Abstract
Purpose:
Little is known about the characteristics and impact of acute pulmonary embolism (PE) during episodes of asthma exacerbation. We aimed to characterize patients diagnosed with acute PE in the setting of asthma exacerbation, develop a prediction model to help identify future patients and assess the impact of acute PE on hospital outcomes.
Methods:
We included 758 patients who were treated for asthma exacerbation and underwent a computed tomographic pulmonary angiography (CTA) during the same encounter at a university-based hospital between June 2011 and October 2018. We compared clinical characteristics of patients with and without acute PE and developed a machine learning prediction model to classify the PE status based on the clinical variables. We used multivariable regression analysis to evaluate the impact of acute PE on hospital outcomes.
Results:
Twenty percent of the asthma exacerbation patients who underwent CTA had an acute PE. Factors associated with acute PE included previous history of PE, high CHA2DS2-VASc score, hyperlipidemia, history of deep vein thrombosis, malignancy, chronic systemic corticosteroids use, high body mass index and atrial fibrillation. Using these factors, we developed a random forest machine learning prediction model which had an 88% accuracy in classifying the acute PE status of the patients (area under the receiver operating characteristic curve=0.899; 95% confidence interval: 0.885–0.913). Acute PE in asthma exacerbation was associated with longer hospital stay and intensive care unit stay.
Conclusion:
It is important to consider acute PE, a potentially life-threatening event, in the setting of asthma exacerbation especially when other risk factors are present.
Keywords: Asthma, Asthma Exacerbation, Pulmonary Embolism, Pulmonary Vascular Disease
Introduction:
Asthma is a chronic inflammatory disease marked by airway hyper-responsiveness and reversible obstruction[1]. Patients with asthma can experience acute worsening of their condition referred to as an asthma exacerbation. Common triggers include viral infection, exposure to allergens and poor compliance with therapy[2]. Severe exacerbations, especially those requiring hospitalization, can be life-threatening and have significant long-term impact in terms of asthma-related morbidity, healthcare and other indirect costs, and can potentially cause progressive decline in lung function[3]. Utilizing a standardized protocol when managing patients with acute exacerbations of asthma is important to improve disease outcomes[4]. However, in the era of protocolized medicine, it may be easy to overlook other causes. One such etiology is acute pulmonary embolism (PE), which should also be considered in the differential diagnosis of asthmatic patients who present with dyspnea[2].
A number of recent studies have reported an association between asthma and venous thromboembolic (VTE) disease[5–7]. While these studies demonstrated an increased lifetime risk of VTE in asthma patients, little is known about patients’ characteristics and the impact of acute PE during episodes of asthma exacerbations. Therefore, we sought to determine clinical characteristics of patients diagnosed with acute PE during episodes of asthma exacerbation and develop a prediction model that would help identify future patients. Additionally, we aimed to assess the impact of acute PE on hospital outcomes in patients treated for asthma exacerbation.
Methods:
We conducted a retrospective cohort study of adult patients presenting with asthma exacerbation who underwent computed tomographic pulmonary angiography (CTA) for suspected PE at the University of Florida (UF) Health System, Gainesville, Florida. We received approval for the study from the UF Institutional Review Board (reference number 201802508).
Data Collection
To identify asthma exacerbation patients, we queried our institutional integrated data repository and compiled a list of all patients with asthma exacerbation who presented between June 2011 and October 2018. Patients were deemed to have asthma exacerbation if they had relevant International Classification of Diseases codes for asthma exacerbation (ICD-9 (493.01, 493.02, 493.11, 493.12, 493.21 493.22, 493.91, 493.92), and ICD-10 (J45.21, J45.22, J45.31, J45.32, J45.41, J45.42, J45.51, J45.52, J45.901, J45.902), as determined by the treating physician in the final hospital discharge diagnosis, and were treated as asthma exacerbation during that encounter (received systemic steroids and albuterol treatment). Thereafter, we identified patients who underwent CTA during the same encounter. The CTA scans were obtained based on clinical suspicion by the treating physician. If a patient had several asthma exacerbation encounters with CTA, the first encounter in the study period was used. We classified the patients into two groups: those who were diagnosed with acute PE in the same encounter based on positive CTA scan and those who were not. Subsequently, we extracted information on demographics, data related to asthma severity and control, history of smoking, co-morbidities, heart rate, oxygen saturation, relevant laboratory results and hospital outcomes.
We used the first value reported in each encounter for laboratory findings, heart rate and oxygen saturation. Data about medication history was based on patients’ self-reported medications, prescriptions and medication refill history. We looked at multiple parameters to assess asthma severity. First, we classified asthma severity based on ICD-10 codes if available (mild: (J45.21, J45.22, J45.31 and J45.32, moderate: J45.41and J45.42, and severe: J45.51 and J45.52). Second, we assessed whether patients were on inhaled corticosteroids or not. Third, we looked at long-term systemic corticosteroid use (defined as corticosteroid use for 30 days or more in the previous year). Fourth, we looked at the number of asthma exacerbations requiring visits to the emergency department (ED) in the previous year. We calculated the CHA2DS2-VASc score for each patient based on the presence or absence of the score components[8].
We compared hospital outcomes between the two groups including: hospital length of stay, need for transfer to the intensive care unit (ICU), need for mechanical ventilation, in-hospital acute kidney injury and in-hospital mortality.
Statistical analysis:
We summarized the distribution of baseline characteristics and outcomes as percentages for categorical variables, and means ±1 standard deviation (SD) for continuous variables. To compare differences between groups, we used independent sample t-test for continuous variables and Chi-square test for categorical variables. We constructed a forest plot to depict the odds ratios of risk factors associated with acute PE based on univariate analysis.
