Abstract
Glypicans are a family of heparan sulfate proteoglycans that are attached to the cell membrane via a glycosylphosphatidylinositol anchor. Glypicans interact with multiple ligands, including morphogens, growth factors, chemokines, ligands, receptors, and components of the extracellular matrix through their heparan sulfate chains and core protein. Therefore, glypicans can function as coreceptors to regulate cell proliferation, cell motility, and morphogenesis. In addition, some glypicans are abnormally expressed in cancers, possibly involved in tumorigenesis, and have the potential to be cancer-specific biomarkers. Here, we provide a brief review focusing on the expression of glypicans in various cancers and their potential to be targets for cancer therapy.
Keywords: biomarker, cancer, extracellular matrix, glycosaminoglycan, glypican, heparan sulfate proteoglycan, immunostaining
Introduction
As one of the leading causes of human death, cancer remains a major health problem worldwide.1 An understanding of molecular changes in cancer development is important to reveal new biomarkers and targets for diagnosis and treatment of the disease. Heparan sulfate proteoglycans (HSPGs), which are mainly found at the cell surface and in the extracellular matrix, have gained increased scientific interest due to their roles in malignant transformation.2,3
Glypicans, one of the HSPG families, are comprised of three key features: a 60- to 70-kDa core protein, heparan sulfate (HS) chains, and a glycosylphosphatidylinositol (GPI) linkage that anchors glypicans to the cell surface.4 The core protein is predicted to form a conserved tertiary structure due to seven intramolecular disulfide bridges caused by 14 conserved cysteine residues. There are six glypican family members in humans, referred to as glypican-1 (GPC1) through glypican-6 (GPC6). In addition to the difference in the primary sequences of their protein cores, they have various numbers of HS chains. Glypicans play important roles in development by modulating morphogen gradient formation5 and cell growth by regulating Wnt6–8 and other signaling pathways. Glypicans are abnormally expressed in multiples types of cancer and are crucial for cancer cell growth and progression.9–11 For example, GPC212,13 and GPC314,15 have highly specific expression in pediatric cancers and hepatocellular carcinoma (HCC), respectively. In particular, GPC1, GPC2, and GPC3 have been studied as potential diagnostic tools and therapeutic targets. This review will summarize the latest findings related to the expression and functions of glypicans in cancers and will emphasize their potentials as emerging biomarkers and promising therapeutic targets to fight cancer.
GPC1
The GPC1 gene on chromosome 2q37 encodes the GPC1 protein, consisting of a 558-amino-acid core protein and three predicted HS chains attached at S486, S488, and S490 (UniProtKB/Swiss-Prot P35052). GPC1 has both a membrane-anchored form and a secreted soluble form.16 It is mainly expressed in the neural and skeletal systems during embryonic development and is expressed at low levels in adult tissues.17 GPC1 is involved in cancer development through interactions with growth factors such as fibroblast growth factor-2 (FGF-2),18,19 vascular endothelial growth factor (VEGF),20,21 and transforming growth factor beta (TGF-β).22
GPC1 Expression in Cancer
Many studies have shown that GPC1 is upregulated in a variety of human cancers. In 1998, Kleeff et al.23 reported that GPC1 was strongly expressed in human pancreatic cancer, by both the cancer cells and the adjacent fibroblasts, whereas its expression was low in normal pancreas and in chronic pancreatitis. Furthermore, high GPC1 expression is significantly related to the perineural invasion of pancreatic cancer and is associated with poor patient prognosis.24 They also observed strong GPC1 immunoreactivity in the fibroblasts surrounding the cancer cells. More recently, Lu et al.25 systematically evaluated GPC1 expression in large-scale clinical samples and detected GPC1 protein in 59.7% (111/186) of pancreatic tumor tissues. However, GPC1 protein was rarely found in normal and adjacent non-cancerous pancreas. They also reported that GPC1 was elevated in 63.6% (34/55) of early-stage cases of pancreatic cancer.
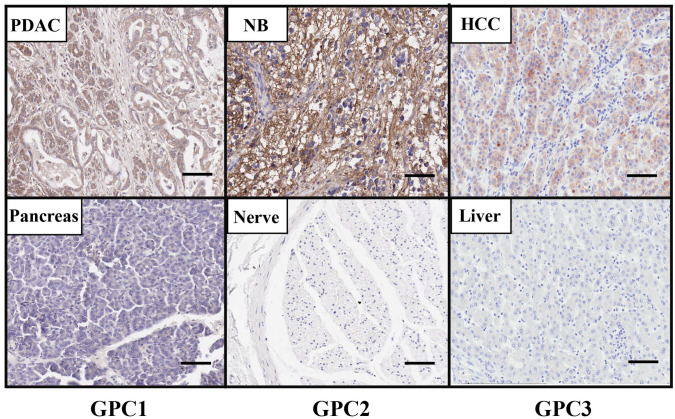
We isolated a high-affinity monoclonal antibody (MAb), HM2, that recognizes the C-lobe of GPC1 by mouse hybridoma technology.26 The HM2 antibody was used to detect GPC1 protein expression in tissues by immunohistochemistry (IHC). As shown in Fig. 1, the GPC1 protein is highly expressed in pancreatic tumor tissue compared with normal pancreas, which is consistent with reports described above. Interestingly, we also detected GPC1 expression in surrounding fibroblasts, which may promote tumor metastasis and angiogenesis by interacting with growth factors.
Figure 1.
The protein expression of GPC1, GPC2, and GPC3 in cancers. GPC1 protein level is increased in PDAC tissue compared with normal pancreas as determined by IHC using the GPC1-specific mouse monoclonal antibody (MAb) HM2. GPC2 protein is presented in NB tissue but not in normal peripheral nerve tissue as determined by IHC using the anti-GPC2 mouse MAb CT3. GPC3 is overexpressed in HCC tissue compared with normal liver tissue using the GPC3-specific mouse MAb YP7 by IHC. Images were obtained under 20 × magnification. Scale bar = 200 µm. Abbreviations: HCC, hepatocellular carcinoma; IHC, immunohistochemistry; NB, neuroblastoma; PDAC, pancreatic ductal adenocarcinoma.
The overexpression of GPC1 has also been described in other cancer types, including breast cancer27 and glioma,28 and is associated with a poor prognosis in glioblastomas.29 Suhovskih et al.30 showed that GPC1 localization was altered, from the epithelial cells in normal prostate tissues, to the stroma surrounding prostate tumors. A recent report demonstrated that the expression of GPC1 and α3(V) collagen, an important factor for mammary tumor progression, was tightly associated in breast cancer.31 These findings suggest that the protumor roles of GPC1 may be mediated by interaction with tumor microenvironment in certain types of cancer. Moreover, 98.8% of esophageal squamous cell carcinoma (ESCC) tissues demonstrated an elevated expression of GPC1 in the IHC assessment of 175 ESCC patients, and the overexpression of GPC1 is associated with poor prognosis and chemoresistance in ESCC.32 In addition, Matsuzaki et al.33 detected high levels of GPC1 in nearly 50% of uterine cervical cancer cases.
Multiple studies have been conducted to reveal the underlying mechanisms of upregulation of GPC1 in various cancers. Tanaka et al.34 reported that KRAS and ecotropic viral integration site 1 (EVI1), two known drivers of pancreatic carcinogenesis, positively regulated GPC1 expression. Another possible mechanism is the promoter hypomethylation in the GPC1 gene in pancreatic cancer, as the methylation status of GPC1 was inversely correlated with its mRNA levels in pancreatic tumor tissues, which explains the reexpression of GPC1 in pancreatic cancer.25
In addition to its membrane-anchored form, GPC1 also has a secreted soluble form and can be detected in the peripheral blood system. Several studies have been carried out to explore its potential as a clinical biomarker. In 2015, Melo et al.35 first reported that GPC1 was enriched in cancer cell–derived exosomes and detected in the early stages of pancreatic cancer with 100% sensitivity and specificity. Nevertheless, some recent studies yielded controversial results. They found that high levels of serum GPC1 may be able to determine pancreatic tumor size and disease burden, but were unable to distinguish pancreatic cancer from benign pancreatic disease.36,37 The discrepancies might originate from different technical approaches to measure exosomal GPC1 levels. Therefore, more thorough studies will be necessary to establish whether GPC1 in the blood can be a biomarker for pancreatic cancer and other cancers.
GPC1 Signaling in Cancer
One of the altered cellular activities induced by GPC1 might be through its modulation of the FGF-2 signaling pathway, which is known to regulate cell growth and survival.38 Qiao et al.18 showed that GPC1 expression enhanced the growth of brain endothelial cells by promoting FGF-2 signaling. GPC1 also modulates VEGF signaling, a key factor for angiogenesis.39 Whipple et al.21 generated a GPC1 null mouse model of pancreatic cancer and found these mice exhibited suppressed angiogenesis in conjunction with the decreased mRNA expression of VEGF-A. In addition, GPC1 is reported to modulate the TGF-β signaling pathway. Downregulation of GPC1 attenuated TGF-β1-induced cell growth inhibition and Smad 2 phosphorylation in pancreatic cancer cells.22 Kayed et al.40 further elucidated the role of GPC1 in the TGF-β signaling pathway, in which they found GPC1 downregulation led to an altered response toward TGF-β1, activin-A, and BMP2 in terms of growth, p21 induction, and Smad2 phosphorylation, resulting in pancreatic cancer cell growth inhibition. Moreover, GPC1 was found to be involved in the activation of the epidermal growth factor receptor (EGFR) signaling pathway in response to EGFR ligands, including heparin-binding epidermal growth factor–like growth factor (HB-EGF) in ESCC cells.41 Finally, Li et al.42 further demonstrated that GPC1 promotes ESCC cell proliferation by regulating the PTEN/Akt/β-catenin pathways. The involvement of GPC1 in the signaling pathways described above is associated with the membrane-bound form of GPC1; whether these activities might be counteracted by the soluble form of GPC1 warrants further investigations.
GPC1 as a Target of Therapy
The anti-GPC1 antibody (clone 1–12) was isolated from chicken and recognized both human and mouse GPC1.41 It induced 70% tumor growth inhibition in both ESCC xenograft models and patient-derived tumor (PDX) xenograft models in immunodeficient mice. The same research group then isolated another anti-GPC1 mouse MAb, clone 01a033, and developed an antibody–drug conjugate (ADC) by conjugating the MAb with monomethyl auristatin F.33 The GPC1-ADC showed potent tumor growth inhibition in xenograft models of uterine cervical cancer and pancreatic cancer.33,43
Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 has shown a remarkable response rate exceeding 80% against B-cell malignancies.44,45 This success has led to many studies evaluating the efficacy of CAR T-cell therapy in solid tumors. Kato et al.46 developed GPC1-specific human and murine CAR T-cells generated from the anti-human/mouse GPC1 antibody (clone 1–12) and demonstrated their antitumor effects in xenogeneic and syngeneic solid tumor mouse models without showing any obvious adverse effects. This study shows the potential of GPC1 as an immunotherapeutic target for solid tumors.
GPC2
The GPC2 gene is located on chromosome 7q22, which encodes the GPC2 protein consisting of a 579-amino-acid core protein and five predicted HS chains at S55, S92, S155, S500, and S502 (UniProtKB/Swiss-Prot Q8N158). GPC2 is exclusively expressed in the developing nervous system.47 GPC2 is expressed on neuronal growth cones, making it likely that it functions in axon growth and guidance.48 After axonal growth has completed, GPC2 is no longer expressed in the nervous system.49
GPC2 Expression in Cancer
Orentas et al.50 initially reported that GPC2 is highly expressed at the mRNA level in at least eight different pediatric cancers. In particular, GPC2 has been highly expressed in neuroblastoma. We and the Maris group showed that GPC2 protein was highly expressed in several neuroblastoma cell lines by Western blot.12,13 GPC2 was detected at the cell surface, shown by IHC, in a majority of neuroblastoma tumors, but is not detectable in adult tissues,12,13 making its expression tumor-specific. Moreover, integration of clinical and genomic information from neuroblastoma patients revealed a correlation between high GPC2 expression and poorer neuroblastoma prognosis, including poorer overall survival.12
We also generated a panel of mouse anti-human GPC2 MAbs.51 Among five hybridoma clones, CT3 demonstrated the best binding affinity to cell surface GPC2 by flow cytometry. As shown in Fig. 1, GPC2 is uniformly overexpressed in neuroblastoma compared with normal peripheral nerve tissue as determined by IHC using the CT3 antibody. Moreover, the CT3 antibody showed the most restricted expression in human normal tissues by IHC among five hybridoma clones. These observations suggest that the CT3 antibody could be useful for neuroblastoma diagnosis.
