Abstract
Background
Cardiovascular disease is a major cause of morbidity and mortality in patients with end-stage renal disease (ESRD). Coronary artery bypass grafting (CABG) is beneficial in selected patients with ESRD. This study investigates the survival outcomes and prognostic factors in ESRD patients who underwent CABG.
Methods
A retrospective analysis was performed for 149 patients with ESRD who underwent isolated CABG between 2006 and 2015.
Results
Mean age was 59.4±8.7 years and 106 patients (71.1%) were male. Operative mortality occurred in 20 patients (13.4%). Overall survival was 81.1%±3.2% at 1 year, 41.5%±4.3% at 5 years and 19.2%±4.2% at 10 years. Median survival was 4.3 years. Multivariable analysis identified age [P=0.001, odds ratio (OR): 1.15 per 1-year increase, 95% confidence interval (CI): 1.06–1.25], preoperative left ventricular ejection fraction (LVEF) (P=0.020, OR: 0.94, 95% CI: 0.89–0.99) and non-elective status of operation (P=0.049, OR: 3.34, 95% CI: 1.00–11.1) as predictors of operative mortality. Cox regression analysis identified age [P<0.001, hazard ratio (HR): 1.05 per 1-year increase, 95% CI: 1.03–1.08], New York Heart Association (NYHA) class III or IV status (P=0.010, HR: 1.75, 95% CI: 1.15–2.67) and the use of a left internal mammary artery (LIMA) to left anterior descending artery (LIMA-LAD) graft (P=0.029, HR: 0.42, 95% CI: 0.19–0.92) as factors influencing long-term survival.
Conclusions
CABG is associated with high operative mortality and poor long-term survival in ESRD patients. Age and NYHA class influenced late survival. LIMA-LAD grafting conferred a long-term survival advantage.
Keywords: End-stage renal disease (ESRD), dialysis-dependent renal failure, coronary artery bypass grafting (CABG), coronary artery disease
Introduction
Cardiovascular disease is prevalent and a major cause of mortality in patients with end-stage renal disease (ESRD). This may be attributed to the presence of pre-existing cardiovascular risk factors and pathological mechanisms present in ESRD, collectively predisposing to atherosclerotic disease (1). ESRD is on the rise in Singapore. According to the Singapore Renal Registry, the age standardized incidence rate of definitive dialysis increased significantly from 165 per million population in 2008 to 180 per million population in 2017. The age standardized prevalence rate of definitive dialysis also increased significantly from 884 per million population to in 2008 to 1,059 per million population in 2017 (2). With the increasing prevalence and life expectancy of patients with ESRD, the number of patients with concomitant cardiovascular disease who could potentially benefit from coronary revascularization is expected to increase. Coronary artery bypass grafting (CABG) is recommended over percutaneous coronary intervention (PCI) for coronary revascularization in appropriately selected ESRD patients with multi-vessel disease (3). Patients with ESRD face increased perioperative risks, due to the presence of multiple comorbidities and severe atherosclerosis causing diffuse coronary artery and aortic wall disease. Our study aimed to investigate the survival outcomes and prognostic factors of patients with ESRD who underwent isolated CABG at our tertiary referral centre. We present the following article in accordance with the STROBE reporting checklist (4) (available at http://dx.doi.org/10.21037/jtd-20-2046).
Methods
This study is a retrospective case-note and database review of all patients with ESRD who underwent isolated CABG between January 2006 and December 2015 at our institution, a tertiary cardiac surgery referral centre in Singapore. The study population included all patients requiring either haemodialysis or peritoneal dialysis for at least 3 months prior to CABG. Patients with acute renal failure, temporary dialysis or chronic renal failure not requiring dialysis were excluded. Prior to surgery, a non-contrasted computed tomography scan of the chest was performed routinely in all patients with ESRD planned for CABG, to assess the burden of calcific plaque in the aorta, in particular the ascending aorta. This information was useful to stratify the risk of thromboembolic complications and to plan the most appropriate surgical strategy. The study was approved by the local institutional review board with a waiver of patient consent (reference: 2016/3132). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Definitions
Early or operative mortality was defined as mortality within 30 days and/or death before discharge from the index hospitalization. Elective procedures were defined as routine admissions for surgery. Urgent procedures were defined as patients who were not electively admitted for but required surgery during the current admission for medical reasons. Emergent procedures were those performed before the beginning of the next working day.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as means with standard deviation and analyzed using Student’s t-test. Categorical data were expressed as percentages and analyzed with the Chi-squared or Fisher’s exact test. Survival function was represented using Kaplan-Meier survival curves with 1-, 3-, 5- and 10-year survival estimates. Logistic regression was used to identify predictors of early mortality. Cox regression analysis was used to identify independent predictors of long-term survival. Preoperative and operative variables with a univariable P<0.10 or those judged to be clinically important were entered into the multivariable Cox model. All two-tailed P values <0.05 were taken as significant.
