Abstract
Background:
Neurophysiologic biomarkers are needed for clinical trials of therapies for myotonic dystrophy (DM1). We characterized muscle properties, spinal reflexes (H-reflexes), and trans-cortical long-latency reflexes (LLRs) in a cohort with mild/moderate DM1.
Methods:
Twenty-four people with DM1 and 25 matched controls underwent assessment of tibial nerve H-reflexes and soleus muscle twitch properties. Quadriceps LLRs were elicited by delivering an unexpected perturbation during a single-limb squat (SLS) visuomotor tracking task.
Results:
DM1 was associated with decreased H-reflex depression. The efficacy of doublet stimulation was enhanced, yielding an elevated double-single twitch ratio. DM1 participants demonstrated greater error during the SLS task. DM1 individuals with the least-robust LLR responses showed the greatest loss of spinal H-reflex depression.
Conclusions:
DM1 is associated with abnormalities of muscle twitch properties. Co-occurring alterations of spinal and trans-cortical reflex properties underscore the central nervous system manifestations of this disorder and may assist in gauging efficacy during clinical trials.
Keywords: excitation-contraction coupling, homosynaptic depression, H-reflex, long-latency reflex, muscle plasticity, perturbation
1 |. INTRODUCTION
The pathophysiology of myotonic dystrophy type 1 (DM1) is now understood as a genetically instigated spliceopathy that involves toxic RNA gain-of-function in several gene pathways, in particular, the muscleblind-like proteins (MBNL).1 The molecular pathomechanism of DM1 has widespread effects in skeletal muscle, cardiac muscle, and the central nervous system (CNS).2,3 Just as in muscle, mutant DM1 protein kinase (DMPK) RNA in brain tissue sequesters MBNL proteins into ribonuclear inclusions and triggers a reversion to a fetal regulatory pattern.4 Although the exact mechanisms are unclear, RNA toxicity and altered splicing in the CNS of patients with DM1 likely contributes to the many neurocognitive sequelae of the disease: gray and white matter irregularities5,6 cognitive function compromise,6,7 and mood disturbance.7
Characterization of DM1 neurological symptom severity is primarily accomplished by means of clinical metrics such as the Muscular Impairment Rating Scale (MIRS),8 manual muscle testing (MMT), quantitative muscle testing (QMT), and the 6-Minute Walk Test.9 Several potential biomarkers for DM1 symptom severity are under study, including measurement of RNA splice defects,10,11 skeletal muscle imaging,12 and brain imaging.13 No gold-standard neurophysiologic biomarker for DM1 severity is currently available. Previous studies have identified alterations in neural activation latency/refractory period14,15 and neuromuscular transmission.16 Although peripheral neuropathy has occasionally been noted, it is frequently mild and sub-clinical17-19 and may be consequent to diabetes, a common clinical correlate of DM1.20
Developing new neurophysiologic biomarkers for DM1 that correlate with neuromotor task performance will provide insight into how therapeutic agents may yield tangible functional outcomes for people living with DM1. Biomarkers that can reveal therapeutic adaptations at the level of discrete organ systems (the periphery [muscle], spinal circuitry, prevolitional neuromuscular control) may provide an enhanced level of mechanistic resolution that is not possible with the behavior-based clinical measures mentioned above. The present study aimed to characterize neuromuscular physiology of the DM1 phenotype at three distinct levels: muscle twitch contractile properties, spinal segmental reflexes, and trans-cortical long-latency reflex (LLR) responses. Trans-cortical reflexes occur between the latency of spinally mediated stretch reflexes and volitional activation. Similar to spinal reflexes, they can be elicited by rapid stretch of skeletal muscle,21 such as during an unexpected weight-bearing perturbation22 Altering cortical excitability through transcranial magnetic stimulation yields changes in the magnitude of LLRs,23 highlighting their potential mutability in response to altered cortical states. Little is understood about the functional correlates of cortical processes in DM1.24 In addition, previous spinal segmental reflex studies had significant methodological limitations.25 Significant knowledge gaps, therefore, exist about the fundamental characteristics of neuromuscular physiology in DM1, particularly as it relates to functional weight-bearing movement control.
The purpose of this study was to characterize muscle twitch properties, spinal segmental reflexes (H-reflexes), and trans-cortical reflexes (LLRs) in a cohort with clinically mild to moderate DM1. We hypothesized that even in these ambulatory, community-dwelling individuals, significant relationships will exist between altered functional task performance and altered muscle and reflex properties. Importantly, muscle twitch and segmental reflex properties were measured using classical electrophysiological measures26,27 that can be carried out in typical clinical neurophysiologic facilities. These measurements may, therefore, serve as practical clinical biomarkers of symptom severity and CNS response to forthcoming DM1 treatments.
2 |. METHODS
Study participants were recruited from the clinical population of our academic medical center and from the Myotonic Dystrophy Family Registry. All participants signed an informed consent document approved by the University of Iowa Human Subjects Institutional Review Board. Inclusion criteria were confirmatory DM1 diagnosis by means of genetic testing including the CTG Allele 1 repeat number; ambulation without assistive devices; MIRS between 1 (no detected impairment) and 5 (severe proximal weakness); and no evidence of peripheral neuropathy. All control participants were assigned a MIRS score of 0 for this study. Exclusion criteria were cardiac pacemaker and any recent trauma or injury to the lower extremities.