In clinical settings, neuroblastoma prognosis is partially determined by the status of the oncogene MYCN, which encodes the protein N-Myc, where the presence of MYCN amplification stratifies neuroblastoma cases into a high-risk group.52 Neuroblastoma tumors already designated as high-risk had significantly higher GPC2 mRNA expression.13 In addition, neuroblastoma cell lines that have high GPC2 protein expression also have high N-Myc protein expression; neuroblastoma cell lines that are GPC2 low-expressing do not express N-Myc, further supporting a possible biological connection between GPC2 and MYCN in neuroblastoma.12
Several other pediatric cancers displayed increased GPC2 levels similar to those of neuroblastoma, including medulloblastoma and retinoblastoma.13 In particular, group 4 medulloblastoma had GPC2 mRNA expression significantly higher than the other groups.13 This group can also have MYCN amplification, where MYCN-amplified cases have a significantly worse outcome.53 In addition, MYCN amplification is also frequently associated with retinoblastoma.54
GPC2 Signaling in Cancer
Glypicans are known to regulate Wnt/β-catenin signaling to affect cell growth and proliferation.6–8 It has been shown that β-catenin can activate the N-Myc promoter during embryonic development.55 It is possible that GPC2 functions as a modulator of Wnt signaling, acting to regulate MYCN in neuroblastoma pathogenesis. Indeed, we found that GPC2 could interact with Wnt3a.12 We demonstrated that genetic silencing of GPC2 inhibited neuroblastoma cell growth by downregulating Wnt/β-catenin signaling to suppress N-Myc expression, indicating that neuroblastoma may be addicted to GPC2 for viability and proliferation. Bosse et al.13 found that depleting GPC2 in neuroblastoma cell lines decreased cell proliferation, whereas overexpressing GPC2 increased cell proliferation. In addition, a pathway analysis showed that the Wnt signaling pathway is GPC2-associated.13
GPC2 as a Target of Therapy
The tumor-specific expression of GPC2 makes it attractive as a therapeutic target, as off-target effects can be avoided. Tumor escape through downregulation of a tumor antigen is a common problem in immunotherapy.56 As neuroblastoma cell proliferation might be dependent on GPC2, a tumor would likely not be able to downregulate its expression. This further supports GPC2 as a promising therapeutic target.
We have developed several antibody-based therapeutics that target GPC2 for the treatment of neuroblastoma. First, anti-GPC2 human single-domain antibodies (e.g., LH7) were isolated via phage display technology. Immunotoxin constructed from LH7 and a 38-kDa fragment of Pseudomonas exotoxin (PE-38) inhibited neuroblastoma tumor growth in mice.12 Moreover, the GPC2-targeted CAR T-cells we produced with LH7 were able to eliminate tumors in a disseminated neuroblastoma mouse model.12
Bosse et al.13 developed an ADC that targets GPC2. They screened a human Fab phage display library with GPC2 and found D3-GPC2-Fab binds GPC2 with high affinity. The ADC was constructed using D3-GPC2-IgG1, a human IgG1 converted from D3-GPC2-Fab, and pyrrolobenzodiazepine, a DNA-damaging agent frequently used in cancer treatment. When tested in seven neuroblastoma cell lines, the D3-GPC2-PBD ADC induced cytotoxicity in an antigen-dependent manner. The ADC also showed antitumor efficacy in a neuroblastoma mouse model.
GPC3
The GPC3 gene is on chromosome Xq26 and encodes the GPC3 protein composed of a 580-amino-acid core protein and two predicted HS chains attached at S495 and S509 (UniProtKB/Swiss-Prot P51654). Three variants have been detected that encode alternatively spliced forms,57 although the distribution and function of GPC3 isoforms remain largely unknown. GPC3 is expressed in various fetal tissues (liver, lung, kidney, placenta), but is absent or expressed at low levels in most adult tissues.58 GPC3 can regulate cell proliferation by modulating Wnt,8,59,60 YAP,15 Hedgehog (Hh),61–63 hepatocyte growth factor (HGF),64 and other signaling pathways.
GPC3 Expression in Cancer
In 1997, Hsu et al.65 first described the abnormal expression of GPC3 mRNA in 74.8% (143/191) of HCC tissues, compared with only 3.2% (5/154) in non-tumor liver tissues. At the protein level, Capurro et al.66 detected GPC3 expression in 72% (21/29) of HCC patients via IHC, whereas no GPC3 expression (0/38) was found in patients with a healthy liver or benign liver diseases. Similar results were confirmed by Baumhoer et al.,67 who found GPC3 expression in 63.6% (140/220) of HCC tissues. In addition, GPC3 was detected in HCC tissues but not in benign liver tumors such as dysplastic nodules.68,69 In patients with hepatitis C virus infection, IHC results showed a 100% positive staining of GPC3 in HCC (n=22) compared with that of dysplastic nodules (0%, n=14).70 Many studies have also reported that GPC3 expression is correlated with a poor prognosis in patients with HCC.71–74 Takai et al.75 reported that the expression of GPC3 in HCC was involved in macrophage recruitment, indicating GPC3 may play a role in tumor microenvironment.
We conducted a high-throughput flow cytometry screening to identify a high-affinity anti-GPC3 MAb, YP7, from mouse hybridoma technology.76 The YP7 antibody recognizes the C-lobe of GPC3. Our immunohistochemical staining using the YP7 antibody confirmed that the level of GPC3 is highly upregulated in HCC tumor compared with normal liver tissue (Fig. 1).
Furthermore, the overexpression of GPC3 was found in the following adult cancers: lung squamous cell carcinoma (LSCC),77 melanoma,78 Merkel cell carcinoma,79 ovarian clear cell carcinoma (OCCC),80 thyroid cancer,81 urothelial carcinoma,82 and salivary gland tumors.83 Moek et al.84 performed a functional genomic mRNA profiling to predict GPC3 overexpression at the protein level in 60 cancer types in 18,055 PDX samples and validated the prediction by comparing with IHC staining of a breast cancer tissue microarray. The percentage of samples with predicted GPC3 overexpression was 77% for HCCs (n=364), 45% for LSCCs (n=405), and 19% for head and neck squamous cell carcinomas (n=344). More recently, GPC3 was found to be expressed in glioblastoma and is a driver of glioma tumorigenesis, network hyperexcitability, and synapse formation.85
As an oncofetal protein, GPC3 is also highly expressed on a variety of pediatric solid embryonal tumors. Multiple studies have demonstrated that GPC3 expression was increased on most hepatoblastomas.86–90 GPC3 was found to be overexpressed in germ cell tumors, particularly yolk sac tumors and choriocarcinomas.90–93 In addition, elevated expression of GPC3 was observed in a significant portion of Wilms tumors compared with adult kidney tumors and normal kidney tissue.86,94,95 Somatic mutations in GPC3 have been identified in some cases of Wilms tumors.96 Moreover, 25% to 35% of rhabdomyosarcoma (RMS) cases showed GPC3-positive staining from three different studies, whereas other pediatric sarcomas often do not express GPC3.89,90,97
Nevertheless, GPC3 was found to act as a potential tumor suppressor in some cancers. In a study done on the loss-of-function mutation of GPC3 in embryonic development, researchers observed an overgrowth of organs in fetal and postnatal development, known as Simpson–Golabi–Behmel overgrowth syndrome (SGBS).98,99 The reduced or even silenced expression of GPC3 was found in other cancer types including renal cell carcinoma, breast cancer, ovarian cancer, and mesothelioma.100–103 In breast cancer, ovarian cancer, and mesothelioma, the loss of GPC3 expression was partially due to hypermethylation of its promoter region, and its expression was restored by treating cells with a demethylating agent.100,102,103 Ectopic expression of GPC3 also induced the apoptosis of lung cancer cells and inhibited tumor growth in mice.104 Overall, these findings suggest that GPC3 plays different roles in a tissue-dependent manner in various cell lineages.
As GPC3 is highly expressed in HCCs, it is important to understand the regulatory mechanisms of GPC3 expression during HCC tumorigenesis. Trinh et al.105 showed that epigenetic alternations including both DNA methylation and histone modifications were critical for the transcriptional regulation of GPC3 in HCC. Li et al.106 demonstrated that GPC3 is a direct transcriptional target of c-Myc and that c-Myc overexpression increased GPC3 levels. Conversely, increased levels of GPC3 also elevated c-Myc expression, thus forming a positive feedback signaling loop. In addition, Maurel et al. screened a library of 876 microRNAs (miRNAs) and identified five miRNAs that regulate GPC3 expression: miR-96 and miR-1271 showed negative regulation, whereas miR-129-1-3p, miR-1291, and miR-1303 played inducing roles.107,108
GPC3 Signaling in Cancer
GPC3 acts as an essential coreceptor and triggers the activation of signaling, including Wnt, Hh, FGF, and HGF pathways in cancer.
Wnt Signaling
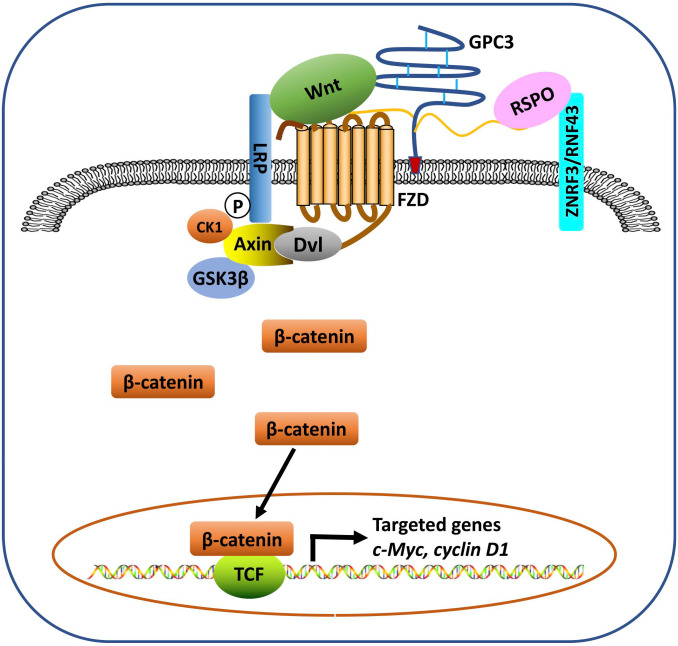
Activation of the Wnt signaling pathway is one of the most frequent molecular events associated with the progression of HCC. Approximately 95% of HCCs exhibit Wnt/β-catenin deregulation.109 Both the core protein and the HS chains may be involved in GPC3-modulated Wnt signaling. It has been shown that GPC3 HS chains may not be required for Wnt binding,59 but are essential for its interaction with frizzled (FZD).6 We identified a Wnt-recognizing motif on the HS chains of GPC3 using the HS20 antibody that binds the HS with high affinity and blocks GPC3 and Wnt3a interaction, and found that 2-O and 6-O sulfations were essential for Wnt binding while 3-O sulfation could enhance Wnt binding.7,110 Evidence of the Wnt binding domain on the GPC3 core protein has been indicated using the HN3 antibody.60 More recently, we identified F41, a key residue within GPC3’s cysteine-rich hydrophobic groove located in the N-lobe of GPC3, which is critical for the GPC3/Wnt interaction.8 We hypothesize that GPC3 serves as a Wnt coreceptor or receiver to attract Wnt to the cell surface and acts as a transmitter through its HS chains to stabilize the Wnt/GPC3/FZD tripartite complex once it is formed, thereby triggering downstream signaling (Fig. 2). Therefore, we believe GPC3 functions as both a receiver and a transmitter on the cell surface for Wnt signals.
Figure 2.
The model for the role of GPC3 in modulating Wnt signaling in HCC. GPC3 functions as a Wnt receiver to attract Wnt to the cell surface and acts as a transmitter through its HS chains to stabilize the Wnt/GPC3/FZD tripartite complex once it is formed, which triggers downstream β-catenin signaling. RSPO binds to the HS chains of glypican (e.g. GPC3) and the E3 ligases RNF43/ZNRF3 to block the degradation of FZD and LRP, thereby potentiating Wnt signaling. Abbreviations: FZD, frizzled; HCC, hepatocellular carcinoma; HS, heparan sulfate; LRP, lipoprotein receptor related protein; RSPO, R-spondin; TCF, T cell factor.
Cell Growth Factors
GPC3 has been demonstrated to interact with growth factors through its HS chains and ultimately stimulate cell growth.111 In HCC cells, GPC3 binds to FGF-2 through the HS chains.112 We demonstrated that GPC3 functions in HCC cell migration and motility through HS chain–mediated cooperation with the HGF/c-Met pathway7; antibody targeting the GPC3 core protein had no effect on HCC motility.64 Sun et al.113 discovered that GPC3 suppression in HCC cells led to enhanced TGF-β2 expression and thereby inhibited cell proliferation, arrested cell cycle progression, and induced replicative senescence. Midorikawa et al.111 showed that GPC3 overexpression inhibited BMP7 signaling and modulated BMP7 activity through the SMAD pathway and thus promoted cell proliferation.
Hh Signaling
GPC3 is a potent inhibitor of Hh signaling. Loss-of-function mutations of GPC3 cause SGBS, partially due to hyperactivation of the Hh pathway.114,115 Capurro et al.62 showed that GPC3 bound to sonic hedgehog (Shh) and Indian hedgehog (Ihh) with high affinity in an HS-independent manner, resulting in a strong competition with Patched (Ptc) for Hh binding. In a later study, they demonstrated that cleavage by convertases is also crucial for GPC3 inhibition of Hh signaling.61 The low-density lipoprotein receptor–related protein 1 (LRP1) was also shown to mediate endocytosis of the GPC3/Hh complex.116
YAP Signaling
HCC tissues are reported to show higher YAP activation, indicating a positive correlation between HCC progression and YAP activity.117 We found that knockdown of GPC3 induced apoptosis of HCC cells and reintroduction of constitutively active mutant YAP (S127A) was able to rescue HCC cells from apoptosis.15 We also demonstrated that the HN3 single-domain antibody inhibited HCC cell growth by inactivating YAP signaling.15,60
GPC3 displays a tissue-specific pattern of expression during tumor progression. It is overexpressed in some cancers (e.g., HCC) but acts as a tumor suppressor in other cancers (e.g., breast cancer). It is speculated that the tissue-specific differences are related to the ability of GPC3 to regulate different growth factors and signaling pathways.115,118 For example, the overexpression of GPC3 in HCC will promote tumor growth by stimulating the Wnt signaling pathway, which plays a vital role in HCC progression. In contrast, GPC3 exhibits an opposite role acting as an inhibitor in Hh signaling. The loss of GPC3 may contribute to cancer progression by inducing the activation of Hh signaling. It has been reported that the Hh signaling pathway is hyperactivated in breast and ovarian cancers.119,120
GPC3 as a Target of Therapy
Given the tumor specificity, GPC3 is an ideal target for cancer immunotherapy. Various therapeutic strategies have been developed to target GPC3, which are described in more detail below.
