Abstract
Immunization with current acellular pertussis (aP) vaccines protects against severe pertussis, but immunity wanes rapidly after vaccination and these vaccines do not prevent nasal colonization with Bordetella pertussis. Studies in mouse and baboon models have demonstrated that Th1 and Th17 responses are integral to protective immunity induced by previous infection with B. pertussis and immunization with whole cell pertussis (wP) vaccines. Mucosal Th17 cells, IL-17 and secretory IgA (sIgA) are particularly important in generating sustained sterilizing immunity in the nasal cavity. Current aP vaccines induce potent IgG and Th2-skewed T cell responses but are less effective at generating Th1 and Th17 responses and fail to prime respiratory tissue-resident memory T (TRM) cells, that maintain long-term immunity at mucosal sites. In contrast, a live attenuated pertussis vaccine, pertussis outer membrane vesicle (OMV) vaccines or aP vaccines formulated with novel adjuvants do induce cellular immune responses in the respiratory tract, especially when delivered by the intranasal route. An increased understanding of the mechanisms of sustained protective immunity, especially the role of respiratory TRM cells, will facilitate the development of next generation pertussis vaccines that not only protect against pertussis disease, but prevent nasal colonization and transmission of B. pertussis.
Keywords: Bordetella pertussis, pertussis vaccine, T cells, Th1 cells, Th17 cells, memory T cells
1. Introduction
Pertussis, or whooping cough, is caused by the Gram-negative bacterium Bordetella pertussis, which infects the upper and lower respiratory tract, causing considerable morbidity in children and adults and severe disease that can be fatal in infants [1]. The clinical features of disease include a paroxysmal cough, creating a characteristic ‘whoop’, leukocytosis and in some cases pneumonia. Dissemination of B. pertussis to other organs occurs very rarely, and only occurs in immunocompromised individuals [2,3]. Whole cell pertussis (wP) vaccines, developed in the 1940s are effective at preventing pertussis, but are associated with significant adverse events, including febrile convulsions [4,5]. This led to their discontinuation in most high-income countries and the development of acellular pertussis (aP) vaccines, now widely used in these countries [6]. aP vaccines contain between 2 and 5 B. pertussis antigens, all of which include the major symptom-causing virulence factor, pertussis toxin (PT), as well as various combinations of filamentous hemagglutinin (FHA), pertactin (PRN) and fimbriae 2 and 3 (FIM2/3) [7,8]. aP vaccines administered as part of the primary immunization of infant and young children also contain diphtheria and tetanus toxoids, termed DTaP, whereas booster vaccines, termed Tdap, have reduced antigen content [9,10]. All current aP vaccines are formulated with alum as the adjuvant. Clinical trials carried out in the 1990s showed that the aP vaccines had excellent safety profiles and were capable of preventing severe disease [11,12,13]. However, recent studies in animal models have indicated that immunization with aP vaccines does not prevent nasal colonization or transmission of B. pertussis [14,15,16].
Pertussis remains a global problem with the highest case rates of any vaccine-preventable disease [17]. Many pertussis-related deaths still occur in low- and middle-income countries, however, these deaths are not attributable to poor efficacy of wP vaccines but to gaps in vaccination coverage and limited access to healthcare in these countries [18,19]. However, a resurgence in pertussis has been observed in the last decades in countries with high aP vaccine coverage, suggesting that immunization with aP vaccines does not generate sustained sterilizing immunity against B. pertussis [20]. Furthermore, epidemiological data have demonstrated that immunization with wP vaccines is more effective at preventing cases of pertussis than aP vaccines [21,22]. Currently, the World Health Organization has recommended that countries still using wP vaccines should not switch to aP vaccines, but it is generally accepted that the reintroduction of wP vaccines would not be possible in countries where it is deemed unsafe.
There are a number of possible reasons for the failure of aP vaccines to generate potent and sustained immunity. In addition to the problem of pertussis in infants too young to be immunized, there are increasing numbers of cases in preadolescents, adolescents and adults, suggesting immunity wanes after immunization with aP vaccines. Indeed, rapid waning of protective immunity has been reported even after five doses of DTaP [23,24]. Although booster vaccination may provide a solution, Tdap boosting only provides significant protection for up to 1 year, and protection substantially wanes 2–3 years later [25]. The emergence of B. pertussis strains with deletions or mutations in the antigens present in aP vaccines, which may be caused by vaccine-driven immune selective pressure, could also provide an explanation for the persistence of B. pertussis in populations immunized with aP vaccines [26,27,28].
Weak or inappropriate cell-mediated immune responses generated by aP vaccines may also be responsible for the resurgence of pertussis in vaccinated populations. Immunization with aP vaccines induces strong serum immunoglobulin G (IgG) and predominantly Th2-type T cell responses [29]. However, extensive studies in mouse and baboon models of B. pertussis infection have demonstrated that robust Th1 and Th17 cell responses are required for optimum protective immunity [30,31,32]. Furthermore, recent reports have indicated that tissue-resident memory T (TRM) cells in the upper and lower respiratory tract induced by previous infection or intranasal (i.n.) immunization play a crucial role in long-term protective immunity in the nose and lungs [15,32,33,34].
While immunization with aP vaccines appears to be capable of protecting against severe pertussis disease in infants, there is increasing evidence from animal models that the immunity generated with aP vaccines does not prevent infection of nasal mucosae [14,15]. Asymptomatic carriage in aP-immunized baboons can lead to the transmission of B. pertussis to naïve animals [14] and mathematical modelling studies have suggested that asymptomatic carriage and transmission may also occur in the human population and may account for the recent resurgence of pertussis cases in certain countries [35]. This poses a significant risk to infants who have not yet received or completed their DTaP immunization program, and it may impede the potential to generate herd immunity in the population [24,36]. Recent studies have suggested that local immunity, especially IL-17 producing TRM responses, as well as mucosal IgA, may be central to the induction of sterilizing protective immunity in the nasal cavity [33,34]. Therefore, strategies for the design of improved pertussis vaccines must consider the induction of mucosal immunity in the respiratory tract.
This review outlines the state of the art in pertussis vaccine research, discusses current licensed vaccines, vaccines in development, as well as those at an experimental stage, and focuses particularly on how immunity is achieved at a molecular and cellular level. We also discuss the mechanism of natural and vaccine-induced immunity and how effective pertussis vaccines can be designed using immunization approaches that induce cellular as well as humoral immunity, especially local T cell responses in the respiratory tract.
2. Immune Responses That Control a Primary Infection with B. pertussis
Natural immunity generated by previous infection is considered to be more effective than immunity generated with aP or wP vaccines. Therefore, an understanding of the mechanism of natural immunity in the respiratory tract should assist in the design of a more effective pertussis vaccine. Following recovery from infection with B. pertussis, convalescent animals and humans have long-term, but not life-long, protection against subsequent infection through the generation of immunological memory [37]. Although correlates of protective immunity have not yet been fully elucidated, or unanimously agreed upon, there is a wealth of knowledge from recent research on the mechanisms of innate and adaptive immunity to B. pertussis that control primary infection and prevent re-infection with B. pertussis.
Primary infection with B. pertussis can broadly be described in three stages, which include the recognition, bactericidal clearance and memory stages [38]. During the recognition stage, bacteria adhere to ciliated epithelia of the upper respiratory mucosa, and B. pertussis is detected by resident innate immune cells, which provide a rapid first line of defense [39,40]. These cells include airway mucosal dendritic cells (DCs), which reside between epithelial cells and survey airway mucosa for pathogens [41]. Alveolar macrophages (AMs), present on the mucosal surface of the lungs, phagocytose and kill bacteria rapidly upon their recognition. Depletion of AM using chlodronate liposomes increases B. pertussis burden in the respiratory tract of mice [42]. The recognition of B. pertussis pathogen-associated molecular patterns (PAMPs), such as pertussis toxin (PT), lipopolysaccharide (LPS) and lipopeptides, by pattern recognition receptors (PRRs) is crucial for macrophage and DC responses. Mice lacking the toll-like-receptor (TLRs) adaptor, MyD88 adaptor-like protein (Mal), have increased infection in the lungs and bacterial dissemination to the liver, with often fatal consequences [43]. These mice also show reduced lung resident AMs following B. pertussis infection, whereas wild-type (WT) mice had increasing numbers of AMs in the lung throughout the course of infection [44]. Airway mucosal DCs process bacterial antigens migrate to the lymph nodes, where they present peptide antigens to naïve T cells [45].
Studies in mouse models have shown that in the bactericidal stage, inflammatory macrophages and DCs infiltrate the lung from the periphery. In addition to their function as antigen presenting cells (APCs), DCs also produce pro-inflammatory cytokines and chemokines in response to PAMPs, including LPS binding to TLR4, that promote the recruitment and activation of other innate immune cells and effector T cells. DCs and macrophages also produce the anti-inflammatory cytokine IL-10, which may protect the lung from excessive inflammation and immunopathology [46]. DCs expressing the integrins CD11c, CD8α and the E-cadherin receptor, CD103, infiltrate the lung early in B. pertussis infection and are required for effective bacterial clearance [47]. IL-17-producing γδ T cells are detected in the mouse lung as early as 2 h post-infection [48]. Inhibition of this early innate IL-17 produced by γδ T cells, by treatment of mice with IL-17 neutralizing antibodies 1 day prior and 2 days after infection, led to increased bacterial burden at 3 and 7 days post bacterial challenge and this was associated with reduced local production of protective antimicrobial peptides (AMPs) in the lungs [48].
IL-17 produced by γδ T cells and Th17 cells helps to promote recruitment of neutrophils to the respiratory tract. Neutrophil numbers peak in the lung at 5-7 days post challenge in mice, and have the capacity to phagocytose and kill B. pertussis, but are not essential for clearance of bacteria from the lungs during primary infection [49,50]. They do, however, play an important role in the protection of immune mice from reinfection, which is believed to be due to bacterial killing via antibody-mediated phagocytosis [49]. Furthermore, we have found that neutrophils play a central role in clearance of primary and secondary infections of the nasal mucosae with B. pertussis (Borkner, Curham and Mills, unpublished data).
NK cells, which infiltrate the lungs of mice around day 7-14 of infection, are an important early source of IFN-γ that activates the bactericidal activity of macrophages [51] and promotes phagolysosomal maturation [52,53]. Macrophages and epithelial cells can act as a niche for intracellular survival of B. pertussis and IFN-γ appears to play a key role in eliminating this intracellular reservoir of live bacteria [54]. IFN-γ produced by NK cells also promotes polarization of Th1 cells, and depletion of NK cells causes a shift from Th1 to Th2 responses and leads to bacterial dissemination to the liver [51].
While innate immune responses can effectively limit bacterial growth in the respiratory tract, they do not clear the infection with B. pertussis. The adaptive arm of the immune system is required for bactericidal clearance of B. pertussis. CD4 T helper (Th) cells are activated by B. pertussis antigens presented by APCs in lymph nodes draining the respiratory tract. These CD4 T cells undergo clonal expansion and are indispensable for clearance of B. pertussis from the lungs and probably the nasal cavity [52,55]. There is strong evidence that Th1 cells are responsible for the effective clearance of B. pertussis from the lungs and there is emerging evidence that Th17 cells mediate clearance from the nasal cavity [16,56]. CD4 T follicular helper (Tfh) cells play an important role in the formation of germinal centers (GCs) and the induction of antibody production and affinity maturation by B cells [57]. Tfh cells are induced by B. pertussis infection and may also play a role in generating of B. pertussis-specific antibodies (Wilk, Allen and Mills, unpublished data).
Finally, in the memory stage, following clearance of B. pertussis from the respiratory tract, the number of effector T and B cells contracts, but populations of memory B and T cells are generated and persist to maintain long-lived immunity against re-infection [37,55,56].
3. Natural and Vaccine-Induced Immunity-Correlates of Protection
3.1. Antibody Responses
3.1.1. Serum IgG
Current aP vaccines generate high levels of antibody and associated Th2 responses (Figure 1), and this is consistent with many alum-adjuvanted vaccines [58,59]. Clinical trials in 1990s demonstrated that immunization of children with aP vaccines induced potent serum IgG responses specific for the vaccine antigens and prevented severe pertussis [13,60]. However, recent evidence suggests that aP-induced immunity does not confer protection against bacterial carriage, which is reflected in an increase in the incidence of pertussis in countries with high vaccine coverage [61,62]. The nature of the antibody isotypes generated, the limited breadth of their antigenic specificity as well as rapid waning of serum antibody concentrations may contribute to this suboptimal protection [23,63].
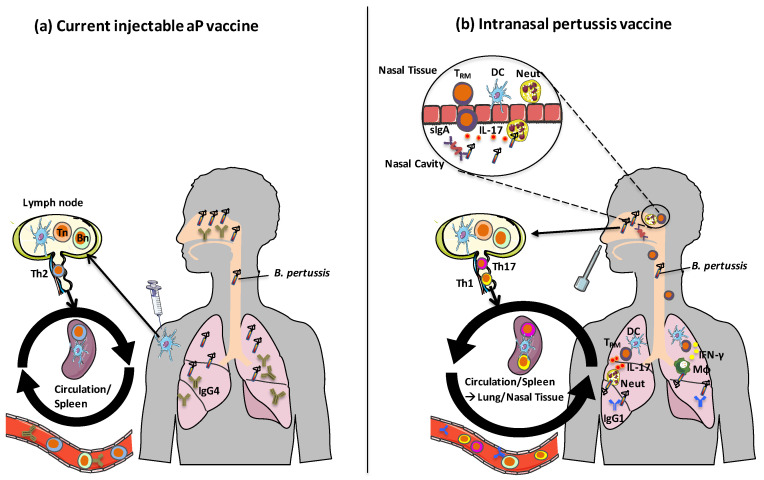
Figure 1.
Mechanisms of action of current and future pertussis vaccines. (a) Following immunization with current injectable aP vaccines formulated with alum, DCs at the site of injection take up antigens and migrate to draining lymph nodes where they activate differentiation of Th2 cells from naive T cells. Memory Th2 cells circulate, but do not home to respiratory tissues. IgG4 (IgG1 in mice) is produced by antigen-specific B cells, which protects against toxin-mediated pertussis disease but does not protect against nasal colonization with B. pertussis. (b) Following intranasal immunization with aP vaccines and novel adjuvants, pertussis OMV vaccines or live attenuated pertussis vaccines, local DCs are activated in the respiratory tract. These DCs migrate to draining lymph nodes and activate differentiation of naïve T cells into Th1 and Th17 cells, which have homing properties that facilitate migration to respiratory tissues. Th1 and Th17-type respiratory TRM cells produce IFN-γ and IL-17, which promote recruitment of macrophages and neutrophil to the lungs and nasal mucosae. Intranasal immunization with these vaccines also leads to B cell activation and the production of IgG1 (IgG2a/c in mice) antibodies as well as sIgA. Images from Servier Medical Art (www.smart.servier.com). Tn: naïve T cell, Bn: naïve B cell, TRM: tissue-resident memory T cell, DC: Dendritic cell, sIgA: secretory immunoglobulin-A, MΦ: macrophage.
