Abstract
Objective:
Acantholimon is a genus of perennial plant within the Plumbaginaceae family. Here, we aimed to investigate anticancer, antioxidant, and antibacterial potential of methanol extract of three Iranian endemic species of Acantholimon including A. austro-iranicum, A. serotinum and A. chlorostegium.
Materials and Methods:
MTT assay was used to evaluate the in vitro cytotoxicity and apoptosis induction was examined by annexin V-PE apoptosis detection kit. Antioxidant activity was reported based on the DPPH-scavenging and DCF-DA assay. Antibacterial activity was measured by disc diffusion and micro-well dilution assay.
Results:
MTT assay showed less cytotoxicity of methanol extracts against the HUVEC normal cell line (IC50 values: 817-900 µg/ml) compared to cancer cell lines MCF-7, HT29, SH-SY5Y, NCCIT and A549 (IC50 values: 213 to 600 µg/ml) that show the specificity of extracts toward cancer cells. Plant extract showed apoptosis induction and cell cycle arrest at the G0/G1 phases documented by annexin V staining and flow cytometry. According to antioxidant tests, extracts exhibited significant DPPH scavenging potential (IC50 values: 30-37 µg/ml) and could protect against H2O2-induced oxidative stress. Antibacterial activities showed a stronger inhibitory effect on Escherichia coli and Pseudomonas aeruginosa as Gram- negative bacteria (diameter of inhibition zone: 11-13 mm and minimal inhibition concentration (MIC): 3.175 to 12.5 mg/ml) compared to Gram-positive bacteria including Enterococcus faecalis and Staphylococcus aureus (diameter of inhibition zone: 3-7 mm and MIC: 25 to 50 mg/ml).
Conclusion:
Our results suggested moderate cytotoxic and antibacterial potential and noteworthy antioxidant activity for the examined Acantholimon species.
Key Words: Acantholimon austro-iranicum, Acantholimon serotinum, Acantholimon chlorostegium, Anticancer, Antioxidant, Antibacterial
Introduction
Recently, medicinal plants have been recognized as a source of biologically active compounds with therapeutic potential and many anti-cancers drugs, antibiotic prototypes and antioxidants have natural sources (Atanasov et al., 2015 ▶; Newman and Cragg, 2016 ▶; Pinto and Silva, 2017 ▶; Thomford et al., 2018 ▶). Plant-derived anticancer drugs can reduce the side effect of chemotherapy drugs on normal cells and improve efficiency of chemical therapeutic agents (Greenwell and Rahman, 2015 ▶; Sameer et al., 2016 ▶; Pinto and Silva, 2017 ▶). Many plant-derived secondary metabolites also have antimicrobial properties (Cowan, 1999 ▶; Dahanukar et al., 2000 ▶; Sher, 2009 ▶). Moreover, many types of natural antioxidants are available in medicinal plants that by scavenging or stabilizing free radicals, can reduce oxidative stress and its adverse effects on human health (Sreejayan and Rao, 1996 ▶; Gerber et al., 2002 ▶; Matteo and Esposito, 2003 ▶). Consequently, medicinal plants can be considered a rich source for herbal drugs for prevention and treatment of many diseases.
Plumbaginaceae is a family of flowering plants that is composed of 30 genera and about 725 species (Christenhusz and Byng, 2016 ▶). In this family, the genus Acantholimon Boiss consists of more than 200 species which are distributed in many different areas of the Irano-Turanian region (Kubitzki, 1993 ▶; Hernández-Ledesma et al., 2015 ▶; Lashgari et al., 2016 ▶; Nasiri et al., 2016 ▶; Gazor et al., 2017 ▶).
Although, pharmacological activities of certain types of the Plumbaginaceae were investigated (Kuo et al., 2002 ▶; Chaung et al., 2003 ▶; Eren, 2016 ▶), considerable research was not done on biological activities and medical use of Acantholimon as a large genus. The present work is the first study dealing with cytotoxicity, antioxidant and antimicrobial potential of methanol extract of A. austro-iranicum, A. serotinum and A. chlorostegium as endemic plants distributed in the south-east of Iran.
Materials and Methods
Plant material
Aerial parts of the following species were gathered, deposited in MIR herbarium and identified according to the standard keys.
A. austro-iranicum. Iran. South-East, Kerman Province, Baft to Khabr, Mirtadzadini 1969 (MIR)
A. serotinum, Iran. South-East, Kerman Province, Baft to Khabr, Mirtadzadini 1968 (MIR)
A. chlorostegium, Iran. South-East, Kerman Province, Baft to Khabr, Mirtadzadini 2043 (MIR)
Preparation of the methanol extracts
The aerial parts (150 g) of Acantholimon were dried in shade and extracted using 80 % methanol (500 ml) by continuous shaking at 200 rpm for 72 hr.
After vacuum filtration, extracts were concentrated to obtain semisolid extracts at a maximum temperature of 40ºC using a rotary evaporator (EYELA SB-1100, JAPAN). The methanol extracts were concentrated to dryness in the oven at 40ºC. The dried extracts were dissolved in DMSO to make a stock of 250 mg/ml and further diluted to a final concentration of 3 mg/ml using complete culture medium.
Cell culture
MCF-7 (human breast cancer), HT29 (human colon cancer), SH-SY5Y (human neuroblastoma), NCCIT (human embryonic carcinoma), A549 (human non-small-cell lung cancer) and HUVEC (human umbilical vein endothelial) cell lines were purchased from Pasteur Institute cell bank (Tehran, Iran). MCF-7, HT29, SH-SY5Y and HUVEC were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco), NCCIT and A549 were cultured in RPMI 1640 (Gibco). All media were supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin. These cells were kept in a humidified atmosphere containing 5% CO2 at 37ºC.
