Abstract
Excessive alcohol consumption is one of the main causes of death and disability worldwide. Alcohol consumption is a heritable complex trait. We conducted a meta-analysis of genome-wide association studies (GWAS) of gram/day (g/d) alcohol consumption in UK-Biobank, AlcGen and CHARGE+ consortia accumulating 480,842 people of European descent to decipher the genetic architecture of alcohol intake. We identified 46 novel, common loci, and investigated their potential functional significance using magnetic resonance imaging data and gene expression studies. Our results identify genetic pathways associated with alcohol consumption and suggest shared genetic mechanisms with neuropsychiatric disorders including schizophrenia.
Excessive alcohol consumption is a major public health problem that is responsible for 2.2% and 6.8% age-standardized deaths for women and men respectively1. Most genetic studies of alcohol use focus on alcohol dependency, although the population burden of alcohol-related disease mainly reflects a broader range of alcohol consumption behaviors2. Small reductions in alcohol consumption could have major public health benefits; even moderate amounts of alcohol/day may have significant impact on mortality3.
Alcohol consumption is a heritable complex trait4, but genetic studies to date have robustly identified only a small number of associated genetic variants 5–8. These include variants in the aldehyde dehydrogenase (ADH) gene family, a group of enzymes that catalyze the oxidation of aldehydes9, including a cluster of genes on chromosome 4q23 (ADH1B, ADH1C, ADH5, ADH6, ADH7)6.
Here, we report a GWAS meta-analysis of alcohol intake (log transformed g/day) among people of European ancestry drawn from UK Biobank (UKB)10, the Alcohol Genome-Wide Consortium (AlcGen) and the Cohorts for Heart and Aging Research in Genomic Epidemiology Plus (CHARGE+) consortia. Briefly, UKB is a prospective cohort study of ~500,000 individuals recruited between the ages of 40 and 69 years. Participants were asked to report their average weekly and monthly alcohol consumption through a self-completed touchscreen questionnaire10. Based on these reports, we calculated the g/d alcohol intake (Methods). Participants were genotyped using a customized array with imputation from the Haplotype Reference Consortium (HRC) panel11, yielding ~7 million common single nucleotide polymorphisms (SNPs) with minor allele frequency (MAF) ≥ 1% and imputation quality score [INFO] ≥ 0.1. After quality control (QC) and exclusions (Methods) we performed GWAS of alcohol consumption using data from 404,731 UKB participants of European descent under an additive genetic model (Methods and Supplementary Table 1). We found that genomic inflation in the UKB analysis was λGC=1.45, but did not adjust for inflation as the LD score regression intercept was 1.05, indicating that this was due to polygenicity rather than to population stratification12. The estimated SNP-wide heritability of alcohol consumption in the UKB data was 0.09.
We also carried out GWAS in 25 independent studies from the AlcGen and CHARGE+ consortia including 76,111 participants of European descent for which alcohol g/d could be calculated (Supplementary Table 2). Various arrays were used for genotyping, with imputations performed using either the 1,000 Genomes Reference Panel or the HRC platforms (Supplementary Table 3). After QC, we applied genomic control at the individual study level and obtained summary results for ~7 million SNPs with imputation quality score ≥ 0.3 (Methods).
We combined the UKB, AlcGen and CHARGE+ results using a fixed effects inverse variance weighted approach for a total of 480,842 individuals13. To maximize power, we performed a single-stage analysis to test common SNPs with MAF ≥ 1%. We set a stringent P-value threshold of P < 5 × 10−9 to denote significance in the combined meta-analysis14, and required signals to be at P < 5 × 10−7 in UKB, with same direction of effect in UKB and AlcGen plus CHARGE+, to minimize false positive findings. We excluded SNPs within 500kb of variants reported as genome-wide significant in previous GWAS of alcohol consumption5,6, identified novel loci by requiring SNPs to be independent of each other (LD r2 < 0.1), and selected the sentinel SNP within each locus according to lowest P-value (Methods).
We then tested for correlations of alcohol-associated SNPs with Magnetic Resonance Imaging (MRI) phenotypes of brain, heart and liver, and gene expression. We tested the sentinel SNPs for association with other traits/diseases and Drosophila mutant models were used to investigate functional effects on ethanol-induced behavior.
RESULTS
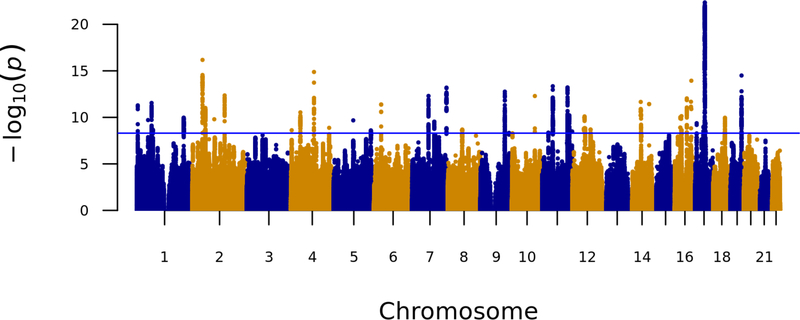
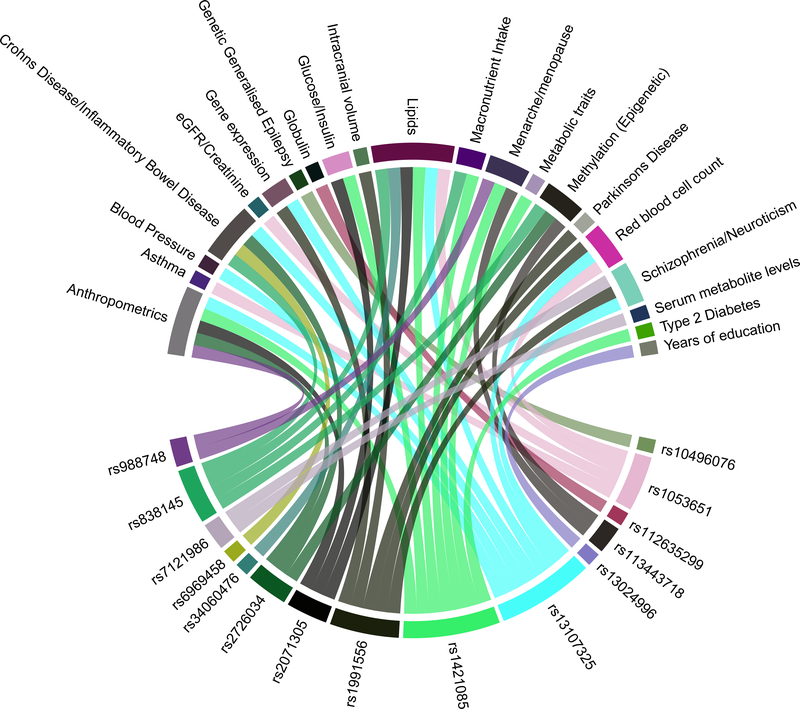
Our meta-analysis identified 46 novel loci associated with alcohol consumption (log transformed g/day) (Fig. 1 and Table 1). All inferential statistics for the novel loci are reported in Table 1 whereas heterogeneity metrics are presented in Supplementary Table 4. In addition, we discovered a further eight variants in the combined analysis at nominal genome-wide significance (P < 1 × 10−8) that may also be associated with alcohol intake (Supplementary Table 5). The most significantly associated variant, rs1991556 (P = 4.5 × 10−23), is an intronic variant in MAPT gene that encodes the microtubule-associated protein tau, and was found through Phenoscanner not only to be associated with dementia15 and Parkinson’s disease16,17, but also with neuroticism, schizophrenia18and other traits19–21 (Methods, Fig. 2 and Supplementary Table 6). The second most significantly associated variant is rs1004787 (P = 6.7 × 10−17), near SIX3 gene, which encodes a member of the sine oculis homeobox transcription factor family involved in eye development22. The third SNP is rs13107325 (P = 1.3 × 10−15), a missense SNP in SLC39A8 (https://www.ncbi.nlm.nih.gov/gene/64116), a gene that encodes a member of the SLC39 family of metal ion transporters, which has been associated with schizophrenia23 as well as inflammatory bowel disease, cardiovascular and metabolic phenotypes 24,25–27 in previous GWAS (Fig. 2 and Supplementary Table 6).
Figure 1. Manhattan plot showing P-values from discovery genome-wide association meta-analysis with alcohol intake (log g/d) among 480,842 individuals across UK Biobank, AlcGen and CHARGE+, excluding known variants.
The P-value was computed using inverse variance fixed effects models. The y axis shows the –log10 P values and the x axis shows their chromosomal positions. Horizontal blue line represents the threshold of P = 5 × 10−9.
