Abstract
Introduction
The prevalence of pregnancy-associated breast cancer is increasing. HER2-positive breast cancers typically have a poor prognosis. The objective of our study was to compare the prognosis of patients with HER2-positive breast cancer diagnosed during pregnancy (HER2-positive BCP) to young women diagnosed with HER2-positive breast cancer outside of pregnancy (HER2 non-BCP).
Methods
Data of patients managed for invasive breast carcinoma between January 2005 and 2020 were retrospectively collected from the database of Tenon University Hospital (Paris, France), part of the “Cancer lié à la Grossesse” network.
Results
Fifty-one patients with HER2-positive BCP were matched on age at diagnosis with 51 HER2-positive non-BCP patients. Locally advanced disease with axillary lymph node involvement were frequent. Tumors were frequently aggressive with high grade (p = 0.57) and high Ki67 (p = 0.15). Among the HER2-positive BCP patients, the mean term at diagnosis was 19.3 week of gestation (WG). Eighty-four percent of the patients continued their pregnancy with a mean term at delivery of 34.2WG. Chemotherapy modalities differed between the two groups: neoadjuvant chemotherapy was more frequent in the HER2-positive BCP group (p = 0.03) and adjuvant chemotherapy more frequent in the HER2 non-BCP group (p = 0.009). The recurrence rate was 10% (n = 5) and 18% (n = 9) in the HER2-positive BCP and HER2 non-BCP groups, respectively, p = 0.25. Breast cancer-free survival was poorer in the HER2-positive BCP group with earlier recurrence, p = 0.008. No difference in type of recurrence was found between the groups (p = 0.58).
Conclusion
This matched case-control study implies that patients with HER2-positive BCP still have a poorer prognosis than non-pregnant HER-positive patients.
Keywords: Pregnancy-associated breast cancer, HER2 positive breast cancer, Prognosis, Breast cancer-free survival, Targeted therapy
Highlights
-
•
Trastuzumab improved survival in HER2-positive patients with early, locally advanced and metastatic breast cancer.
-
•
Administration of trastuzumab is contraindicated during pregnancy.
-
•
Patients with HER2-positive breast cancer during pregnancy have a poorer prognosis than non-pregnant HER-positive patients.
-
•
The prognosis of patients with HER2-positive breast cancer is not related to the term of diagnosis.
1. Introduction
The prevalence of breast cancer associated with pregnancy (BCP) is increasing as women are increasingly choosing to become mothers later in life [1,2]. BCP constitutes a major medical challenge due to the impact of treatments on both maternal and fetal outcomes [3]. In view of the clinical complexity of cancers occurring during pregnancy, a national network - the CALG (Cancer Associé à La Grossesse) network - was created in France in 2008. National and international guidelines recommend that BCP treatment should be as similar as possible to patients with breast cancer who are not pregnant [3]. However, therapeutic strategies and prognoses differ widely according to the molecular classification of breast cancer [4]. This raises specific issues especially for patients with HER2-positive BCP who require targeted therapies the specific impact of which on pregnancy, maternal and newborn outcomes remains unclear.
Overexpression of HER2 is observed in 20–30% of breast carcinomas [5,6] and HER2-positive breast cancers typically have a poor prognosis compared to luminal A and B breast cancers [7,8]. The paradigm for treating HER2-positive breast cancer changed in 1998 when the FDA approved the use of trastuzumab, a humanized recombinant monoclonal antibody to HER2 [9]. Trastuzumab improved overall survival and progression-free survival in HER2-positive patients with early, locally advanced and metastatic breast cancer [10,11]. However, administration of trastuzumab is contraindicated during pregnancy because of the risk of oligohydramnios [12] meaning that women with HER2-positive BCP have a potentially poorer prognosis.
Therefore, the objectives of our study were to describe the management of HER2-positive BCP as well as maternal and neonatal outcomes, and to compare the prognosis of HER2-positive patients with and without associated pregnancy.
2. Material and methods
2.1. Study population
Between January 2005 and January 2020, data of patients with histologically proven invasive breast carcinoma <46 years old at diagnosis were retrospectively collected from the prospective database of Tenon University Hospital (Paris, France) from the CALG cancer network. From this data set, we selected breast cancer patients with HER2 overexpression. The age of 46 years was set as the threshold as it corresponded to the oldest patient with HER2-positive BCP in our cohort. The Ethics Committee (CEROG) of the Collège National des Gynécologues et Obstétriciens Français (CNGOF) approved the study (CEROG 2019-GYN-603).
Although BCP is defined as the diagnosis of breast cancer during pregnancy and the year following the delivery, to avoid biases linked to the inclusion of patients diagnosed with BCP in the postpartum period, only patients with HER2-positive BCP diagnosed during pregnancy (HER2-positive BCP group) were included. The control group was composed of patients matched on age with HER2-positive breast cancer not associated with pregnancy (HER2 non-BCP group).
For all the patients, epidemiological data (age at diagnosis, genetic mutation, familial or personal history of cancer), histological and immunohistochemical data (histological grade according to Ellis and Easton, hormonal-receptor status (estrogen receptors (ER) and progesterone receptors (PR)), HER2 overexpression, Ki67 expression), and treatment modalities were recorded. The histological data corresponded to surgical specimens except when neoadjuvant chemotherapy was performed in which case biopsy data and initial imaging data were used. For patients undergoing neoadjuvant chemotherapy, lymph node status was determined on the result of axillary lymph node cytology before chemotherapy and on analysis of a surgical specimen using the Sataloff criteria [13].