Cell viability assay
Different cancer cell lines including MCF-7, HT29, SH-SY5Y, NCCIT and A549 were treated with different concentration of the methanol extract of A. austro-iranicum, A. serotinum and A. chlorostegium for 48 h and cytotoxic activity was evaluated using MTT assay. HUVEC human umbilical vein endothelial cell line was selected to examine the toxic effect of the extracts on normal cells. Briefly, 5×103 cells were seeded in 96-well tissue culture plates for overnight incubation and treated with increasing concentrations of the extracts (10 to 1250 μg/ml) for 48 hr. The cells were then incubated with medium containing MTT (3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide) at a final concentration of 0.5 mg/ml for 3 hr. After this period, media was removed and 100 µl DMSO was added to each well to dissolve formazan crystals and finally, enzyme-linked immunosorbent assay (ELISA) reader (BioTek-ELx800, USA) at 490 nm was used to measure optical densities (ODs). Mean absorbance for each concentration was divided to the mean absorbance of its controls (control samples were incubated with an equivalent amount of DMSO as the solvent of plant extracts) to calculate percentage of cell viability. The IC50 values of each extract were calculated using Prism 6.0 (Graph Pad Software, Inc., San Diego, California, USA).
Colony formation assay
The inhibitory effect of plant extract on cellular proliferation was also determined using a colony-forming assay. NCCIT cells were plated in 6well dishes at 1x103 cells per well and treated with various concentration (25, 50, 100, 200 µg/ml) of A. serotinum methanol extract for 14 days. The colonies were fixed with methanol and stained with crystal violet. Stained colonies containing>50 cells were counted and plating efficiency was calculated according to the following formula: colony number/total cell number) × 100%.
Apoptosis assay by flow cytometry
Apoptosis assay was performed on NCCIT cells treated with A. serotinum methanol extract. Briefly, cells at a density of 3×105 were grown in 60-mm petri dishes and allowed to attach for 24 hr and then they were treated with 700 μg/ml of the plant extract. After 24 hr incubation, cells were washed with phosphate buffered saline (PBS) and centrifuged at 300 g for 5 min. Afterward, the cells were suspended in 100 μl annexin V binding buffer and 5 µl of PE Annexin V and 5 µl of 7-AAD and incubated in the dark at room temperature for 15 min. Annexin/7-AAD was evaluated by Becton Dickinson FACScan instrument using fl2 and fl3 filters for detection of annexin-PE and 7-AAD.
Cell cycle analysis
NCCIT cells (5x106) were seeded into 60 mm dishes and subjected to 300 µg/ml of A. serotinum extract for 72 hr. Adherent cells were detached by trypsinization and washed twice with PBS. Cells were fixed using 70% ice-cold ethanol for 30 min and incubated with 20 µg/ml propidium iodide and 10 μg/ml RNase A for 1 hr at 37°C in the dark. The stained cells were subsequently analyzed using BD FACSCalibur flow cytometer at a wavelength of 488 nm, equipped with Cell Quest 3.3 software.
DPPH free radical scavenging assay
To determine the antioxidant activity of methanol extract of three Acantholimon species, 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay was used. The antioxidant agents can convert DPPH with purple color into the yellow molecule, 1-1diphenyl-2-picryl hydrazine by donating electron or hydrogen (Thambiraj et al., 2012 ▶). In detail, the extract (50 μl) at concentrations ranging from 1.5 to 2000 µg/ml was added to 150 μl of 0.04 mg/ml DPPH (Sigma–Aldrich; St-Louis, USA) solution in methanol and incubated in the dark for 30 min. DPPH in methanol without the extracts served as a control. The blank was prepared in the same manner except that methanol was used instead DPPH solution to eliminate interference in each sample. The reduction of DPPH absorbance was measured at 515 nm using a plate reader (BioTek-ELx800, USA). Butylated hydroxytoluene (BHT) was used as a positive control. All determinations were performed in triplicate.
The percentage inhibition (I%) was calculated in the following way: I%= [Absorbance of control-(Absorbance of sample-Absorbance of blank)]/Absorbance of control×100. The concentration of the plant extract that scavenge 50% of the total DPPH radical (IC50 value) was calculated from the graph plotting inhibition percentage against the extract concentrations.
Intracellular reactive oxygen species scavenging activity
Intracellular reactive oxygen species (ROS) levels of living cells were determined using 2′, 7′-dichlorodihydrofluorescein diacetate (DCF-DA; Sigma-Aldrich, USA). DCFH-DA can be deacetylated by cellular esterases to the non-fluorescent DCFH. DCFH is oxidized to fluorescent DCF (2′, 7′-dichlorofluorescein) in the presence of ROS, which can be readily detected by a spectrofluorometer. Briefly, 25×103 NCCIT cells were cultured in 96-well microplates for 24 hr. Then, the medium was removed and cells were exposed to PBS containing 20 μM of DCFH-DA (Sigma-Aldrich, USA) and kept in a humidified atmosphere (with 5% CO2 at 37°C) for 45 min. Next, cells were treated with H2O2 (200 μM, Sigma-Aldrich, Germany) in the absence/presence of each plant extract (50, 100, 200, and 400 µg/ml). After 3 hr, the fluorescence intensity was quantified using a fluorescence plate reader at an excitation of 485 nm and an emission of 538 nm (FLX 800; BioTek).
Antimicrobial activity
Bacterial strains
The antibacterial potency of A. austro-iranicum, A. serotinum and A. chlorostegium methanol extracts was individually examined against 4 bacteria strains that were obtained from Iranian Biological Resource Center. Enterococcus faecalis (ATCC 29212) and Staphylococcus aureus (ATCC 25838) as Gram-positive strains and Pseudomonas aeruginosa (ATCC 27853) and Escherichia coli (ATCC 11333) as Gram-negative strains, were tested in this study.