During natural infection with B. pertussis, antigen-specific IgG is only detectable in the serum late in infection when the bacteria are almost cleared from the lung, suggesting that circulating antibodies do not play a major role clearing a primary infection with B. pertussis, but may play a role in preventing re-infection [55]. Infection with B. pertussis or immunization with wP vaccines induce IgG2a/IgG2c in mice and a corresponding IgG1 response in humans. However, the strong serum antibody response generated by aP vaccines is predominantly of the IgG1 subclass in mice, or, its functional equivalent, IgG4 in humans [64,65]. IgG subclasses are determined by class switching of mature B cells, which occurs through cell–cell interaction between B and T cells, as well as by cytokines secreted by T cells [66]. IgG2a/IgG2c-skewed B cell class switching is associated with Th1-derived IFN-γ [67,68], whereas IgG1 antibodies are associated with Th2 interactions and associated cytokine signaling [69].
In addition to overall levels and specificity, antibody function is an important determinant of effective bacterial clearance, especially opsonizing antibodies which bind B. pertussis and promote bacterial uptake and killing by macrophages and neutrophils [54,70]. Phagocytosis can also occur by binding of the complement receptor 3 (CR3), but this mechanism of entry is hijacked by B. pertussis to promote intracellular survival [70]. In contrast, uptake via the IgG Fc receptor, FcγRIII, leads to increased B. pertussis clearance when compared to CR3-mediated phagocytosis [70]. The IgG subclass can influence opsonization and downstream phagocyte effector function [71]. IgG1 and IgG3, present in serum from subjects vaccinated with the live attenuated pertussis vaccine BPZE1, effectively opsonized B. pertussis, whereas IgG2 and IgG4 were the dominant subclass, but opsonizing antibodies were low or absent in the serum from individuals immunized with an aP vaccine [72]. Taken together, these studies show that the ratio of different IgG subclasses generated following vaccination may influence the outcome of infection, as a result of functional differences in the antibodies and their associated T cell responses.
3.1.2. Secretory IgA
During primary infection of mice and humans with B. pertussis, secretory IgA (sIgA) is detected in nasopharyngeal secretions, before the detection of IgG [55,73]. IgA can provide early defense against bacterial adherence to epithelial cells [73], can opsonize B. pertussis and enhance binding, uptake and killing of bacteria in human polymorphonuclear cells, the latter being mediated by the myeloid-expressed IgA receptor, FcαRI [74]. IL-17 production by Th17 cells has been shown to be important for the generation of sIgA. The induction of Th17 cells by parenteral priming followed by an intranasal (i.n.) boosting with a Group A streptococcal C5a peptidase (ScpA) vaccine correlated with increased local sIgA-producing B cells [75]. This IgA-inducing function may be mediated by Th17-driven expression of the poly-Ig receptor (pIgR), which is involved in sIgA secretion from airway lumen epithelial cells [75,76]. A study using the live-attenuated B. pertussis vaccine, BPZE1, demonstrated that IL-17-dependent sIgA is not only important in the primary response to B. pertussis, but also in vaccine-induced immunity [34]. Transfer of nasal washes from BPZE1-immunized mice conferred protection against B. pertussis infection; however, this protection could not be conferred by transfer of nasal washes from IgA or pIgR deficient mice [34].
3.2. CD4 Th Cell Subtypes
3.2.1. Th1 Cells Protect in the Lung
The importance of T cell responses in generating long-lived immunity to many pathogens, including B. pertussis, is becoming increasingly evident, and natural infection with B. pertussis generates strong Th1 and Th17 responses which mediate effective protection against respiratory infection in animal models [30,55,56]. A similar, albeit weaker Th1/Th17-skewed response and associated protection is generated by immunization with wP vaccines [14,15,16]. Conversely, aP vaccines induce strongly Th2-skewed responses, which are associated with the production of the cytokines IL-4, IL-5 and IL-13 [14,16]. Th1 responses have a well-established role in protective immunity in the lungs induced by natural infection with B. pertussis in animal models [30,55]. Th1 cells characteristically produce the pleiotropic inflammatory cytokine, IFN-γ, and its signaling is crucial for protection against lung infection with B. pertussis in mice [30,52]. Ablation of IFN-γ signaling, using IFN-γ receptor knockout mice (IFN-γR-/-), leads to severe disseminating infection and in some cases fatal disease [30]. Th1 cells also play an essential role in protection conferred with wP vaccines; IFN-γR-/- mice immunized with wP vaccines have 100-1000 fold higher B. pertussis load in the lung at days 3, 7 and 10 post challenge, when compared with WT mice immunized with the same vaccine [16,77].
Further evidence supporting the crucial role of Th1 cells in host immunity against B. pertussis is provided by the immune evasion mechanisms employed by B. pertussis to evade the protective role of IFN-γ [78]. IL-10 is an anti-inflammatory cytokine, which can be produced by regulatory T (Treg) cells as well as macrophages and DCs, and myeloid cell-derived IL-10 can promote the induction of Treg cells [79,80]. IL-10-producing Tr1-type Treg cells are induced in the lung during B. pertussis infection driven by innate IL-10 induced by FHA [81] and possibly other B. pertussis virulence factors. These B. pertussis-specific Tr1 cells suppress protective Th1 cells, highlighting the selective advantage gained by B. pertussis in subverting host immunity through skewing the T cell response towards a more Treg phenotype [81]. Although the weight of evidence suggests that CD4 Th cells are the major protective T cells in B. pertussis infection, CD8 cytotoxic T cells (CTLs), which also produce IFN-γ, may also play a role, especially against intracellular bacteria [82,83].
There is a bias toward Th2 differentiation in neonates [58,84]. This may reflect high expression of IL-4 and the Th2 transcription factor, GATA3 and hypomethylation of the Th2 locus, which includes il4, il13 and il5 genes [85,86,87]. Nevertheless, there is evidence that both natural infection and immunization with wP vaccines in infants as young as 2–6 months can generate Th1 responses [29,88]. These studies show that the predilection for Th2 immunity in neonates can be overcome through immunization with wP vaccines, suggesting that these vaccines may include appropriate components that stimulate innate immune responses that direct the induction of Th1 cells. This is an important consideration for the design of novel aP vaccines for administration to neonates.
3.2.2. Th17 Cells Protect Against Nasal Colonization
Th17 cells have more recently been identified as key players in protecting mucosal surfaces against infection with candida and extracellular bacteria [89,90,91]. Many early studies on B. pertussis focused primarily on the mechanism of immunity in the lung, but recent studies from mouse and baboon models have shown that IL-17 and Th17 cells in the nasal mucosae may play pivotal roles in natural and vaccine-induced immunity in the nasopharynx [33,56,92].
Th17 cells are polarized by the inflammatory cytokines IL-1β, IL-23 and IL-6. Early evidence for the role of Th17 cells in protection against B. pertussis came from studies that showed that immunization with a wP vaccine generated a population of IL-17-producing CD4 T cells [93]. These Th17 cells played a role in wP vaccine-induced protection against lung infection and their induction was dependent on innate TLR4 signaling and the production of IL-23 and IL-1, highlighting the importance of vaccine-induced innate immune activation in promoting effective adaptive immune responses [93]. The generation of B. pertussis-specific Th17 cells is dependent on NLRP3 inflammasome-driven IL-1β production in macrophages or DCs and this is activated by the pore-forming capacity of the B. pertussis virulence factor, adenylate cyclase toxin (ACT) [94].
Th17 cells are necessary for the effective clearance of B. pertussis from the lungs by promoting recruitment of neutrophils and inflammatory macrophages to the respiratory tract [16,93]. Mice defective in IL-17 have impaired clearance of B. pertussis from the lung, which is associated with reduced neutrophil recruitment following B. pertussis challenge [16,94]. Adoptive transfer of Th1 or Th17 cells from convalescent to naïve mice enhanced clearance of B. pertussis from the lung, but the greatest protection was observed when a combination of Th1 and Th17 cells were transferred, indicating a protective role in the lungs for both cell types [16].
While Th1 and Th17 cells contribute to protective immunity generated by immunization with wP vaccines, studies in IL-4-/- mice demonstrated that the strong Th2-skewed cellular responses generated following immunization with aP vaccines were dispensable for the protection in the lungs [16]. However, aP-induced protection in the lungs was significantly impaired in IL-17-defective mice providing further evidence of a role for Th17 in pertussis vaccine-induced immunity [16].
In the baboon model, sterilizing immunity is generated by previous infection with B. pertussis and this is associated with production of mucosal IL-17 and the generation of long-lived memory Th17 cells [56]. High concentrations of IL-17 were found in nasal washes on days 5 and 7 of B. pertussis infection and this was accompanied by high levels of Th17-polarising cytokines IL-1β, IL-23, IL-6 [56]. Long-lived CD95+ CD4+ memory T cells that secreted IL-17 or IFN-γ were detected in baboons up to two years following challenge with B. pertussis [56]. Furthermore, immunization with wP vaccines protected against nasopharyngeal colonization and disease [14]. However, while natural infection or immunization with a wP vaccine generated Th1/Th17 responses, aP vaccines generated predominantly Th2 responses [14]. Furthermore, immunization of baboons with an aP vaccine protected against clinical disease, but was ineffective at protecting against nasal colonization with B. pertussis or transmission of bacteria to naive baboons [14]. While natural infection or immunization with either vaccine generates strong IgG responses against PT, FHA, PRN and FIM, the inability of aP vaccines to prevent nasal infection may be attributed to their limited ability to induce an effective cellular immune response, especially memory Th17 cells in the respiratory tract.
In humans, vaccination with wP vaccines has also been shown to generate Th17 as well as Th1 responses [95]. The nature of the initial priming dose of the vaccine dictates long term polarization of memory T cells [59,95,96]. A recent study reported that boosting with Tdap more than 15 years after the primary vaccination with five doses of DTwP generated greater PT-specific CD4 T cell responses, enhanced proliferative capacity of memory T cells and induced higher numbers of Th17 and Th1 cells than in individuals primed with DTaP vaccines [95]. Transcriptomic analysis showed upregulation of IL-17 and IFN-γ gene expression in the DTwP-primed individuals, and upregulation of IL-4 and IL-9 in the DTaP-primed individuals [95]. A study in children primed with aP or wP vaccines and boosted with an aP vaccine at 9 years of age revealed lower B. pertussis-specific cellular immune responses and reduced Th1/Th2 ratio in the aP-primed individuals [96]. Together, these studies indicate that the priming vaccine is of utmost importance in determining enduring T cell differentiation and memory responses.
3.3. TRM Cells Maintain Sustained Protective Immunity in the Respiratory Tract
Circulating memory T cells have classically been subdivided into effector memory (TEM) and central memory (TCM) cells based on both their homing and effector function [97]. More recently, a distinct subset of memory T cells, tissue-resident memory T (TRM) cells have been identified, which remain in tissues following infection, with little or no recirculation [98,99,100]. CD4 and CD8 TRM cells in humans and mice express the transmembrane C-type lectin, CD69, which plays a role in their retention within peripheral tissues [101,102,103]. CD8 and some CD4 TRM cells also express the αE integrin CD103. CD4 TRM cells that co-express CD103 and CD69 are found in the lungs of mice following B. pertussis infection [32].
TRM cells maintain immunological memory at mucosal surfaces and are poised to act rapidly as first responders at mucosal sites [104], recruiting innate and adaptive immune cells to the site of infection [105,106]. TRM cells possess innate-like properties and can be activated by cytokines, in the absence of TCR ligation with a cognate antigen [107]. CD8 TRM cells are particularly important in viral infections and have been studied in the greatest detail [99,108,109]. However, CD4 TRM cells also play a role in protection against viral infections at mucosal sites [103,110]. CD4 TRM cells are retained in the lungs of mice following influenza challenge and traffic back to the lungs of naïve mice following adoptive transfer [103].
There is growing evidence that local memory T cells in the respiratory tract may mediate protective immunity against certain pathogens independent of antibody responses. Natural infection to respiratory syncytial virus (RSV) generates both lung and airway CD8 TRM cells, which protect in the absence of antibodies [111]. CD4 TRM cells induced in the airways following intranasal immunization with Venezuelan equine encephalitis replicon particles expressing the SARS-CoV nucleocapsid protein produced IFN-γ that mobilized CD8 T cells and protected against SARS-CoV challenge in mice [112]. Respiratory CD4 and CD8 TRM, which are induced by the infection of mice with influenza virus or through i.n. immunization with a live-attenuated virus, mediate protection against challenge with heterosubtypic influenza strains in mice [113,114]. These TRM cells have been shown to confer protection independently of antibody responses [113,115]. IL-17-producing CD4 TRM cells expanded in the lungs following Streptococcus pneumoniae infection were capable of preventing reinfection with a heterotypic pneumococcus [116]. Interestingly, virus-specific, lung CD8 TRM cells have been shown to be protective against bacterial pneumonia challenge through bystander activation [117]. Lung resident CD4 TRM, cells induced by various tuberculosis (TB) vaccines mediate protection against M. tuberculosis challenge in animal models [118,119,120].
The recent understanding that B. pertussis can asymptomatically colonize the nasal cavity has focused attention on the role of local immunity in the respiratory tract. CD4 TRM cells are expanded in the lung and nasal tissue during infection of mice with B. pertussis [15,32]. Blocking accumulation of TRM cells in the lungs during primary infection with B. pertussis, using the sphingosine-1-phosphate (S1P) receptor agonist, FTY720, which blocks T cell egress from lymph nodes, enhanced the bacterial burden in the lungs [32]. However, when mice were treated with FTY720 prior to and during secondary infection, the bacterial burden was similar to untreated convalescent mice, indicating that TRM cells are retained in the lung tissue after the clearance of B. pertussis, and proliferate locally in response to re-infection. These protective CD4 TRM cells secreted IL-17 or both IL-17 and IFN-γ, and expressed CD69, with or without CD103 [32].
IL-17 and IFN-γ secreting CD4 TRM cells accumulate in the lung parenchyma and nasal tissue soon after B. pertussis challenge of mice immunized with a wP vaccine [15]. Mice immunized with a wP vaccine rapidly cleared bacteria from the lungs following B. pertussis challenge. In contrast, bacterial clearance from the lungs was delayed in mice immunized with an aP vaccine. However, a striking difference was observed in the nasal cavity. Mice immunized with aP vaccines had similar or greater bacterial burden in the nasal mucosae than naïve mice following challenge with B. pertussis. In contrast, mice immunized with a wP vaccine had significantly reduced bacterial counts by day 3 post challenge and cleared the infection completely by day 14. CD4 TRM cells transferred from the lungs of convalescent to naïve mice one day before B. pertussis challenge migrated to the lungs and conferred significant protection against challenge. Conversely, transfer of splenic memory T cell from previously infected, aP or wP-immunized mice did not lead to an accumulation of TRM cells in the lung, or confer any protection in recipient mice [15].
B. pertussis-specific CD69+ CD4 TRM cells were found to persist in the mouse lungs for at least 7 months after B. pertussis challenge. Sustained protective immunity in the nasal cavity, generated by natural infection or immunization with a wP vaccine, was strongly correlated with IFN-γ-producing and, to an even greater extent, IL-17-producing CD69+ CD4 TRM cells [15]. Therefore, there is growing evidence to suggest that the induction of respiratory TRM cells may be central to the generation of sterilizing immunity and long-term protection against B. pertussis infection of the lung and nasal mucosae.
4. Experimental Vaccines
It is now evident from studies in mice and baboons that immunization with aP vaccines is ineffective at generating protective immunity against nasal colonization with B. pertussis [17] (Figure 1). Although wP vaccines do confer some protection against nasal colonization, they are not as effective as previous infection with B. pertussis [14,15] and are associated with significant side effects. Therefore, new pertussis vaccines are in development based on live attenuated B. pertussis, pertussis outer membrane vesicles (OMVs) or aP vaccines formulated with novel adjuvants.
