Abstract
Background
High sensitivity cardiac troponin-T (hs-TnT) has been associated with mortality in patients hospitalized with COVID-19. We aimed to determine if hs-TnT levels and their timing are independent predictors of adverse events in these patients.
Design
Retrospective chart review was performed for all patients hospitalized at our institution between 23 March 2020 and 13 April 2020 who were found to be COVID-19-positive. Clinical, demographic, and laboratory variables including initial and peak hs-TnT were recorded. Univariable and multivariable analyses were completed for a primary composite endpoint of in-hospital death, intubation, need for critical care, or cardiac arrest.
Results
In the 276 patients analysed, initial hs-TnT above the median (≥17 ng/L) was associated with increased length of stay, need for vasoactive medications, and death, along with the composite endpoint (OR 3.92, p < 0.001). Multivariable analysis demonstrated that elevated initial hs-TnT was independently associated with the primary endpoint (OR 2.92, p = 0.01). Late-peaking hs-TnT (OR 2.19 for each additional day until peak, p < 0.001) was also independently associated with the composite endpoint.
Conclusions
In patients hospitalized with COVID-19, hs-TnT identifies patients at high risk for adverse in-hospital events, and trends of hs-TnT over time, particularly during the first day, provide additional prognostic information.
Keywords: Troponin, COVID-19, biomarker, risk stratification, cardiac outcomes
Introduction
The rapid spread of the novel coronavirus disease (COVID-19) has resulted in over 18 million cases worldwide and over 600,000 deaths (World Health Organization 2020). The continued accrual of cases has significantly impacted health care expenditures and resource utilization. Methods to identify those who are likely to have morbid outcomes may help point these patients towards more intensive monitoring and aggressive medical therapy (Bartsch et al. 2020).
Even in the absence of an acute coronary syndrome, troponin elevation indicative of myocardial injury has been identified in 20–30% of hospitalized patients with COVID-19 and has been associated with increased risk of mortality in retrospective studies (Aikawa et al. 2020, Hendren et al. 2020, Shi et al. 2020). However, given the non-specific nature of troponin elevations, some have argued against its routine use unless there is clinical concern for an acute coronary plaque rupture (Imazio et al. 2020, Januzzi 2020). The objective of this study is to determine the predictive value of high sensitivity cardiac troponin-T (hs-TnT) as compared to traditional inflammatory markers for adverse outcomes that include the need for critical care, respiratory failure requiring intubation, cardiac arrest, and in-hospital mortality.
Clinical significance
Elevated troponin values have been associated with increased risk of adverse events in patients hospitalized with COVID-19.
Unlike pure inflammatory markers (CRP, ferritin, LDH, etc.) which are elevated in nearly all patients with COVID-19 regardless of outcome, a normal hs-TnT is common in these patients and predictive of freedom from adverse events, so this biomarker is a better discriminator for overall prognosis.
The time it takes for hs-TnT to peak is also independently predictive. Checking hs-TnT upon presentation to the hospital and trending it for the first day could allow for effective stratification, by the end of that first day, of the patient population into those who will likely survive without need for escalation of care and those who are at high risk for critical illness, intubation, arrest, and death.
Methods
Study participants
We performed a single-centre, retrospective, observational study of all patients over 18 years of age who presented to the Emergency Department (ED) at the University of Chicago Medical Centre between 23 March 2020 and 13 April 2020 and were found to be COVID-19 positive by nasopharyngeal swab polymerase chain reaction (PCR) testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Retrospective chart review was performed following Institutional Review Board approval. De-identified study data were collected from the electronic health record system (Epic, Madison, WI) and managed using the secure, web-based platform REDCap (Research Electronic Data Capture), an electronic data capture tool hosted at the University of Chicago (Harris et al. 2009, 2019).
Patients with multiple presentations for COVID-related illness during the study period were considered as single entries, and first hospitalization following confirmed diagnosis was used for data collection and analysis. Patients who were found to be COVID-19 positive in the ED but did not require admission during this time period (n = 11) and those who were still hospitalized at the time of chart review (n = 17) were excluded from analysis. Of the initial 304 patients considered, 276 remained for final analysis after application of these exclusions (Supplemental Figure).
Patient data
Demographic information on age, gender, race (white, black, Hispanic, other), body mass index (BMI), and insurance status (Medicare, Medicaid, Private, or None/Other) were collected for all patients. Race was self-disclosed by each patient. Baseline clinical variables including presence of hypertension (HTN), hyperlipidaemia (HLD), diabetes (DM), cerebrovascular disease, vascular disease, heart failure, lung disease, renal impairment, and history of malignancy were recorded. Smoking status was recorded and further stratified into current, prior (documented quit date prior to admission), or never. Hyperlipidaemia was defined by history or use of lipid-lowering agents at time of admission. Diabetes was defined by documented history and haemoglobin A1c value ≥6.5% or current use of anti-hyperglycemic medications. Cerebrovascular disease was defined by documented history of stroke, transient ischaemic attack (TIA), or imaging evidence of prior cerebral infarct, regardless of residual effects. Vascular disease was defined by documented history of coronary artery disease (CAD), peripheral arterial disease (PAD), or a history of peripheral or coronary revascularization. Heart failure was defined by documented clinical history of heart failure, irrespective of left ventricular ejection fraction. Lung disease was defined as a documented history of interstitial lung disease, asthma, chronic obstructive lung disease, or use of inhalers for greater than one month prior to admission. Renal impairment was defined by an estimated glomerular filtration rate (eGFR) of less than 30 mL/min/1.73m2 by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation or dialysis dependence (Levey et al. 2009, Matsushita et al. 2012).