Table 1:
Association results of 46 novel alcohol variants identified through the meta-analysis of UK Biobank and AlcGen and CHARGE+. Results are ordered by P-value of combined analysis.
| leadSNP |
Combined |
UKB |
AlcGen and CHARGE+ |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nearest_Gene | Annotated Gene | rsID_LEAD_SNP | CP | EA | EAF | BETA | SE | P | BETA | SE | P | BETA | SE | P |
| MAPT | STH | rs1991556 | 17:44083402 | A | 0.22 | −0.012 | 0.001 | 4.5E-23 | −0.013 | 0.001 | 2.4E-21 | −0.011 | 0.004 | 4.0E-03 |
| RP11–89K21.1 | SIX3 | rs1004787 | 2:45159091 | A | 0.54 | 0.009 | 0.001 | 6.7E-17 | 0.009 | 0.001 | 1.1E-15 | 0.007 | 0.003 | 1.4E-02 |
| SLC39A8 | SLC39A8 | rs13107325 | 4:103188709 | T | 0.07 | −0.016 | 0.002 | 1.3E-15 | −0.017 | 0.002 | 4.8E-16 | −0.006 | 0.006 | 3.6E-01 |
| IZUMO1, RASIP1, FUT1 | IZUMO1 | rs838145 | 19:49248730 | A | 0.55 | −0.008 | 0.001 | 3.2E-15 | −0.009 | 0.001 | 2.4E-15 | −0.004 | 0.003 | 1.7E-01 |
| Na | PSMD7 | rs1104608 | 16:73912588 | C | 0.43 | −0.008 | 0.001 | 1.2E-14 | −0.009 | 0.001 | 4.9E-15 | −0.003 | 0.003 | 2.5E-01 |
| MYBPC3 | MYBPC3 | rs2071305 | 11:47370957 | A | 0.69 | 0.009 | 0.001 | 4.5E-14 | 0.009 | 0.001 | 3.9E-13 | 0.007 | 0.003 | 3.1E-02 |
| Na | DRD2 | rs7121986 | 11:113355444 | T | 0.37 | −0.008 | 0.001 | 6.2E-14 | −0.008 | 0.001 | 1.3E-13 | −0.005 | 0.003 | 1.1E-01 |
| Na | DPP6 | rs6969458 | 7:153489725 | A | 0.47 | 0.008 | 0.001 | 6.4E-14 | 0.008 | 0.001 | 1.3E-12 | 0.007 | 0.003 | 1.5E-02 |
| RP11–308N19.1 | ZNF462 | rs74424378 | 9:109331094 | T | 0.76 | 0.009 | 0.001 | 1.7E-13 | 0.009 | 0.001 | 4.5E-13 | 0.006 | 0.003 | 8.4E-02 |
| ARHGAP15, AC096558.1, RP11–570L15.2 | ARHGAP15 | rs13024996 | 2:144225215 | A | 0.37 | −0.008 | 0.001 | 4.4E-13 | −0.008 | 0.001 | 6.6E-13 | −0.004 | 0.003 | 1.4E-01 |
| MLXIPL | MLXIPL | rs34060476 | 7:73037956 | A | 0.87 | −0.011 | 0.002 | 5.0E-13 | −0.012 | 0.002 | 1.4E-13 | −0.004 | 0.004 | 4.1E-01 |
| Na | FAM178A | rs61873510 | 10:102626510 | T | 0.33 | −0.008 | 0.001 | 5.1E-13 | −0.008 | 0.001 | 9.8E-12 | −0.008 | 0.003 | 1.7E-02 |
| FTO | FTO | rs1421085 | 16:53800954 | T | 0.60 | 0.008 | 0.001 | 9.2E-13 | 0.007 | 0.001 | 1.7E-10 | 0.010 | 0.003 | 9.2E-04 |
| Na | PMFBP1 | rs11648570 | 16:72356964 | T | 0.89 | −0.012 | 0.002 | 2.1E-12 | −0.011 | 0.002 | 1.5E-10 | −0.013 | 0.005 | 3.4E-03 |
| OTX2, RP11–1085N6.6 | OTX2 | rs2277499 | 14:57271127 | T | 0.34 | −0.008 | 0.001 | 2.2E-12 | −0.007 | 0.001 | 2.4E-09 | −0.012 | 0.003 | 9.1E-05 |
| PDE4B | PDE4B | rs2310752 | 1:66392405 | A | 0.43 | −0.007 | 0.001 | 2.8E-12 | −0.008 | 0.001 | 1.8E-11 | −0.006 | 0.003 | 4.2E-02 |
| SERPINA1 | SERPINA1 | rs112635299 | 14:94838142 | T | 0.02 | −0.025 | 0.004 | 3.7E-12 | −0.027 | 0.004 | 9.8E-12 | −0.017 | 0.010 | 9.9E-02 |
| Na | AJAP1 | rs780569 | 1:4569436 | A | 0.71 | −0.008 | 0.001 | 5.2E-12 | −0.008 | 0.001 | 1.1E-11 | −0.005 | 0.003 | 1.2E-01 |
| Na | VRK2 | rs10496076 | 2:57942987 | T | 0.37 | −0.007 | 0.001 | 9.7E-12 | −0.007 | 0.001 | 1.3E-09 | −0.009 | 0.003 | 1.6E-03 |
| ACTR10, C14orf37 | ACTR10 | rs71414193 | 14:58685301 | A | 0.19 | −0.009 | 0.001 | 1.8E-11 | −0.008 | 0.001 | 5.8E-09 | −0.013 | 0.004 | 4.5E-04 |
| BEND4 | BEND4 | rs16854020 | 4:42117559 | A | 0.13 | 0.010 | 0.002 | 2.9E-11 | 0.010 | 0.002 | 5.8E-09 | 0.016 | 0.005 | 6.4E-04 |
| Na | SORL1 | rs485425 | 11:121544984 | C | 0.45 | −0.007 | 0.001 | 6.1E-11 | −0.007 | 0.001 | 7.3E-11 | −0.004 | 0.003 | 1.9E-01 |
| SEZ6L2 | SEZ6L2 | rs113443718 | 16:29892184 | A | 0.31 | −0.007 | 0.001 | 7.4E-11 | −0.008 | 0.001 | 4.5E-11 | −0.003 | 0.003 | 2.9E-01 |
| CBX5, RP11–968A15.2 | CBX5 | rs57281063 | 12:54660427 | A | 0.41 | 0.007 | 0.001 | 7.9E-11 | 0.007 | 0.001 | 1.8E-09 | 0.007 | 0.003 | 1.2E-02 |
| Na | TNRC6A | rs72768626 | 16:24693048 | A | 0.94 | 0.014 | 0.002 | 9.7E-11 | 0.015 | 0.002 | 1.7E-09 | 0.014 | 0.006 | 1.8E-02 |
| SYT14 | SYT14 | rs227179 | 1:210216731 | A | 0.59 | −0.007 | 0.001 | 1.1E-10 | −0.007 | 0.001 | 1.4E-09 | −0.006 | 0.003 | 2.8E-02 |
| TCF4 | TCF4 | rs9320010 | 18:53053897 | A | 0.60 | 0.007 | 0.001 | 1.1E-10 | 0.007 | 0.001 | 1.6E-09 | 0.007 | 0.003 | 2.2E-02 |
| SBK1 | NPIPB6 | rs2726034 | 16:28336882 | T | 0.68 | 0.007 | 0.001 | 1.4E-10 | 0.007 | 0.001 | 1.1E-09 | 0.006 | 0.003 | 4.7E-02 |
| ANKRD36 | ANKRD36 | rs13390019 | 2:97797680 | T | 0.87 | 0.010 | 0.002 | 1.6E-10 | 0.011 | 0.002 | 7.0E-11 | 0.004 | 0.005 | 4.5E-01 |
| Na | ELAVL4 | rs7517344 | 1:50711961 | A | 0.17 | 0.009 | 0.001 | 1.9E-10 | 0.008 | 0.001 | 2.5E-07 | 0.016 | 0.004 | 2.1E-05 |
| LINC00461 | MEF2C | rs4916723 | 5:87854395 | A | 0.58 | 0.007 | 0.001 | 2.1E-10 | 0.007 | 0.001 | 5.1E-10 | 0.005 | 0.003 | 1.1E-01 |
| ARPC1B, ARPC1A | ARPC1B | rs10249167 | 7:98980879 | A | 0.87 | 0.010 | 0.002 | 2.9E-10 | 0.009 | 0.002 | 8.1E-08 | 0.015 | 0.004 | 3.8E-04 |
| EFNB3, WRAP53 | EFNB3 | rs7640 | 17:7606722 | C | 0.80 | 0.008 | 0.001 | 4.3E-10 | 0.009 | 0.001 | 1.3E-09 | 0.006 | 0.004 | 9.9E-02 |
| RP11–501C14.5 | IGF2BP1 | rs4794015 | 17:47067826 | A | 0.41 | 0.007 | 0.001 | 4.3E-10 | 0.006 | 0.001 | 5.4E-08 | 0.009 | 0.003 | 1.2E-03 |
| TCAP, PNMT, STARD3 | TCAP | rs1053651 | 17:37822311 | A | 0.27 | −0.007 | 0.001 | 1.1E-09 | −0.008 | 0.001 | 8.4E-10 | −0.003 | 0.003 | 2.8E-01 |
| Na | AADAT | rs7698119 | 4:171070910 | A | 0.49 | −0.006 | 0.001 | 1.3E-09 | −0.006 | 0.001 | 1.6E-07 | −0.009 | 0.003 | 1.6E-03 |
| STAT6, AC023237.1 | STAT6 | rs12312693 | 12:57511734 | T | 0.55 | −0.006 | 0.001 | 1.5E-09 | −0.006 | 0.001 | 9.5E-09 | −0.005 | 0.003 | 5.6E-02 |
| SCN8A | SCN8A | rs7958704 | 12:51984349 | T | 0.41 | −0.006 | 0.001 | 1.6E-09 | −0.006 | 0.001 | 1.7E-08 | −0.006 | 0.003 | 3.5E-02 |
| ACSS3 | ACSS3 | rs11114787 | 12:81595700 | T | 0.27 | 0.007 | 0.001 | 2.0E-09 | 0.007 | 0.001 | 2.7E-08 | 0.007 | 0.003 | 2.4E-02 |
| RP11–32K4.1 | BHLHE22 | rs2356369 | 8:64956882 | T | 0.52 | −0.006 | 0.001 | 2.0E-09 | −0.006 | 0.001 | 4.1E-08 | −0.007 | 0.003 | 1.6E-02 |
| ZRANB2-AS2 | ZRANB2 | rs12031875 | 1:71585097 | A | 0.82 | −0.008 | 0.001 | 2.2E-09 | −0.008 | 0.001 | 7.6E-08 | −0.010 | 0.004 | 8.7E-03 |
| MSANTD1, HTT | MSANTD1 | rs12646808 | 4:3249828 | T | 0.66 | 0.007 | 0.001 | 2.4E-09 | 0.007 | 0.001 | 1.1E-09 | 0.002 | 0.003 | 4.7E-01 |
| TENM2 | TENM2 | rs10078588 | 5:166816176 | A | 0.52 | 0.006 | 0.001 | 2.5E-09 | 0.006 | 0.001 | 4.3E-08 | 0.007 | 0.003 | 1.9E-02 |
| IGSF9B | IGSF9B | rs748919 | 11:133783232 | T | 0.79 | 0.008 | 0.001 | 3.3E-09 | 0.008 | 0.001 | 1.0E-08 | 0.005 | 0.003 | 1.1E-01 |