4.1. Live Attenuated Pertussis Vaccine-BPZE1
Locht and colleagues developed a live attenuated B. pertussis from the Tohama-1 strain, termed BPZE1, in which three virulence factors, PT, dermonecrotic factor (DNT) and tracheal cytotoxin (TCT) are genetically modified or removed [121]. Studies in mice showed that a single i.n. immunization with BPZE1 effectively colonized the respiratory tract without generating pathology in the lung and conferred superior protection against infection with virulent B. pertussis compared with two doses of an aP vaccine [121]. BPZE1 has also been shown to be safe in humans in phase 1 clinical trials [122]. Furthermore, studies in baboons showed that a single i.n. dose of BPZE1 protected against nasopharyngeal colonization and pertussis disease [14,92].
BPZE1 also generates long-lived protective immunity to B. pertussis in mice and this has been attributed to antibody and Th1/Th17 responses [123,124,125]. Transfer of serum antibodies or splenic CD4 T cells from BPZE1-immunized mice provided protection against infection in the lung with virulent B. pertussis, but did not protect in the nasal cavity [34,124]. However, transfer of nasal washes from BPZE1-immunized mice to naïve mice did protect against nasal colonization [34]. BPZE1-induced protection against nasopharyngeal colonization was dependent on the production of local sIgA and IL-17. BPZE1 immunization also generated nasal CD4 TRM cells, which produced IL-17 and, to a lesser extent, IFN-γ, and protection was greatly diminished in IL-17-/- mice [34]. This study also showed that long-term protection was maintained in the nasal cavity for up to 10 months post immunization. However, nasal washes transferred at the later time point did not protect naïve mice, suggesting that sustained protective immunity generated by BPZE1 in the nasal cavity may be due to local IL-17-producing TRM cells [34].
It was also reported that BPZE1 could induce rapid protection against B. pertussis infection in mice soon after immunization [126]. Mice were significantly protected at 1 week after immunization and this was dependent on TLR4 and the TLR adaptor protein, MyD88, and was completely independent of adaptive immune responses [126]. These findings suggest that BPZE1 is effective at stimulating protective innate immune responses. The studies in mouse models were translated to humans through the demonstration that human monocyte-derived DCs, cultured in vitro with BPZE1, mature similarly to those cultured with virulent B. pertussis and, when co-cultured with naïve T cells, these DCs promoted Th1 and Th17 differentiation [127]. A vaccine study in humans showed that immunization with BPZE1 induced strong Th1 responses, with IgG1/IgG3 serum antibodies, whereas volunteers immunized with an aP vaccine had Th2-biased responses [72]. Serum antibodies from the BPZE1-immunized individuals promoted neutrophil reactive oxygen species (ROS) production and had bactericidal killing capacity [72]. Systemic Th17 responses were not detected in this study, however mucosal T cell responses were not assessed, thus, it remains to be seen whether local airway Th17 cells are generated by immunization with BPZE1 in humans [72]. BPZE1 is currently being tested in Phase 2 clinical trials as a single i.n. immunization (NCT03541499) or comparison with aP vaccine in various prime-boost vaccination strategies (NCT03942406).
4.2. OMV Vaccines
OMVs are spherical structures derived from the cellular envelope of Gram-negative bacteria, which bud from actively growing cells and are composed of their outer membrane containing periplasmic components [128]. An advantage of pertussis OMV over aP vaccines is that they contain a wider variety of bacterial antigens in their natural conformation, which can elicit a broader immune response, without the same risk of over-exuberant inflammatory responses observed with wP vaccines [129,130]. Studies in mice suggest that pertussis OMV vaccines delivered parenterally generate mixed Th1/Th2/Th17 responses as well as CD4 TRM cells, conferring protection greater than that induced with aP vaccines, and similar to that induced with wP vaccines, whilst displaying a better safety profile [129,131,132]. Zurita et al. showed that immunization of mice with a pertussis OMV vaccine conferred greater protection than an aP vaccine against a PRN(−) strain of B. pertussis. Immunization with the pertussis OMV vaccine also induced B. pertussis-specific splenic Th1 and Th17 memory responses, as well as IL-17- and IFN-γ-producing CD4 TRM cells in the lungs [131]. Long-term protection conferred by OMV vaccines may be mediated by cytokine-secreting TRM cells in the lung [131,133].
An OMV vaccine produced from the B. pertussis Tohama-1 strain, which expresses the B. bronchoseptica gene pagL encoding lipid A 3-deacylase PagL (OMVBpPagL), enhanced protection in the lungs induced with a Tdap vaccine, and provided long-term protection against multiple B. pertussis strains at 9 months post-immunization [133]. Studies in mice suggested that the OMVBpPagL vaccine was safer than WT OMVs; mice immunized i.n. with OMVBpPagL had less weight loss and reduced IL-6 and IL-1 production compared with mice immunized with WT OMVs [134]. OMVs generated from the B. pertussis B1917 strain provided comparable protection to a wP vaccine in mice, but induced lower serum IL-1β and IL-6 concentrations. Induction of IL-1β in particular has been associated with wP-induced neurological effects [135].
Immunization with pertussis OMV vaccine generates antibody responses with a higher IgG2a/IgG1 ratio and stronger opsonizing activity when compared with antibodies induced with aP vaccines [132]. The breadth of the antibody responses is also greater after immunization with pertussis OMV vaccines, with antibodies specific for both aP and non-aP antigens, such as GroEL, Vag8 and BrkA [44,132].
Pulmonary administration was found to be an effective route for the delivery of a pertussis OMV vaccine and induced Th17 and Th1 responses locally in the lung and systemically, as well as antigen-specific IgA production [136]. However, pulmonary immunization did not prevent nasal colonization with B. pertussis [136]. In contrast, immunization with OMV by the i.n. but not the s.c. route conferred significant protection in the nasal cavity [137]. Protection was associated with the induction of mucosal IL-17 and IFN-γ, increased lung and nasal IgA as well as strong systemic Th17 responses [137].
4.3. aP Vaccines with New Antigens
One explanation for the failure of aP vaccines to confer long lasting protection in immunized individuals, is the limited number of antigens, which results in a narrower range of B. pertussis-specific immune responses [72]. In addition, there is evidence that aP vaccines with a limited number of antigens may have facilitated immune selective pressure that has led to the emergence of PRN(−) strains of B. pertussis [26,138]. PRN(−) strains have been shown to outcompete PRN(+) strains in aP-immunized mice [28]. Strains with mutations in the promoter region of the ptx gene which encodes PT have also been identified [139]. B. pertussis strains lacking both PRN and PT are rarer, but have also been documented, as have reports of FHA and FIM2/3 deficient strains [140,141,142,143]. Therefore, addition of new antigens to aP vaccines may increase protection and reduce the effects of immune selective pressure.
FIM2/3 are currently included in five-component aP vaccines, but not in two- and three-component vaccines, and clinical trials have shown that aP vaccines which contain FIM2/3 may be more efficacious in preventing pertussis disease [12,13]. Studies in mice showed that the addition of high doses of FIM2/3 to commercial, two-, three- and five-component aP vaccines increased protection in the lungs following challenge with a Prn(−) strain of B. pertussis.
B. pertussis virulence is regulated by the BvgAS system, which includes two components, the histidine kinase, BvgS, and the response regulator BvgA [144]. The BvgAS system positively regulates almost all B. pertussis virulence factors, including current aP antigens PT, FHA, PRN and FIM2/3 [145]. Adenylate cyclase toxin (ACT) is encoded by the cyaA gene, activated by BvgA [146], and plays a central role in inhibiting the host immune response to B. pertussis and promotes bacterial survival [147]. Although ACT is an important B. pertussis virulence factor, it is not currently included in any commercial aP vaccine. Early evidence that ACT may be an immunodominant antigen came from studies showing that B. pertussis-infected individuals had high levels of anti-ACT antibodies [148]. Anti-ACT antibodies are also generated following immunization with wP vaccines and are present in newborn cord blood [149,150]. Native and recombinant ACT have been shown to have protective function in animal models [151,152,153,154]. Addition of active or enzymatically inactive ACT to aP vaccines promoted a shift from Th2 to a mixed Th1/Th2 response and enhanced protection against lung infection with B. pertussis in mice, however, immunization with ACT alone did not confer protection [155]. ACT in its native form, undergoes quick proteolytic degradation within the cell, complicating its potential use in aP vaccines [156,157]. However, RTX antigens, which are more stable than full-length ACT generate strong ACT neutralizing antibody responses [158]. Addition of C-terminal repeats-in-toxin domain (RTX) of ACT to a low dose (1/80 of human dose) of an aP vaccine enhanced its protective efficacy against lung infection with B. pertussis in mice [159].
Bordetella resistance to killing A (BrkA), a virulence factor activated by phosphorylated BvgA, which has autotransporter function and plays a role in bacterial adherence to lung epithelial cells, as well as in evading complement-mediated phagocytosis [160] is another candidate antigen that may improve the efficacy of aP vaccines. Immunization of mice with BrkA alone by the s.c. route did not confer protection against lung infection in mice, but it did enhance protection when added to an aP vaccine comprising FHA and PT antigens [161]. However, another study showed that when compared with control mice immunized with alum only, mice immunization with an alum-adjuvanted mono-component BrkA vaccine by the i.p. route had lower numbers of bacteria in the lungs 7 days post B. pertussis challenge [162].
Immunization by the s.c. route with either recombinant BvgA-activated autotransporter, Vag8 or SphB1, adjuvanted with alum conferred some protection against lung infection with B. pertussis B1917. However, vaccines containing these antigens did not confer protection in the upper respiratory tract [163]. Although the addition of new antigens may indeed enhance protection in the lung, it is evident that generating protection in the upper respiratory tract will prove more challenging and may not be achievable solely through the addition of antigens to parenterally administered aP vaccines.
4.4. aP Vaccines with New Adjuvants
Adjuvants function by activating innate immune cells, which in turn direct adaptive immune responses to antigenic components in a vaccine, and this not only increases the quantity of the immune response but can improve its quality [164]. Natural components of many live attenuated and killed whole-cell vaccines have adjuvant properties, but this can result in excessive unwanted inflammation, as observed in some instances with wP vaccines [4,165]. Subunit vaccines, on the other hand, are often poorly immunogenic without the addition of an appropriate adjuvant [166]. Live B. pertussis and wP vaccines contain LPS, which activates DCs through TLR4, and although effective in eliciting strong Th1 and Th17 responses, LPS is not a safe adjuvant for human use [93,167]. However, there are a number of alternative adjuvants based on PAMPs, including TLR agonists, and particulate delivery systems that are safe for use in humans (Figure 2).
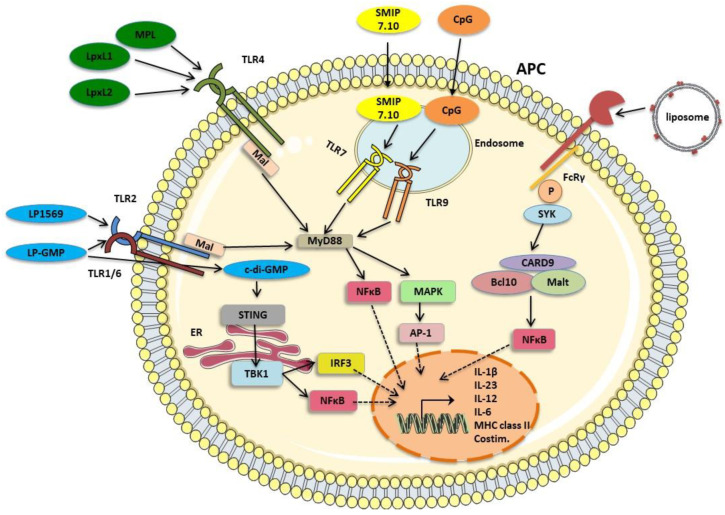
Figure 2.
Mechanisms of action of novel adjuvants under investigation in experimental aP vaccines. Activation of signaling pathways in APC by various adjuvants can be harnessed to generate Th1/Th17 polarizing conditions following immunization. These include the TLR2 agonist LP1569 and the TLR4 agonists MPL, LpxL1 and LpxL2 which are synthetic analogues of LPS. SMIP7.10 and CpG ligate the endosomal TLRs, TLR7 and TLR 9 respectively. TLR 2, 4, 7 and 9 signal through the adaptor molecule MyD88, with TLR2 and TLR4 requiring the bridging adaptor Mal to activate MyD88-mediated signaling. MyD88 signaling results in downstream activation of NFκB and MAPKs. MAPKs activate transcription factors such as AP-1. NFκB and AP-1 translocate to the nucleus leading to the transcription of pro-inflammatory and T cell-polarizing cytokines, chemokines, MHC class II and costimulatory molecules. The CLR, Mincle, is ligated by the TDB component (shown in red) of liposomes, which signals via SYK, and subsequently activates CARD9-Bcl10-Malt signalosome and NFκB. c-di-GMP activates the intracellular DNA sensor, STING, located at the ER, and complexed with TBK1 translocates to perinuclear regions, activating NFκB and IRF3. LP-GMP, which combines LP1569 and c-di-GMP adjuvants, signals through TLR2 and STING respectively, resulting in synergistic induction of Th1/Th17 responses. Block arrows denote activation and dashed arrows denote translocation of transcription factors to the nucleus. Images from Servier Medical Art (www.smart.servier.com). APC: antigen presenting cell, TLR: Toll-like receptor, Th: T helper cell, MPL: Monophosphoryl lipid A, LPS: Lipopolysaccharide, MyD88: Myeloid differentiation primary response gene 88, Mal: MyD88 adaptor-like, NF-κB: Nuclear Factor kappa B, MAPK: Mitogen-activated tyrosine kinase, TDB: α,α trehalose 6,6’-dibehenate, SYK: Spleen tyrosine kinase, CARD9: Caspase recruitment domain family member 9, Bcl10: B-cell lymphoma 10, Malt1: Mucosa-associated lymphoid tissue 1, AP1: Activator protein 1, IRF3: Interferon response factor 3, STING: Stimulator of interferon genes, TBK1: TANK-binding kinase 1, MHC Class II: Major histocompatibility complex class II, Costim: costimulatory molecules.
4.4.1. Alum
All licensed aP vaccines utilize alum as the adjuvant. Alum has been in common use since the 1920s, when it was first shown to enhance immunogenicity of diphtheria toxoid [168]. Alum or aluminum salts are safe and non-toxic adjuvants, and promote strong antibody and Th2 response, but also induce IL-10 production by DCs and macrophages, which can inhibit polarization of Th1 cells [169] Its beneficial role was initially believed to be due to depot formation, where antigens are sequestered within an alum aggregate at the site of injection, allowing slow release of antigens [170,171]. Furthermore, the particulate antigens generated with alum can be phagocytosed more readily by APCs [172]. Alum can also activate the NLRP3 inflammasome, leading to IL-1β processing [173,174] and this can promote the generation of Th17 responses in mice immunized with aP vaccines [16]. Other studies have shown that NLRP3 may be dispensable for its activity as an adjuvant, suggesting multiple mechanisms of immune activation [175].