ER and/or PR status was considered positive when ≥10%. If an intermediary HER2 expression (i.e., score 2) was detected, a fluorescent in situ hybridization test (FISH) was performed. A tumor was considered proliferative when Ki67 was ≥15% but an analysis with a Ki67 ≥ 20% was also performed as this cut-off is routinely used in France. The treatment modalities for BCP followed the recommendations for non-pregnant patients as closely as possible [3]. In both groups, patients received the same chemotherapy (anthracyclines (adriamycin or epirubicine), taxanes and cyclophosphamide), trastuzumab, tamoxifen as endocrine therapy in cases of positive hormonal receptors. However, none of the patients in the HER2-positive BCP received either tamoxifen or trastuzumab during the pregnancy. Radiotherapy was also postponed until after delivery.
Recurrent disease was assessed by physical examination, histological findings, clinical follow-up and imaging. Breast cancer-free survival was defined as time from diagnosis to breast cancer recurrence and was censored at the date of the last follow-up or the date of death without recurrence. Breast cancer-specific survival was defined as time from diagnosis to breast cancer-related death. Recurrence events were defined as: i) local when recurrence was ipsilateral; ii) regional for ipsilateral axillary recurrence, and iii) distant when metastasis to bone, liver, lung, brain or peritoneum was observed, and for contralateral axillary recurrence.
2.2. Statistical analysis
The population was divided into two groups HER2-positive BCP and non-pregnant patients with HER2-positive breast cancer (HER2 non-BCP). HER2-positive BCP patients were matched for age at diagnosis with HER2 non-BCP patients and compared. Statistical analysis was based on the Student’s t-test or ANOVA test as appropriate for continuous variables, and the Chi-square test or Fisher’s exact test as appropriate for categorical variables. Values of p < 0.05 were considered to denote significant differences. The Kaplan Meier method was used to estimate the cumulative rates (CRs), and comparisons of CRs were made using the log-rank test. Data were analyzed using R 3.0.1 software, available online.
3. Results
During the study period, 256 patients with BCP were extracted from the database of Tenon University Hospital from the CALG network. Among these, 144 patients were excluded because of non-HER2 BCP and 61 because the breast cancer was diagnosed during the postpartum period. Therefore, 51 HER2-positive BCP patients were retained for analysis. These patients were matched for age at diagnosis with HER2 non-BCP patients giving an overall study population of 102 patients: 51 HER2-positive BCP and 51 HER2 non-BCP.
3.1. Epidemiological characteristics of the study population
The epidemiological characteristics of the groups are summarized in Table 1. Mean age at diagnosis was 35.2 years and 34.7 years in HER2-positive BCP and HER2 non-BCP groups respectively, p = 0.59.
Table 1.
Comparison of clinical characteristics between patients with HER2-positive BCP and HER2 non-BCP groups.
| HER2-positive BCP (n = 51) | HER2 non-BCP (n = 51) | p | |
|---|---|---|---|
| Age at diagnosis (mean) | 35.2 | 34.7 | 0.59 |
| BMI (mean) (kg/m2) | 27 | 26.3 | 0.67 |
| Gestity (mean) | 2 | 1 | 0.68 |
| Parity (mean) | 1 | 0 | 0.73 |
| Personal breast cancer history, n(%) | 3 (6) | 0 (0) | 0.10 |
| Familial breast cancer history, n(%) | 11 (22) | 13 (28.9) | 0.44 |
TNM stages, histological and immunohistochemical data at diagnosis are reported in Table 2. No differences in the tumor size (p = 0.33), lymph node involvement (p = 0.83), distant metastasis (p = 0.39), tumor type (p = 1) or multifocality (p = 0.82) were noted between the groups. There was a high number of aggressive and high grade tumors in both groups: 70.6% and 60.8% grade SBR 3 (p = 0.57) and high Ki67 (96.1% and 92.2% Ki67 ≥ 15% (p = 0.15) in HER2-positive BCP and HER2 non-BCP groups, respectively.
Table 2.
Comparison of histological and immunohistochemical characteristics between HER2-positive BCP and HER2 non-BCP groups.