| AC010967.2 | GPR75-ASB3 | rs785293 | 2:53023304 | A | 0.57 | −0.006 | 0.001 | 3.3E-09 | −0.006 | 0.001 | 3.2E-08 | −0.006 | 0.003 | 3.8E-02 |
| BDNF, RP11–587D21.4 | BDNF | rs988748 | 11:27724745 | C | 0.21 | −0.008 | 0.001 | 4.4E-09 | −0.007 | 0.001 | 1.2E-07 | −0.010 | 0.004 | 8.3E-03 |
SNP: Single Nucleotide polymorphism; LocusName: Nearest Gene; rsID_LEAD_SNP: Rs ID number of the lead SNP; CP: Chromosome/Position (build hg19/37); EA: Effect allele of the discovered SNP; EAF: Frequency of the effect allele; BETA_comb: Effect size in meta-analysis; SE_comb; Standard Error of the effect in meta-analysis; P_comb: Meta-analysis P-value; BETA_UKB: Effect size in UK Biobank analysis; SE_UKB: Standard Error of the effect in the UK Biobank analysis; P_UKB: UK Biobank analysis P-value;BETA_AlcGenCHARGE+: Effect size in the AlcGen meta-analysis; SE_AlcGenCHARGE+: Standard Error of the effect in the AlcGen meta-analysis; P_AlcGenCHARGE+: AlcGen meta-analysis P-value
Figure 2. Association of alcohol intake loci with other traits.
Plot shows results from associations with other traits which were extracted from the PhenoScanner database for the 46 novel sentinel SNPs including proxies in Linkage Disequilibrium (r2 ≥ 0.8) with genome-wide significant associations. Each colored line connects a specific variant with the associated traits and diseases.
Another of our most significant variants, an intronic SNP rs7121986 (P = 6.2 × 10−14) in DRD2 (https://www.ncbi.nlm.nih.gov/gene/1813), encodes the dopamine receptor D2 that has been associated with cocaine addiction, neuroticism and schizophrenia18. We also found significant associations with SNP rs988748 (P = 4.4 × 10−9) in the BDNF gene (https://www.ncbi.nlm.nih.gov/gene/627, that encodes a member of the nerve growth factor family of proteins and rs7517344, which is near ELAVL4 (https://www.ncbi.nlm.nih.gov/gene/1996) (P = 2.0 × 10−10), the gene product of which is involved in BDNF regulation28. Previous studies have suggested that a variant in BDNF is associated with alcohol consumption and that alcohol consumption modulates BDNF expression29.
Additionally, we found association of alcohol consumption with SNP rs838145 (P = 3.2 × 10−15), which has been associated with macronutrient intake in a previous GWAS30. This variant is nearest IZUMO (https://www.ncbi.nlm.nih.gov/gene/284359) in a locus of around 50kb that spans a number of genes including FGF21 (https://www.ncbi.nlm.nih.gov/gene/26291), whose gene product FGF21 is a liver hormone involved in the regulation of alcohol preference, glucose and lipid metabolism31. We previously reported significant association of alcohol intake with SNP rs11940694 in KLB (https://www.ncbi.nlm.nih.gov/gene/152831), an obligate receptor of FGF21 in the brain5, and we strongly replicated that finding here (P = 3.3 × 10−68).
As well as variants in KLB and in the alcohol dehydrogenase locus (smallest P = 1.2 × 10−125), we found support (P = 1 × 10−5) for association of common variants in the three other alcohol intake-related loci previously reported in GWAS (Supplementary Table 7), including SNP rs6943555 in AUTS2 (https://www.ncbi.nlm.nih.gov/gene/26053) (P = 2.9 × 10−6). In addition, we found a novel alcohol intake-related SNP rs1421085 in FTO (https://www.ncbi.nlm.nih.gov/gene/79068) in high LD (r2 = 0.92) with a variant reported previously as genome-wide significant for association with alcohol dependence32.
Conditional analysis using Genome-wide Complex Trait Analysis (GCTA) did not reveal any independent secondary signals related to alcohol consumption. Among ~14,000 individuals in the independent Airwave cohort33 (Methods), 7% of the variance in alcohol consumption was explained by the novel and known common variants. Using weights from our analysis, we constructed an unbiased weighted genetic risk score (GRS) in Airwave (Methods) and found a strong association of the novel and known variants on alcohol consumption levels (P = 2.75 × 10−14), with mean difference in sex-adjusted alcohol intake of 2.6 g/d comparing the top vs the bottom quintile of the GRS (Supplementary Table 8).
Associations with MRI imaging phenotypes
We functionally characterized novel variants by carrying out single-SNP analyses of the imaging phenotypes in UKB (Methods), focusing on brain (N=9,702), heart (N=10,706) and liver (N=8,479).
With Bonferroni correction (corrected P-value 6.6 × 10−6, corresponding to 0.05/46 SNPs*164 imaging phenotypes), we found significant positive associations between SNP rs13107325 in SLC39A8 and the volumes of multiple brain regions; All inferential statistics for these associations are reported in Supplementary Table 9. The strongest associations were with putamen (left: P = 2.5 × 10−45, right: P = 2.8 × 10−47), ventral striatum (left: P = 9.5 × 10−53, right: P = 9.6 × 10−51) and cerebellum (strongest association for left I-IV volume; P = 1.2 × 10−9) (Supplementary Table 9); similar findings were recently reported in a GWAS on brain imaging in UKB34. The other significant association was for rs1991556 with the parahippocampal gyrus (P = 1.2 × 10−6).
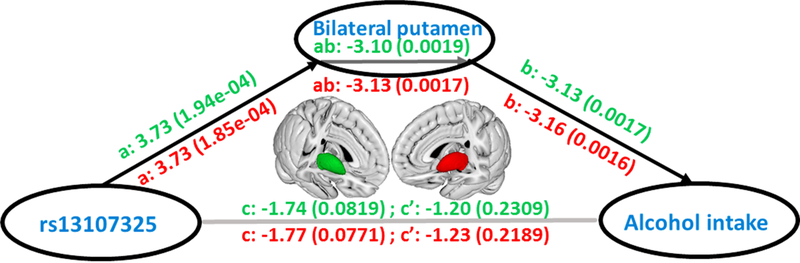
We then tested these brain regions for association with alcohol consumption and found a significant effect for the left (t8601 = −3.7; beta ± SE = −0.0019 ± 0.0005; P = 2.0 × 10−4) and right (t8601 = −3.65; beta ± SE = −0.0070 ± 0.0005; P = 2.6 × 10−4) putamen. Finally, we used data from N= 8,610 individuals and performed a mediation analysis using a standard three-variable path model, bootstrapping 10,000 times to calculate the significance of the mediation effect of putamen volume for genetic influences on alcohol consumption (Methods). We found evidence that the effect of SNP rs13107325 in SLC39A8 on alcohol intake is partially mediated via its association with left (t8601 = −3.03; beta ± SE = −0.27 ± 0.09; P = 1.9 × 10−3) and right (t8601 = −2.82; beta ± SE = −0.27 ± 0.09; P = 1.7 × 10−3) putamen volume (Fig. 3 and Supplementary Table 10). To exclude the possibility of an inverse causal pathway we performed additional analyses in UKB non-drinkers (N =589). With 10,000 random permutations, associations of rs13107325 with both left and right putamen remained significant (left putamen: t541=1.06; P = 0.02; right putamen: t541=0.38; P = 0.04) indicating that the association between rs13107325 and putamen regions is not mediated by alcohol intake.