Disc diffusion method
Antibacterial potential of methanol extracts was determined by agar disc diffusion method (Lehmann, 1999 ▶). The dried plant extracts were dissolved in DMSO to a final concentration of 200 mg/ml. Bacterial strains were sub cultured from original culture stored at -80ºC and grown on Mueller-Hinton Broth at 37°C for 24 hr. To obtain a lawn culture, 100 µl of the bacterial suspension containing 108 CFU/ml of bacteria was spread on Mueller Hinton agar. The 6 mm disc impregnated with extract solution (200 mg/ml), DMSO (as negative control) and ciprofloxacin (25 mg/ml, as positive control) were placed on the lawn cultures. After 24 hr incubation at 37ºC, antibacterial activity was measured as the diameter of the inhibition zone produced by the extract around the disc.
Determination of minimal inhibition concentration (MIC)
MIC values were calculated for bacterial strains using micro-well dilution assay method (Baron, 1999 ▶). Three replicates of the serial two-fold dilutions of each extract (0.003 to 200 mg/ml) were prepared in Mueller Hinton Broth medium in 96-well plates. Next, 10 µl of bacterial suspension (108 CFU/ml) was added into each well. The plates were placed in an incubator at 37°C for 24 hr. The wells containing 200 µl of the culture media and 10 µl of bacterial suspension without the test materials, were used as the negative control. The microorganism growth was shown by turbidity and the lowest concentration of the plant extracts required for inhibiting the growth of microorganisms (MIC value) was obtained. All tests were repeated twice.
Minimum bactericidal concentration (MBC)
MBC value is defined as the lowest concentration of extract, at which no growth was observed. MBC was measured after MIC determination. It is determined by sub culturing broth dilutions that inhibit growth of a bacterial organism (i.e., those at or above the MIC). The broth dilutions are streaked onto agar and incubated for 24 hr at 37oC.
Statistical analysis
Data are expressed as mean±SD of three experiments. Statistical analyses were performed using SPSS version 16. Statistical significance was determined using Student’s t-test for comparisons between treated versus control cells and a p<0.05 was considered statistically significant.
Results
Cytotoxicity evaluation of methanol extracts on human cancer and normal cells
The IC50 values of all three extracts were determined against both tumor and non-tumor cells (Figure 1).
Figure 1.

Anti-proliferative effects of methanol extract of A. austro-iranicum (a), A. serotinum (b) and A. chlorostegium (c) on various tumor cells (MCF-7, HT29, NCCIT, SH-SY5Y and A549) and one normal (HUVEC) cell line. Cells were treated with various concentrations of the extracts (10, 20, 40, 80, 160, 320, 640, and 1280 µg/ml) for 48 hr and subjected to MTT assay. IC50 values are expressed as mean±SD of three independent experiments
NCCIT was the most susceptible cell to treatment with all three extracts with an IC50 value of 213 µg/ml for A. serotinum, 257 µg/ml for A. austro-iranicum and 277 µg/ml for A. chlorostegium. The lowest cytotoxic effect was found against normal cell line compared to cancer cell lines with IC50 values of 858 µg/ml for A. austro-iranicum, 817 µg/ml for A. chlorostegium and 900 µg/ml for A. serotinum. The MTT assay showed that methanol extracts are more toxic to human cancer cell lines (MCF-7, A549, HT29, SH-SY5Y and NCCIT) compared to the normal cell line HUVEC. Since A. serotinum extract showed higher cytotoxic activity against NCCIT cells, it was used for further investigation of in vitro anticancer activity, apoptosis induction and cell cycle arrest.
Methanol extract of A. serotinum suppressed colony formation of NCCIT cells
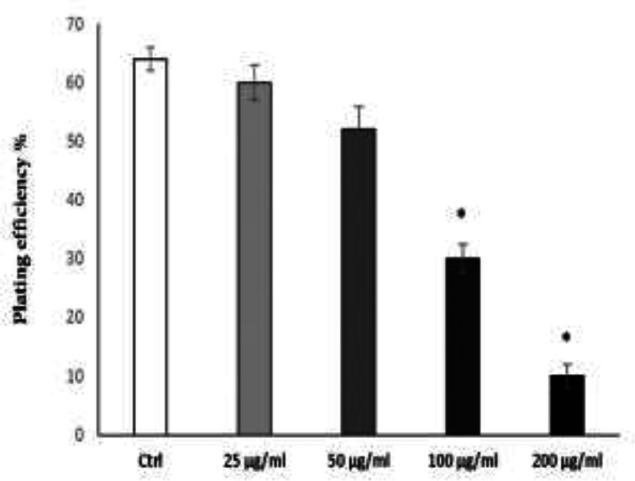
Capacity of a single cell to proliferate into a colony can be tested by clonogenic assays. We tested clonogenicity of NCCIT cells as embryonal carcinoma cells, after treatment with methanol extract of A. serotinum. Methanol extract dose-dependently inhibited colony formation ability of NCCIT cells. The minimum concentration of the extract (25 µg/ml) caused a reduction of approximately 4% in terms of colony formation and higher concentrations (100 and 200 µg/ml) significantly abrogated colony formation of NCCIT cells (Figure 2). Therefore, the inhibitory effect of A. serotinum extract on colony formation of NCCIT, confirmed its cytotoxic effect evaluated by MTT test.
Figure 2.
Methanol extract of A. serotinum decreased the colony formation of NCCIT cells. NCCIT cells were seeded into 6-well plates at a density of 1000 cells/well. After 24 hr, the cells were treated with the indicated concentrations of extract for 14 days. Cells were fixed and stained with crystal violet to visualize the colonies for counting. Colony numbers (≥50 cells per colony) were counted. The experiments were independently repeated 3 times and plating efficiency ±SEM was calculated. *p<0.05 shows significant differences as compared to the control (ctrl) as tested by the student’s t-test
Methanol extract of A. serotinum promoted cell death by apoptosis
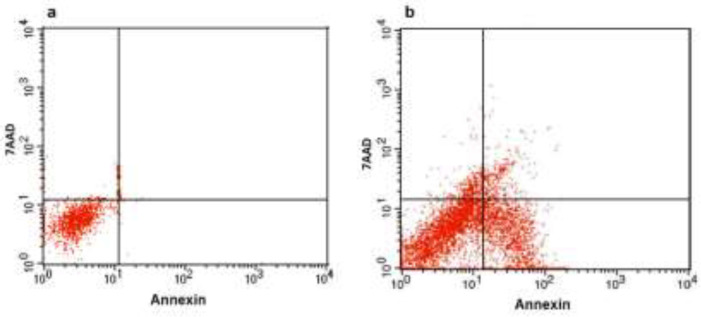
Apoptosis induction in NCCIT cells after 48 hr treatment with methanol extract of A. serotinum was monitored by flow cytometry using annexin V-PE/7-AAD kit. Marker for early apoptosis is translocation of phosphatidylserine to the exterior surfaces of the plasma membrane which can be detected by annexin V-PE binding. Late apoptotic or necrotic cells are permeable for 7-AAD, and DNA stains with this reagent. After treatment with 700 μg/mL A. serotinum extract for 48 hr, the early apoptotic rate was 36% and late apoptotic rate was 5% (Figure 3).
