Abstract
Parkinson’s disease (PD) is a systemic brain disorder where the cortical cholinergic network begins to degenerate early in the disease process. Readily accessible, quantitative, and specific behavioral markers of the cortical cholinergic network are lacking. Although degeneration of the dopaminergic network may be responsible for deficits in cardinal motor signs, the control of gait is a complex process and control of higher-order aspects of gait, such as gait variability, may be influenced by cognitive processes attributed to cholinergic networks. We investigated whether swing time variability, a metric of gait variability that is independent from gait speed, was a quantitative behavioral marker of cortical cholinergic network integrity in PD.
Twenty-two individuals with PD and subthalamic nucleus (STN) deep brain stimulation (PD-DBS cohort) and twenty-nine age-matched controls performed a validated stepping-in-place (SIP) task to assess swing time variability off all therapy. The PD-DBS cohort underwent structural MRI scans to measure gray matter volume of the Nucleus Basalis of Meynert (NBM), the key node in the cortical cholinergic network. In order to determine the role of the dopaminergic system on swing time variability, it was measured ON and OFF STN DBS in the PD-DBS cohort, and on and off dopaminergic medication in a second PD cohort of thirty-two individuals (PD-med). A subset of eleven individuals in the PD-DBS cohort completed the SIP task again off all therapy after three years of continuous DBS to assess progression of gait impairment.
Swing time variability was significantly greater (i.e., worse) in PD compared to controls and greater swing time variability was related to greater atrophy of the NBM, as was gait speed. STN DBS significantly improved cardinal motor signs and gait speed but did not improve swing time variability, which was replicated in the second cohort using dopaminergic medication. Swing time variability continued to worsen in PD, off therapy, after three years of continuous STN DBS, and NBM atrophy showed a trend for predicting the degree of increase. In contrast, cardinal motor signs did not progress. These results demonstrate that swing time variability is a reliable marker of cortical cholinergic health, and support a framework in which higher-order aspects of gait control in PD are reliant on the cortical cholinergic system, in contrast to other motor aspects of PD that rely on the dopaminergic network.
Keywords: Gait, Nucleus Basalis of Meynert, Parkinson’s disease, Cholinergic, Deep brain stimulation, Basal forebrain, Acetylcholine, Dopaminergic, Gait variability
1. Introduction
Neuronal degeneration and Lewy body formation manifest in the basal forebrain early on in the progression of Parkinson’s disease (PD), specifically in the Nucleus Basalis of Meynert (NBM) (Nakano and Hirano, 1984; Rogers et al., 1985). Close to 90% of cell makeup of the NBM is cholinergic and it serves as the primary source of cholinergic innervation to the cortex (Mesulam et al., 1983; Mesulam, 2013). Atrophy of the NBM leads to major reductions in cortical acetylcholine (ACh) levels (Johnston et al., 1979; Mufson et al., 1986), and has been associated with a wide range of cognitive deficits in PD, as well as with the worsening of cognition over time (Barrett et al., 2019; Gang et al., 2020; Pereira et al., 2020; Ray et al., 2018; Schulz et al., 2018). Medications to enhance cortical ACh are usually only implemented in later stages of PD when the cortical cholinergic tone may be so low that these are largely ineffective. This missed therapeutic strategy may be partly due to the lack of behavioral biomarkers of cortical cholinergic integrity.
Gait impairment and freezing of gait (FOG) affect nearly 75% of individuals with advanced PD (Panisset, 2004). Gait variability is associated with FOG and both have been linked to deficits in attention switching, visuospatial processing, and aspects of executive function (Amboni et al., 2008, 2013; Nantel et al., 2012; Yao et al., 2017), which have all been shown to be influenced by the cortical cholinergic system (Ballinger et al., 2016; Bohnen et al., 2006; Gratton et al., 2017; Klinkenberg et al., 2011). This suggests that the NBM-cortical cholinergic system may pay a role in higher-order aspects of gait control, such as gait variability, in PD but direct evidence for this is lacking. There is evidence that the cholinergic system may be related to gait speed in PD (Bohnen et al., 2013; Rochester et al., 2012). However, gait speed is also linked to dopaminergic networks since it and other lower order aspects of gait control such as step amplitude are consistently improved by dopaminergic medical therapy and subthalamic nucleus (STN) deep brain stimulation (DBS) (Blin et al., 1991; Roper et al., 2016; Yttri and Dudman, 2018). In contrast higher-order aspects of gait control, such as gait variability, tend to be more resistant to dopaminergic treatment, which may explain the often observed progressive decline in gait variability in PD since current therapies do not address deficits related to the cholinergic system (Blin et al., 1991; Smulders et al., 2016).
The goal of this study was to determine whether higher order aspects of gait, such as gait variability, may be useful markers of the health of the cortical cholinergic network in PD. We questioned whether the structural integrity of the NBM was related to the degree and longitudinal progression of gait variability in PD. We specifically chose swing time variability as our primary measure of gait variability since it is a metric that is independent from gait speed, unlike stride and step time variability (Beauchet et al., 2009; Callisaya et al., 2010; Frenkel-Toledo et al., 2005). Additionally, we looked at speed and step time variability as secondary gait measures, which have previously been linked to the cortical cholinergic system (Bohnen et al., 2013; Henderson et al., 2016; Rochester et al., 2012). We used a validated stepping-in-place task in a harnessed environment to provide both a safe environment for stepping and to minimize the role of optic flow in order to highlight the potential higher-order contributions to gait variability (Nantel et al., 2011). As NBM atrophy occurs early in PD, we hypothesized that swing time variability would be greater in moderate stage PD compared to age-matched controls. We hypothesized that atrophy of the NBM would be associated with greater impairment of swing time variability and that this relationship would not be seen in a size-matched portion of the visual cortex. Additionally, we hypothesized that dopaminergic therapy, either in the form of STN DBS or dopaminergic medication, would improve cardinal motor signs and gait speed which are reliant on the dopaminergic system, but would not improve swing time variability due to its reliance on the cortical cholinergic system. If these hypotheses were supported, we anticipated that swing time variability would continue to decline over time despite successful, continuous STN DBS, and we investigated whether the degree of decline would be related to the amount of atrophy of the NBM present at the pre-operative timepoint. Meanwhile, we hypothesized that cardinal motor signs would be slower in their decline due to the efficacy of STN DBS in targeting the dopaminergic system.