Results
Between 2006 and 2015, 149 consecutive patients with ESRD underwent isolated CABG at our institution. Preoperative characteristics are shown in Table 1. The mean age of patients was 59.4±8.7 years (range, 35–80 years). The mean EuroSCORE II was 3.8±3.5, ranging from 0.6 to 26.8. Operative variables are summarized in Table 2. Thirty-seven patients (24.8%) underwent urgent [29 patients (19.4%)] or emergent surgery [8 patients (5.4%)]. In the urgent surgery group, the indications for surgery were recurrent unstable angina in 24 patients (82.8%), critical left main artery stenosis in 3 patients (10.3%), and hypotension during dialysis in 2 patients (6.9%). In the emergent surgery group, surgery was indicted for critical left main artery stenosis with ongoing myocardial ischaemia in 6 patients (75.0%) and ongoing myocardial ischaemia with recurrent ventricular arrhythmias in 2 patients (25.0%). Eleven surgeries (7.4%) were performed off-pump, without the use of cardiopulmonary bypass (CPB). Due to significant ascending aortic calcifications, 21 surgeries (14.1%) were performed on-pump without the use of an aortic cross-clamp and cardioplegia. On-pump beating surgery was the preferred technique in patients where there was sufficient plaque-free area to perform aortic cannulation safely but insufficient safe area for cross-clamping of the aorta. Off-pump surgery was reserved for patients without safe areas for both aortic cannulation and cross-clamping. The in-situ pedicled left internal mammary artery (LIMA) was grafted to the left anterior descending artery (LIMA-LAD) in 140 (94.0%) patients. Nine patients (6.0%) received a saphenous vein graft to the LAD. Within this group, the LIMA was not used in view of haemodynamic stability in 7 patients (77.8%), insufficient LIMA length in 1 patient (11.1%) and poor LIMA quality in 1 patient (11.1%). The mean CPB duration was 95.3±26.8 minutes and the mean aortic cross-clamp time was 50.1±14.4 minutes. The mean number of distal anastomoses was 3.0±0.9.
Table 1. Preoperative variables.
| Variables | All patients (n=149) |
|---|---|
| General characteristics | |
| Age (years) | 59.4±8.7 |
| Male/female | 106 (71.1)/43 (28.9) |
| Body surface area (m2) | 1.7±0.2 |
| Co-morbidities | |
| Diabetes mellitus | 109 (73.2) |
| Hypertension | 141 (94.6) |
| Hyperlipidaemia | 113 (75.8) |
| Peripheral vascular disease | 36 (24.2) |
| Smoking history | 48 (32.2) |
| Cardiac profile | |
| Number of diseased coronary arteries | 2.8±0.4 |
| 1 | 4 (2.7) |
| 2 | 18 (12.1) |
| 3 | 127 (85.2) |
| Left main coronary artery stenosis ≥50% | 63 (42.3) |
| Previous myocardial infarction | 107 (71.8) |
| Left ventricular ejection fraction (%) | 43.0±12.6 |
| New York Heart Association Class III or IV | 48 (32.2) |
| EuroSCORE II | 3.8±3.5 |
| Renal profile | |
| Cause of end-stage renal disease | |
| Diabetes mellitus | 106 (71.1) |
| Hypertension | 16 (10.7) |
| Glomerulonephritis | 5 (3.4) |
| Polycystic kidney disease | 3 (2.0) |
| Systemic lupus erythematosus | 3 (2.0) |
| Renal cell carcinoma | 1 (0.7) |
| Obstructive uropathy | 1 (0.7) |
| Unknown | 14 (9.4) |
| Mode of renal replacement therapy | |
| Haemodialysis | 135 (90.6) |
| Peritoneal dialysis | 14 (9.4) |
Values for parametric continuous variables are expressed as mean ± standard deviation. Values for categorical variables are expressed as numbers (%).