2.1 |. Soleus force and electromyography
Participants sat in a padded chair with the right foot on a soleus torque measurement apparatus.27 Straps secured the knee and ankle in 90° of flexion. This position minimized the contribution of the gastrocnemius muscle to plantar flexion force.28 Isometric plantar flexion force was measured by a force transducer (Interface Inc., 1500ASK, Scottsdale, AZ) mounted to the device's rigid metal foot plate. An electromyographic (EMG) electrode (Therapeutics Unlimited, Iowa City, IA) was placed over the belly of the soleus muscle. The reference electrode was positioned over the anterior distal aspect of the tibia. Force and EMG responses were monitored by means of an oscilloscope. The force transducer and EMG signals were amplified and sampled at 2000 Hz using an analog-to-digital data acquisition board and a custom Labview application. Signals were analyzed offline using a custom DIAdem script (National Instruments, v.2012).
M-waves and H-reflexes were assessed by means of transcutaneous stimulation of the tibial nerve in the popliteal fossa (Digitimer DS7A, Welwyn Garden City, Herts, UK). The stimulating electrode was positioned at the location where an H-reflex could be most reliably elicited with a small M wave at very low stimulus intensities. The stimulating electrode was then secured with a strap to prevent movement during testing.
2.2 |. Experimental protocol
The three-phase experimental protocol appears in Supporting Information Figure S1, which is available online. The protocol required approximately 60 min to complete. In phase 1 (Supporting Information Figure S1A), with subjects seated in the soleus force assessment apparatus, M-wave stimulus intensity was incrementally increased and three supra-maximal M-waves were obtained. Maximal M-wave amplitude was identified as the maximal peak-to-peak difference of the compound muscle action potential. All subsequent H-reflex responses were normalized to this value.
Maximal H-reflex amplitude was determined (1000-μs pulse width), and then the stimulus intensity was reduced to obtain H-reflexes of approximately 50% maximum H amplitude. Next, we ensured that H-reflex responses were on the ascending limb of the recruitment curve26 and that H responses corresponded to ~15–20% of the maximal M-wave response. Using this intensity, 15 doublet stimulation pulses with an inter-pulse interval of 500 ms (2 Hz) were administered to elicit paired H-reflexes (termed H1 and H2) (Supporting Information Figure S1A). The influence of homosynaptic depression upon each H2 (H-reflex depression) was calculated as (H2/H1). Doublet pairs were separated by 15 s to avoid the effects of homosynaptic depression on H1 amplitude.29
In the second experiment phase (Supporting Information Figure S1B), participants remained seated in the soleus force assessment apparatus. Soleus contractions were evoked by means of selfadhesive carbon electrodes adhered to the skin over the posterior aspect of the calf. Single twitches were given at incrementally higher intensities until maximal twitch torque was observed. The stimulus intensity was then increased an additional 10% to ensure supramaximal stimulation throughout this phase of the experiment. A doublet was first given (160 HZ; 6.25-ms inter-pulse interval), followed 5 s later by a single twitch. Five such double-single twitch pairs were obtained, spaced by 5 s each. The double-single ratio (DSR) was calculated by dividing the torque developed during the doublet twitch by the torque developed by the following single twitch. Average DSR was obtained for the five double-single pairs. Participants then received a soleus repetitive stimulation protocol, during which they received 1000 stimulus pulses at a 3 Hz frequency (5.55 min of stimulation). Previous work indicated that repetitive 3 Hz stimulation induces fatigue in muscle that has converted to a fast-glycolytic phenotype, such as in patients with spinal cord injury (SCI).30 In the present study, 3 Hz repetitive stimulation was used as a probe to determine whether such a functional phenotypic shift occurred in the DM1 group. After the 3 Hz protocol, participants repeated the double-single twitch assessment (five double-single pairs) and average DSR was again calculated.
Phase III of the experiment (Supporting Information Figure S1C) consisted of movement performance testing designed to examine trans-cortical sensorimotor reflex pathways (trans-cortical LLRs). The study used a well-validated functional weight-bearing task that elicits LLRs after unexpected perturbations in single limb stance.22,31-35 Subjects stood with the right knee positioned against a padded plate that moved horizontally on a rack and pinion system.22 An electromagnetic brake resisted linear displacement of the rack during knee flexion and extension. Subjects viewed a sinusoid waveform on a display monitor, paired with a line that represented their instantaneous knee position. As the sinusoid progressed across the screen, subjects performed a single-limb squat (SLS) maneuver (~0 to 25 degrees of knee flexion), attempting to entrain their user signal to the target signal (Supporting Information Figure S1C). Subjects received two practice trials (five sinusoid cycles each) to learn the position-matching task. Subjects then performed the position-matching task across nine conditions that varied the brake resistance (5%, 10%, and 15% of body weight) and sinusoid speed (0.2, 0.4, 0.6 Hz).22 Each condition consisted of five sine-wave cycles. Position error during the SLS task was calculated as the difference between the target signal and the user signal at each instantaneous sampling point. Absolute error (AE) was calculated as the mean of all position error samples.