Vaccines
Peptide vaccines of GPC3 have been extensively investigated in preclinical and clinical studies. Initially, several reports showed that peptide-specific cytotoxic T-lymphocytes (CTLs) were induced in mice immunized with HLA-A24- and HLA-A2-restricited GPC3-derived peptides, which exerted antitumor effects without causing an autoimmune response.121,122 The first-in-human study of GPC3 peptide vaccine was conducted in Japan, which confirmed the safety of vaccines and demonstrated the GPC3 peptide–specific immune responses.123,124 In a later phase II clinical trial, Sawada et al.125 demonstrated that postsurgical administration of GPC3 peptide vaccine extended the recurrence-free survival period, and overall survival was prolonged in cases where CTL induction was observed. The same group developed GPC3-derived long peptides (GPC3-LPs) carrying human leukocyte antigen class II–restricted T-helper cell epitopes, which induced peptide-specific CD4+ T-cell responses from most healthy donors.126 In addition, clinical trials with a GPC3-derived peptide vaccine were conducted in patients with OCCC and hepatoblastoma.127,128 In both cancer types, vaccines induced GPC3-specific CTL responses in patients and extended recurrence-free survival.
Monoclonal Antibodies
Several therapeutic anti-GPC3 antibodies have been developed in recent years. GC33, a mouse MAb against GPC3, has been shown to induce antibody-dependent cell-mediated cytotoxicity against GPC3-positive HCC cell lines and inhibit tumor growth in HCC xenograft mouse models.129,130 In a first-in-human phase I trial for patients with advanced HCC, GC33 was well tolerated and antitumor effects were observed in some patients with high GPC3 expression.131 However, it did not show significant clinical benefit in a randomized phase II clinical trial,132 suggesting that antibodies alone may have limited therapeutic effects in HCC patients.
Our laboratory has isolated several anti-GPC3 antibodies for cancer therapy, including YP7, HN3 and HS20, which have been widely used preclinically. Although YP7 recognizes the C-lobe of GPC3 protein, it has a distinct binding epitope from GC33 based on our recent study.133 We also humanized the YP7 antibody (hYP7) using antibody engineering134 and showed that both YP7 and hYP7 antibodies had considerable antitumor activity in HCC xenograft mouse models.76,134 HN3, a human single-domain antibody that targets the N-lobe of GPC3, induced cell cycle arrest in HCC cells via inhibition of Wnt and YAP signaling.15,60 HS20 is a human MAb isolated by phage display technology, which recognizes HS but not chondroitin sulfate.7,110,135 The binding of the HS20 antibody to HS requires sulfation at both C2 position and C6 position. HS20 could disrupt the GPC3/Wnt interaction, thereby inhibiting Wnt/β-catenin signaling and suppressing the growth of HCC xenografts in mice.7,110
Immunotoxins
To improve the potency of MAb, we have made numerous efforts to develop novel GPC3-targeted immunotoxins. Our first study showed that HN3-PE38 immunotoxin regressed HCC xenograft tumors in mice at 0.6 mg/kg dosage.60 To reduce the side effects and immunogenicity, we constructed two deimmunized immunotoxins, HN3-mPE24 and HN3-T20, that contain only domain III of the PE38 toxin. In one approach, we removed B-cell epitopes on domain III and constructed HN3-mPE24 which was well tolerated in mice even at 5 mg/kg dosage and induced significant tumor regression in mice bearing HCC xenograft.136 In another approach, we engineered domain III by removing T-cell epitopes, creating the immunotoxin HN3-T20, which showed a greater increase in overall survival than HN3-mPE24.137 To enhance serum retention, we then added a streptococcal albumin-binding domain (ABD) into HN3-T20 and found that HN3-ABD-T20 had a 45-fold higher serum half-life than HN3-T20. Although further clinical investigation is required, the current data identify GPC3-targeted immunotoxins as promising candidates for HCC therapy.
CARs
The GPC3-targeted CARs based on the GC33 single-chain variable fragment (scFv) were initially reported by Gao et al.138 They demonstrated that GPC3-targeted CAR T-cells could inhibit the growth of orthotopic HCC xenografts in mice. Li et al.139 compared the contribution of CD28 and 4-1BB by evaluating the antitumor activities of T-cells expressing GC33-based CAR targeting GPC3. T-cell signaling through CD28 had higher cytotoxicity than the signaling through 4-1BB in vitro; however, 4-1BB induced the superior expansion of CAR T-cells and demonstrated potent antitumor activity in vivo. Jiang et al.140 found that GPC3-targeted CAR T-cells effectively eliminated tumors in PDX model of HCC. In addition, Wang et al.141 used the PiggyBac transposon system to deliver the GPC3-targeted CAR into T-cells, which reduced tumor burden in an HCC xenograft mouse model. Moreover, Chen et al. found that dual-targeted CAR T-cells coexpressing GPC3 and asialoglycoprotein receptor 1, a liver tissue–specific protein, reduced the risk of on-target/off-tumor toxicity in vivo. Furthermore, the same research group demonstrated that disruption of PD-1 enhanced the antitumor activity of GPC3-targeted CAR T-cells against HCC in mice and improved the persistence of infiltration of CAR T-cells.142,143 Based on these findings, Shi et al.144 have begun a phase I trial of HCC patients in China and demonstrated that GPC3-targeted CAR T-cell therapy was feasible for the first time. Early signs of antitumor activity were also observed in patients with advanced HCC.
We have developed CARs based on the hYP7 and HN3 antibodies, which have high affinities for the C-lobe and N-lobe of GPC3, respectively.133 Injection of CAR (hYP7) T-cells eliminated tumors in mice bearing orthotopic HCC xenografts, whereas CAR (HN3) T-cells did not reduce tumor burden. Mice injected with CAR (hYP7) T-cells had persistent expansion of T-cells that were found in spleen for up to 7 weeks after infusion and in subsets of polyfunctional CAR T-cells via antigen-induced selection. In the same study, we analyzed the integration sites of CAR (hYP7) in mice to investigate candidate factors contributing to CAR T-cell expansion and persistence. Many shared integrated genes were observed in different tissues on the same mouse, likely due to the expansion of selected clones. Interestingly, several integration events (e.g., NUPL1, DENND1B) became highly abundant in multiple mice at weeks 5 and 7 after infusion, indicating certain CAR-integrated sites or genes might play roles in CAR (hYP7) T-cell activation, survival, and expansion in vivo. The clinical development of CAR T-cell therapy against GPC3 based on our research is underway in China (NCT04121273) and the United States (NIH Clinical Center).
ADCs
In addition to immunotoxins and CARs, we constructed the GPC3-specific ADCs using the hYP7 antibody.145 HCC is known to have resistance to cancer chemotherapeutics.146,147 To determine the payload for ADCs against liver cancer, we and collaborators screened three large drug libraries (>9000 compounds) against HCC cell lines and found that the most potent drugs are DNA-damaging agents. We chose duocarmycin SA and pyrrolobenzodiazepine dimer as the payloads to construct two GPC3-specific ADCs: hYP7-DC and hYP7-PC. Encouragingly, single treatment of hYP7-PC induced tumor regression in multiple HCC xenograft mouse models. Lipovsek et al.148 conjugated an Adnectin that binds GPC3 to a cytotoxic derivative of tubulysin and proved its fast clearance from normal tissues and efficacy against GPC3-positive xenograft mouse models.
Other Approaches
MAbs can also be conjugated to the photosensitizing phthalocyanine dye IR700 to target cancer cells through exposure to near-infrared light. The conjugation of IR700 with our YP7 and HN3 antibodies, IR700-YP7 and IR700-HN3, showed an antitumor effect in GPC3-positive tumor-bearing mice.149 Furthermore, the combination of IR700-YP7 and nab-paclitaxel drastically increased nab-paclitaxel delivery and enhanced the therapeutic efficacy.150
ERY974, an anti-GPC3/CD3 bispecific T-cell-redirecting antibody regressed GPC3-positive solid tumors in mice.151 Intriguingly, ERY974 was effective even against tumors with non-immunogenic features by causing inflammation in the local tumor microenvironment. Bi et al.152 also reported a bispecific T-cell engager (BiTE) targeting GPC3, and CD3 could efficiently mediate the T-cell killing of GPC3-positive HCC cells in vitro and in vivo.
A dominant T-cell receptor (TCR), P1-1, targeting an abundantly displayed HLA-A2-specific GPC3 peptide was cloned from CTLs that were induced by dendritic cells overexpressing HLA-A2 and GPC3.153 CTLs overexpressing P1-1 inhibited GPC3-positive xenograft tumor growth at early stages, but its effect decreased in vivo in later phases. In addition, GPC3-specific CAR natural killer cells inhibited 90% of GPC3-expressing HCC xenograft growth.154
GPC4
GPC4 is composed of a 556-amino-acid core protein with the attachment of three HS chains at S494, S498, and S500 (UniProtKB/Swiss-Prot O75487). Interestingly, GPC4 is adjacent to the 3′ end of GPC3 on chromosome Xq26.155 GPC4 shows a predominant expression in developing brain and kidney.156
GPC4 Expression in Cancer
The increased expression of GPC4 has been found in pancreatic cancer. It is also involved in 5-fluorouracil resistance and pancreatic cancer stemness,157 suggesting that GPC4 may serve as a strategy for pancreatic cancer therapy. Fang et al.158 found that GPC4 protein level was significantly upregulated in colorectal tumor tissues compared with matched adjacent normal mucosa. In addition, recent studies demonstrated that GPC4 gene polymorphism was associated with susceptibilities to Epstein-Barr virus (EBV)-associated gastric carcinoma159 and EBV-positive nasopharyngeal carcinoma in Northern China.160 Moreover, Varma et al.161 showed that GPC4 mRNA level was downregulated in oxaliplatin-resistant ovarian carcinoma cell line A2780/C10. Recently, Munir et al.162 demonstrated that GPC4 expression was downregulated in metastatic breast tumors compared with non-metastatic breast tumors. Their results also indicate that GPC4 acts as a tumor suppressor in breast cancer by decreasing migration and proliferation.
Although mutations in GPC3 have been causatively and genetically linked to the SGBS, it has been speculated that GPC4, which is close to GPC3 on the X chromosome, might also be involved in the SGBS or other X-linked overgrowth or undergrowth syndromes. In one study, analysis of DNA samples from eight SGBS patients identified one individual with a deletion of both 3′ end of GPC3 and the entire GPC4 gene.155 In another report, a duplication GPC4 was identified in SGBS patients with no mutation found in GPC3,163 indicating GPC4 might independently play a role in growth control.
GPC4 Signaling in Cancer
Like other glypicans (e.g., GPC2 and GPC3), GPC4 could also be a regulator of Wnt signaling. Sakane et al.164 demonstrated that GPC4 is colocalized with different Wnts to lipid raft and non-lipid raft microdomains and thereby regulates distinct Wnt signaling pathways. Recently, Fang et al.158 reported that GPC4 interacted with CD36, a membrane glycoprotein involved in diverse cellular functions,165 in colorectal cancer. CD36–GPC4 interaction promoted the proteasome-dependent ubiquitination of GPC4, followed by inhibition of Wnt/β-catenin signaling.
R-spondins (RSPOs) are secreted proteins that act as potent amplifiers of the Wnt/β-catenin signaling. Lebensohn and Rohatgi166 found that HSPGs such as GPC4 and GPC6 are required for RSPO-enhanced Wnt signaling in cells lacking leucine-rich repeat-containing G-protein-coupled receptors (LGRs) (LGR4/5/6 knockout). Knockout of exostosin-like 3 (encoded by the EXTL3 gene), a glycosyltransferase required for the biosynthesis of HS chains on HSPGs, reduced ~80% of LGR-independent Wnt signaling. The TSP/BR domain of RSPO3 is proposed as a binding site for HSPGs because the deletion of this domain significantly reduced Wnt signaling activity. A more recent study showed that mutations of key residues in the TSP/BR domain of RSPO3 for HSPG binding also largely reduced Wnt signaling. Furthermore, the loss of Wnt activity in the presence and absence of LGRs by deletion or mutations of the TSP/BR domain of RSPO3 was rescued by replacing the entire TSP/BR domain with the HS20 scFv antibody64,135 that recognizes HS chains of GPC3 and GPC4.167 The fact that HSPGs can potentiate Wnt signaling in the absence of LGR (LGR-independent pathway) further supports the important role of GPC4 and other HSPGs as potent Wnt modulators (Fig. 2). Further studies will be needed to analyze precise and potentially differential roles of glypicans in cells containing and lacking LGRs.
GPC5
The GPC5 gene is located on chromosome 13q32, and encodes the GPC5 protein, which consists of a 572-amino-acid core protein and five predicted HS chains attached at S441, S486, S495, S507 and S509 (UniProtKB/Swiss-Prot P78333). GPC5 is expressed in kidney, limb, and brain during development; its expression in brain persists into adulthood.168,169
GPC5 Expression in Cancer
It has been previously reported that the GPC5 gene was amplified and its mRNA expression was upregulated in RMS.170 Nishimura et al.171 further confirmed that GPC5 was located in the frequently amplified region in RMS cases, suggesting aberrant GPC5 activity might play a role in RMS oncogenesis.
