Vagts A, Vandlen R, Watanabe C, Wieand D, Woods K, Xie M-H, Yansura D, Yi S, Yu G, Yuan J, Zhang M, Zhang Z, Goddard A, Wood WI, Godowski P. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res. 2003;13(10):2265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schar CR, Jensen JK, Christensen A, Blouse GE, Andreasen PA, Peterson CB. Characterization of a site on PAI-1 that binds to vitronectin outside of the somatomedin B domain. J Biol Chem. 2008;283:28487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sivakumar A, Mahadevan A, Lauer ME, Narvaez RJ, Ramesh S, Demler CM, Souchet NR, Hascall VC, Midura RJ, Garantziotis S, Frank DB, Kimata K, Kurpios NA. Midgut laterality is driven by hyaluronan on the right. Dev Cell. 2018;46(5):533–51.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hull RL, Johnson PY, Braun KR, Day AJ, Wight TN. Hyaluronan and hyaluronan binding proteins are normal components of mouse pancreatic islets and are differentially expressed by islet endocrine cell types. J Histochem Cytochem. 2012;60(10):749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Iida S, Peck AB, Byer KJ, Khan SR. Expression of bikunin mRNA in renal epithelial cells after oxalate exposure. J Urol. 1999;162(4):1480–6. [PubMed] [Google Scholar]
- 139. Selbi W, Day AJ, Rugg MS, Fülöp C, de la Motte CA, Bowen T, Hascall VC, Phillips AO. Overexpression of hyaluronan synthase 2 alters hyaluronan distribution and function in proximal tubular epithelial cells. J Am Soc Nephrol. 2006;17(6):1553–67. [DOI] [PubMed] [Google Scholar]
- 140. Powers RW, Chen L, Russell PT, Larsen WJ. Gonadotropin-stimulated regulation of blood-follicle barrier is mediated by nitric oxide. Am J Physiol. 1995;269:E290–8. [DOI] [PubMed] [Google Scholar]
- 141. Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16(6):1154–67. [DOI] [PubMed] [Google Scholar]
- 142. Chen X, Bonfiglio R, Banerji S, Jackson DG, Salustri A, Richter RP. Micromechanical analysis of the hyaluronan-rich matrix surrounding the oocyte reveals a uniquely soft and elastic composition. Biophys J. 2016;110(12):2779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179(3):325–35. [DOI] [PubMed] [Google Scholar]
- 144. Hettinger AM, Allen MR, Zhang BR, Goad DW, Malayer JR, Geisert RD. Presence of the acute phase protein, bikunin, in the endometrium of gilts during estrous cycle and early pregnancy. Biol Reprod. 2001;65(2):507–13. [DOI] [PubMed] [Google Scholar]
- 145. Melrose J, Shu C, Whitelock JM, Lord MS. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biol. 2016;52–54:363–83. [DOI] [PubMed] [Google Scholar]
- 146. Shakya S, Mack JA, Maytin EV. Cutaneous wounds in mice lacking tumor necrosis factor-stimulated gene-6 exhibit delayed closure and an abnormal inflammatory response. bioRxiv. 2019:676411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Coulson-Thomas VJ, Lauer ME, Soleman S, Zhao C, Hascall VC, Day AJ, Fawcett JW. Tumor necrosis factor-stimulated gene-6 (TSG-6) is constitutively expressed in adult central nervous system (CNS) and associated with astrocyte-mediated glial scar formation following spinal cord injury. J Biol Chem. 2016;291(38):19939–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Harry GJ. Neuroinflammation: a need to understand microglia as resident cells of the developing brain. Neurotoxicology. 2012;33(3):558–9. [DOI] [PubMed] [Google Scholar]
- 149. Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49(6):377–91. [DOI] [PubMed] [Google Scholar]
- 150. Vivien D, Buisson A. Serine protease inhibitors: novel therapeutic targets for stroke? J Cereb Blood Flow Metab. 2000;20(5):755–64. [DOI] [PubMed] [Google Scholar]