Table 2. Operative variables.
| Variables | All patients (n=149) |
|---|---|
| Urgency of operation | |
| Elective | 112 (75.2) |
| Urgent | 29 (19.5) |
| Emergent | 8 (5.3) |
| Number of grafts | 3.0±0.9 |
| Left internal mammary artery to left anterior descending artery graft | 140 (94.0) |
| Number of venous grafts | 2.1±0.9 |
| Aortic cross clamp time (min) (n=117) | 50.1±14.4 |
| Cardiopulmonary bypass time (min) (n=138) | 95.3±26.8 |
Values for parametric continuous variables are expressed as mean ± standard deviation. Values for categorical variables are expressed as numbers (%).
Early postoperative outcomes
Operative mortality occurred in 20 patients (13.4%) and was higher in patients undergoing non-elective (emergent and urgent) surgery [27% (10 of 37 patients) vs. 8.9% (10 of 112 patients), P=0.005]. Amongst the 37 patients who underwent non-elective surgery, patients suffering early mortality had a higher EuroSCORE II compared to those who survived (10.4 vs. 5.2, P=0.008). Operative mortality was 40% (2 of 5 patients) in patients receiving only vein grafts (non-LIMA-LAD group) compared to 25% (8 of 32 patients) in LIMA-LAD graft recipients (P=0.482). The median duration of postoperative hospitalization was 11 days (25th percentile: 7 days, 75th percentile: 18 days). Causes of death included multi-organ failure in 11 patients (55.0%), sepsis in 5 (25.0%) and mesenteric ischaemia in 4 patients (20.0%). No patients suffered stroke or limb ischaemia.
Low cardiac output syndrome occurred in 36 patients (24.2%). Postoperative low cardiac output syndrome was associated with lower preoperative LVEF (38.2% vs. 44.5%, P=0.009), higher EuroSCORE II (6.3 vs. 3.0, P<0.001) and higher inpatient mortality [52.8% (19 of 36 patients) vs. 0.9% (1 of 113 patients), P<0.001], when compared to patients without this complication. Seventeen patients (11.4%) required insertion of an intra-aortic balloon pump for haemodynamic support.
Sepsis occurred in 32 patients (21.5%). The most common sources of sepsis were pneumonia in 21 patients (14.1%), intra-abdominal in 4 patients (2.7%), central venous catheter in 4 patients (2.7%), superficial sternal wound infection in 3 patients (2.0%), saphenous vein graft harvest site infection in 2 patients (1.3%) and urinary tract infection in 2 patients (1.3%). One patient (0.7%) developed deep sternal wound infection and mediastinitis.
Univariable analysis (Table 3) identified age (P<0.001), hyperlipidaemia (P=0.026), LVEF (P=0.005), NYHA class III or IV (P=0.019), left main coronary artery stenosis ≥50% (P=0.007), non-elective status of operation (P=0.01), and EuroSCORE II (P=0.002) as predictors of operative mortality. Multivariable analysis (Table 4) showed that age [P=0.001, odds ratio (OR): 1.15, 95% confidence interval (CI): 1.06–1.25], preoperative LVEF (P=0.020, OR: 0.94, 95% CI: 0.89–0.99) and non-elective status of operation (P=0.049, OR: 3.34, 95% CI: 1.01–11.1) were significant risk factors for operative mortality. Being a derived variable, EuroSCORE II (P=0.002) was excluded from multivariable analysis.
Table 3. Univariable analysis of factors contributing to operative mortality.