| HER2-positive BCP (n = 51) | HER2 non-BCP (n = 51) | P | |
|---|---|---|---|
| Tumor size (mean) (mm) | 40.6 | 36.4 | 0.49 |
| Tumor size (TNM classification) | |||
| T1 | 10 (20.4) | 17 (34) | 0.33 |
| T2 | 20 (40.8) | 21 (42) | |
| T3 | 11 (22.4) | 7 (14) | |
| T4 | 8 (16.3) | 5 (10) | |
| N+, n(%) | 34 (68) | 33 (66) | 0.83 |
| M+, n(%) | 8 (16) | 5 (10.2) | 0.39 |
| Tumor type, n(%) | |||
| Invasive ductal carcinoma | 50 (98) | 50 (98) | 1 |
| Invasive lobular carcinoma | 1 (2) | 1 (2) | |
| Focality, n(%) | |||
| Unifocal tumor | 39 (76.5) | 37 (72.5) | 0.82 |
| Multifocal tumor | 12 (23.5) | 14 (27.5) | |
| Grade SBR, n(%) | |||
| 1 | 1 (2) | 1 (2) | 0.57 |
| 2 | 14 (27.5) | 19 (37.2) | |
| 3 | 36 (70.6) | 31 (60.8) | |
| HR status, n(%) | |||
| Negative | 17 (33.3) | 12 (23.5) | 0.27 |
| Positive | 34 (66.7) | 39 (76.5) | |
| Ki67 rate, n(%) | |||
| <15% | 0 (0) | 2 (3.9) | 0.15 |
| ≥15% | 49 (96.1) | 47 (92.2) | |
| NC | 2 (3.9) | 2 (3.9) | |
| <20% | 3 (5.9) | 8 (15.7) | 0.11 |
| ≥20% | 46 (90.2) | 41 (80.4) | |
| NC | 2 (3.9) | 2 (3.9) | |
3.2. Characteristics of the pregnancies associated with HER2-positive BCP
Among the HER2-positive BCP patients (n = 51), the mean term at diagnosis was 19.3 weeks of gestation (WG)). Fourteen patients (27%) were diagnosed during the first trimester (<15 WG), 21 (41%) during the second trimester (≥15 WG - <28 WG) and 13 (25%) during the third trimester (≥28 WG). Data were missing for three patients (6%).
Among the patients diagnosed during the first trimester of pregnancy (n = 14), the pregnancy outcomes were: one (7%) abortion, three (21%) medical terminations of pregnancy, nine (64%) patients continued their pregnancies, and one (7%) patient with missing data.
The abortion was performed at 6WG for a 36-year-old patient after diagnosis of unifocal T1N0M0, grade SBR3, Ki>20%, HR-. The medical terminations were performed for patients with locally advanced breast carcinoma with axillary lymph node involvement: at 7 WG for a 29-year-old patient with unifocal T3N1M0, grade SBR3, Ki>20%, HR-who received neoadjuvant chemotherapy with anti-HER2 immediately after the termination with complete histological response (Sataloff TANA, pCR); at 13 WG for a 41-year-old patient with unifocal T4BN1M0, grade SBR3, Ki>20%, HR+; and at 12 WG for a 39-year-old patient with unifocal T3N1M0, grade SBR3, Ki>20%, HR-who received neoadjuvant chemotherapy with anti-HER2 with complete histological response (pCR)). No recurrence was observed in these four cases.
All the patients diagnosed in the second (n = 21) and third trimesters (n = 13) continued their pregnancies. In the whole HER2-positive BCP group, when pregnancy was continued (n = 43) the mean term at delivery was 34.2 WG. All the children were healthy at birth.
3.3. Treatments received by the study population
Chemotherapy modalities differed between the two groups: neoadjuvant chemotherapy was more frequent in the HER2-positive BCP group (72% vs 51%, p = 0.03) whereas adjuvant chemotherapy was more frequent in the HER2 non-BCP group (27% vs 49%, respectively, p = 0.04) (Table 3). Axillary lymphadenectomy was performed in 84% of patients in the HER2-positive BCP group vs 62% in the HER2 non-BCP group, p = 0.01. Radiotherapy and antihormonal treatment did not differ between the two groups. Chemotherapy regimens based on anthracyclines and taxanes were similar in HER2-positive BCP and HER2 non-BCP. However, for patients in the HER2-positive BCP group, anti-HER2 treatment was always administered in the postpartum period. Hence, 12 patients had delayed trastuzumab therapy linked to pregnancy varying between 2 and 22 weeks. No other anti-HER2 therapy was used in both groups.
Table 3.
Treatments received in patients with HER2-positive BCP and HER2 non-BCP group.
| HER2-positive BCP (n = 51) | HER2 non-BCP (n = 51) | p | |
|---|---|---|---|
| Chemotherapy | |||
| Neoadjuvant | 36 (72) | 26 (51) | 0.03 |
| Adjuvant | 14 (27) | 25 (49) | 0.04 |
| Mammary surgery | |||
| Partial mastectomy | 17 (34) | 23 (45) | 0.25 |
| Total mastectomy | 29 (58) | 32 (63) | 0.63 |
| None | 5 (9.8) | 0 | 1 |
| Axillary surgery, n(%) | |||
| Sentinel lymph node (SLN) | 13 (26) | 13 (26) | 1 |
| Lymphadenectomy | 31 (62) | 43 (84) | 0.01 |
| None | 7 (13.7) | 0 | 1 |
| Postpartum radiotherapy | 43 (86) | 47 (92) | 0.32 |
| Antihormonal treatment | 34 (66.7) | 39 (76.5) | 0.27 |
During pregnancy, 39 patients received chemotherapy (Table 5), 21 received anthracyclines only, and 12 received anthracyclines and taxanes.
Table 5.
