Abstract
Background
Severe pneumonia is pathological manifestation of Coronavirus Disease 2019 (COVID-19), however complications have been reported in COVID-19 patients with a worst prognosis. Aim of this study was to evaluate the role of high sensitivity cardiac troponin I (hs-TnI) in patients with SARS-CoV-2 infection.
Methods
we retrospectively analysed hs-TnI values measured in 523 patients (median age 64 years, 68% men) admitted to a university hospital in Milan, Italy, and diagnosed COVID-19.
Results
A significant difference in hs-TnI concentrations was found between deceased patients (98 patients) vs discharged (425 patients) [36.05 ng/L IQR 16.5–94.9 vs 6.3 ng/L IQR 2.6–13.9, p < 0.001 respectively]. Hs-TnI measurements were independent predictors of mortality at multivariate analysis adjusted for confounding parameters such as age (HR 1.004 for each 10 point of troponin, 95% CI 1.002–1.006, p < 0.001). The survival rate, after one week, in patients with hs-TnI values under 6 ng/L was 97.94%, between 6 ng/L and the normal value was 90.87%, between the normal value and 40 ng/L was 86.98, and 59.27% over 40 ng/L.
Conclusion
Increase of hs-TnI associated with elevated mortality in patients with COVID-19. Troponin shows to be a useful biomarker of disease progression and worse prognosis in COVID-19 patients.
Keywords: COVID-19, mortality, cardiac troponin, serially measurements of hs-TnI, hospitalization
Introduction
The World Health Organization defined Coronavirus Disease 2019 (COVID-19) as a serious infective condition caused by the new coronavirus SARS-CoV-2 (WHO 2020). Detected in China in late 2019, the COVID-19 epidemics spread from Wuhan, China, to all over the word, as a pandemia. As of June 4, total affected people in Italy 234,013 with more than 33,689 deaths, and 6,416,828 infected worldwide with more than 382,867 deaths (World Health Organizzazion 2019, Livingston and Bucher 2020, WHO 2020). SARS-CoV-2 infection may present with no or mild symptoms. In a small percentage of cases, less than 20%, the disease evolves and severe critical manifestations appear. In symptomatic patients, symptoms mostly begin with fever (83–98%), cough (76–82%) and fatigue. Other common clinical signs are lymphopenia, diarrhoea, nausea and increased levels of inflammatory markers. In up to 15% of COVID-19 patients the clinical course is complicated by the onset of a severe form of interstitial pneumonia, which may evolve towards acute respiratory distress syndrome (ARDS) and/or multi organ failure (MOF) and death (Guan et al. 2020, Mattiuzzi and Lippi 2020). Decreased oxygen saturation, blood gas deviations, changes of CT scan imaging are the main signs of SARS-CoV-2 pneumonia. COVID19 mortality is higher in elderly patients, while the role of any ‘predisposing’ conditions such as hypertension and diabetes is still under discussion. The viral mortality rate is around 3.4%, according to the WHO, but this data could be underestimated (World Health Organizzazion 2019). Since major cardiac complications were reported to develop in a considerable number of COVID-19 patients (Huang et al. 2020, Inciardi et al. 2020, Madjid et al. 2020, Wang et al. 2020) worsening severe prognosis and possibly leading to death, we performed a study to investigate whether determination of high sensitivity cardiac troponin I (hs-TnI) levels, during hospitalization, are predictive of mortality in patients with COVID-19.
Clinical significance
In this cohort study of 523 consecutive patients with confirmed COVID-19, 18.7% patients died whereas 81.3% were successfully treated. A significant difference in hs-TnI concentrations was found between deceased patients vs discharged.
Increased hs-TnI release during hospitalization was associated with elevated mortality.
Measurements of Cardiac Troponin may help to predict clinical severity in patients with COVID-19.
