This cross-sectional study describes electrocardiographic and echocardiographic findings in healthy elite US soccer players.
Key Points
Question
What are normal electrocardiographic and echocardiographic findings in elite female and male US soccer players?
Findings
In this cross-sectional study of 238 elite US soccer players, we found that male athletes frequently displayed common training-related electrocardiography changes, whereas female athletes had significantly more abnormal electrocardiograms per the International Recommendations for Electrocardiographic Interpretation in Athletes. Both female and male athletes frequently exceeded American Society of Echocardiography normative value standards for basic echocardiographic parameters.
Meaning
Elite US soccer players frequently present with training-related electrocardiography patterns and echocardiographic parameters that are above guideline-defined normal ranges.
Abstract
Importance
Population-specific normative data are essential for the evaluation of competitive athletes. At present, there are limited data defining normal electrocardiographic (ECG) and echocardiographic values among elite US soccer players.
Objective
To describe ECG and echocardiographic findings in healthy elite US soccer players.
Design, Setting, and Participants
This cross-sectional study analyzed Fédération Internationale de Football Association–mandated screening sessions performed at US Soccer National Team training locations from January 2015 to December 2019. US women’s and men’s national team soccer players undergoing mandated cardiovascular screening were included.
Main Outcomes and Measures
Normal training-related and abnormal ECG findings were reported using the International Recommendations for Electrocardiographic Interpretation in Athletes. Echocardiographic measurements of structural and functional parameters relevant to cardiovascular remodeling were assessed relative to American Society of Echocardiography guideline–defined normal ranges.
Results
A total of 238 athletes (122 [51%] female; mean [SD] age, 20 [4] years; age range, 15-40 years) were included. Male athletes demonstrated a higher prevalence of normal training-related ECG findings, while female athletes were more likely to have abnormal ECG patterns (14 [11%] vs 0 in male cohort), largely accounted for by abnormal T-wave inversions. Echocardiography revealed no pathologic findings meeting criteria for sport restriction, but athletes frequently exceeded normal ranges for structural cardiac parameters responsive to exercise-induced remodeling including body surface area–indexed left ventricular (LV) mass (58 of 113 female athletes [51%] and 67 of 114 male athletes [59%]), indexed LV volume (89 of 115 female athletes [77%] and 76 of 111 male athletes [68%]), and LV wall thickness (37 of 122 female athletes [30%] and 47 of 116 male athletes [41%]). Age-stratified analysis revealed age-dependent increases in LV wall thickness, mass, and volumes among female athletes and LV wall thickness and mass among male athletes.
Conclusions and Relevance
These data represent the first set of comprehensive normative values for elite US soccer players and one of the largest sport-specific echocardiographic remodeling studies in female athletes. Abnormal ECG findings were more common in female athletes, while both female and male athletes frequently exceeded clinical normality cut points for remodeling-associated echocardiographic parameters.
Introduction
Soccer, internationally referred to as football, is the world’s most popular sport, and soccer players are increasingly encountered in clinical cardiovascular practice. Clinicians are commonly asked to assess soccer players with symptoms suggestive of cardiovascular disease and to oversee preparticipation screening of asymptomatic athletes. In response to numerous highly publicized cardiac arrest cases occurring during soccer matches,1 the Fédération Internationale de Football Association (FIFA) mandates preparticipation screening with electrocardiography (ECG) and echocardiography prior to international competition.2,3 It is anticipated that this practice will become more widely adopted during return to competitive soccer in the wake of the coronavirus disease 2019 (COVID-19) pandemic given concerns about cardiac complications of infection.4,5,6
Exercise-induced cardiac remodeling is the process by which the cardiovascular system adapts to the hemodynamic stress imposed by athletic training.7 Determinants of the structural, functional, and electrophysiologic manifestations of exercise-induced cardiac remodeling include age,8 duration of sport exposure,9 sex,10 ethnicity,11 and sport type.12,13,14 Accurate differentiation of benign exercise-induced cardiovascular adaptations from high-risk pathology, a process that requires sport- and sex-specific normative data, underlies the successful clinical evaluation of symptomatic and asymptomatic athletes. To date, normative data integrating ECG and echocardiography among elite US soccer players, particularly women, have been lacking.
Accordingly, we conducted a prospective 5-year study of US national team soccer players participating in FIFA-mandated preparticipation screening with several discrete objectives. First, we sought to develop normative data characterizing ECG and echocardiographic findings among asymptomatic elite soccer players. Next, we aimed to determine the prevalence of abnormal ECG findings and their association with underlying pathology as determined by concomitant echocardiography. It is anticipated that the results of this study will provide valuable reference standards during future clinical evaluations among the large global population of competitive female and male soccer players.
Methods
This prospective cross-sectional study included female and male members of US Soccer national teams who underwent FIFA-mandated preparticipation screening prior to international World Cup competitions during a 5-year study period (January 2015-December 2019). Repeat evaluation was performed in a subset of individuals participating in multiple competitions. Screening was performed at US Soccer training sites by experienced clinicians affiliated with the Massachusetts General Hospital Cardiovascular Performance Program. The Mass General Brigham institutional review board approved this study and waived the need for informed consent based on use of deidentified data obtained during routine clinical care.
Data Acquisition
Demographic data including race was self-reported by participants. ECGs were performed by experienced cardiologists (T.W.C. and A.L.B.) using standard 12-lead placement and commercially available ECG software (Cardea 20/20 ECG; Cardiac Insight) after 5 to 10 minutes of quiet rest. Two-dimensional transthoracic echocardiography was performed using a commercially available system (Vivid-Q; GE Healthcare). Two-dimensional, pulsed-Doppler, and color tissue Doppler imaging from parasternal, apical, and subcostal transducer positions was performed with 3-beat acquisition.15 Two-dimensional frame rate of 50 to 75 seconds and tissue Doppler frame rate of more than 120 seconds were maintained for all images.
ECG Data Analysis
ECGs were reviewed in a digital printable format, which included athlete age, sex, and ethnicity by 1 of 2 readers (T.W.C. and B.J.P.) with experience in athlete ECG interpretation. Readers were blinded to all other athlete characteristics. ECGs were coded to define the presence/absence of all components of the International Recommendations for Electrocardiographic Interpretation in Athletes (henceforth referred to as the international criteria).16 ECGs were defined as abnormal if they contained 1 or more abnormal findings or 2 or more borderline findings.16 Quantitative measurements including heart rate, PR, QT, corrected QT intervals (calculated by the Bazett formula17), QRS duration, and P and R wave axis were calculated automatically and confirmed by visual inspection. Corrected QT intervals of 450 milliseconds or more were manually confirmed using the tangent method on the lead with the longest absolute QT interval.18
ECG definitions were as defined in the international criteria,16 with additional criteria as follows in cases where exact definitions were not present. Voltage criteria for left ventricular (LV) hypertrophy was quantified as a dichotomous variable based on either Romhilt Estes19 score more than 4 or fulfilment of Sokolow Lyon criteria20 (S in V1 + R in V5 or V6 ≥ 35 mm). Right ventricular hypertrophy was defined based on the Sokolow Lyon criteria (R wave in V1 + S wave in V5 or V6 > 10.5 mm).21 Early repolarization was defined as a J point elevation of more than 1 mm in 2 leads in either lateral (V4-V6, I, aVL) and/or inferior territories (II, III, aVF).22 Sinus arrhythmia was defined as PP interval variation more than 120 milliseconds. T-wave inversions (TWI) were defined as 1 mm or more in depth in 2 or more continuous leads excluding III, aVR, and V1.