2. Materials and methods
2.1. Human subjects
The first cohort, the PD-DBS cohort, comprised twenty-three individuals (16 males, 7 females) with clinically established Parkinson’s disease, who underwent bilateral implantation of DBS leads (model 3389, Medtronic., Inc) in the sensorimotor region of the STN, and implantation of an investigative sensing neurostimulator (Activa™ PC + S, Medtronic PLC, FDA Investigational Device Exemption (IDE) approved). Description of the preoperative selection criteria and surgical technique are provided in the supplementary materials, and included confirmation of no significant cognitive deficits. Post-operative CT scans were acquired as part of the standard clinical protocol (Brontë-Stewart et al., 2010). A second cohort, the PD-med cohort, comprised thirty-two individuals with PD (21 males, 11 females) took part in the study to test the effects of dopaminergic medication on gait. All off-therapy experimental testing was done in the off-medication state for the PD cohorts in which long- and short-acting medications were withdrawn over 24 to 48 and 12 h, respectively, and DBS therapy was turned off for at least 15 min before testing began (Trager et al., 2016). No participants were taking cholinergic medications. A third cohort comprised twenty-nine age-matched controls (16 males, 13 females). All participants gave written informed consent to participate in the study, which was approved by the Stanford University Institutional Review Board.
2.2. Experimental protocols
Quantitative measures of gait were captured using the stepping-in-place (SIP) task, which is a validated metric of gait impairment and freezing (Nantel et al., 2011) (Fig. 1A). In the SIP task, participants completed 90 s of alternating, self-paced stepping on dual force plates while harnessed, Fig. 1A and B. Age-matched controls completed only the SIP task at their visit. The PD-DBS cohort completed the SIP task off all therapy at their Initial Programming (IP) visit, prior to activation of the DBS system, and ON and OFF STN DBS on the same day at a timepoint within the first three years after the IP visit. A subset of the PD-DBS cohort performed the SIP task off all therapy after three years of continuous DBS. The PD-med cohort performed the task on and off their usual dopaminergic medication on two separate days. At each timepoint, clinical motor impairment was assessed using part III of the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS III) (Goetz et al., 2007).
Fig. 1.
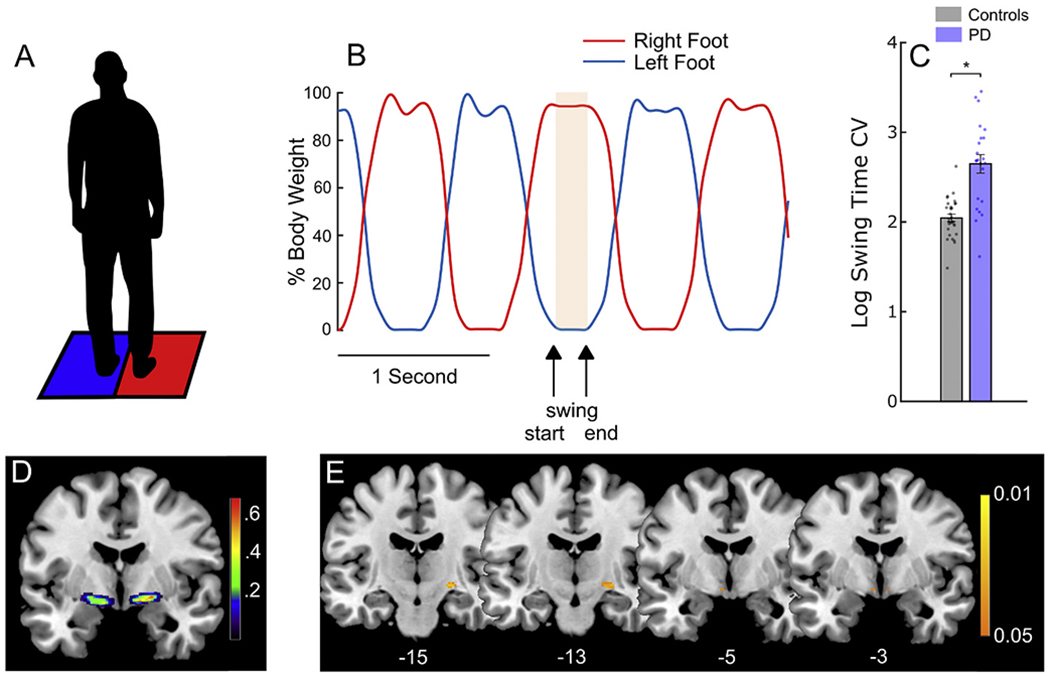
NBM atrophy was associated with greater swing time variability. (A) Depiction of the stepping-in-place (SIP) task in which the patient alternately steps on two force plates. (B) A representative trace from the SIP task showing the stepping cycles. The red line represents the stepping trace of the right foot and the blue line represents that of the left foot. The highlighted portion represents the period of time when the left foot was in the swing phase: from when the foot left the force plate to the point when it returned (arrows). The swing time coefficient of variation (standard deviation/mean) was calculated as a measure of gait variability. (C) Comparison of swing time variability between age-matched controls and the PD cohort during the SIP task. Individuals with PD showed significantly greater (i.e., worse) swing time variability. (D) Probabilistic mask of the Nucleus Basalis of Meynert (NBM) in MNI space as determined previously by Zaborszky et al., 2008. The colour bar represents the probability that the voxel was identified as NBM in that cohort. (E) Voxels showing a significant negative correlation between NBM gray matter volume and swing time variability on the SIP task at Initial Programming (IP). Individuals with lower gray matter volume in the NBM show greater (i.e., worse) swing time variability. The colour bar represents p-values (FWE-corrected). MNI coordinates of each slice are shown at the bottom. Error bars represent SEM. * indicates significant differences. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3. Kinetic data acquisition and analysis
During the SIP task, ground reaction forces were captured at either 100 or 1000 Hz, using the Smart Equitest or Bertec systems, respectively (Neurocom Inc., Clackamas, OR, USA; Bertec Corporation, Columbus, OH, USA). Swing time was defined as the duration between when one foot left the force plate and when the same foot contacted the force plate again, Fig. 1B. Swing time variability was defined as the mean swing time coefficient of variation (CV = standard deviation/mean) and was averaged between legs. A larger swing time CV is indicative of more variable gait and has been shown to be independent of gait speed and shows high reliability (Arcolin et al., 2019; Frenkel-Toledo et al., 2005). Stride time, the time between initial contact of two consecutive steps of the same foot, was measured as a surrogate for gait speed since there is no forward motion component in the SIP task. Step time CV, the variability of the time between initial contact of one foot to initial contact with the opposite foot, was calculated as a secondary measure of gait variability. Data were log transformed to conform to normality. A previously validated computerized algorithm was used to identify freezing episodes (Nantel et al., 2011) and swing time variability was calculated during non-freezing stepping.
2.4. MRI acquisition and analysis
Pre-operative MRI scans were acquired in the PD-DBS cohort as part of the standard clinical protocol previously described (Brontë-Stewart et al., 2010). Tl-structural scans were performed at Stanford Hospital and Clinics on a 3 T Discovery MR750 scanner with a 32-channel head coil. The scans had an isotropic voxel resolution of 1x1x1 mm3 (TR = 8.24 ms, TE = 3.24 ms, FOV = 240x240mm2).