Treatments received in HER2-positive BCP diagnosed during first trimester versus HER2 BCP diagnosed during second and third trimester.
| HER2-positive BCP First Trimester (n = 14) | HER2 BCP Second and third Trimester (n = 34) | p | |
|---|---|---|---|
| Chemotherapy, n(%) Neoadjuvant Adjuvant | 6 (43) 8 (57) | 28 (82) 6 (18) | 0.01 |
| Chemotherapy modalities received during pregnancy, n(%) | n = 10 patients | n = 29 patients | |
| 1 AC | 0 (0) | 2 (7) | 0.06 |
| 2 AC | 0 (0) | 6 (21) | |
| 3 AC | 1 (10) | 6 (21) | |
| 4 AC | 0 (0) | 6 (21) | |
| 4 AC + 1 taxol | 0 (0) | 1 (3) | |
| 4 AC + 2 taxol | 4 (40) | 4 (14) | |
| 4 AC + 3 taxol | 2 (20) | 1 (3) | |
| 4 AC + 4 taxol | 0 (0) | 0 (0) | |
| Missing data | 3 (30) | 3 (10) | |
| Total only AC | 1 (10) | 20 (70) | 0.004 |
| Total AC + taxol | 6 (60) | 6 (20) | |
| Surgery during pregnancy∗, n(%) | 7 (50) | 5 (15) | 0.02 |
| Mammary surgery, n(%) | |||
| Partial mastectomy | 5 (36) | 11 (32) | 1 |
| Total mastectomy | 8 (57) | 19 (56) | |
| None | 1 (7) | 4 (12) | |
| Axillary surgery, n(%) | |||
| Sentinel lymph node (SLN) | 5 (36) | 8 (24) | 0.33 |
| Lymphadenectomy | 8 (57) | 20 (59) | |
| None | 1 (7) | 6 (18) | |
| Post-operative radiotherapy, n(%) | 11 (79)∗∗ | 31 (91) | 0.33 |
| Antihormonal treatment, n(%) | 11 (79) | 20 (59) | 0.32 |
AC: anthracyclines.
∗first surgery, before chemotherapy.
∗∗ post-partum radiotherapy.
3.4. Survival of the study population
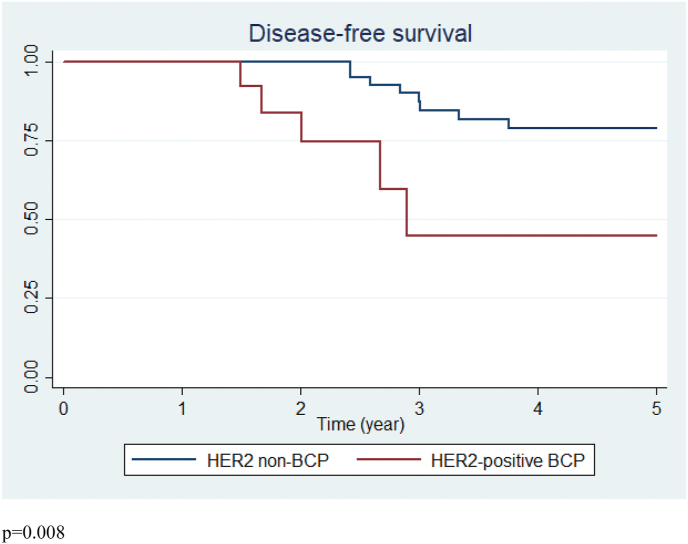
The mean follow-up was 3.7 years. At 3 years, five recurrences (10%) occurred in the HER2-positive BCP group and six (12%) in the HER2 non-BCP group (p = 1). In the HER2 non-BCP group, three recurrences occurred more than 3 years after diagnosis (3.3 years, 3.75 years and 5.3 years). The recurrence rate was 10% (n = 5) and 18% (n = 9) in the HER2-positive BCP and HER2 non-BCP groups, respectively (p = 0.25). Breast cancer-free survival was poorer in the HER2-positive BCP group with earlier recurrences (p = 0.008) (Fig. 1).
Fig. 1.
Breast cancer-free survival of HER2-positive BCP (n = 51) and HER2 non-BCP groups (n = 51).
There were two cases of local recurrence and three of distant recurrence in the HER2-positive BCP group compared to two and seven cases in the HER2 non-BCP group, respectively. No difference in the type of recurrence was found between the groups (p = 0.58). Cerebral metastasis occurred in one HER2-positive BCP patient and in three HER2 non-BCP patients (p = 1). The disease-related death rate was 4% (n = 2) in the HER2-positive BCP and 12% (n = 6) in the HER2 non-BCP group (p = 0.25). Breast cancer specific survival was similar in both groups (p = 0.20).
Sub-analysis of HER2-positive BCP diagnosed during the first trimester (n = 14) versus HER2-positive BCP diagnosed during second-third trimester of pregnancy (n = 34)
3.5. Epidemiological characteristics and treatments (Table 4, Table 5)
Table 4.
Comparison of histological and immunohistochemical characteristics between HER2-positive BCP diagnosed during first trimester versus HER2 BCP diagnosed during second and third trimester.