- 151. Turgeon VL, Houenou LJ. The role of thrombin-like (serine) proteases in the development, plasticity and pathology of the nervous system. Brain Res Brain Res Rev. 1997;25(1):85–95. [DOI] [PubMed] [Google Scholar]
- 152. Reuther C, Ganjam GK, Dolga AM, Culmsee C. The serine protease inhibitor TLCK attenuates intrinsic death pathways in neurons upstream of mitochondrial demise. Apoptosis. 2014;19(11):1545–58. [DOI] [PubMed] [Google Scholar]
- 153. Jortani SA, Pugia MJ, Elin RJ, Thomas M, Womack EP, Cast T, Valdes R., Jr. Sensitive noninvasive marker for the diagnosis of probable bacterial or viral infection. J Clin Lab Anal. 2004;18(6):289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kida D, Yoneda M, Miyaura S, Ishimaru T, Yoshida Y, Ito T, Ishiguro N, Iwata H, Kimata K. The SHAP-HA complex in sera from patients with rheumatoid arthritis and osteoarthritis. J Rheumatol. 1999;26(6):1230–8. [PubMed] [Google Scholar]
- 155. Bogdani M, Johnson PY, Potter-Perigo S, Nagy N, Day AJ, Bollyky PL, Wight TN. Hyaluronan and hyaluronan-binding proteins accumulate in both human type 1 diabetic islets and lymphoid tissues and associate with inflammatory cells in insulitis. Diabetes. 2014;63(8):2727–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Nagy N, Kaber G, Johnson PY, Gebe JA, Preisinger A, Falk BA, Sunkari VG, Gooden MD, Vernon RB, Bogdani M, Kuipers HF, Day AJ, Campbell DJ, Wight TN, Bollyky PL. Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis. J Clin Invest. 2015;125(10):3928–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lepedda AJ, Nieddu G, Rocchiccioli S, Ucciferri N, Idini M, De Muro P, Formato M. Levels of urinary trypsin inhibitor and structure of its chondroitin sulphate moiety in type 1 and type 2 diabetes. J Diabetes Res. 2018;2018:9378515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mizon C, Mairie C, Balduyck M, Hachulla E, Mizon J. The chondroitin sulfate chain of bikunin-containing proteins in the inter-alpha-inhibitor family increases in size in inflammatory diseases. Eur J Biochem. 2001;268(9):2717–24. [DOI] [PubMed] [Google Scholar]
- 159. Mahoney DJ, Swales C, Athanasou NA, Bombardieri M, Pitzalis C, Kliskey K, Sharif M, Day AJ, Milner CM, Sabokbar A. TSG-6 inhibits osteoclast activity via an autocrine mechanism and is functionally synergistic with osteoprotegerin. Arthritis Rheum. 2011;63(4):1034–43. [DOI] [PubMed] [Google Scholar]
- 160. Wisniewski HG, Colón E, Liublinska V, Karia RJ, Stabler TV, Attur M, Abramson SB, Band PA, Kraus VB. TSG-6 activity as a novel biomarker of progression in knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bayliss MT, Howat SL, Dudhia J, Murphy JM, Barry FP, Edwards JC, Day AJ. Up-regulation and differential expression of the hyaluronan-binding protein TSG-6 in cartilage and synovium in rheumatoid arthritis and osteoarthritis. Osteoarthritis Cartilage. 2001;9(1):42–8. [DOI] [PubMed] [Google Scholar]
- 162. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chou CH, Attarian DE, Wisniewski HG, Band PA, Kraus VB. TSG-6—a double-edged sword for osteoarthritis (OA). Osteoarthritis Cartilage. 2018;26(2):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-alpha-trypsin inhibitor is crucial to structure and function. Am J Pathol. 2003;163(1):121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bandyopadhyay SK, de la Motte CA, Kessler SP, Hascall VC, Hill DR, Strong SA. Hyaluronan-mediated leukocyte adhesion and dextran sulfate sodium-induced colitis are attenuated in the absence of signal transducer and activator of transcription 1. Am J Pathol. 2008;173(5):1361–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Petrey AC, de la Motte CA. Thrombin cleavage of inter-α-inhibitor heavy chain 1 regulates leukocyte binding to an inflammatory hyaluronan matrix. J Biol Chem. 2016;291(47):24324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ohba T, Takase Y, Ohhara M, Kasukawa R. Thrombin in the synovial fluid of patients with rheumatoid arthritis mediates proliferation of synovial fibroblast-like cells by induction of platelet derived growth factor. J Rheumatol. 1996;23(9):1505–11. [PubMed] [Google Scholar]