2.5. MRI preprocessing
Voxel-based morphometry was applied to T1 images using SPM12 software (Statistical Parametric Mapping 12; Wellcome Trust Centre for Neuroimaging, UCL, London, UK). Images were automatically segmented into gray matter, white matter, and cerebrospinal fluid with 1.5 mm3 resolution. The extracted gray and white matter were non-linearly registered to Montreal Neurological Institute (MNI) space using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra algorithm (DARTEL). The gray matter was then warped using the individual flow fields resulting from the DARTEL registration, and voxel values were modulated for volumetric changed introduced by the high dimensional normalization to ensure that the total amount of gray matter volume was preserved. Images were then smoothed (FWHM: 4 mm) to account for anatomical variability across individuals and potential misregistrations to facilitate statistical comparison. The NBM was identified and masked using probabilistic anatomical maps available in the Anatomy Toolbox. These maps were created from histological post-mortem analysis of 10 brains (Zaborszky et al., 2008). A size-matched portion of the visual cortex was used as a control region of interest (Supplementary Fig. S1).
2.6. Localization of DBS leads
Locations of DBS leads were determined by the Lead-DBS toolbox (Horn et al., 2019) based on preoperative T1 and T2 MRIs and post-operative CT scans. Postoperative CT scans and preoperative T2 scans were co-registered to preoperative T1 scans, which were then normalized into MNI space using SPM12 and Advanced Normalization Tools (Avants et al., 2008). DBS electrode localizations were corrected for brain-shift in the postoperative CT scan (Horn and Kühn, 2015). DBS electrodes were then localized in template space using the PaCER algorithm (Husch et al., 2018) and projected onto the DISTAL Atlas to visualize overlap with the STN (Ewert et al., 2018).
2.7. Statistical analysis
A Two-sample t-test was used to compare swing time variability on the SIP task in controls compared to the PD-DBS cohort at the IP visit. Voxel-wise statistics for associations between swing time variability in the SIP task in the PD-DBS cohort and gray matter volume within the NBM were computed using a general linear model in SPM12. Total intracranial volume (i.e., total white matter, gray matter, and CSF) was calculated for each individual as a global normalization to deal with brains of different sizes. A multiple regression model was used with threshold-free cluster enhancement (doi: https://doi.org/10.5281/zenodo.2641381) to assess any association between gray matter volume of the NBM and swing time variability in the SIP task, considering age, sex, and disease duration as covariates and using a TFCE extent value of 0.5 and height of 2.0, as used previously (Smith and Nichols, 2009). P-values for the TFCE values were obtained based on 5000 permutations. Significance was set at p < 0.05 (family-wise error corrected; FWE) with a cluster threshold of 10 voxels. The same test was run for a size-matched region of the visual cortex (Supplementary Fig. S1). In addition to the main outcome of swing time variability, multiple regression models were also run for stride time (e.g., speed) and step time CV. Paired t-tests were used to compare the effect of DBS/meds (ON vs. OFF) on swing time variability on the SIP task for the two PD cohorts, as well as change in UPDRS scores. Paired t-tests were also used to compare changes in swing time variability and UPDRS from IP to 3 years later in the PD-DBS cohort. Significance was set at p < 0.05 for the t-tests. Finally, a second general linear model was computed for the percent change in swing time variability from IP to 3 years later and gray matter volume within the NBM, as well as for the secondary outcomes stride time and step time CV, and the control region in the visual cortex.
3. Results
3.1. Demographic characteristics
Demographic information for the PD-DBS cohort is shown in Table 1 and for the control and PD-med cohort in Supplementary Table 1. The PD-DBS cohort had a mean pre-op MDS-UPDRS III score of 42.5 ± 11.9 off medication, whereas the PD-med cohort had a mean score of 20.6 ± 11.3 off medication. Both the PD-DBS cohort (age: 59.2 ± 10.1 yrs) and the PD-med cohort (age: 65.7 ± 7.3 yrs) were age-matched to the control cohort (age: 62.5 ± 8.0 yrs).
Table 1.
DBS Patient Demographics
| Patient | Age (Years) | Sex | Time Since Diagnosis (Years) | Pre-Op MDS-UPDRS III (off meds) | Pre-Op MDS-UPDRS III (on meds) | MDS-UPDRS III (ON STN DBS, off meds) | LEDD (mg) |
|---|---|---|---|---|---|---|---|
| 1* | 72.9 | M | 9.9 | 54 | 41 | 24 | 1098 |
| 2** | 52.8 | M | 4.9 | 24 | 8 | 5 | 1314 |
| 3** | 62.5 | M | 3.0 | 35 | 26 | 11 | 400 |
| 4** | 65.7 | M | 5.2 | 29 | 16 | 12 | 500 |
| 5** | 58.1 | M | 3.4 | 39 | 23 | 19 | 1923 |
| 6** | 42.2 | M | 4.5 | 58 | 12 | 9 | 1200 |
| 7* | 69.0 | M | 10.6 | 41 | 28 | 14 | 1116 |
| 8** | 53.5 | M | 7.6 | 52 | 29 | 17 | 2405 |
| 9 | 73.1 | F | 8.8 | 37 | 22 | 18 | 1588 |
| 10** | 72.2 | M | 8.9 | 47 | 23 | 29 | 750 |
| 11** | 58.6 | F | 10.0 | 33 | 6 | 18 | 1000 |
| 12* | 54.5 | M | 11.0 | 56 | 20 | 20 | 1710 |
| 13 | 34.3 | M | 2.1 | 59 | 22 | 35 | 950 |
| 14* | 57.3 | M | 6.1 | 62 | 23 | 19 | 1075 |
| 15** | 67.6 | F | 6.1 | 44 | 25 | 2 | 825 |
| 16* | 50.6 | F | 9.2 | 50 | 26 | 14 | 920 |
| 17* | 61.9 | M | 14.3 | 42 | 24 | 8 | 1200 |
| 18** | 52.0 | F | 12.5 | 34 | 6 | 6 | 650 |
| 19 | 66.1 | M | 7.6 | 51 | 10 | 16 | 875 |
| 20** | 56.8 | F | 11.6 | 38 | 18 | 8 | 1125 |
| 21 | 75.7 | F | 8.5 | 44 | 19 | 23 | 800 |
| 22* | 55.0 | M | 10.4 | 11 | 7 | 2 | 917 |
| 23* | 50.1 | M | 10.2 | 38 | 31 | 17 | 375 |
|
| |||||||
| Average ± SD | 59.2 ± 10.1 | 8.1 ± 3.2 | 42.5 ± 11.9 | 20.2 ± 8.7 | 15.0 ± 8.1 | 1074.6 ± 479.5 | |
indicates that the participant performed the SIP ON DBS.
indicates that the participant performed the SIP ON DBS and completed the three-year follow-up visit. LEDD: Levodopa Equivalent daily dose
3.2. NBM atrophy was associated with greater swing time variability and increased stride time
Of the PD-DBS cohort of twenty-three individuals, one was excluded due to movement artifacts in their T1 scan. For the remaining twenty-two individuals, we compared swing time variability in the SIP task at the Initial Programming (IP) visit with age-matched controls. The PD-DBS cohort showed significantly greater swing time variability compared to controls, Fig. 1C (t(49) = 5.96, p = 2.69e-7).