Figure 3. Mediation effect of the grey matter volume of bilateral putamen on the relationship between SNP rs13107325 and alcohol intake.
The green is for left putamen, and, the red is for the right one. We use ‘a’ for the relationship between rs13107325 and putamen, ‘b’ for the relationship between putamen and alcohol consumption, ‘c’ for the relationship between rs13107325 and alcohol consumption, ‘c” for the relationship between rs13107325 and alcohol consumption after excluding the effect of putamen, and ‘ab’ as the mediation effect. The significance tests are based on the bootstrapping method (10,000 times). Z- statistics and the corresponding P values are provided in parentheses. The brain icon was created using Mango software, version 4.1 (http://ric.uthscsa.edu/mango/).
We did not find any significant associations of novel SNPs with either cardiac (left ventricular mass or end diastolic volume or right ventricular end diastolic volume) (Supplementary Table 11) or liver fat measures on MRI (Supplementary Table 12), after adjustment for multiple testing.
Effects of SNPs on gene expression
We carried out expression quantitative trait loci eQTL analyses using the Genotype-Tissue Expression (GTEx) and the UK Brain Expression Consortium (UKBEC) datasets; 34 of the 53 novel and known SNPs associated with alcohol consumption have a significant effect on gene expression in at least one tissue, including 33 SNPs that affect gene expression in the brain (Supplementary Tables 13 and 14, and Supplementary Figures 1–3). We found that the most significant eQTLs often do not involve the nearest gene and that several of the SNPs affect expression of different genes in different tissues. For example, SNP rs1991556 in the MAPT gene (https://www.ncbi.nlm.nih.gov/gene/4137) affects expression of 33 genes overall, with most significant effects on the expression of the non-protein coding genes CRHR1-IT1 (also known as C17orf69 or LINC02210) (https://www.ncbi.nlm.nih.gov/gene/147081) and LRRC37A4P (https://www.ncbi.nlm.nih.gov/gene/?term=LRRC37A4P), near MAPT, across a wide range of tissues including brain, adipose tissue and skin (P = 7.2 × 10−126 to P = 2.5 × 10−6) (Supplementary Figure 2). Similarly, the A-allele at SNP rs2071305 within MYBPC3 (https://www.ncbi.nlm.nih.gov/gene/4607) affects the expression of several genes and is most significantly associated with increased expression of C1QTNF4 (https://www.ncbi.nlm.nih.gov/gene/114900) across several tissues (P = 1.9 × 10−25 to P = 8.4 × 10−5).
Several of these eQTLs were found to affect expression of genes known to be involved in reward and addiction. SNP rs1053651 in the TCAP-PNMT-STARD3 gene cluster affects expression of the PPP1R1B gene (also known as DARPP-32) (https://www.ncbi.nlm.nih.gov/gene/84152) which encodes a protein that mediates the effects of dopamine in the mesolimbic reward pathway35. Other known addiction-related genes include ANKK1 (https://www.ncbi.nlm.nih.gov/gene/255239) and DRD2 (expression affected by SNP rs7121986) implicated in alcohol and nicotine dependence36,37, CRHR1 (https://www.ncbi.nlm.nih.gov/gene/1394) (affected by SNP rs1991556) involved in stress-mediated alcohol dependence38,39 and PPM1G (SNP rs1260326) (https://www.ncbi.nlm.nih.gov/gene/5496) whose epigenetic modification was reported to be associated with alcohol abuse40.
Over-representation enrichment analyses based on functional annotations and disease-related terms indicated that genes whose expressions are affected by the identified eQTLs are most significantly enriched for terms related to abdominal (n=91) and other malignant cancers, motor function (n= 5) and cellular homeostasis (n= 22) (Supplementary Figure 4). We performed a gene-based analysis and repeated the over-representation enrichment analysis adding the new set of identified genes (Supplementary Table 15). The results were similar supporting an enrichment for abdominal (n=100) and other cancers, as well as motor function (n=5) and cellular homeostasis (n=24) (Supplementary Figure 5).
Other traits and diseases
Using LD score regression12, we assessed genetic correlations between alcohol consumption and 235 complex traits and diseases from publicly available summary GWAS statistics (Methods). All results including their statistics (i.e. rg, standard errors, z value and P value) are included in Supplementary Table 16. The strongest positive genetic correlations based on false discovery rate P < 0.02 were found for smoking (rg= 0.42, P = 1.0 × 10−23) and HDL cholesterol levels (rg= 0.26, P = 5.1 × 10−13). We also found negative correlations for sleep duration (rg= −0.14, P = 3.8 × 10−7) and fasting insulin levels (rg= −0.25, P = 4.5 × 10−6). A significant genetic correlation was also found with schizophrenia (rg= 0.07, P = 3.9 × 10−3) and bipolar disorder (rg= 0.15, P = 5.0 × 10−4) (Supplementary Table 16). Over-representation enrichment analysis using WebGestalt41 (http://www.webgestalt.org) showed that our list of novel and known variants is significantly enriched for several diseases and traits including developmental disorder in children (P = 7.3 × 10−5), epilepsy (P = 1.4 × 10−4), heroin dependence (P = 5.7 ×10−4) and schizophrenia (P = 8.4 × 10−4) (Supplementary Figure 6). The result of the Mendelian randomization analysis (Methods) to assess a potential causal effect of alcohol on schizophrenia risk, using the inverse variance weighted approach, was not significant (P = 0.089), with large heterogeneity of the estimates of the tested variants.
Functional studies in Drosophila
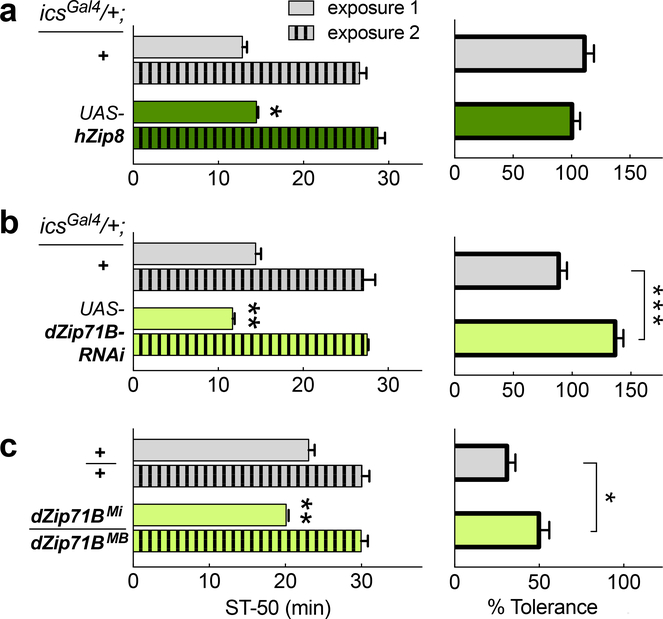
Based on our GWAS and brain imaging findings we took forward SNP rs13107325 in SLC39A8 (alias Zip8 gene) for additional testing in Drosophila, which employ conserved mechanisms to modulate ethanol-induced behaviors42,43. First, we overexpressed human Zip8 using a Gal4-driver that included expression in neurons involved in multiple ethanol-induced behaviors43. Flies carrying icsGal4/+ UAS-hZip8/+ showed a slight, but significant, resistance to ethanol-induced sedation compared to control flies (t30 = 2.3; Hedge’s g = 0.80; 95% CI: 0.08 – 1.53; P = 0.026; N = 16 per genotype). Ethanol tolerance, induced with repeat exposures spaced by a 4-hour recovery, was unchanged in these flies (t = 1.0; P = 0.33; Fig. 4a). We next used the same Gal4-driver to knock down the endogenous Drosophila ortholog of hZip8, namely dZip71B. This caused the flies to display naïve sensitivity to ethanol-induced sedation (t14 = 3.98; Hedge’s g = −1.84; 95% CI: −0.67 – −3.01; P = 0.0014; N = 8 per genotype), and in addition, these flies developed greater tolerance to ethanol upon repeat exposure (t14 = 4.80; Hedge’s g = 2.29; 95% CI: 1.03 – 3.55; P = 0.0003; Fig. 4b). To corroborate this phenotype, we then tested flies transheterozygous for two independent transposon-insertions in the middle of the dZip71B gene (Supplementary Figure 7) and found that these dZip71BMi / MB flies also displayed naïve sensitivity (t14 = 3.23; Hedge’s g = −1.54; 95% CI: −0.42 – −2.65; P = 0.006) and increased ethanol-induced tolerance (t14 = 2.39; Hedge’s g = 1.13; 95% CI: 0.07 – 2.18; P = 0.032) compared to controls (N = 8 each) (Fig. 4c).
Figure 4. Comparison of Zip8 alcohol phenotypes in Drosophila.
Flies were exposed to 100/50 Ethanol/Air vapor for 30 min for exposure 1, and the time to 50% loss of righting was determined (ST-50, sedation time). After recovery on food for 4 hours, flies were re-exposed to the same vapors, and the second ST-50 recorded (left side). The resulting increase in ST-50, i.e. tolerance, is shown on the right. In a) overexpressed human hZIP8 in ics-expressing cells flies are compared against controls whereas in b) knockdown of the fly ortholog dZip71B is compared against controls. In c) flies carrying two transposon insertions in the endogenous dZip71B gene are compared against controls. Significance levels: ***P <0.001, **P <0.01, *P <0.05. Exact P-values are presented in the text.