Figure 3.
Methanol extract of A. serotinum stimulated apoptosis in NCCIT cells. Apoptosis cells were evaluated using flow cytometry following annexin V-PE and 7-AAD staining in non-treated control cells (a) and plant extract-treated cells (b). Cells in the lower left quadrant are viable, those in the lower right quadrant are early apoptotic and those in the upper right and left quadrant are late apoptotic and necrotic
Methanol extract of A. serotinum induced G1 cell cycle arrest in NCCIT cells
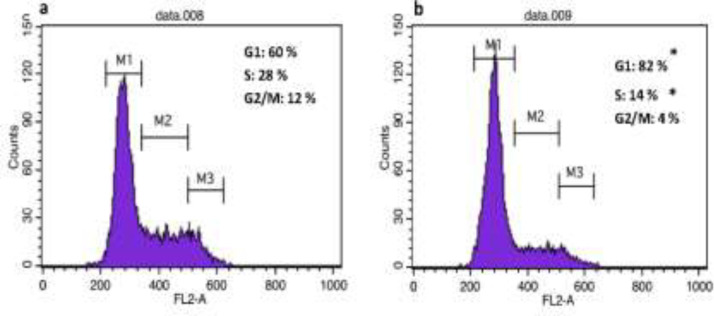
To investigate whether methanol extract could induce cell cycle perturbations in cancer cells, propidium iodide-stained nuclei were analyzed using flow cytometry. As shown in Figure 4, 48 hr treatment with extract of A. serotinum (300 µg/ml) increased the proportion of NCCIT cells in G0/G1 phase (20%) and reduced the proportion of S-phase cells (14%) compared to the control, indicating that the extract induces G0/G1 cell cycle arrest in NCCIT cells.
Figure 4.
The effect of A. serotinum methanol extract on cell cycle progression in NCCIT cells. Untreated cells (a) and cell incubated for 48 hr in the presence of A. serotinum extract (b) were harvested by trypsinization, fixed, and stained with propidium iodide to determine the profile of the cell cycle by flow cytometry. Results are expressed as mean±SD of 3 independent experiments. *p<0.05 shows significant differences as compared to the control as tested by the Student’s t-test. Each DNA histogram represents one of the three independent experiments
Antioxidant activity
The scavenging activity of the antioxidants can decrease oxidative stress and control some diseases including cancer, AIDS and neurodegenerative conditions. To assess antioxidant potential of the examined plant extracts, both DPPH scavenging assay and measurement of intracellular ROS as commonly used assays for antioxidant studies of specific compounds or extracts, were used (Amarowicz et al., 2004 ▶).
DPPH Free Radical Scavenging Activity
DPPH assay is the most commonly method to determine the antioxidant activity of different components (Kumar et al., 2008 ▶). As shown in Table 1, DPPH test confirmed that methanol extracts of A. austro-iranicum, A. serotinum and A. chlorostegium exerted approximately the same free radical-scavenging activity with IC50 values of 30-37 µg/ml.
Table 1.
The inhibitory concentration 50% (IC50) of methanol extract of A. austroiranicum, A. serotinum and A. chlorostegium in DPPH test (Mean±SD).
| A. austroiranicum (µg/ml) | A. serotinum (µg/ml) | A. chlorostegium (µg/ml) | BHT (µg/ml) | |
|---|---|---|---|---|
| IC 50 | 37±3.5 | 30±1.5 | 34±1.08 | 7.45±1.08 |
Extracts that possess IC50 values ranging between 10 and 50 mg/ml are considered to have strong antioxidant activities (Phongpaichit et al., 2007 ▶; Jadid et al., 2017 ▶). Therefore, methanol extracts of three species of Acantholimon that were tested in this study, possess strong antioxidant activities.