Echocardiographic Data Analysis
Echocardiographic measurements were performed by a single experienced echocardiographer (A.L.B.) on commercially available software (EchoPac, version 6.5; GE Healthcare). All measurements were made according to American Society of Echocardiography guidelines.23 LV relative wall thickness (RWT) was calculated as 2 × (posterior wall thickness) / LV end-diastolic dimension. LV volumes and LV ejection fraction (LVEF) were measured using the modified Simpson biplane method of disks. LV mass was calculated using the area-length technique. Chamber dimensions and LV mass were indexed to body surface area, which was calculated using the Mosteller formula.24 Tissue velocities were measured offline from 2-dimensional pulsed-wave Doppler images. Tissue and spectral Doppler measurements were performed in triplicate. As most individuals lacked sufficient tricuspid regurgitation for Doppler evaluation, pulmonary artery systolic pressure was estimated from pulmonary artery acceleration time.25
Intraobserver variability analysis was performed through blinded reassessment of 2 key metrics (LV mass and LV end-diastolic volume [LVEDV]) for 10 randomly selected individuals from the female and male cohorts (n = 20). Correlation for each measurement was assessed using linear regression with the following results: LV mass (R2 = 0.97) and LVEDV (R2 = 0.97).
Statistical Analysis
Continuous variables are presented as mean (SD), with the range (minimum − maximum) included for ECG and echocardiographic measurements. Categorical variables are presented as number (%). Significance of differences across groups was assessed using t test, χ2 testing, or 1-way analysis of variance. Distributions of important echocardiographic parameters were compared with sex-specific, general population reference values.23 Two-sided P values had a significance threshold of .05.
Results
Study Population
A total of 238 athletes (122 female athletes [51.3%] and 116 male athletes [48.7%]) from the US National Soccer teams were included in this study (Table 1). The mean (SD) age was 20 (4) years (range, 15-40 years). Female athletes were predominantly White (87 [71%]) with fewer Black (23 [19%]) and Hispanic (8 [7%]) players, whereas male players were evenly divided between Black (40 [34%]), Hispanic (38 [33%]), and White individuals (37 [32%]). Compared with male soccer players, the female soccer players in this sample were older and had smaller body size (height, weight, body mass index, and body surface area).
Table 1. Clinical and Electrocardiographic Characteristics by Sex.
| Clinical characteristic | Mean (SD) | |
|---|---|---|
| Female athletes (n = 122) | Male athletes (n = 116) | |
| Age, y | 21 (5.4) | 18 (1.2)a |
| Height, in | 66 (2.6) | 71 (2.9)a |
| Weight, lb | 136 (16.2) | 160 (16.2)a |
| BMI | 22 (1.8) | 22 (1.6)a |
| Body surface area, m2 | 1.7 (0.1) | 1.9 (0.1)a |
| Race/ethnicity, No. (%) | ||
| Hispanic | 8 (7) | 38 (33)a |
| Black | 23 (19) | 40 (34)a |
| White | 87 (71) | 37 (32)a |
| Otherb/unknown | 4 (3) | 1 (1) |
| ECG data | ||
| Heart rate, beats/min | 56 (9) | 58 (9) |
| Intervals, mean (SD) [range], ms | ||
| PR | 154 (26) [104 to 244] | 166 (24) [114 to 252]a |
| QRS | 87 (8) [66 to 108] | 94 (9) [72 to 122]a |
| QTc | 415 (23) [361 to 481] | 403 (21) [349 to 460]a |
| QRS axis, mean (SD) [range], ° | 73 (23) [−69 to 147] | 75 (21) [−40 to 119] |
| International ECG criteria, No. (%) | ||
| Normal ECG findings | ||
| Increased QRS voltage for LVH | 13 (11) | 74 (64)a |
| Increased QRS voltage for RVH | 1 (1) | 17 (15)a |
| Incomplete right bundle branch block | 5 (4) | 15 (13)a |
| Early repolarization | 35 (29) | 97 (84)a |
| Black athlete repolarization pattern | 3 (3) | 7 (6) |
| Sinus bradycardia | 85 (70) | 74 (64) |
| Sinus arrhythmia | 57 (47) | 50 (43) |
| Ectopic atrial rhythm | 5 (4) | 13 (11) |
| Junctional rhythm | 2 (2) | 0 |
| First degree AV block | 8 (7) | 12 (10) |
| Mobitz type 1 2° AV block | 0 | 0 |
| Borderline ECG findings | ||
| Left axis deviation | 1 (1) | 1 (1) |
| Left atrial enlargement | 2 (2) | 4 (3) |
| Right axis deviation | 1 (1) | 0 |
| Right atrial enlargement | 2 (2) | 2 (2) |
| Complete right bundle branch block | 0 | 1 (1) |
| Abnormal ECG findings | ||
| T-wave inversion | 9 (7) | 0a |
| ST-segment depression | 1 (1) | 0 |
| Pathologic Q waves | 2 (2) | 0 |
| Complete left bundle branch block | 0 | 0 |
| QRS ≥140 ms duration | 0 | 0 |
| Epsilon wave | 0 | 0 |
| Ventricular pre-excitation | 0 | 0 |
| Prolonged QT interval | 1 (1) | 0 |
| Brugada type 1 pattern | 0 | 0 |
| Profound sinus bradycardia (<30 beats per min) | 0 | 0 |
| PR interval ≥400 ms | 0 | 0 |
| Mobitz type II 2° AV block | 0 | 0 |
| 3° AV block | 0 | 0 |
| ≥2 Premature ventricular contractions | 0 | 0 |
| Tachyarrhythmia | ||
| Atrial | 0 | 0 |
| Ventricular | 0 | 0 |
Abbreviations: AV, atrioventricular; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ECG, electrocardiography; LVH, left ventricular hypertrophy; RVH, right ventricular hypertrophy.
P < .05.
The other category includes people who self-identified as Asian/Pacific Islander, >1 race/ethnicity, and/or indicated that the available options failed to adequately describe them.