We compared swing time variability, stride time, and step time variability in the PD-DBS cohort with NBM gray matter volume, Fig. 1D. The IP visit occurred 63.9 ± 31.9 days after the MRI scan. Lower gray matter volume was significantly associated with greater swing time variability (Fig 1E) and increased stride time (Supplementary Fig. S2) (p < 0.05; FWE corrected for both). Information on each significant cluster is shown in Table 2.
Table 2.
Correlations between NBM Gray Matter Volume and Swing Time Variability and Stride Time at Initial Programming
| Gait Metric | MNI Coordinates | Cluster Size (Voxels) | TFCE Value | p value* |
|---|---|---|---|---|
| Swing Time Variability | x = 23, y = −15, z = −6 | 31 voxels | 173 | p = 0.02 |
| Stride Time | x = 23, y = −12, z = −8 | 47 voxels | 187 | p = 0.013 |
Family-wise error (FWE) corrected
There was no association for step variability or positive correlations for any gait metric, or for the control region of interest in the visual cortex. Scatter plots comparing NBM volume and each gait metric confirmed the voxel relationship and are shown in Supplementary Fig. S3. Of note, swing time variability was independent of stride time, whereas stride time and step variability were correlated (r = 0.46, p = 0.01), confirming that swing time variability is a measure of gait variability that is independent from gait speed.
3.3. Swing time variability was resistant to STN DBS
Nineteen individuals from the PD-DBS cohort were assessed with the MDS-UPDRS III and completed the SIP task OFF and ON STN DBS. Fig. 2A demonstrates that the STN DBS leads were located within the STN for all individuals.
Fig. 2.

STN DBS improved cardinal motor signs but did not improve swing time variability. (A) DBS electrode lead placement in the STN (orange) for 19 participants. (B) Comparison of total MDS-UPDRS III and (C) its sub-scores OFF (blue) and ON (pink) DBS. STN DBS reduced total MDS-UPDRS-III and all of its sub-scores. (D) Comparison of swing time variability during the SIP task OFF and ON DBS. STN DBS did not improve swing time variability. Error bars represent SEM. * indicates significant differences. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
All cardinal motor signs improved ON compared to OFF STN DBS, as shown by the reduced total MDS-UPDRS III scores, Fig. 2B (t(22) = 9.64, p = 2.33e-9) and all of its sub-scores, Fig. 2C, which included axial (t(22) = 5.00, p = 5.27e-5), tremor (t(22) = 5.46, p = 1.75e-5), rigidity (t(22) = 6.38. p = 2.01e-6, and bradykinesia (t(22) = 6.61, p = 1.21e-6). Of the gait metrics, stride time improved in the SIP task ON compared to OFF DBS (t(18) = 2.37, p = 0.029) but swing time variability did not improve, Fig. 2D (t(18) = 0.89, p = 0.39).
3.4. Medication did not improve swing time variability
The lack of improvement in swing time variability with dopaminergic therapy was confirmed in the PD-med cohort. Swing time variability did not improve on medication compared to off medication (t(31) = 0.74, p = 0.47), and was still worse on medication than that of age-matched controls (t(59) = 2.10, p = 0.04), Supplementary Fig. S4.
3.5. NBM atrophy showed a trend for predicting the degree of increase in swing time variability over three years
After three years of continuous STN DBS, a subset of eleven individuals in the PD-DBS cohort were assessed off all therapy. A portion of a SIP trace from a representative individual at IP and at the three-year timepoint is depicted in Fig. 3A and B.
Fig. 3.

Swing time variability increased over time, off therapy, and the degree of increase was related to initial levels of NBM atrophy. (A) Example of a kinetic trace during the stepping-in-place (SIP) task at Initial Programming (IP) and (B) three years later in the same individual. (C) Comparison of swing time variability during the SIP task at IP (blue) and 3 years (pink). Swing time variability significantly increased (i.e., worsened) over time. (D) Voxels showing a negative correlation between gray matter volume and percent change in swing time variability on the SIP task from IP to 3 years later. Individuals with lower gray matter volume showed a trend of greater increases in swing time variability over time. The colour bar represents p-values (uncorrected). MNI coordinates of each slice are shown at the bottom. Error bars represent SEM. * indicates significant differences. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
This individual demonstrated more variable stepping at the 3-year timepoint compared to their IP visit. The demonstration of gait deterioration in this individual was supported by the group statistic demonstrating that swing time variability during non-freezing stepping significantly increased (worsened), over time, Fig. 3C (t(10) = 4.42, p = 0.001). Stride time (e.g., speed) also decreased over time (t(10) = 2.62, p = 0.025) but to a lesser extent than swing time variability.
A cluster within the NBM where lower gray matter volume showed a trend towards an association with greater increase of swing time variability from IP to three years, Fig. 3D (p = 0.001; uncorrected), but that did not survive FWE correction. Information on this cluster is shown in Table 3. Meanwhile, there was no association for either stride time or step variability or positive correlation for any gait metric, or for the control region of interest in the visual cortex. Scatter plots comparing NBM volume and the percent change over time of each gait metric are shown in Supplementary Fig. S5.
Table 3.
Correlation between NBM Gray Matter Volume and Worsening of Swing Time Variability at 3 Years
| MNI Coordinates | Cluster Size (Voxels) | TFCE Value | p Value* |
|---|---|---|---|
| x = 24, y = −2, z = −6 | 17 voxels | 229 | p = 0.001 |
Uncorrected
3.6. Continuous DBS stalled progression of cardinal motor signs measured off therapy
In contrast to the worsening of swing time variability over time, there was no change in MDS-UPDRS III, off therapy, between IP and the three-year timepoint, Fig. 4A (t(10) = 0.36, p = 0.73). This held true for all sub-scores, Fig. 4B, including axial (t(10) = 0.54, p = 0.60), tremor (t(10) = 1.31, p = 0.22), rigidity (t(10) = 1.27, p = 0.23), and bradykinesia (t(10) = 0.89, p = 0.40) indicating that there was no observable decline in cardinal motor signs over time, off therapy, after three years of continuous STN DBS.
Fig. 4.

Cardinal motor signs did not worsen over time, measured off therapy, after three years of continuous STN DBS. (A) Total MDS-UPDRS III and (B) its sub-scores at IP (blue) and 3 years later (pink) tested off of all therapy. Error bars represent SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The current study investigated the relationship between gray matter volume of the Nucleus Basalis of Meynert (NBM), the central node in the cortical cholinergic system, and gait impairment in individuals with PD through a combination of structural imaging, quantitative gait analysis, and dopaminergic therapy. We demonstrated, for the first time, that atrophy of the NBM was associated with greater swing time variability in individuals with PD, and confirmed its role in gait speed. STN DBS, which targets the degenerating dopaminergic system in PD, did not improve deficits in swing time variability despite overall improvements in tremor, rigidity, bradykinesia, axial motor function and gait speed. This was further confirmed using dopaminergic medication in a second separate cohort. It was evident that, off therapy, swing time variability continued to worsen over three years, and the amount of atrophy of NBM, measured pre-operatively, showed a trend towards predicting the degree of worsening of swing time variability. In contrast, there was no progression of cardinal motor signs, off therapy, after three years of continuous STN DBS. Together, these results point towards a critical role of the cortical cholinergic system in higher order aspects of gait control, such as gait variability in PD, and give insight into the limitations of STN DBS and dopaminergic medication in ameliorating specific domains of gait impairment.