At one randomly determined sinusoid cycle during each of the nine SLS conditions (but never during the first cycle), the brake resistance was eliminated for 400 ms. This created an unexpected perturbation to knee flexion (downward-going movement) that elicited compensatory reflex responses. Vastus medialis EMG was recorded with wireless EMG electrodes (Delsys Trigno, Natick, MA) and was then digitized at 2000 Hz by a custom Labview application. The peak root mean square EMG magnitude of the long-latency (trans-cortical) reflex response was analyzed between 50 and 200 ms after the onset of the perturbation,36 then normalized to the preperturbation time bin (−50 to 0 ms before perturbation).
2.3 |. Statistical analysis
For phase 1 of the experiment, soleus H2/H1 ratio (H-reflex depression) was contrasted for the DM1 and Control groups by means of one-way analysis of variance (ANOVA). In phase 2, soleus twitch amplitude was compared between groups and between time periods (pre/post-fatigue) by means of a two-way repeated-measures ANOVA. Soleus doublet amplitude and soleus DSR were analyzed in the same manner. In phase 3, overall SLS AE across all nine test conditions was compared between groups by means of one-way ANOVA. For the analysis of perturbations, previous work indicated that among the nine conditions, the 0.2 Hz, 15% body weight resistance condition comprised a moderate-difficulty task that could be successfully performed even by participants with underlying sensorimotor impairment.22,33 All perturbation analyses used data obtained from this test condition. The effect of the perturbation was assessed in two ways: first, by comparing AE for a perturbed cycle to its immediately preceding nonperturbed cycle (two-way repeated measures [ Perturbation × Group] ANOVA). Second, a similar two-way repeated measures ANOVA tested the effect of the perturbation on normalized LLR amplitude in the LLR latency bin (50–200 ms) vs EMG at the same region of the sinusoid cycle in nonperturbed trials.
Pearson product-moment correlation coefficients were computed to evaluate relationships between: (1) MIRS level and physiologic outcome measures; (2) CTG repeat number and physiologic outcome measures, and; (3) the LLR EMG response versus H-reflex depression and the double-single twitch ratio. Significance for all tests was set at P = .05.
3 |. RESULTS
Twenty-four people with DM1 (9 women and 15 men) and 25 people with no known neurologic compromise (CTRL, 11 women and 14 men) (Table 1) took part in the study.
TABLE 1.
Participant characteristics
| Population | N | Age (yr) | Gender | CTG Repeats | MIRS | WAIS-IV IQ |
|---|---|---|---|---|---|---|
| CTRL | 25 | 39.4 ± 13.6 | 11 F / 14 M | 14.5 ± 5.5 | 0.0 ± 0.0 | 115.4 ± 9.1 |
| DM1 | 24 | 45.6 ± 12.9 | 9 F / 15 M | 288.5 ± 237.3 | 2.0 ± 1.1 | 108.4 ± 13.1 |
| P-value | .112 | .64 | < .001 | .00 | .035 |
Abbreviation: WAIS-IV IQ, Wechsler Adult Intelligence Scale-IV Full Scale IQ.
Figure 1 illustrates between-group differences in H2/H1 ratio. H2 was significantly larger as a function of H1 for the DM group than the control group (P = .05). Neither MIRS score (P = .26; R2 = 0.066) nor the number of CTG repeats (P = .82; R2 = 0.003) was significantly associated with H2/H1 ratio for participants with DM1.
FIGURE 1.
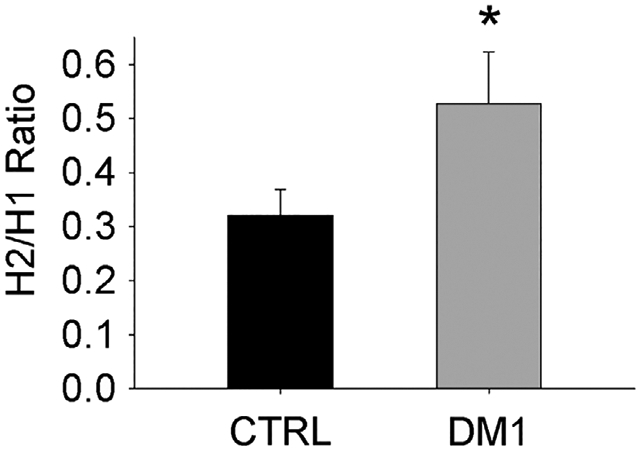
H2/H1 ratio. *Significant difference CTRL vs DM1
In phase 2 of the study, all participants tolerated the supramaximal stimulus intensity required to achieve full soleus recruitment during muscle twitch testing. Doublet and single twitch force for people with DM1 were lower, on average, than for controls (31.4% and 30.0% lower, respectively) (Figure 2A). A significant interaction existed for subject group and time pre/post fatigue (P = .026 and .028 for single and doublet twitch force, respectively). Analysis of pairwise contrasts indicated that both single twitch and doublet forces were lower for DM1 than for CTRL at both Pre and at Post (all P < .002). In addition, single twitch and doublet forces were higher for CTRL at Post than at Pre (both P < .009), indicating that CTRL subjects demonstrated potentiation of soleus twitch and doublet force after repetitive 3 Hz stimulation. For DM1 participants, the uniformity of single twitch and doublet force before and after 3 Hz stimulation (P = .622 and .605, respectively) indicates that repetitive stimulation caused neither fatigue nor potentiation of force in this group.
FIGURE 2.