Intracellular reactive oxygen species scavenging activity
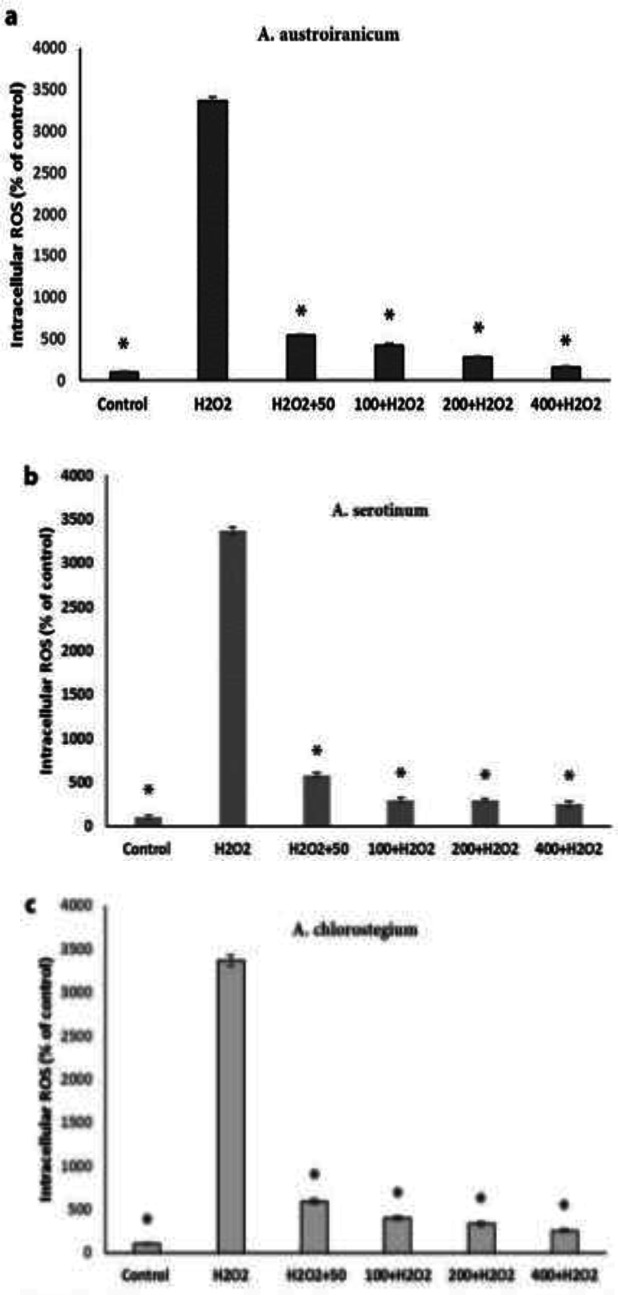
To investigate antioxidant potential of methanol extracts of three species of Acantholimon, the intracellular ROS levels were measured in control, H2O2-treated and H2O2-treated cells incubated with different concentrations of the plant extracts. As shown in Figure 5, exposure of NCCIT cells to H2O2 (200 µM) led to an increase in the fluorescent intensity (33-fold) as compared to the control cells which showed production of ROS by H2O2.
Figure 5.
Effect of the methanol extracts from A. austro-iranicum, A. serotinum and A. chlorostegium on ROS production in NCCIT cells exposed to H2O2. Cells were treated with H2O2 (200 μM) in the presence or absence of each plant extract at different concentrations (50, 100, 200 and 400 µg/ml). Data are means±SEM of three independent experiments. *p<0.05 as compared to H2O2-treated cells
However, co-treating with H2O2 (200 µM) and different concentrations (50, 100, 200 and 400 µM) of each plant extract, reduced fluorescent intensity around to 13 folds as compared to H2O2-treated cells that indicated that methanol extracts act as ROS scavenger and have significant antioxidant activities.
Antibacterial activity
The antibacterial activity of A. austro-iranicum, A. serotinum and A. chlorostegium methanol extract was examined both qualitatively and quantitatively against two Gram-positive and two Gram-negative bacteria. The results presented in Table 2 were compared with ciprofloxacin. Inhibition zones and MIC values for bacterial strains were in the range of 3-13 mm and 0.39-50 mg/ml, respectively. The extracts showed higher inhibitory effects against E. coli and P. aeruginosa as Gram-negative bacteria with inhibition zones of 11-13 mm and MIC range of 0.39-12.5 mg/ml and weaker antibacterial activity against Gram-positive bacteria E. faecalis and S. aureus with inhibition zones of 3-7 mm and MIC range of 25-50 mg/ml. Variation in the cell wall structure between Gram-negative and Gram-positive may have led to different inhibitory effects.
Table 2.
Antibacterial activity of the methanol extract of A. austro-iranicum, A. festucaceum and A. chlorostegium
| A. austroiranicum | A. serotinum | A. chlorostegium | ciprofloxacin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test Bacteria | D.D | MIC | MBC | D.D | MIC | MBC | D.D | MIC | MBC | D.D | MIC | MBC |
| E. faecalis | 7 | 50 | 100 | 7 | 50 | 100 | 6 | 25 | 50 | 26 | 8 | 16 |
| S. aureus | 3 | 25 | 50 | 4 | 25 | 50 | 4 | 25 | 50 | 28 | 8 | 16 |
| P. aeruginosa | 13 | 6.25 | 12.5 | 11 | 12.5 | 25 | 11 | 3.175 | 6.25 | 32 | 8 | 16 |
| E. coli | 11 | 0.39 | 0.78 | 13 | 0.78 | 1.56 | 12 | 3.175 | 6.25 | 30 | 0.25 | 0.5 |
D.D: Inhibition zone in diameter (mm) around the impregnated discs. MIC: Minimal inhibition concentrations (as mg/ml). MBC: minimal bactericidal concentration (as mg/ml)
Discussion
Pharmacological properties that were described for the Plumbaginaceae family plants include anticancer, anti-inflammatory, antioxidant, anti-mycobacterial, antimicrobial, antiatherogenic, cardiotonic, neuroprotective and insecticidal activities (Oyedapo, 1996 ▶; Kuo et al., 2002 ▶; Chaung et al., 2003 ▶; Mossa et al., 2004 ▶; Nguyen et al., 2004 ▶; SivaKumar et al., 2005 ▶; Yang et al., 2010 ▶; Dhale and Markandeya, 2011 ▶; Eren, 2016 ▶; Lashgari et al., 2016 ▶; Sundari et al., 2017 ▶).