Alum can inhibit IL-12 production by DCs thereby generating Th2-skewed CD4 T cell responses [176]. Th2 polarization along with strong antibody responses are effective for vaccines against certain infectious diseases, where their protection clearly correlates with humoral immunity [177]. However, this may not be the case for pertussis, where there is a clear protective role for T cells. An alternative strategy for inducing protective cellular immune responses and memory is through the addition of novel adjuvants to aP vaccines, either replacing alum completely, or adding them to vaccines already formulated with alum.
4.4.2. TLR4 Agonists
The TLR4 agonist LPS is a natural component of the outer membrane of B. pertussis and is present in wP vaccines, functioning as a natural adjuvant, which contributes to protective immunity induced with this vaccine [93,178]. TLR4 signaling is crucial for early innate responses and airway neutrophil recruitment during B. pertussis infection, and is essential for protection generated with the BPZE1 vaccine [126,179]. LPS is a potent activator of TLR4-dependent pro-inflammatory responses, but because of its toxicity is not safe for use as an adjuvant for human subunit vaccines [180]. To overcome toxicity issue while retaining its adjuvant capacity, LPS derivatives, such as monophosphoryl lipid-A (MPL), have been developed which can bind TLR4, and induce downstream signaling without the risk of adverse events [181]. MPL is an approved adjuvant in vaccines against hepatitis B virus and human papilloma virus [182,183]. Mice immunized with an aP vaccine formulated with MPL had lower bacterial load in the lung post B. pertussis challenge, compared to mice immunized with alum-adjuvanted aP [184]. These mice also had higher anti-PT IgG in serum, and reduced B. pertussis-specific Th2 responses [184].
An alternative LPS derivative from Neisseria meningitis, LpxL2, promoted protective immunity comparable to MPL. The aP vaccines formulated with MPL or LpxL2 induced less IL-6 production in mice than a wP vaccine, suggesting that they may promote protective inflammatory response with a reduced risk of immunopathology [184]. The LPS derivative, LpxL1, also derived from N. meningitis, has been shown to shift CD4 T cell responses from a Th2 to Th1/Th17 profile in mice when added to a commercial alum-adjuvanted aP vaccine [185]. Using MHC class II PRN tetramers, the authors showed that the addition of LpxL1 to aP vaccine led to increased PRN-specific CD4 T cells and a greater number of T cells with a TCM phenotype in the draining lymph nodes and blood of immunized mice [185]. Transcriptomic analysis revealed that genes involved in Th2 and Treg polarization were upregulated in mice immunized with the aP vaccine alone, whereas the addition of LpxL1 to an aP vaccine led to an increase in il17 and ifnγ mRNA expression, as well as increasing expression of Th1 and Th17 transcription factor genes, tbx21 and rorc. This demonstrates how the addition of a TLR4 agonist can alter immune responses at a gene expression level [186].
4.4.3. TLR 9 Agonists
Unmethylated single-stranded CpG DNA motifs, which are released from bacteria during infection, activate TLR9 signaling [187]. Synthetic CpG oligodeoxynucleotides (ODNs) that mimic this process have adjuvant capacity, generate strong Th1 responses in mice and humans and are capable of overriding the alum-driven Th2 bias when used in combination with alum [188,189,190]. The addition of CpG ODNs to an aP vaccine, either with or without alum, increased anti-PT IgG2a serum antibody titers [191] and robustly induce IFN-γ-producing splenic T cells [192]. Ross et al. showed that an experimental aP vaccine formulated with CpG alone induced B. pertussis-specific Th1 and Th17 responses, and IgG2 antibody responses in mice [16]. This was associated with significant protection against lung infection with B. pertussis, with full clearance of bacteria by day 10 post challenge, compared with day 15 for mice immunized with an aP vaccine formulated with alum [16].
A study by Asokanathan and colleagues demonstrated that addition of CpG to an alum-adjuvanted aP vaccine enhanced protection against lung infection of mice with B. pertussis. This was associated with increased IFN-γ production in the spleen and increased nitric oxide production in peritoneal macrophages stimulated in vitro with heat-killed B. pertussis [190]. However, in this study, substituting CpG for alum did not enhance protection against lung infection.
4.4.4. TLR7 Agonists
TLR7 agonists have adjuvant activity, enhance vaccine efficacy and generate Th1/Th17 responses in a number of infectious diseases [193,194,195]. The adsorption of small molecule TLR7 agonists to alum increases their safety for use as adjuvants, due to reduced systemic inflammation and attenuated cytokine release into the blood, while also improving adjuvant function [196]. Addition of the synthetic TLR7 agonist, SMIP-7.10 to an alum-absorbed aP vaccine significantly enhanced its efficacy and conferred protection against lung infection of mice with B. pertussis, comparable to that generated with a wP vaccine [197]. The addition of SMIP-7.10 resulted in a shift from a Th2 response induced with the alum-adjuvanted vaccine to the induction of Th1 and Th17 responses. This was accompanied by an increase in IgG2a and IgG2c antibodies as well as enhanced functional activity, including antibodies that neutralized PT and inhibited FHA binding to human lung epithelial cells [197]. These findings suggest that it may be possible to enhance the efficacy of existing alum-adjuvanted aP vaccines by addition of small molecule TLR agonists that promote protective T cell responses.
4.4.5. TLR2 Agonists
B. pertussis expresses a number of lipoproteins that are TLR2 agonists and also have antigenic properties [198]. BP1569 and a synthetic lipopeptide derivative LP1569 have immunomodulatory activity, activating innate immune responses and acted as adjuvants for an experimental aP vaccine in mice. LP1569 induced the production of T cell polarising cytokines, IL-1β, IL-12, IL-23 and enhanced expression of costimulatory molecules, CD80 and CD86, and MHC class II on murine and human DCs [198]. Immunization of mice with an aP vaccine formulated with LP1569 enhanced protection against lung and trachea infection compared with an alum-adjuvanted aP vaccine [198]. Furthermore, strong FHA-specific Th1 and Th17 responses were observed in spleen of mice immunized with the aP vaccine formulated with LP1569, whereas strong Th2 responses were observed in mice immunized with B. pertussis antigens alone or with alum [198].
4.4.6. STING and TLR Agonists
Stimulator of interferon genes (STING), which binds cyclic dinucleotides (CDNs), functions as an adaptor molecule for the cytosolic DNA receptor cyclic-GMP-AMP synthase (cGAS), which converts double-stranded (ds)DNA to the second messenger, cyclic GMP-AMP (cGAMP) [199]. STING acts as a PRR and detects bacterial second messengers, such as cyclic-di-GMP (c-di-GMP) and c-di-AMP (c-di-AMP) [200,201]. Ligation of STING by CDNs activates signaling pathways leading to the activation of the transcription factors NF-κB and IRF3 and type 1 interferon gene expression [202,203].
Administration of c-di-GMP to mice by the i.n. route 24 h before B. pertussis challenge enhanced bacterial clearance from the lungs of mice [204]. C-di-GMP treatment increased pulmonary neutrophil and macrophage recruitment and induced early macrophage inflammatory protein-2 (MIP-2), which is important for neutrophil recruitment, and macrophage chemotactic protein 1 (MCP-1), which recruits a range of lymphoid and myeloid cells [204,205,206,207]. DC activation and Th1 cytokine production was also enhanced before and after B. pertussis challenge in mice treated with c-di-GMP [204]. These findings demonstrated the central role of innate immune stimulation in generating a protective immunity against B. pertussis and suggested that c-di-GMP could be a viable adjuvant for a pertussis vaccine.
The adjuvant activity of STING agonists is enhanced when combined with TLR agonists. The combination of agonists for STING and TLR2 or TLR9 induced IL-12 and IL-23 production by innate immune cells, which promoted induction of Th1 and Th17 cells [33,208]. The STING agonist c-di-GMP combined with the TLR2 agonist LP1569, termed LP-GMP, has potent adjuvant activity and when added to an experimental 3-component aP vaccine, containing FHA, PT and PRN, promoted protective immunity against B. pertussis in the lower and upper respiratory tract of mice [33]. The most dramatic adjuvant effect was observed when LP-GMP-adjuvanted aP was administered by the i.n. route; this combination conferred sustained protection against nasal colonization and lung infection of mice when challenged with B. pertussis up to 10 months after immunization [33]. In contrast, the same aP vaccine formulated with alum did not confer any protection against nasal colonization [14,15,33]. The aP vaccine formulated with LP-GMP induced potent Th17 responses, especially when delivered by the i.n. route. However, parenteral immunization generated stronger systemic B. pertussis-specific IFN-γ, indicating stronger Th1 responses [33]. Immunization with LP-GMP-formulated aP vaccine also promoted accumulation of CD69+ CD103+/- CD4 TRM cells in the lung and nasal tissue; induction of respiratory TRM cells was most effective following i.n. administration of the vaccine. These respiratory TRM cells predominantly produced IL-17 and the number of these cells strongly correlated with long-term protective immunity in the nasal cavity [33]. This highlights the potential power of combining adjuvants to generate a robust Th1/Th17 response and TRM cells, and demonstrates how mucosal immunization can further enhance protective immunity in the respiratory tract.
4.4.7. Particulate Antigen Delivery Systems, Live Vectors and Nucleic Acid Vaccines
Vaccine delivery vehicles have also been used as tools to improve the efficacy of pertussis vaccines. Microparticles made from the biodegradable polymer poly(lactide-co-glycolide) (PLG) have been utilized as a delivery vehicle for aP vaccines. A single parenteral dose of PLG microparticle-encapsulated FHA and detoxified PT was shown to induce robust Th1 responses and to protect against B. pertussis infection in the lungs of mice [209]. Liposomes also have potential as particulate delivery systems for aP vaccines, having already shown promise in experimental vaccines against other infectious diseases, notably Mycobacterium tuberculosis [210]. Furthermore, recombinant vector vaccines and mRNA and DNA vaccines are in development for viruses such as SARS-CoV-2 [211] and have potential in the development of new vaccines against B. pertussis.
5. Route of Vaccine Administration—A Case for an Intranasal Pertussis Vaccine
Most vaccines approved for use in humans are delivered via parenteral routes, mostly intramuscular (i.m.), but also s.c. or intradermal (i.d.) [212]. It is now evident that pathogens that infect mucosal surfaces require effective local immunity and immune memory at the mucosal site of infection. Recent studies in mouse models have demonstrated that the induction of mucosal TRM cells is key to sustained protective immunity against many mucosal pathogens, and immunization at mucosal site of infection appears to be the most effective strategy to achieve this [213]. Currently, the only approved mucosal vaccines are a live attenuated influenza vaccine delivered by nasal spray and a number of oral vaccines against enteric pathogens [214].
Studies that examined the route of administration of TB vaccines have demonstrated that efficacy is often enhanced when a vaccine is delivered by a mucosal route [215,216,217,218,219]. Protection against M. tuberculosis conferred by the attenuated M. bovis vaccine BCG is more effective following i.n. or intratracheal (i.t.) delivery compared with parenteral administration [220,221,222]. Mucosal immunization of mice with BCG induces a higher frequency of CD4 TRM cells in the lungs when compared with immunization by a parenteral route, and this is associated with enhanced protection against infection in the lungs [215,216].
In the context of B. pertussis infection, it is now established that generating strong mucosal responses in the lung and nasal cavity, including the generation of respiratory TRM cells, local IL-17 and sIgA are important for sustained protection against lung infection and nasal colonization [14,32,33,34,131]. However, parenterally delivered alum-adjuvanted aP vaccines do not generate local immunity in the respiratory tract, but instead rely on circulating antibodies driven by systemic T cell responses to prevent severe disease mediated by the B. pertussis toxins [14,15,29,223]. Delivery of pertussis vaccines by the i.n. route has the potential to enhance local immune responses in the nasopharynx, and may provide a solution to the problem of asymptomatic colonization and transmission of B. pertussis [224]. The live attenuated vaccine, BPZE1, was specifically developed to be administered by the i.n. route and animal studies have shown that it can generate protective TRM cells that produce IL-17 and IFN-γ, as well as local sIgA in the respiratory tract, and can confer durable protection against nasal colonization with B. pertussis [34,92,121,123]. The first-in-human trials showed that the i.n. administration of BPZE1 is safe [122], and it is now being assessed in Phase 2 clinical trials (NCT03541499; NCT03942406).
A pertussis OMV vaccine delivered by the i.n. route generated superior protection and stronger Th1/Th17 response compared with parenteral administration of the same vaccine [137]. Delivery of aP vaccines by the i.n. route also has the capacity to enhance the efficacy of aP vaccines, especially when formulated with adjuvants that stimulate local immunity in the respiratory tract. We have reported that i.n. administration of a three-component aP vaccine, adjuvanted combined with the potent T cell-inducing adjuvant LP-GMP promoted induction of IL-17+ TRM cells in the lungs and nasal tissue and conferred long-lived protection against nasal colonization as well as lung infection [33].
6. Concluding Remarks
Great strides have been made in recent years on our understanding of the mechanisms of protective immunity against B. pertussis, which will assist in the design of next generation pertussis vaccines. Most people in the field now accept that the current alum-adjuvanted patenterally-delivered aP vaccine is suboptimal and needs to be supplemented or replaced with a more effective pertussis vaccine. The discovery from baboon and mouse models that current aP vaccines do not generate protective immunity against nasal colonization has been a game-changer. Together with epidemiological studies in humans, the findings suggest asymptomatic transmission is occurring in vaccinated populations. The demonstration that the current aP vaccines fail to generate CD4 TRM cells in the lung and nasal tissue, alongside the well-established fact that they do not induce mucosal IgA, has focused attention on the need for new vaccine approaches that induce local T cells and antibodies in the respiratory tract. There is strong evidence that IFN-γ-secreting Th1 cells play a key role in clearance of a primary infection and preventing re-infection of the lungs with B. pertussis. In contrast, protection in the nasal cavity appears to be more dependent on IL-17 produced by Th17 and possibly also from other sources, including γδ T cells.
The induction of potent Th1- and Th17-type CD4 TRM cells is likely to be a key factor in the design of more effective vaccines. The use of novel adjuvants and vaccine delivery systems in aP vaccines, as well as the development of live attenuated and OMV vaccines for pertussis, are already showing promising results in animal models and more limited early clinical trials. The implementation of new vaccines will not be straightforward, as unbundling of the current pediatric combination would be logistically difficult. The first steps may see the implementation of new booster vaccines involving the existing aP vaccine antigens but with a novel adjuvant. However, emerging data suggest that it may not be easy to shift the Th2 responses set following immunization with current alum-adjuvanted aP vaccine. Therefore, an alternative approach may be to introduce a priming dose with stand-alone pertussis vaccine formulation that induces the desired cellular immune responses in neonates. If B. pertussis-specific Th1 and Th17 responses are set early in life prior to immunization with routine pediatric vaccine (including pertussis) formulated with alum, this may be the road to a more effective vaccine that will induce sterilizing immunity in the nasal cavity as well as the lungs. However, a longer term objective, which is likely to be more effective, should be to remove the aP vaccine from the current pediatric combination and replace it with an easily administered nasally-delivered pertussis vaccine, which promotes respiratory TRM cells that confer sustained protective immunity in the nasal mucosae as well as the lungs.
Acknowledgments
We are grateful to Lisa Borkner, Pauline Schmitt and Mieszko Wilk for helpful comments on the manuscript.