Methods
Data collection
Consecutive patients admitted to Humanitas Clinical and Research Hospital (Rozzano, Milan, Italy) from 1 March 2020 to 14 April 2020 were included in this retrospective cohort study. All patients were tested for SARS-CoV-2 on admission of emergency department or on the day of initial hospital admission. A diagnosis of COVID-19 was based on SARS-CoV-2 real-time reverse-transcriptase polymerase-chain-reaction (RT-qPCR) in respiratory samples, clinical, and radiological criteria according to in-house, national and international recommendations and guidelines (European Center for Desease Prevention and Control, n.d; World Health Organizzazion, 28 Jan 2020). We retrospectively analysed laboratory records of patients, treated and discharged or died during the hospitalization, regarding to hs-TnI evaluation measured on admission of emergency department, and during the hospital stay. The demographic characteristics (age and sex), clinical data (outcome) for participants were collected on admission at the emergency department and during the hospitalization. The ethical committee of the Humanitas Hospital approved this study.
Laboratory methods
Serum hs-TnI levels, serially required and included in the laboratory routine for patient monitoring, were assessed by a high-sensitivity cardiac troponin I chemiluminescent immunoassay (ACCESS hs-TnI, Beckman Coulter) using the DXI800 platform. The upper reference limit (URL) of hs-TnI, defined, as the 99th percentile of hs-TnI distribution in a reference population, was 11.6 ng/L for women and 19.8 ng/L for men respectively. Limit of blank and limit of detection have been determined to be 1.7 ng/L and 2.3 ng/L, respectively.
Statistical analysis
Data were described as number and percentage, if categorical, or mean ± standard deviation if continuous with distribution approximately symmetrical, or median and interquartile interval (IQR) if continuous with asymmetrical distribution or discrete. After checking for normality of distribution with Shapiro Wilk test, differences between groups were explored with Mann–Whitney test. Differences among time were calculated with Wilcoxon test for paired data. hs-TnI at admission was defined as the first measured troponin, hs-TnI near dead or at discharge was defined as the last measured troponin before dead or discharge, and hs-TnI during hospitalization was defined as the second measurement of hs-TnI after different admission from the last one. Comparison with second measure of hs-TnI was done only for patients with at least 3 hs-TnI measurement. Impact of troponin on patients’ status was explored with logistic regression analysis, and possible cut-off was determined on ROC curve. Association with status at discharge was evaluated with Cox regression analysis. A p < to 0.05 was considered as significant. All analyses were made with Stata15.
Results
From 1055 consecutive patients admitted to emergency department and tested for COVID-19, 527 were diagnosed COVID-19 and had serial hsTnI determination available. Four patients were excluded as they had been admitted at the emergency department with a diagnosis of acute myocardial infarction, and only later on, during the hospitalization, showed evidence of COVID-19. Five hundred and twenty-three patients fulfilled the inclusion criteria and represented the present study population (Figure 1). Demographic characteristics and laboratory results of the study patients are summarized in Table 1. During the hospitalization, 98 patients (18.7%) died and 425 patients (81.3%) were successfully treated and discharged. There was a significant difference in mean age between deceased and cured patients (76.2 ± 9.9 vs 61.7 ± 13.6 years, respectively, p < 0.001) but no significant difference in the sex ratio was found in the two groups (p = 0.722).
Figure 1.
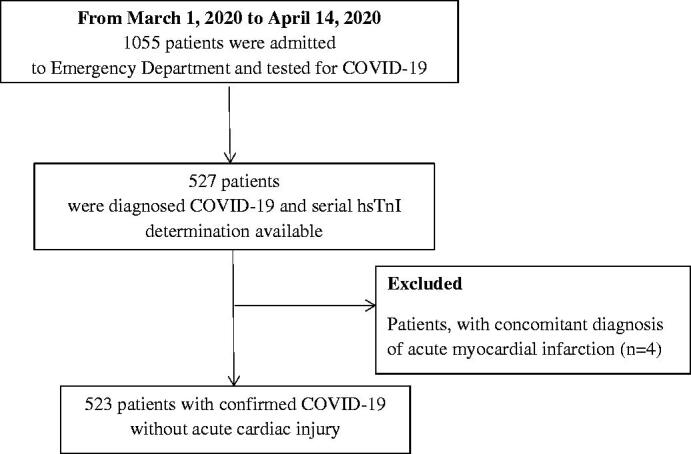
Flowchart of Patients Recruitment.