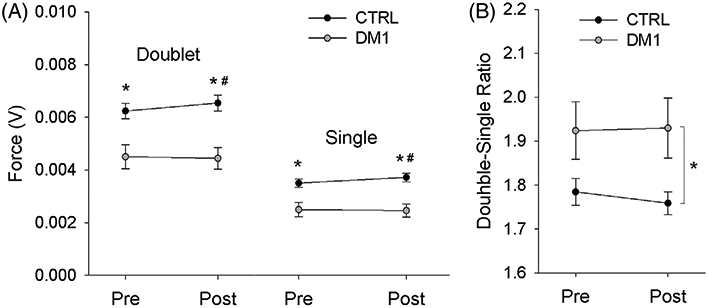
A,B, Soleus muscle twitch properties before (Pre) and after (Post) a fatigue protocol. *Significant difference CTRL vs DM1. #Significant difference CTRL Pre vs Post
No significant interaction existed for DSR between subject group and time pre/post fatigue (P = .0710) (Figure 2B). A significant main effect existed for subject group (CTRL vs DM1; P = .034) but not for time (Pre vs Post, p = 0.257). A significant linear relationship existed between DSR and the MIRS score for people with DM1 (P < .001, R2 = 0.625) (Figure 3). However, no significant relationship existed between DSR and the number of CTG repeats for this cohort (P = .37; R2 = 0.05).
FIGURE 3.
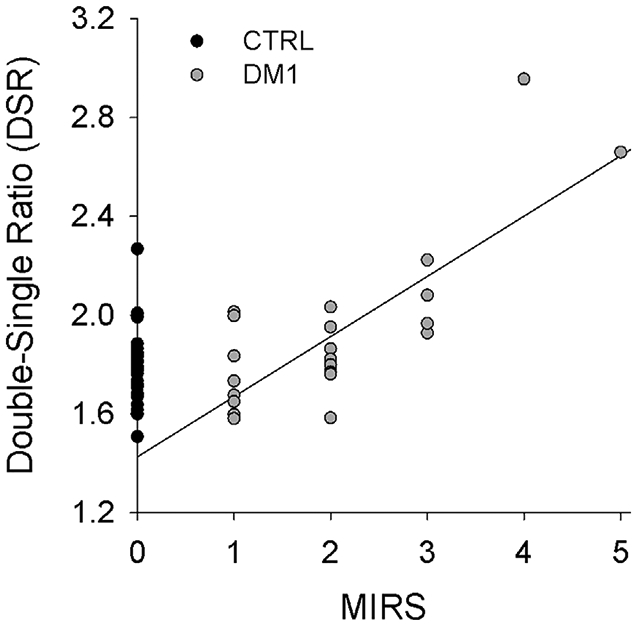
Linear regression relationship between DSR and the MIRS score for people with DM1
In phase 3 of the study, people with DM1 demonstrated significantly higher overall AE during the SLS visual-motor task (P < .001) (Figure 4). For both perturbation and nonperturbation trials, AE was higher for people with DM1 than for controls (P < .001). (Figure 5A). A perturbation significantly increased AE for people with and without DM1 (P = .002). A perturbation also significantly increased peak EMG response during the LLR time bin (50–200 ms) for perturbation trials, compared with EMG from the same sinusoid position in nonperturbation trials (P = .002; Figure 5B). The LLR response did not vary by subject group (P = .466), nor was there a significant interaction between perturbation and group (P = .250). A significant relationship existed between the LLR magnitude (normalized to nonperturbed trials) and the magnitude of depression of the H-reflex in people with DM1 (P = .05; R2 = 0.248) (Figure 5C). No such relationship existed for controls (P = .73; R2 = 0.0074). For participants with DM1, the LLR response did not correlate significantly with MIRS score (P = .64) or with the number of CTG repeats (P = .16).
FIGURE 4.
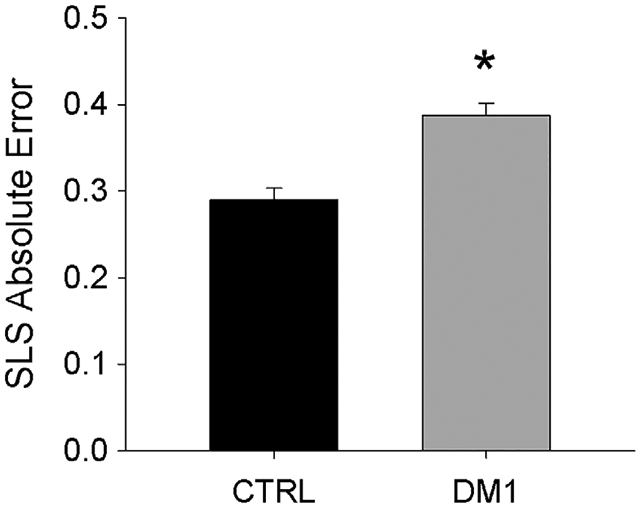
SLS AE (mean/SD across nine test conditions). *Significant difference CTRL vs DM1
FIGURE 5.