- 169. Scavenius C, Poulsen EC, Thøgersen IB, Roebuck M, Frostick S, Bou-Gharios G, Yamamoto K, Deleuran B, Enghild JJ. Matrix-degrading protease ADAMTS-5 cleaves inter-α-inhibitor and releases active heavy chain 2 in synovial fluids from arthritic patients. J Biol Chem. 2019;294(42):15495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kissin E, Korn JH. Apoptosis and myofibroblasts in the pathogenesis of systemic sclerosis. Curr Rheumatol Rep. 2002;4(2):129–35. [DOI] [PubMed] [Google Scholar]
- 171. Matuska B, Comhair S, Farver C, Chmiel J, Midura RJ, Bonfield T, Lauer ME. Pathological hyaluronan matrices in cystic fibrosis airways and secretions. Am J Respir Cell Mol Biol. 2016;55(4):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. He H, Li W, Chen SY, Zhang S, Chen YT, Hayashida Y, Zhu YT, Tseng SC. Suppression of activation and induction of apoptosis in RAW264.7 cells by amniotic membrane extract. Invest Ophthalmol Vis Sci. 2008;49(10):4468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Li W, He H, Chen YT, Hayashida Y, Tseng SC. Reversal of myofibroblasts by amniotic membrane stromal extract. J Cell Physiol. 2008;215(3):657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Garantziotis S, Zudaire E, Trempus CS, Hollingsworth JW, Jiang D, Lancaster LH, Richardson E, Zhuo L, Cuttitta F, Brown KK, Noble PW, Kimata K, Schwartz DA. Serum inter-alpha-trypsin inhibitor and matrix hyaluronan promote angiogenesis in fibrotic lung injury. Am J Respir Crit Care Med. 2008;178(9):939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Shay E, He H, Sakurai S, Tseng SC. Inhibition of angiogenesis by HC.HA, a complex of hyaluronan and the heavy chain of inter-alpha-inhibitor, purified from human amniotic membrane. Invest Ophthalmol Vis Sci. 2011;52(5):2669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhu L, Zhuo L, Kimata K, Yamaguchi E, Watanabe H, Aronica MA, Hascall VC, Baba K. Deficiency in the serum-derived hyaluronan-associated protein-hyaluronan complex enhances airway hyperresponsiveness in a murine model of asthma. Int Arch Allergy Immunol. 2010;153(3):223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lilly CM, Tateno H, Oguma T, Israel E, Sonna LA. Effects of allergen challenge on airway epithelial cell gene expression. Am J Respir Crit Care Med. 2005;171(6):579–86. [DOI] [PubMed] [Google Scholar]
- 178. Getting SJ, Mahoney DJ, Cao T, Rugg MS, Fries E, Milner CM, Perretti M, Day AJ. The link module from human TSG-6 inhibits neutrophil migration in a hyaluronan- and inter-α-inhibitor-independent manner. J Biol Chem. 2002;277(52):51068–76. [DOI] [PubMed] [Google Scholar]
- 179. Swaidani S, Cheng G, Lauer ME, Sharma M, Mikecz K, Hascall VC, Aronica MA. TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J Biol Chem. 2013;288(1):412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Stober VP, Johnson CG, Majors A, Lauer ME, Cali V, Midura RJ, Wisniewski HG, Aronica MA, Garantziotis S. TNF-stimulated gene 6 promotes formation of hyaluronan-inter-α-inhibitor heavy chain complexes necessary for ozone-induced airway hyperresponsiveness. J Biol Chem. 2017;292(51):20845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hirose J, Ozawa T, Miura T, Isaji M, Nagao Y, Yamashiro K, Nii A, Kato K, Uemura A. Human neutrophil elastase degrades inter-alpha-trypsin inhibitor to liberate urinary trypsin inhibitor related proteins. Biol Pharm Bull. 1998;21(7):651–6. [DOI] [PubMed] [Google Scholar]