Author Contributions
C.N.C. and K.H.G.M.; writing, review and editing, K.H.G.M.; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Science Foundation Ireland (grant number 16/IA/4468) and the Irish Research Council (grant number GOIPG/2020/1241).
Conflicts of Interest
Kingston Mills is inventor on a patent application on B. pertussis-derived TLR2 agonists as novel adjuvants and has collaborative research funding from and acts as consultant to Vaccine manufacturers. The funders had no role in the writing of the manuscript or in the decision to publish this review.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mattoo S., Cherry J.D. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewlett E.L., Burns D.L., Cotter P.A., Harvill E.T., Merkel T.J., Quinn C.P., Stibitz E.S. Pertussis Pathogenesis—What We Know and What We Don’t Know. J. Infect. Dis. 2014;209:982–985. doi: 10.1093/infdis/jit639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janda W.M., Santos E., Stevens J., Celig D., Terrile L., Schreckenberger P.C. Unexpected isolation of Bordetella pertussis from a blood culture. J. Clin. Microbiol. 1994;32:2851–2853. doi: 10.1128/JCM.32.11.2851-2853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cody C.L., Baraff L.J., Cherry J.D., Marcy S.M., Manclark C.R. Nature and Rates of Adverse Reactions Associated with DTP and DT Immunizations in Infants and Children. Pediatrics. 1981;68:650–660. [PubMed] [Google Scholar]
- 5.Decker M.D., Edwards K.M., Steinhoff M.C., Rennels M.B., Pichichero M.E., Englund J.A., Anderson E.L., Deloria M.A., Reed G.F. Comparison of 13 acellular pertussis vaccines: Adverse reactions. Pediatrics. 1995;96:557–566. [PubMed] [Google Scholar]
- 6.Edwards K.M. Unraveling the challenges of pertussis. Proc. Natl. Acad. Sci. USA. 2014;111:575–576. doi: 10.1073/pnas.1321360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorringe A.R., Vaughan T.E. Bordetella pertussis fimbriae (Fim): Relevance for vaccines. Expert Rev. Vaccines. 2014;13:1205–1214. doi: 10.1586/14760584.2014.930667. [DOI] [PubMed] [Google Scholar]
- 8.Edwards K.M., Meade B.D., Decker M.D., Reed G.F., Rennels M.B., Steinhoff M.C., Anderson E.L., Englund J.A., Pichichero M.E., Deloria M.A. Comparison of 13 acellular pertussis vaccines: Overview and serologic response. Pediatrics. 1995;96:548–557. [PubMed] [Google Scholar]
- 9.World Health Organization Pertussis vaccines: WHO position paper, August 2015—Recommendations. Vaccine. 2016;34:1423–1425. doi: 10.1016/j.vaccine.2015.10.136. [DOI] [PubMed] [Google Scholar]
- 10.Broder K.R., Cortese M.M., Iskander J.K., Kretsinger K., Slade B.A., Brown K.H., Mijalski C.M., Tiwari T., Weston E.J., Cohn A.C., et al. Preventing tetanus, diphtheria, and pertussis among adolescents: Use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2006;55:1–34. [PubMed] [Google Scholar]
- 11.Lambert L.C. Pertussis Vaccine Trials in the 1990s. J. Infect. Dis. 2014;209:S4–S9. doi: 10.1093/infdis/jit592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olin P., Rasmussen F., Gustafsson L., Hallander H.O., Heijbel H. Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Lancet. 1997;350:1569–1577. doi: 10.1016/S0140-6736(97)06508-2. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson L., Hallander H.O., Olin P., Reizenstein E., Storsaeter J. A Controlled Trial of a Two-Component Acellular, a Five-Component Acellular, and a Whole-Cell Pertussis Vaccine. N. Engl. J. Med. 1996;334:349–356. doi: 10.1056/NEJM199602083340602. [DOI] [PubMed] [Google Scholar]
- 14.Warfel J.M., Zimmerman L.I., Merkel T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA. 2014;111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilk M.M., Borkner L., Misiak A., Curham L., Allen A.C., Mills K.H.G. Immunization with whole cell but not acellular pertussis vaccines primes CD4 T RM cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerg. Microbes Infect. 2019;8:169–185. doi: 10.1080/22221751.2018.1564630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross P.J., Sutton C.E., Higgins S., Allen A.C., Walsh K., Misiak A., Lavelle E.C., McLoughlin R.M., Mills K.H.G. Relative Contribution of Th1 and Th17 Cells in Adaptive Immunity to Bordetella pertussis: Towards the Rational Design of an Improved Acellular Pertussis Vaccine. PLoS Pathog. 2013;9:e1003264. doi: 10.1371/journal.ppat.1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapil P., Merkel T.J. Pertussis vaccines and protective immunity. Curr. Opin. Immunol. 2019;59:72–78. doi: 10.1016/j.coi.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arsenault C., Johri M., Nandi A., Mendoza Rodríguez J.M., Hansen P.M., Harper S. Country-level predictors of vaccination coverage and inequalities in Gavi-supported countries. Vaccine. 2017;35:2479–2488. doi: 10.1016/j.vaccine.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Sobanjo-ter Meulen A., Duclos P., McIntyre P., Lewis K.D.C., Van Damme P., O’Brien K.L., Klugman K.P. Assessing the Evidence for Maternal Pertussis Immunization: A Report From the Bill & Melinda Gates Foundation Symposium on Pertussis Infant Disease Burden in Low- and Lower-Middle-Income Countries. Clin. Infect. Dis. 2016;63:S123–S133. doi: 10.1093/cid/ciw530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diavatopoulos D.A., Mills K.H.G., Kester K.E., Kampmann B., Silerova M., Heininger U., van Dongen J.J.M., van der Most R.G., Huijnen M.A., Siena E., et al. PERISCOPE: Road towards effective control of pertussis. Lancet Infect. Dis. 2019;19:e179–e186. doi: 10.1016/S1473-3099(18)30646-7. [DOI] [PubMed] [Google Scholar]
- 21.Klein N.P., Bartlett J., Fireman B., Rowhani-Rahbar A., Baxter R. Comparative Effectiveness of Acellular Versus Whole-Cell Pertussis Vaccines in Teenagers. Pediatrics. 2013;131:e1716–e1722. doi: 10.1542/peds.2012-3836. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan S.L., Ware R.S., Grimwood K., Lambert S.B. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. 2012;308:454–456. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- 23.Klein N.P., Bartlett J., Rowhani-Rahbar A., Fireman B., Baxter R. Waning Protection after Fifth Dose of Acellular Pertussis Vaccine in Children. N. Engl. J. Med. 2012;367:1012–1019. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- 24.Winter K., Harriman K., Zipprich J., Schechter R., Talarico J., Watt J., Chavez G. California Pertussis Epidemic, 2010. J. Pediatr. 2012;161:1091–1096. doi: 10.1016/j.jpeds.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Klein N.P., Bartlett J., Fireman B., Baxter R. Waning Tdap Effectiveness in Adolescents. Pediatrics. 2016;137:e20153326. doi: 10.1542/peds.2015-3326. [DOI] [PubMed] [Google Scholar]
- 26.Martin S.W., Pawloski L., Williams M., Weening K., DeBolt C., Qin X., Reynolds L., Kenyon C., Giambrone G., Kudish K., et al. Pertactin-Negative Bordetella pertussis Strains: Evidence for a Possible Selective Advantage. Clin. Infect. Dis. 2014;60:223–227. doi: 10.1093/cid/ciu788. [DOI] [PubMed] [Google Scholar]
- 27.Octavia S., Maharjan R.P., Sintchenko V., Stevenson G., Reeves P.R., Gilbert G.L., Lan R. Insight into Evolution of Bordetella pertussis from Comparative Genomic Analysis: Evidence of Vaccine-Driven Selection. Mol. Biol. Evol. 2010;28:707–715. doi: 10.1093/molbev/msq245. [DOI] [PubMed] [Google Scholar]
- 28.Safarchi A., Octavia S., Luu L.D.W., Tay C.Y., Sintchenko V., Wood N., Marshall H., McIntyre P., Lan R. Pertactin negative Bordetella pertussis demonstrates higher fitness under vaccine selection pressure in a mixed infection model. Vaccine. 2015;33:6277–6281. doi: 10.1016/j.vaccine.2015.09.064. [DOI] [PubMed] [Google Scholar]
- 29.Ryan M., Murphy G., Gothefors L., Nilsson L., Storsaeter J., Mills K.H.G. Bordetella pertussis Respiratory Infection in Children Is Associated with Preferential Activation of Type 1 T Helper Cells. J. Infect. Dis. 1997;175:1246–1250. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 30.Mahon B.P., Sheahan B.J., Griffin F., Murphy G., Mills K.H. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J. Exp. Med. 1997;186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warfel J.M., Zimmerman L.I., Merkel T.J. Comparison of Three Whole-Cell Pertussis Vaccines in the Baboon Model of Pertussis. Clin. Vaccine Immunol. 2015;23:47–54. doi: 10.1128/CVI.00449-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilk M.M., Misiak A., McManus R.M., Allen A.C., Lynch M.A., Mills K.H.G. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol. 2017;199:233–243. doi: 10.4049/jimmunol.1602051. [DOI] [PubMed] [Google Scholar]
- 33.Allen A.C., Wilk M.M., Misiak A., Borkner L., Murphy D., Mills K.H.G. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol. 2018;11:1763–1776. doi: 10.1038/s41385-018-0080-x. [DOI] [PubMed] [Google Scholar]
- 34.Solans L., Debrie A.-S., Borkner L., Aguiló N., Thiriard A., Coutte L., Uranga S., Trottein F., Martín C., Mills K.H.G., et al. IL-17-dependent SIgA-mediated protection against nasal Bordetella pertussis infection by live attenuated BPZE1 vaccine. Mucosal Immunol. 2018;11:1753–1762. doi: 10.1038/s41385-018-0073-9. [DOI] [PubMed] [Google Scholar]
- 35.Althouse B.M., Scarpino S.V. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 2015;13:146. doi: 10.1186/s12916-015-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi Y.H., Campbell H., Amirthalingam G., van Hoek A.J., Miller E. Investigating the pertussis resurgence in England and Wales, and options for future control. BMC Med. 2016;14:121. doi: 10.1186/s12916-016-0665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burdin N., Handy L.K., Plotkin S.A. What is wrong with pertussis vaccine immunity? The problem of waning effectiveness of pertussis vaccines. Cold Spring Harb. Perspect. Biol. 2017;9:a029454. doi: 10.1101/cshperspect.a029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brummelman J., Wilk M.M., Han W.G.H., van Els C.A.C.M., Mills K.H.G. Roads to the development of improved pertussis vaccines paved by immunology. Pathog. Dis. 2015;73:ftv067. doi: 10.1093/femspd/ftv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coutte L., Alonso S., Reveneau N., Willery E., Quatannens B., Locht C., Jacob-Dubuisson F. Role of adhesin release for mucosal colonization by a bacterial pathogen. J. Exp. Med. 2003;197:735–742. doi: 10.1084/jem.20021153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishibashi Y., Nishikawa A. Bordetella pertussis infection of human respiratory epithelial cells up-regulates intercellular adhesion molecule-1 expression: Role of filamentous hemagglutinin and pertussis toxin. Microb. Pathog. 2002;33:115–125. doi: 10.1006/mpat.2002.0517. [DOI] [PubMed] [Google Scholar]
- 41.Jahnsen F.L., Strickland D.H., Thomas J.A., Tobagus I.T., Napoli S., Zosky G.R., Turner D.J., Sly P.D., Stumbles P.A., Holt P.G. Accelerated Antigen Sampling and Transport by Airway Mucosal Dendritic Cells following Inhalation of a Bacterial Stimulus. J. Immunol. 2006;177:5861–5867. doi: 10.4049/jimmunol.177.9.5861. [DOI] [PubMed] [Google Scholar]
- 42.Carbonetti N.H., Artamonova G.V., Van Rooijen N., Ayala V.I. Pertussis toxin targets airway macrophages to promote Bordetella pertussis infection of the respiratory tract. Infect. Immun. 2007;75:1713–1720. doi: 10.1128/IAI.01578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernard N.J., Finlay C.M., Tannahill G.M., Cassidy J.P., O’Neill L.A., Mills K.H. A critical role for the TLR signaling adapter Mal in alveolar macrophage-mediated protection against Bordetella pertussis. Mucosal Immunol. 2015;8:982–992. doi: 10.1038/mi.2014.125. [DOI] [PubMed] [Google Scholar]
- 44.Raeven R.H.M., van der Maas L., Tilstra W., Uittenbogaard J.P., Bindels T.H.E., Kuipers B., van der Ark A., Pennings J.L.A., van Riet E., Jiskoot W., et al. Immunoproteomic Profiling of Bordetella pertussis Outer Membrane Vesicle Vaccine Reveals Broad and Balanced Humoral Immunogenicity. J. Proteome Res. 2015;14:2929–2942. doi: 10.1021/acs.jproteome.5b00258. [DOI] [PubMed] [Google Scholar]
- 45.Lambrecht B.N., Prins J.B., Hoogsteden H.C. Lung dendritic cells and host immunity to infection. Eur. Respir. J. 2001;18:692–704. [PubMed] [Google Scholar]
- 46.Higgins S.C., Lavelle E.C., McCann C., Keogh B., McNeela E., Byrne P., O’Gorman B., Jarnicki A., McGuirk P., Mills K.H.G. Toll-Like Receptor 4-Mediated Innate IL-10 Activates Antigen-Specific Regulatory T Cells and Confers Resistance to Bordetella pertussis by Inhibiting Inflammatory Pathology. J. Immunol. 2003;171:3119–3127. doi: 10.4049/jimmunol.171.6.3119. [DOI] [PubMed] [Google Scholar]
- 47.Dunne P.J., Moran B., Cummins R.C., Mills K.H.G. CD11c + CD8α + Dendritic Cells Promote Protective Immunity to Respiratory Infection with Bordetella pertussis. J. Immunol. 2009;183:400–410. doi: 10.4049/jimmunol.0900169. [DOI] [PubMed] [Google Scholar]
- 48.Misiak A., Wilk M.M., Raverdeau M., Mills K.H.G. IL-17–Producing Innate and Pathogen-Specific Tissue Resident Memory γδ T Cells Expand in the Lungs of Bordetella pertussis–Infected Mice. J. Immunol. 2016;198:363–374. doi: 10.4049/jimmunol.1601024. [DOI] [PubMed] [Google Scholar]
- 49.Andreasen C., Carbonetti N.H. Role of neutrophils in response to Bordetella pertussis infection in mice. Infect. Immun. 2009;77:1182–1188. doi: 10.1128/IAI.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boehm D.T., Varney M.E., Wong T.Y., Nowak E.S., Sen-Kilic E., Hall J., Bradford S.D., DeRoos K., Bevere J., Epperly M., et al. Characterizing the innate and adaptive responses of immunized mice to Bordetella pertussis infection using in vivo imaging and transcriptomic analysis. bioRxiv. 2019 doi: 10.1101/674408. [DOI] [Google Scholar]
- 51.Byrne P., McGuirk P., Todryk S., Mills K.H.G. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur. J. Immunol. 2004;34:2579–2588. doi: 10.1002/eji.200425092. [DOI] [PubMed] [Google Scholar]
- 52.Barbic J., Leef M.F., Burns D.L., Shahin R.D. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect. Immun. 1997;65:4904–4908. doi: 10.1128/IAI.65.12.4904-4908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacMicking J.D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 2012;12:367–382. doi: 10.1038/nri3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higgs R., Higgins S.C., Ross P.J., Mills K.H.G. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 2012;5:485–500. doi: 10.1038/mi.2012.54. [DOI] [PubMed] [Google Scholar]
