Abstract
We describe two cases of patients with Steinert’s dystrophy or myotonic dystrophy type 1 (DM1) who presented with frequent respiratory exacerbations and pneumonia. They did not report any risk factors for asthma, allergy, bronchopathy or dysphagia in their history. The Videofluoroscopic swallow study test allowed to highlight post-swallowing aspiration phenomena responsible for respiratory exacerbations.
Key words: dysphagia, Steinert disease, respiratory exacerbation
Introduction
Myotonic dystrophy type 1 (DM1) is characterized by highly variable clinical manifestations that affect specific tissues, such as distal limbs and facial muscles, smooth muscles, eye (primarily the lens), brain (especially the anterior temporal and frontal lobes), and endocrine function (testosterone deficiency, insulin resistance, thyroid dysfunction). Cardiac involvement is noticed in about 80% of cases 1.
A respiratory dysfunction, predominantly a restrictive ventilatory pattern, is common in myotonic dystrophy and is associated with alveolar hypoventilation, chronic hypercapnia, and sleep disturbance in the form of sleep apnoea and sleep related disordered breathing 2.
Aspiration caused by dysphagia is possible in DM1 3. Pulmonary aspiration syndromes refer to a wide spectrum of pulmonary disorders resulting from aspiration of foreign material into the lung. Although aspiration generally triggers coughing, it can be silent, causing difficulties in recognizing aspiration as the cause of undiagnosed respiratory diseases. The severity of aspiration is related to the volume and nature of the aspirate, the chronicity, and the host responses.
Aspiration and its consequences can be divided into 3 forms: a) aspiration pneumonia and diffuse aspiration bronchiolitis; b) aspiration pneumonitis; and c) a foreign body obstruction of a central airway. The first form generally occurs in elderly, debilitated patients with dysphagia, and usually presents as a ‘community acquired pneumonia’, which tends to be recurrent in patients with diffuse aspiration bronchiolitis. Treatment consists of broad-spectrum antibiotics and management of the underlying dysphagia.
The aspiration pneumonitis is the consequence of the aspiration of regurgitated gastric contents, usually occurring in patients with a marked decreased level of consciousness. Treatment in this form is essentially supportive; however, corticosteroids and other immunomodulating agents may have some effect in these patients 4.
Generally, neuromuscular disorders, esophageal diseases, and the presence of a nasogastric (NG) tube or an endotracheal (ET) tube are factors that may increase the risk of aspiration. Focal or multifocal consolidations in a dependent location is the most common finding on chest X ray 5.
Case report
We describe two patients affected by myotonic dystrophy type 1 (DM1) or Steinert disease, followed at our department for respiratory problems. Both patients - #1 aged 64 and # 2 aged 39 years, were females and on nocturnal mechanical ventilation due to respiratory insufficiency secondary to sleep apnea. The patient #1 had experienced in the previous six months two episodes of pneumonia requiring hospitalization. The patient #2 presented one episode of respiratory exacerbation/month in the past 4 months, the last of which was slowly resolving. When the patients returned to our observation for a clinical-functional re-assessment, both presented a moderate restrictive ventilatory deficit at spirometry, stationary compared to the last checks. The cough peak was 240 L/min in patient # 1, and 220 L/min in patient #2. Both patients did not report dysphagia, difficulty in swallowing, nor did they complain of coughing fits during meals. Allergic tests were negative, as well as the spit test. Both patients were already on home therapy with a cough machine.
Chest CT scan showed in patient #1 the lamellar dysventilation -consolidation of the lower lobe, middle lobe and lingula and incipient tubular ectasias, mainly cleansed, of the air lumen of the segmental bronchi of the middle lobe. In patient # 2 CT scan showed right consolidation of lower lobe and bronchiolitis. Both patients underwent videofluoroscopic swallow study (VFSS). The investigation was performed by digital image acquisition after oral administration of an opaque bolus liquid (gastrografin diluted to 80%), semi-solid (yogurt + gastrografin), and solid (biscuits + gastrografin).
In patient # 1 the VFSS showed stagnation in the pharyngeal valleys and in the piriform sinuses, minimal penetration in the liquid phase of the examination (Fig. 1); in patient # 2, stagnation of the radiological contrast was in the pharyngeal valleys and in the piriform sinuses and determined a sensation of encumbrance, cough and expectoration of the ingested material especially in the solid phase of the examination. The material stagnated after breathing and opening of the epiglottis could be aspirated with post-aspiration swallowing.
After the test confirmed the suspicion of dysphagia in both patients, a nutritional counseling was activated to modify the diet, and a course of logotherapy prescribed to re-educate the swallowing muscles. No further episodes of respiratory exacerbation occurred during the following 12 months.
Discussion
Dysphagia is often not recognized in its early stages. It can cause even serious clinical pictures that last in silence for many months 6.
VFSS allows to examine all stages of swallowing, from the preparation stage to the onset of swallowing, the passage of the opaque meal in the oropharynx, and of the bolus in the hypopharynx, so it is considered as the procedure of choice in neuromuscular and NIV-treated patients 2.