- 182. Lim Y-P, Bendelja K, Opal SM, Siryaporn E, Hixson DC, Palardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis. 2003;188(6):919–26. [DOI] [PubMed] [Google Scholar]
- 183. Opal SM, Lim Y-P, Siryaporn E, Moldawer LL, Pribble JP, Palardy JE, Souza S. Longitudinal studies of inter-alpha inhibitor proteins in severely septic patients: a potential clinical marker and mediator of severe sepsis. Crit Care Med. 2007;35(2):387–92. [DOI] [PubMed] [Google Scholar]
- 184. Mizon C, Piva F, Queyrel V, Balduyck M, Hachulla E, Mizon J. Urinary bikunin determination provides insight into proteinase/proteinase inhibitor imbalance in patients with inflammatory diseases. Clin Chem Lab Med. 2002;40(6):579. [DOI] [PubMed] [Google Scholar]
- 185. Wu R, Cui X, Lim YP, Bendelja K, Zhou M, Simms HH, Wang P. Delayed administration of human inter-a inhibitor proteins reduces mortality in sepsis. Crit Care Med. 2004;32(8):1747–52. [DOI] [PubMed] [Google Scholar]
- 186. Chaaban H, Keshari RS, Silasi-Mansat R, Popescu NI, Mehta-D’Souza P, Lim Y-P, Lupu F. Inter-α inhibitor protein and its associated glycosaminoglycans protect against histone-induced injury. Blood. 2015;125(14):2286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. McDonald B, McAvoy EF, Lam F, Gill V, de la Motte C, Savani RC, Kubes P. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J Exp Med. 2008;205(4):915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Stober VP, Lim Y-P, Opal S, Zhuo L, Kimata K, Garantziotis S. Inter-α-inhibitor ameliorates endothelial inflammation in sepsis. Lung. 2019;197(3):361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kaya G, Augsburger E, Stamenkovic I, Saurat JH. Decrease in epidermal CD44 expression as a potential mechanism for abnormal hyaluronate accumulation in superficial dermis in lichen sclerosus et atrophicus. J Invest Dermatol. 2000;115(6):1054–58. [DOI] [PubMed] [Google Scholar]
- 190. Kuroda K, Fujimoto N, Tajima S. Abnormal accumulation of inter-alpha-trypsin inhibitor and hyaluronic acid in lichen sclerosus. J Cutan Pathol. 2005;32(2):137–40. [DOI] [PubMed] [Google Scholar]
- 191. Spasova MS, Chen X, Sadowska GB, Horton ER, Lim YP, Stonestreet BS. Ischemia reduces inter-alpha inhibitor proteins in the brain of the ovine fetus. Dev Neurobiol. 2017;77(6):726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Chen X, Nakada S, Donahue JE, Chen RH, Tucker R, Qiu J, Lim Y-P, Stopa EG, Stonestreet BS. Neuro-protective effects of inter-alpha inhibitor proteins after hypoxic-ischemic brain injury in neonatal rats. Exp Neurol. 2019;317:244–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Gaudet CM, Lim YP, Stonestreet BS, Threlkeld SW. Effects of age, experience and inter-alpha inhibitor proteins on working memory and neuronal plasticity after neonatal hypoxia-ischemia. Behav Brain Res. 2016;302:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003;98(2):465–73. [DOI] [PubMed] [Google Scholar]