Table 1.
Clinical and demographic characteristics of 523 patients with COVID-19.
| All | Deceased | Discharged | p Value | |
|---|---|---|---|---|
| N (%) | 523 | 98 (18.7%) | 425 (81.3%) | |
| Sex (M) | 355 (67.9%) | 68 (69.4%) | 287 (67.5%) | 0.722 |
| Age, years (mean ± SD) |
64.4 ± 14.2 | 76.2 ± 9.9 | 61.7 ± 13.6 | <0.001 |
| Troponin, ng/L (first measurement) (median, IQI) |
8.2 (3.1–22.1) | 36.1 (16.5–94.9) | 6.3 (2.6–13.9) | <0.001 |
| Troponin, ng/L (max value during hospitalization) (median, IQI) | 10.5 (4.3–35.1) | 59.1 (26.5–192.7) | 7.6 (3.5–20.3) | <0.001 |
| Time of maximum troponin value, days (median, IQI) |
3 (1–6) | 2 (1–5) | 3 (1–6) | 0.005 |
A total of 2239 hs-TnI measurements were performed during the hospital stay (2.7 ± 1.9 vs 4.6 ± 3.1 in deceased vs discharged patients, respectively) with a follow-up of 4.5 ± 3.8 days in patients who died vs 10.6 ± 7.9 days in discharged patients (p < 0.001).
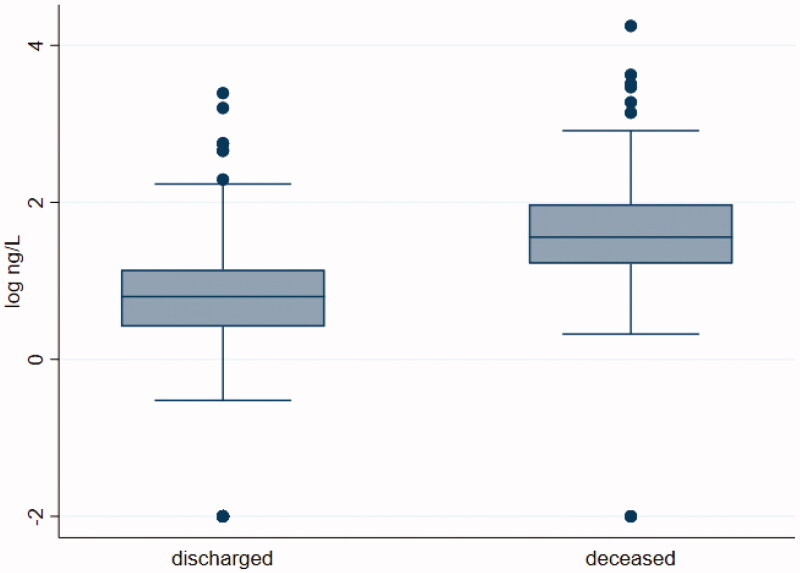
Laboratory results showed median values of troponin at admission in all included patients, of 8.2 ng/L [IQR 3.1–22.1] with concentrations markedly higher in the deceased patients vs survivors [36.05 ng/L IQR 16.5–94.9 vs 6.3 ng/L IQR 2.6–13.9, p < 0.001], Figure 2. The distribution of hs-TnI maximum value measured, showed a peak on the third hospitalization day (median time, 3 days IQR 1–6, p < 0.001) with a concentration significantly higher in the deceased patients vs survivors [59.1 ng/L IQR 26.5–192.7 vs 7.6 ng/L IQR 3.5–20.3, p < 0.001].
Figure 2.
Median values of hs-TnI in discharged and died COVID-19 patients during the hospital stay.