For example, study on anti- proliferative effects of some species of the Plumbaginaceae (e.g. Acantholimon longiscapum, Plumbago rosea and Plumbago zeylanica) indicated good and moderate levels of tumor inhibition and cancer cell cytotoxicity (Ahmad et al., 2008 ▶; Nabi et al., 2013 ▶; Anuf et al., 2014 ▶; Sharma and Kaushik, 2014 ▶; Roy et al., 2017 ▶; Sundari et al., 2017 ▶). Antioxidant potential was also detected in some species of the genus Plumbago (Plumbaginaceae) such as P. zeylanica and P. rosea which have a high level of phenolic content (Nahak and Sahu, 2011 ▶; Sundari et al., 2017 ▶). Antimicrobial activity P. zeylanica, P. scandens and P. indica which belong to the family Plumbaginaceae, toward drug resistant and pathogenic bacteria were also indicated in some reports (Paiva et al., 2003 ▶; Rahman and Anwar, 2007 ▶; Dhale and Markandeya, 2011 ▶; Saha and Paul, 2014 ▶; Sharma and Kaushik, 2014 ▶; Shukla et al., 2016 ▶). Different parts of these plants are used to treat various diseases (Thakur et al., 1989 ▶; Tilak et al., 2004 ▶). Moreover, the hepatoprotective effect and their usage in the treatment of liver disease have also been demonstrated in some researches (Girish and Pradhan, 2012 ▶; Rahmatullah et al., 2012 ▶; Nasiri et al., 2016 ▶; Gazor et al., 2017 ▶). According to the literature, plants from the Plumbaginaceae contain various secondary metabolites and active constituents such as alkaloids, glycoside, reducing sugars, simple phenolic, tannins, lignin, saponins, anthocyanin, quinines and flavonoids that contribute to their biological activities (Asen and Plimmer, 1972 ▶; Gunaherath et al., 1983 ▶; Duffey and Stout, 1996 ▶; Sreelatha et al., 2010 ▶; Dhale and Markandeya, 2011 ▶; Trabelsi et al., 2012 ▶; Pavela, 2013 ▶). For example, it was observed that one of the major secondary metabolites present in members of the Plumbaginaceae is plumbagin (Babula et al., 2005 ▶; Jeyachandran et al., 2009 ▶; Sharma and Kaushik, 2014 ▶). Several pharmacological activities, e. g. antitumor, antimicrobial, insecticidal, would healing, anti- inflammatory and antifertility actions, have been identified for plumbagin (Kavimani et al., 1996 ▶; Kini et al., 1997 ▶; Reddy et al., 2002 ▶; Sheeja et al., 2010 ▶). The genus Acantholimon may also contain plumbagin which needs to be determined by phytochemical screening.
In conclusion, our study can be considered the first study on anti-cancer, antioxidant and antibacterial potential of three Iranian endemic species of Acantholimon including A. austro-iranicum, A. serotinum and A. chlorostegium. The results showed a specific cytotoxic effect for the extracts against cancerous cells. Moreover, the significant antioxidant potential shown by the extracts demonstrates their ability to protect the non-target (normal) cells against oxidative stress and antibacterial tests indicated good activity of extracts against Gram-negative bacteria. Unexplored genus Acantholimon needs more attention for detailed scientific investigations. For instance, there is no evidence in the literature about chemical constituents of Acantholimon. Therefore, phytochemical studies for identification and elucidation of active constituents of the extracts from the three examined species of Acantholimon are needed.
Acknowledgment
The authors thank, firoozeh bordbar at Department of Biology, Faculty of science, Shahid Bahonar University of Kerman for plant collection and identification. This study was funded by Kerman University of Medical Science, Kerman, Iran (grant number: 98000046)
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Ahmad MS, Hussain M, Hanif M, Ali S, Qayyum M, Mirza B. Di‐and Triorganotin (IV) Esters of 3, 4‐Methylenedioxyphenylpropenoic Acid: Synthesis, Spectroscopic Characterization and Biological Screening for Antimicrobial, Cytotoxic and Antitumor Activities. Chem Biol Drug Des. 2008;71:568–576. doi: 10.1111/j.1747-0285.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Amarowicz R, Pegg R, Rahimi-Moghaddam P, Barl B, Weil J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. [Google Scholar]
- Anuf AR, Ramachandran R, Krishnasamy R, Gandhi PS, Periyasamy S. Antiproliferative effects of Plumbago rosea and its purified constituent plumbagin on SK-MEL 28 melanoma cell lines. Pharmacognosy Res. 2014;6:312. doi: 10.4103/0974-8490.138280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asen S, Plimmer J. 4, 6, 4′-Trihydroxyaurone and other flavonoids from Limonium. Phytochemistry. 1972;11:2601–2603. [Google Scholar]
- Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babula P, Mikelova R, Potesil D, Adam V, Kizek R, Havel L, Sladky Z. Simultaneous determination of 1, 4-naphtoquinone, lawsone, juglone and plumbagin by liquid chromatography with UV detection. Biomed. Papers. 2005;149:25–28. [Google Scholar]
- Baron E. Bacteriology. In: Murray PR, Baron EJ, editors. Manual of Clinical Microbiology. Washington DC: ASM Press; 1999. pp. 150–171. [Google Scholar]
- Chaung SS, Lin CC, Lin J, Yu KH, Hsu YF, Yen MH. The hepatoprotective effects of Limonium sinense against carbon tetrachloride and β‐D‐galactosamine intoxication in rats. Phytother RES. 2003;17:784–791. doi: 10.1002/ptr.1236. [DOI] [PubMed] [Google Scholar]