A genome-wide association study identified a single-nucleotide polymorphism (SNP) in the GPC5 gene associated with susceptibility to lung cancer in never-smokers.172 Downregulation of GPC5 may contribute to the development of lung cancer in never-smokers. Additional report also demonstrated that GPC5 expression was decreased in non–small cell lung cancer (NSCLC), and overexpression of GPC5 suppressed the migration, invasion, and proliferation of NSCLC cells.173 Yuan et al.174 further confirmed the low expression of GPC5 was significantly associated with poor outcome in NSCLC. They also found that the GPC5 promoter was hypermethylated in lung cancer tissues compared with normal lung tissues, which explains the loss of GPC5 expression.
However, another study found that GPC5 was upregulated in NSCLC and was associated with poor overall survival.175 Thus, the role of GPC5 in tumorigenesis of lung cancer needs to be further investigated.
In addition, a growing number of studies have demonstrated that GPC5 expression is downregulated in multiple cancers beyond lung cancer. For example, reduced GPC5 expression was found in breast cancer and was associated with poor survival.176 Zhang et al.177 recently reported that the level of GPC5 in prostate cancer tissues was significantly lower than that in normal controls, especially in high-risk prostate cancer. The reduced expression of GPC5 has been observed in pancreatic cancer,178 HCC,179 and glioma,180 indicating the tumor suppressive role of GPC5.
GPC5 Signaling in Cancer
GPC5 is reported to be involved in regulating various pathways, including Wnt, Hh, and FGF signaling. GPC5 has been shown to promote RMS proliferation through increasing the intracellular signaling of FGF-2 and HGF.170 Li et al.181 showed that GPC5 stimulates RMS cell proliferation by promoting Hh signaling. In particular, the tumor suppressive role of GPC5 is extensively associated with its inhibitory effect on Wnt/β-catenin signaling.174,182,183 Yuan et al.174 demonstrated that GPC5 could block the activation of Wnt/β-catenin signaling by binding to Wnt3a at the cell surface, which mediates its function as a tumor suppressor. In addition, Wang et al.182 reported that GPC5 suppressed lung cancer cell metastasis by competitively binding to Wnt3a. Moreover, GPC5 is shown to inhibit Wnt/β-catenin signaling in glioma, prostate, and pancreatic cancer.178,180,184
Table 1.
Expression of Glypicans in Cancers.
| Glypican | Cancer Type | Sample Type | Aberrant Expression |
Sites and Localization | Ref. | |
|---|---|---|---|---|---|---|
| mRNA | Protein | |||||
| GPC1 | Pancreatic cancer | Cells, tissues | Increase | Increase | Cancer cell, fibroblast | Kleeff et al., 199823 |
| Tissues | NA | Increase 56% (35/62) |
Cancer cell, fibroblast | Duan et al., 201324 | ||
| Tissues | NA | Increase 60% (111/186) |
Cell membrane and cytoplasm | Lu et al., 201725 | ||
| Breast cancer | Cells, tissues | Increase | Increase | Cell membrane | Matsuda et al., 200127 | |
| Glioma | Cells | Increase | NA | NA | Su et al., 200628 | |
| Glioblastoma | Tissue | NA | Increase 51% (27/53) |
NA | Saito et al., 201729 | |
| Prostate cancer | Cells, tissues | Increase | Increase | Tumor stroma | Suhovskih et al., 201330 | |
| ESCC | Cells, tissues | NA | Increase 99% (172/175) |
Cell membrane | Hara et al., 201632 | |
| Uterine cervical cancer | Cells, tissues |
NA | Increase | Cell membrane | Matsuzaki et al., 201833 | |
| GPC2 | Pediatric cancers | Tissues | Increase | NA | Cell membrane | Orentas et al., 201250 |
| Neuroblastoma | Cells, tissues | NA | Increase 52% (13/25) |
Cell membrane | Li et al., 201712 | |
| Neuroblastoma | Cells, tissues | Increase | Increase 93% (91/98) |
Cell membrane | Bosse et al., 201713 | |
| Medulloblastoma | Cells, tissues | Increase | Increase 90% (57/63) |
Cell membrane | Bosse et al., 201713 | |
| GPC3 | HCC | Tissues | Increase 75% (143/191) |
NA | NA | Hsu et al., 199765 |
| Tissues | NA | Increase 72% (21/29) |
Cell membrane | Capurro et al., 200366 | ||
| Tissues | NA | Increase 64% (140/220) |
NA | Baumhoer et al., 200867 | ||
| Tissues | Increase | Increase 76% (36/47) |
Cell membrane and cytoplasm | Libbrecht et al., 200669 | ||
| Tissues | NA | Increase 60% (24/40) |
Cell membrane and cytoplasm | Li et al., 2020133 | ||
| LSCC | Tissues | Increase | Increase 55% (17/31) |
Cell membrane and cytoplasm | Aviel-Ronen et al., 200877 | |
| Melanoma | Cells, tissues | Increase | Increase | Cytoplasm | Nakatsura et al., 200478 | |
| Merkel cell carcinoma | Tissues | NA | Increase 55% (17/31) |
Cell membrane and cytoplasm | He et al., 200979 | |
| OCCC | Tissues | NA | Increase 44% (41/94) |
Cell membrane and cytoplasm | Maeda et al., 200980 | |
| Thyroid cancer | Tissues | Increase | Increase | Cell membrane and cytoplasm | Yamanaka et al., 200781 | |
| Urothelial carcinoma | Tissues | NA | Increase 35% (38/108) |
NA | Aydin et al., 201582 | |
| GPC3 | Salivary gland tumors | Tissues | NA | Increase | Cell membrane and cytoplasm | Andisheh-Tadbir et al., 201983 |
| Glioblastoma | Tissues | NA | Increase | NA | Yu et al., 202085 | |
| Hepatoblastoma | Tissues | NA | Increase 100% (9/9) |
NA | Chan et al., 201389 | |
| Tissues | NA | Increase 60% (6/9) |
NA | Kinoshita et al., 201590 | ||
| Tissues | Increase | NA | NA | Toretsky et al., 200186 | ||
| Tissues | NA | Increase 100% (65/65) |
Cytoplasm | Zynger et al., 200888 | ||
| YST | Tissues | NA | Increase 91% (10/11) |
NA | Kinoshita et al., 201590 | |
| Tissues | NA | Increase 97% (31/32) |
Cell membrane and cytoplasm | Esheba et al., 200891 | ||
| Tissues | NA | Increase 100% (24/24) |
Cell membrane and cytoplasm | Zynger et al., 200692 | ||
| Tissues | NA | Increase 100% (33/33) |
Cell membrane and cytoplasm | Zynger et al., 200893 | ||
| choriocarcinoma | Tissues | NA | Increase 100% (7/7) |
Cell membrane and cytoplasm | Zynger et al., 200692 | |
| Wilms tumor | Tissues | Increase | NA | NA | Toretsky et al., 200186 | |
| Tissues | Increase | NA | NA | Saikali et al., 200094 | ||
| Tissues | Increase | Increase 77% (23/30) |
Cell membrane and cytoplasm | Tretiakova et al., 201595 | ||
| RMS | Tissues | NA | Increase 23% (19/82) |
NA | Chan et al., 201389 | |
| Tissues | NA | Increase 25% (14/56) |
NA | Kinoshita et al., 201590 | ||
| Tissues | Increase | Increase 35% (74/213) |
Cell membrane and cytoplasm | Thway et al., 201197 | ||
| Mesothelioma | Cells, tissues | Decrease | NA | NA | Murthy et al., 2000100 | |
| Renal CCC | Cells, tissues | Decrease | NA | NA | Valsechi et al., 2014101 | |
| GPC4 | Pancreatic cancer | Tissues | NA | Increase | NA | Cao et al., 2018157 |
| Colorectal cancer | Tissues | NA | Increase | NA | Fang et al., 2019158 | |
| Drug-resistant ovarian cancer | Cells | Decrease | NA | NA | Varma et al., 2005161 | |
| Breast cancer | Cells | Decrease | Decrease | NA | Munir et al., 2020162 | |
| GPC5 | RMS | Tissues | Increase | NA | NA | Williamson et al., 2007170 |
| NSCLC | Cells, tissues | Increase | Increase 46% (58/127) |
Cell membrane | Li et al., 2013175 | |
| Cells, tissues | Decrease | Decrease | NA | Yang et al., 2013173 | ||
| Cells, tissues | Decrease | Decrease | NA | Yuan et al., 2016174 | ||
| GPC5 | Breast cancer | Tissues | Decrease | NA | NA | Zhang et al., 2011176 |
| Prostate cancer | Tissues | NA | Decrease | NA | Zhang et al., 2016177 | |
| Pancreatic cancer | Cells | NA | Decrease | NA | Yuan et al., 2018178 | |
| Glioma | Cells | NA | Decrease | NA | Hong et al., 2019180 | |
| GPC6 | Gastric cancer | Tissues | Increase | NA | NA | Dinccelik-Aslan et al., 2015186 |
| Ovarian cancer | Cells, tissues | Increase | Increase | NA | Karapetsas et al., 2015187 | |
| Melanoma | Cells | Increase | NA | NA | Li et al., 2019189 | |
| Drug-resistant ovarian cancer | Cells | Decrease | NA | NA | Januchowski et al., 2014190 | |
| Retinoblastoma | Tissues | Decrease | NA | NA | Lau et al., 2010191 | |
Abbreviations: GPC, glypican; CCC, clear cell carcinoma; ESCC, esophageal squamous cell carcinoma; HCC, hepatocellular carcinoma; LSCC, lung squamous cell carcinoma; NA, not available; NSCLC, non-small cell lung cancer; OCCC, ovarian clear cell carcinoma; RMS, rhabdomyosarcoma; YST, yolk sac tumor.
GPC6
The GPC6 gene is colocalized with GPC5 on chromosome 13q32. It encodes the GPC6 protein composed of a 555-amino-acid core protein and an unknown number of HS chains (UniProtKB/Swiss-Prot Q9Y625). GPC6 is most homologous to GPC4 (63%). Like other glypicans that are expressed during embryonic development, GPC6 shows a high expression in human fetal kidney.185 It is also widely expressed in adult tissues with the most abundance in ovary.185
GPC6 Expression in Cancer
The role of GPC6 has been implicated in cancer. The expression of GPC6 mRNA was found to be significantly higher in gastric cancer tissues compared with that in normal tissues; however, there was no statistically significant association between GPC6 expression and pathological parameters studied.186 Karapetsas et al.187 reported that GPC6 mRNA was overexpressed in early-stage ovarian cancer and its expression level correlated with CD8+ T-cell infiltration. They also found that the mRNA level of GPC6 was associated with overall survival of patients with early-stage ovarian cancer. A recent study reported that hypoxia-induced HIF1α in melanocytes directly regulated the expression of GPC6 and that increased expression of GPC6 was positively associated with poor survival in melanoma.188 Li et al.189 further identified GPC6 as one of the top genes whose expression levels distinguished primary melanoma from metastatic melanoma, indicating GPC6 could be a biomarker for melanoma progression. In addition, GPC6 expression has been reported to be reduced in certain cancer types. Januchowski et al.190 showed that GPC6 was strongly downregulated in chemoresistant variants of the A2780 ovarian cancer cell line. Lau et al.191 found that a reduction of GPC6 mRNA level in retinoblastoma was associated with the non-random allelic loss at chromosome 13q31, which could contribute to retinoblastoma development.
Alternations of GPC6 DNA in cancer have also been identified in recent years. Kumar et al.192 identified that GPC6 was recurrently mutated across individual prostate tumors from different patients. In another large-scale genome-wide association study, Amankwah et al.193 identified an SNP (rs17702471) in GPC6/GPC5 that was associated with an increased risk of invasive epithelial ovarian cancer.
GPC6 Signaling in Cancer
Similar to the interaction between GPC4 and RSPO, GPC6 is also required for RSPO-enhanced Wnt signaling at low levels of Wnt194 or in the absence of LGRs.166 In addition, Yiu et al.195 found that nuclear factor of activated T-cells (NFAT) transcriptionally regulates GPC6 induction in breast cancer, which leads to increased breast cancer cell invasion and migration by upregulating non-canonical Wnt5a signaling.
In conclusion, glypicans are very important in the modulation of growth factors and signaling pathways, such as Wnt signaling. They are expressed abnormally in various cancerous tissues and involved in tumorigenesis. In this review, we summarized the abnormal expression of glypicans in cancers and discussed their potentials as diagnostic tools and therapeutic targets in cancers. Glypicans have also emerged as novel tumor antigens. The therapeutic options involving glypican-targeted therapies are promising. There are currently multiple ongoing preclinical and clinical studies of glypicans-targeted therapies, which will validate and corroborate the role of glypicans as emerging cancer therapeutic targets.
Acknowledgments
We thank the National Institutes of Health Fellows Editorial Board for editorial assistance. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The YP7, CT3, and HM2 antibodies are available for licensing, in a wide range of fields of use, from the National Cancer Institute. If you are interested in obtaining a license, please contact the principal investigator at homi@mail.nih.gov.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: NL and MS reviewed the literature and wrote the manuscript; MH revised and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Intramural Research Program of National Institutes of Health, National Cancer Institute (Z01 BC010891 and ZIA BC010891 to M.H).