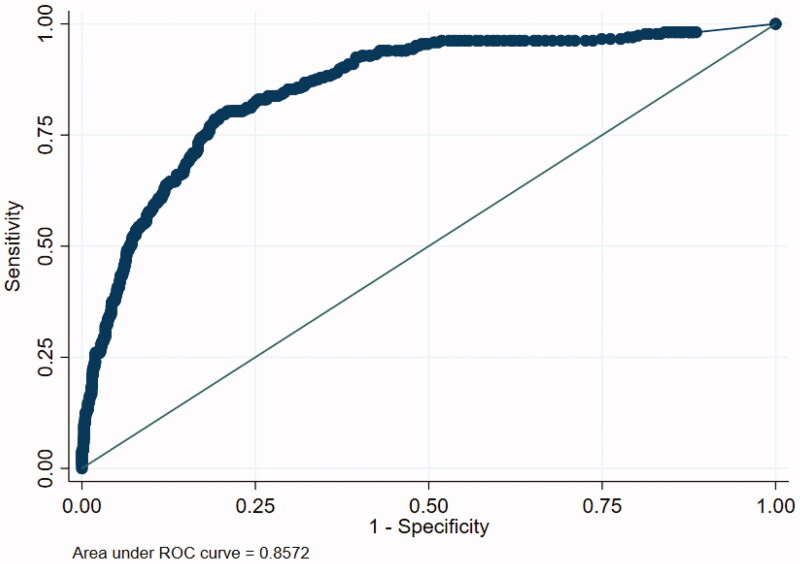
To evaluate the discriminative capacity for mortality of hs-TnI, and to calculate the positive and negative predictive values of this biomarker a ROC curve was calculated (Figure 3). The AUC for hs-TnI was 0.8572 (95% CI 0.8145–0.8958). In order to maximize sensitivity and specificity, two cut-offs were identified: at 6 ng/L sensitivity was 94.3% and specificity 52.3%; at 40 ng/L sensitivity and specificity were 47.9% and 93.6%, respectively.
Figure 3.
ROC curve for hs-TnI mesurement as a predictor of mortality.
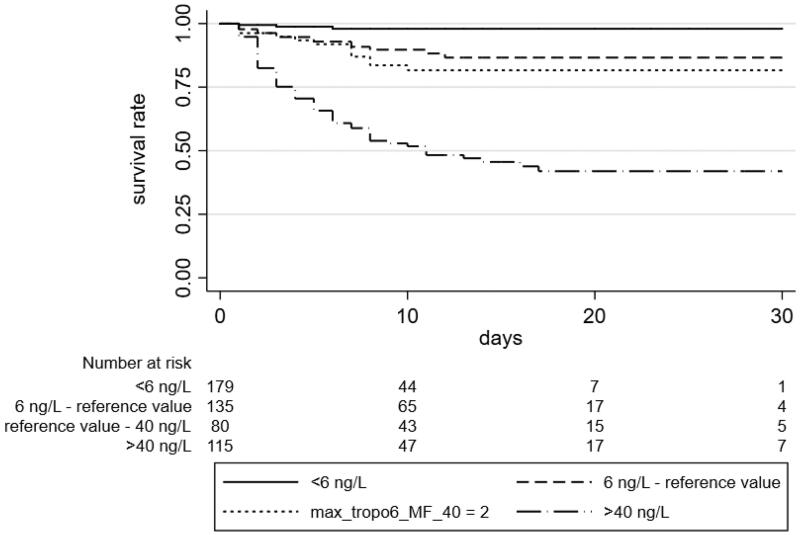
The median survival was 8 days (range 0–38). The mortality rate raised with increasing troponin concentration: higher troponin values were associated with a higher risk of mortality. Furthermore, compared with surviving group, deceased patients were older and showed hs-TnI values significantly higher during the follow-up. At a multivariate analysis, after adjustment for confounding parameters such as age (Hazard Ratio (HR) 1.10, 95% CI 1.08–1.13, p < 0.001), troponin values still were independent predictors of mortality (HR 1.004 for each 10 point of hs-TnI, 95% CI 1.002–1.006, p < 0.001). We stratified patients according to the cut-off suggested by the manufacturer (11.6 ng/L for women and 19.8 ng/L for men), and the thresholds of 6 ng/L and 40 ng/L calculated from the ROC curve analysis. After one week, the survival rate in patients with values of hs-TnI under 6 ng/L was 97.94%, between 6 ng/L and the normal value was 90.87%, between the normal value and 40 ng/L was 86.98, and for over 40 ng/L patients was 59.27% (Figure 4).