We further developed a prediction model to predict the patients’ PE status using the random forest machine learning method based on the observed clinical variables [9]. Specifically, we included the CHA2DS2-VASc score plus clinical variables associated with acute PE with a univariate p-value of <0.10. We then adopted a 10-fold cross-validation scheme by randomly splitting the total sample of patients into as-even-as-possible 10 non-overlap data subsets for training (9 folds) and testing (1-fold) alternatively. Two-thousands trees were grown in each cross-validation classification. Based on the trained model, we predicted the PE status of patients in the testing set and compared the results with the actual observed PE status. With this, we obtained the accuracy of the prediction model presented as ROC curve. We also evaluated the prediction performance in terms of the area under the receiver operating characteristic curve (AUC) metrics and its 95% confidence interval (CI) via bootstrapping with replacement of 100 replications. We then repeated the same steps and incorporated all 22 variables in another random forest model including all observed variables. A relative importance prediction score for each variable was extracted and summarized in increasing order.
To evaluate the independent impact of acute PE on hospital outcomes, we used multivariable logistic regression analyses. We adjusted for demographics, body mass index, smoking history, comorbidities (hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation (AF), coronary artery or peripheral vascular disease, cerebral vascular disease, heart failure, history of cancer, lower limb fracture or general anesthesia in the past month) and baseline creatinine level.
We conducted statistical analyses using IBM SPSS Statistics for Windows, Version 23.0 (released 2015, IBM Cor, Armonk, NY) and open source statistical software package R version 3.6.1.
Results:
Our initial database query based on ICD-9 and ICD-10 revealed 7280 asthma exacerbation encounters (3660 unique patients). The final study sample included 758 patients who underwent CTA in the same encounter. Of these, 145 (19.1%) were diagnosed with acute PE during the same encounter.
Clinical Characteristics
Acute PE patients were generally middle-aged (54.1 ± 15.3 years), predominantly women (69%) and of Caucasian ancestry (53%). There were no significant differences between the two groups in their demographics (Table 1).
Table 1:
Baseline demographics and clinical characteristics features of asthma exacerbation patients with PE compared with asthma exacerbation patients with no PE
| Patients with PE (n=145) | Patients with no PE (n=613) | P value | |
|---|---|---|---|
| Demographics | |||
| Age years (years ± SD) | 54.1 ± 15.3 | 51.7 ± 16.4 | 0.111 |
| Women, n (%) | 100 (69) | 432 (70.5) | 0.721 |
| Race, n (%) | |||
| Caucasians | 77 (53) | 351 (57) | 0.364 |
| African-Americans | 61 (42) | 229 (37) | 0.293 |
| Hispanics | 3 (2) | 18 (3) | 0.567 |
| Other races | 4 (3) | 15 (2) | 0.829 |
| Clinical Characteristics | |||
| Body mass index (kg/m2 ± SD) | 33.1 ± 9.7 | 31.1 ± 9.1 | 0.020 |
| Heart rate (bpm ± SD) | 100.2 ± 19.3 | 100.5 ± 19 | 0.867 |
| Oxygen saturation (percent± SD) * | 95.5 ±4.3 | 95.5 ±4.4 | 0.928 |
| Cigarette smoker, n (%) * | 85 (63) | 347 (62) | 0.834 |
| Systemic hypertension, n (%) | 112 (77) | 375 (61) | <0.001 |
| Diabetes mellitus, n (%) | 63 (43) | 184 (30) | 0.002 |
| Hyperlipidemia, n (%) | 69 (48) | 238 (39) | 0.053 |
| Atrial fibrillation, n (%) | 28 (19) | 73 (12) | 0.018 |
| Coronary artery disease or peripheral vascular disease, n (%) | 48 (33) | 141 (23) | 0.011 |
| Cerebral vascular disease, n (%) | 13 (9) | 24 (4) | 0.011 |
| Heart failure, n (%) | 35 (24) | 102 (17) | 0.035 |
| History of cancer, n (%) | 62 (43) | 200 (33) | 0.021 |
| Pregnancy, n (%) | 0 | 1 (0.2) | 0.809 |
| Oral contraceptives use, n (%) | 2 (1) | 11 (2) | 0.729 |
| Lower limbs fracture or general anesthesia in the past month, n (%) | 2 (1) | 3 (0.5) | 0.234 |
| History of pulmonary embolism, n, (%) | 65 (45) | 15 (3) | <0.001 |
| History of deep vein thrombosis, n (%) | 19 (13) | 23 (4) | <0.001 |
| Previous use of anticoagulation, n (%) | 36 (25) | 126 (21) | 0.259 |
| Warfarin, n (%) | 24 (17) | 92 (15) | 0.642 |
| Dabigatran, n (%) | 3 (2) | 15 (2) | 0.788 |
| Apixaban, n (%) | 6 (4) | 14 (2) | 0.210 |
| Edoxaban, n (%) | 3 (2) | 5 (1) | 0.184 |
| Hemoptysis, n (%) | 1 (1) | 8 (1) | 0.538 |
| CHA2DS2-VASc score, ± SD | 3 ± 1.7 | 2.4 ± 1.7 | <0.001 |
| Laboratory findings | |||
| Creatinine mg/dl, ± SD† | 1.2 ± 1.4 | 0.9 ± 0.7 | 0.010 |
| Brain natriuretic peptide pg/ml, ± SD† | 1251 ± 1945 | 661.1 ± 1303.2 | 0.006 |
| Platelets count k/cu mm, ± SD† | 256.6 ± 85.6 | 256.7 ± 105.4 | 0.988 |
| INR, ± SD† | 1.2 ± 0.4 | 1.1 ± 0.3 | 0.056 |
| INR ≥ 2, n (%) | 4 (3) | 11 (2) | |
| D-Dimer, ± SD† | 2 ±1.8 | 1.1 ± 1.1 | 0.006 |
INR= International Normalized Ratio; LMWH= Low Molecular Weight Heparin; PE= Pulmonary Embolism; SD = Standard Deviation
Data on oxygen saturation and cigarette smoking history could not be ascertained in 11.9% and 7.7% of participants respectively.