ECG Findings
ECG findings including basic normative data and the prevalence of specific ECG findings as defined by the international criteria are presented in Table 1. Compared with male athlethes, female athletes had shorter PR (mean [95% CI], 154 [149-159] vs 166 [162-171] milliseconds; P < .001) and QRS intervals (mean [95% CI], 87 [85-88] vs 94 [93-96] milliseconds; P < .001) but longer corrected QT intervals (mean [95% CI], 415 [411-419] vs 403 [399-407] milliseconds; P < .001). Male athletes demonstrated a higher prevalence of normal training-related ECG findings including voltage criteria for LV hypertrophy (74 [64%] vs 13 [11%]; P < .001) and right ventricular hypertrophy (17 [15%] vs 1 [1%]; P < .001), incomplete right bundle branch block (15 [13%] vs 5 [4%]; P = .03), and early repolarization pattern (97 [84%] vs 35 [29%]; P < .001). The prevalence of abnormal ECGs was higher in female athletes than among male athletes (14 of 122 [11%] vs 0 of 116; P < .001). The most common abnormal ECG pattern was pathologic TWI (9 of 14 [69%]), which were most commonly isolated to the anterior leads (7 of 9) and less often involved anterolateral (V3-V6) and inferior (II, III, aVF) leads (2 of 9). Additional abnormal ECG findings included septal Q waves (2 of 14 [14.3%]), inferior ST-segment depressions (1 of 14 [7.1%]), and a mildly prolonged corrected QT interval (481 milliseconds; 1 of 14 [7.1%]).
Echocardiographic Findings
Echocardiographic data are presented in Table 2. Compared with female athletes, male athletes had greater maximum wall thickness (mean [95% CI], 10.0 [9.8-10.3] vs 8.9 [8.7-9.1] mm; P < .001), RWT (mean [95% CI], 0.38 [0.37-0.39] vs 0.36 [0.35-0.37]; P = .04), indexed LVEDV (mean [95% CI], 81 [79-84] vs 70 [68-72] mL/m2; P < .001), and indexed LV mass (mean [95% CI], 108 [105-111] vs 91 [88-93] g/m2; P < .001). Only 1 athlete (a 28-year old woman) had an LV end-diastolic dimension more than 60 mm, measuring 61 mm. As shown in Figure 1A, 37 of 122 female athletes (30%) and 47 of 116 male athletes (41%) exceeded American Society of Echocardiography upper limits of normal for LV wall thickness. LV wall thickness of 12 mm or more was seen in 14 male athletes (12%) but only in 1 female athlete (1%), and maximum LV wall thickness among all individuals was 13 mm (present in only 2 male athletes).
Table 2. Echocardiographic Findings by Sex.
| Characteristic | Mean (SD) [range] | P value | |
|---|---|---|---|
| Female athletes (n = 122) | Male athletes (n = 116) | ||
| Structural | |||
| Interventricular septum, mm | 8.3 (1.2) [6-12] | 9.3 (1.3) [6-13]a | <.001 |
| Posterior wall, mm | 8.6 (1.2) [6-11] | 9.8 (1.4) [7-13]a | <.001 |
| Relative wall thickness | 0.36 (0.06) [0.23-0.54] | 0.38 (0.06) [0.26-0.56]a | .04 |
| Maximum wall thickness, mm | 8.9 (1.2) [6-12] | 10 (1.3) [7-13]a | <.001 |
| LV end-diastolic dimension, mm | 48 (3.6) [39-61] | 52 (3.7) [39-60]a | <.001 |
| LV end-systolic dimension, mm | 31 (3.0) [24-40] | 34 (3.7) [25-45]a | <.001 |
| LV outflow tract, mm | 19 (1.7) [15-22] | 21 (1.9) [16-28]a | <.001 |
| Aortic root: sinuses of valsalva, mm | 26 (2.5) [20-33] | 29 (2.7) [23-36]a | <.001 |
| Left atrium antero-posterior dimension, mm | 33 (4.0) [22-44] | 37 (3.6) [28-47]a | <.001 |
| Right ventricular basal diameter, end-diastole, mm | 38 (4.2) [27-51] | 42 (4.4) [32-54]a | <.001 |
| LV length, mm | 83 (6.6) [48-101] | 89 (5.7) [73-101]a | <.001 |
| LV mass, g | 155 (27) [105-225] | 205 (34) [130-309]a | <.001 |
| LV mass/BSA, g/cm2 | 91 (13) [64-122] | 108 (16) [74-156]a | <.001 |
| Volumes and ventricular function | |||
| LV end-diastolic volume, mL | 120 (22) [74-180] | 155 (26) [105-228]a | <.001 |
| LV end-diastolic volume/BSA, mL/cm2 | 70 (11) [46-96] | 81 (14) [58-127]a | <.001 |
| LV end-systolic volume, mL | 43 (10) [23-87] | 58 (12) [34-98]a | <.001 |
| LV ejection fraction, % | 64 (5.5) [47-75] | 63 (5.3) [49.6-77]a | .03 |
| Spectral and tissue doppler | |||
| Mitral inflow velocity | |||
| Early (E wave), cm/s | 95 (18) [57-133] | 92 (17) [59-143] | .25 |
| Late (A wave), cm/s | 40 (10) [22-73] | 42 (11) [20-68] | .09 |
| E/A ratio | 2.5 (0.72) [1.3-5.6] | 2.3 (0.68) [1.3-6.0]a | .04 |
| Lateral e′, cm/s | 18 (2) [14-20] | 21 (3) [17-27]a | <.001 |
| Pulmonary artery acceleration time, ms | 173 (20) [122-214] | 166 (19) [121-210]a | .02 |
| Estimated pulmonary artery systolic pressure, mm Hgb | 26.1 (4.8) [18-41] | 27.7 (4.8) [18-41]a | .02 |
Abbreviations: BSA, body surface area; LV, left ventricular.
P < .05.
Estimated pulmonary artery systolic pressure was calculated using pulmonary artery acceleration time according to published formula.25
Figure 1. Left Ventricular (LV) Wall Thickness and Remodeling Patterns Among Female and Male Elite Soccer Players.