Initial and peak laboratory data of interest were collected starting from time of admission. Interleukin-6 testing was sent to the Mayo Clinic (Mayo Clinic Department of Medicine and Pathology, 3050 Superior Drive NW, Rochester, MN 55901) for analysis, with values ≤1.8 pg/mL being considered within normal range. All other laboratory testing was completed on-site at the University of Chicago Medical Centre. Hs-TnT values were assessed using the Roche cobas e601 system, which is an automated immunoassay system using electrochemiluminescence. It has a coefficient of variation (CV) of <10% at the reported 99th percentile of a normal, healthy population (U.S. Food and Drug Administration 2017). Per manufacturer datasheets, a value of 14.0 ng/L was considered the 99th percentile of a normal population (Bagai et al. 2017, U.S. Food and Drug Administration 2020). No distinction was made by the manufacturer with regards to sex-specific cut-offs. In those without an acute myocardial infarction, hs-TnT values using the Roche system have demonstrated significant variability between studies and manufacturer claims, which may be related to differences in populations studied (Monneret et al. 2018). Additionally, black patients were not well represented in these studies, and the true 99th percentile values are unknown. For these reasons, we used the median value in our study population for the hs-TnT analysis.
The clinical outcomes recorded during the index hospitalization included length of stay (LOS), need for intubation, need for critical care as defined by admission or transfer to the intensive care unit (ICU), cardiac arrest, and all-cause mortality.
Statistical analyses
For baseline clinical characteristics, continuous variables were expressed as mean ± standard deviation or median with interquartile range and compared with either Student t-test or Mann–Whitney U (Wilcoxon) tests depending upon normality as determined by Shapiro–Wilk tests. Initial biomarker measurements were dichotomized using their median values. Categorical variables were expressed as relative counts and percentages and compared with Chi-square tests of association or Fisher exact tests. There was no imputation of missing data. Multivariable logistic regression was conducted to determine which baseline and clinical characteristics were associated with the outcome variables. Relevant clinical parameters that did not correlate with each other and which had a p-value <0.01 in the univariable logistic regression were included in the multivariable logistic regression model, and results were presented as odds ratio (OR) and 95% confidence interval (CI). Independent parameters were checked for multicollinearity using Spearman rank correlations, and there were no multicollinearity issues between the independent parameters. Tests were two-tailed, and a p-value <0.05 was considered statistically significant. All statistical analyses were performed using STATA MP version 15 (College Station, TX).
Results
The patients in the study population were primarily black (84%), with a majority being female (53%) and obese (median BMI 31 [IQR 26–37]); median age was 62 years (Table 1). Medicare was the most common form of insurance (46.7%). Median initial hs-TnT value was 17 ng/L, with 261 (95%) of patients having recorded values at presentation. The median initial hs-TnT was 14 ng/L for women and 21 ng/L for men. Compared to those with low values, patients with initial hs-TnT values above the median were older (median age 71 years [IQR 62–81] vs 53 years [IQR 45–63]) and had higher rates of traditional cardiovascular comorbidities and risk factors including hypertension, hyperlipidaemia, renal insufficiency, cerebrovascular disease, heart failure, vascular disease, and tobacco use.
Table 1.
Demographic and clinical description of study population stratified by median initial hs-TnT value.