Creatinine level, brain natriuretic peptide, platelets count, INR and D-Dimer were not available in 4.4%, 42.7%, 2.9%, 43.9 and 64.6% respectively.
Acute PE patients had higher body mass index (p = 0.02) but similar heart rate and oxygen saturation. Patients with acute PE had more baseline comorbidities (Figure 1 and Table 1). The acute PE group had higher serum creatinine (p=0.01), brain natriuretic peptide (BNP) (p=0.006), D-Dimer (p=0.006) and slightly higher INR but this was not statistically significant (p=0.06). (Table 1).
Figure 1.
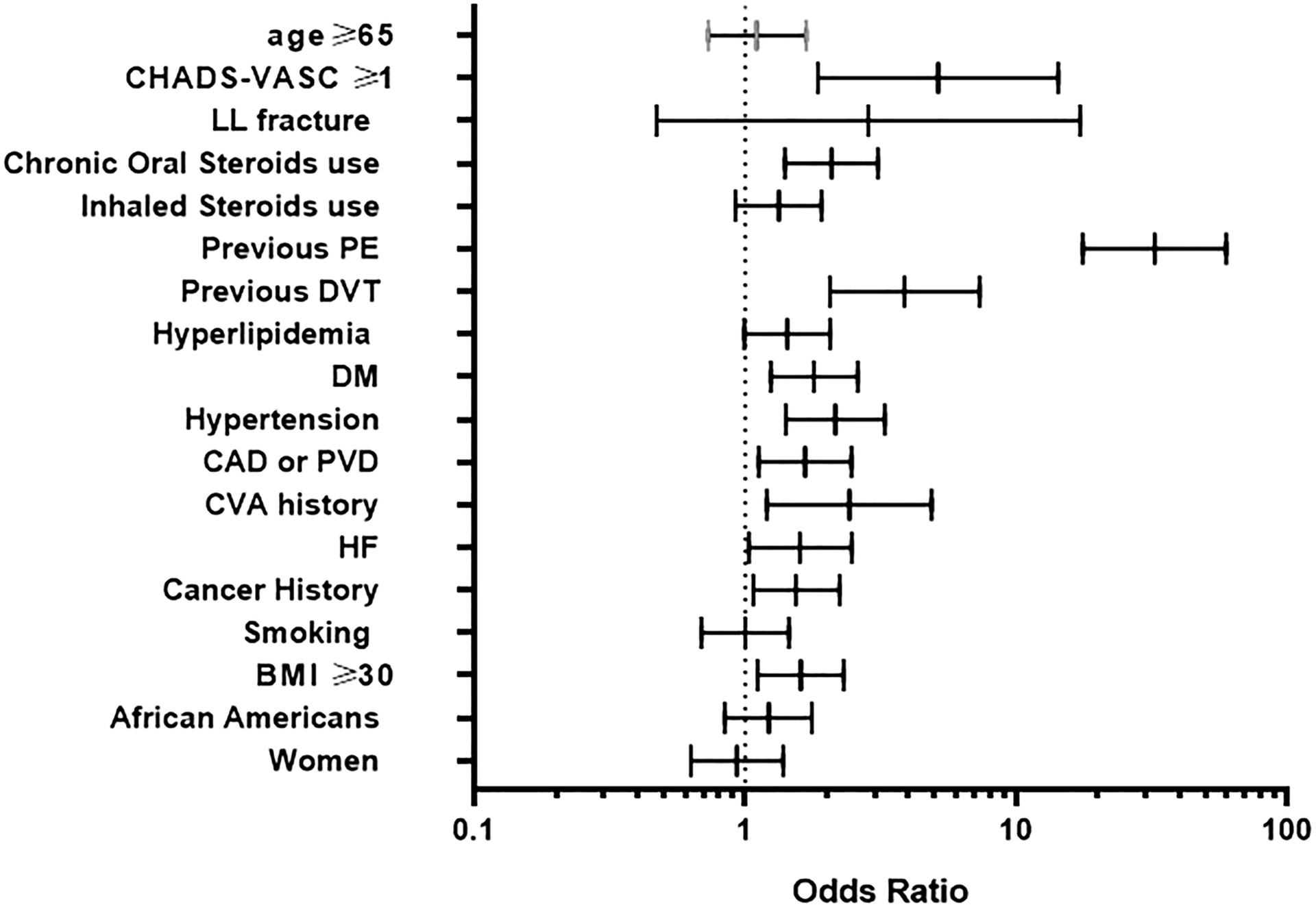
Odds ratios of risk factors associated with acute pulmonary embolism in asthma exacerbation. The diagram depicts univariate odds ratio for each risk factor when comparing patients with acute pulmonary embolism in the setting of asthma exacerbation to those with no pulmonary embolism
Twenty-five percent of the acute PE group were on anticoagulation prior to their presentation as compared to 21% of the non-PE group (p = 0.26). Warfarin was the most commonly prescribed anti-coagulant in both groups prior to admission (Table 1). Among the 116 patients who were previously prescribed warfarin from both groups, INR was available in 90 patients; of those only 9 patients had INR ≥2 (data not shown). Among patients with previous history of PE or DVT, 47% had an active anticoagulation order at the time of their presentation whereas 55% of patients with AF were on anticoagulation (data not shown).