- 55.Mills K.H., Barnard A., Watkins J., Redhead K. Cell-mediated immunity to Bordetella pertussis: Role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 1993;61:399–410. doi: 10.1128/IAI.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warfel J.M., Merkel T.J. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol. 2013;6:787–796. doi: 10.1038/mi.2012.117. [DOI] [PubMed] [Google Scholar]
- 57.Vinuesa C.G., Linterman M.A., Yu D., MacLennan I.C.M. Follicular Helper T Cells. Annu. Rev. Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 58.Rowe J., Macaubas C., Monger T.M., Holt B.J., Harvey J., Poolman J.T., Sly P.D., Holt P.G. Antigen-Specific Responses to Diphtheria-Tetanus-Acellular Pertussis Vaccine in Human Infants Are Initially Th2 Polarized. Infect. Immun. 2000;68:3873–3877. doi: 10.1128/IAI.68.7.3873-3877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bancroft T., Dillon M.B.C., da Silva Antunes R., Paul S., Peters B., Crotty S., Lindestam Arlehamn C.S., Sette A. Th1 versus Th2 T cell polarization by whole-cell and acellular childhood pertussis vaccines persists upon re-immunization in adolescence and adulthood. Cell. Immunol. 2016;304–305:35–43. doi: 10.1016/j.cellimm.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greco D., Salmaso S., Mastrantonio P., Giuliano M., Tozzi A.E., Anemona A., Ciofi degli Atti M.L., Giammanco A., Panei P., Blackwelder W.C., et al. A Controlled Trial of Two Acellular Vaccines and One Whole-Cell Vaccine against Pertussis. N. Engl. J. Med. 1996;334:341–349. doi: 10.1056/NEJM199602083340601. [DOI] [PubMed] [Google Scholar]
- 61.Sealey K.L., Belcher T., Preston A. Bordetella pertussis epidemiology and evolution in the light of pertussis resurgence. Infect. Genet. Evol. 2016;40:136–143. doi: 10.1016/j.meegid.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 62.Gambhir M., Clark T.A., Cauchemez S., Tartof S.Y., Swerdlow D.L., Ferguson N.M. A change in vaccine efficacy and duration of protection explains recent rises in pertussis incidence in the United States. PLoS Comput. Biol. 2015;11:e1004138. doi: 10.1371/journal.pcbi.1004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cherry J.D., Heininger U., Richards D.M., Storsaeter J., Gustafsson L., Ljungman M., Hallander H.O. Antibody response patterns to Bordetella pertussis antigens in vaccinated (primed) and unvaccinated (unprimed) young children with pertussis. Clin. Vaccine Immunol. 2010;17:741–747. doi: 10.1128/CVI.00469-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hendrikx L.H., Schure R.-M., Öztürk K., de Rond L.G.H., de Greeff S.C., Sanders E.A.M., Berbers G.A.M., Buisman A.-M. Different IgG-subclass distributions after whole-cell and acellular pertussis infant primary vaccinations in healthy and pertussis infected children. Vaccine. 2011;29:6874–6880. doi: 10.1016/j.vaccine.2011.07.055. [DOI] [PubMed] [Google Scholar]
- 65.Mills K.H.G., Ryan M., Ryan E., Mahon B.P. A Murine Model in Which Protection Correlates with Pertussis Vaccine Efficacy in Children Reveals Complementary Roles for Humoral and Cell-Mediated Immunity in Protection againstBordetella pertussis. Infect. Immun. 1998;66:594–602. doi: 10.1128/IAI.66.2.594-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coffman R.L., Seymour B.W.P., Lebman D.A., Hiraki D.D., Christiansen J.A., Shrader B., Cherwinski H.M., Savelkoul H.F.J., Finkelman F.D., Bond M.W., et al. The Role of Helper T Cell Products in Mouse B Cell Differentiation and Isotype Regulation. Immunol. Rev. 1988;102:5–28. doi: 10.1111/j.1600-065X.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 67.Bossie A., Vitetta E.S. IFN-γ enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: Implications for the role of IFN-γ in class switching. Cell. Immunol. 1991;135:95–104. doi: 10.1016/0008-8749(91)90257-C. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z., Goldschmidt T., Salter H. Possible allelic structure of IgG2a and IgG2c in mice. Mol. Immunol. 2012;50:169–171. doi: 10.1016/j.molimm.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Moon H.B., Severinson E., Heusser C., Johansson S.G.O., Möller G., Persson U. Regulation of IgG1 and IgE Synthesis by Interleukin 4 in Mouse B Cells. Scand. J. Immunol. 1989;30:355–361. doi: 10.1111/j.1365-3083.1989.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 70.Hellwig S.M.M., van Oirschot H.F.L.M., Hazenbos W.L.W., van Spriel A.B., Mooi F.R., van de Winkel J.G.J. Targeting to Fcγ Receptors, But Not CR3 (CD11b/CD18), Increases Clearance of Bordetella pertussis. J. Infect. Dis. 2001;183:871–879. doi: 10.1086/319266. [DOI] [PubMed] [Google Scholar]
- 71.Vidarsson G., Dekkers G., Rispens T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin A., Apostolovic D., Jahnmatz M., Liang F., Ols S., Tecleab T., Wu C., van Hage M., Solovay K., Rubin K., et al. Live attenuated pertussis vaccine BPZE1 induces a broad antibody response in humans. J. Clin. Investig. 2020;130:2332–2346. doi: 10.1172/JCI135020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tuomanen E.I., Zapiain L.A., Galvan P., Hewlett E.L. Characterization of antibody inhibiting adherence of Bordetella pertussis to human respiratory epithelial cells. J. Clin. Microbiol. 1984;20:167–170. doi: 10.1128/JCM.20.2.167-170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hellwig S.M., van Spriel A.B., Schellekens J.F., Mooi F.R., van de Winkel J.G. Immunoglobulin A-mediated protection against Bordetella pertussis infection. Infect. Immun. 2001;69:4846–4850. doi: 10.1128/IAI.69.8.4846-4850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christensen D., Mortensen R., Rosenkrands I., Dietrich J., Andersen P. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol. 2017;10:260–270. doi: 10.1038/mi.2016.28. [DOI] [PubMed] [Google Scholar]
- 76.Jaffar Z., Ferrini M.E., Herritt L.A., Roberts K. Cutting Edge: Lung Mucosal Th17-Mediated Responses Induce Polymeric Ig Receptor Expression by the Airway Epithelium and Elevate Secretory IgA Levels. J. Immunol. 2009;182:4507–4511. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Redhead K., Watkins J., Barnard A., Mills K.H. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect. Immun. 1993;61:3190–3198. doi: 10.1128/IAI.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagamatsu K., Kuwae A., Konaka T., Nagai S., Yoshida S., Eguchi M., Watanabe M., Mimuro H., Koyasu S., Abe A. Bordetella evades the host immune system by inducing IL-10 through a type III effector, BopN. J. Exp. Med. 2009;206:3073–3088. doi: 10.1084/jem.20090494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mosser D.M., Zhang X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saraiva M., O’Garra A., Saraiva M., O’Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 81.McGuirk P., McCann C., Mills K.H.G. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: A novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dirix V., Verscheure V., Vermeulen F., De Schutter I., Goetghebuer T., Locht C., Mascart F. Both CD4+ and CD8+ lymphocytes participate in the IFN-γ response to filamentous hemagglutinin from Bordetella pertussis in infants, children, and adults. Clin. Dev. Immunol. 2012;2012:795958. doi: 10.1155/2012/795958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raeven R.H.M., Brummelman J., Pennings J.L.A., Nijst O.E.M., Kuipers B., Blok L.E.R., Helm K., van Riet E., Jiskoot W., van Els C.A.C.M., et al. Molecular signatures of the evolving immune response in mice following a Bordetella pertussis infection. PLoS ONE. 2014;9:e104548. doi: 10.1371/journal.pone.0104548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Argondizo-Correia C., Rodrigues A.K.S., de Brito C.A. Neonatal Immunity to Bordetella pertussis Infection and Current Prevention Strategies. J. Immunol. Res. 2019;2019:7134168. doi: 10.1155/2019/7134168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Webster R.B., Rodriguez Y., Klimecki W.T., Vercelli D. The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. J. Biol. Chem. 2007;282:700–709. doi: 10.1074/jbc.M609501200. [DOI] [PubMed] [Google Scholar]
- 86.Hebel K., Weinert S., Kuropka B., Knolle J., Kosak B., Jorch G., Arens C., Krause E., Braun-Dullaeus R.C., Brunner-Weinzierl M.C. CD4+ T cells from human neonates and infants are poised spontaneously to run a nonclassical IL-4 program. J. Immunol. 2014;192:5160–5170. doi: 10.4049/jimmunol.1302539. [DOI] [PubMed] [Google Scholar]
- 87.Adkins B., Yoshimoto M. Epigenetic Regulation of the Th2 Locus in Fetal and Neonatal T Cells. Adv. Neuroimmune Biol. 2014;5:69–73. doi: 10.3233/NIB-140078. [DOI] [Google Scholar]
- 88.Mascart F., Verscheure V., Malfroot A., Hainaut M., Piérard D., Temerman S., Peltier A., Debrie A.-S., Levy J., Del Giudice G., et al. Bordetella pertussis Infection in 2-Month-Old Infants Promotes Type 1 T Cell Responses. J. Immunol. 2003;170:1504–1509. doi: 10.4049/jimmunol.170.3.1504. [DOI] [PubMed] [Google Scholar]
- 89.Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mills K.H.G. Induction, function and regulation of IL-17-producing T cells. Eur. J. Immunol. 2008;38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 91.Kolls J.K., Khader S.A. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Locht C., Papin J.F., Lecher S., Debrie A.-S., Thalen M., Solovay K., Rubin K., Mielcarek N. Live Attenuated Pertussis Vaccine BPZE1 Protects Baboons against Bordetella pertussis Disease and Infection. J. Infect. Dis. 2017;216:117–124. doi: 10.1093/infdis/jix254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Higgins S.C., Jarnicki A.G., Lavelle E.C., Mills K.H.G. TLR4 Mediates Vaccine-Induced Protective Cellular Immunity to Bordetella pertussis: Role of IL-17-Producing T Cells. J. Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 94.Dunne A., Ross P.J., Pospisilova E., Masin J., Meaney A., Sutton C.E., Iwakura Y., Tschopp J., Sebo P., Mills K.H.G. Inflammasome Activation by Adenylate Cyclase Toxin Directs Th17 Responses and Protection against Bordetella pertussis. J. Immunol. 2010;185:1711–1719. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- 95.da Silva Antunes R., Babor M., Carpenter C., Khalil N., Cortese M., Mentzer A.J., Seumois G., Petro C.D., Purcell L.A., Vijayanand P., et al. Th1/Th17 polarization persists following whole-cell pertussis vaccination despite repeated acellular boosters. J. Clin. Investig. 2018;128:3853–3865. doi: 10.1172/JCI121309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van der Lee S., Hendrikx L.H., Sanders E.A.M., Berbers G.A.M., Buisman A.-M. Whole-Cell or Acellular Pertussis Primary Immunizations in Infancy Determines Adolescent Cellular Immune Profiles. Front. Immunol. 2018;9:51. doi: 10.3389/fimmu.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 98.Mueller S.N., Mackay L.K. Tissue-resident memory T cells: Local specialists in immune defence. Nat. Rev. Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 99.Gebhardt T., Wakim L.M., Eidsmo L., Reading P.C., Heath W.R., Carbone F.R. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 100.Masopust D., Choo D., Vezys V., Wherry E.J., Duraiswamy J., Akondy R., Wang J., Casey K.A., Barber D.L., Kawamura K.S., et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar B.V., Ma W., Miron M., Granot T., Guyer R.S., Carpenter D.J., Senda T., Sun X., Ho S.-H., Lerner H., et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017;20:2921–2934. doi: 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mackay L.K., Braun A., Macleod B.L., Collins N., Tebartz C., Bedoui S., Carbone F.R., Gebhardt T. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 103.Teijaro J.R., Turner D., Pham Q., Wherry E.J., Lefrançois L., Farber D.L. Cutting Edge: Tissue-Retentive Lung Memory CD4 T Cells Mediate Optimal Protection to Respiratory Virus Infection. J. Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pham O.H., McSorley S.J. Divergent behavior of mucosal memory T cells. Mucosal Immunol. 2015;8:731–734. doi: 10.1038/mi.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schenkel J.M., Fraser K.A., Beura L.K., Pauken K.E., Vezys V., Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schenkel J.M., Fraser K.A., Vezys V., Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holmkvist P., Roepstorff K., Uronen-Hansson H., Sandén C., Gudjonsson S., Patschan O., Grip O., Marsal J., Schmidtchen A., Hornum L., et al. A major population of mucosal memory CD4+ T cells, coexpressing IL-18Rα and DR3, display innate lymphocyte functionality. Mucosal Immunol. 2015;8:545–558. doi: 10.1038/mi.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masopust D., Jiang J., Shen H., Lefrançois L. Direct Analysis of the Dynamics of the Intestinal Mucosa CD8 T Cell Response to Systemic Virus Infection. J. Immunol. 2001;166:2348–2356. doi: 10.4049/jimmunol.166.4.2348. [DOI] [PubMed] [Google Scholar]
- 109.Khanna K.M., Bonneau R.H., Kinchington P.R., Hendricks R.L. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/S1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iijima N., Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kinnear E., Lambert L., McDonald J.U., Cheeseman H.M., Caproni L.J., Tregoning J.S. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol. 2018;11:249–256. doi: 10.1038/mi.2017.46. [DOI] [PubMed] [Google Scholar]
- 112.Zhao J., Zhao J., Mangalam A.K., Channappanavar R., Fett C., Meyerholz D.K., Agnihothram S., Baric R.S., David C.S., Perlman S. Airway Memory CD4+ T Cells Mediate Protective Immunity against Emerging Respiratory Coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zens K.D., Chen J.K., Farber D.L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1:e85832. doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McMaster S.R., Wilson J.J., Wang H., Kohlmeier J.E. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-γ Production. J. Immunol. 2015;195:203–209. doi: 10.4049/jimmunol.1402975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Paik D.H., Farber D.L. Influenza infection fortifies local lymph nodes to promote lung-resident heterosubtypic immunity. J. Exp. Med. 2020;218:e20200218. doi: 10.1084/jem.20200218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith N.M., Wasserman G.A., Coleman F.T., Hilliard K.L., Yamamoto K., Lipsitz E., Malley R., Dooms H., Jones M.R., Quinton L.J., et al. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol. 2018;11:220–235. doi: 10.1038/mi.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ge C., Monk I.R., Pizzolla A., Wang N., Bedford J.G., Stinear T.P., Westall G.P., Wakim L.M. Bystander Activation of Pulmonary Trm Cells Attenuates the Severity of Bacterial Pneumonia by Enhancing Neutrophil Recruitment. Cell Rep. 2019;29:4236–4244.e3. doi: 10.1016/j.celrep.2019.11.103. [DOI] [PubMed] [Google Scholar]