DISCUSSION
Our discovery utilizing data on common variants from over 480,000 people of European descent extends our knowledge of the genetic architecture of alcohol intake, increasing the number of identified loci to 46. We found loci involved in neuropsychiatric conditions such as schizophrenia, Parkinson’s disease and dementia, as well as BDNF where gene expression is affected by alcohol abuse. Our findings illustrate that large-scale studies of genetic associations with alcohol intake in the general population, rather than on alcohol dependency alone, can provide additional insights into genetic mechanisms regulating alcohol consumption.
We highlight the role of the highly pleiotropic MAPT and SLC39A8 genes in the genetics of alcohol consumption. MAPT plays a key role in tau-associated dementia44 and both genes are also implicated in other neuropsychiatric conditions including neuroticism, schizophrenia and Parkinson’s disease16–18. The SLC39A8 gene encodes a member of the SLC39 family of metal ion transporters. The encoded protein is glycosylated and found in plasma membrane and mitochondria, and is involved in the cellular transport of zinc, modulation of which could affect microglial inflammatory responses45. Our gain- and loss-of-function studies in Drosophila indicate a potential causal role of SLC39A8 in alcohol drinking behavior, even though results should be interpreted with caution due to small sample size in our experiment. The MRI brain imaging demonstrates a significant association of SNP rs13107325 in the SLC39A8 gene and putamen volume differences, and these structural differences appear to partially mediate associations of rs13107325 with alcohol consumption. The putamen has been associated with alcohol consumption and the withdrawal syndrome after chronic administration to rodents and non-human primates46. Our mediation analysis is suggestive of a plausible causal pathway linking rs13107325 in SLC39A8 with alcohol intake via an effect on putamen volume, but follow-up work is needed to conclusively demonstrate causal links. Putamen volume differences have also been associated with both schizophrenia and psychosis47,48 and robust association between SNP rs13107325 in SLC39A8 and schizophrenia was reported in a previous GWAS23.
We also report SNP rs7121986 near DRD2 as a novel alcohol intake variant in GWAS. The gene product of DRD2, D2 dopamine receptor, is a G protein-coupled receptor on post-synaptic dopaminergic neurons that has long been implicated in alcoholism49. In addition, we identify SNP rs988748 in BDNF as a novel alcohol intake variant; BDNF expression is differentially affected by alcohol exposure in animal models50,51. Both genes (along with PPP1R1P) are centrally involved in reward-mediating mesocortico-limbic pathways and both are implicated in the development of schizophrenia. For example, there is a robust GWAS association between schizophrenia and SNP rs4938021 in DRD2 (in perfect LD with our novel alcohol intake-related variant rs7121986) and DRD2 appears to be pivotal in network analyses of genes involved in schizophrenia52. Taken together, our results suggest that there are shared genetic mechanisms between the regulation of alcohol intake and susceptibility to schizophrenia, as well as other neuropsychiatric disorders. In this regard, large prospective epidemiological studies report a three-fold risk of schizophrenia in relation to alcohol abuse53.
We previously reported genome-wide significant associations of alcohol intake with KLB, and identified a liver-brain axis linking the liver hormone FGF21 with central regulation of alcohol intake involving β-Klotho receptor (the gene product of KLB) in the brain5. Here, we identify a significant variant near FGF21 gene and strongly replicate the previously reported KLB gene variant, strengthening the genetic evidence for the importance of this pathway in regulating alcohol consumption.
The LD score regression analysis showed a positive genetic correlation between alcohol consumption, smoking and HDL cholesterol levels. This confirms previous findings that reported an almost identical genetic correlation of alcohol consumption with number of cigarettes per day54. Furthermore, the observed genetic correlation with HDL levels is consistent with previous observations of an association between alcohol consumption and HDL55,56, including results of a Mendelian randomization study that suggested a possible causal role linking alcohol intake with increased HDL levels57. Furthermore, we found a genetic correlation (inverse) between sleep duration and alcohol consumption, an association previously reported only in a few small epidemiological studies58. We also found a significant genetic correlation with schizophrenia and bipolar disorder, a result that is supported by a recently published trans-ethnic meta-analysis of case-control studies on alcohol dependence59. We could not test for a genetic association between alcohol and risk of alcohol-related cancers60 because of limited availability of summary data. However, our gene-set enrichment analysis showed a significant enrichment for genes related to abdominal as well as other cancers.
Strengths of our study include its size, detailed attention to the alcohol phenotype, dense coverage of the genome through imputation, and incorporation of brain and other imaging data to explore potential mechanisms. Over 80% of the data came from UKB, which combines high-quality phenotypic data and imputed genome-wide genetic data with strict attention to quality control61. We adopted a stringent approach to claim novel variants involving a conservative P-value threshold, internal replication in UKB and consistent direction of effect with the other studies, to minimize the reporting of false positive signals.
However, since alcohol intake is socio-culturally as well as genetically determined, it is influenced by other lifestyle and environmental factors which may modify or dilute the genetic signal. A key limitation is that assessment of alcohol intake relies on self-report, which is prone to errors and biases including recall bias and systematic under-reporting by heavy drinkers62,63. Furthermore, questionnaires on alcohol intake covered a short duration (e.g. day or week) at a single period, which may not be representative of broader drinking patterns of cohort participants. We harmonized data across cohorts by converting alcohol intake into a common metric of g/d, with imputation as necessary in UKB for participants reporting consumption of small amounts of alcohol. Taking this approach, we were able to detect strong genetic associations with alcohol intake that explained 7% of the variance in alcohol in an independent cohort, while our GRS analysis indicates that individuals in the lower fifth of the GRS distribution were consuming daily approximately one third of a standard drink (2.6 g/d alcohol) less compared with those in the upper fifth.
We should also point out that our eQTL analyses are a first step in the identification of causal genes. Yet, as the most significant eQTLs affected expression of many genes, not necessarily the nearest, there is a need to further prioritize potential causal genes. Unbiased strategies that leverage information from multiple data sets including extensive genomic annotations and high-throughput functional screening in a broad range of tissues will be essential for effective prioritization of genes and uncovering of underlying causal mechanisms64. Establishing confidence in the prioritized genes in such a way is a prerequisite for performing functional follow-up studies in appropriate model systems, as demonstrated by the identification of the causal genes and potential disease mechanisms at the obesity- associated FTO locus65.
In summary, in this large study of genetic associations with alcohol consumption, we identified common variants in 46 novel loci, with several of the genes expressed in the brain as well as other tissues. Our findings suggest that there may be shared genetic mechanisms underpinning regulation of alcohol intake and development of a neuropsychiatric disorders including schizophrenia. This may form the basis for greater understanding of observed associations between alcohol consumption, schizophrenia66 and other disorders.
METHODS
UK Biobank data
We conducted a Genome Wide Association Study (GWAS) analysis among 458,577 UKB participants of European descent, identified from a combination of self-reported and genetic data. The details of the selection of the participants has been described elsewhere14. These comprise 408,951 individuals from UKB genotyped at 825,927 variants with a custom Affymetrix UK Biobank Axiom Array chip and 49,626 individuals genotyped at 807,411 variants with a custom Affymetrix UK BiLEVE Axiom Array chip from the UK BiLEVE study, which is a subset of UKB. For our analyses, we used SNPs imputed centrally by UKB using the Haplotype Reference Consortium (HRC) panel.
Alcohol intake
We calculated the alcohol intake as grams of alcohol per day (g/d) based on self-reported alcohol drinking from the touch-screen questionnaire. The quantity of each type of drink (red wine, white wine, beer/cider, fortified wine, spirits) was multiplied by its standard drink size and reference alcohol content. Drink-specific intake during the reported drinking period (a week for frequent drinkers defined as: daily or almost daily/once or twice a week/three or four times a week; or a month for occasional drinkers defined as: one to three times a month/special occasions only) was summed up and converted to g/d alcohol intake for all participants with complete response to the quantitative drinking questions. The alcohol intake for participants with incomplete response was imputed by bootstrap resampling from the complete responses, stratified by drinking frequency (occasional or frequent) and sex.
Participants were defined as life-time non-drinkers if they reported ‘never’ on the question on alcohol drinking frequency (UKB field 1558) and ‘no’ for the question on former drinker (UKB field 3731); they were excluded from further analysis. We considered participants with alcohol consumption > 500 g/d as outliers and they were dropped from the analyses. We also excluded participants with missing covariates, leaving data on 404,732 individuals. We log10 transformed g/d alcohol and sex-specific residuals were derived from the regression of log10 transformed g/d alcohol on age, age2, genotyping chip and weight.
UKB genetic analysis
We performed linear mixed modeling using BOLT-LMM software67, under an additive genetic model, for associations of measured and imputed SNPs with alcohol consumption (sex-specific residuals of the log10 transformed g/d variable). Model building was based on SNPs with MAF > 5%, call rate > 98.5% and HWE P > 1 × 10−6. SNPs were imputed using the HRC panel with imputation quality INFO score > 0.1. We estimated the LD score regression (LDSR) intercept to assess the degree of genomic inflation beyond polygenicity as well as the lambda inflation factor λGC68.