Asthma Severity and Pulmonary Embolism:
Patients with acute PE tended to have higher prevalence of moderate to severe asthma (85% vs 76%) and higher prevalence of inhaled corticosteroid use (55% vs 48%) but the difference did not reach statistical significance (p=0.1 and 0.13, respectively). Systemic steroid use was more common in the previous year in patients with acute PE compared to the non-PE group (34% vs 20%, p<0.001). There was no difference in the number of asthma related ED visits in the prior year between the two groups (Table 2).
Table 2:
Asthma severity in patients with asthma exacerbation and acute PE compared to asthma exacerbation patients with no PE
| Patients with PE (n=145) | Patients with no PE (n=613) | P value | |
|---|---|---|---|
| Moderate to severe asthma, n, (%)* | 63 (85) | 184 (76) | 0.097 |
| Moderate persistent asthma, n (%) | 15 (20) | 45 (19) | 0.748 |
| Severe persistent asthma, n (%) | 48 (65) | 139 (57) | 0.255 |
| Status asthmaticus presentation, n (%) | 1 (1) | 5 (1) | 0.677 |
| Use of inhaled corticosteroids, n (%) | 80 (55) | 295 (48) | 0.127 |
| Chronic oral corticosteroids use, n (%)† | 49 (34) | 121 (20) | <0.001 |
| Emergency department visits in previous year, n (%)§ | |||
| 0–1 ED visits | 135 (93) | 561 (92) | 0.531 |
| 2–4 ED visits | 7 (5) | 38 (6) | 0.530 |
| >4 ED visits | 3 (2) | 14 (2) | 0.875 |
ED= Emergency Department; PE= Pulmonary Embolism; SD = Standard Deviation
Moderate to severe asthma classification is based on ICD-10 coding which was available in 58.6% of the patients.
Chronic corticosteroids use was defined as need for oral steroids ≥ 30 days in the year prior to the encounter based on patients’ self-report, physicians’ orders and prescription refill history.
The information on number of emergency department visits is limited to University of Florida health care system.
Prediction model:
Using CHA2DS2-VASc score in addition to 7 other clinical factors with univariate P-value less than 0.1, the average accuracy of prediction on testing data was 0.875 represented as AUROC (AUC=0.899; 95% CI: 0.885–0.913) (Figure 2). The confusion matrix is presented in (Supplementary Table 1). Previous history of PE followed by CHA2DS2-VASc were the most important factors in predicting acute PE, while history of AF had the lowest ranking (Table 3). Using 22 variables in the prediction model, the average accuracy of classification on the testing data was 0.867 (AUC=0.941; 95% CI: 0.931–0.951). The ROC curve based on the testing data classification is shown in (supplementary Figure 1). The importance score ranking (in increasing order) among the 22 variables for predicting the pulmonary embolism status accuracy is summarized in (Supplementary Table 2).
Figure 2.
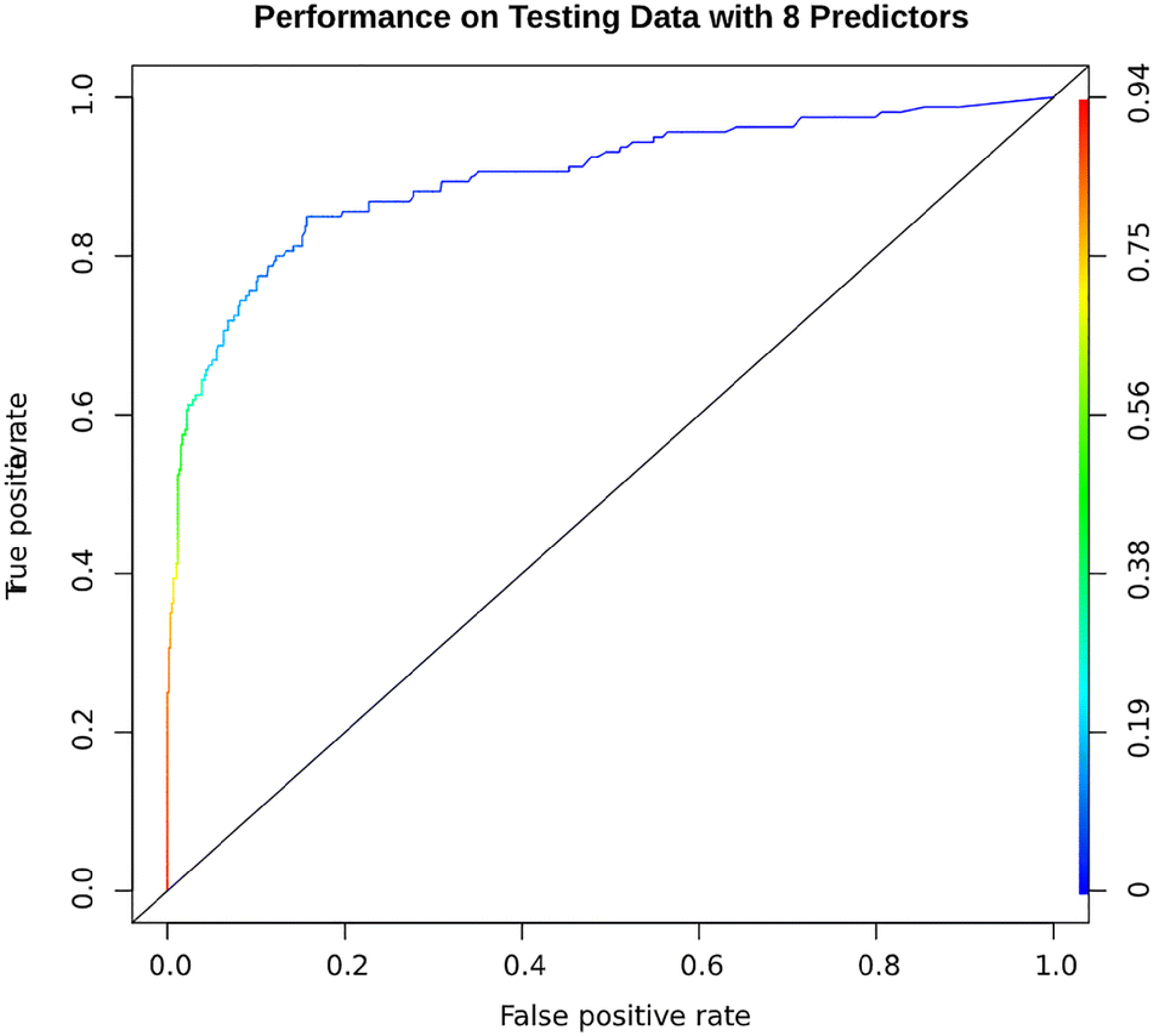
AUROC curve for predicting future cases of acute pulmonary embolism in asthma exacerbation patients. This model was built using random forest machine learning method based on the observed clinical variables. Specifically, we included the CHA2DS2-VASc score plus clinical variables associated with acute PE with a univariate p-value of <0.10 (history of pulmonary embolism, hyperlipidemia, history of deep vein thrombosis, cancer, chronic systemic steroids use, body mass index and atrial fibrillation). This was validated by adopting10-fold cross-validation scheme by randomly splitting the total sample of patients into as-even-as-possible 10 non-overlap data subsets for training (9 folds) and testing (1-fold) alternatively. Two-thousands trees were grown in each cross-validation classification. Based on the trained model, we predicted the PE status of patients in the testing set and compared the results with the actual observed PE status. With this, we obtained the accuracy of the prediction model presented as ROC curve
Table 3:
The average importance score ranking * (in increasing order) among the 8 variables for predicting acute pulmonary embolism in asthma exacerbation patients.
| Variable | Importance Score |
|---|---|
| Atrial fibrillation | −6.9 |
| Body mass index | 0.6 |
| Chronic systemic corticosteroids use† | 3.3 |
| Cancer | 6 |
| History of DVT | 13.9 |
| Hyperlipidemia | 29.3 |
| CHA2DS2-VASc score | 30.6 |
| History of PE | 116.1 |
DVT= deep vein thrombosis; PE= pulmonary embolism
The importance score is calculated as the average aggregated out-of-bag sample classification error rate across the whole forest after permuting the corresponding predictor variable in 10-fold cross-validation. That is, on average the out-of-bag sample classification error will increase by 116.1/2000 (5.8%) compared with pre-permutation classification of predictor variable “History of PE” for each tree. On the other hand, on average the out-of-bag sample classification error will decrease by 6.878/2000(0.34%)compared with pre-permutation classification of predictor variable “atrial fibrillation” for each tree. Specifically, “History of PE” is the most important variable for predicting the pulmonary embolism status among the 8 variables, while “atrial fibrillation” is the least important variable for predicting the pulmonary embolism status.
Chronic corticosteroids use was defined as need for oral steroids ≥ 30 days in the year prior to the encounter based on patients’ self-report, physicians’ orders and prescription refill history.
Hospital outcomes:
CTA was performed within the first 72 hours of the hospitalization in the majority (90%) of cases. In multivariable analyses adjusting for demographics and comorbidities, the acute PE patients had longer length of stay (p=0.001), were transferred more frequently to the ICU (p<0.001), and tended to have higher in-hospital mortality but did not reach statistical significance (0.073). The acute PE group developed acute kidney injury more frequently but this was not statistically significant on multivariable analysis (p=0.99). There was no difference in the need for mechanical ventilation (p=0.97) (Table 4).
Table 4:
Hospital stay characteristics and outcomes in asthma exacerbation patients with acute PE compared to asthma exacerbation patients with no acute PE
| Encounters with PE (n=145) | Encounters with no PE (n=613) | P-value | Adjusted p-value* | |
|---|---|---|---|---|
| Time to obtaining chest CTA (days± SD) | 2.5 ± 3.5 | 2 ± 3 | 0.142 | 0.158 |
| Hospital length of stay (days± SD) | 8.5 ± 10.4 | 5.2 ± 7 | <0.001 | <0.001 |
| Need for ICU transfer, n (%) | 38 (26) | 80 (13) | <0.001 | 0.001 |
| Need for mechanical ventilation, n (%) | 6 (4) | 24 (4) | 0.902 | 0.457 |
| In-hospital acute kidney injury, n (%) | 27 (19) | 0 | <0.001 | 0.990 |
| In-hospital mortality, n (%) | 6 (4) | 10 (2) | 0.059 | 0.073 |
CTA = Computed Tomography Angiography; ICU= Intensive Care Unit; PE= Pulmonary Embolism; SD = Standard Deviation.
Based on multivariable regression analysis adjusted for demographics, body mass index, smoking history, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, coronary artery or peripheral vascular disease, cerebral vascular disease, heart failure, history of cancer, lower limb fracture or general anesthesia in the past month and baseline creatinine level.
Discussion:
One-fifth of asthma exacerbation patients who underwent CTA in a hospital-based cohort were diagnosed with acute PE. Previous history of PE had the strongest association with the development of acute PE. Other associated factors included high CHA2DS2-VASc score, hyperlipidemia, history of DVT, cancer, use of systemic corticosteroids, obesity and history of AF. Acute PE was independently associated with higher in-hospital mortality, need for ICU admission, and longer hospital length of stay in asthma exacerbation patients.