| Variables | Survivors (n=129) | Non-survivors (n=20) | P value |
|---|---|---|---|
| General characteristics | |||
| Age | 58.2±8.2 | 67.3±8.1 | <0.001 |
| Gender | |||
| Male | 92 (71.3) | 14 (70.0) | 0.904 |
| Female | 37 (28.7) | 6 (30.0) | |
| Body surface area (m2) | 1.70±0.19 | 1.70±0.20 | 0.876 |
| Comorbidities | |||
| Diabetes mellitus | 94 (72.9) | 15 (75.0) | 0.841 |
| Hypertension | 122 (94.6) | 19 (95.0) | 0.937 |
| Hyperlipidaemia | 102 (79.1) | 11 (55.0) | 0.026 |
| History of smoking | 42 (32.6) | 6 (30.0) | 0.820 |
| Peripheral vascular disease | 29 (22.5) | 7 (35.0) | 0.262 |
| Cardiac profile | |||
| Prior myocardial infarction | 91 (70.5) | 16 (80.0) | 0.382 |
| Left ventricular ejection fraction (%) | 44.1±12.5 | 35.8±10.9 | 0.005 |
| New York Heart Association Class III or IV | 37 (28.7) | 11 (55.0) | 0.019 |
| Left main coronary artery stenosis ≥50% | 49 (38.0) | 14 (70.0) | 0.007 |
| Number of diseased coronary arteries | 2.8±0.5 | 2.8±0.4 | 0.785 |
| EuroSCORE II | 3.2±2.6 | 7.8±5.6 | 0.002 |
| Renal profile | |||
| Mode of dialysis (haemodialysis/peritoneal dialysis) | 116 (89.9)/13 (10.1) | 19 (95.0)/1(5.0) | 0.694 |
| Operative variables | |||
| Non-elective surgery | 27 (20.9) | 10 (50.0) | 0.010 |
| Off-pump surgery | 10 (7.8) | 1 (5.0) | 1.000 |
| Cardiopulmonary bypass time (min) | 95.7±27.5 | 93.0±23.1 | 0.696 |
| Aortic cross clamp time (min) | 50.5±14.8 | 47.8±12.3 | 0.460 |
| Number of distal anastomoses | 3.0±0.9 | 3.0±0.9 | 0.943 |
| LIMA to LAD graft | 122 (94.6) | 18 (90.0) | 0.346 |
| Number of venous grafts | 2.1±0.9 | 2.1±0.9 | 0.888 |
Values for parametric continuous variables are expressed as mean ± standard deviation. Values for categorical variables are expressed as numbers (%). LAD, left anterior descending artery; LIMA, left internal mammary artery; CI, confidence interval.
Table 4. Multivariable analysis of factors contributing to operative mortality.
| Parameter | 95% CI | Odds ratio | P value |
|---|---|---|---|
| Age | 1.06–1.25 | 1.15 | 0.001 |
| Hyperlipidaemia | 0.12–1.45 | 0.42 | 0.170 |
| Peripheral vascular disease | 0.45–5.47 | 1.57 | 0.477 |
| Left ventricular ejection fraction | 0.89–0.99 | 0.94 | 0.020 |
| New York Heart Association Class III or IV | 0.63–6.92 | 2.10 | 0.225 |
| Left main coronary artery stenosis ≥50% | 0.55–6.80 | 1.94 | 0.301 |
| Non-elective operation | 1.01–11.1 | 3.34 | 0.049 |
CI, confidence interval.
Long-term prognosis
Data regarding follow-up was obtained by direct assessment during scheduled clinic reviews at our institution. Clinical follow-up was 100% complete for the 129 patients who survived to hospital discharge. The mean period of follow-up was 4.0±3.1 years. Ninety-nine patients (66.4%) died during the follow-up period. Kaplan-Meier analysis of long-term overall survival is shown in Figure 1. Overall survival was 81.1%, 63.5%, 41.5% and 19.2% at 1, 3, 5 and 10 years, respectively. The median survival was 4.3 years. Multivariable Cox-regression analysis (Table 5) showed that age (P<0.001, HR: 1.05, 95% CI: 1.03–1.08), NYHA Class III or IV status (P=0.010, HR: 1.75, 95% CI: 1.15–2.67) and the use of a LIMA-LAD graft (P=0.029, HR: 0.42, 95% CI: 0.19–0.92) were factors influencing long-term survival.
Figure 1.
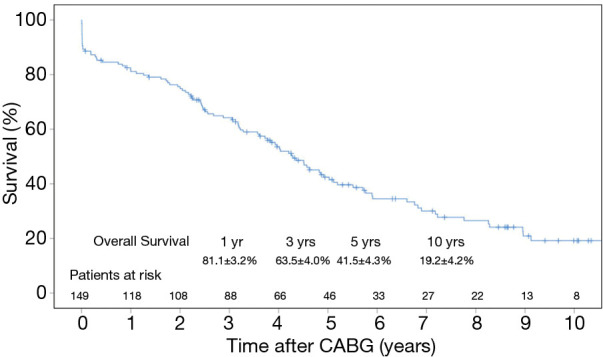
Overall survival of ESRD patients after CABG. CABG, coronary artery bypass grafting; ESRD, end-stage renal disease.