The defects mainly detected at VFSS are reduced pharyngeal peristalsis, hypopharyngeal stasis and fragmented swallowing 5. In recent studies, the aspiration was seen in half of the patients affected by Steinert disease, both adults and infants, and mostly during swallowing 7,8.
In both cases here described, VFSS documented the presence of a swallowing disorder; the correction of the dietary regimen led to no episode of respiratory exacerbation after 12 months.
Data recently appeared in the literature 9 document how the food material stagnated in the pharynx can be aspirated in the post-swallowing phase, even after a few minutes. Aspirated food debris can mimic respiratory exacerbations, and the condition can go silent for many months until it leads to severe respiratory failure.
We agree that a systematic screening of dysphagia should be recommended 10 to achieve an early diagnosis and to set up appropriate dietary modifications and rehabilitation interventions, to avoid aspiration and to prevent severe respiratory complications in these patients.
Figures and tables
Figure 1.
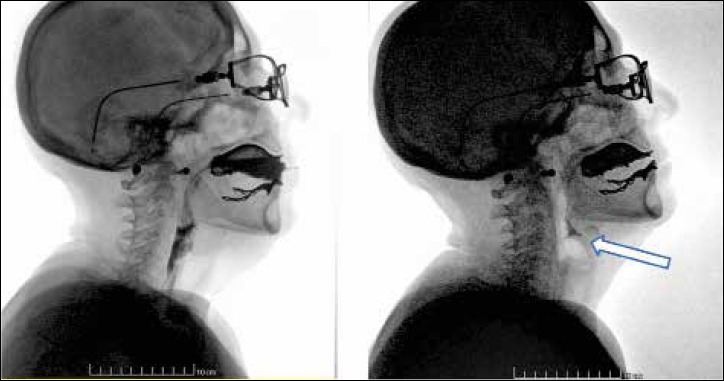
Patient #1. VFSS showing stagnation of the radiological contrast in the pharyngeal valleys and in the piriform sinuses (see arrow).
References
- 1.Nigro G, Papa AA, Politano L. The heart and cardiac pacing in Steinert disease. Acta Myol 2012;31:110-6. [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkins AM, Hawkins CL, Abdul Razak K, et al. Respiratory dysfunction in myotonic dystrophy type 1: a systematic review. Neuromuscul Disord 2019;29:198-212. https://doi.org/10.1016/j.nmd.2018.12.002 10.1016/j.nmd.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 3.Pilz W, Passos VL, Verdonschot RJ, et al. Swallow-related quality of life and oropharyngeal dysphagia in myotonic dystrophy. Eur Arch Otorhinolaryngol 2020;277:2357-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. doi: 10.1007/s00405-020-05964-2. https://doi.org/10.1007/s00405-020-05964-2. [DOI] [Google Scholar]
- 5.Jones K, Pitceathly RD, Rose MR, et al. Interventions for dysphagia in long-term, progressive muscle disease. Cochrane Database Syst Rev 2016;2:CD004303. https://doi.org/10.1002/14651858.CD004303.pub4. 10.1002/14651858.CD004303.pub4 PMID: 26859621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu X, Lee JS, Pianosi PT, et al. Aspiration-related pulmonary syndromes. Chest 2015;147:815-23. https://doi.org/10.1378/chest.14-1049 10.1378/chest.14-1049 [DOI] [PubMed] [Google Scholar]
- 7.Leonard RJ, Kendall KA, Johnson R, et al. Swallowing in myotonic muscular dystrophy: a videofluoroscopic study. Arch Phys Med Rehabil 2001;82:979-85. https://doi.org/10.1053/apmr.2001.23962 10.1053/apmr.2001.23962 [DOI] [PubMed] [Google Scholar]
- 8.Berggren KN, Hung M, Dixon MM, et al. Orofacial strength, dysarthria, and dysphagia in congenital myotonic dystrophy. Muscle Nerve 2018;58:413-7. https://doi.org/10.1002/mus.26176 10.1002/mus.26176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willaert A, Jorissen M, Goeleven A. Swallowing dysfunction in myotonic dystrophy: a retrospective study of symptomatology and radiographic findings. B-ENT 2015;11:249-56. [PubMed] [Google Scholar]
- 10.Johnson NE, Butterfield R, Berggren K, et al. Disease burden and functional outcomes in congenital myotonic dystrophy: a cross-sectional study. Neurology 2016;87:160-7. https://doi.org/10.1212/WNL.0000000000002845 10.1212/WNL.0000000000002845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teuschl Y, Trapl M, Ratajczak P, et al. Systematic dysphagia screening and dietary modifications to reduce stroke-associated pneumonia rates in a stroke-unit. PLoS One 2018;13:e0192142. https://doi.org/10.1371/journal.pone.0192142. 10.1371/journal.pone.0192142 PMID: 29389984; ; PMCID: PMC5794132 [DOI] [PMC free article] [PubMed] [Google Scholar]