- 195. Al-Mubarak B, Abouelhoda M, Omar A, AlDhalaan H, Aldosari M, Nester M, Alshamrani HA, El-Kalioby M, Goljan E, Albar R, Subhani S, Tahir A, Asfahani S, Eskandrani A, Almusaiab A, Magrashi A, Shinwari J, Monies D, Al Tassa N. Whole exome sequencing reveals inherited and de novo variants in autism spectrum disorder: a trio study from Saudi families. Sci Rep. 2017;7(1):5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Meier S, Bell M, Lyons DN, Ingram A, Chen J, Gensel JC, Zhu H, Nelson PT, Abisambra JF. Identification of novel Tau interactions with endoplasmic reticulum proteins in Alzheimer’s disease brain. J Alzheimers Dis. 2015;48(3):687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Reed MJ, Damodarasamy M, Pathan JL, Chan CK, Spiekerman C, Wight TN, Banks WA, Day AJ, Vernon RB, Keene CD. Increased hyaluronan and TSG-6 in association with neuropathologic changes of Alzheimer’s disease. J Alzheimers Dis. 2019;67:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Brandl EJ, Lett TA, Chowdhury NI, Tiwari AK, Bakanidze G, Meltzer HY, Potkin SG, Lieberman JA, Kennedy JL, Müller DJ. The role of the ITIH3 rs2535629 variant in antipsychotic response. Schizophr Res. 2016;176(2–3):131–5. [DOI] [PubMed] [Google Scholar]
- 199. Khan SR. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol. 2014;3(3):256–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Dawson CJ, Grover PK, Kanellos J, Pham H, Kupczyk G, Oates A, Ryall RL. Inter-alpha-inhibitor in calcium stones. Clin Sci. 1998;95(2):187–93. [PubMed] [Google Scholar]
- 201. Roberts SD, Resnick MI. Glycosaminoglycans content of stone matrix. J Urol. 1986;135(5):1078–83. [DOI] [PubMed] [Google Scholar]
- 202. Evan AP, Bledsoe S, Worcester EM, Coe FL, Lingeman JE, Bergsland KJ. Renal inter-alpha-trypsin inhibitor heavy chain 3 increases in calcium oxalate stone-forming patients. Kidney Int. 2007;72(12):1503–11. [DOI] [PubMed] [Google Scholar]
- 203. Eguchi Y, Inoue M, Iida S, Matsuoka K, Noda S. Heparan sulfate (HS)/heparan sulfate proteoglycan (HSPG) and bikunin are up-regulated during calcium oxalate nephrolithiasis in rat kidney. Kurume Med J. 2002;49(3):99–107. [DOI] [PubMed] [Google Scholar]
- 204. Sekikawa S, Onda T, Miura N, Nomura T, Takano N, Shibahara T, Honda K. Underexpression of α-1-microglobulin/bikunin precursor predicts a poor prognosis in oral squamous cell carcinoma. Int J Oncol. 2018;53(6):2605–14. [DOI] [PubMed] [Google Scholar]
- 205. Bayraktar E, Igci M, Erturhan S, Igci YZ, Karakok M, Gogebakan B, Ulasli M, Cakmak EA, Arslan A. Reduced gene expression of bikunin as a prognostic marker for renal cell carcinoma. Exp Oncol. 2014;36(2):107–11. [PubMed] [Google Scholar]
- 206. Rose M, Gaisa NT, Antony P, Fiedler D, Heidenreich A, Otto W, Denzinger S, Bertz S, Hartmann A, Karl A, Knüchel R, Dahl E. Epigenetic inactivation of ITIH5 promotes bladder cancer progression and predicts early relapse of pT1 high-grade urothelial tumours. Carcinogenesis. 2014;35(3):727–36. [DOI] [PubMed] [Google Scholar]
- 207. Kloten V, Rose M, Kaspar S, von Stillfried S, Knuchel R, Dahl E. Epigenetic inactivation of the novel candidate tumor suppressor gene ITIH5 in colon cancer predicts unfavorable overall survival in the CpG island methylator phenotype. Epigenetics. 2014;9(9):1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Oing C, Jost E, Dahl E, Wilop S, Brummendorf TH, Galm O. Aberrant DNA hypermethylation of the ITIH5 tumor suppressor gene in acute myeloid leukemia. Clin Epigenetics. 2011;2(2):419–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Sasaki K, Kurahara H, Young ED, Natsugoe S, Ijichi A, Iwakuma T, Welch DR. Genome-wide in vivo RNAi screen identifies ITIH5 as a metastasis suppressor in pancreatic cancer. Clin Exp Metastas. 2017;34(3):229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Rose M, Meurer SK, Kloten V, Weiskirchen R, Denecke B, Antonopoulos W, Deckert M, Knüchel R, Dahl E. ITIH5 induces a shift in TGF-β superfamily signaling involving Endoglin and reduces risk for breast cancer metastasis and tumor death. Mol Carcinog. 2018;57(2):167–81. [DOI] [PubMed] [Google Scholar]