Table 5. Multivariable analysis of long-term survival.
| Parameter | 95% CI | Hazard ratio | P value |
|---|---|---|---|
| Age | 1.03–1.08 | 1.05 | <0.001 |
| Gender (male) | 0.50–1.20 | 0.80 | 0.277 |
| Peripheral vascular disease | 0.90–2.10 | 1.30 | 0.203 |
| Prior myocardial infarction | 0.60–1.70 | 1.00 | 0.865 |
| New York Heart Association Class III or IV | 1.15–2.67 | 1.75 | 0.010 |
| Mode of renal replacement therapy (peritoneal dialysis) | 0.98–3.50 | 1.80 | 0.071 |
| Non-elective surgery | 0.97–2.40 | 1.50 | 0.064 |
| LIMA to LAD graft | 0.19–0.92 | 0.42 | 0.029 |
CI, confidence interval; LAD, left anterior descending artery; LIMA, left internal mammary artery.
Discussion
There is a rising trend of ESRD worldwide, including in Singapore. Coronary artery disease is prevalent in these patients, with 35.9% of deaths in ESRD patients attributable to cardiac causes (2). This may be attributed to the presence of co-existing cardiovascular risk factors such as hypertension, hyperlipidaemia and diabetes. Pathological mechanisms in ESRD such as chronically elevated inflammatory markers and electrolyte derangements, in particular hypercalcaemia and hyperphosphataemia, predispose to vascular calcification and coronary atherosclerosis. Medical treatment of ESRD patients is challenging due to the presence of multiple concomitant comorbidities and drug interactions.
According to the Singapore Renal Registry, the overall survival of ESRD patients without ischaemic heart disease (IHD) was 92.7% at 1 year, 67.6% at 5 years and 44.9% at 10 years. The median survival was 8.6 years (2). IHD was associated with poorer survival in patients with ESRD (HR 1.50, 95% CI: 1.42–1.57, P<0.001) (2). For appropriately selected patients with ESRD and multi-vessel coronary artery disease, the 2014 ESC/EACTS guidelines recommends CABG over PCI for revascularization (3). Chang et al. analyzed data from the United States Renal Data system and found CABG to be associated with lower risks of death and cardiac events, when compared to PCI (5).
Compared with CABG in patients without ESRD, the perioperative risk and mortality is increased in ESRD patients, due to co-existing extracardiac comorbidities such as diabetes mellitus, cerebrovascular disease, peripheral vascular disease, uraemia, anaemia, mineral bone disease and electrolyte disturbances. Extensive coronary artery calcifications are often encountered in patients with ESRD, making CABG technically difficult. The risks of systemic embolism from aortic manipulation, bleeding and postoperative infections are also higher in patients with ESRD (6). Perioperative fluid management in ESRD patients is particularly challenging, and requires close collaboration between the surgical, intensive care and renal medicine teams.
There are limited studies reporting the long-term outcomes of isolated CABG in patients with ESRD. Sample sizes range from 24 to 152 patients in single-centre reports and larger cohorts of 326 to 14,316 patients from multicenter studies or data from national databases (5,7-14). Some of these studies include heterogenous populations of patients undergoing both CABG and concomitant valvular or aortic surgery (5,10,14). In a cohort of 6,874 non-ESRD patient and 174 ESRD patients who underwent CABG, Bianco et al. identified dialysis dependence as an independent predictor of mortality at 30 days [hazard ratio (HR), 3.86; 95% confidence interval (CI), 2.96, 5.03; P<0.001] (7). In patients with ESRD undergoing CABG, age, diabetes mellitus, peripheral vascular disease, a history of stroke, mode of dialysis, emergent surgery, LVEF, NYHA class, use of CPB and postoperative continuous haemofiltration have been identified as predictors of inpatient mortality and long-term survival (8,11,15-18).