| HER2-positive BCP First Trimester (n = 14) | HER2 BCP Second and third Trimester (n = 34) | P | |
|---|---|---|---|
| Tumor size (mean) (mm) | 38.2 | 42.3 | 0.35 |
| Tumor size (TNM classification) | |||
| T1 | 4 (28) | 6 (18) | 0.79 |
| T2 | 4 (28) | 14 (41) | |
| T3 | 3 (21) | 7 (21) | |
| T4 | 2 (14) | 6 (18) | |
| T4B | 2 (14) | 0 (0) | |
| T4D | 0 (0) | 6 (18) | 0.16 |
| Missing data | 1 (7) | 1 (3) | |
| N+, n(%) | 8 (57) | 24 (71) | 0.50 |
| M+, n(%) | 0 (0) | 8 (24) | 0.08 |
| Tumor type, n(%) | 0.29 | ||
| Invasive ductal carcinoma | 13 (93) | 34 (100) | |
| Invasive lobular carcinoma | 1 (7) | 0 (0) | |
| Focality, n(%) | 0.14 | ||
| Unifocal tumor | 13 (93) | 24 (71) | |
| Multifocal tumor | 1 (7) | 10 (29) | |
| Grade SBR, n(%) | 0.33 | ||
| 1 | 1 (7) | 0 (0) | |
| 2 | 3 (21) | 11 (32) | |
| 3 | 10 (71) | 23 (68) | |
| HR status, n(%) | 0.32 | ||
| Negative | 3 (21) | 14 (41) | |
| Positive | 11 (78) | 20 (59) | |
| Ki67 rate, n(%) | |||
| <15% | 0 (0) | 0 (0) | 0.08 |
| ≥15% | 12 (86) | 34 (100) | |
| NC | 2 (14) | 0 (0) | |
| <20% | 1 (7) | 2 (6) | 0.10 |
| ≥20% | 11(79) | 32 (94) | |
| NC | 2 (14) | 0 (0) | |
No differences were observed in the tumor size (p = 0.79), lymph node involvement (p = 0.50), distant metastasis (p = 0.08), tumor type (p = 29), multifocality (p = 0.14), SBR grade (p = 0.33), HR status (p = 0.32), or Ki67 rate (p = 0.10) according to when the breast cancer was diagnosed (first versus second-third trimesters) (Table 4).
Patients with HER2-positive BCP diagnosed during the first trimester received less neoadjuvant chemotherapy (43% vs 82%, p = 0.01) and were more often operated on during pregnancy (50% vs 15%, p = 0.02) than those diagnosed during second-third trimesters (Table 5).
Chemotherapy modalities differed between the two groups: patients with HER2-positive BCP diagnosed during the first trimester received more combined chemotherapies with anthracyclines and taxanes whereas single chemotherapy with anthracyclines was more frequent in the HER2-positive BCP group diagnosed during second-third trimesters (p = 0.004) (Table 5).
Other treatment modalities – breast surgery (p = 1), axillary lymphadenectomy (p = 0.33), postpartum radiotherapy (p = 0.33) and antihormonal treatment (p = 0.32) – did not differ between the groups according to pregnancy trimesters (Table 5).
When the pregnancy was continued, the mean term at delivery was 38.4 WG for patients diagnosed during the first trimester and 37.7 WG for patients diagnosed during second-third trimesters (p = 0.16). All the children were healthy at birth.
3.6. Survival
The recurrence rate was 7% (n = 1) for HER2-positive BCP patients diagnosed during the first trimester and 6% (n = 2) for those diagnosed during second-third trimesters (p = 1). One distant recurrence occurred in an HER2-positive BCP patient diagnosed during the first trimester, 1.5 years after diagnosis: unifocal T2N1M0, grade SBR3, Ki>20%, HR + diagnosed at 13WG who underwent surgery during pregnancy at 21WG followed by adjuvant chemotherapy during pregnancy with delivery at 37 WG. In the group of HER2-positive BCP patients diagnosed during the second-third trimesters, one local recurrence occurred 2 years after diagnosis (unifocal TXN1M1, grade SBR 2, Ki>20%, HR – diagnosed at 16 WG with chemotherapy started at 18 WG and delivered at 33.3 WG because of maternal clinical deterioration secondary to lymphangitis), and one distant recurrence occurred 1.7 years after diagnosis (multifocal T1N1M0, grade SBR3, Ki>20%, HR-diagnosed at 36 WG followed by vaginal delivery at 37.5 WG and started chemotherapy 1 week later). Two deaths related to breast cancer occurred in the patients with HER2-positive BCP diagnosed during the second-third trimesters at 3.5 and 6.6 years after diagnosis. No deaths occurred in the HER-2 positive BCP patients diagnosed during first trimester (p = 1). There was no difference in disease-free and overall survival for HER2-positive BCP patients diagnosed during the first trimester who underwent medical termination compared to those who continued their pregnancy (p = 1).
4. Discussion
The present study demonstrates that the prognosis of patients with HER2-positive breast cancer associated with pregnancy have a lower recurrence-free survival rate but similar overall survival rates compared to non-pregnant patients. No difference in survival was noted between patients diagnosed with HER2-positive breast cancer during the first vs second-third trimesters of pregnancy. Finally, no difference in outcome was noted between patients who underwent medical termination vs those who continued their pregnancy.
In the current study, one-quarter of the population with BCP had HER2-positive breast cancer. This incidence of over-expression of HER is similar to that observed in non-pregnant patients [5,6]. Moreover, this rate is in agreement with that observed in a previous study involving more than 300 patients with BCP [14] and with another study which reported that over-expression of HER2 was found in 32% of tumors in young non-pregnant patients [15]. To the best of our knowledge, the present study was the first to evaluate the prognosis of women with HER2-positive BCP using a matched-case control study design. Previous studies have suggested that BCP patients exhibit more advanced stages and more lymph node metastases than young non-pregnant patients with breast cancer [[16], [17], [18]]. In contrast, the present study found no differences in tumor size, TNM classification, histologic grades, or in hormonal status and Ki67 expression between the BCP and non-BCP patients. Differences found in previous series may be because they were mainly based on case control [16,17,19,20]. Our data reinforce the need for suitable methodology to rule out confounding prognostic factors. For example, a recent study showed that recurrence and survival in patients with BCP were similar to non-pregnant patients after adjustment for known prognostic factors [21].