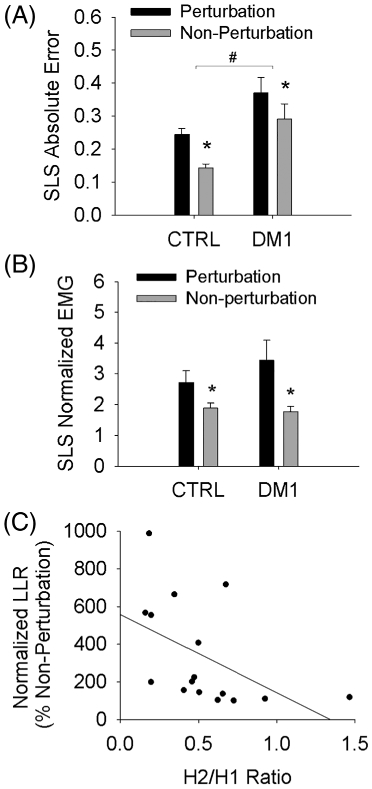
Position error and LLR responses to an unexpected perturbation during SLS. A,B, #Significant difference CTRL vs DM1 for perturbation and nonperturbation trials. *Significant difference perturbation vs nonperturbation within each group. C, For people with DM1, a significant negative relationship existed between LLR magnitude and magnitude of depression of the H-reflex (H2/H1 ratio)
4 |. DISCUSSION
4.1 |. Summary of results
The first key finding in the present study is that participants with DM1 demonstrated reduced depression of the H-reflex during repetitive tibial nerve stimulation. Secondly, we observed reduced overall twitch force amplitude, but elevated doublet force relative to the twitch force in the DM1 group (DSR). Unlike CTRL participants, the DM1 group did not demonstrate enhancement of twitch or doublet force (potentiation) after repetitive 3 HZ stimulation. And finally, participants with DM1 had poorer overall performance on a functional weight bearing task (SLS) than controls. A significant negative correlation existed between trans-cortical reflex responses (LLRs) and the magnitude of spinal reflex homonymous depression (H2/H1 ratio). This co-variance of spinal and cortical reflex responses may serve as a meaningful biomarker for disease progression or recovery in future clinical trials.
4.2 |. Spinal circuitry adaptations
Limited information has been previously reported about spinal reflex adaptations in DM1. Two early papers reported hypotonia in these patients, implicating reduced excitability of the monosynaptic stretch reflex arc (muscle spindle receptors, afferent fibers, and alpha motor neurons).25,37 These reports predated genetic confirmatory diagnosis for DM1. As has been recently emphasized38 the H-reflex is often mistakenly presumed to be a monosynaptic response that provides a simple probe into excitability of the stretch reflex arc. In reality, the H-reflex is modulated by a host of homonymous and heteronymous pathways39-41 that reveal facilitatory and inhibitory processes at the spinal level and in higher centers.42 Diminution of the H-reflex in response to repetitive stimulation is an important window into homosynaptic depression of the spinal reflex system.29,43
In this phenomenon, activation of the Ia afferent / alpha motor neuron arc reduces the probability of subsequent activation of the same pathway within the following ~500 ms.29 Substantial loss of H2 depression is frequently observed in neurologic disease and injury. In SCI, H2 is suppressed by only 20–30%, a process that can be partially reversed by daily electrical stimulation training.44 In neurologically intact subjects, partial loss of H2 depression was observed after limb immobilization (casting) and was reversible with re-mobilization.45 As all DM1 participants in the present study were ambulatory, we do not suspect that lack of physical mobility caused the observed reduction in H2 depression. Rather, we hypothesize that altered H-reflex depression is, like hypotonia, a characteristic adaptation of interneuronal spinal circuitry in people with DM1.
4.3 |. Contractile adaptations
The second phase of the study used repetitive 3 Hz stimulation as a probe to determine the relative fatigue resistance of muscle in DM1. This same protocol has been shown to elicit significant fatigue in the soleus muscle of patients with SCI,30 who undergo a phenotypic shift to predominantly fast-fatigable (type II) muscle fibers.27 DM1 skeletal muscle continues to express fatigue-resistant (type I) fibers, but these fibers undergo extensive, selective atrophy.46 Participants in the present study demonstrated the expected degree of overall reduction in evoked muscle force, compared with controls. However, they did not demonstrate a reduction of twitch or doublet force after repetitive 3 Hz stimulation. Thus, for ambulatory people with DM1, no evidence emerged for a broad shift in muscle physiologic phenotype toward a more fatigable profile.
Excitation-contraction (E-C) coupling alterations were suggested by the significantly elevated DSR in the DM1 group, a finding that was previously noted in the upper extremity of DM1 patients.47 E-C compromise reflects impaired Ca2+ release from the sarcoplasmic reticulum,48 and it can be overcome by the rapid increase in Ca2+ concentration created by doublet stimulation.49 The enhanced efficacy of doublet stimulation, relative to twitch force, suggests that DM1 muscle may exist in a physiologic state of partial E-C coupling compromise. This physiologic presentation is consistent with current knowledge about the effects of DM1 spliceopathy upon genes that govern E-C coupling. Key E-C proteins such as ryanodine receptor 1 (RYR1), sarco/endoplasmic Ca2+ ATPase (SERCA), and the Cav1.1 voltage-sensitive calcium channel all undergo alternative splicing in DM1 and appear to contribute to skeletal muscle dysfunction.50-52
If future work confirms that E-C un-coupling is indeed a causative factor of muscle dysfunction in DM1, then future therapies are likely to target the transcription/translation processes that produce these mis-spliced E-C proteins. Effective therapies would be expected to reduce the expression of mis-spliced proteins and increase the expression of correctly functioning proteins. Physiologic biomarkers like twitch and doublet properties are needed to reveal the functional importance of adaptations in these proteins. By providing a link to muscle function, neurophysiologic biomarkers such as twitch and doublet properties can complement spliceopathy-related biomarkers and yield a more clinically relevant understanding of adaptations to therapies.