The Alcohol Genome-Wide Consortium (AlcGen) and the Cohorts for Heart and Aging Research in Genomic Epidemiology Plus (CHARGE+) consortia
We analyzed available GWAS data from 25 independent studies (N=76,111) from the AlcGen and the CHARGE+ consortia. All study participants were of reported European ancestry and data were imputed to either the 1000 Genome Project or the HRC panel. Alcohol intake in g/d was computed and the log10 transformed residuals were analyzed as described above. Study names, cohort information and general study methods are included in Supplementary Table 2 and 3.
All studies were centrally quality-controlled using easyQC69 including filtering for MAF. Finally, we analyzed data on ~7.1 M SNPs at MAF >1% and imputation quality score (Impute [Info score] or Mach [r2]) > 0.3. Genomic control (GC) was applied at study level. We synthesized the available GWAS using a fixed effects inverse variance weighted meta-analysis and summary estimates were derived for AlcGen and CHARGE+.
One-stage meta-analysis
We performed a one-stage meta-analysis applying a fixed-effects inverse variance weighted meta-analysis using METAL70 to obtain summary results from the UKB and and the AlcGen plus CHARGE+ GWAS, for up to N=480,842 participants and ~7.1 M SNPs with MAF ≥ 1% for variants present in both the UKB data and AlcGen and CHARGE+ meta-analysis. We assessed the observed heterogeneity using Cochran’s Q and we quantified this using the I2 metric. We considered a Cochran’s Q P < 1 10−4 as significant. The LDSR intercept (standard error), in the discovery meta-analysis was 1.05 and no further correction was applied. QQ plots of the combined meta-analysis summary results, UK Biobank only as well as AlcGen and CHARGE+ only, are presented in Supplementary Figure 8.
Previously reported (known) SNPs
We looked up in the GWAS catalog (http://www.ebi.ac.uk/gwas/) and identified 17 SNPs associated with alcohol consumption at genome-wide significance level (P < 5 10−8). We enhanced the list by reference to a recent GWAS by Clarke et al6 that was not covered by the GWAS catalog at the time of the analysis, reporting 14 additional rare and common SNPs. Together with a SNP in RASGRF2 shown to be associated with alcohol-induced reinforcement71, we found 31 previously reported alcohol consumption related SNPs.
Novel loci
According to locus definition of i) SNPs within ±500kb distance of each other; ii) SNPs in linkage disequilibrium LD (r2 > 0.1) calculated with PLINK, we augmented the list of known SNPs with all SNPs present within our data, not contained within the previously published loci. We further excluded SNPs in the HLA region (chromosome 6, 25–34Mb) due to its complex LD structure. We performed LD clumping in PLINK on 4,515 unknown SNPs with P < 1 ×10−8 using an r2 > 0.1 and distance threshold of 500kb. We further grouped the lead SNPs within 500kb from each other into the same loci and selected the SNP with smallest P-value from the locus as sentinel SNP.
To report a SNP as novel signal of association with alcohol consumption:
the sentinel SNP has P < 5 × 10−9 in the one-stage meta-analysis;
the sentinel SNP is strongly associated (P < 5 × 10−7) in the UKB GWAS alone;
the sentinel SNP has concordant direction of effect between UKB and AlcGen and CHARGE+ datasets;
The sentinel SNP is not located within any of the previously reported loci
We selected the above criteria i) to iii) to minimize false positive findings including use of a conservative one-stage P-value threshold that is an order of magnitude more stringent than a genome-wide significance P-value. (The threshold of P < 5 × 10−9 has been proposed e.g. for whole-genome sequencing-based studies.) This approach led us to the identification of 46 sentinel SNPs in total. Regional plots for all 46 sentinel SNPs are presented in Supplementary Figure 9.
Conditional analysis
We conducted locus-specific conditional analysis using the GCTA (Genome-wide Complex Trait Analysis) software (http://cnsgenomics.com/software/gcta). For each of the 46 novel sentinel SNPs, we obtained conditional analysis results for the SNPs with MAF>1% and within 500kb from the sentinel SNP after conditioning on the sentinel SNP. The meta-analysis results of the GWAS in UKB, AlcGen and CHARGE+ were used as input summary statistics and the individual-level genetic data from UKB were used as the reference sample. Results for a SNP were considered conditionally significant if the difference between the conditional P-value and the original P-value is greater than 1.5-fold (-log10P/-log10(P_conditional) >1.5) and the conditional P-value is smaller than 5 × 10−8.
Gene-based analysis
We performed a gene-based analysis using fastBAT, a method that performs a set-based association analysis using summary-level data from GWAS. We used the UKB dataset as a reference set for the LD calculation72. Gene-based associations with P < 5 × 10−9 were considered significant.
Gene expression analyses
To analyze the impact of genetic variants on expression of neighboring genes and identify expression quantitative trait loci (cis-eQTLs; i.e., SNPs associated with differences in local gene expression), we used two publicly available databases, the Genotype-Tissue Expression (GTEx) database73 (www.gtexportal.org) and the UK Brain Expression Consortium (UKBEC) dataset74 (http://www.braineac.org). We searched these databases for significant variant-transcripts pairs for genes within 1Mb of each input SNP.
With the GTEx database, we tested for cis-eQTL effects in 48 tissues from 620 donors. The data described herein were obtained from the GTEx Portal, Release: V7 and used FastQTL75, to map SNPs to gene-level expression data and calculate q-values based on beta distribution-adjusted empirical P-values76. A false discovery rate (FDR) threshold of ≤0.05 was applied to identify genes with a significant eQTL. The effect size, defined as the slope of the linear regression, was computed in a normalized space (normalized effect size (NES)), where magnitude has no direct biological interpretation. Here, NES reflects the effects of our GWAS A1 alleles (that are not necessarily the alternative alleles relative to the reference alleles, as reported in the GTEx database). Supplementary Table 13 lists transcripts-SNPs associations with significant eQTL effects.
With the UKBEC dataset that comprises 134 brains (http://www.braineac.org/), we searched for cis-eQTLs in 10 brain regions, including the cerebellar cortex (CRBL), frontal cortex (FCTX), hippocampus (HIPP), medulla (specifically inferior olivary nucleus, MEDU), occipital cortex (specifically primary visual cortex, OCTX), putamen (PUTM), substantia nigra (SNIG), thalamus (THAL), temporal cortex (TCTX) and intralobular white matter (WHMT), as well as across all brain tissues (aveALL). MatrixEQTL77 generated P-values for each expression profile (either exon-level or gene-level) against the respective SNP were obtained for the 10 different tissues and overall (aveALL). Supplementary Table 14 lists transcripts-SNPs associations with a eQTL P-value < 0.0045 in at least one brain tissue. Subsequent data analysis was performed in R (http://www.R-project.org/).
We carried out over-representation enrichment analysis using a list of 146 GTEx eQTL genes that were derived from the single-variant analysis and a list of 160 eQTL genes that were derived from both single-variant and gene-based analysis. Ingenuity pathway analysis (IPA®, QIAGEN Inc.) was performed on these lists using ontology annotations from all available databases except those derived from low-confidence computational predictions.
Magnetic Resonance Imaging Data
We used the most recent release of magnetic resonance imaging (MRI) data on brain, heart and liver for UKB participants to investigate genetic associations with the 46 novel SNPs for alcohol consumption.
Brain imaging
Brain MRI acquisition and pre-processing
We used the T1 data from UKB to elucidate volumetric brain structures, including the cortical and the sub-cortical areas. The T1 data were acquired and pre-processed centrally by UKB. The brain regions were defined by combining the Harvard-Oxford cortical and subcortical atlases78 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) and the Diedrichsen cerebellar atlas79 (http://www.diedrichsenlab.org/imaging/propatlas.htm). FAST (FMRIB’s Automated Segmentation Tool)80 was then used to estimate the grey matter partial volume within each brain region. Subcortical region volumes were also modelled by using FIRST (FMRIB’s Integrated Registration and Segmentation Tool). More details about the MRI scanning protocol and pre-processing has been provided in UKB documentation (https://biobank.ctsu.ox.ac.uk/crystal/docs/brain_mri.pdf).
Association Analyses
We performed association analyses on N = 9,702 individuals between all novel SNPs and the grey matter volume of brain regions using Pearson correlation, adjusting for age, age2, sex, age × sex, age2 × sex, and head size. All, brain volume features, log transformed alcohol intake data (g/d), and the confounders were firstly transformed by using a rank-based inverse Gaussian transformation. Significance levels were set at P < 0.05 adjusted using the false-discovery rate method for multiple comparisons.
Mediation analysis
To assess if the effect of a SNP on alcohol consumption is mediated through a brain region, we performed a single-level mediation analysis based on a standard three-variable path model (SNP-brain region-alcohol consumption) with corrected and accelerated percentile bootstrapping 10,000 times to calculate the significance of the mediation effect. We considered as mediator variable the grey matter volume of brain regions that had a significant association on alcohol consumption. We calculated the significance of path a, path b and a*b mediation (SNP-brain region-alcohol consumption) using a multilevel mediation and moderation (M3) toolbox81,82. To exclude the possibility of an inverse causal pathway we performed additional analyses in UKB non-drinkers (N =589). performing 10,000 random permutations, associations of rs13107325 with both left and right putamen.