4.1. The influence of the cholinergic system on gait variability
Gait variability is a metric that requires higher-order control and differentiates PD from healthy controls. Although both healthy controls and individuals with PD will decrease speed in response to a demanding dual task, only PD show increases in gait variability (Yogev et al., 2005). This suggests that individuals with PD show deficits in the automaticity of gait, and increasingly rely on cognitive-related functions to compensate. Additionally, greater gait variability during walking is associated with greater sensorimotor activity, suggesting a role of top-down processing (Berger et al., 2019; Kurz et al., 2012). The stepping-in-place task used here, may be especially effective at highlighting the higher-order contributions to gait variability considering optic flow is minimized, so sensory-related guidance of stepping is more limited compared to forward walking. It has been shown that increased gait variability is associated with impairment in certain cognitive domains in PD, some of which may be related to the cholinergic system (Amboni et al., 2008; Morris et al., 2017; Nantel et al., 2012; Yao et al., 2017). However, there has been no link between gait variability and the cholinergic system until now, despite theoretical predictions (Morris et al., 2019). The results of this study demonstrate for the first time that atrophy of the NBM, the key node in the cortical cholinergic network, is related to greater (i.e., worse) swing time variability in PD, thus providing evidence that the cholinergic system plays a significant role in the underlying mechanism contributing to impairment of higher-order aspects of gait.
The observed relationship between the cholinergic system and swing time variability continues to build on the evidence that PD is not merely a hypodopaminergic disease, but rather a multisystem neurodegenerative disorder (Bohnen et al., 2013). This points to the reliance of higher-order aspects of gait (stepping control) on the cholinergic system, possibly through its effects on attention and other cognitive processes that may become increasingly critical as the automaticity of gait becomes more impaired. Previously, it has been shown that levels of cortical ACh were related to gait speed during forward walking in PD (Bohnen et al., 2013; Rochester et al., 2012). Our current results support and build on this finding, as we found that the structure of NBM was related to gait speed. However, gait speed is also intertwined with the dopaminergic network and we confirmed previous reports that STN DBS improved gait speed (Ferrarin et al., 2005; Roper et al., 2016; Stolze et al., 2001). Swing time variability, a gait variability metric independent of gait speed, was related to the integrity of the NBM and was resistant to dopaminergic therapy from DBS or medication, suggesting that it may be a better marker of cortical cholinergic integrity. We propose that a compromised cholinergic system negatively impacts higher-order aspects of gait control. This idea is supported by pharmacological evidence showing that administration of acetylcholinesterase inhibitors improved both attention (Wesnes et al., 2005) and step variability in PD (Henderson et al., 2016). Within this framework, the cholinergic system appears to be the common denominator in the interplay between higher order control of gait and cognition, leaving both processes susceptible to decline in PD as the NBM deteriorates.
Swing time variability is not the only metric of gait variability. Step time, step length, and stride time variability are also all different accepted metrics of gait variability and may also be related to the cholinergic system (Henderson et al., 2016). However, swing time variability is the only speed-independent measure of gait variability of this group (Beauchet et al., 2009; Callisaya et al., 2010; Frenkel-Toledo et al., 2005). We chose to use this as our primary measure of gait variability in order to limit potential confounds of the effect of DBS or natural aging on gait variability due to changes in speed and better separate from speed-related contributions of the dopaminergic system. There was no significant association of step time variability with NBM integrity that survived controlling for age, sex, and disease duration.
Although the NBM is the key node in the cortical cholinergic system, the pedunculopontine nucleus (PPN) provides cholinergic input at the level of the brainstem and the thalamus and is implicated in gait impairment in PD (Craig et al., 2020; Jenkinson et al., 2009; Lau et al., 2015; Thevathasan et al., 2018). Impaired cholinergic integrity of the PPN is strongly related to falls and posture (Müller et al., 2013), and its position in the brainstem allows it to serve as a final outflow block influencing gait (Lewis and Shine, 2016). Whereas the PPN modulates bottom-up processing (Kim et al., 2017), the cortical cholinergic system strongly correlates with cognitive deficits (Bohnen et al., 2012, 2015) and other higher-order top-down processes (Kim et al., 2019). The observed results are not incongruous with a significant role for the PPN in aspects of gait control as it is also possible that the two systems interact at the level of the thalamus or brainstem to impact aspects of gait and postural control (Hallanger et al., 1987; Parent et al., 1988).
4.2. Differentiating the networks involved in cardinal motor signs and gait variability in PD with STN DBS and dopaminergic medication
STN DBS and dopaminergic medications improved the cardinal motor signs of PD but did not improve swing time variability in two separate cohorts. These results shed insight into the inconsistency of the efficacy of STN DBS and dopaminergic medication on different metrics of gait variability and swing time asymmetry (Anidi et al., 2018; Blin et al., 1991; Bryant et al., 2011; Curtze et al., 2015; Lord et al., 2011; Rochester et al., 2017). Gait is a highly complex process that encompasses motor and non-motor mechanisms, including executive function, attention, and visuospatial processing (Amboni et al., 2013). STN DBS and dopaminergic medication may successfully address certain motor aspects of gait such as gait speed in PD by targeting the basal ganglia dopaminergic network; however, it would not be expected to address aspects of gait control that rely more heavily on other networks. The lack of improvement in swing time variability from neuromodulation of dopaminergic networks, demonstrates that the control of higher order or non-motor aspects of gait is likely strongly influenced by other networks.
4.3. Long-term progression of gait variability and cardinal motor signs
Swing time variability, measured after withdrawal of STN DBS, continued to worsen over three years, and to a greater extent than gait speed, whereas cardinal motor signs off therapy did not decline. Although this result does not specifically implicate the cholinergic system, it provides further evidence that the worsening of swing time variability over time is a manifestation of progressing neuropathology in non-dopaminergic networks. It is unlikely that the observed worsening of swing time variability in the current study is merely due to aging as previous findings in a cohort of older healthy adults showed no worsening of swing time variability, as measured by standard deviation, over a three year period (Rochester et al., 2017), while we observed a 17% worsening in our PD cohort.
Continuous STN DBS appeared to alter the expected decline of cardinal motor signs, measured off all therapy, over three years. This finding builds on previous work from our lab that continuous neuromodulation of the sensorimotor network via STN DBS may alter the progression of cardinal motor signs (Trager et al., 2016). Any disease-modifying effect of neuromodulation of the dopaminergic network would not extend to the cholinergic network and therefore would not modify the progression of higher-order aspects of gait reliant on cholinergic function.