4.4 |. Trans-cortical reflex responses
Brain imaging studies in DM1 have primarily focused on identifying cortical changes that underlie cognitive symptoms such as executive function impairment. Few previous studies have investigated the potential cortical basis for sensorimotor impairment53,54 and we are aware of only one study that has directly examined cortical excitability in humans with DM1.24 Widespread brain structural abnormalities have been reported, including white matter lesions and reduction of gray matter volume in the sensorimotor cortex and corticospinal tract,54 providing support for deterioration of this pathway in DM1. In the present study, we examined adaptations in trans-cortical reflexes in a way that replicated a realistic motor challenge; an unexpected perturbation that caused knee buckling. We have previously used this test paradigm to characterize motor responses in healthy subjects, patients with neurologic disease, elders at risk of falls, and as part of a dual-task cognitive challenge.22,31-35
Despite having relatively mild/moderate clinical symptoms and being community ambulators, the DM1 cohort demonstrated significantly poorer motor performance on the functional weight bearing task than controls. This deficit was not accompanied by differences in LLR amplitude after an unexpected perturbation. However, closer inspection of the data indicated that DM1 participants with the least-robust LLR responses tended to have a high H2/H1 ratio (Figure 5C); that is, they tended to fail to suppress the H2 response. As previously mentioned, the loss of H2 depression in the DM1 cohort is suggestive of adaptations in spinal neural networks that mediate homosynaptic depression of the stretch reflex arc. Co-occurring adaptations in cortical and spinal motor reflex responses is a novel finding in DM1 that highlights the probable widespread CNS sequelae of DM1 mis-splicing. These neural adaptations may also play a role in the decline of motor function with the progression of DM1 symptoms. Weight-bearing tasks such as balance, gait, and transfers use both spinal and trans-cortical reflexes to tune motor output. A co-occurring loss of homosynaptic reflex depression and transcortical LLR magnitude may undermine lower extremity motor control in ways that have serious sequelae for patients’ independent mobility and function.
4.5 |. Clinical application and study considerations
Neurophysiologic biomarkers such as those included in the present study can offer insight into the specific locus of adaptation for future DM1 therapies: the muscle, the spinal circuitry, and trans-cortical (but prevolitional) sensorimotor loops. This insight would be complementary to the broad behavior-based endpoint measures currently used (manual muscle test, 6-Minute Walk Test, etc). The necessary technology to conduct H-reflex and doublet/single twitch tests is present in many clinical neurophysiology laboratories. Such clinical facilities would be well-positioned to explore the utility of H-reflexes and doublets as neurophysiologic biomarkers in the context of clinical trials. Future trials would benefit from including corroborating functional metrics, analogous to the SLS test used in the present study. Trans-cortical LLRs can be evoked in several ways, including simple clinic-based systems such as balance boards and unstable surfaces. Any clinical facility with the ability to measure the latency and amplitude of EMG after unexpected perturbations has the potential to engage in the exploration of aberrant sensorimotor control in DM1. As the present study included only individuals with clinically mild to moderate DM1, a need exists for further research into neurophysiologic biomarkers in patients with more advanced disease. In addition, larger studies of more symptomatically heterogeneous groups are needed to examine relationships among neurophysiologic biomarkers, functional task performance, and biological factors such as CTG repeat number.
5 |. CONCLUSIONS
Neurophysiologic biomarkers of DM1 motor impairment are needed to provide a mechanistic link between spliceopathy-related biomarkers and clinical measures of physical function. They will also be essential tools to gauge the efficacy of future therapies upon the CNS manifestations of DM1. H-reflex homosynaptic depression and spinal trans-cortical reflexes are clinically feasible to test and they warrant further investigation as correlates of motor function in DM1.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Jason Wu, BS, MS; Claire Johnson, BS; Ashley Cochran, BS; Kristin Johnson, DPT; and Patrick McCue, DPT, for their assistance with the study.