Cardiac Imaging
Cardiac MRI acquisition and pre-processing
Details of the cardiac image acquisition in UKB are reported previously83. Cardiac MRI was acquired using a clinical wide bore 1.5T scanner (MAGNETOM Aera, Syngo Platform VD13A, Siemens Healthcare, Erlangen, Germany) with 48 receiver channels, a 45 mT/m and 200 T/m/s gradient system, an 18-channel anterior body surface coil used in combination with 12 elements of an integrated 32 element spine coil and electrocardiogram gating for cardiac synchronization. A two-dimensional short-axis cardiac MRI was obtained using a balanced steady state free precession to cover the entire left and right ventricle (echo time, 1.10msec; repetition time, 2.6msec; flip angle, 80°; slice thickness, 8mm with 2mm gap; typical field of view, 380×252mm; matrix size, 208×187, acquisition of 1 slice per breath-hold).
The cardiac images were segmented to provide left ventricular mass (LVM), left end-diastolic (LVEDV), left end-systolic volume (LVESV), and right end-diastolic (RVEDV) and right end-systolic volume (RVESV) using a fully convolutional network as described previously84. Left (LVEF) and right ventricular ejection fraction (RVEF) were derived from (LVEDV–LVESV)/LVEDV×100 and (RVEDV–RVESV)/RVEDV×100, respectively.
Association Analyses
To test associations between cardiac MRI measures and alcohol consumption-related SNPs, we carried out a regression of LVM, LVEDV, LVEF, RVEDV, and RVEF onto each of the 46 SNPs adjusting for age, sex, height, weight, hypertension (defined as systolic blood pressure >140mmHg and or diastolic blood pressure >90mmHg or under antihypertensive treatment), diabetes, and smoking history on N=10,706 participants. Significance levels were set at P < 0.05 adjusted using the false-discovery rate method for multiple comparisons.
Liver Imaging
Liver MRI acquisition and pre-processing
Details of the liver image acquisition protocol have been reported previously85. Briefly, all participants were scanned in a Siemens MAGNETOM Aera 1.5-T MRI scanner (Siemens Healthineers, Erlangen, Germany) using a 6-minute dual-echo Dixon Vibe protocol, providing a water and fat separated volumetric data set for fat and muscle covering neck to knees. For liver proton density fat fraction (PDFF) quantification, an additional single multi-echo gradient slice was acquired over the liver. Liver images were analysed by computing specific ROI for water, fat and T2* by magnitude-based chemical shift technique with a 6-peak lipid model, correcting for T1 and T2*.
Association Analyses
We performed association analyses between 46 alcohol consumption-related SNPs and liver PDFF (%), from 8,479 samples, using a linear regression model adjusting for age, age2, sex, T2D, BMI, genotyping chip and first three PCs. Liver PDDF was firstly transformed by using a rank-based inverse transformation. Significance levels were set at P < 0.05 adjusted using the false-discovery rate method for multiple comparisons.
Drosophila experiments
Flies were kept on standard cornmeal/molasses fly food in a 12:12hr light:dark cycle at 25°C. Transgenc flies were obtained from the Bloomington Drosophila Stock Center: UAS-hZip8 BL#66125, UAS-dZIP71B-TRiP-RNAiHMC04064 BL#55376, dZip71BMI13940 BL#59234, and dZip71BMB11703 BL#29928. For behavioral experiments, crosses were set up such that experimental and control flies were sibling progeny from a cross, and both were therefore in the same hybrid genetic background (w Berlin / unknown). Flies aged 1–5 days of adult age were collected, exposed to 100/50 (flowrates) ethanol/air vapor in the Booze-o-Mat 2 days later, and their loss of righting determined by slight tapping, as described86. For tolerance, flies were put back onto regular food after a 30-min initial exposure and were then re-exposed to the same vapor 4 hours later. Note that tolerance is not connected to initial sensitivity, and flies naively sensitive to ethanol-induced sedation can have no, or a reduced tolerance phenotype. Flies overexpressing hZip8 (and their sibling controls) were placed at 28°C for two days to increase the expression levels of the transgene, as we did not detect a phenotype when they were kept at 25°C (data not shown). Data from experimental and control flies were compared by two-sided Student’s t-tests. Data were normally distributed according to Shapiro-Wilk testing with Bonferroni adjustment for each of the three experiments.
Effects on other traits and diseases
We queried SNPs against GWAS results included in PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk), to investigate cross-trait effects, extracting all association results with genome-wide significance at P < 5 × 10−8 for all SNPs in high LD (r2 ≥ 0.8) with the 46 sentinel novel SNPs, to highlight the loci with strongest evidence of association with other traits. At the gene level, overrepresentation enrichment analysis (ORA) with WebGestalt41 on the nearest genes to all alcohol consumption loci was carried out.
The genetic correlations between alcohol consumption and 235 other traits and diseases were obtained in the online software LD Hub. LD hub is a centralized database of summary-level GWAS results and a web interface for LD score regression analysis
To estimate the potential causal effect of alcohol consumption-related variants on schizophrenia, we performed a Mendelian randomization analysis utilizing publicly available GWAS data on schizophrenia and the Mendelian randomization package in R. The effect was estimated using the inverse-variance weighted (IVM) method. Pleiotropy was tested by applying the MR-Egger regression method and heterogeneity statistics were obtained. In presence of heterogeneity the random effects inverse-variance method was applied87.
Genetic risk scores and percentage of variance explained
We calculated an unbiased weighted GRS in 14,004 unrelated participants in Airwave, an independent cohort with high quality HRC imputed genetic data33. All previously reported and novel variants were used for the construction of the GRS. We weighted the alcohol-increasing alleles by the beta coefficients of the meta-analysis. We assessed the association of the GRS with alcohol intake and calculated the alcohol consumption levels for individuals in the top vs the bottom quintiles of the distribution. To calculate the percent of variance of alcohol consumption explained by genetic variants, we generated the residuals from a regression of alcohol consumption in Airwave. We then fit a second linear model for the trait residuals with all novel and known variants plus the top 10 principal components and estimated the percentage variance of the dependent variable explained by the variants.
Statistical analysis
All inferential statistics for the analyses described above are provided in the text or in tables and figures. All performed tests were two-sided.
Data availability statement
The UKB GWAS data can be assessed from the UK Biobank data repository (http://biota.osc.ox.ac.uk/). The genetic and phenotypic UKB data are available upon application to the UK Biobank (https://www.ukbiobank.ac.uk). Summary GWAS data data can be assessed by request to the corresponding authors and will be available via LDHub (http://ldsc.broadinstitute.org/ldhub/).
Supplementary Material
Acknowledgements
H.G. was funded by the NIHR Imperial College Health Care NHS Trust and Imperial College London Biomedical Research Centre. I.K. was supported by the EU PhenoMeNal project (Horizon 2020, 654241) and the UK Dementia Research Institute, which is supported by the MRC, the Alzheimer’s Society and Alzheimer’s Research UK. S.Thériault was supported by the Canadian Institutes of Health Research and Université Laval (Quebec City, Canada). L.R. was supported by Forschungs- und Förder-Stiftung INOVA, Vaduz, Liechtenstein. D.C. holds a McMaster University Department of Medicine Mid-Career Research Award. M.B. is supported by NIH grant R01-DK062370. P.v.d.H. was supported by ICIN-NHI and Marie Sklodowska-Curie GF (call: H2020-MSCA-IF-2014, Project ID: 661395). C.H. was supported by a core MRC grant to the MRCHGU QTL in Health and Disease research programme. N.V. was supported by Marie Sklodowska-Curie GF grant (661395) and ICIN-NHI. P.E. acknowledges support from the NIHR Biomedical Research Centre at Imperial College Healthcare NHS Trust and Imperial College London, the NIHR Health Protection Research Unit in Health Impact of Environmental Hazards (HPRU-2012–10141), the Medical Research Council (MRC) and Public Health England (PHE) Centre for Environment and Health (MR/L01341X/1) and Health Data Research (HDR) UK. P.E. is a UK Dementia Research Institute (DRI) professor, UK DRI at Imperial College London, funded by the MRC, Alzheimer’s Society and Alzheimer’s Research UK. This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007–037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (695313), ERANID (Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways) (PR-ST-0416–10004), BRIDGET (JPND: BRain Imaging, cognition Dementia and next generation GEnomics) (MR/N027558/1), the FP7 projects IMAGEMEND(602450; IMAging GEnetics for MENtal Disorders) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300–2), the Medical Research Council Grant ‘c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the Swedish Research Council FORMAS, the Medical Research Council, the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL 01EE1406A, 01EE1406B), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7–2, SFB 940/2), the Medical Research Foundation and Medical research council (grant MR/R00465X/1), the Human Brain Project (HBP SGA 2). Further support was provided by grants from: ANR (project AF12-NEUR0008–01 - WM2NA, and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772–01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence.
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing Interests
B.M.P. serves on the DSMB of a clinical trial funded by the manufacturer (Zoll LifeCor) and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson.
B.W.J.H.P. has received research funding (non-related to the work reported here) from Jansen Research and Boehringer Ingelheim.