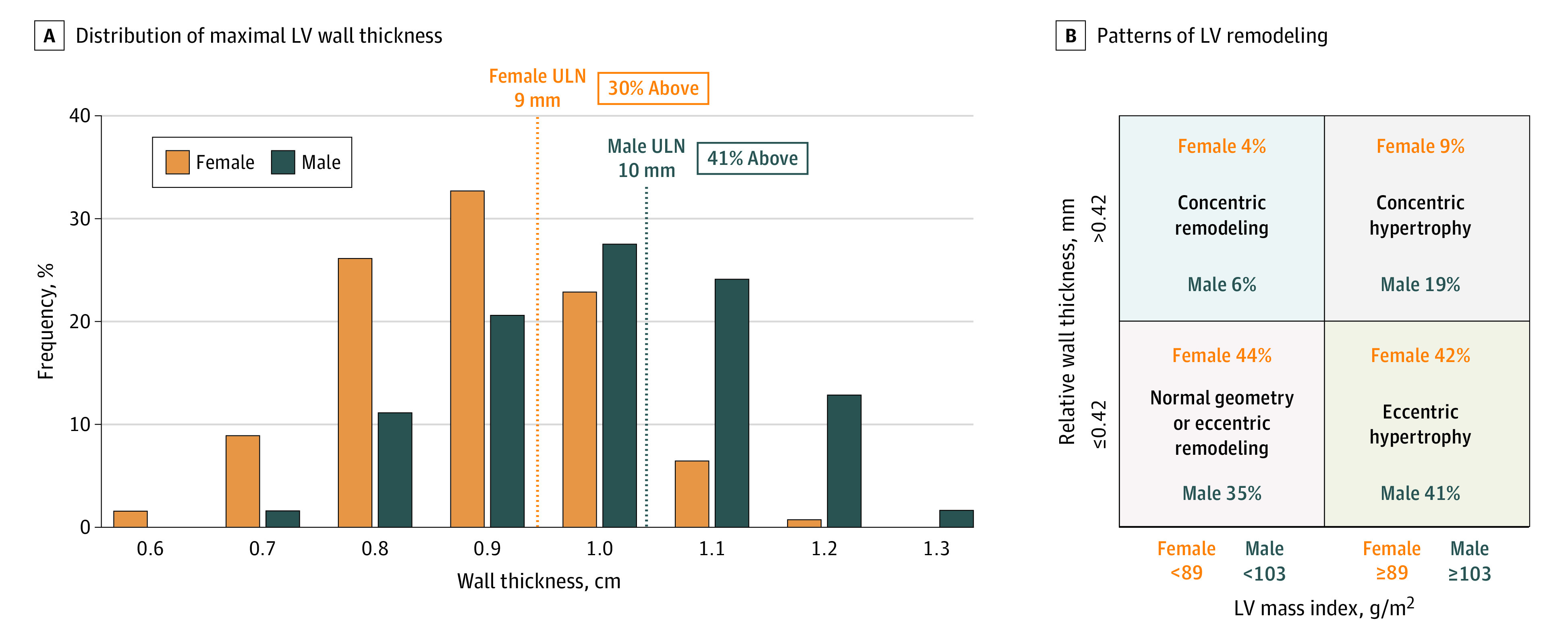
A, The distribution of maximal LV wall thickness is shown for female and male soccer players. Overall, 31% of female athletes and 41% of male athletes had wall thickness above the American Society of Echocardiography–defined normal range.23 The maximal wall thickness observed was 1.3 cm. B, Patterns of LV remodeling in the study population are shown, stratified based on relative wall thickness and sex-specific LV mass index, according to guideline framework.23 ULN indicates upper limit of normal.
Assessment of LV hypertrophy/remodeling geometry according to clinical guideline definitions23 (Figure 1B) revealed that a majority of athletes had either eccentric hypertrophy (RWT ≤0.42 and LV mass index above sex-specific normal ranges; 48 of 113 female athletes [42%] and 46 of 114 male athletes [41%]) or normal LV geometry (RWT ≤0.42 and normal LV mass index; 50 of 113 female athletes [44%] and 40 of 114 male athletes [35%]). Concentric remodeling patterns (either concentric remodeling or concentric hypertrophy) were more common in male athletes (28 of 114 [25%]) than in female athletes (15 of 113 [13%]) (P < .05). Diastolic function was normal in all athletes, but female athletes had lower lateral LV early tissue Doppler velocity (E′) (mean [95% CI], 18 [17-19] vs 21 [20-22] cm/s; P < .001) than their male counterparts. None of the athletes with abnormal TWI (all female) had evidence of pathology by echocardiography, but compared with female athletes without TWI, this group had higher indexed LV mass (mean [95% CI], 102.0 [92.8-111.3] vs 90.1 [87.7-92.6] g/m2; P = .02) and indexed LVEDV (mean [95% CI], 77.5 [68.8-86.2] vs 69.2 [67.2-71.2] mL/m2; P = .03). Four players (2 male athletes and 2 female athletes) had bicuspid aortic valves, but none had clinically relevant valvular dysfunction or aortic enlargement. There were no cases identified of significant valvular regurgitation (≥moderate in severity) or valvular stenosis.
The distribution of echocardiographic parameters compared with clinical normative cut points is shown in Figure 2. A total of 58 of 113 female athletes (51%) and 97 of 114 male athletes (59%) demonstrated elevated body surface area–indexed LV mass, and an even higher proportion of athletes were found to have increased indexed LVEDV (89 of 115 female athletes [77%] and 76 of 111 male athletes [68%]). Increased RV basal diameter was more common in male than female athletes (60 of 112 [54%] vs 16 of 118 [14%]; P < .001). While the mean LVEF was in the normal range and slightly higher for female athletes (mean [95% CI], 64 [63-65] vs 63 [62-64]; P = .03), 17 of 231 athletes with measured LVEF (7.4%; 8 female and 9 male) had an LVEF less than 55%, with 9 below the guideline-defined normal ranges of less than 54% for female athletes (n = 7) and less than 52% for male athletes (n = 2). Selected LV structural parameters stratified by age groups are shown in Figure 3. Among female athletes, there was a significant increase in mean LV wall thickness, indexed LVEDV, and LV mass index with increasing age. In contrast, male athletes showed age-dependent increases in LV wall thickness and LV mass index but not indexed LVEDV.
Figure 2. Structural and Functional Parameters Among Female and Male Elite Soccer Players.