The mean age of our study cohort (59.4 years) is similar to other similar studies, ranging from 63.0 to 65.4 years (5,7,8,11). Early postoperative mortality rates range between 7.8% to 18.7% (7,8,10,11,14,15). The operative mortality rate of 13.4% at our centre is comparable with previous reports. In the present study, age, LVEF and non-elective status of surgery were associated with a higher risk of early mortality. This is consistent with other studies (8,11). Five-year survival of ESRD patients undergoing CABG range widely from 25% to 60% (5,10,11). Takami et al. reported a 10-year survival of 36% (11). Results from our study show that the long-term prognosis of patients with ESRD remains guarded despite CABG. Long-term survival following CABG were 81.1%, 41.5%, 19.2% at 1, 5 and 10 years respectively, with a median survival of 4.3 years.
From our study, advanced age and NYHA class III or IV were negative predictors of long-term survival. Other predictors of death during long-term follow-up reported previously include age, peripheral vascular disease, diabetes mellitus, and emergency surgery (10,11). The proportion of patients with diabetes mellitus in studies comprising patients with ESRD undergoing isolated CABG ranges from 54% to 70% (7,8,11). Although a large proportion (73.2%) of the patients in our study had diabetes mellitus, we did not demonstrate an association with poorer long-term survival.
The presence of an IMA graft significantly improves survival of patients undergoing CABG (19). In the present study, multivariable analysis identified LIMA-LAD grafting as a factor conferring a long-term survival benefit in patients with ESRD. Bechtel et al. reported results from a retrospective multi-centre study of 522 patients with ESRD undergoing CABG, of which 326 patients underwent isolated CABG. In this study, the authors found that the use of an IMA graft was associated with a survival benefit in ESRD patients (10). Subsequent renal transplantation was also associated with improved long-term survival (10,12).
The proportion of patients undergoing off-pump surgery in previous reports ranges from 2.7% to 64.4% (7-9,11-13). In the present study, only 7.4% of our cohort underwent off-pump CABG. Several studies have compared off-pump and on-pump CABG in ESRD patients, with varying results. Ariyoshi et al. reported significant differences in morbidity rates amongst patients who underwent off-pump CABG compared to those who underwent conventional CABG (16). Ueki et al. reported significantly lower surgical mortality (OR: 0.68 95% CI: 0.51–0.90) favouring off-pump CABG (17). Chen et al. did not find a significant difference in survival between on and off-pump groups (9).
Limitations
This is a retrospective observational study with inherent biases in data collection. There is a likelihood of selection bias, as LIMA-LAD grafting may not have been performed in patients who were in a more critical preoperative state. Due to the small sample size, statistical analyses may have been underpowered, especially in the subgroups of patients undergoing off-pump CABG and those not receiving a LIMA-LAD graft. Being conducted at a single centre, the surgical technique and postoperative care adopted were standardized. The same group of surgeons performed the surgeries throughout the study period. However, our results may not be generalizable to all centres. We did not compare outcomes of our surgical cohort with ESRD patients with coronary artery disease who had undergone PCI or received medical therapy alone.
Conclusions
This study investigates the survival outcomes and prognostic factors of 149 patients with ESRD who underwent isolated CABG. Operative mortality was 13.4%. Five and ten-year survival rates were 41.5% and 19.2% respectively. Age, NYHA class, urgency of surgery and preoperative LVEF could be useful prognostic factors for risk stratification. LIMA-LAD grafting conferred a long-term survival advantage and should be the standard of care in ESRD patients undergoing CABG.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors thank Clara Zhang and Selena Chew for their editorial assistance. Oral presentation (2nd prize) at the 28th Annual Congress of the Association of the Thoracic and Cardiovascular Surgeons of Asia, 26 Oct 2018, Indonesia.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the local institutional review board with a waiver of patient consent (reference: 2016/3132). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2046
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2046
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-2046
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2046). The authors have no conflicts of interest to declare.