The other authors declare no competing interests.
References
- 1.GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 5, 987–1012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global status report on alcohol and health 2018. Eds: Poznyak V and Rekve D, https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/ (2018).
- 3.Wood AM et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 391, 1513–1523 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verhulst B, Neale MC & Kendler KS The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med 45, 1061–72 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schumann G et al. KLB is associated with alcohol drinking, and its gene product beta-Klotho is necessary for FGF21 regulation of alcohol preference. Proc Natl Acad Sci U S A 113, 14372–14377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke TK et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117). Mol Psychiatry 22, 1376–1384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgenson E et al. Genetic contributors to variation in alcohol consumption vary by race/ethnicity in a large multi-ethnic genome-wide association study. Mol Psychiatry 22, 1359–1367 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baik I, Cho NH, Kim SH, Han BG & Shin C Genome-wide association studies identify genetic loci related to alcohol consumption in Korean men. Am J Clin Nutr 93, 809–16 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Jackson B et al. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Hum Genomics 5, 283–303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudlow C et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12, e1001779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy S et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 48, 1279–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulik-Sullivan BK et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 47, 291–5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evangelou E & Ioannidis JP Meta-analysis methods for genome-wide association studies and beyond. Nat Rev Genet 14, 379–89 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Evangelou E et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet 50, 1412–1425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desikan RS et al. Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol Psychiatry 20, 1588–95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do CB et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet 7, e1002141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pankratz N et al. Meta-analysis of Parkinson’s disease: identification of a novel locus, RIT2. Ann Neurol 71, 370–84 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okbay A et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet 48, 624–33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couch FJ et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 9, e1003212 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikram MA et al. Common variants at 6q22 and 17q21 are associated with intracranial volume. Nat Genet 44, 539–44 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Harst P et al. Seventy-five genetic loci influencing the human red blood cell. Nature 492, 369–75 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuel A et al. Six3 regulates optic nerve development via multiple mechanisms. Sci Rep 6, 20267 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu JZ et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 47, 979–986 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Consortium for Blood Pressure Genome-Wide Association Studies et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speliotes EK et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42, 937–48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teslovich TM et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim CS & Alkon DL Protein kinase C stimulates HuD-mediated mRNA stability and protein expression of neurotrophic factors and enhances dendritic maturation of hippocampal neurons in culture. Hippocampus 22, 2303–19 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Barker JM, Taylor JR, De Vries TJ & Peters J Brain-derived neurotrophic factor and addiction: Pathological versus therapeutic effects on drug seeking. Brain Res 1628, 68–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka T et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am J Clin Nutr 97, 1395–402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talukdar S et al. FGF21 Regulates Sweet and Alcohol Preference. Cell Metab 23, 344–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant SF et al. Association analysis of the FTO gene with obesity in children of Caucasian and African ancestry reveals a common tagging SNP. PLoS One 3, e1746 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott P et al. The Airwave Health Monitoring Study of police officers and staff in Great Britain: rationale, design and methods. Environ Res 134, 280–5 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Elliott LT et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562, 210–216 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stipanovich A et al. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature 453, 879–84 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang BZ et al. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Hum Mol Genet 16, 2844–53 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Gelernter J et al. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet 15, 3498–507 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Treutlein J et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry 11, 594–602 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Timpl P et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet 19, 162–6 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Ruggeri B et al. Association of Protein Phosphatase PPM1G With Alcohol Use Disorder and Brain Activity During Behavioral Control in a Genome-Wide Methylation Analysis. Am J Psychiatry 172, 543–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Vasaikar S, Shi Z, Greer M & Zhang B WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res 45, W130–W137 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez DA et al. The Arf6 activator Efa6/PSD3 confers regional specificity and modulates ethanol consumption in Drosophila and humans. Mol Psychiatry 23, 621–628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ojelade SA et al. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc Natl Acad Sci U S A 112, E4085–93 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rademakers R, Cruts M & van Broeckhoven C The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat 24, 277–95 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Higashi Y et al. Influence of extracellular zinc on M1 microglial activation. Sci Rep 7, 43778 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G et al. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcohol Clin Exp Res 35, 1739–48 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada N et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry 21, 1460–6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Erp TG et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 21, 547–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyers JL et al. The association between DRD2/ANKK1 and genetically informed measures of alcohol use and problems. Addict Biol 18, 523–36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Logrip ML, Barak S, Warnault V & Ron D Corticostriatal BDNF and alcohol addiction. Brain Res 1628, 60–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boschen KE, Criss KJ, Palamarchouk V, Roth TL & Klintsova AY Effects of developmental alcohol exposure vs. intubation stress on BDNF and TrkB expression in the hippocampus and frontal cortex of neonatal rats. Int J Dev Neurosci 43, 16–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monaco A et al. A complex network approach reveals a pivotal substructure of genes linked to schizophrenia. PLoS One 13, e0190110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen SM, Toftdahl NG, Nordentoft M & Hjorthoj C Association between alcohol, cannabis, and other illicit substance abuse and risk of developing schizophrenia: a nationwide population based register study. Psychol Med 47, 1668–1677 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Nivard MG et al. Connecting the dots, genome-wide association studies in substance use. Mol Psychiatry 21, 733–5 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Gaziano JM et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med 329, 1829–34 (1993). [DOI] [PubMed] [Google Scholar]
- 56.Linn S et al. High-density lipoprotein cholesterol and alcohol consumption in US white and black adults: data from NHANES II. Am J Public Health 83, 811–6 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vu KN et al. Causal Role of Alcohol Consumption in an Improved Lipid Profile: The Atherosclerosis Risk in Communities (ARIC) Study. PLoS One 11, e0148765 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaput JP, McNeil J, Despres JP, Bouchard C & Tremblay A Short sleep duration is associated with greater alcohol consumption in adults. Appetite 59, 650–5 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Walters RK et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci 21, 1656–1669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bagnardi V et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 112, 580–93 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bycroft C et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boniface S, Kneale J & Shelton N Drinking pattern is more strongly associated with under-reporting of alcohol consumption than socio-demographic factors: evidence from a mixed-methods study. BMC Public Health 14, 1297 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenfield TK & Kerr WC Alcohol measurement methodology in epidemiology: recent advances and opportunities. Addiction 103, 1082–99 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grotz AK, Gloyn AL & Thomsen SK Prioritising Causal Genes at Type 2 Diabetes Risk Loci. Curr Diab Rep 17, 76 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Claussnitzer M et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N Engl J Med 373, 895–907 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hambrecht M & Hafner H Substance abuse and the onset of schizophrenia. Biol Psychiatry 40, 1155–63 (1996). [DOI] [PubMed] [Google Scholar]
- 67.Loh PR et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet 47, 284–90 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Georgiopoulos G & Evangelou E Power considerations for lambda inflation factor in meta-analyses of genome-wide association studies. Genet Res (Camb) 98, e9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winkler TW et al. Quality control and conduct of genome-wide association meta-analyses. Nat Protoc 9, 1192–212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willer CJ, Li Y & Abecasis GR METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stacey D et al. RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release. Proc Natl Acad Sci U S A 109, 21128–33 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bakshi A et al. Fast set-based association analysis using summary data from GWAS identifies novel gene loci for human complex traits. Sci Rep 6, 32894 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Consortium GTEx. The Genotype-Tissue Expression (GTEx) project. Nat Genet 45, 580–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramasamy A et al. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci 17, 1418–1428 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ongen H, Buil A, Brown AA, Dermitzakis ET & Delaneau O Fast and efficient QTL mapper for thousands of molecular phenotypes. Bioinformatics 32, 1479–85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Storey JD & Tibshirani R Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100, 9440–5 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shabalin AA Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics 28, 1353–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown CA et al. Development, validation and application of a new fornix template for studies of aging and preclinical Alzheimer’s disease. Neuroimage Clin 13, 106–115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diedrichsen J et al. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage 54, 1786–94 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Brady M & Smith S Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20, 45–57 (2001). [DOI] [PubMed] [Google Scholar]
- 81.Wager TD, Davidson ML, Hughes BL, Lindquist MA & Ochsner KN Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–50 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wager TD et al. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage 47, 821–35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petersen SE et al. UK Biobank’s cardiovascular magnetic resonance protocol. J Cardiovasc Magn Reson 18, 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bai W et al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. J Cardiovasc Magn Reson 20, 65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Linge J et al. Body Composition Profiling in the UK Biobank Imaging Study. Obesity (Silver Spring) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peru Y.C.d.P.R.L. et al. Adult neuronal Arf6 controls ethanol-induced behavior with Arfaptin downstream of Rac1 and RhoGAP18B. J Neurosci 32, 17706–13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dimou NL & Tsilidis KK A Primer in Mendelian Randomization Methodology with a Focus on Utilizing Published Summary Association Data. Methods Mol Biol 1793, 211–230 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The UKB GWAS data can be assessed from the UK Biobank data repository (http://biota.osc.ox.ac.uk/). The genetic and phenotypic UKB data are available upon application to the UK Biobank (https://www.ukbiobank.ac.uk). Summary GWAS data data can be assessed by request to the corresponding authors and will be available via LDHub (http://ldsc.broadinstitute.org/ldhub/).