Funding information
CTSA, Grant/Award Number: UL1TR002537; National Institutes of Health, Grant/Award Numbers: R01 HD082109, R01 HD084645, R01 NS094387
Abbreviations:
- AE
absolute error
- ANOVA
analysis of variance
- CNS
central nervous system
- CTRL
control subjects
- DM1
myotonic dystrophy type 1
- DMPK
myotonic dystrophy type 1 protein kinase
- DSR
double-single ratio
- E-C
excitation-contraction
- EMG
electromyographic
- LLR
long-latency reflex
- MBNL
muscleblind-like proteins
- MIRS
Muscular Impairment Rating Scale
- RYR1
ryanodine receptor 1
- SCI
spinal cord injury
- SERCA
sarco/endoplasmic Ca2+ ATPase
- SLS
single-limb squat
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Fardaei M, Rogers MT, Thorpe HM, et al. Three proteins, MBNL, MBLL and MBXL, co-localize in vivo with nuclear foci of expanded-repeat transcripts in DM1 and DM2 cells. Hum Mol Genet. 2002;11: 805–814. [DOI] [PubMed] [Google Scholar]
- 2.Wenninger S, Montagnese F, Schoser B. Core clinical phenotypes in myotonic dystrophies. Front Neurol. 2018;9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braz SO, Acquaire J, Gourdon G, Gomes-Pereira M. Of mice and men: advances in the understanding of neuromuscular aspects of myotonic dystrophy. Front Neurol. 2018;9:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodwin M, Mohan A, Batra R, et al. MBNL Sequestration by Toxic RNAs and RNA Misprocessing in the Myotonic Dystrophy Brain. Cell Rep. 2015;12:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo WK, Park YG, Choi YC, Kim SM. Cortical thickness and white matter integrity are associated with CTG expansion size in myotonic dystrophy type I. Yonsei Med J. 2017;58:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanigni S, Evangelisti S, Giannoccaro MP, et al. Relationship of white and gray matter abnormalities to clinical and genetic features in myotonic dystrophy type 1. Neuroimage Clin. 2016;11:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton MJ, McLean J, Cumming S, et al. Outcome measures for central nervous system evaluation in myotonic dystrophy type 1 may be confounded by deficits in motor function or insight. Front Neurol. 2018;9:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathieu J, Boivin H, Meunier D, Gaudreault M, Begin P. Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology. 2001;56:336–340. [DOI] [PubMed] [Google Scholar]
- 9.Sedehizadeh S, Brook JD, Maddison P. Body composition and clinical outcome measures in patients with myotonic dystrophy type 1. Neuromuscul Disord. 2017;27:286–289. [DOI] [PubMed] [Google Scholar]
- 10.Nakamori M, Sobczak K, Puwanant A, et al. Splicing biomarkers of disease severity in myotonic dystrophy. Ann Neurol. 2013;74: 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoury L, Hu N, Balaj L, et al. Analysis of extracellular mRNA in human urine reveals splice variant biomarkers of muscular dystrophies. Nat Commun. 2018;9:3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiba B, Richard N, Hebert LJ, et al. Quantitative assessment of skeletal muscle degeneration in patients with myotonic dystrophy type 1 using MRI. J Magn Reson Imaging. 2012;35:678–685. [DOI] [PubMed] [Google Scholar]
- 13.Serra L, Silvestri G, Petrucci A, et al. Abnormal functional brain connectivity and personality traits in myotonic dystrophy type 1. JAMA Neurol. 2014;71:603–611. [DOI] [PubMed] [Google Scholar]
- 14.Boland-Freitas R, Lee J, Howells J, et al. Sarcolemmal excitability in the myotonic dystrophies. Muscle Nerve. 2018;57:595–602. [DOI] [PubMed] [Google Scholar]
- 15.Esposito F, Ce E, Rampichini S, et al. Electromechanical delay components during skeletal muscle contraction and relaxation in patients with myotonic dystrophy type 1. Neuromuscul Disord. 2016;26:60–72. [DOI] [PubMed] [Google Scholar]
- 16.Bombelli F, Lispi L, Porrini SC, et al. Neuromuscular transmission abnormalities in myotonic dystrophy type 1: a neurophysiological study. Clin Neurol Neurosurg. 2016;150:84–88. [DOI] [PubMed] [Google Scholar]
- 17.Nojszewska M, Lusakowska A, Szmidt-Salkowska E, et al. Peripheral nerve involvement in myotonic dystrophy type 2 - similar or different than in myotonic dystrophy type 1? Neurol Neurochir Pol. 2015;49: 164–170. [DOI] [PubMed] [Google Scholar]
- 18.Hermans MC, Faber CG, Vanhoutte EK, et al. Peripheral neuropathy in myotonic dystrophy type 1. J Peripher Nerv Syst. 2011;16:24–29. [DOI] [PubMed] [Google Scholar]
- 19.Bae JS, Kim OK, Kim SJ, Kim BJ. Abnormalities of nerve conduction studies in myotonic dystrophy type 1: primary involvement of nerves or incidental coexistence? J Clin Neurosci. 2008;15:1120–1124. [DOI] [PubMed] [Google Scholar]
- 20.Takeshima K, Ariyasu H, Ishibashi T, et al. Myotonic dystrophy type 1 with diabetes mellitus, mixed hypogonadism and adrenal insufficiency. Endocrinol Diabetes Metab Case Rep. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol. 1984;349: 249–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng SC, Cole KR, Shaffer MA, Petrie MA, Yen CL, Shields RK. Speed, resistance, and unexpected accelerations modulate feed forward and feedback control during a novel weight bearing task. Gait Posture. 2017;52:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature. 2011;478:387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boerio D, Lefaucheur JP, Bassez G, Hogrel JY. Central and peripheral components of exercise-related fatigability in myotonic dystrophy type 1. Acta Neurol Scand. 2012;125:38–46. [DOI] [PubMed] [Google Scholar]
- 25.Sica RE, Rey RC. Spinal reflex activity in patients with Duchenne, Limb-Girdle and myotonic muscular dystrophies. Medicina (B Aires). 1985;45:501–507. [PubMed] [Google Scholar]
- 26.Knikou M The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008;171:1–12. [DOI] [PubMed] [Google Scholar]