References
- 1.Hori D, Yamaguchi A, Adachi H. Coronary artery bypass surgery in end-stage renal disease patients. Ann Vasc Dis 2017;10:79-87. 10.3400/avd.ra.17-00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singapore Renal Registry Annual Report 2017. Available online: https://www.nrdo.gov.sg/docs/librariesprovider3/publications---kidney-failure/srr-annual-report-2017.pdf
- 3.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2014;35:2541-619. 10.1093/eurheartj/ehu278 [DOI] [PubMed] [Google Scholar]
- 4.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573-7. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 5.Chang TI, Shilane D, Kazi DS, et al. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol 2012;23:2042-9. 10.1681/ASN.2012060554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takami Y, Tajima K, Kato W, et al. Effects of the side of arteriovenous fistula on outcomes after coronary artery bypass surgery in hemodialysis-dependent patients. J Thorac Cardiovasc Surg 2014;147:619-24. 10.1016/j.jtcvs.2013.01.031 [DOI] [PubMed] [Google Scholar]
- 7.Bianco V, Kilic A, Gleason TG, et al. Longitudinal outcomes of dialysis-dependent patients undergoing isolated coronary artery bypass grafting. J Card Surg 2019;34:110-7. 10.1111/jocs.13991 [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi T, Miyata H, Sakaguchi T, et al. Coronary artery bypass grafting in hemodialysis-dependent patients: analysis of Japan Adult Cardiovascular Surgery Database. Circ J 2012;76:1115-20. 10.1253/circj.CJ-11-1146 [DOI] [PubMed] [Google Scholar]
- 9.Chen JJ, Lin LY, Yang YH, et al. On pump versus off pump coronary artery bypass grafting in patients with end-stage renal disease and coronary artery disease - A nation-wide, propensity score matched database analyses. Int J Cardiol 2017;227:529-34. 10.1016/j.ijcard.2016.10.108 [DOI] [PubMed] [Google Scholar]
- 10.Bechtel JF, Detter C, Fischlein T, et al. Cardiac surgery in patients on dialysis: decreased 30-day mortality, unchanged overall survival. Ann Thorac Surg 2008;85:147-53. 10.1016/j.athoracsur.2007.08.048 [DOI] [PubMed] [Google Scholar]
- 11.Takami Y, Tajima K, Kato W, et al. Predictors for early and late outcomes after coronary artery bypass grafting in hemodialysis patients. Ann Thorac Surg 2012;94:1940-5. 10.1016/j.athoracsur.2012.07.037 [DOI] [PubMed] [Google Scholar]
- 12.Mourad F, Cleve N, Nowak J, et al. Long-term single centre outcomes of patients with chronic renal dialysis undergoing cardiac surgery. Ann Thorac Surg 2020;109:1442-8. 10.1016/j.athoracsur.2019.08.042 [DOI] [PubMed] [Google Scholar]
- 13.Kurazumi H, Takahashi M, Ikenaga S. Outcomes of cardiovascular surgery for chronic dialysis patients in current Japan. Asian Cardiovasc Thorac Ann 2019;27:464-70. 10.1177/0218492319859147 [DOI] [PubMed] [Google Scholar]
- 14.Rahmanian PB, Adams DH, Castillo JG, at al. Early and late outcome of cardiac surgery in dialysis-dependent patients: single-center experience with 245 consecutive patients. J Thorac Cardiovasc Surg 2008;135:915-22. 10.1016/j.jtcvs.2007.09.027 [DOI] [PubMed] [Google Scholar]
- 15.Li HY, Chang CH, Lee CC, et al. Risk analysis of dialysis-dependent patients who underwent coronary artery bypass grafting: Effects of dialysis modes on outcomes. Medicine (Baltimore) 2017;96:e8146. 10.1097/MD.0000000000008146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariyoshi T, Eishi K, Yamachika S, et al. Perioperative and mid-term results of coronary bypass surgery in patients undergoing chronic dialysis. Ann Thorac Cardiovasc Surg 2006;12:257-64. [PubMed] [Google Scholar]
- 17.Ueki C, Miyata H, Motomura N, et al. Off-pump technique reduces surgical mortality after elective coronary artery bypass grafting in patients with preoperative renal failure. J Thorac Cardiovasc Surg 2018;156:976-83. 10.1016/j.jtcvs.2018.03.145 [DOI] [PubMed] [Google Scholar]
- 18.Horst M, Mehlhorn U, Hoerstrup SP, et al. Cardiac surgery in patients with end-stage renal disease: 10-year experience. Ann Thorac Surg 2000;69:96-101. 10.1016/S0003-4975(99)01133-9 [DOI] [PubMed] [Google Scholar]
- 19.Cameron A, Davis KB, Green G, et al. Coronary bypass surgery with internal-thoracic-artery grafts--effects on survival over a 15-year period. N Engl J Med 1996;334:216-9. 10.1056/NEJM199601253340402 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