The management of patients with HER2-positive BCP raises several specific issues. Although there is a consensus supporting that the association of pregnancy with breast cancer should not be a reason for postponing antineoplastic therapy, none of our patients with BCP underwent an anti-HER2 therapy during pregnancy [22]. However, the issue of prognosis on the non-use of anti-HER2 therapy during pregnancy is questionable. In the current study, chemotherapy regimen differed between HER2-positive BCP and HER2 non-BCP groups. Although no difference in overall survival was noted, a decrease in DFS was noted in pregnancy women suggesting a potential negative impact to delay HER2 targeted therapy. Previous randomized trials have demonstrated the major impact of neoadjuvant anti-HER2 treatment on histological complete response rate correlated with a higher recurrence-free survival in locally advanced breast cancer [8,10,11,13,[23], [24], [25]]. This was the case for most of our patients. Hence, to postpone this therapy in pregnant women might have negative effects. In this specific setting, while our results underline a lower recurrence-free survival for HER-positive BCP patients, no difference in overall survival was found when compared to non-pregnant patients. In the HERA trial, a 24% reduction in the risk of relapse was observed in women administered trastuzumab versus those who were not [24]. Previous studies have demonstrated transplacental transfer of trastuzumab [26]. However, no pregnancy complications or anomalies were reported in the 16 pregnant patients of the HERA study [24]. Nonetheless, 11 abortions were recorded: four spontaneous and seven pregnancy terminations [24]. It is important to note that the reasons for the pregnancy terminations were not attributed to complications, but rather because both the patients and the attending physicians felt a degree of uncertainty and anxiety with regard to fetal trastuzumab exposure [27]. No congenital anomalies were observed in fetuses exposed to trastuzumab. In contrast to the HERA study, a recent overview of the literature on trastuzumab in BCP patients reported oligohydramnios in 13/20 pregnancies and fetal intrauterine growth retardation in two cases. Fifteen of the 20 children were delivered via cesarean section, on average in the 34 WG. Birth weight was below 2700 g for 11/17 children. Neonatal complications were observed in 10/20 children including eight children of women who developed oligo- or anhydramnios during pregnancy. All of these eight children suffered from respiratory complications; three had concomitant renal failure, four died within the first 4 months of life, and 10 children had normal development at a mean follow-up of 22 months (2–84 months) [28]. While many of these complications could be attributed to the preterm birth, it raises the issue of the safety of anti-HER2 agents administered during pregnancy. Trastuzumab placental crossing is poor early in pregnancy as active placental transport is needed which is not effective before 14 WG [29]. This mechanism potentially explains why no offspring anomalies were observed, despite early exposure to trastuzumab, in all the adjuvant trials [30]. A high risk of oligo- or anhydramnios has been reported with trastuzumab exposure starting second trimester when transplacental transport has become efficient. Being HER2 expressed on fetal kidney, this could potentially explain the increased risk of oligo- or anhydramnios [31].
Another crucial issue is whether the pregnancy should be terminated to allow targeted anti-HER2 therapy. In the present study, only 21% of BCP patients diagnosed during the first trimester underwent pregnancy termination. The use of chemotherapy during pregnancy is possible during the second-third trimesters of pregnancy with good neonatal outcomes when a term delivery is possible [22,31,32]. Conversely, chemotherapy is contraindicated during the first trimester of pregnancy due to major teratogenic risks. The American register of the National Toxicology Program reported that the prevalence of malformations after chemotherapy in the first trimester was 14% vs 3% when delivered later [33]. Moreover, seven patients (50%) diagnosed during the first trimester underwent first intention surgery compared to five (15%) diagnosed during the second-third trimesters. To date, no data are available about the prognosis of patients with HER2-positive BCP diagnosed during the first pregnancy trimester. The median term of diagnosis for patients during the first trimester was 10.5 WG. For patients whose pregnancies were terminated, the median term at diagnosis was 9.5 WG (range 5–11). For those who continued their pregnancy, the median term at diagnosis was 12 WG (range 8–14). As chemotherapy is systematically started after 14 WG, it can be deduced that the treatment was postponed by a median of 2 weeks (max 6 weeks). In our series, patients with HER2-positive BCP diagnosed during the first trimester did not undergo the same management as those diagnosed during the second-third trimesters: primary surgery and chemotherapy with anthracyclines and taxane-based therapy without anti-HER2 treatment were more frequently used. In the present study, similar recurrence-free and overall survivals were observed for patients who continued their pregnancy when diagnosed during the first trimester as those diagnosed during the second-third trimesters, with similar tumor characteristics (Table 4). This suggests that while pregnancy per se is a prognostic factor for HER2-positive BCP, it is not related to the term of pregnancy. This finding is in full agreement with the results of Larouzee et al. which showed that HER2 status in women with BCP was an independent risk factor of recurrence [34].
Pertuzumab, another monoclonal antibody, is used in combination with trastuzumab and docetaxel as first-line therapy for metastatic breast cancer [35]. It prevents dimerization of HER2 with other HER family receptors by binding to a different site to trastuzumab [36]. The NeoSphere trial also highlighted the superiority of the combination of pertuzumab and trastuzumab on recurrence-free survival compared to three other chemotherapy regimens [25]. However, there are still no data available about the use of pertuzumab in pregnancy.