- 211. Dittmann J, Ziegfeld A, Jansen L, Gajda M, Kloten V, Dahl E, Runnebaum IB, Dürst M, Backsch C. Gene expression analysis combined with functional genomics approach identifies ITIH5 as tumor suppressor gene in cervical carcinogenesis. Mol Carcinog. 2017;56(6):1578–89. [DOI] [PubMed] [Google Scholar]
- 212. Dötsch MM, Kloten V, Schlensog M, Heide T, Braunschweig T, Veeck J, Petersen I, Knüchel R, Dahl E. Low expression of ITIH5 in adenocarcinoma of the lung is associated with unfavorable patients’ outcome. Epigenetics. 2015;10(10):903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Mai C, Zhao J-J, Tang X-F, Wang W, Pan K, Pan Q-Z, Zhang X-F, Jiang S-S, Zhao B-W, Li Y-F, Xia J-C, Zhou Z-W. Decreased ITIH5 expression is associated with poor prognosis in primary gastric cancer. Med Oncol. 2014;31(7):53. [DOI] [PubMed] [Google Scholar]
- 214. Kobayashi H, Fujie M, Shinohara H, Ohi H, Sugimura M, Terao T. Effects of urinary trypsin inhibitor on the invasion of reconstituted basement membranes by ovarian cancer cells. Int J Cancer. 1994;57(3):378–84. [DOI] [PubMed] [Google Scholar]
- 215. Kobayashi H, Shinohara H, Fujie M, Gotoh J, Itoh M, Takeuchi K, Terao T. Inhibition of metastasis of Lewis lung carcinoma by urinary trypsin inhibitor in experimental and spontaneous metastasis models. Int J Cancer. 1995;63(3):455–62. [DOI] [PubMed] [Google Scholar]
- 216. Kobayashi H, Sugino D, She MY, Ohi H, Hirashima Y, Shinohara H, Fujie M, Shibata K, Terao T. A bifunctional hybrid molecule of the amino-terminal fragment of urokinase and domain II of bikunin efficiently inhibits tumor cell invasion and metastasis. Eur J Biochem. 1998;253(3):817–26. [DOI] [PubMed] [Google Scholar]
- 217. Zhang T, Guo J, Gu J, Wang Z, Wang G, Li H, Wang J. Identifying the key genes and microRNAs in colorectal cancer liver metastasis by bioinformatics analysis and in vitro experiments. Oncol Rep. 2019;41(1):279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Suzuki M, Kobayashi H, Tanaka Y, Hirashima Y, Terao T. Structure and function analysis of urinary trypsin inhibitor (UTI): identification of binding domains and signaling property of UTI by analysis of truncated proteins. Biochim Biophys Acta. 2001;1547(1):26–36. [DOI] [PubMed] [Google Scholar]
- 219. Choi-Miura NH, Tobe T, Sumiya J, Nakano Y, Sano Y, Mazda T, Tomita M. Purification and characterization of a novel hyaluronan-binding protein (PHBP) from human plasma: it has three EGF, a kringle and a serine protease domain, similar to hepatocyte growth factor activator. J Biochem. 1996;119(6):1157–65. [DOI] [PubMed] [Google Scholar]
- 220. Matsuzaki H, Kobayashi H, Yagyu T, Wakahara K, Kondo T, Kurita N, Sekino H, Inagaki K, Suzuki M, Kanayama N, Terao T. Plasma bikunin as a favorable prognostic factor in ovarian cancer. J Clin Oncol. 2005;23(7):1463–72. [DOI] [PubMed] [Google Scholar]
- 221. Huang H, Han Y, Gao J, Feng J, Zhu L, Qu L, Shen L, Shou C. High level of serum AMBP is associated with poor response to paclitaxel-capecitabine chemotherapy in advanced gastric cancer patients. Med Oncol. 2013;30(4):748. [DOI] [PubMed] [Google Scholar]
- 222. Boccellino M, Pinto F, Ieluzzi V, Giovane A, Quagliuolo L, Fariello C, Coppola M, Carlucci A, Santini M, Ferati K, Bexheti-Ferati A, Giordano A, Di Domenico M. Proteomics analysis of human serum of patients with non-small-cell lung cancer reveals proteins as diagnostic biomarker candidates. J Cell Physiol. 2019;234(12):23798–806. [DOI] [PubMed] [Google Scholar]
- 223. Kanayama N, el Maradny E, Halim A, Liping S, Maehara K, Kajiwara Y, Terao T. Urinary trypsin inhibitor prevents uterine muscle contraction by inhibition of Ca++ influx. Am J Obstet Gynecol. 1995;173(1):192–9. [DOI] [PubMed] [Google Scholar]