Figure 4.
Survival of COVID-19 patients according to hs-TnI levels. Higher hs-TnI values were followed by a lower survival. The thresholds of hs-TnI used coming from the reference value suggested by manufacturer, as the 99th percentile of TnI distribution in a reference population (reference value), and from the two cut-off resulting from the ROC analysis.
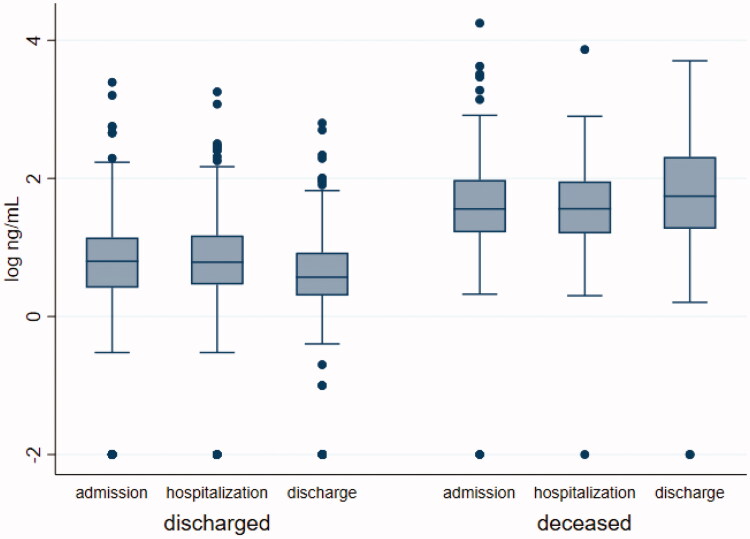
Figure 5 shows distribution and level changes of hs-TnI in patients who died and in those discharged. In died patients hs-TnI levels remained unchanged during the course of hospitalization [median value of hs-TnI at the admission 36.05 ng/L (16.5–94.9 ng/L) vs 36.3 ng/L (15.9–90.4 ng/L) during the hospital stay p = 0.9135], with a partially significant increase only near the death [55.3 ng/L (18.6–205.05 ng/L) at the end of follow-up p = 0.0531]. On the contrary significant decreases in hs-TnI levels were evident in survivors at each time point evaluated [median value of hs-TnI at the admission 6.3 ng/L (2.6–13.9 ng/L) vs 6.1 ng/L (2.9–14.9 ng/L) during the hospitalization (p = 0.0022) and 3.7 ng/L (2–8.4 ng/L) p= <0.0001 at the end of follow-up].
Figure 5.
Distribution and dynamic levels changes of hs-TnI during the hospitalization. Median value of hs-TnI in discharged and dead patients during the admission, the hospitalization and at the end of follow-up. Discharged patients: median value of hs-TnI at the admission vs median value of hs-TnI during the hospital stay p = 0.0033 and p Bonferroni= 0.0098, median value of hs-TnI at the admission vs median value at the end of follow-up p < 0.0001 and p Bonferroni <0.0001; median value of hs-TnI during the hospitalization vs median value at the end of follow-up p < 0.0001 and p Bonferroni <0.0001. Dead patients: median value of hs-TnI at the admission vs median value of hs-TnI during the hospital stay p = 0.9135 and p Bonferroni= 0.9135, median value of hs-TnI at the admission vs median value at the end of follow-up p = 0.0531 and p Bonferroni = 0.1593, median value of hs-TnI during the hospitalization vs median value at the end of follow-up p = 0.0131 and p Bonferroni= 0.0393.
Discussion
The present study demonstrates the significant association between cardiac injury detected through hs-TnI measurement and mortality in patients with COVID-19. To the best of our knowledge, this report is the first study conducted in Italy in a large group of COVID-19 patients showing a statistically significant association between myocardial injury, elevated levels of hs-TnI and mortality. Our data clearly show that values of cardiac troponin, repeatedly elevated, accurately identify patients with a higher risk of death.