- 118.Woodworth J.S., Cohen S.B., Moguche A.O., Plumlee C.R., Agger E.M., Urdahl K.B., Andersen P. Subunit vaccine H56/CAF01 induces a population of circulating CD4 T cells that traffic into the Mycobacterium tuberculosis-infected lung. Mucosal Immunol. 2017;10:555–564. doi: 10.1038/mi.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hansen S.G., Zak D.E., Xu G., Ford J.C., Marshall E.E., Malouli D., Gilbride R.M., Hughes C.M., Ventura A.B., Ainslie E., et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat. Med. 2018;24:130–143. doi: 10.1038/nm.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bull N.C., Kaveh D.A., Garcia-Pelayo M.C., Stylianou E., McShane H., Hogarth P.J. Induction and maintenance of a phenotypically heterogeneous lung tissue-resident CD4+ T cell population following BCG immunisation. Vaccine. 2018;36:5625–5635. doi: 10.1016/j.vaccine.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mielcarek N., Debrie A.-S., Raze D., Bertout J., Rouanet C., Younes A.B., Creusy C., Engle J., Goldman W.E., Locht C. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2006;2:e65. doi: 10.1371/journal.ppat.0020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thorstensson R., Trollfors B., Al-Tawil N., Jahnmatz M., Bergstrom J., Ljungman M., Torner A., Wehlin L., Van Broekhoven A., Bosman F., et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine--BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS ONE. 2014;9:e83449. doi: 10.1371/journal.pone.0083449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feunou P.F., Kammoun H., Debrie A.-S., Mielcarek N., Locht C. Long-term immunity against pertussis induced by a single nasal administration of live attenuated B. pertussis BPZE1. Vaccine. 2010;28:7047–7053. doi: 10.1016/j.vaccine.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 124.Feunou Feunou P., Bertout J., Locht C. T- and B-Cell-Mediated Protection Induced by Novel, Live Attenuated Pertussis Vaccine in Mice. Cross Protection against Parapertussis. PLoS ONE. 2010;5:e10178. doi: 10.1371/journal.pone.0010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Skerry C.M., Mahon B.P. A Live, Attenuated Bordetella pertussis Vaccine Provides Long-Term Protection against Virulent Challenge in a Murine Model. Clin. Vaccine Immunol. 2011;18:187–193. doi: 10.1128/CVI.00371-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Debrie A.-S., Mielcarek N., Lecher S., Roux X., Sirard J.-C., Locht C. Early Protection against Pertussis Induced by Live Attenuated Bordetella pertussis BPZE1 Depends on TLR4. J. Immunol. 2019;203:3293–3300. doi: 10.4049/jimmunol.1901102. [DOI] [PubMed] [Google Scholar]
- 127.Fedele G., Bianco M., Debrie A.-S., Locht C., Ausiello C.M. Attenuated Bordetella pertussis Vaccine Candidate BPZE1 Promotes Human Dendritic Cell CCL21-Induced Migration and Drives a Th1/Th17 Response. J. Immunol. 2011;186:5388–5396. doi: 10.4049/jimmunol.1003765. [DOI] [PubMed] [Google Scholar]
- 128.Schwechheimer C., Kuehn M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015;13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raeven R.H.M., Brummelman J., Pennings J.L.A., van der Maas L., Tilstra W., Helm K., van Riet E., Jiskoot W., van Els C.A.C.M., Han W.G.H., et al. Bordetella pertussis outer membrane vesicle vaccine confers equal efficacy in mice with milder inflammatory responses compared to a whole-cell vaccine. Sci. Rep. 2016;6:38240. doi: 10.1038/srep38240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roberts R., Moreno G., Bottero D., Gaillard M.E., Fingermann M., Graieb A., Rumbo M., Hozbor D. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine. 2008;26:4639–4646. doi: 10.1016/j.vaccine.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 131.Zurita M.E., Wilk M.M., Carriquiriborde F., Bartel E., Moreno G., Misiak A., Mills K.H.G., Hozbor D. A Pertussis Outer Membrane Vesicle-Based Vaccine Induces Lung-Resident Memory CD4 T Cells and Protection Against Bordetella pertussis, Including Pertactin Deficient Strains. Front. Cell. Infect. Microbiol. 2019;9:125. doi: 10.3389/fcimb.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bottero D., Gaillard M.E., Zurita E., Moreno G., Martinez D.S., Bartel E., Bravo S., Carriquiriborde F., Errea A., Castuma C., et al. Characterization of the immune response induced by pertussis OMVs-based vaccine. Vaccine. 2016;34:3303–3309. doi: 10.1016/j.vaccine.2016.04.079. [DOI] [PubMed] [Google Scholar]
- 133.Gaillard M.E., Bottero D., Errea A., Ormazábal M., Zurita M.E., Moreno G., Rumbo M., Castuma C., Bartel E., Flores D., et al. Acellular pertussis vaccine based on outer membrane vesicles capable of conferring both long-lasting immunity and protection against different strain genotypes. Vaccine. 2014;32:931–937. doi: 10.1016/j.vaccine.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 134.Asensio C.J.A., Gaillard M.E., Moreno G., Bottero D., Zurita E., Rumbo M., van der Ley P., van der Ark A., Hozbor D. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine. 2011;29:1649–1656. doi: 10.1016/j.vaccine.2010.12.068. [DOI] [PubMed] [Google Scholar]
- 135.Armstrong M.E., Loscher C.E., Lynch M.A., Mills K.H.G. IL-1β-dependent neurological effects of the whole cell pertussis vaccine: A role for IL-1-associated signalling components in vaccine reactogenicity. J. Neuroimmunol. 2003;136:25–33. doi: 10.1016/S0165-5728(02)00468-X. [DOI] [PubMed] [Google Scholar]
- 136.Raeven R.H.M., Brummelman J., Pennings J.L.A., van der Maas L., Helm K., Tilstra W., van der Ark A., Sloots A., van der Ley P., van Eden W., et al. Molecular and cellular signatures underlying superior immunity against Bordetella pertussis upon pulmonary vaccination. Mucosal Immunol. 2018;11:979–993. doi: 10.1038/mi.2017.81. [DOI] [PubMed] [Google Scholar]
- 137.Raeven R.H.M., Rockx-Brouwer D., Kanojia G., van der Maas L., Bindels T.H.E., ten Have R., van Riet E., Metz B., Kersten G.F.A. Intranasal immunization with outer membrane vesicle pertussis vaccine confers broad protection through mucosal IgA and Th17 responses. Sci. Rep. 2020;10:7396. doi: 10.1038/s41598-020-63998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pawloski L.C., Queenan A.M., Cassiday P.K., Lynch A.S., Harrison M.J., Shang W., Williams M.M., Bowden K.E., Burgos-Rivera B., Qin X., et al. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin. Vaccine Immunol. 2014;21:119–125. doi: 10.1128/CVI.00717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mooi F.R., van Loo I.H.M., van Gent M., He Q., Bart M.J., Heuvelman K.J., de Greeff S.C., Diavatopoulos D., Teunis P., Nagelkerke N., et al. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg. Infect. Dis. 2009;15:1206–1213. doi: 10.3201/eid1508.081511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Williams M.M., Sen K., Weigand M.R., Skoff T.H., Cunningham V.A., Halse T.A., Tondella M.L., CDC Pertussis Working Group Bordetella pertussis Strain Lacking Pertactin and Pertussis Toxin. Emerg. Infect. Dis. 2016;22:319–322. doi: 10.3201/eid2202.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bouchez V., Brun D., Cantinelli T., Dore G., Njamkepo E., Guiso N. First report and detailed characterization of B. pertussis isolates not expressing pertussis toxin or pertactin. Vaccine. 2009;27:6034–6041. doi: 10.1016/j.vaccine.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 142.Barkoff A.-M., Mertsola J., Pierard D., Dalby T., Hoegh S.V., Guillot S., Stefanelli P., van Gent M., Berbers G., Vestrheim D., et al. Pertactin-deficient Bordetella pertussis isolates: Evidence of increased circulation in Europe, 1998 to 2015. Euro Surveill. 2019;24:1700832. doi: 10.2807/1560-7917.ES.2019.24.7.1700832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miyaji Y., Otsuka N., Toyoizumi-Ajisaka H., Shibayama K., Kamachi K. Genetic Analysis of Bordetella pertussis Isolates from the 2008–2010 Pertussis Epidemic in Japan. PLoS ONE. 2013;8:e77165. doi: 10.1371/journal.pone.0077165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dorji D., Mooi F., Yantorno O., Deora R., Graham R.M., Mukkur T.K. Bordetella Pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance. Med. Microbiol. Immunol. 2018;207:3–26. doi: 10.1007/s00430-017-0524-z. [DOI] [PubMed] [Google Scholar]
- 145.Decker K.B., James T.D., Stibitz S., Hinton D.M. The Bordetella pertussis model of exquisite gene control by the global transcription factor BvgA. Microbiology. 2012;158:1665–1676. doi: 10.1099/mic.0.058941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karimova G., Bellalou J., Ullmann A. Phosphorylation-dependent binding of BvgA to the upstream region of the cyaA gene of Bordetella pertussis. Mol. Microbiol. 1996;20:489–496. doi: 10.1046/j.1365-2958.1996.5231057.x. [DOI] [PubMed] [Google Scholar]
- 147.Kamanova J., Kofronova O., Masin J., Genth H., Vojtova J., Linhartova I., Benada O., Just I., Sebo P. Adenylate Cyclase Toxin Subverts Phagocyte Function by RhoA Inhibition and Unproductive Ruffling. J. Immunol. 2008;181:5587–5597. doi: 10.4049/jimmunol.181.8.5587. [DOI] [PubMed] [Google Scholar]
- 148.Arciniega J.L., Hewlett E.L., Johnson F.D., Deforest A., Wassilak S.G., Onorato I.M., Manclark C.R., Burns D.L. Human serologic response to envelope-associated proteins and adenylate cyclase toxin of Bordetella pertussis. J. Infect. Dis. 1991;163:135–142. doi: 10.1093/infdis/163.1.135. [DOI] [PubMed] [Google Scholar]
- 149.Farfel Z., Könen S., Wiertz E., Klapmuts R., Addy P.A., Hanski E. Antibodies to Bordetella pertussis adenylate cyclase are produced in man during pertussis infection and after vaccination. J. Med. Microbiol. 1990;32:173–177. doi: 10.1099/00222615-32-3-173. [DOI] [PubMed] [Google Scholar]
- 150.Arciniega J.L., Hewlett E.L., Edwards K.M., Burns D.L. Antibodies to Bordetella pertussis adenylate cyclase toxin in neonatal and maternal sera. FEMS Immunol. Med. Microbiol. 1993;6:325–330. doi: 10.1111/j.1574-695X.1993.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 151.Guiso N., Rocancourt M., Szatanik M., Alonso J. Bordetella adenylate cyclase is a virulent associated factor and an immunoprotective antigen. Microb. Pathog. 1989;7:373–380. doi: 10.1016/0882-4010(89)90040-5. [DOI] [PubMed] [Google Scholar]
- 152.Hormozi K., Parton R., Coote J. Adjuvant and protective properties of native and recombinant Bordetella pertussis adenylate cyclase toxin preparations in mice. FEMS Immunol. Med. Microbiol. 1999;23:273–282. doi: 10.1111/j.1574-695X.1999.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 153.Guiso N., Szatanik M., Rocancourt M. Protective activity of Bordetella adenylate cyclase-hemolysin against bacterial colonization. Microb. Pathog. 1991;11:423–431. doi: 10.1016/0882-4010(91)90038-C. [DOI] [PubMed] [Google Scholar]
- 154.Khelef N., Sakamoto H., Guiso N. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb. Pathog. 1992;12:227–235. doi: 10.1016/0882-4010(92)90057-U. [DOI] [PubMed] [Google Scholar]
- 155.Cheung G.Y.C., Xing D., Prior S., Corbel M.J., Parton R., Coote J.G. Effect of different forms of adenylate cyclase toxin of Bordetella pertussis on protection afforded by an acellular pertussis vaccine in a murine model. Infect. Immun. 2006;74:6797–6805. doi: 10.1128/IAI.01104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gray M.C., Donato G.M., Jones F.R., Kim T., Hewlett E.L. Newly secreted adenylate cyclase toxin is responsible for intoxication of target cells by Bordetella pertussis. Mol. Microbiol. 2004;53:1709–1719. doi: 10.1111/j.1365-2958.2004.04227.x. [DOI] [PubMed] [Google Scholar]
- 157.Hanski E., Farfel Z. Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. J. Biol. Chem. 1985;260:5526–5532. [PubMed] [Google Scholar]
- 158.Wang X., Gray M.C., Hewlett E.L., Maynard J.A. The Bordetella adenylate cyclase repeat-in-toxin (RTX) domain is immunodominant and elicits neutralizing antibodies. J. Biol. Chem. 2015;290:3576–3591. doi: 10.1074/jbc.M114.585281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boehm D.T., Hall J.M., Wong T.Y., DiVenere A.M., Sen-Kilic E., Bevere J.R., Bradford S.D., Blackwood C.B., Elkins C.M., DeRoos K.A., et al. Evaluation of Adenylate Cyclase Toxoid Antigen in Acellular Pertussis Vaccines by Using a Bordetella pertussis Challenge Model in Mice. Infect. Immun. 2018;86:e00857-17. doi: 10.1128/IAI.00857-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barnes M.G., Weiss A.A. BrkA Protein of Bordetella pertussis Inhibits the Classical Pathway of Complement after C1 Deposition. Infect. Immun. 2001;69:3067–3072. doi: 10.1128/IAI.69.5.3067-3072.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Marr N., Oliver D.C., Laurent V., Poolman J., Denoel P., Fernandez R.C. Protective activity of the Bordetella pertussis BrkA autotransporter in the murine lung colonization model. Vaccine. 2008;26:4306–4311. doi: 10.1016/j.vaccine.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 162.Gasperini G., Biagini M., Arato V., Gianfaldoni C., Vadi A., Norais N., Bensi G., Delany I., Pizza M., Aricò B., et al. Outer Membrane Vesicles (OMV)-based and Proteomics-driven Antigen Selection Identifies Novel Factors Contributing to Bordetella pertussis Adhesion to Epithelial Cells. Mol. Cell. Proteom. 2018;17:205–215. doi: 10.1074/mcp.RA117.000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.de Gouw D., de Jonge M.I., Hermans P.W.M., Wessels H.J.C.T., Zomer A., Berends A., Pratt C., Berbers G.A., Mooi F.R., Diavatopoulos D.A. Proteomics-Identified Bvg-Activated Autotransporters Protect against Bordetella pertussis in a Mouse Model. PLoS ONE. 2014;9:e105011. doi: 10.1371/journal.pone.0105011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coffman R.L., Sher A., Seder R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baraff L.J., Shields W.D., Beckwith L., Strome G., Marcy S.M., Cherry J.D., Manclark C.R. Infants and children with convulsions and hypotonic-hyporesponsive episodes following diphtheria-tetanus-pertussis immunization: Follow-up evaluation. Pediatrics. 1988;81:789–794. [PubMed] [Google Scholar]
- 166.McKee A.S., MacLeod M.K.L., Kappler J.W., Marrack P. Immune mechanisms of protection: Can adjuvants rise to the challenge? BMC Biol. 2010;8:37. doi: 10.1186/1741-7007-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Villa P., Ghezzi P. Tumor Necrosis Factor. Humana Press; Totowa, NJ, USA: 2004. Animal Models of Endotoxic Shock; pp. 199–206. [DOI] [PubMed] [Google Scholar]