- 27.Shields RK. Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol. 1995;73:2195–2206. [DOI] [PubMed] [Google Scholar]
- 28.Sale D, Quinlan J, Marsh E, McComas AJ, Belanger AY. Influence of joint position on ankle plantarflexion in humans. J Appl Physiol. 1982; 52:1636–1642. [DOI] [PubMed] [Google Scholar]
- 29.Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. [DOI] [PubMed] [Google Scholar]
- 30.Petrie MA, Suneja M, Faidley E, Shields RK. Low force contractions induce fatigue consistent with muscle mRNA expression in people with and without spinal cord injury. Physiol Rep. 2014;2:e00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abode-Iyamah KO, Viljoen SV, McHenry CL, et al. Effect of surgery on gait and sensory motor performance in patients with cervical spondylotic myelopathy. Neurosurgery. 2016;79:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole KR, Shields RK. Age and cognitive stress influences motor skill acquisition, consolidation, and dual-task effect in humans. J Mot Behav. 2019;51:622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madhavan S, Burkart S, Baggett G, et al. Influence of age on neuromuscular control during a dynamic weight-bearing task. J Aging Phys Act. 2009;17:327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madhavan S, Shields RK. Weight-bearing exercise accuracy influences muscle activation strategies of the knee. J Neurol Phys Ther. 2007;31: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madhavan S, Shields RK. Movement accuracy changes muscle-activation strategies in female subjects during a novel single-leg weight-bearing task. PM R. 2009;1:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mrachacz-Kersting N, Grey MJ, Sinkjaer T. Evidence for a supraspinal contribution to the human quadriceps long-latency stretch reflex. Exp Brain Res. 2006;168:529–540. [DOI] [PubMed] [Google Scholar]
- 37.Messina C, Tonali P, Scoppetta C. The lack of deep reflexes in myotonic dystrophy. J Neurol Sci. 1976;30:303–311. [DOI] [PubMed] [Google Scholar]
- 38.Burke D Clinical uses of H reflexes of upper and lower limb muscles. Clin Neurophysiol Pract. 2016;1:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oza PD, Dudley-Javoroski S, Shields RK. Dynamic fatigue does not alter soleus H-reflexes conditioned by homonymous or heteronymous pathways. Motor Control. 2017;21:345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oza PD, Dudley-Javoroski S, Shields RK. Modulation of H-reflex depression with paired-pulse stimulation in healthy active humans. Rehabil Res Pract. 2017;2017:5107097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oza PD, Dudley-Javoroski S, Shields RK. Sustained submaximal contraction yields biphasic modulation of soleus Post-activation depression in healthy humans. Scand J Med Sci Sports. 2019;29:944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolpaw JR, Chen XY. The cerebellum in maintenance of a motor skill: a hierarchy of brain and spinal cord plasticity underlies H-reflex conditioning. Learn Mem. 2006;13:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohn AF, Floeter MK, Hallett M. Presynaptic inhibition compared with homosynaptic depression as an explanation for soleus H-reflex depression in humans. Exp Brain Res. 1997;116:375–380. [DOI] [PubMed] [Google Scholar]
- 44.Shields RK, Dudley-Javoroski S, Oza PD. Low-frequency H-reflex depression in trained human soleus after spinal cord injury. Neurosci Lett. 2011;499:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundbye-Jensen J, Nielsen JB. Immobilization induces changes in presynaptic control of group Ia afferents in healthy humans. J Physiol. 2008;586:4121–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vihola A, Bassez G, Meola G, et al. Histopathological differences of myotonic dystrophy type 1 (DM1) and PROMM/DM2. Neurology. 2003;60:1854–1857. [DOI] [PubMed] [Google Scholar]
- 47.Dillmann U, Hopf HC, Luder G, Schimrigk K. Isometric muscle contractions after double pulse stimulation. comparison of healthy subjects and patients with myotonic dystrophy. Eur J Appl Physiol Occup Physiol. 1996;74:219–226. [DOI] [PubMed] [Google Scholar]
- 48.Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol. 1993;75:382–388. [DOI] [PubMed] [Google Scholar]
- 49.Bakker AJ, Cully TR, Wingate CD, Barclay CJ, Launikonis BS. Doublet stimulation increases Ca(2+) binding to troponin C to ensure rapid force development in skeletal muscle. J Gen Physiol. 2017;149: 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura T, Nakamori M, Lueck JD, et al. Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum Mol Genet. 2005;14:2189–2200. [DOI] [PubMed] [Google Scholar]
- 51.Santoro M, Piacentini R, Masciullo M, et al. Alternative splicing alterations of Ca2+ handling genes are associated with Ca2+ signal dys-regulation in myotonic dystrophy type 1 (DM1) and type 2 (DM2) myotubes. Neuropathol Appl Neurobiol. 2014;40:464–476. [DOI] [PubMed] [Google Scholar]
- 52.Tang ZZ, Yarotskyy V, Wei L, et al. Muscle weakness in myotonic dystrophy associated with misregulated splicing and altered gating of Ca(V)1.1 calcium channel. Hum Mol Genet. 2012;21:1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JS, Seo J, Cha H, et al. Altered power spectral density in the resting-state sensorimotor network in patients with myotonic dystrophy type 1. Sci Rep. 2018;8:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park JS, Song H, Jang KE, et al. Diffusion tensor imaging and voxel-based morphometry reveal corticospinal tract involvement in the motor dysfunction of adult-onset myotonic dystrophy type 1. Sci Rep. 2018;8:15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