COVID-19 has emerged as a pandemic and public health crisis of global proportions. At the time of writing this article, there have been a total of more than 6,400,000 SARS-CoV-2 infected people, causing nearly 390,000 related deaths (World Health Organizzazion 2019). Italy has been the first non-Asian country to report autochthonous cases of COVID-19, and Lombardy, where this study was conducted, is the region more severely affected by SARS-CoV-2. Data from China, as well as from Italy, indicate that in the majority of affected individuals, COVID-19 is a relatively mild condition, whereas, in others can develop as a severe illness requiring hospitalization and leading to death (Chen et al. 2020, Guan et al. 2020, Huang et al. 2020, Wang et al. 2020, Wu and McGoogan 2020). According to studies on the large clinical sample in China, a severe pneumonia is the most frequent pathological manifestation and the severe respiratory distress syndrome is usually considered the main cause of coronavirus-induced death. However, many aspects of this disease are yet unexplored. In contrast with data from SARS, in which tachycardic cardiovascular complications were common and self-limiting, but not associated with risk of death (Peiris et al. 2003), recent reports (Bonow et al. 2020, Guo et al. 2020, Huang et al. 2020, Inciardi et al. 2020, Lang et al. 2020, Liu et al. 2020, Lodigiani et al. 2020, Madjid et al. 2020, Nishiga et al. 2020, Ruan et al. 2020, Shi et al. 2020, Yang et al. 2020, Wang et al. 2020, Zhou et al. 2020) suggested that cardiovascular complications (myocardial injury, arrhythmia, acute coronary syndrome and venous thromboembolism) are a frequent comorbidity, probably associated with a worse clinical outcome in SARS-CoV-2 affected patients. Yang et al. (2020) in 52 critically COVID-19 patients admitted to the intensive care unit observed a cardiac injury in 23% of patients, with a higher prevalence of cardiac disease in non-survivor patients (28%) than in survivor patients (15%). Wang et al. (2020) found a cardiac arrhythmia in 44% of 36 patients admitted to the intensive care unit . A very recent report by Shi et al. (2020) showed that cardiological impairment was a common condition among patients with COVID-19 in Wuhan, and that, more than half of the patients with cardiac injury died. An elevated cardiac troponin values, suggesting myocardial injury as a possible mechanism contributing to severe illness and mortality has been reported in some studies (Guo et al. 2020, Huang et al. 2020, Liu et al. 2020, Ruan et al. 2020, Shi et al. 2020). From these evidences it was developed the hypothesis that initial measurement of cardiac damage biomarkers immediately after hospitalization for SARS-CoV-2 infection, followed by a longitudinal monitoring during hospital stay, could help clinicians to identify a subset of patients with possible cardiac injury and thereby predict the progression of COVID-19 towards a worse outcome. Shi et al. (2020) reported that approximately 20% of COVID-19 patients had evidence of a cardiac injury manifested by a significantly elevation of hs-TnI and this finding was associated with a significantly higher in-hospital mortality [51.2% vs. 4.5% respectively; p<.001]. Similarly, to Shi et al., we found, in our study, that about 19% of patients with SARS-CoV-2 infection died during the hospital stay, and that hs-TnI levels were significantly higher in these patients respect to successfully treated ones. Moreover patients showing hs-TnI levels >40 ng/L presented a more complicated clinical course with a higher mortality rate. Given the high specificity of hs-TnI (93.6% at the predicted cut-off of 40 ng/L) we may suggest to plan prioritized treatment and more aggressive therapeutic strategies in selected high-risk patients, namely those with an elevated hs-TnI during the hospital-stay, to prevent the occurrence of mortality. On the contrary, the lower the value of hs-TnI the lower the risk of death. Therefore, initial measurement of hs-TnI immediately after hospitalization for SARS-CoV-2, followed by a longitudinal monitoring, can help clinicians to intercept dynamic changes in the release of hs-TnI as evidence of onset myocardial injury.