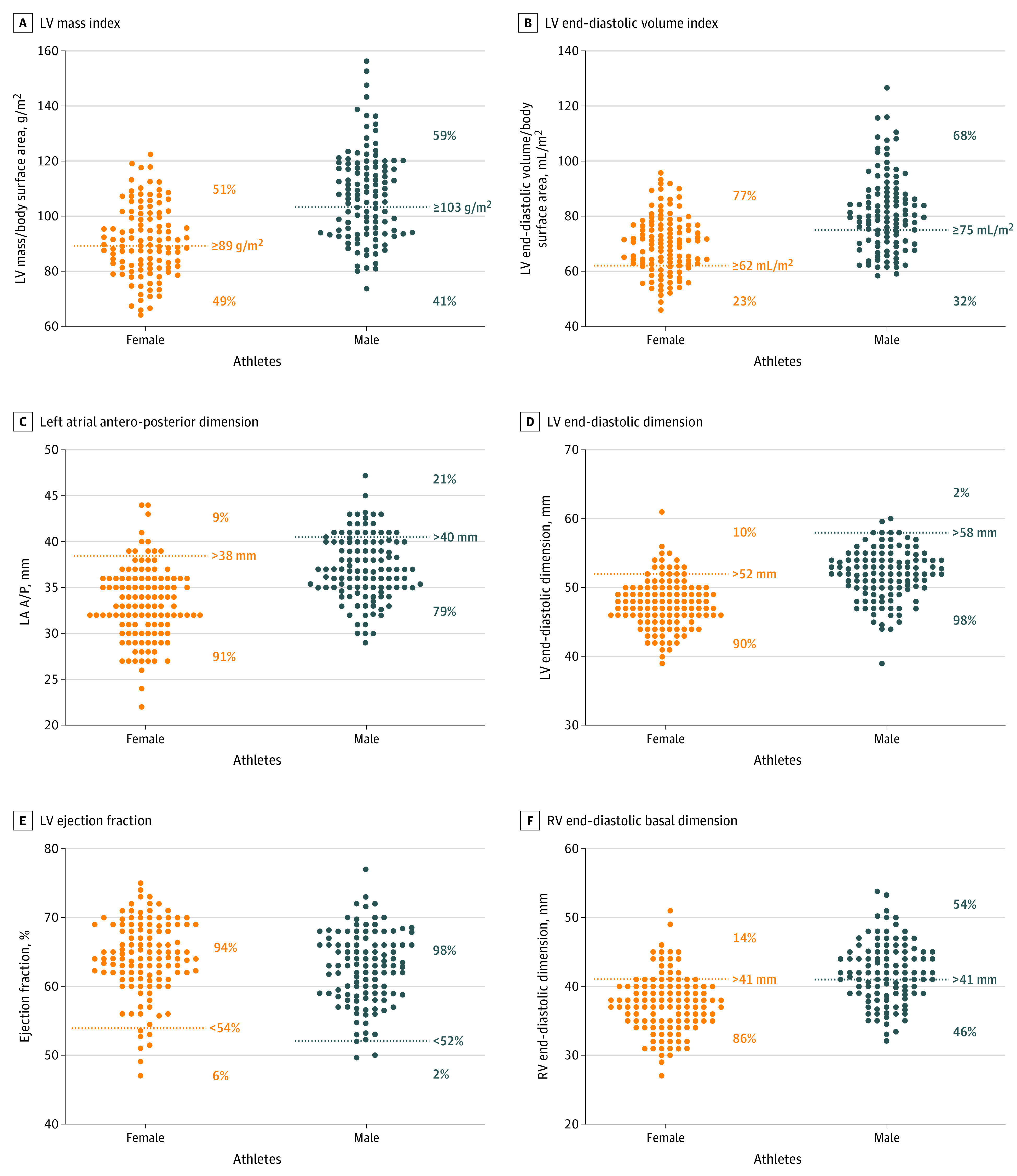
Sex-specific distributions of 6 key structural and functional echocardiographic parameters are shown for members of the US Women’s and Men’s national soccer teams, along with American Society of Echocardiography–defined normal ranges and the percentage falling within and outside the normal range. BSA indicates body surface area; LA A/P, left atrial antero-posterior dimension; LV, left ventricular; LVEDD, left ventricular end-diastolic dimension; LVM, left ventricular mass; RV, right ventricular; RVEDD, right ventricular end-diastolic dimension.
Figure 3. Differences in Key Left Ventricular (LV) Structural Parameters Across Different Age Groups.
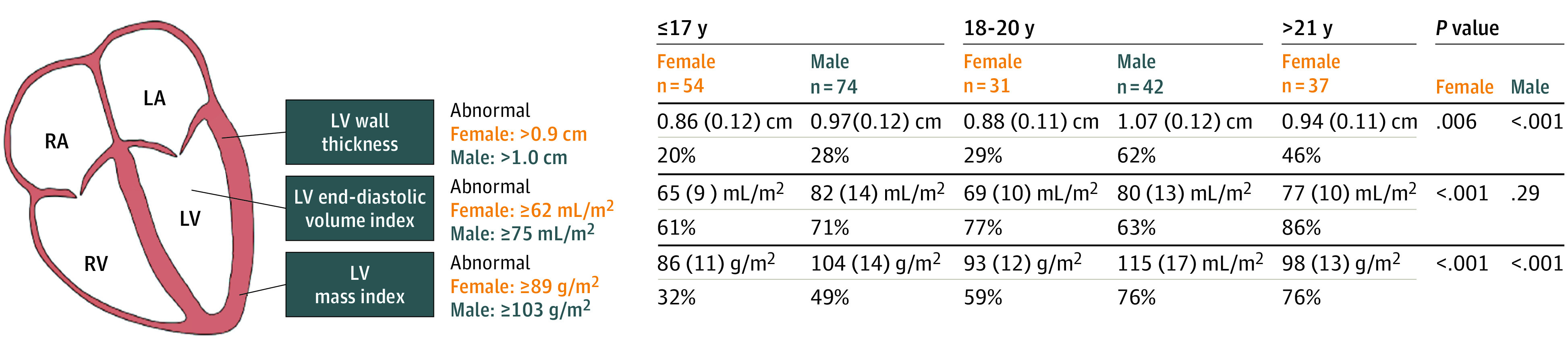
Three key structural echocardiographic parameters tightly linked to the process of exercise-induced cardiac remodeling are shown stratified by sex and age group in this sample of elite US soccer players. Values are presented as mean (SD) and percentage of individuals meeting criteria for abnormal measurements (% abnormal). Senior Men’s national team did not participate in screening program during study period. LA indicates left axis; RA, right axis; RV, right ventricular.
A total of 34 individuals (24 female, 8 male) underwent repeat evaluation after a mean (SD) of 2.4 (0.9) years. Within individuals, LV wall thickness and LV mass both increased by a mean (SD) of 0.08 (0.13) cm (95% CI, 0.03-0.12; P = .001) and 9.6 (14) g (95% CI, 5.0-14.2; P < .001), respectively, while LV volumes remained stable. Of this group, 4 of 34 (all female) athletes (7.1%) had ECG abnormalities on initial evaluation (3 with abnormal TWI and 1 meeting criteria for 2 borderline findings), all of whom had normal ECG findings on subsequent evaluation. Echocardiograms performed at the time of the initial abnormal ECG findings were only notable for increased LV mass above ASE normality thresholds in 3 of 4 and increased indexed LVEDV in 2 of 4.