To our knowledge, this is the first study to describe the risk factors and outcomes of acute PE in asthma exacerbation. A number of reports in recent years found that asthma increases the risk of DVT and PE; Majoor et al. studied an outpatient cohort of patients referred to three asthma clinics in Netherlands and found that severe asthma and oral steroid use conferred a 3-fold greater odds of PE[5]. In a nationwide population-based study from Taiwan, Chung et al. found that asthma conferred a 3-fold increased risk of PE after adjusting for age, sex and comorbidities. They also found an increase in PE hazard ratio proportional to the number of asthma related ED visits and hospital admissions suggesting that uncontrolled asthma increases the risk of developing PE[6]. Another Taiwanese study found a 2-fold increased risk of PE in patients with asthma-COPD overlap[10]. Similarly, Zöller et al. conducted a nationwide case-control study in Sweden and found that asthma carried around a 1.5-fold increase in the risk of DVT and PE. Interestingly, they found that the incidence rate of DVT or PE was highest within 3 months of asthma-related hospital admission[7].
It is unclear whether or not an acute PE triggers bronchospasm, resulting in an asthma exacerbation, or if the inflammatory response associated with an exacerbation predisposes the patient to acute PE. In one single-center study, 12% of acute PE cases were diagnosed in asthmatics, the vast majority of whom (92%) presented with an acute exacerbation [11]. Multiple case reports also document asthma exacerbation, bronchospasm, and wheezing in the setting of acute PE[12–17]. The pathophysiology underlying pulmonary embolism in asthma exacerbation is yet to be elucidated. However, it has been well demonstrated that inflammation and hemostasis are closely linked processes[18–20]. The coagulation system in asthmatic patients is skewed to favor a procoagulant state both in the airways as well as in the vascular compartment. Studies have demonstrated increased sputum and bronchoalveolar fluid levels of tissue factor, thrombinantithrombin complexes and the plasminogen activator inhibitor-1 (PAI-1), as well as decreased levels of the anticoagulant protein C[21]. Similarly, serum levels of hemostatic factors have also been shown to increase in correlation to asthma severity[22] as well as in the setting of an asthma exacerbation when compared to stable disease[23]. Majoor et al. found that rhinovirus infection, a common cause of asthma exacerbation, led to significantly higher plasma PAI-1 levels in mild asthma patients compared to healthy controls[24]. These findings lend weight to the reasoning that an already dysfunctional endothelium in asthmatic patients[25, 26] will become activated (e.g., by a virus) during an exacerbation. The resulting release of hemostatic factors such as Von-Willebrand Factor and PAI-1 in turn fosters a pro-coagulant state, potentially leading to pulmonary embolism in this setting.
Our prediction model had 88% accuracy in classifying PE vs non-PE patients. Previous history of PE was the strongest predicting factor. However, in both groups, only 10% of patients prescribed warfarin had INR≥2 which indirectly reflects the patients’ probable non-compliance. The second most important factor was the CHA2DS2-VASc score, which is a widely used tool in predicting the risk of stroke in AF patients. Several studies have demonstrated applicability of the CHA2DS2-VASc score in predicting risk of ischemic stroke, thromboembolic events, and death extending beyond AF for which it was proposed[27–29]. It is therefore recognized that the cluster of risk factors included in the CHA2DS2-VASc score also increase the risk of thromboembolic events irrespective of the presence or absence of AF. Chronic oral corticosteroid use carried a 2-fold increase in the risk of PE during asthma exacerbation. This finding is in agreement with previous reports in which oral corticosteroid use was associated with an increased risk of PE [5] and development of a prothrombotic state[30].
The mortality rate of acute PE can range from 1% to more than 20% depending on the severity of the PE and presence or absence of right heart strain [31, 32]. In our cohort, the in-hospital mortality rate of asthma exacerbation patients with acute PE was 4%. Acute PE was associated with increased length of stay and higher need for ICU admission. The average time to obtaining the CTA was 2.5 days. It is possible that earlier suspicion and diagnosis of PE could have led to better outcomes.
The main strengths of our study include examination of asthma exacerbation patients over 7 years with a relatively good sample size. Also, we developed a model to help predict acute PE in the setting of asthma exacerbation using a powerful machine learning method, i.e. random forest, which is good for regression as well as classification of cases. Our study has limitations. First, it is a single center study which limits the generalizability of our prediction model and requires external validation. However, the cluster of risk factors we identified is similar to those previously published in the literature, which is reassuring. Although the rate of acute PE is relatively high in our cohort, the prevalence rate of PE reported in literature can be as high as 21% in a group of patients selected based on clinical suspicion[33]. Second, the retrospective nature of our data collection made it difficult to ascertain more details about asthma control and medication use history. Additionally, the accuracy of ICD-10 codes to assess asthma severity is not fully validated. In one study, ICD-10 coding was accurate around 80% of the time[34]. To address this limitation, we looked at multiple parameters to define asthma severity including oral steroid use and admission history for recurrent exacerbations. Third, our analysis was performed on what is almost certainly a higher risk sub-population. As such, the reported incidence rate of PE is among the patients who actually underwent CTA based on individual clinical judgment; as opposed to all other patients with asthma exacerbation seen during the study period, leading to a selection bias. Although our study does not reflect the actual incidence rate of PE in asthma exacerbation, it describes the clinical characteristics of the asthma exacerbation patients who are actually diagnosed with PE. Fourth, we identified the study subjects using ICD codes with potential misclassification. However, the ICD codes for asthma have specificity approaching 98%[35, 36] and we identified the cases if they were actually treated for asthma exacerbation. Fifth, some of our patients may have presented with PE which can sometime present with wheezy dyspnea[14, 15] raising a concern for potential reverse association. Nevertheless, all of these patients were labelled to have asthma exacerbation by the treating physician. Lastly, we did not have the full components of previously published PE prediction scores, like the Wells’ criteria[37] or the revised Geneva score[38] to assess the performance of these scores in the setting of asthma exacerbation.