- Christenhusz MJ, Byng JW. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;261:201–217. [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar S, Kulkarni R, Rege N. Pharmacology of medicinal plants and natural products. Indian J Pharmacol. 2000;32:S81–S118. [Google Scholar]
- Dhale D, Markandeya S. Antimicrobial and phytochemical screening of Plumbago zeylanica Linn (Plumbaginaceae) leaf. J EXP BIOL. 2011;2:4–6. [Google Scholar]
- Duffey SS, Stout MJ. Antinutritive and toxic components of plant defense against insects. Arch Insect Biochem Physiol. 1996;32:3–37. [Google Scholar]
- Eren Y. Mutagenic and cytotoxic activities of Limonium globuliferum methanol extracts. Cytotechnology. 2016;68:2115–2124. doi: 10.1007/s10616-016-9951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazor R, Asgari M, Pasdaran A, Mohammadghasemi F, Nasiri E, Roushan ZA. Evaluation of Hepatoprotective Effect of Acantholimon Gilliati Eerial Part Methanolic Extract. Iran J Pharm Res. 2017;16:135. [PMC free article] [PubMed] [Google Scholar]
- Gerber M, Boutron-Ruault M-C, Hercberg S, Riboli E, Scalbert A, Siess M-H. Food and cancer: state of the art about the protective effect of fruits and vegetables. Bull Cancer. 2002;89:293–312. [PubMed] [Google Scholar]
- Girish C, Pradhan SC. Indian herbal medicines in the treatment of liver diseases: problems and promises. Fundam Clin Pharmacol. 2012;26:180–189. doi: 10.1111/j.1472-8206.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- Greenwell M, Rahman P. Medicinal plants: their use in anticancer treatment. Int. J Pharm Sci Rev Res. 2015;6:4103. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaherath GKB, Gunatilaka AL, Sultanbawa MUS, Balasubramaniam S. 1, 2 (3)-Tetrahydro-3, 3′-biplumbagin: A naphthalenone and other constituents from Plumbago zeylanica. Phytochemistry. 1983;22:1245–1247. [Google Scholar]
- Hernández-Ledesma P, Berendsohn WG, Borsch T, Mering SV, Akhani H, Arias S, Castañeda-Noa I, Eggli U, Eriksson R, Flores-Olvera H. A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales. Willdenowia. 2015;45:281–383. [Google Scholar]
- Jadid N, Hidayati D, Hartanti SR, Arraniry BA, Rachman RY, Wikanta W. Antioxidant activities of different solvent extracts of Piper retrofractum Vah using DPPH assay AIP Conference Proceedings. AIP Publishing. 2017;1854:020019. [Google Scholar]
- Jeyachandran R, Mahesh A, Cindrella L, Sudhakar S, Pazhanichamy K. Antibacterial activity of plumbagin and root extracts of Plumbago zeylanica L. Acta Biol Crac Ser. 2009;51:17–22. [Google Scholar]
- Kavimani S, Ilango R, Madheswaran M, Jayakar B, Gupta M, Majumdar U. Antitumour Activity Of Pulmbagin Against Dalton's Ascitic Lymphoma. Indian J Pharm Sci. 1996;58:194. [Google Scholar]
- Kini D, Pandey S, Shenoy B, Singh U, Udupa N, Umadevi P, Kamath R, Ramanarayan K. Antitumor and antifertility activities of plumbagin controlled release formulations. Indian J Exp Biol. 1997;35:374–379. [PubMed] [Google Scholar]
- Kubitzki K. Plumbaginaceae. Flowering Plants· Dicotyledons. Berlin Heidelberg: Springer-Verlag ; 1993. pp. 523–530. [Google Scholar]
- Kumar PS, Sucheta S, Deepa VS, Selvamani P, Latha S. Antioxidant activity in some selected Indian medicinal plants. Afr J Biotechnol. 2008;7:1826–1828. [Google Scholar]
- Kuo Y-C, Lin L-C, Tsai W-J, Chou C-J, Kung S-H, Ho Y-H. Samarangenin B from Limonium sinense suppresses herpes simplex virus type 1 replication in Vero cells by regulation of viral macromolecular synthesis. Antimicrob Agents Chemother. 2002;46:2854–2864. doi: 10.1128/AAC.46.9.2854-2864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashgari AP, Delazar A, Afshar FH, Parsa D. Contact Toxicity and Chemical Composition of Essential Oil of Acantholimon scorpius. Pharmaceutical Sciences. 2016;22:138. [Google Scholar]
- Lehmann PF. PR Murray, EJ Baron, MA Pfaller, FC Tenover and RH Yolken, eds. Manual of Clinical Microbiology. Mycopathologia. 1999;146:107–108. [Google Scholar]
- Matteo V, Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. CNS Neurol DISORD-DR. 2003;2:95–107. doi: 10.2174/1568007033482959. [DOI] [PubMed] [Google Scholar]
- Mossa JS, El‐Feraly FS, Muhammad I. Antimycobacterial constituents from Juniperus procera, Ferula communis and Plumbago zeylanica and their in vitro synergistic activity with isonicotinic acid hydrazide. Phytother R. 2004;18:934–937. doi: 10.1002/ptr.1420. [DOI] [PubMed] [Google Scholar]
- Nabi S, Baloch NU, Bashir S, Rabbani T, Al-Kahraman YM. In Vitro Antimicrobial, Antitumor and Cytotoxic activities and their phytochemical analysis of Methanolic Extract and its Fractions of Acantholimon longiscapum leaves. Int J Phytopharm. 2013;4:179–183. [Google Scholar]
- Nahak G, Sahu RK. Antioxidant activity of Plumbago zeylanica and Plumbago rosea belonging to family plumbaginaceae. Natural Product: An Indian Journal. 2011;7:51–56. [Google Scholar]
- Nasiri E, Naserirad S, Pasdaran LA, Gazor R, Mohammadghasemi F, Atrkar RZ. Hepatoprotective effect of Acantholimon bracteatum (Girard) Boiss on formaldehyde-induced liver injury in adult male mice. RJP. 2016;3:55–61. [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nguyen AT, Malonne H, Duez P, Vanhaelen-Fastre R, Vanhaelen M, Fontaine J. Cytotoxic constituents from Plumbago zeylanica. Fitoterapia. 2004;75:500–504. doi: 10.1016/j.fitote.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Oyedapo O. Studies on bioactivity of the root extract of Plumbago zeylanica. Int j pharmacogn. 1996;34:365–369. [Google Scholar]