4.4. NBM atrophy may predict worsening of gait variability over time
The degree of atrophy of the NBM showed a trend towards predicting the increase in (worsening of) swing time variability three years after initial evaluation, but not the other gait metrics in a small subset of the PD-DBS cohort. It has been shown that atrophy of the NBM and its microstructure predicted cognitive decline several years later in PD (Pereira et al., 2020; Ray et al., 2018; Schulz et al., 2018). Considering that NBM degeneration occurs early on in the disease process in PD (Braak et al., 2003; Shimada et al., 2009), there is a growing need to find ways of intervening before any accumulating effects start significantly impacting behavior. The link between NBM, cognition, and gait may point towards a potential role for gait variability as a readily accessible metric that will serve as a “canary in the coal mine” to signal impairment in cortical cholinergic networks and to serve as a measure of efficacy of novel therapeutic interventions. Such a metric could be crucial to allow for identification of patients who may benefit from early interventions to prevent consequences of increasing gait variability, such as freezing of gait and falls (Morris et al., 2019).
4.5. Limitations
The number of participants for the IP to 3-year comparison was small. This cohort was limited by the fact that these individuals were part of a larger trial using an investigative sensing neurostimulator (Activa™ PC + S, Medtronic PLC, FDA Investigational Device Exemption (IDE) approved). This is the largest cohort of individuals with this stimulator at any individual site in the world. The cohort examined here also had a relatively long disease duration at the time of their MRI scan (8.1 ± 3.2 years). It is unclear whether individuals with PD earlier in their disease state would demonstrate similar NBM atrophy. However, it is significant that atrophy was already present despite no clear cognitive deficits during the DBS preoperative evaluation. Another possible limitation is that different Parkinsonian signs may return at different rates after discontinuing STN DBS. For instance, axial symptoms return more gradually than tremor (Temperli et al., 2003). During the DBS visit and at the three-year follow-up, DBS was turned off at least 15 min prior to testing; we have shown that beta band power, a neural biomarker of hypokinetic aspects of PD, returned to baseline within one minute after DBS was turned off (Trager et al., 2016).
The SIP task provides a safe environment for assessment of gait due to the presence of a harness, has been validated as a measure of gait impairment in PD, and minimizes the role of visual feedback. One drawback is that one cannot directly measure speed or step length, which have both been associated with the cholinergic system, since there is no forward motion component to the task. However, the goal of this study was to measure a marker of gait variability independent of speed to better separate from possible dopaminergic contributions. Another limitation is that the analyses were restricted to a region of interest analysis rather than a whole-brain analysis due to the small size of the NBM. However, this was an a priori decision based on previous findings for the NBM’s role in cognitive decline in PD. We found no associations between swing time variability and our control regions of interest, a size-matched region of the visual cortex. Finally, there was no follow-up MRI to track the change in NBM atrophy alongside the decline in swing time variability or MRIs for the age-matched control cohort. Future studies should combine both quantitative metrics of gait with longitudinal MRIs to further investigate the predictive power of the NBM for gait impairment and vice versa. Additionally, the effect of acetylcholinesterase inhibitors on swing time variability could be used in the future to confirm the role of the cholinergic system on gait variability.
5. Conclusions
This study demonstrated that deficits in swing time variability, a measure of gait variability that is independent of gait speed, in PD were related to a degenerating cortical cholinergic system: greater swing time variability was associated with lower gray matter volume in the NBM, the key node in the cortical cholinergic network. Swing time variability was greater in the PD cohort compared to a group of age-matched controls and was not associated with atrophy of the visual cortex. Neuromodulation of the dopaminergic basal ganglia-cortical network, via STN DBS, improved cardinal motor signs of tremor, rigidity, bradykinesia, and axial motor function, but did not improve swing time variability, which was further confirmed in a second cohort using dopaminergic medication. Furthermore, swing time variability continued to decline over three years of continuous STN DBS, and a subset analysis showed that pre-operative NBM atrophy tended to be associated with worsening swing time variability. In contrast, after three years of continuous STN DBS, cardinal motor signs measured off therapy were no different from those prior to activation of the DBS system. The above results support a framework in which higher-order aspects of gait control may be reliant on cortical cholinergic processing. Swing time variability is a readily accessible measure of impairment that could provide insight into the health of cortical cholinergic networks in PD and could identify individuals, who may benefit from early interventions to prevent the devastating behavioral manifestations of the degeneration of the cortical cholinergic system over the course of PD.
Supplementary Material
Acknowledgements
We would like to thank Dr. Jaimie Henderson, the members of the Human Motor Control and Neuromodulation lab, Varsha Prabhakar and Talora Martin for their help in collecting the control data, and, most importantly, the participants who dedicated their time to this study.
Funding
This work was supported in part by the following: Parkinson’s Foundation-Postdoctoral Fellowship (PF-FBS-2024), NINDS UH3NS107709, NINDS R21 NS096398-02, Michael J. Fox Foundation (9605), Robert and Ruth Halperin Foundation, John A. Blume Foundation, Smart Family Foundation, John E Cahill Family Foundation, and Medtronic Inc. who provided the devices used in this study but no additional financial support.
Abbreviations:
- ACh
Acetylcholine
- CV
Coefficient of Variation
- DBS
Deep brain stimulation
- FWE
Family-wise error
- IP
Initial Programming
- MDS-UPDRS
Movement Disorders Society Unified Parkinson’s Disease Rating Scale
- NBM
Nucleus Basalis of Meynert
- PD
Parkinson’s Disease
- PPN
Pedunculopontine nucleus
- SIP
Stepping-In-Place
- STN
Subthalamic Nucleus
Footnotes
Data access and responsibility
HMBS takes responsibility for the integrity of the data and the accuracy of the data analysis. Data will be made available on request when possible.
Declaration of Competing Interest
Dr. Bronte-Stewart serves on a clinical advisory board for Medtronic, Inc.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbd.2020.105134.