Conclusion:
This study showed that one fifth of the asthma exacerbation patients who underwent CTA had an acute PE, with significant impact on hospital outcomes. It is important not to overlook PE, a potentially life-threatening event, in the setting of asthma exacerbation especially when associated with risk factors like previous history of DVT or PE, chronic oral corticosteroid use and other comorbidities.
Supplementary Material
Supplementary Figure 1 AUROC curve for predicting future cases of acute pulmonary embolism in asthma exacerbation patients. This model was built using random forest machine learning method based on 22 observed clinical variables. This was validated by adopting10-fold cross-validation scheme by randomly splitting the total sample of patients into as-even-as-possible 10 non-overlap data subsets for training (9 folds) and testing (1-fold) alternatively. Twothousands trees were grown in each cross-validation classification. Based on the trained model, we predicted the PE status of patients in the testing set and compared the results with the actual observed PE status. With this, we obtained the accuracy of the prediction model presented as ROC curve.
Acknowledgement
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AF
atrial fibrillation
- CTA
computed tomographic pulmonary angiography
- DVT
deep vein thrombosis
- ED
emergency department
- ICD
International Classification of Diseases
- ICU
intensive care unit
- PAI-1
plasminogen activator inhibitor-1
- PE
pulmonary embolism
- UF
University of Florida
- VTE
venous thromboembolic
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest: None of the authors have conflict of interest
Availability of Data and Material: Data Available on request from authors (I.F.) if approved by University of Florida Institutional Review Board.
Code availability: not applicable
References:
- 1.Martinez FD, Vercelli D (2013) Asthma. Lancet (London, England) 382:1360–1372. 10.1016/S0140-6736(13)61536-6 [DOI] [PubMed] [Google Scholar]
- 2.Global Strategy for Asthma Management and Prevention. https://ginasthma.org/gina-reports. Updated 2019. Date last accessed: December 6 2019.
- 3.Bai TR, Vonk JM, Postma DS, Boezen HM (2007) Severe exacerbations predict excess lung function decline in asthma. Eur Respir J 30:452–456. 10.1183/09031936.00165106 [DOI] [PubMed] [Google Scholar]
- 4.Castillo JR, Peters SP, Busse WW (2017) Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J allergy Clin Immunol Pract 5:918–927. 10.1016/j.jaip.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majoor CJ, Kamphuisen PW, Zwinderman AH, et al. (2013) Risk of deep vein thrombosis and pulmonary embolism in asthma. Eur Respir J 42:655–661. 10.1183/09031936.00150312 [DOI] [PubMed] [Google Scholar]
- 6.Chung WS, Lin CL, Ho FM, et al. (2014) Asthma increases pulmonary thromboembolism risk: A nationwide population cohort study. Eur Respir J 43:801–807. 10.1183/09031936.00043313 [DOI] [PubMed] [Google Scholar]
- 7.Zöller B, Pirouzifard M, Memon AA, et al. (2017) Risk of pulmonary embolism and deep venous thrombosis in patients with asthma: A nationwide case-control study from Sweden. Eur Respir J 49:. 10.1183/13993003.01014-2016 [DOI] [PubMed] [Google Scholar]
- 8.Lip GYH, Nieuwlaat R, Pisters R, et al. (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137:263–272. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 9.Breiman L (2001) Random Forests. Otras caracteristicas. 1–33. 10.1017/CBO9781107415324.004 [DOI] [Google Scholar]
- 10.Yeh J-J, Wang Y-C, Kao C-H (2016) Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome Associated with Risk of Pulmonary Embolism. PLoS One 11:e0162483 10.1371/journal.pone.0162483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreto I, Teles Martins C, Manique A, Bárbara C (2016) Asthma and the risk of pulmonary embolism. Eur Respir J 48:PA2459. 10.1183/13993003.congress-2016.PA2459 [DOI] [Google Scholar]
- 12.Lee P-H, Fu P-K (2019) Pulmonary Embolism and Severe Asthma: Case Report and Literature Review. Medicina (Kaunas). 55 (10). pii: E647 10.3390/medicina55100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bansal DP, Maazuddin M, Viquasuddin M (2018) Pulmonary Embolism Mimicking Acute Severe Asthma. J Assoc Physicians India 66:11–12. [PubMed] [Google Scholar]
- 14.Komaki C, Niwa T, Tatsuoka H, Inoue Y (2011) [Bronchial asthma-like symptoms induced by pulmonary embolism]. Nihon Kokyuki Gakkai Zasshi 49:756–759 [PubMed] [Google Scholar]
- 15.Broux R, Bury J, Marcelle R, et al. (1976) [Bronchospasm, a sign revealing pulmonary embolism]. Arch Mal Coeur Vaiss 69:419–425 [PubMed] [Google Scholar]
- 16.Windebank WJ, Boyd G, Moran F (1973) Pulmonary thromboembolism presenting as asthma. Br Med J 1:90–94. 10.1136/bmj.1.5845.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto T, Ando M, Kan T, et al. (2019) Asthma Exacerbation Coincident with Saddle Pulmonary Embolism and Paradoxical Embolism. Tohoku J Exp Med 248:137–141. 10.1620/tjem.248.137 [DOI] [PubMed] [Google Scholar]
- 18.Ramagopalan SV, Wotton CJ, Handel AE, et al. (2011) Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: Record-linkage study. BMC Med 9:1–8. 10.1186/1741-7015-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoller B, Li X, Sundquist J, Sundquist K (2012) Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet (London, England) 379:244–249. 10.1016/S0140-6736(11)61306-8 [DOI] [PubMed] [Google Scholar]