- Paiva SRd, Figueiredo MR, Aragão TV, Kaplan MAC. Antimicrobial activity in vitro of plumbagin isolated from Plumbago species. MEM I OSWALDO CRUZ. 2003;98:959–961. doi: 10.1590/s0074-02762003000700017. [DOI] [PubMed] [Google Scholar]
- Pavela R. Efficacy of naphthoquinones as insecticides against the house fly, Musca domestica L. IND CROP PROD. 2013;43:745–750. [Google Scholar]
- Phongpaichit S, Nikom J, Rungjindamai N, Sakayaroj J, Hutadilok-Towatana N, Rukachaisirikul V, Kirtikara K. Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS IMMUNOL MED MIC. 2007;51:517–525. doi: 10.1111/j.1574-695X.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- Pinto DC, Silva A. Anticancer natural coumarins as lead compounds for the discovery of new drugs. CURR TOP MED CHEM. 2017;17:3190–3198. doi: 10.2174/1568026618666171215095750. [DOI] [PubMed] [Google Scholar]
- Rahman MS, Anwar MN. Antimicrobial activity of crude extract obtained from the root of Plumbago zeylanica. Bangladesh j microbiol. 2007;24:73–75. [Google Scholar]
- Rahmatullah M, Khatun Z, Hasan A, Parvin W, Moniruzzaman M, Khatun A, Mahal MJ, Bhuiyan S, Mou SM, Jahan R. Survey and scientific evaluation of medicinal plants used by the Pahan and Teli tribal communities of Natore district, Bangladesh. Afr J Tradit Complement Altern Med. 2012;9:366–373. doi: 10.4314/ajtcam.v9i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy JS, Rao PR, Reddy MS. Wound healing effects of Heliotropium indicum, Plumbago zeylanicum and Acalypha indica in rats. J Ethnopharmacol. 2002;79:249–251. doi: 10.1016/s0378-8741(01)00388-9. [DOI] [PubMed] [Google Scholar]
- Roy A, Attre T, Bharadvaja N. Anticancer agent from medicinal plants: A Review. New aspects in medicinal plants and pharmacognos. Poland: JB Books Publisher; 2017. pp. 1–159. [Google Scholar]
- Saha D, Paul S. Antibacterial activity of Plumbago indica. Turk J Pharm Sci. 2014;11:217–222. [Google Scholar]
- Sameer R, Nidhi S, Tarun V, Charan S, Jyoti G. A review on naturally derived compounds for potential anticancer activity. Indian j drugs. 2016;4:75–86. [Google Scholar]
- Sharma N, Kaushik P. Medicinal, biological and pharmacological aspects of Plumbago zeylanica (Linn ) J Pharmacogn Phytochem. 2014;3:117–120. [Google Scholar]
- Sheeja E, Joshi S, Jain D. Bioassay-guided isolation of anti-inflammatory and antinociceptive compound from Plumbago zeylanica leaf. Pharm. Biol. 2010;48:381–387. doi: 10.3109/13880200903156424. [DOI] [PubMed] [Google Scholar]
- Sher A. Antimicrobial activity of natural products from medicinal plants. Gomal J Med Sci. 2009;7:72–78. [Google Scholar]
- Shukla R, Sharma DC, H Baig M, Bano S, Roy S, Provazník I, A Kamal M. Antioxidant, Antimicrobial Activity and Medicinal Properties of Grewia asiatica L. Med Chem. 2016;12:211–216. doi: 10.2174/1573406411666151030110530. [DOI] [PubMed] [Google Scholar]
- SivaKumar V, Prakash R, Murali M, Devaraj H, Niranjali Devaraj S. In vivo micronucleus assay and GST activity in assessing genotoxicity of plumbagin in Swiss albino mice. DRUG CHEM TOXICOL. 2005;28:499–507. doi: 10.1080/01480540500263019. [DOI] [PubMed] [Google Scholar]
- Sreejayan N, Rao M. Free radical scavenging activity of curcuminoids. Arzneimittel-forschung. 1996;46:169–171. [PubMed] [Google Scholar]
- Sreelatha T, Hymavathi A, Murthy JM, Rani P, Rao JM, Babu KS. Bioactivity-guided isolation of mosquitocidal constituents from the rhizomes of Plumbago capensis Thunb. Bioorg Med Chem Lett. 2010;20:2974–2977. doi: 10.1016/j.bmcl.2010.02.107. [DOI] [PubMed] [Google Scholar]
- Sundari BKR, Telapolu S, Bilikere S, Thyagarajan SP. Cytotoxic and antioxidant effects in various tissue extracts of Plumbago zeylanica: Implications for anticancer potential. Phcog J. . 2017;9:706–712. [Google Scholar]
- Thakur R, Puri H, Hussain A. Major Medicinal Plants of India, Central Institute of Medicinal and Aromatic Plants. India: Lucknow; 1989. pp. 1–100. [Google Scholar]
- Thambiraj J, Paulsamy S, Sevukaperumal R. Evaluation of in vitro antioxidant activity in the traditional medicinal shrub of western districts of Tamilnadu, India, Acalypha fruticosa Forssk. (Euphorbiaceae). Asian Pac J Trop Biomed. 2012;2:S127–S130. [Google Scholar]
- Thomford N, Senthebane D, Rowe A, Munro D, Seele P, Maroyi A, Dzobo K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int J Mol Sci. 2018;19:1578. doi: 10.3390/ijms19061578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilak JC, Adhikari S, Devasagayam TP. Antioxidant properties of Plumbago zeylanica, an Indian medicinal plant and its active ingredient, plumbagin. Redox report. 2004;9:219–227. doi: 10.1179/135100004225005976. [DOI] [PubMed] [Google Scholar]
- Trabelsi N, Oueslati S, Falleh H, Waffo-Téguo P, Papastamoulis Y, Mérillon J-M, Abdelly C, Ksouri R. Isolation of powerful antioxidants from the medicinal halophyte Limoniastrum guyonianum. Food Chem. 2012;135:1419–1424. doi: 10.1016/j.foodchem.2012.05.120. [DOI] [PubMed] [Google Scholar]
- Yang S-J, Chang SC, Wen HC, Chen CY, Liao JF, Chang CH. Plumbagin activates ERK1/2 and Akt via superoxide, Src and PI3-kinase in 3T3-L1 cells. Eur J Pharmacol. 2010;638:21–28. doi: 10.1016/j.ejphar.2010.04.016. [DOI] [PubMed] [Google Scholar]