References
- Amboni M, Cozzolino A, Longo K, Picillo M, Barone P, 2008. Freezing of gait and executive functions in patients with Parkinson’s disease. Mov. Disord 10.1002/mds.21850. [DOI] [PubMed] [Google Scholar]
- Amboni M, Barone P, Hausdorff JM, 2013. Cognitive contributions to gait and falls: evidence and implications. Mov. Disord 10.1002/mds.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anidi C, O’Day JJ, Anderson RW, Afzal MF, Syrkin-Nikolau J, Velisar A, Bronte-Stewart HM, 2018. Neuromodulation targets pathological not physiological beta bursts during gait in Parkinson’s disease. Neurobiol. Dis 120, 107–117. 10.1016/j.nbd.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcolin I, Corna S, Giardini M, Giordano A, Nardone A, Godi M, 2019. Proposal of a new conceptual gait model for patients with Parkinson’s disease based on factor analysis. Biomed. Eng. Online 10.1186/s12938-019-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC, 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger EC, Ananth M, Talmage DA, Role LW, 2016. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron. 10.1016/j.neuron.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett MJ, Sperling SA, Blair JC, Freeman CS, Flanigan JL, Smolkin ME, Manning CA, Druzgal TJ, 2019. Lower volume, more impairment: reduced cholinergic basal forebrain grey matter density is associated with impaired cognition in Parkinson disease. J. Neurol. Neurosurg. Psychiatry 10.1136/jnnp-2019-320450. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Annweiler C, Lecordroch Y, Allali G, Dubost V, Herrmann FR, Kressig RW, 2009. Walking speed-related changes in stride time variability: effects of decreased speed. J. Neuroeng. Rehabil 10.1186/1743-0003-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Horst F, Steinberg F, Thomas F, Müller-Eising C, Schöllhorn WI, Doppelmayr M, 2019. Increased gait variability during robot-assisted walking is accompanied by increased sensorimotor brain activity in healthy people. J. Neuroeng. Rehabil 10.1186/s12984-019-0636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin O, Ferrandez AM, Pailhous J, Serratrice G, 1991. Dopa-sensitive and Dopa-resistant gait parameters in Parkinson’s disease. J. Neurol. Sci 10.1016/0022-510X(91)90283-D. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Constantine GM, Mathis CA, Davis JG, Moore RY, DeKosky ST, 2006. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J. Neurol 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Müller MLTM, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, Albin RL, Frey KA, 2012. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J. Cereb. Blood Flow Metab 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Scott PJH, Albin RL, Müller MLTM, 2013. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology. 10.1212/WNL.0b013e3182a9f558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL, Müller MLTM, Petrou M, Kotagal V, Koeppe RA, Scott PJH, Frey KA, 2015. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of parkinson disease and evidence of interaction effects. JAMA Neurol. 10.1001/jamaneurol.2014.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E, 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brontë-Stewart H, Louie S, Batya S, Henderson JM, 2010. Clinical motor outcome of bilateral subthalamic nucleus deep-brain stimulation for Parkinson’s disease using image-guided frameless stereotaxy. Neurosurgery. 10.1227/NEU.0b013e3181ecc887. [DOI] [PubMed] [Google Scholar]
- Bryant MS, Rintala DH, Hou JG, Charness AL, Fernandez AL, Collins RL, Baker J, Lai EC, Protas EJ, 2011. Gait variability in Parkinson’s disease: influence of walking speed and dopaminergic treatment. Neurol. Res 10.1179/1743132811Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK, 2010. Ageing and gait variability-a population-based study of older people. Age Ageing. 10.1093/ageing/afp250. [DOI] [PubMed] [Google Scholar]
- Craig CE, Jenkinson NJ, Brittain JS, Grothe MJ, Rochester L, Silverdale M, Alho ATDL, Alho EJL, Holmes PS, Ray NJ, 2020. Pedunculopontine nucleus microstructure predicts postural and gait symptoms in Parkinson’s disease. Mov. Disord 10.1002/mds.28051. [DOI] [PubMed] [Google Scholar]
- Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB, 2015. Levodopa is a double-edged sword for balance and gait in people with Parkinson’s disease. Mov. Disord, 10.1002/mds.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert S, Plettig P, Li N, Chakravarty MM, Collins DL, Herrington TM, Kühn AA, Horn A, 2018. Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage. 10.1016/j.neuroimage.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Ferrarin M, Rizzone M, Bergamasco B, Lanotte M, Recalcati M, Pedotti A, Lopiano L, 2005. Effects of bilateral subthalamic stimulation on gait kinematics and kinetics in Parkinson’s disease. Exp. Brain Res 10.1007/s00221-004-2036-5. [DOI] [PubMed] [Google Scholar]
- Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM, 2005. Effect of gait speed on gait rhythmieity in Parkinson’s disease: variability of stride time and swing time respond differently. J. Neuroeng. Rehabil 10.1186/1743-0003-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang M, Baba T, Hosokai Y, Nishio Y, Kikuchi A, Hirayama K, Hasegawa T, Aoki M, Takeda A, Mori E, Suzuki K, 2020. Clinical and cerebral metabolic changes in Parkinson’s disease with basal forebrain atrophy. Mov. Disord 10.1002/mds.27988. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, LaPelle N, 2007. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov. Disord 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- Gratton C, Yousef S, Aarts E, Wallace DL, D’Esposito M, Silver MA, 2017. Cholinergic, but not dopaminergic or noradrenergic, enhancement sharpens visual spatial perception in humans. J. Neurosci 10.1523/JNEUROSCI.2405-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH, 1987. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J. Comp. Neurol 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Henderson EJ, Lord SR, Brodie MA, Gaunt DM, Lawrence AD, Close JCT, Whone AL, Ben-Shlomo Y, 2016. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 10.1016/S1474-4422(15)00389-0. [DOI] [PubMed] [Google Scholar]
- Horn A, Kühn AA, 2015. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage 10.1016/j.neuroimage.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Horn A, Li N, Dembek TA, Kappel A, Boulay C, Ewert S, Tietze A, Husch A, Perera T, Neumann WJ, Reisert M, Si H, Oostenveld R, Rorden C, Yeh FC, Fang Q, Herrington TM, Vorwerk J, Kühn AA, 2019. Lead-DBS v2: towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 10.1016/j.neuroimage.2018.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husch A, Petersen VM, Gemmar P, Goncalves J, Hertel F, 2018. PaCER - A fully automated method for electrode trajectory and contact reconstruction in deep brain stimulation. Neuroimage Clin 10.1016/j.nicl.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, Stein JF, Aziz TZ, 2009. Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov. Disord 10.1002/mds.22189. [DOI] [PubMed] [Google Scholar]
- Johnston MV, McKinney M, Coyle JT, 1979. Evidence for a cholinergic projection to neocortex from neurons in basal forebrain. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.76.10.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Müller MLTM, Bohnen NI, Sarter M, Lustig C, 2017. Thalamic cholinergic innervation makes a specific bottom-up contribution to signal detection: evidence from Parkinson’s disease patients with defined cholinergic losses. Neuroimage. 10.1016/j.neuroimage.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Müller MLTM, Bohnen NI, Sarter M, Lustig C, 2019. The cortical cholinergic system contributes to the top-down control of distraction: evidence from patients with Parkinson’s disease. Neuroimage. 10.1016/j.neuroimage.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I, Sambeth A, Blokland A, 2011. Acetylcholine and attention. Behav. Brain Res 10.1016/j.bbr.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Wilson TW, Arpin DJ, 2012. Stride-time variability and sensorimotor cortical activation during walking. Neuroimage. 10.1016/j.neuroimage.2011.08.084. [DOI] [PubMed] [Google Scholar]