Contributor Information
Nan Li, Laboratory of Molecular Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Madeline R. Spetz, Laboratory of Molecular Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Mitchell Ho, Laboratory of Molecular Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Literature Cited
- 1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019. April 15;144(8):1941–53. [DOI] [PubMed] [Google Scholar]
- 2. Knelson EH, Nee JC, Blobe GC. Heparan sulfate signaling in cancer. Trends Biochem Sci. 2014. June;39(6):277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagarajan A, Malvi P, Wajapeyee N. Heparan sulfate and Heparan sulfate proteoglycans in cancer initiation and progression. Front Endocrinol (Lausanne). 2018;9:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. [DOI] [PubMed] [Google Scholar]
- 5. Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011. July 1;3(7):a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Capurro M, Martin T, Shi W, Filmus J. Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. J Cell Sci. 2014. April 1;127(Pt 7):1565–75. [DOI] [PubMed] [Google Scholar]
- 7. Gao W, Kim H, Feng M, Phung Y, Xavier CP, Rubin JS, Ho M. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology. 2014. August;60(2):576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li N, Wei L, Liu X, Bai H, Ye Y, Li D, Li N, Baxa U, Wang Q, Lv L, Chen Y, Feng M, Lee B, Gao W, Ho M. A Frizzled-like cysteine-rich domain in Glypican-3 mediates Wnt binding and regulates hepatocellular carcinoma tumor growth in mice. Hepatology. 2019. October;70(4):1231–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fico A, Maina F, Dono R. Fine-tuning of cell signaling by glypicans. Cell Mol Life Sci. 2011. March;68(6):923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9(5):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li N, Gao W, Zhang YF, Ho M. Glypicans as cancer therapeutic targets. Trends Cancer. 2018. November;4(11):741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li N, Fu H, Hewitt SM, Dimitrov DS, Ho M. Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma. Proc Natl Acad Sci U S A. 2017. August 8;114(32):E6623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bosse KR, Raman P, Zhu Z, Lane M, Martinez D, Heitzeneder S, Rathi KS, Kendsersky NM, Randall M, Donovan L, Morrissy S, Sussman RT, Zhelev DV, Feng Y, Wang Y, Hwang J, Lopez G, Harenza JL, Wei JS, Pawel B, Bhatti T, Santi M, Ganguly A, Khan J, Marra MA, Taylor MD, Dimitrov DS, Mackall CL, Maris JM. Identification of GPC2 as an oncoprotein and candidate immunotherapeutic target in high-risk neuroblastoma. Cancer Cell. 2017. September 11;32(3):295–309.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ho M, Kim H. Glypican-3: a new target for cancer immunotherapy. Eur J Cancer. 2011. February;47(3):333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng M, Gao W, Wang R, Chen W, Man YG, Figg WD, Wang XW, Dimitrov DS, Ho M. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2013. March 19;110(12):E1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Truong Q, Justiniano IO, Nocon AL, Soon JT, Wissmueller S, Campbell DH, Walsh BJ. Glypican-1 as a biomarker for prostate cancer: isolation and characterization. J Cancer. 2016;7(8):1002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Awad W, Logan D, Mani K. GPC1 (glypican 1). Atlas Genet Cytogenet Oncol Haematol. 2014;18(7):461–4. [Google Scholar]
- 18. Qiao D, Meyer K, Mundhenke C, Drew SA, Friedl A. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 signaling in brain endothelial cells. Specific role for glypican-1 in glioma angiogenesis. J Biol Chem. 2003. May 2;278(18):16045–53. [DOI] [PubMed] [Google Scholar]
- 19. Chamorro-Jorganes A, Araldi E, Rotllan N, Cirera-Salinas D, Suarez Y. Autoregulation of glypican-1 by intronic microRNA-149 fine tunes the angiogenic response to FGF2 in human endothelial cells. J Cell Sci. 2014. March 15;127(Pt 6):1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aikawa T, Whipple CA, Lopez ME, Gunn J, Young A, Lander AD, Korc M. Glypican-1 modulates the angiogenic and metastatic potential of human and mouse cancer cells. J Clin Invest. 2008. January;118(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whipple CA, Young AL, Korc M. A KrasG12D-driven genetic mouse model of pancreatic cancer requires glypican-1 for efficient proliferation and angiogenesis. Oncogene. 2012. May 17;31(20):2535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Kleeff J, Kayed H, Felix K, Penzel R, Buchler MW, Korc M, Friess H. Glypican-1 antisense transfection modulates TGF-beta-dependent signaling in Colo-357 pancreatic cancer cells. Biochem Biophys Res Commun. 2004. August 6;320(4):1148–55. [DOI] [PubMed] [Google Scholar]
- 23. Kleeff J, Ishiwata T, Kumbasar A, Friess H, Buchler MW, Lander AD, Korc M. The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J Clin Invest. 1998. November 1;102(9):1662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duan L, Hu XQ, Feng DY, Lei SY, Hu GH. GPC-1 may serve as a predictor of perineural invasion and a prognosticator of survival in pancreatic cancer. Asian J Surg. 2013. January;36(1):7–12. [DOI] [PubMed] [Google Scholar]
- 25. Lu H, Niu F, Liu F, Gao J, Sun Y, Zhao X. Elevated glypican-1 expression is associated with an unfavorable prognosis in pancreatic ductal adenocarcinoma. Cancer Med. 2017. June;6(6):1181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li N, Li D, Ren H, Torres M, Ho M. Chimeric antigen receptor T-cell therapy targeting glypican-1 in pancreatic cancer. Proceedings of the American Association for Cancer Research Annual Meeting; 2019 Mar 29–Apr 3; Atlanta. Philadelphia: American Association for Cancer Research; 2019. [Google Scholar]
- 27. Matsuda K, Maruyama H, Guo F, Kleeff J, Itakura J, Matsumoto Y, Lander AD, Korc M. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 2001. July 15;61(14):5562–9. [PubMed] [Google Scholar]
- 28. Su G, Meyer K, Nandini CD, Qiao D, Salamat S, Friedl A. Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am J Pathol. 2006. June;168(6):2014–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saito T, Sugiyama K, Hama S, Yamasaki F, Takayasu T, Nosaka R, Onishi S, Muragaki Y, Kawamata T, Kurisu K. High expression of glypican-1 predicts dissemination and poor prognosis in glioblastomas. World Neurosurg. 2017. September;105:282–88. [DOI] [PubMed] [Google Scholar]
- 30. Suhovskih AV, Mostovich LA, Kunin IS, Boboev MM, Nepomnyashchikh GI, Aidagulova SV, Grigorieva EV. Proteoglycan expression in normal human prostate tissue and prostate cancer. ISRN Oncol. 2013;2013:680136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang G, Ge G, Izzi V, Greenspan DS. alpha3 chains of type V collagen regulate breast tumour growth via glypican-1. Nat Commun. 2017. Jan 19;8:14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hara H, Takahashi T, Serada S, Fujimoto M, Ohkawara T, Nakatsuka R, Harada E, Nishigaki T, Takahashi Y, Nojima S, Miyazaki Y, Makino T, Kurokawa Y, Yamasaki M, Miyata H, Nakajima K, Takiguchi S, Morii E, Mori M, Doki Y, Naka T. Overexpression of glypican-1 implicates poor prognosis and their chemoresistance in oesophageal squamous cell carcinoma. Br J Cancer. 2016. Jun 28;115(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuzaki S, Serada S, Hiramatsu K, Nojima S, Matsuzaki S, Ueda Y, Ohkawara T, Mabuchi S, Fujimoto M, Morii E, Yoshino K, Kimura T, Naka T. Anti-glypican-1 antibody-drug conjugate exhibits potent preclinical antitumor activity against glypican-1 positive uterine cervical cancer. Int J Cancer. 2018. Mar 1;142(5):1056–66. [DOI] [PubMed] [Google Scholar]
- 34. Tanaka M, Ishikawa S, Ushiku T, Morikawa T, Isagawa T, Yamagishi M, Yamamoto H, Katoh H, Takeshita K, Arita J, Sakamoto Y, Hasegawa K, Kokudo N, Fukayama M. EVI1 modulates oncogenic role of GPC1 in pancreatic carcinogenesis. Oncotarget. 2017. November 21;8(59):99552–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015. July 9;523(7559):177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017. May 1;393:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frampton AE, Prado MM, Lopez-Jimenez E, Fajardo-Puerta AB, Jawad ZAR, Lawton P, Giovannetti E, Habib NA, Castellano L, Stebbing J, Krell J, Jiao LR. Glypican-1 is enriched in circulating-exosomes in pancreatic cancer and correlates with tumor burden. Oncotarget. 2018. April 10;9(27):19006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017. May;17(5):318–32. [DOI] [PubMed] [Google Scholar]
- 39. Olsson AK, Dimberg A, Kreuger J, Claesson—Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006. May;7(5):359–71. [DOI] [PubMed] [Google Scholar]
- 40. Kayed H, Kleeff J, Keleg S, Jiang X, Penzel R, Giese T, Zentgraf H, Buchler MW, Korc M, Friess H. Correlation of glypican-1 expression with TGF-beta, BMP, and activin receptors in pancreatic ductal adenocarcinoma. Int J Oncol. 2006. November;29(5):1139–48. [PubMed] [Google Scholar]
- 41. Harada E, Serada S, Fujimoto M, Takahashi Y, Takahashi T, Hara H, Nakatsuka R, Sugase T, Nishigaki T, Saito Y, Hiramatsu K, Nojima S, Mitsuo R, Ohkawara T, Morii E, Mori M, Doki Y, Kaneda Y, Naka T. Glypican-1 targeted antibody-based therapy induces preclinical antitumor activity against esophageal squamous cell carcinoma. Oncotarget. 2017. April 11;8(15):24741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li J, Chen Y, Zhan C, Zhu J, Weng S, Dong L, Liu T, Shen X. Glypican-1 promotes tumorigenesis by regulating the PTEN/Akt/beta-catenin signaling pathway in esophageal squamous cell carcinoma. Dig Dis Sci. 2019. June;64(6):1493–502. [DOI] [PubMed] [Google Scholar]
- 43. Nishigaki T, Takahashi T, Serada S, Fujimoto M, Ohkawara T, Hara H, Sugase T, Otsuru T, Saito Y, Tsujii S, Nomura T, Tanaka K, Miyazaki Y, Makino T, Kurokawa Y, Nakajima K, Eguchi H, Yamasaki M, Mori M, Doki Y, Naka T. Anti-glypican-1 antibody-drug conjugate is a potential therapy against pancreatic cancer. Br J Cancer. 2020. April;122(9):1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011. August 25;365(8):725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012. March 22;119(12):2709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kato D, Yaguchi T, Iwata T, Katoh Y, Morii K, Tsubota K, Takise Y, Tamiya M, Kamada H, Akiba H, Tsumoto K, Serada S, Naka T, Nishimura R, Nakagawa T, Kawakami Y. GPC1 specific CAR-T cells eradicate established solid tumor without adverse effects and synergize with anti-PD-1 Ab. Elife. 2020. March 31;9:e49392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stipp CS, Litwack ED, Lander AD. Cerebroglycan: an integral membrane heparan sulfate proteoglycan that is unique to the developing nervous system and expressed specifically during neuronal differentiation. J Cell Biol. 1994. January;124(1–2):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ivins JK, Litwack ED, Kumbasar A, Stipp CS, Lander AD. Cerebroglycan, a developmentally regulated cell-surface heparan sulfate proteoglycan, is expressed on developing axons and growth cones. Dev Biol. 1997;184:320–32. [DOI] [PubMed] [Google Scholar]
- 49. Stipp CS, Litwack ED, Lander AD. Cerebroglycan: an integral membrane heparan sulfate proteoglycan that is unique to the developing nervous system and expressed specifically during neuronal differentiation. J Cell Biol. 1994;124:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Orentas RJ, Yang JJ, Wen X, Wei JS, Mackall CL, Khan J. Identification of cell surface proteins as potential immunotherapy targets in 12 pediatric cancers. Front Oncol. 2012;2:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ho M, Li N, Fleming B. High affinity monoclonal antibodies targeting glypican-2 and uses thereof. 2019. Available from: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020033430
- 52. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuro-blastoma. Lancet. 2007;369(9579):2106–20. [DOI] [PubMed] [Google Scholar]
- 53. Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Backlund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012. April;123(4):473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu N, Jia D, Bates B, Basom R, Eberhart CG, MacPherson D. A mouse model of MYCN-driven retinoblastoma reveals MYCN-independent tumor reemergence. J Clin Invest. 2017. March 1;127(3):888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005. July 1;283(1):226–39. [DOI] [PubMed] [Google Scholar]