Understanding the effects of COVID-19 on cardiovascular system should be crucial for providing comprehensive medical care. Several hypothesis have been proposed regarding the mechanism of cardiac damage among patients affected by COVID-19 ( Tersalvi et al. 2020). A hypothesis based looked at the role of the Spike protein of SARS-CoV-2 presents a strong binding affinity with the angiotensin-converting enzyme 2 (ACE2) human cell receptor, and ACE2 is also highly expressed in heart. Consequently, an induced COVID-19 myocardial damage could be mediated by ACE2 (Chen et al. 2020, Wrapp et al. 2020, Zou et al. 2020) and the expression and distribution of this receptor could be key determinant for the entry of virus. Another hypothesis (Chen et al. 2020, Corrales-Medina et al. 2013, Huang et al. 2020, Madjid et al. 2007, Yang and Jin 2020, Zhang et al. 2020) suggests that a key role is played by a systemic inflammation and multiple organ failure. SARS-CoV-2 can in fact prompt an intense release of multiple cytokines and chemokines causing an uncontrolled and dysfunctional immune response involving the continuous activation and proliferation of lymphocytes and macrophages which could lead to a deteriorating of heart condition until the development of an overt heart damage. Additionally, the occurrence of myocardial infarction (MI) and acute coronary syndrome (ACS) in infected patients was described during the SARS outbreak (Peiris et al. 2003, Chong et al. 2004). The imbalance between oxygen supply and demand in an acute setting, hypoxaemia, the infectious states often accompanied by fever, tachycardia, endocrine dysregulation, as well as a direct viral vascular infection and inflammation, typically observed in COVID-19 patients, could cause a MI (Zou et al. 2020). Nevertheless, up to now no substantial higher numbers of AMI have been described with COVID-19. Finally, hospitalized patients with COVID-19 were characterized by a high rate of thromboembolic complications primarily represented by pulmonary embolism (PE) (Lang et al. 2020, Lodigiani et al. 2020, Nishiga et al. 2020). The mechanisms involved in possible thrombotic events in COVID-19 are uncertain. However, proinflammatory cytokine/chemokine release, increased endothelial dysfunction/damage, potential sepsis, multiorgan dysfunction, and critical illness may be all condition promoting coagulation activation contributing to increased risk venous thromboembolic event. Studies have shown an increase in cardiac troponins is a common finding in the course of PE (Giannitsis et al. 2000, Meyer et al. 2000) therefore it is possible to speculate that elevated levels of cardiac biomarkers in COVID-19 patients could be due also to a cellular damage related to wall stress caused by acute overload of the right ventricle.
In our study we excluded from the study population, four patients because they had been admitted at the emergency department with a diagnosis of acute myocardial infarction, and only later on, during the hospitalization, showed evidence of COVID-19; three patients recovered and showed rapidly decreasing levels of hs-TnI, while only one died and showed hs-TnI levels persistently elevated.
Our study has some limitations, first of all, this study was conducted in a single centre. However, the number of patients included is the highest compared to other published studies and the first reporting data from an European country. Moreover, unfortunately, we could not evaluate whether there was a pre-existing cardiovascular disease. There have been evidences (Guo et al. 2020) that the presence of cardiac impairment could be associated with a hiker risk of death, and that this is increased when troponin is elevated. Concluding, COVID-19 can cause a viral pneumonia with additional extra-pulmonary manifestations and complications. Cardiac injury, is commonly observed in severe cases, and is strongly associated with mortality. Our results should suggest to consider not only the respiratory symptoms, but also to triage patients with COVID-19 according to serially measurements of high-sensitivity troponin I, for identifying patients at higher risk of in-hospital mortality to assign for more stringent strategies of treatment.