Discussion
This study provides the first normative data set integrating ECG and echocardiography derived from a sex- and race-balanced group of elite United States soccer players. Importantly, our data represent one of the largest sport-specific studies of female athletes to date. Key findings can be summarized as follows. First, normal training-related ECG changes are highly prevalent in US male soccer players but are less common among female athletes. In contrast, female soccer players are more likely to demonstrate potentially pathologic ECG findings, which are most commonly accounted for by pathologic anterior precordial TWIs. Independent of ECG findings, female and male soccer players demonstrate a high prevalence of age-dependent structural LV parameters (ie, LV wall thickness, chamber volume, and mass) that exceed clinical cut points for normality. In aggregate, these data define normative ECG and echocardiographic parameters among healthy elite adolescent and young adult US soccer players that can be applied in clinical practice.
Normative cardiac parameter reference ranges in athletes are essential for differentiating training-related cardiovascular adaptations from potentially pathologic phenotypes. This process, the basis for determining athletic eligibility and for providing guideline-directed medical therapy for those with confirmed pathology, represents a common and often complex challenge for cardiovascular and sports medicine practitioners. Consensus documents delineating the use and interpretation of ECG16 and multimodality imaging among athletes26 acknowledge both the critical importance and the current dearth of sport- and sex-specific normative data for this purpose. With important exceptions derived from men playing in the National Football League27,28 and among women and men participating in the National Basketball Association,12,13,29 prior series defining normal reference ranges in competitive athletes have comprised mixed sport cohorts and have focused almost exclusively on men. Results from the current study add to the existing literature by providing sex-specific ECG and echocardiographic data derived from elite athletes participating in the world’s most popular competitive sport.
Several insights regarding our ECG data are noteworthy. Elite soccer players of both sexes demonstrated a high prevalence of training-related ECG patterns. This finding underscores the value of this classification strategy as a way to identify adaptive ECG findings while minimizing the burden of false-positive testing. The overall rate of overtly abnormal ECG findings in this current study (5.9%), as defined by the most recent international criteria, was similar to previous cohorts of trained athletes from other sports (1.5%-7.5%).30,31,32,33,34 However, we found a notably higher prevalence of female athletes with abnormal ECG findings (11.5%), which was largely attributable to pathologic TWI confined to the anterior precordial lead distribution (V1-V3). Notably, the prevalence of anterior TWI in female athletes was similar in our cohort to a recent study of 895 female athletes from multiple sporting disciplines35 (5.7% vs 6.5%, respectively), and follow-up testing for female athletes in both cohorts did not reveal explanatory cardiac pathology. Mechanisms underlying the high prevalence of anterior TWI among women remain uncertain, but prior hypotheses have included variation in sympathetic innervation and anatomical differences including increased breast tissue in female athletes.35 At present, the international criteria define TWI confined to anterior precordial leads as a normal juvenile ECG pattern in adolescent White athletes younger than 16 years.16,36 While anterior TWI have previously been shown to be more common in young female than male athletes,35 our data suggest that they may represent a benign finding across a broader age range of female soccer players. Confirmation of this finding among similar cohorts of elite female athletes represents an important area of future work. Finally, a unique aspect of this study was the opportunity to examine serial cardiac data among athletes who attended multiple training camps. In this small but instructive subgroup, we identified 4 female athletes with abnormal ECG findings on initial evaluation that normalized on repeat testing 2 to 4 years later; notably 2 of these had lateral precordial (V4-V6) TWI, a finding previously shown to have variable but significant associations with underlying myopathic diagnoses.37,38,39,40 Each of these athletes had normal corollary echocardiograms at both study points that only were noteworthy for elevated LV mass and volumes. In aggregate, this observation highlights the underappreciated plasticity of the athlete’s ECG despite the apparent absence of underlying pathology.
Echocardiographic data obtained in this study are similarly instructive and clinically relevant. Among a relatively young population of individuals (63% younger than 19 years), we observed a high prevalence of cardiac structural indices that exceeded clinical normality cut points. This finding is consistent with prior data derived from soccer athletes from primarily male cohorts41,42,43,44,45,46,47,48 and underscores the limitations of applying general population-based normative data to elite athlete populations. When compared with other sport-specific data, elite female and male soccer players had smaller unadjusted average chamber dimensions than professional basketball12,13 and American-style football athletes,27 an unsurprising finding given associations of LV chamber diameter with body size. Wall thickness was also slightly lower in the soccer athletes, making clear again the importance of sport-specific normative data. When compared against 2 larger, multisport samples of female athletes (n = 60049 and n = 43910), elite female soccer players had similar LV dimensions (mean [SD], 49 [4] vs 48 [3.6] and 49 [4] mm in the 2 larger studies, respectively) and wall thickness (mean [SD], 8.2 [0.9] vs 8.9 [1.2] and 8.7 [1.2] mm), although it is difficult to draw firm conclusions here given the diversity of the larger samples. Finally, although cross-sectional, our data also suggest that optimal normative data should account for athlete age. The finding of incremental, age-dependent increases in LV mass, wall thickness, and chamber volumes in this adolescent and young adult population suggest that exercise-induced remodeling is a progressive process that continues throughout the duration of an athletic career. Future prospective, repeated-measures studies will be required to clarify the temporal nature and clinical implications of long-term exposure to elite athletics.
The normative reference data derived from this study are of particular clinical relevance in the setting of the COVID-19 pandemic. Emerging data suggest that individuals infected with COVID-19 are at risk for cardiovascular involvement including myocarditis.50,51 Accordingly, multiple expert consensus return-to-sport testing algorithms that rely heavily on the use of ECG and echocardiography have been proposed.4,5,6 In this context, we anticipate that preparticipation screening incorporating these modalities will occur with increasing frequency. The availability of comprehensive normative data derived from healthy athletes in the pre–COVID-19 era will serve as valuable frame of reference to differentiate athletes with clinically relevant COVID-19 complications from those with benign exercise-induced adaptation.
Limitations
We acknowledge several important limitations of this study. First, this study contains the largest database of elite US soccer players and is among the largest cohorts of single-sport female athletes, a group previously underrepresented in the literature.52 However, our sample size, deliberately confined to US National Team players preparing for World Cup play, may be underpowered to permit conclusive subgroup analyses. We further acknowledge the relative dearth of older men in this cohort, which was attributable to the US men’s senior national team’s failure to qualify for World Cup play during the study period. Third, individuals with ECG abnormalities but normal echocardiograms did not undergo further noninvasive imaging (ie, cardiac magnetic resonance imaging) as echocardiographic imaging was deemed to be of sufficient quality to exclude pathology in all cases. Similarly, the small group of athletes (7%) with mildly reduced LVEF did not undergo subsequent testing to assess the LV response to exercise because they were completely asymptomatic and demonstrated robust LV diastolic function. In both cases, we nonetheless acknowledge the small possibility of undetected occult pathology. Fourth, we note that normative data derived from elite athletes may be incompletely generalizable to athletes at subelite competition levels. However, cardiac parameters characteristic of healthy elite competitors likely represent the upper limits of adaptive remodeling among same-sport athletes at lower levels of competition. Finally, serial cardiac data were only available in a limited subset of athletes, rendering conclusions about the evolution and plasticity of ECG and echocardiographic data in this population preliminary.