- 168.Glenny A.T., Pope C.G., Waddington H., Wallace U. Immunological notes. XVII–XXIV. J. Pathol. Bacteriol. 1926;29:31–40. doi: 10.1002/path.1700290106. [DOI] [Google Scholar]
- 169.Oleszycka E., McCluskey S., Sharp F.A., Muñoz-Wolf N., Hams E., Gorman A.L., Fallon P.G., Lavelle E.C. The vaccine adjuvant alum promotes IL-10 production that suppresses Th1 responses. Eur. J. Immunol. 2018;48:705–715. doi: 10.1002/eji.201747150. [DOI] [PubMed] [Google Scholar]
- 170.Glenny A.T., Buttle G.A.H., Stevens M.F. Rate of disappearance of diphtheria toxoid injected into rabbits and guinea - pigs: Toxoid precipitated with alum. J. Pathol. Bacteriol. 1931;34:267–275. doi: 10.1002/path.1700340214. [DOI] [Google Scholar]
- 171.Harrison W.T. Some Observations on the Use of Alum Precipitated Diphtheria Toxoid. Am. J. Public Health Nation’s Health. 1935;25:298–300. doi: 10.2105/AJPH.25.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morefield G.L., Sokolovska A., Jiang D., HogenEsch H., Robinson J.P., Hem S.L. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine. 2005;23:1588–1595. doi: 10.1016/j.vaccine.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 173.Li H., Willingham S.B., Ting J.P.-Y., Re F. Cutting Edge: Inflammasome Activation by Alum and Alum’s Adjuvant Effect Are Mediated by NLRP3. J. Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li H., Nookala S., Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J. Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 175.Franchi L., Núñez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur. J. Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mori A., Oleszycka E., Sharp F.A., Coleman M., Ozasa Y., Singh M., O’Hagan D.T., Tajber L., Corrigan O.I., McNeela E.A., et al. The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses. Eur. J. Immunol. 2012;42:2709–2719. doi: 10.1002/eji.201242372. [DOI] [PubMed] [Google Scholar]
- 177.Plotkin S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Banus S., Stenger R.M., Gremmer E.R., Dormans J.A.M.A., Mooi F.R., Kimman T.G., Vandebriel R.J. The role of Toll-like receptor-4 in pertussis vaccine-induced immunity. BMC Immunol. 2008;9:21. doi: 10.1186/1471-2172-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moreno G., Errea A., Van Maele L., Roberts R., Leger H., Sirard J.C., Benecke A., Rumbo M., Hozbor D. Toll-like receptor 4 orchestrates neutrophil recruitment into airways during the first hours of Bordetella pertussis infection. Microbes Infect. 2013;15:708–718. doi: 10.1016/j.micinf.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 180.Opal S.M. Endotoxemia and Endotoxin Shock. Volume 167. KARGER; Basel, Switzerland: 2010. Endotoxins and Other Sepsis Triggers; pp. 14–24. [DOI] [PubMed] [Google Scholar]
- 181.Ireton G.C., Reed S.G. Adjuvants containing natural and synthetic Toll-like receptor 4 ligands. Expert Rev. Vaccines. 2013;12:793–807. doi: 10.1586/14760584.2013.811204. [DOI] [PubMed] [Google Scholar]
- 182.Garçon N., Morel S., Didierlaurent A., Descamps D., Wettendorff M., Van Mechelen M. Development of an AS04-Adjuvanted HPV Vaccine with the Adjuvant System Approach. BioDrugs. 2011;25:217–226. doi: 10.2165/11591760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 183.Leroux-Roels G., Van Belle P., Vandepapeliere P., Horsmans Y., Janssens M., Carletti I., Garçon N., Wettendorff M., Van Mechelen M. Vaccine Adjuvant Systems containing monophosphoryl lipid A and QS-21 induce strong humoral and cellular immune responses against hepatitis B surface antigen which persist for at least 4 years after vaccination. Vaccine. 2015;33:1084–1091. doi: 10.1016/j.vaccine.2014.10.078. [DOI] [PubMed] [Google Scholar]
- 184.Geurtsen J., Banus H.A., Gremmer E.R., Ferguson H., de la Fonteyne-Blankestijn L.J.J., Vermeulen J.P., Dormans J.A.M.A., Tommassen J., van der Ley P., Mooi F.R., et al. Lipopolysaccharide Analogs Improve Efficacy of Acellular Pertussis Vaccine and Reduce Type I Hypersensitivity in Mice. Clin. Vaccine Immunol. 2007;14:821–829. doi: 10.1128/CVI.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brummelman J., Helm K., Hamstra H.-J., van der Ley P., Boog C.J.P., Han W.G.H., van Els C.A.C.M. Modulation of the CD4+ T cell response after acellular pertussis vaccination in the presence of TLR4 ligation. Vaccine. 2015;33:1483–1491. doi: 10.1016/j.vaccine.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 186.Brummelman J., Raeven R.H.M., Helm K., Pennings J.L.A., Metz B., van Eden W., van Els C.A.C.M., Han W.G.H. Transcriptome signature for dampened Th2 dominance in acellular pertussis vaccine-induced CD4+ T cell responses through TLR4 ligation. Sci. Rep. 2016;6:25064. doi: 10.1038/srep25064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vollmer J., Krieg A.M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 188.Weeratna R.D., Brazolot Millan C.L., McCluskie M.J., Davis H.L. CpG ODN can re-direct the Th bias of established Th2 immune responses in adult and young mice. FEMS Immunol. Med. Microbiol. 2001;32:65–71. doi: 10.1111/j.1574-695X.2001.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 189.Bode C., Zhao G., Steinhagen F., Kinjo T., Klinman D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines. 2011;10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Asokanathan C., Corbel M., Xing D. A CpG-containing oligodeoxynucleotide adjuvant for acellular pertussis vaccine improves the protective response against Bordetella pertussis. Hum. Vaccines Immunother. 2013;9:325–331. doi: 10.4161/hv.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sugai T., Mori M., Nakazawa M., Ichino M., Naruto T., Kobayashi N., Kobayashi Y., Minami M., Yokota S. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria–tetanus–pertussis vaccine. Vaccine. 2005;23:5450–5456. doi: 10.1016/j.vaccine.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 192.Bakhshaei P., Kazemi M.H., Golara M., Abdolmaleki S., Khosravi-Eghbal R., Khoshnoodi J., Judaki M.A., Salimi V., Douraghi M., Jeddi-Tehrani M., et al. Investigation of the Cellular Immune Response to Recombinant Fragments of Filamentous Hemagglutinin and Pertactin of Bordetella pertussis in BALB/c Mice. J. Interf. Cytokine Res. 2018;38:161–170. doi: 10.1089/jir.2017.0060. [DOI] [PubMed] [Google Scholar]
- 193.Buonsanti C., Balocchi C., Harfouche C., Corrente F., Galli Stampino L., Mancini F., Tontini M., Malyala P., Bufali S., Baudner B., et al. Novel adjuvant Alum-TLR7 significantly potentiates immune response to glycoconjugate vaccines. Sci. Rep. 2016;6:29063. doi: 10.1038/srep29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mancini F., Monaci E., Lofano G., Torre A., Bacconi M., Tavarini S., Sammicheli C., Arcidiacono L., Galletti B., Laera D., et al. One Dose of Staphylococcus aureus 4C-Staph Vaccine Formulated with a Novel TLR7-Dependent Adjuvant Rapidly Protects Mice through Antibodies, Effector CD4+ T Cells, and IL-17A. PLoS ONE. 2016;11:e0147767. doi: 10.1371/journal.pone.0147767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bagnoli F., Fontana M.R., Soldaini E., Mishra R.P.N., Fiaschi L., Cartocci E., Nardi-Dei V., Ruggiero P., Nosari S., De Falco M.G., et al. Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc. Natl. Acad. Sci. USA. 2015;112:3680–3685. doi: 10.1073/pnas.1424924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wu T.Y.-H., Singh M., Miller A.T., De Gregorio E., Doro F., D’Oro U., Skibinski D.A.G., Mbow M.L., Bufali S., Herman A.E., et al. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 2014;6:263ra160. doi: 10.1126/scitranslmed.3009980. [DOI] [PubMed] [Google Scholar]
- 197.Misiak A., Leuzzi R., Allen A.C., Galletti B., Baudner B.C., D’Oro U., O’Hagan D.T., Pizza M., Seubert A., Mills K.H.G. Addition of a TLR7 agonist to an acellular pertussis vaccine enhances Th1 and Th17 responses and protective immunity in a mouse model. Vaccine. 2017;35:5256–5263. doi: 10.1016/j.vaccine.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 198.Dunne A., Mielke L.A., Allen A.C., Sutton C.E., Higgs R., Cunningham C.C., Higgins S.C., Mills K.H.G. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol. 2015;8:607–617. doi: 10.1038/mi.2014.93. [DOI] [PubMed] [Google Scholar]
- 199.Xiao T.S., Fitzgerald K.A. The cGAS-STING Pathway for DNA Sensing. Mol. Cell. 2013;51:135–139. doi: 10.1016/j.molcel.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Burdette D.L., Vance R.E. STING and the innate immune response to nucleic acids in the cytosol. Nat. Immunol. 2013;14:19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- 201.Burdette D.L., Monroe K.M., Sotelo-Troha K., Iwig J.S., Eckert B., Hyodo M., Hayakawa Y., Vance R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tanaka Y., Chen Z.J. STING Specifies IRF3 Phosphorylation by TBK1 in the Cytosolic DNA Signaling Pathway. Sci. Signal. 2012;5:ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Elahi S., Van Kessel J., Kiros T.G., Strom S., Hayakawa Y., Hyodo M., Babiuk L.A., Gerdts V. c-di-GMP enhances protective innate immunity in a murine model of pertussis. PLoS ONE. 2014;9:e109778. doi: 10.1371/journal.pone.0109778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gschwandtner M., Derler R., Midwood K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019;10:2759. doi: 10.3389/fimmu.2019.02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Allavena P., Bianchi G., Zhou D., Van Damme J., Jílek P., Sozzani S., Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein−1, −2 and −3. Eur. J. Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 207.Andreasen C., Carbonetti N.H. Pertussis toxin inhibits early chemokine production to delay neutrophil recruitment in response to Bordetella pertussis respiratory tract infection in mice. Infect. Immun. 2008;76:5139–5148. doi: 10.1128/IAI.00895-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Temizoz B., Kuroda E., Ohata K., Jounai N., Ozasa K., Kobiyama K., Aoshi T., Ishii K.J. TLR9 and STING agonists synergistically induce innate and adaptive type-II IFN. Eur. J. Immunol. 2015;45:1159–1169. doi: 10.1002/eji.201445132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Conway M.A., Madrigal-Estebas L., McClean S., Brayden D.J., Mills K.H.G. Protection against Bordetella pertussis infection following parenteral or oral immunization with antigens entrapped in biodegradable particles: Effect of formulation and route of immunization on induction of Th1 and Th2 cells. Vaccine. 2001;19:1940–1950. doi: 10.1016/S0264-410X(00)00433-3. [DOI] [PubMed] [Google Scholar]
- 210.Lindenstrøm T., Moguche A., Damborg M., Agger E.M., Urdahl K., Andersen P. T Cells Primed by Live Mycobacteria Versus a Tuberculosis Subunit Vaccine Exhibit Distinct Functional Properties. EBioMedicine. 2018;27:27–39. doi: 10.1016/j.ebiom.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020 doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 212.Wallis J., Shenton D.P., Carlisle R.C. Novel approaches for the design, delivery and administration of vaccine technologies. Clin. Exp. Immunol. 2019;196:189–204. doi: 10.1111/cei.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wilk M.M., Mills K.H.G. CD4 TRM Cells Following Infection and Immunization: Implications for More Effective Vaccine Design. Front. Immunol. 2018;9:1860. doi: 10.3389/fimmu.2018.01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Miquel-Clopés A., Bentley E.G., Stewart J.P., Carding S.R. Mucosal vaccines and technology. Clin. Exp. Immunol. 2019;196:205–214. doi: 10.1111/cei.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Perdomo C., Zedler U., Kühl A.A., Lozza L., Saikali P., Sander L.E., Vogelzang A., Kaufmann S.H.E., Kupz A. Mucosal BCG Vaccination Induces Protective Lung-Resident Memory T Cell Populations against Tuberculosis. MBio. 2016;7:e01686-16. doi: 10.1128/mBio.01686-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bull N.C., Stylianou E., Kaveh D.A., Pinpathomrat N., Pasricha J., Harrington-Kandt R., Garcia-Pelayo M.C., Hogarth P.J., McShane H. Enhanced protection conferred by mucosal BCG vaccination associates with presence of antigen-specific lung tissue-resident PD-1+ KLRG1− CD4+ T cells. Mucosal Immunol. 2019;12:555–564. doi: 10.1038/s41385-018-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Van Dis E., Sogi K.M., Rae C.S., Sivick K.E., Surh N.H., Leong M.L., Kanne D.B., Metchette K., Leong J.J., Bruml J.R., et al. STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium tuberculosis Infection. Cell Rep. 2018;23:1435–1447. doi: 10.1016/j.celrep.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Santosuosso M., Zhang X., McCormick S., Wang J., Hitt M., Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: Adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J. Immunol. 2005;174:7986–7994. doi: 10.4049/jimmunol.174.12.7986. [DOI] [PubMed] [Google Scholar]
- 219.Dietrich J., Andersen C., Rappuoli R., Doherty T.M., Jensen C.G., Andersen P. Mucosal Administration of Ag85B-ESAT-6 Protects against Infection with Mycobacterium tuberculosis and Boosts Prior Bacillus Calmette-Guérin Immunity. J. Immunol. 2006;177:6353–6360. doi: 10.4049/jimmunol.177.9.6353. [DOI] [PubMed] [Google Scholar]
- 220.Chen L., Wang J., Zganiacz A., Xing Z. Single Intranasal Mucosal Mycobacterium bovis BCG Vaccination Confers Improved Protection Compared to Subcutaneous Vaccination against Pulmonary Tuberculosis. Infect. Immun. 2004;72:238–246. doi: 10.1128/IAI.72.1.238-246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Aguilo N., Toledo A.M., Lopez-Roman E.M., Perez-Herran E., Gormley E., Rullas-Trincado J., Angulo-Barturen I., Martin C. Pulmonary Mycobacterium bovis BCG Vaccination Confers Dose-Dependent Superior Protection Compared to That of Subcutaneous Vaccination. Clin. Vaccine Immunol. 2014;21:594–597. doi: 10.1128/CVI.00700-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Barclay W.R., Busey W.M., Dalgard D.W., Good R.C., Janicki B.W., Kasik J.E., Ribi E., Ulrich C.E., Wolinsky E. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am. Rev. Respir. Dis. 1973;107:351–358. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- 223.Storsaeter J., Hallander H.O., Gustafsson L., Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16:1907–1916. doi: 10.1016/S0264-410X(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 224.Solans L., Locht C. The Role of Mucosal Immunity in Pertussis. Front. Immunol. 2019;9:3068. doi: 10.3389/fimmu.2018.03068. [DOI] [PMC free article] [PubMed] [Google Scholar]