- 56. Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018. October;8(10):1219–26. [DOI] [PubMed] [Google Scholar]
- 57. Ho M. Advances in liver cancer antibody therapies: a focus on glypican-3 and mesothelin. BioDrugs. 2011. October 1;25(5):275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Iglesias BV, Centeno G, Pascuccelli H, Ward F, Peters MG, Filmus J, Puricelli L, de Kier Joffe EB. Expression pattern of glypican-3 (GPC3) during human embryonic and fetal development. Histol Histopathol. 2008. November;23(11):1333–40. [DOI] [PubMed] [Google Scholar]
- 59. Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005. July 15;65(14):6245–54. [DOI] [PubMed] [Google Scholar]
- 60. Gao W, Tang Z, Zhang YF, Feng M, Qian M, Dimitrov DS, Ho M. Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signalling and protein synthesis. Nat Commun. 2015. March 11;6:6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Capurro M, Shi W, Izumikawa T, Kitagawa H, Filmus J. Processing by convertases is required for glypican-3-induced inhibition of Hedgehog signaling. J Biol Chem. 2015. March 20;290(12):7576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J. Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell. 2008. May;14(5):700–11. [DOI] [PubMed] [Google Scholar]
- 63. Filmus J, Capurro M. The role of glypicans in Hedgehog signaling. Matrix Biol. 2014. April;35:248–52. [DOI] [PubMed] [Google Scholar]
- 64. Gao W, Kim H, Ho M. Human monoclonal antibody targeting the heparan sulfate chains of glypican-3 inhibits HGF-mediated migration and motility of hepatocellular carcinoma cells. PLoS ONE. 2015;10(9):e0137664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 1997. November 15;57(22):5179–84. [PubMed] [Google Scholar]
- 66. Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003. July;125(1):89–97. [DOI] [PubMed] [Google Scholar]
- 67. Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008. June;129(6):899–906. [DOI] [PubMed] [Google Scholar]
- 68. Zhu ZW, Friess H, Wang L, Abou-Shady M, Zimmermann A, Lander AD, Korc M, Kleeff J, Buchler MW. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001. April;48(4):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Libbrecht L, Severi T, Cassiman D, Vander Borght S, Pirenne J, Nevens F, Verslype C, van Pelt J, Roskams T. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 2006. November;30(11):1405–11. [DOI] [PubMed] [Google Scholar]
- 70. Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, Thung SN, Khitrov G, Zhang W, Villanueva A, Battiston C, Mazzaferro V, Bruix J, Waxman S, Friedman SL. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006. December;131(6):1758–67. [DOI] [PubMed] [Google Scholar]
- 71. Kaseb AO, Hassan M, Lacin S, Abdel-Wahab R, Amin HM, Shalaby A, Wolff RA, Yao J, Rashid A, Vennapusa B, Feng J, Ohtomo T. Evaluating clinical and prognostic implications of Glypican-3 in hepatocellular carcinoma. Oncotarget. 2016. October 25;7(43):69916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu H, Yang C, Lu W, Zeng Y. Prognostic significance of glypican-3 expression in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2018. January;97(4):e9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, Kinoshita T, Nakatsura T. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009. August;100(8):1403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang J, Zhang M, Ma H, Song X, He L, Ye X, Li X. Overexpression of glypican-3 is a predictor of poor prognosis in hepatocellular carcinoma: an updated meta-analysis. Medicine (Baltimore). 2018. June;97(24):e11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Takai H, Kato A, Kato C, Watanabe T, Matsubara K, Suzuki M, Kataoka H. The expression profile of glypican-3 and its relation to macrophage population in human hepatocellular carcinoma. Liver Int. 2009. August;29(7):1056–64. [DOI] [PubMed] [Google Scholar]
- 76. Phung Y, Gao W, Man YG, Nagata S, Ho M. High-affinity monoclonal antibodies to cell surface tumor antigen glypican-3 generated through a combination of peptide immunization and flow cytometry screening. MABs. 2012. Sep-Oct;4(5):592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Aviel-Ronen S, Lau SK, Pintilie M, Lau D, Liu N, Tsao MS, Jothy S. Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Mod Pathol. 2008. July;21(7):817–25. [DOI] [PubMed] [Google Scholar]
- 78. Nakatsura T, Kageshita T, Ito S, Wakamatsu K, Monji M, Ikuta Y, Senju S, Ono T, Nishimura Y. Identification of glypican-3 as a novel tumor marker for melanoma. Clin Cancer Res. 2004. October 1;10(19):6612–21. [DOI] [PubMed] [Google Scholar]
- 79. He H, Fang W, Liu X, Weiss LM, Chu PG. Frequent expression of glypican-3 in Merkel cell carcinoma: an immunohistochemical study of 55 cases. Appl Immuno-histochem Mol Morphol. 2009. January;17(1):40–6. [DOI] [PubMed] [Google Scholar]
- 80. Maeda D, Ota S, Takazawa Y, Aburatani H, Nakagawa S, Yano T, Taketani Y, Kodama T, Fukayama M. Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod Pathol. 2009. June;22(6):824–32. [DOI] [PubMed] [Google Scholar]
- 81. Yamanaka K, Ito Y, Okuyama N, Noda K, Matsumoto H, Yoshida H, Miyauchi A, Capurro M, Filmus J, Miyoshi E. Immunohistochemical study of glypican 3 in thyroid cancer. Oncology. 2007;73(5–6):389–94. [DOI] [PubMed] [Google Scholar]
- 82. Aydin O, Yildiz L, Baris S, Dundar C, Karagoz F. Expression of glypican 3 in low and high grade urothelial carcinomas. Diagn Pathol. 2015. April 21;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Andisheh-Tadbir A, Ashraf MJ, Gudarzi A, Zare R. Evaluation of glypican-3 expression in benign and malignant salivary gland tumors. J Oral Biol Craniofac Res. 2019. Jan-Mar;9(1):63–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Moek KL, Fehrmann RSN, van der Vegt B, de Vries EGE, de Groot DJA. Glypican 3 overexpression across a broad spectrum of tumor types discovered with functional genomic mRNA profiling of a large cancer database. Am J Pathol. 2018. September;188(9):1973–81. [DOI] [PubMed] [Google Scholar]
- 85. Yu K, Lin CJ, Hatcher A, Lozzi B, Kong K, Huang-Hobbs E, Cheng YT, Beechar VB, Zhu W, Zhang Y, Chen F, Mills GB, Mohila CA, Creighton CJ, Noebels JL, Scott KL, Deneen B. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature. 2020. February;578(7793):166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Toretsky JA, Zitomersky NL, Eskenazi AE, Voigt RW, Strauch ED, Sun CC, Huber R, Meltzer SJ, Schlessinger D. Glypican-3 expression in Wilms tumor and hepatoblastoma. J Pediatr Hematol Oncol. 2001. November;23(8):496–9. [DOI] [PubMed] [Google Scholar]
- 87. Yamauchi N, Watanabe A, Hishinuma M, Ohashi K, Midorikawa Y, Morishita Y, Niki T, Shibahara J, Mori M, Makuuchi M, Hippo Y, Kodama T, Iwanari H, Aburatani H, Fukayama M. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005. December;18(12):1591–8. [DOI] [PubMed] [Google Scholar]
- 88. Zynger DL, Gupta A, Luan C, Chou PM, Yang GY, Yang XJ. Expression of glypican 3 in hepatoblastoma: an immunohistochemical study of 65 cases. Hum Pathol. 2008. February;39(2):224–30. [DOI] [PubMed] [Google Scholar]
- 89. Chan ES, Pawel BR, Corao DA, Venneti S, Russo P, Santi M, Sullivan LM. Immunohistochemical expression of glypican-3 in pediatric tumors: an analysis of 414 cases. Pediatr Dev Pathol. 2013. Jul-Aug;16(4):272–7. [DOI] [PubMed] [Google Scholar]
- 90. Kinoshita Y, Tanaka S, Souzaki R, Miyoshi K, Kohashi K, Oda Y, Nakatsura T, Taguchi T. Glypican 3 expression in pediatric malignant solid tumors. Eur J Pediatr Surg. 2015. February;25(1):138–44. [DOI] [PubMed] [Google Scholar]
- 91. Esheba GE, Pate LL, Longacre TA. Oncofetal protein glypican-3 distinguishes yolk sac tumor from clear cell carcinoma of the ovary. Am J Surg Pathol. 2008. April;32(4):600–7. [DOI] [PubMed] [Google Scholar]
- 92. Zynger DL, Dimov ND, Luan C, Teh BT, Yang XJ. Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol. 2006. December;30(12):1570–5. [DOI] [PubMed] [Google Scholar]
- 93. Zynger DL, Everton MJ, Dimov ND, Chou PM, Yang XJ. Expression of glypican 3 in ovarian and extragonadal germ cell tumors. Am J Clin Pathol. 2008. August;130(2):224–30. [DOI] [PubMed] [Google Scholar]
- 94. Saikali Z, Sinnett D. Expression of glypican 3 (GPC3) in embryonal tumors. Int J Cancer. 2000. September 20;89(5):418–22. [PubMed] [Google Scholar]
- 95. Tretiakova M, Zynger DL, Luan C, Andeen NK, Finn LS, Kocherginsky M, Teh BT, Yang XJ. Glypican 3 overexpression in primary and metastatic Wilms tumors. Virchows Arch. 2015. January;466(1):67–76. [DOI] [PubMed] [Google Scholar]
- 96. White GR, Kelsey AM, Varley JM, Birch JM. Somatic glypican 3 (GPC3) mutations in Wilms’ tumour. Br J Cancer. 2002. June 17;86(12):1920–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Thway K, Selfe J, Missiaglia E, Fisher C, Shipley J. Glypican-3 is expressed in rhabdomyosarcomas but not adult spindle cell and pleomorphic sarcomas. J Clin Pathol. 2011. July;64(7):587–91. [DOI] [PubMed] [Google Scholar]
- 98. Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nature Genetics. 1996;12(3):241–47. [DOI] [PubMed] [Google Scholar]
- 99. Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S, Sedlackova L, Tanswell AK, Mak TW, Yeger H, Lockwood GA, Rosenblum ND, Filmus J. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol. 1999. July 12;146(1):255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Murthy SS, Shen T, De Rienzo A, Lee WC, Ferriola PC, Jhanwar SC, Mossman BT, Filmus J, Testa JR. Expression of GPC3, an X-linked recessive overgrowth gene, is silenced in malignant mesothelioma. Oncogene. 2000. January 20;19(3):410–6. [DOI] [PubMed] [Google Scholar]
- 101. Valsechi MC, Oliveira AB, Conceicao AL, Stuqui B, Candido NM, Provazzi PJ, de Araujo LF, Silva WA, Jr, de Freitas Calmon M, Rahal P. GPC3 reduces cell proliferation in renal carcinoma cell lines. BMC Cancer. 2014. August 29;14:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Xiang YY, Ladeda V, Filmus J. Glypican-3 expression is silenced in human breast cancer. Oncogene. 2001. November 1;20(50):7408–12. [DOI] [PubMed] [Google Scholar]
- 103. Lin H, Huber R, Schlessinger D, Morin PJ. Frequent silencing of the GPC3 gene in ovarian cancer cell lines. Cancer Res. 1999. February 15;59(4):807–10. [PubMed] [Google Scholar]
- 104. Kim H, Xu GL, Borczuk AC, Busch S, Filmus J, Capurro M, Brody JS, Lange J, D’Armiento JM, Rothman PB, Powell CA. The heparan sulfate proteoglycan GPC3 is a potential lung tumor suppressor. Am J Respir Cell Mol Biol. 2003. December;29(6):694–701. [DOI] [PubMed] [Google Scholar]
- 105. Trinh T, Puszyk W, Liu C. Epigenetic regulation of glypican-3 in hepatocellular carcinoma. Cancer Res. 2014;74(Suppl 19):418. [Google Scholar]
- 106. Li L, Jin R, Zhang X, Lv F, Liu L, Liu D, Liu K, Li N, Chen D. Oncogenic activation of glypican-3 by c-Myc in human hepatocellular carcinoma. Hepatology. 2012. October;56(4):1380–90. [DOI] [PubMed] [Google Scholar]
- 107. Jalvy-Delvaille S, Maurel M, Majo V, Pierre N, Chabas S, Combe C, Rosenbaum J, Sagliocco F, Grosset CF. Molecular basis of differential target regulation by miR-96 and miR-182: the Glypican-3 as a model. Nucleic Acids Res. 2012. February;40(3):1356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Maurel M, Jalvy S, Ladeiro Y, Combe C, Vachet L, Sagliocco F, Bioulac-Sage P, Pitard V, Jacquemin-Sablon H, Zucman-Rossi J, Laloo B, Grosset CF. A functional screening identifies five microRNAs controlling glypican-3: role of miR-1271 down-regulation in hepatocellular carcinoma. Hepatology. 2013. January;57(1):195–204. [DOI] [PubMed] [Google Scholar]
- 109. Bengochea A, de Souza MM, Lefrancois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, Scoazec JY, Vitvitski L, Merle P. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008. July 8;99(1):143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gao W, Xu Y, Liu J, Ho M. Epitope mapping by a Wnt-blocking antibody: evidence of the Wnt binding domain in heparan sulfate. Sci Rep. 2016. May 17;6:26245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Midorikawa Y, Ishikawa S, Iwanari H, Imamura T, Sakamoto H, Miyazono K, Kodama T, Makuuchi M, Aburatani H. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. Int J Cancer. 2003. February 10;103(4):455–65. [DOI] [PubMed] [Google Scholar]