- Lau B, Welter ML, Belaid H, Fernandez Vidal S, Bardinet E, Grabli D, Karachi C, 2015. The integrative role of the pedunculopontine nucleus in human gait. Brain. 10.1093/brain/awv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJG, Shine JM, 2016. The next step: a common neural mechanism for freezing of gait. Neuroscientist. 10.1177/1073858414559101. [DOI] [PubMed] [Google Scholar]
- Lord S, Baker K, Nieuwboer A, Burn D, Rochester L, 2011. Gait variability in Parkinson’s disease: an indicator of non-dopaminergic contributors to gait dysfunction? J. Neurol 10.1007/s00415-010-5789-8. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, 2013. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J. Comp. Neurol 10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH, 1983. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (Substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Morris R, Lord S, Lawson RA, Coleman S, Galna B, Duncan GW, Khoo TK, Yarnall AJ, Burn DJ, Rochester L, 2017. Gait rather than cognition predicts decline in specific cognitive domains in early Parkinson’s disease. J. Gerontol. - Ser. A Biol. Sci. Med. Sci 10.1093/gerona/glx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R, Martini DN, Madhyastha T, Kelly VE, Grabowski TJ, Nutt J, Horak F, 2019. Overview of the cholinergic contribution to gait, balance and falls in Parkinson’s disease. Parkinsonism Relat. Disord 10.1016/j.parkreldis.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Martin TL, Mash DC, Wainer BH, Mesulam MM, 1986. Cholinergic projections from the parabigeminal nucleus (Ch8) to the superior colliculus in the mouse: a combined analysis of horseradish peroxidase transport and choline acetyl-transferase immunohistochemistry. Brain Res. 10.1016/0006-8993(86)91114-5. [DOI] [PubMed] [Google Scholar]
- Müller MLTM, Albin RL, Kotagal V, Koeppe RA, Scott PJH, Frey KA, Bohnen NI, 2013. Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain. 10.1093/brain/awt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano I, Hirano A, 1984. Parkinson’s disease: neuron loss in the nucleus basalis without concomitant Alzheimer’s disease. Ann. Neurol 10.1002/ana.410150503. [DOI] [PubMed] [Google Scholar]
- Nantel J, de Solages C, Bronte-Stewart H, 2011. Repetitive stepping in place identifies and measures freezing episodes in subjects with Parkinson’s disease. Gait Posture. 10.1016/j.gaitpost.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Nantel J, McDonald JC, Tan S, Bronte-Stewart H, 2012. Deficits in visuospatial processing contribute to quantitative measures of freezing of gait in Parkinson’s disease. Neuroscience. 10.1016/j.neuroscience.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Panisset M, 2004. Freezing of gait in Parkinson’s disease. Neurol. Clin 10.1016/j.ncl.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Parent A, Pare D, Smith Y, Steriade M, 1988. Basal forebrain cholinergic and non-cholinergic projections to the thalamus and brainstem in cats and monkeys. J. Comp. Neurol 10.1002/cne.902770209. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Hall S, Jalakas M, Grothe MJ, Strandberg O, Stomrud E, Westman E, van Westen D, Hansson O, 2020. Longitudinal degeneration of the basal forebrain predicts subsequent dementia in Parkinson’s disease. Neurobiol. Dis 10.1016/j.nbd.2020.104831. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Bradburn S, Murgatroyd C, Toseeb U, Mir P, Kountouriotis GK, Teipel SJ, Grothe MJ, 2018. In vivo cholinergic basal forebrain atrophy predicts cognitive decline in de novo Parkinson’s disease. Brain. 10.1093/brain/awx310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester L, Yarnall AJ, Baker MR, David RV, Lord S, Galna B, Burn DJ, 2012. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain. 10.1093/brain/aws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester L, Galna B, Lord S, Yarnall AJ, Morris R, Duncan G, Khoo TK, Mollenhauer B, Burn DJ, 2017. Decrease in Αβ42 predicts dopa-resistant gait progression in early Parkinson disease. Neurology. 10.1212/WNL.0000000000003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JD, Brogan D, Mirra SS, 1985. The nucleus basalis of Meynert in neurological disease: a quantitative morphological study. Ann. Neurol 10.1002/ana.410170210. [DOI] [PubMed] [Google Scholar]
- Roper JA, Kang N, Ben J, Cauraugh JH, Okun MS, Hass CJ, 2016. Deep brain stimulation improves gait velocity in Parkinson’s disease: a systematic review and meta-analysis. J. Neurol 10.1007/s00415-016-8129-9. [DOI] [PubMed] [Google Scholar]
- Schulz J, Pagano G, Fernandez Bonfante JA, Wilson H, Politis M, 2018. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain. 10.1093/brain/awy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, Ota T, Asahina M, Fukushi K, Kuwabara S, Hattori T, Suhara T, Irie T, 2009. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 10.1212/WNL.0b013e3181ab2b58. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smulders K, Dale ML, Carlson-Kuhta P, Nutt JG, Horak FB, 2016. Pharmacological treatment in Parkinson’s disease: effects on gait. Parkinsonism Relat. Disord 10.1016/j.parkreldis.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolze H, Klebe S, Poepping M, Lorenz D, Herzog J, Hamel W, Schrader B, Raethjen J, Wenzelburger R, Mehdorn HM, Deuschl G, Krack P, 2001. Effects of bilateral subthalamic nucleus stimulation on parkinsonian gait. Neurology. 10.1212/WNL.57.1.144. [DOI] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJG, 2003. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 10.1212/WNL.60.1.78. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Debu B, Aziz T, Bloem BR, Blahak C, Butson C, Czernecki V, Foltynie T, Fraix V, Grabli D, Joint C, Lozano AM, Okun MS, Ostrem J, Pavese N, Schrader C, Tai CH, Krauss JK, Moro E, 2018. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: a clinical review. Mov. Disord 10.1002/mds.27098. [DOI] [PubMed] [Google Scholar]
- Trager MH, Koop MM, Velisar A, Blumenfeld Z, Nikolau JS, Quinn EJ, Martin T, Bronte-Stewart H, 2016. Subthalamic beta oscillations are attenuated after withdrawal of chronic high frequency neurostimulation in Parkinson’s disease. Neurobiol. Dis 10.1016/j.nbd.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Wesnes KA, McKeith I, Edgar C, Emre M, Lane R, 2005. Benefits of rivastigmine on attention in dementia associated with Parkinson disease. Neurology. 10.1212/01.wnl.0000184517.69816.e9. [DOI] [PubMed] [Google Scholar]
- Yao Z, Shao Y, Han X, 2017. Freezing of gait is associated with cognitive impairment in patients with Parkinson disease. Neurosci. Lett 10.1016/j.neulet.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM, 2005. Dual tasking, gait rhythmieity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur. J. Neurosci 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- Yttri EA, Dudman JT, 2018. A proposed circuit computation in Basal Ganglia: history-dependent gain. Mov. Disord 10.1002/mds.27321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K, 2008. Stereotaxic probabilistic maps of the magnoeellular cell groups in human basal forebrain. Neuroimage 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