Some limits of the present study deserve to be underlined. First, although based on a prospective database, the retrospective nature of the study cannot rule out all biases. Second, only breast cancers with more than 10% of ER positive cells were considered positive, in line with French recommendations [37]. Recent ASCO/CAP Guideline Update underlined that breast cancers with 1–100% of tumor nuclei positive should be interpreted as ER positive [38]. However, in this report, it has been underlined that the clinical relevance of this threshold remains a matter of debate with unknown endocrine therapy benefit [38]. Third, many data about the neonates were missing meaning that we cannot draw conclusions about the impact of HER2-positive BCP on newborn outcomes. Fourth, given our small population, we could not stratify survival on the number of cycles of anthracyclines, taxanes and on the impact to delay trastuzumab therapy. Finally, we were unable to find any difference in prognosis between patients diagnosed in the first trimester of pregnancy and those diagnosed later in pregnancy which could also be explained by the low number of cases and events.
In conclusion, this matched case-control study implies that HER2-positive breast cancer associated with pregnancy remains a disease with poor prognosis. However, we are unable to draw a conclusion as to whether a pregnancy should be terminated to begin targeted therapy. Further research about the impact of target therapies during pregnancy are needed to improve outcomes of these patients.
Funding details
None.
Declarations of competing interest
None.
References
- 1.Matthews T.J., Hamilton B.E. First births to older women continue to rise. NCHS Data Brief. 2014 May;(152):1–8. [PubMed] [Google Scholar]
- 2.Andersson T.M.-L., Johansson A.L.V., Hsieh C.-C., Cnattingius S., Lambe M. Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet Gynecol. 2009 Sep;114(3):568–572. doi: 10.1097/AOG.0b013e3181b19154. [DOI] [PubMed] [Google Scholar]
- 3.Loibl S., Schmidt A., Gentilini O., Kaufman B., Kuhl C., Denkert C. Breast cancer diagnosed during pregnancy: adapting recent advances in breast cancer care for pregnant patients. JAMA Oncol. 2015 Nov;1(8):1145–1153. doi: 10.1001/jamaoncol.2015.2413. [DOI] [PubMed] [Google Scholar]
- 4.Perou C.M., Sørlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A. Molecular portraits of human breast tumours. Nature. 2000 Aug 17;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.Nami B., Maadi H., Wang Z. Mechanisms underlying the action and synergism of trastuzumab and pertuzumab in targeting HER2-positive breast cancer. Cancers. 2018 Sep 20;10(10) doi: 10.3390/cancers10100342. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6210751/ [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rimawi M.F., Schiff R., Osborne C.K. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111–128. doi: 10.1146/annurev-med-042513-015127. [DOI] [PubMed] [Google Scholar]
- 7.Ross J.S., Slodkowska E.A., Symmans W.F., Pusztai L., Ravdin P.M., Hortobagyi G.N. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncol. 2009 Apr;14(4):320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 8.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 9.Monoclonal antibody approved for metastatic breast cancer. Oncol Williston Park N. 1998 Dec;12(12):1727. [PubMed] [Google Scholar]
- 10.Balduzzi S., Mantarro S., Guarneri V., Tagliabue L., Pistotti V., Moja L. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014 Jun 12;(6) doi: 10.1002/14651858.CD006242.pub2. CD006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moja L., Tagliabue L., Balduzzi S., Parmelli E., Pistotti V., Guarneri V. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012 Apr 18;(4) doi: 10.1002/14651858.CD006243.pub2. CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zagouri F., Sergentanis T.N., Chrysikos D., Papadimitriou C.A., Dimopoulos M.-A., Bartsch R. Trastuzumab administration during pregnancy: a systematic review and meta-analysis. Breast Canc Res Treat. 2013 Jan;137(2):349–357. doi: 10.1007/s10549-012-2368-y. [DOI] [PubMed] [Google Scholar]
- 13.Sataloff D.M., Mason B.A., Prestipino A.J., Seinige U.L., Lieber C.P., Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg. 1995 Mar;180(3):297–306. [PubMed] [Google Scholar]
- 14.Amant F., von Minckwitz G., Han S.N., Bontenbal M., Ring A.E., Giermek J. Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol Off J Am Soc Clin Oncol. 2013 Jul 10;31(20):2532–2539. doi: 10.1200/JCO.2012.45.6335. [DOI] [PubMed] [Google Scholar]
- 15.Colleoni M., Rotmensz N., Robertson C., Orlando L., Viale G., Renne G. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol Off J Eur Soc Med Oncol. 2002 Feb;13(2):273–279. doi: 10.1093/annonc/mdf039. [DOI] [PubMed] [Google Scholar]
- 16.Ishida T., Yokoe T., Kasumi F., Sakamoto G., Makita M., Tominaga T. Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: analysis of case-control study in Japan. Jpn J Cancer Res Gann. 1992 Nov;83(11):1143–1149. doi: 10.1111/j.1349-7006.1992.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guinee V.F., Olsson H., Möller T., Hess K.R., Taylor S.H., Fahey T. Effect of pregnancy on prognosis for young women with breast cancer. Lancet Lond Engl. 1994 Jun 25;343(8913):1587–1589. doi: 10.1016/s0140-6736(94)93054-6. [DOI] [PubMed] [Google Scholar]