- 112. Song HH, Shi W, Filmus J. OCI-5/rat glypican-3 binds to fibroblast growth factor-2 but not to insulin-like growth factor-2. J Biol Chem. 1997. March 21;272(12):7574–7. [DOI] [PubMed] [Google Scholar]
- 113. Sun CK, Chua MS, He J, So SK. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-beta2. Neoplasia. 2011. August;13(8):735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Capurro MI, Li F, Filmus J. Overgrowth of a mouse model of Simpson-Golabi-Behmel syndrome is partly mediated by Indian hedgehog. EMBO Rep. 2009. August;10(8):901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Filmus J, Capurro M. The role of glypican-3 in the regulation of body size and cancer. Cell Cycle. 2008. September 15;7(18):2787–90. [DOI] [PubMed] [Google Scholar]
- 116. Capurro MI, Shi W, Filmus J. LRP1 mediates Hedgehog-induced endocytosis of the GPC3-Hedgehog complex. J Cell Sci. 2012. July 15;125(Pt 14):3380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li H, Wolfe A, Septer S, Edwards G, Zhong X, Abdulkarim AB, Ranganathan S, Apte U. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012. January;32(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Filmus J. Glypicans in growth control and cancer. Glycobiology. 2001. March;11(3):19R–23R. [DOI] [PubMed] [Google Scholar]
- 119. Chen X, Horiuchi A, Kikuchi N, Osada R, Yoshida J, Shiozawa T, Konishi I. Hedgehog signal pathway is activated in ovarian carcinomas, correlating with cell proliferation: it’s inhibition leads to growth suppression and apoptosis. Cancer Sci. 2007. January;98(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kubo M, Nakamura M, Tasaki A, Yamanaka N, Nakashima H, Nomura M, Kuroki S, Katano M. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004. September 1;64(17):6071–4. [DOI] [PubMed] [Google Scholar]
- 121. Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, Fukuma D, Yokomine K, Harao M, Beppu T, Matsui M, Torigoe T, Sato N, Baba H, Nishimura Y. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res. 2006. May 1;12(9):2689–97. [DOI] [PubMed] [Google Scholar]
- 122. Nakatsura T, Komori H, Kubo T, Yoshitake Y, Senju S, Katagiri T, Furukawa Y, Ogawa M, Nakamura Y, Nishimura Y. Mouse homologue of a novel human oncofetal antigen, glypican-3, evokes T-cell-mediated tumor rejection without autoimmune reactions in mice. Clin Cancer Res. 2004. December 15;10(24):8630–40. [DOI] [PubMed] [Google Scholar]
- 123. Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Takayama T, Yamao K, Uesaka K, Furuse J, Kinoshita T, Nakatsura T. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012. July 1;18(13):3686–96. [DOI] [PubMed] [Google Scholar]
- 124. Yoshikawa T, Nakatsugawa M, Suzuki S, Shirakawa H, Nobuoka D, Sakemura N, Motomura Y, Tanaka Y, Hayashi S, Nakatsura T. HLA-A2-restricted glypican-3 peptide-specific CTL clones induced by peptide vaccine show high avidity and antigen-specific killing activity against tumor cells. Cancer Sci. 2011. May;10 2(5):918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, Nobuoka D, Gotohda N, Takahashi S, Kato Y, Konishi M, Kinoshita T, Ikeda M, Nakachi K, Yamazaki N, Mizuno S, Takayama T, Yamao K, Uesaka K, Furuse J, Endo I, Nakatsura T. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016. May;5(5):e1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sayem MA, Tomita Y, Yuno A, Hirayama M, Irie A, Tsukamoto H, Senju S, Yuba E, Yoshikawa T, Kono K, Nakatsura T, Nishimura Y. Identification of glypican-3-derived long peptides activating both CD8(+) and CD4(+) T cells; prolonged overall survival in cancer patients with Th cell response. Oncoimmunology. 2016;5(1):e1062209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Suzuki S, Sakata J, Utsumi F, Sekiya R, Kajiyama H, Shibata K, Kikkawa F, Nakatsura T. Efficacy of glypican-3-derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma. Oncoimmunology. 2016;5(11):e1238542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tsuchiya N, Hosono A, Yoshikawa T, Shoda K, Nosaka K, Shimomura M, Hara J, Nitani C, Manabe A, Yoshihara H, Hosoya Y, Kaneda H, Kinoshita Y, Kohashi K, Yoshimura K, Fujinami N, Saito K, Mizuno S, Nakatsura T. Phase I study of glypican-3-derived peptide vaccine therapy for patients with refractory pediatric solid tumors. Oncoimmunology. 2017;7(1):e1377872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H, Sugo I, Ohizumi I, Aburatani H, Hamakubo T, Kodama T, Tsuchiya M, Yamada-Okabe H. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008. December 1;68(23):9832–8. [DOI] [PubMed] [Google Scholar]
- 130. Nakano K, Orita T, Nezu J, Yoshino T, Ohizumi I, Sugimoto M, Furugaki K, Kinoshita Y, Ishiguro T, Hamakubo T, Kodama T, Aburatani H, Yamada-Okabe H, Tsuchiya M. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009. January 9;378(2):279–84. [DOI] [PubMed] [Google Scholar]
- 131. Zhu AX, Gold PJ, El-Khoueiry AB, Abrams TA, Morikawa H, Ohishi N, Ohtomo T, Philip PA. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2013. February 15;19(4):920–8. [DOI] [PubMed] [Google Scholar]
- 132. Yen C-J, Daniele B, Kudo M, Merle P, Park J-W, Ross PJ, Peron J-M, Ebert O, Chan SL, Poon RTP, Colombo M, Okusaka T, Ryoo B-Y, Minguez B, Tanaka T, Ohtomo T, Rutman O, Chen Y-C, Lee R-M, Abou-Alfa GK. Randomized phase II trial of intravenous RO5137382/GC33 at 1600 mg every other week and placebo in previously treated patients with unresectable advanced hepatocellular carcinoma (HCC; NCT01507168). J Clin Oncol. 2014;32(Suppl 15):4102.25403208 [Google Scholar]
- 133. Li D, Li N, Zhang YF, Fu H, Feng M, Schneider D, Su L, Wu X, Zhou J, Mackay S, Kramer J, Duan Z, Yang H, Kolluri A, Hummer AM, Torres MB, Zhu H, Hall MD, Luo X, Chen J, Wang Q, Abate-Daga D, Dropublic B, Hewitt SM, Orentas RJ, Greten TF, Ho M. Persistent polyfunctional chimeric antigen receptor T cells that target glypican 3 eliminate orthotopic hepatocellular carcinomas in mice. Gastroenterology. 2020. June;158(8):2250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhang YF, Ho M. Humanization of high-affinity antibodies targeting glypican-3 in hepatocellular carcinoma. Sci Rep. 2016. September 26;6:33878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kim H, Ho M. Isolation of antibodies to heparan sulfate on glypicans by phage display. Curr Protoc Protein Sci. 2018. November;94(1):e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wang C, Gao W, Feng M, Pastan I, Ho M. Construction of an immunotoxin, HN3-mPE24, targeting glypican-3 for liver cancer therapy. Oncotarget. 2017. May 16;8(20):32450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Fleming BD, Urban DJ, Hall MD, Longerich T, Greten TF, Pastan I, Ho M. Engineered anti-GPC3 immunotoxin, HN3-ABD-T20, produces regression in mouse liver cancer xenografts through prolonged serum retention. Hepatology. 2020. May;71(5):1696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, Kong J, Wang H, Yang S, Gu J, Li Z. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014. December 15;20(24):6418–28. [DOI] [PubMed] [Google Scholar]
- 139. Li W, Guo L, Rathi P, Marinova E, Gao X, Wu MF, Liu H, Dotti G, Gottschalk S, Metelitsa LS, Heczey A. Redirecting T cells to glypican-3 with 4-1BB zeta chimeric antigen receptors results in Th1 polarization and potent antitumor activity. Hum Gene Ther. 2017. May;28(5):437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jiang Z, Jiang X, Chen S, Lai Y, Wei X, Li B, Lin S, Wang S, Wu Q, Liang Q, Liu Q, Peng M, Yu F, Weng J, Du X, Pei D, Liu P, Yao Y, Xue P, Li P. Anti-GPC3-CAR T cells suppress the growth of tumor cells in patient-derived xenografts of hepatocellular carcinoma. Front Immunol. 2016;7:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang P, Qin W, Liu T, Jiang D, Cui L, Liu X, Fang Y, Tang X, Jin H, Qian Q. PiggyBac-engineered T cells expressing a glypican-3-specific chimeric antigen receptor show potent activities against hepatocellular carcinoma. Immunobiology. 2020. January;225(1):151850. [DOI] [PubMed] [Google Scholar]
- 142. Guo X, Jiang H, Shi B, Zhou M, Zhang H, Shi Z, Du G, Luo H, Wu X, Wang Y, Sun R, Li Z. Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front Pharmacol. 2018;9:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pan Z, Di S, Shi B, Jiang H, Shi Z, Liu Y, Wang Y, Luo H, Yu M, Wu X, Li Z. Increased antitumor activities of glypican-3-specific chimeric antigen receptor-modified T cells by coexpression of a soluble PD1-CH3 fusion protein. Cancer Immunol Immunother. 2018. October;67(10):1621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, Lu Q, Gao H, Jiang H, Wang H, Yuan D, Ma H, Wang H, Li Z, Zhai B. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: Results of phase 1 trials. Clin Cancer Res. 2020. May 5. [DOI] [PubMed] [Google Scholar]
- 145. Fu Y, Urban DJ, Nani RR, Zhang YF, Li N, Fu H, Shah H, Gorka AP, Guha R, Chen L, Hall MD, Schnermann MJ, Ho M. Glypican-3-specific antibody drug conjugates targeting hepatocellular carcinoma. Hepatology. 2019. August;70(2):563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010. August 26;29(34):4741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang PY, Zhang JB, Wang L, Wu WZ, Qin LX, Tang ZY. Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin Cancer Res. 2009. August 1;15(15):4838–46. [DOI] [PubMed] [Google Scholar]
- 148. Lipovsek D, Carvajal I, Allentoff AJ, Barros A, Jr., Brailsford J, Cong Q, Cotter P, Gangwar S, Hollander C, Lafont V, Lau WL, Li W, Moreta M, O’Neil S, Pinckney J, Smith MJ, Su J, Terragni C, Wallace MA, Wang L, Wright M, Marsh HN, Bryson JW. Adnectin-drug conjugates for Glypican-3-specific delivery of a cytotoxic payload to tumors. Protein Eng Des Sel. 2018. May 1;31(5):159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hanaoka H, Nagaya T, Sato K, Nakamura Y, Watanabe R, Harada T, Gao W, Feng M, Phung Y, Kim I, Paik CH, Choyke PL, Ho M, Kobayashi H. Glypican-3 targeted human heavy chain antibody as a drug carrier for hepatocellular carcinoma therapy. Mol Pharm. 2015. June 1;12(6):2151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hanaoka H, Nakajima T, Sato K, Watanabe R, Phung Y, Gao W, Harada T, Kim I, Paik CH, Choyke PL, Ho M, Kobayashi H. Photoimmunotherapy of hepatocellular carcinoma-targeting glypican-3 combined with nanosized albumin-bound paclitaxel. Nanomedicine (Lond). 2015;10(7):1139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ishiguro T, Sano Y, Komatsu SI, Kamata-Sakurai M, Kaneko A, Kinoshita Y, Shiraiwa H, Azuma Y, Tsunenari T, Kayukawa Y, Sonobe Y, Ono N, Sakata K, Fujii T, Miyazaki Y, Noguchi M, Endo M, Harada A, Frings W, Fujii E, Nanba E, Narita A, Sakamoto A, Wakabayashi T, Konishi H, Segawa H, Igawa T, Tsushima T, Mutoh H, Nishito Y, Takahashi M, Stewart L, ElGabry E, Kawabe Y, Ishigai M, Chiba S, Aoki M, Hattori K, Nezu J. An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci Transl Med. 2017. October 4;9(410):eaal4291. [DOI] [PubMed] [Google Scholar]
- 152. Bi Y, Jiang H, Wang P, Song B, Wang H, Kong X, Li Z. Treatment of hepatocellular carcinoma with a GPC3-targeted bispecific T cell engager. Oncotarget. 2017. August 8;8(32):52866–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Dargel C, Bassani-Sternberg M, Hasreiter J, Zani F, Bockmann JH, Thiele F, Bohne F, Wisskirchen K, Wilde S, Sprinzl MF, Schendel DJ, Krackhardt AM, Uckert W, Wohlleber D, Schiemann M, Stemmer K, Heikenwalder M, Busch DH, Richter G, Mann M, Protzer U. T cells engineered to express a T-cell receptor specific for glypican-3 to recognize and kill hepatoma cells in vitro and in mice. Gastroenterology. 2015. October;149(4):1042–52. [DOI] [PubMed] [Google Scholar]
- 154. Yu M, Luo H, Fan M, Wu X, Shi B, Di S, Liu Y, Pan Z, Jiang H, Li Z. Development of GPC3-specific chimeric antigen receptor-engineered natural killer cells for the treatment of hepatocellular carcinoma. Mol Ther. 2018. February 7;26(2):366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Veugelers M, Vermeesch J, Watanabe K, Yamaguchi Y, Marynen P, David G. GPC4, the gene for human K-glypican, flanks GPC3 on xq26: deletion of the GPC3-GPC4 gene cluster in one family with Simpson-Golabi-Behmel syndrome. Genomics. 1998. October 1;53(1):1–11. [DOI] [PubMed] [Google Scholar]