Conclusions
In conclusion, this study provides the first comprehensive normative data set of ECG and echocardiographic data derived from elite female and male US soccer players. Our data demonstrate a high prevalence of benign training-related ECG findings and identify several specific abnormal ECG patterns with suboptimal specificity for true underlying pathology. Similar to other cohorts of elite athletes, our data confirm an elevated prevalence of adaptative cardiac structural remodeling findings that exceed general population-based clinical imaging cut points. We anticipate that the data presented in this article will serve as a valuable reference during future clinical assessments of elite female and male soccer players.
References
- 1.Higgins JP, Andino A. Soccer and sudden cardiac death in young competitive athletes: a review. J Sports Med (Hindawi Publ Corp). 2013;2013:967183. doi: 10.1155/2013/967183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer EB, Dvorak J, Schmied C, Meyer T. F-MARC: promoting the prevention and management of sudden cardiac arrest in football. Br J Sports Med. 2015;49(9):597-598. doi: 10.1136/bjsports-2015-094764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FIFA. Pre-competition medical assessment (PCMA). Accessed August 22, 2020. https://www.fifa.com/who-we-are/official-documents/#fifa-medical
- 4.Baggish A, Drezner JA, Kim J, Martinez M, Prutkin JM. Resurgence of sport in the wake of COVID-19: cardiac considerations in competitive athletes. Br J Sports Med. 2020;54(19):1130-1131. doi: 10.1136/bjsports-2020-102516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggish AL, Levine BD. Icarus and sports after COVID 19: too close to the sun? Circulation. 2020;142(7):615-617. doi: 10.1161/CIRCULATIONAHA.120.048335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phelan D, Kim JH, Chung EH. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol. Published online May 13, 2020. doi: 10.1001/jamacardio.2020.2136 [DOI] [PubMed] [Google Scholar]
- 7.Baggish AL, Wang F, Weiner RB, et al. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol (1985). 2008;104(4):1121-1128. doi: 10.1152/japplphysiol.01170.2007 [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Maron BJ, Whyte G, Firoozi S, Elliott PM, McKenna WJ. Physiologic limits of left ventricular hypertrophy in elite junior athletes: relevance to differential diagnosis of athlete’s heart and hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40(8):1431-1436. doi: 10.1016/S0735-1097(02)02270-2 [DOI] [PubMed] [Google Scholar]
- 9.Churchill TW, Groezinger E, Kim JH, et al. Association of ascending aortic dilatation and long-term endurance exercise among older masters-level athletes. JAMA Cardiol. 2020;5(5):522-531. doi: 10.1001/jamacardio.2020.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finocchiaro G, Dhutia H, D’Silva A, et al. Effect of sex and sporting discipline on LV adaptation to exercise. JACC Cardiovasc Imaging. 2017;10(9):965-972. doi: 10.1016/j.jcmg.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 11.Rawlins J, Carre F, Kervio G, et al. Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation. 2010;121(9):1078-1085. doi: 10.1161/CIRCULATIONAHA.109.917211 [DOI] [PubMed] [Google Scholar]
- 12.Engel DJ, Schwartz A, Homma S. Athletic cardiac remodeling in US professional basketball players. JAMA Cardiol. 2016;1(1):80-87. doi: 10.1001/jamacardio.2015.0252 [DOI] [PubMed] [Google Scholar]
- 13.Shames S, Bello NA, Schwartz A, et al. Echocardiographic characterization of female professional basketball players in the US. JAMA Cardiol. 2020;5(9):991-998. doi: 10.1001/jamacardio.2020.0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasfy MM, Weiner RB, Wang F, et al. Endurance exercise-induced cardiac remodeling: not all sports are created equal. J Am Soc Echocardiogr. 2015;28(12):1434-1440. doi: 10.1016/j.echo.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 15.Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32(1):1-64. doi: 10.1016/j.echo.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 16.Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol. 2017;69(8):1057-1075. doi: 10.1016/j.jacc.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 17.Bazett H. An analysis of the time-relations of electrocardiograms. Heart. 1920:353-370. [Google Scholar]
- 18.Postema PG, De Jong JS, Van der Bilt IA, Wilde AA. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm. 2008;5(7):1015-1018. doi: 10.1016/j.hrthm.2008.03.037 [DOI] [PubMed] [Google Scholar]
- 19.Romhilt DW, Bove KE, Norris RJ, et al. A critical appraisal of the electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. Circulation. 1969;40(2):185-195. doi: 10.1161/01.CIR.40.2.185 [DOI] [PubMed] [Google Scholar]
- 20.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37(2):161-186. doi: 10.1016/0002-8703(49)90562-1 [DOI] [PubMed] [Google Scholar]
- 21.Sokolow M, Lyon TP. The ventricular complex in right ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;38(2):273-294. doi: 10.1016/0002-8703(49)91335-6 [DOI] [PubMed] [Google Scholar]
- 22.Noseworthy PA, Weiner R, Kim J, et al. Early repolarization pattern in competitive athletes: clinical correlates and the effects of exercise training. Circ Arrhythm Electrophysiol. 2011;4(4):432-440. doi: 10.1161/CIRCEP.111.962852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 24.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717 [DOI] [PubMed] [Google Scholar]
- 25.Yared K, Noseworthy P, Weyman AE, McCabe E, Picard MH, Baggish AL. Pulmonary artery acceleration time provides an accurate estimate of systolic pulmonary arterial pressure during transthoracic echocardiography. J Am Soc Echocardiogr. 2011;24(6):687-692. doi: 10.1016/j.echo.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 26.Baggish AL, Battle RW, Beaver TA, et al. Recommendations on the use of multimodality cardiovascular imaging in young adult competitive athletes: a report from the American Society of Echocardiography in collaboration with the Society of Cardiovascular Computed Tomography and the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2020;33(5):523-549. doi: 10.1016/j.echo.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 27.Abernethy WB, Choo JK, Hutter AM Jr. Echocardiographic characteristics of professional football players. J Am Coll Cardiol. 2003;41(2):280-284. doi: 10.1016/S0735-1097(02)02633-5 [DOI] [PubMed] [Google Scholar]