- 18.Peccatori F.A., Lambertini M., Scarfone G., Del Pup L., Codacci-Pisanelli G. Biology, staging, and treatment of breast cancer during pregnancy: reassessing the evidences. Cancer Biol Med. 2018 Feb;15(1):6–13. doi: 10.20892/j.issn.2095-3941.2017.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartman E.K., Eslick G.D. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: a meta-analysis. Breast Canc Res Treat. 2016;160(2):347–360. doi: 10.1007/s10549-016-3989-3. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal J., Amir E., Rochon P.A., Giannakeas V., Sun P., Narod S.A. Association of the timing of pregnancy with survival in women with breast cancer. JAMA Oncol. 2017 May 1;3(5):659–665. doi: 10.1001/jamaoncol.2017.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boudy A.-S., Naoura I., Selleret L., Zilberman S., Gligorov J., Richard S. Propensity score to evaluate prognosis in pregnancy-associated breast cancer: analysis from a French cancer network. Breast Edinb Scotl. 2018 Aug;40:10–15. doi: 10.1016/j.breast.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Amant F., Vandenbroucke T., Verheecke M., Fumagalli M., Halaska M.J., Boere I. Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med. 2015 Nov 5;373(19):1824–1834. doi: 10.1056/NEJMoa1508913. [DOI] [PubMed] [Google Scholar]
- 23.Untch M., Fasching P.A., Konecny G.E., Hasmüller S., Lebeau A., Kreienberg R. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol Off J Am Soc Clin Oncol. 2011 Sep 1;29(25):3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 24.Cameron D., Piccart-Gebhart M.J., Gelber R.D., Procter M., Goldhirsch A., de Azambuja E. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet Lond Engl. 2017 25;389(10075):1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianni L., Pienkowski T., Im Y.-H., Tseng L.-M., Liu M.-C., Lluch A. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016 Jun;17(6):791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 26.Loibl S. New therapeutic options for breast cancer during pregnancy. Breast Care Basel Switz. 2008;3(3):171–176. doi: 10.1159/000136002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azim H.A., Metzger-Filho O., de Azambuja E., Loibl S., Focant F., Gresko E. Pregnancy occurring during or following adjuvant trastuzumab in patients enrolled in the HERA trial (BIG 01-01) Breast Canc Res Treat. 2012 May;133(1):387–391. doi: 10.1007/s10549-012-1996-6. [DOI] [PubMed] [Google Scholar]
- 28.Goller S.S., Markert U.R., Fröhlich K. Trastuzumab in the treatment of pregnant breast cancer patients - an overview of the literature. Geburtshilfe Frauenheilkd. 2019 Jun;79(6):618–625. doi: 10.1055/a-0880-9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pentsuk N., van der Laan J.W. An interspecies comparison of placental antibody transfer: new insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Res B Dev Reprod Toxicol. 2009 Aug;86(4):328–344. doi: 10.1002/bdrb.20201. [DOI] [PubMed] [Google Scholar]
- 30.Lambertini M., Martel S., Campbell C., Guillaume S., Hilbers F.S., Schuehly U. Pregnancies during and after trastuzumab and/or lapatinib in patients with human epidermal growth factor receptor 2-positive early breast cancer: analysis from the NeoALTTO (BIG 1-06) and ALTTO (BIG 2-06) trials. Cancer. 2019 15;125(2):307–316. doi: 10.1002/cncr.31784. [DOI] [PubMed] [Google Scholar]
- 31.Lambertini M., Peccatori F.A., Azim H.A. Targeted agents for cancer treatment during pregnancy. Canc Treat Rev. 2015 Apr;41(4):301–309. doi: 10.1016/j.ctrv.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 32.de Haan J., Verheecke M., Van Calsteren K., Van Calster B., Shmakov R.G., Mhallem Gziri M. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19(3):337–346. doi: 10.1016/S1470-2045(18)30059-7. [DOI] [PubMed] [Google Scholar]
- 33.National Toxicology Program NTP monograph: developmental effects and pregnancy outcomes associated with cancer chemotherapy use during pregnancy. NTP Monogr. 2013 May;(2):i–214. [PubMed] [Google Scholar]
- 34.Larouzee E., Allegre L., Boudy A.-S., Ilenko A., Selleret L., Zilberman S. Predicting the likelihood of recurrence of pregnancy-associated breast cancer: nomogram based on analysis of the French cancer network: cancer Associé à La Grossesse. J Gynecol Obstet Hum Reprod. 2020 Apr 20:101766. doi: 10.1016/j.jogoh.2020.101766. [DOI] [PubMed] [Google Scholar]
- 35.Niikura N., Liu J., Hayashi N., Mittendorf E.A., Gong Y., Palla S.L. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol Off J Am Soc Clin Oncol. 2012 Feb 20;30(6):593–599. doi: 10.1200/JCO.2010.33.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franklin M.C., Carey K.D., Vajdos F.F., Leahy D.J., de Vos A.M., Sliwkowski M.X. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Canc Cell. 2004 Apr;5(4):317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 37.Goldhirsch A., Wood W.C., Coates A.S., Gelber R.D., Thürlimann B., Senn H.-J. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the st. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol Off J Eur Soc Med Oncol. 2011 Aug;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allison K.H., Hammond M.E.H., Dowsett M., McKernin S.E., Carey L.A., Fitzgibbons P.L. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol Off J Am Soc Clin Oncol. 2020 Apr 20;38(12):1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]