- 156. Watanabe K, Yamada H, Yamaguchi Y. K-glypican: a novel GPI-anchored heparan sulfate proteoglycan that is highly expressed in developing brain and kidney. J Cell Biol. 1995. September;130(5):1207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cao J, Ma J, Sun L, Li J, Qin T, Zhou C, Cheng L, Chen K, Qian W, Duan W, Wang F, Wu E, Wang Z, Ma Q, Han L. Targeting glypican-4 overcomes 5-FU resistance and attenuates stem cell-like properties via suppression of Wnt/beta-catenin pathway in pancreatic cancer cells. J Cell Biochem. 2018. November;119(11):9498–512. [DOI] [PubMed] [Google Scholar]
- 158. Fang Y, Shen ZY, Zhan YZ, Feng XC, Chen KL, Li YS, Deng HJ, Pan SM, Wu DH, Ding Y. CD36 inhibits beta-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat Commun. 2019. September 4;10(1):3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhao D, Liu S, Sun L, Zhao Z, Liu S, Kuang X, Shu J, Luo B. Glypican-4 gene polymorphism (rs1048369) and susceptibility to Epstein-Barr virus-associated and -negative gastric carcinoma. Virus Res. 2016. July 15;220:52–6. [DOI] [PubMed] [Google Scholar]
- 160. Liu S, Liu W, Zhao D, Zhang Y, Zhao Z, Luo B. The glypican-4 gene polymorphism rs1048369 and susceptibility to Epstein-Barr virus-positive and -negative nasopharyngeal carcinoma in Northern China. Oncol Res Treat. 2019;42(11):572–9. [DOI] [PubMed] [Google Scholar]
- 161. Varma RR, Hector SM, Clark K, Greco WR, Hawthorn L, Pendyala L. Gene expression profiling of a clonal isolate of oxaliplatin-resistant ovarian carcinoma cell line A2780/C10. Oncol Rep. 2005. October;14(4):925–32. [PubMed] [Google Scholar]
- 162. Munir J, Van Ngu T, Na Ayudthaya PD, Ryu S. Downregulation of glypican-4 facilitates breast cancer progression by inducing cell migration and proliferation. Biochem Biophys Res Commun. 2020. May 21;526(1):91–7. [DOI] [PubMed] [Google Scholar]
- 163. Waterson J, Stockley TL, Segal S, Golabi M. Novel duplication in glypican-4 as an apparent cause of Simpson-Golabi-Behmel syndrome. Am J Med Genet A. 2010. December;152A(12):3179–81. [DOI] [PubMed] [Google Scholar]
- 164. Sakane H, Yamamoto H, Matsumoto S, Sato A, Kikuchi A. Localization of glypican-4 in different membrane microdomains is involved in the regulation of Wnt signaling. J Cell Sci. 2012. January 15;125(Pt 2):449–60. [DOI] [PubMed] [Google Scholar]
- 165. Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009. May 26;2(72):re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lebensohn AM, Rohatgi R. R-spondins can potentiate WNT signaling without LGRs. Elife. 2018. February 6;7:e33126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Dubey R, van Kerkhof P, Jordens I, Malinauskas T, Pusapati GV, McKenna JK, Li D, Carette JE, Ho M, Siebold C, Maurice M, Lebensohn AM, Rohatgi R. R-spondins engage heparan sulfate proteoglycans to potentiate WNT signaling. Elife. 2020. May 20;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Saunders S, Paine-Saunders S, Lander AD. Expression of the cell surface proteoglycan glypican-5 is developmentally regulated in kidney, limb, and brain. Dev Biol. 1997. October 1;190(1):78–93. [DOI] [PubMed] [Google Scholar]
- 169. Veugelers M, Vermeesch J, Reekmans G, Steinfeld R, Marynen P, David G. Characterization of glypican-5 and chromosomal localization of human GPC5, a new member of the glypican gene family. Genomics. 1997. February 15;40(1):24–30. [DOI] [PubMed] [Google Scholar]
- 170. Williamson D, Selfe J, Gordon T, Lu YJ, Pritchard-Jones K, Murai K, Jones P, Workman P, Shipley J. Role for amplification and expression of glypican-5 in rhabdomyosarcoma. Cancer Res. 2007. January 1;67(1):57–65. [DOI] [PubMed] [Google Scholar]
- 171. Nishimura R, Takita J, Sato-Otsubo A, Kato M, Koh K, Hanada R, Tanaka Y, Kato K, Maeda D, Fukayama M, Sanada M, Hayashi Y, Ogawa S. Characterization of genetic lesions in rhabdomyosarcoma using a high-density single nucleotide polymorphism array. Cancer Sci. 2013. July;104(7):856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Li Y, Sheu CC, Ye Y, de Andrade M, Wang L, Chang SC, Aubry MC, Aakre JA, Allen MS, Chen F, Cunningham JM, Deschamps C, Jiang R, Lin J, Marks RS, Pankratz VS, Su L, Li Y, Sun Z, Tang H, Vasmatzis G, Harris CC, Spitz MR, Jen J, Wang R, Zhang ZF, Christiani DC, Wu X, Yang P. Genetic variants and risk of lung cancer in never smokers: a genome-wide association study. Lancet Oncol. 2010. April;11(4):321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yang X, Zhang Z, Qiu M, Hu J, Fan X, Wang J, Xu L, Yin R. Glypican-5 is a novel metastasis suppressor gene in non-small cell lung cancer. Cancer Lett. 2013. December 1;341(2):265–73. [DOI] [PubMed] [Google Scholar]
- 174. Yuan S, Yu Z, Liu Q, Zhang M, Xiang Y, Wu N, Wu L, Hu Z, Xu B, Cai T, Ma X, Zhang Y, Liao C, Wang L, Yang P, Bai L, Li Y. GPC5, a novel epigenetically silenced tumor suppressor, inhibits tumor growth by suppressing Wnt/beta-catenin signaling in lung adenocarcinoma. Oncogene. 2016. November 24;35(47):6120–31. [DOI] [PubMed] [Google Scholar]
- 175. Li Y, Miao L, Cai H, Ding J, Xiao Y, Yang J, Zhang D. The overexpression of glypican-5 promotes cancer cell migration and is associated with shorter overall survival in non-small cell lung cancer. Oncol Lett. 2013. December;6(6):1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhang C, Zhang S, Zhang D, Zhang Z, Xu Y, Liu S. A lung cancer gene GPC5 could also be crucial in breast cancer. Mol Genet Metab. 2011. May;10 3(1):104–5. [DOI] [PubMed] [Google Scholar]
- 177. Zhang C, Liu Z, Wang L, Qiao B, Du E, Li L, Xu Y, Zhang Z. Prognostic significance of GPC5 expression in patients with prostate cancer. Tumour Biol. 2016. May;37(5):6413–8. [DOI] [PubMed] [Google Scholar]
- 178. Yuan Q, Zhang Y, Li J, Cao G, Yang W. High expression of microRNA-4295 contributes to cell proliferation and invasion of pancreatic ductal adenocarcinoma by the down-regulation of Glypican-5. Biochem Biophys Res Commun. 2018. February 26;497(1):73–9. [DOI] [PubMed] [Google Scholar]
- 179. Liu T, Zhang X, Sha K, Liu X, Zhang L, Wang B. miR-709 up-regulated in hepatocellular carcinoma, promotes proliferation and invasion by targeting GPC5. Cell Prolif. 2015. June;48(3):330–7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 180. Hong X, Zhang Z, Pan L, Ma W, Zhai X, Gu C, Zhang Y, Bi X, Huang W, Pei H, Liu Z. MicroRNA-301b promotes the proliferation and invasion of glioma cells through enhancing activation of Wnt/beta-catenin signaling via targeting Glypican-5. Eur J Pharmacol. 2019. July 5;854:39–47. [DOI] [PubMed] [Google Scholar]
- 181. Li F, Shi W, Capurro M, Filmus J. Glypican-5 stimulates rhabdomyosarcoma cell proliferation by activating Hedgehog signaling. J Cell Biol. 2011. February 21;192(4):691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wang S, Qiu M, Xia W, Xu Y, Mao Q, Wang J, Dong G, Xu L, Yang X, Yin R. Glypican-5 suppresses Epithelial-Mesenchymal Transition of the lung adenocarcinoma by competitively binding to Wnt3a. Oncotarget. 2016. November 29;7(48):79736–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wang W, Liu M, Guan Y, Wu Q. Hypoxia-responsive mir-301a and mir-301b promote radioresistance of prostate cancer cells via downregulating NDRG2. Med Sci Monit. 2016. June 21;22:2126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Sun Y, Xu K, He M, Fan G, Lu H. Overexpression of Glypican 5 (GPC5) inhibits prostate cancer cell proliferation and invasion via suppressing Sp1-mediated EMT and activation of Wnt/beta-catenin signaling. Oncol Res. 2018. May 7;26(4):565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Veugelers M, De Cat B, Ceulemans H, Bruystens AM, Coomans C, Durr J, Vermeesch J, Marynen P, David G. Glypican-6, a new member of the glypican family of cell surface heparan sulfate proteoglycans. J Biol Chem. 1999. September 17;274(38):26968–77. [DOI] [PubMed] [Google Scholar]
- 186. Dinccelik-Aslan M, Gumus-Akay G, Elhan AH, Unal E, Tukun A. Diagnostic and prognostic significance of glypican 5 and glypican 6 gene expression levels in gastric adenocarcinoma. Mol Clin Oncol. 2015. May;3(3):584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Karapetsas A, Giannakakis A, Dangaj D, Lanitis E, Kynigopoulos S, Lambropoulou M, Tanyi JL, Galanis A, Kakolyris S, Trypsianis G, Coukos G, Sandaltzopoulos R. Overexpression of GPC6 and TMEM132D in early stage ovarian cancer correlates with CD8+ T-lymphocyte infiltration and increased patient survival. Biomed Res Int. 2015;2015:712438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Loftus SK, Baxter LL, Cronin JC, Fufa TD, Program NCS, Pavan WJ. Hypoxia-induced HIF1alpha targets in melanocytes reveal a molecular profile associated with poor melanoma prognosis. Pigment Cell Melanoma Res. 2017. May;30(3):339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Li Y, Li M, Shats I, Krahn JM, Flake GP, Umbach DM, Li X, Li L. Glypican 6 is a putative biomarker for metastatic progression of cutaneous melanoma. PLoS ONE. 2019;14(6):e0218067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Januchowski R, Zawierucha P, Rucinski M, Nowicki M, Zabel M. Extracellular matrix proteins expression profiling in chemoresistant variants of the A2780 ovarian cancer cell line. Biomed Res Int. 2014;2014:365867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Lau CS, Yu CB, Wong HK, Fan DS, Mak HT, Wong KW, Lam DS, Pang CP, Choy KW. Allelic imbalance at 13q31 is associated with reduced GPC6 in Chinese with sporadic retinoblastoma. Br J Ophthalmol. 2010. March;94(3):357–62. [DOI] [PubMed] [Google Scholar]
- 192. Kumar A, White TA, MacKenzie AP, Clegg N, Lee C, Dumpit RF, Coleman I, Ng SB, Salipante SJ, Rieder MJ, Nickerson DA, Corey E, Lange PH, Morrissey C, Vessella RL, Nelson PS, Shendure J. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci U S A. 2011. October 11;108(41):17087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Amankwah EK, Lin HY, Tyrer JP, Lawrenson K, Dennis J, Chornokur G, Aben KK, Anton-Culver H, Antonenkova N, Bruinsma F, Bandera EV, Bean YT, Beckmann MW, Bisogna M, Bjorge L, Bogdanova N, Brinton LA, Brooks-Wilson A, Bunker CH, Butzow R, Campbell IG, Carty K, Chen Z, Chen YA, Chang-Claude J, Cook LS, Cramer DW, Cunningham JM, Cybulski C, Dansonka-Mieszkowska A, du Bois A, Despierre E, Dicks E, Doherty JA, Dork T, Durst M, Easton DF, Eccles DM, Edwards RP, Ekici AB, Fasching PA, Fridley BL, Gao YT, Gentry-Maharaj A, Giles GG, Glasspool R, Goodman MT, Gronwald J, Harrington P, Harter P, Hasmad HN, Hein A, Heitz F, Hildebrandt MA, Hillemanns P, Hogdall CK, Hogdall E, Hosono S, Iversen ES, Jakubowska A, Jensen A, Ji BT, Karlan BY, Jim H, Kellar M, Kiemeney LA, Krakstad C, Kjaer SK, Kupryjanczyk J, Lambrechts D, Lambrechts S, Le ND, Lee AW, Lele S, Leminen A, Lester J, Levine DA, Liang D, Lim BK, Lissowska J, Lu K, Lubinski J, Lundvall L, Massuger LF, Matsuo K, McGuire V, McLaughlin JR, McNeish I, Menon U, Milne RL, Modugno F, Moysich KB, Ness RB, Nevanlinna H, Eilber U, Odunsi K, Olson SH, Orlow I, Orsulic S, Weber RP, Paul J, Pearce CL, Pejovic T, Pelttari LM, Permuth-Wey J, Pike MC, Poole EM, Risch HA, Rosen B, Rossing MA, Rothstein JH, Rudolph A, Runnebaum IB, Rzepecka IK, Salvesen HB, Schernhammer E, Schwaab I, Shu XO, Shvetsov YB, Siddiqui N, Sieh W, Song H, Southey MC, Spiewankiewicz B, Sucheston-Campbell L, Teo SH, Terry KL, Thompson PJ, Thomsen L, Tangen IL, Tworoger SS, van Altena AM, Vierkant RA, Vergote I, Walsh CS, Wang-Gohrke S, Wentzensen N, Whittemore AS, Wicklund KG, Wilkens LR, Wu AH, Wu X, Woo YL, Yang H, Zheng W, Ziogas A, Kelemen LE, Berchuck A. Georgia Chenevix-Trench on behalf of the Amg Schildkraut JM Ramus SJ, Goode EL, Monteiro AN, Gayther SA, Narod SA, Pharoah PD, Sellers TA, Phelan CM. Epithelial-mesenchymal transition (EMT) gene variants and epithelial ovarian cancer (EOC) risk. Genet Epidemiol. 2015. December;39(8):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Lebensohn AM, Dubey R, Neitzel LR, Tacchelly-Benites O, Yang E, Marceau CD, Davis EM, Patel BB, Bahrami-Nejad Z, Travaglini KJ, Ahmed Y, Lee E, Carette JE, Rohatgi R. Comparative genetic screens in human cells reveal new regulatory mechanisms in WNT signaling. Elife. 2016. December 20;5:e21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Yiu GK, Kaunisto A, Chin YR, Toker A. NFAT promotes carcinoma invasive migration through glypican-6. Biochem J. 2011. November 15;440(1):157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]