- 28.Choo JK, Abernethy WB III, Hutter AM Jr. Electrocardiographic observations in professional football players. Am J Cardiol. 2002;90(2):198-200. doi: 10.1016/S0002-9149(02)02454-2 [DOI] [PubMed] [Google Scholar]
- 29.Waase MP, Mutharasan RK, Whang W, et al. Electrocardiographic findings in National Basketball Association athletes. JAMA Cardiol. 2018;3(1):69-74. doi: 10.1001/jamacardio.2017.4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhutia H, Malhotra A, Finocchiaro G, et al. Impact of the international recommendations for electrocardiographic interpretation on cardiovascular screening in young athletes. J Am Coll Cardiol. 2017;70(6):805-807. doi: 10.1016/j.jacc.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 31.Hyde N, Prutkin JM, Drezner JA. Electrocardiogram interpretation in NCAA athletes: comparison of the ‘Seattle’ and ‘International’ criteria. J Electrocardiol. 2019;56:81-84. doi: 10.1016/j.jelectrocard.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 32.Malhotra A, Dhutia H, Yeo T-J, et al. Accuracy of the 2017 international recommendations for clinicians who interpret adolescent athletes’ ECGs: a cohort study of 11 168 British white and black soccer players. Br J Sports Med. 2020;54:739-745. doi: 10.1136/bjsports-2017-098528 [DOI] [PubMed] [Google Scholar]
- 33.McClean G, Riding NR, Pieles G, et al. Diagnostic accuracy and Bayesian analysis of new international ECG recommendations in paediatric athletes. Heart. 2019;105(2):152-159. doi: 10.1136/heartjnl-2018-313466 [DOI] [PubMed] [Google Scholar]
- 34.Petek BJ, Drezner JA, Prutkin JM, Owens DS, Tran T, Harmon KG. Electrocardiogram interpretation in college athletes: local institution versus sports cardiology center interpretation. J Electrocardiol. 2020;62:49-56. doi: 10.1016/j.jelectrocard.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 35.Malhotra A, Dhutia H, Gati S, et al. Anterior T-wave inversion in young white athletes and nonathletes: prevalence and significance. J Am Coll Cardiol. 2017;69(1):1-9. doi: 10.1016/j.jacc.2016.10.044 [DOI] [PubMed] [Google Scholar]
- 36.Papadakis M, Basavarajaiah S, Rawlins J, et al. Prevalence and significance of T-wave inversions in predominantly Caucasian adolescent athletes. Eur Heart J. 2009;30(14):1728-1735. doi: 10.1093/eurheartj/ehp164 [DOI] [PubMed] [Google Scholar]
- 37.Pelliccia A, Di Paolo FM, Quattrini FM, et al. Outcomes in athletes with marked ECG repolarization abnormalities. N Engl J Med. 2008;358(2):152-161. doi: 10.1056/NEJMoa060781 [DOI] [PubMed] [Google Scholar]
- 38.Schnell F, Riding N, O’Hanlon R, et al. Recognition and significance of pathological T-wave inversions in athletes. Circulation. 2015;131(2):165-173. doi: 10.1161/CIRCULATIONAHA.114.011038 [DOI] [PubMed] [Google Scholar]
- 39.Calò L, Sperandii F, Martino A, et al. Echocardiographic findings in 2261 peri-pubertal athletes with or without inverted T waves at electrocardiogram. Heart. 2015;101(3):193-200. doi: 10.1136/heartjnl-2014-306110 [DOI] [PubMed] [Google Scholar]
- 40.Migliore F, Zorzi A, Michieli P, et al. Prevalence of cardiomyopathy in Italian asymptomatic children with electrocardiographic T-wave inversion at preparticipation screening. Circulation. 2012;125(3):529-538. doi: 10.1161/CIRCULATIONAHA.111.055673 [DOI] [PubMed] [Google Scholar]
- 41.Muir DF, MacGregor GD, McCann GP, Hillis WS. The prevalence of left ventricular hypertrophy in elite professional footballers. Int J Cardiol. 1999;71(2):129-134. doi: 10.1016/S0167-5273(99)00133-3 [DOI] [PubMed] [Google Scholar]
- 42.Somauroo JD, Pyatt JR, Jackson M, Perry RA, Ramsdale DR. An echocardiographic assessment of cardiac morphology and common ECG findings in teenage professional soccer players: reference ranges for use in screening. Heart. 2001;85(6):649-654. doi: 10.1136/heart.85.6.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pambo P, Adu-Adadey M, Ankrah PT, Agbodzakey H, Scharhag J. Electrocardiographic and echocardiographic findings in Ghanaian female soccer players. Clin J Sport Med. Published online June 5, 2020. doi: 10.1097/JSM.0000000000000851 [DOI] [PubMed] [Google Scholar]
- 44.Pambo P, Adu-Adadey M, Agbodzakey H, Scharhag J. Electrocardiographic and echocardiographic findings in elite Ghanaian male soccer players. Clin J Sport Med. Published online December 24, 2019. doi: 10.1097/JSM.0000000000000801 [DOI] [PubMed] [Google Scholar]
- 45.Di Paolo FM, Schmied C, Zerguini YA, et al. The athlete’s heart in adolescent Africans: an electrocardiographic and echocardiographic study. J Am Coll Cardiol. 2012;59(11):1029-1036. doi: 10.1016/j.jacc.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 46.Galanti G, Stefani L, Mascherini G, Di Tante V, Toncelli L. Left ventricular remodeling and the athlete’s heart, irrespective of quality load training. Cardiovasc Ultrasound. 2016;14(1):46. doi: 10.1186/s12947-016-0088-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gjerdalen GF, Hisdal J, Solberg EE, Andersen TE, Radunovic Z, Steine K. The Scandinavian athlete’s heart; echocardiographic characteristics of male professional football players. Scand J Med Sci Sports. 2014;24(5):e372-e380. doi: 10.1111/sms.12178 [DOI] [PubMed] [Google Scholar]
- 48.Sansonio de Morais A, Ferreira GA, Lima-Silva AE, Gomes Filho A. Gender-related cardiac dimension differences between female and male professional soccer players. J Sports Med Phys Fitness. 2018;58(9):1354-1359. doi: 10.23736/S0022-4707.17.07422-9 [DOI] [PubMed] [Google Scholar]
- 49.Pelliccia A, Maron BJ, Culasso F, Spataro A, Caselli G. Athlete’s heart in women: echocardiographic characterization of highly trained elite female athletes. JAMA. 1996;276(3):211-215. doi: 10.1001/jama.1996.03540030045030 [DOI] [PubMed] [Google Scholar]
- 50.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. Published online July 27, 2020. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. Published online September 11, 2020. doi: 10.1001/jamacardio.2020.4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petek BJ, Wasfy MM. Cardiac adaption to exercise training: the female athlete. Curr Treat Options Cardiovasc Med. 2018;20(8):68. doi: 10.1007/s11936-018-0659-2 [DOI] [PubMed] [Google Scholar]