- 224. El Maradny E, Kanayama N, Halim A, Maehara K, Kobayashi T, Terao T. Effects of urinary trypsin inhibitor on myometrial contraction in term and preterm deliveries. Gynecol Obstet Invest. 1996;41(2):96–102. [DOI] [PubMed] [Google Scholar]
- 225. Borzychowski AM, Sargent IL, Redman CW. Inflam-mation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11(5):309–16. [DOI] [PubMed] [Google Scholar]
- 226. van den Berg CB, Duvekot JJ, Guzel C, Hansson SR, de Leeuw TG, Steegers EA, Versendaal J, Luider TM, Stoop MP. Elevated levels of protein AMBP in cerebrospinal fluid of women with preeclampsia compared to normotensive pregnant women. Prot Clin Appl. 2017;11(1–2):1600082. [DOI] [PubMed] [Google Scholar]
- 227. Park J, Cha DH, Lee SJ, Kim YN, Kim YH, Kim KP. Discovery of the serum biomarker proteins in severe preeclampsia by proteomic analysis. Exp Mol Med. 2011;43(7):427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Zhang X, Zhu Z, Jiao W, Liu W, Liu F, Zhu X. Ulinastatin treatment for acute respiratory distress syndrome in China: a meta-analysis of randomized controlled trials. BMC Pulm Med. 2019;19:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Wang H, Liu B, Tang Y, Chang P, Yao L, Huang B, Lodato RF, Liu Z. Improvement of sepsis prognosis by ulinastatin: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2019;10:1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Lagoo JY, D’Souza MC, Kartha A, Kutappa AM. Role of Ulinastatin, a trypsin inhibitor, in severe acute pancreatitis in critical care setting: a retrospective analysis. J Crit Care. 2018;45:27–32. [DOI] [PubMed] [Google Scholar]
- 231. Balduyck M, Albani D, Jourdain M, Mizon C, Tournoys A, Drobecq H, Fourrier F, Mizon J. Inflammation-induced systemic proteolysis of inter-alpha-inhibitor in plasma from patients with sepsis. J Lab Clin Med. 2000;135:188–98. [DOI] [PubMed] [Google Scholar]
- 232. Nuijens JH, Abbink JJ, Wachtfogel YT, Colman RW, Eerenberg AJ, Dors D, Kamp AJ, Strack van Schijndel RJ, Thijs LG, Hack CE. Plasma elastase alpha 1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. J Lab Clin Med. 1992;119:159–68. [PubMed] [Google Scholar]
- 233. Hällgren R, Samuelsson T, Laurent TC, Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis. 1989;139(3):682–7. [DOI] [PubMed] [Google Scholar]
- 234. Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Bell TJ, Brand OJ, Morgan DJ, Salek-Ardakani S, Jagger C, Fujimori T, Cholewa L, Tilakaratna V, Östling J, Thomas M, Day AJ, Snelgrove RJ, Hussell T. Defective lung function following influenza virus is due to prolonged, reversible hyaluronan synthesis. Matrix Biol. 2019;80:14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D’Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kanai T, Ishiwata T, Kobayashi T, Sato H, Takizawa M, Kawamura Y, Tsujimoto H, Nakatani K, Ishibashi N, Nishiyama M, Hatai Y, Asano Y, Kobayashi T, Takeshita S, Nonoyama S. Ulinastatin, a urinary trypsin inhibitor, for the initial treatment of patients with Kawasaki disease: a retrospective study. Circulation. 2011;124(25):2822–8. [DOI] [PubMed] [Google Scholar]
- 238. Singh K, Zhang LX, Bendelja K, Heath R, Murphy S, Sharma S, Padbury JF, Lim Y-P. Inter-alpha inhibitor protein administration improves survival from neonatal sepsis in mice. Pediatr Res. 2010;68(3):242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Opal SM, Lim Y-P, Cristofaro P, Artenstein AW, Kessimian N, Delsesto D, Parejo N, Palardy JE, Siryaporn E. Inter-α inhibitor proteins: a novel therapeutic strategy for experimental anthrax infection. Shock. 2011;35(1):42–4. [DOI] [PMC free article] [PubMed] [Google Scholar]