- 20.van der Poll T, de Boer JD, Levi M (2011) The effect of inflammation on coagulation and vice versa. Curr Opin Infect Dis 24:273–278. 10.1097/QCO.0b013e328344c078 [DOI] [PubMed] [Google Scholar]
- 21.de Boer JD, Majoor CJ, van ‘t Veer C, et al. (2012) Asthma and coagulation. Blood 119:3236–3244. 10.1182/blood-2011-11-391532 [DOI] [PubMed] [Google Scholar]
- 22.Sneeboer M, Majoor C, De Kievit A, et al. (2015) Coagulation activity increases with disease severity in patients with asthma. Eur Respir J 46:PA4016. 10.1183/13993003.congress-2015.PA4016 [DOI] [Google Scholar]
- 23.Manuyakorn W, Mairiang D, Sirachainan N, et al. (2016) Blood Coagulation and Asthma Exacerbation in Children. Int Arch Allergy Immunol 170:75–83. 10.1159/000446775 [DOI] [PubMed] [Google Scholar]
- 24.Majoor CJ, van de Pol MA, Kamphuisen PW, et al. (2014) Evaluation of coagulation activation after rhinovirus infection in patients with asthma and healthy control subjects: an observational study. Respir Res 15:14 10.1186/1465-9921-15-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yildiz P, Oflaz H, Cine N, et al. (2004) Endothelial dysfunction in patients with asthma: the role of polymorphisms of ACE and endothelial NOS genes. J Asthma 41:159–166. 10.1081/jas-120026073 [DOI] [PubMed] [Google Scholar]
- 26.Wanner A, Mendes ES (2010) Airway endothelial dysfunction in asthma and chronic obstructive pulmonary disease: a challenge for future research. Am J Respir Crit Care Med 182:1344–1351. 10.1164/rccm.201001-0038PP [DOI] [PubMed] [Google Scholar]
- 27.Lip GYH, Lin H-J, Chien K-L, et al. (2013) Comparative assessment of published atrial fibrillation stroke risk stratification schemes for predicting stroke, in a non-atrial fibrillation population: the Chin-Shan Community Cohort Study. Int J Cardiol 168:414–419. 10.1016/j.ijcard.2012.09.148 [DOI] [PubMed] [Google Scholar]
- 28.Mitchell LB, Southern DA, Galbraith D, et al. (2014) Prediction of stroke or TIA in patients without atrial fibrillation using CHADS2 and CHA2DS2-VASc scores. Heart 100:1524–1530. 10.1136/heartjnl-2013-305303 [DOI] [PubMed] [Google Scholar]
- 29.Melgaard L, Gorst-Rasmussen A, Lane DA, et al. (2015) Assessment of the CHA2DS2-VASc Score in Predicting Ischemic Stroke, Thromboembolism, and Death in Patients With Heart Failure With and Without Atrial Fibrillation. JAMA 314:1030–1038. 10.1001/jama.2015.10725 [DOI] [PubMed] [Google Scholar]
- 30.Sneeboer MMS, Majoor CJ, de Kievit A, et al. (2016) Prothrombotic state in patients with severe and prednisolone-dependent asthma. J Allergy Clin Immunol 137:1727–1732. 10.1016/j.jaci.2015.10.038 [DOI] [PubMed] [Google Scholar]
- 31.Agnelli G, Becattini C Anticoagulant treatment for acute pulmonary embolism : a pathophysiology-based clinical approach. 1142–1149. 10.1183/09031936.00164714 [DOI] [PubMed]
- 32.Task A, Members F, Konstantinides SV, et al. (2019) 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) The Task Force for the diagnosis and management of acute. 1–61. 10.1093/eurheartj/ehz405 [DOI] [Google Scholar]
- 33.Righini M, Le Gal G, Aujesky D, et al. (2008) Diagnosis of pulmonary embolism by multidetector CT alone or combined with venous ultrasonography of the leg: a randomised non-inferiority trial. Lancet (London, England) 371:1343–1352. 10.1016/S0140-6736(08)60594-2 [DOI] [PubMed] [Google Scholar]
- 34.Horsky J, Drucker EA, Ramelson HZ (2017) Accuracy and Completeness of Clinical Coding Using ICD-10 for Ambulatory Visits. AMIA. Annu Symp proceedings AMIA Symp 2017:912–920 [PMC free article] [PubMed] [Google Scholar]
- 35.Juhn Y, Kung A, Voigt R, Johnson S (2011) Characterisation of children’s asthma status by ICD-9 code and criteria-based medical record review. Prim Care Respir J 20:79–83. 10.4104/pcrj.2010.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raita Y, Camargo CAJ, Faridi MK, et al. (2019) Risk of Acute Myocardial Infarction and Ischemic Stroke in Patients with Asthma Exacerbation: A Population-Based, Self-Controlled Case Series Study. J allergy Clin Immunol Pract. 10.1016/j.jaip.2019.06.043 [DOI] [PubMed] [Google Scholar]
- 37.Wells PS, Anderson DR, Rodger M, et al. (2001) Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med 135:98–107. 10.7326/0003-4819-135-2-200107170-00010 [DOI] [PubMed] [Google Scholar]
- 38.Le Gal G, Righini M, Roy P-M, et al. (2006) Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 144:165–171. 10.7326/0003-4819-144-3-200602070-00004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 AUROC curve for predicting future cases of acute pulmonary embolism in asthma exacerbation patients. This model was built using random forest machine learning method based on 22 observed clinical variables. This was validated by adopting10-fold cross-validation scheme by randomly splitting the total sample of patients into as-even-as-possible 10 non-overlap data subsets for training (9 folds) and testing (1-fold) alternatively. Twothousands trees were grown in each cross-validation classification. Based on the trained model, we predicted the PE status of patients in the testing set and compared the results with the actual observed PE status. With this, we obtained the accuracy of the prediction model presented as ROC curve.