Acknowledgements
The authors acknowledge all medical team for their clinical activities in the diagnosis and treatment of COVID-19. The authors thank the staff of the laboratory for assistance through test performance and data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bonow, R.O., et al. , 2020. Association of Coronavirus Disease 2019 (COVID-19) with myocardial injury and mortality. JAMA cardiology, 5 (7), 751. [DOI] [PubMed] [Google Scholar]
- Chen, L., et al. , 2020. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovascular research, 116 (6), 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N., et al. , 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England), 395 (10223), 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, P.Y., et al. , 2004. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch pathol lab med, 128, 195–204. [DOI] [PubMed] [Google Scholar]
- Corrales-Medina, V.F., et al. , 2013. Acute pneumonia and the cardiovascular system. Lancet (London, England), 381 (9865), 496–505. [DOI] [PubMed] [Google Scholar]
- European Center for Desease Prevention and Control , n.d. Available from: https://www.ecdc.europa.eu/en
- Giannitsis, E., et al. , 2000. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation, 102 (2), 211–217. [DOI] [PubMed] [Google Scholar]
- Guan, W.J., et al. , 2020. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of Coronavirus Disease 2019 in China. New England journal of medicine, 382 (18), 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, T., et al. , 2020. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19). JAMA cardiology, 5 (7), 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C., et al. , 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. Lancet (London, England), 395 (10223), 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciardi, R.M., et al. , 2020. Cardiac involvement in a patient with Coronavirus Disease 2019 (COVID-19). JAMA cardiology, 5 (7), 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, J.P., et al. , 2020. A current review of COVID-19 for the cardiovascular specialist. American heart journal, 226, 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., et al. , 2020. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Science China. Life sciences, 63 (3), 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston, E., and Bucher, K., 2020. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA, 323 (14), 1335. [DOI] [PubMed] [Google Scholar]
- Lodigiani, C., et al. , 2020. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis research, 191, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjid, M., et al. , 2007. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Texas heart institute journal, 34 (1), 11–18. [PMC free article] [PubMed] [Google Scholar]
- Madjid, M., et al. , 2020. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA cardiology, 5 (7), 831. [DOI] [PubMed] [Google Scholar]
- Mattiuzzi, C., and Lippi, G., 2020. Which lessons shall we learn from the 2019 novel coronavirus outbreak? Annals of translational medicine, 8 (3), 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, T., et al. , 2000. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. Journal of the American College of Cardiology, 36 (5), 1632–1636. [DOI] [PubMed] [Google Scholar]
- Nishiga, M., et al. , 2020. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nature reviews. Cardiology, 17 (9), 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris, J.S., et al. , 2003. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. The lancet, 361 (9371), 1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, Q., et al. , 2020. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive care medicine, 46 (6), 1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, S., et al. , 2020. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA cardiology, 5 (7), 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersalvi, G., et al. , 2020. Elevated troponin in patients with Coronavirus Disease 2019 (COVID-19): possible mechanisms. Journal of cardiac failure, 26 (6), 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D., et al. , 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA, 323 (11), 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO , 12 Jan 2020. Novel coronavirus-China. Available from: http://wwwhoint/csr/don/12-january-2020-novel-coronavirus-china/en/
- World Health Organizzazion , 2019. Novel Coronavirus (2019-nCoV) situation reports. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ [Accessed 4 June 2020].
- World Health Organizzazion , 28 Jan 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCOV) infection is suspected interim guidance. [Google Scholar]
- Wrapp, D., et al. , 2020. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (New York, N.Y.), 367 (6483), 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z., and McGoogan, J.M., 2020. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA, 323 (13), 1239. [DOI] [PubMed] [Google Scholar]
- Yang, C., and Jin, Z., 2020. An acute respiratory infection runs into the most common noncommunicable epidemic-COVID-19 and cardiovascular diseases. JAMA cardiology, 5 (7), 743. [DOI] [PubMed] [Google Scholar]
- Yang, X., et al. , 2020. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The lancet respiratory medicine, 8 (5), 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J.J., et al. , 2020. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan. Allergy, 75 (7), 1730–1741. [DOI] [PubMed] [Google Scholar]
- Zhou, F., et al. , 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet, 395 (10229), 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, X., et al. , 2020. The single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to Wuhan 2019-nCoV infection. Frontiers in medicine, 14, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]