| Variable | Overall population | hs-TnT ≥ 17ng/L | hs-TnT < 17ng/L | p Value |
|---|---|---|---|---|
| (n = 276) | (n = 132) | (n = 129) | ||
| Age | 62 (50–73) | 71 (62–81) | 53 (45–63) | <0.01 |
| Male | 130 (47.1) | 70 (53.0) | 54 (41.9) | 0.07 (NS) |
| BMI | 30.8 | 27.7 | 32.6 | <0.01 |
| (25.5–36.6) | (23.7–34.7) | (27.7–40.6) | ||
| Race | ||||
| Black | 232 (84.1) | 113 (85.6) | 107 (83.0) | 0.56 (NS) |
| White | 24 (8.7) | 14 (10.6) | 9 (7.0) | 0.30 (NS) |
| Hispanic | 6 (2.2) | 1 (0.8) | 5 (3.9) | 0.09 (NS) |
| Other | 14 (5.1) | 4 (3.0) | 8 (6.2) | 0.22 (NS) |
| Insurance | ||||
| Medicare | 129 (46.7) | 92 (69.7) | 33 (25.6) | <0.01 |
| Medicaid | 65 (23.6) | 21 (15.9) | 38 (29.5) | 0.01 |
| Private | 77 (27.9) | 20 (15.2) | 52 (40.3) | <0.01 |
| History of smoking | ||||
| Current | 18 (6.5) | 6 (4.6) | 9 (7.0) | 0.40 (NS) |
| Former | 98 (35.5) | 56 (42.4) | 38 (29.5) | 0.03 |
| Never | 160 (58.0) | 70 (53.0) | 82 (63.6) | 0.08 (NS) |
| Diabetes | 117 (42.4) | 63 (47.7) | 50 (38.8) | 0.14 (NS) |
| Hypertension | 201 (72.8) | 117 (88.6) | 76 (58.9) | <0.01 |
| Hyperlipidaemia | 130 (47.1) | 83 (62.9) | 44 (34.1) | <0.01 |
| Renal impairment | 44 (15.9) | 40 (30.3) | 3 (2.3) | <0.01 |
| Lung disease | 79 (28.6) | 35 (26.5) | 42 (32.6) | 0.28 (NS) |
| Vascular disease | 49 (17.8) | 35 (26.5) | 14 (10.9) | <0.01 |
| Heart failure | 56 (20.3) | 41 (31.2) | 14 (10.9) | <0.01 |
| Cerebrovascular disease | 37 (13.4) | 28 (21.2) | 8 (6.2) | <0.01 |
| Malignancy | 41 (14.9) | 29 (22.0) | 11 (8.5) | <0.01 |
Continuous variables reported as median (interquartile range). Categorial variables reported as number (percentage). Fifteen patients in the overall population did not have hs-TnT measured.
Patients with initial hs-TnT above the median had longer hospital LOS (median 9 vs 4 days, p < 0.001) and were less often discharged to home (55% vs 92%, p < 0.001), either because of death or facility placement (Table 2). Patients with elevated hs-TnT also had higher rates of ICU admission (48% vs 19%, p < 0.001), intubation (22% vs 8%, p = 0.001), and shock requiring vasoactive medications (21% vs 5%, p < 0.001). These patterns persisted using sex-specific thresholds based on median hs-TnT (Supplemental Table 1).
Table 2.
In-hospital clinical outcomes of study population stratified by median initial hs-TnT value.
| Variable | Overall population (n = 276) | hs-TnT ≥ 17ng/L (n = 132) |
hs-TnT < 17ng/L (n = 129) |
p Value |
|---|---|---|---|---|
| Length of stay (days) | 6 (3–11) | 9 (5–14) | 4 (3–8) | <0.01 |
| Discharged home | 202 (73.2) | 72 (54.6) | 118 (91.5) | <0.01 |
| Discharged to facility | 48 (17.4) | 40 (30.3) | 6 (4.7) | <0.01 |
| Deceased inpatient | 26 (9.4) | 20 (15.2) | 5 (3.9) | <0.01 |
| Intubation | 41 (14.9) | 29 (22.0) | 10 (7.8) | <0.01 |
| Shock requiring vasoactive agents | 36 (13.0) | 27 (20.5) | 7 (5.4) | <0.01 |
| ICU admission | 93 (33.7) | 63 (47.7) | 25 (19.4) | <0.01 |
| Cardiac arrest | 9 (3.3) | 7 (5.3) | 2 (1.6) | 0.17 (NS) |
Continuous variables reported as median (interquartile range). Categorial variables reported as number (percentage).
Using median initial biomarker values as cut-offs, univariable analysis showed that all recorded serum biomarker elevations, except for erythrocyte sedimentation rate (ESR), showed association with the primary composite endpoint of in-hospital death, critical care, intubation, or cardiac arrest (Table 3). This included elevated hs-TnT ≥ 17 ng/L (OR 3.92; 95% CI 2.25–6.81, p < 0.001). Additionally, time to peak hs-TnT on a continuous scale was also associated with the primary composite endpoint (OR 1.61; 95% CI 1.25–2.07 for each additional hospital day to peak, p < 0.001). Apart from hyperlipidaemia and diabetes, no recorded clinical or demographic variables of interest correlated with the composite endpoint. A similar pattern was seen for the composite endpoint of in-hospital death, intubation, or cardiac arrest, except diabetes was no longer significantly associated. Furthermore, for both composite endpoints, none of the variables measured demonstrated a negative correlation (i.e. OR < 1) suggestive of ‘low risk’.
Table 3.
Univariate analysis of clinical outcomes of interest.
| Variable | In-hospital mortality |
In-hospital mortality, intubation, cardiac arrest |
In-hospital mortality, intubation, need for critical care, cardiac arrest |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CÌ) | p Value | OR (95% CI) | p Value | |
| Hs-TnT ≥ 17 ng/L | 4.43 (1.61–12.19) | <0.01 | 4.29 (2.02–9.11) | <0.01 | 3.92 (2.25–6.81) | <0.01 |
| CRP ≥ 79 mg/L | 2.42 (1.01–5.77) | 0.05 | 3.12 (1.57–6.23) | <0.01 | 3.75 (2.19–6.43) | <0.01 |
| ESR ≥ 81 mm/hr | 0.75 (0.31–1.78) | 0.51 (NS) | 1.21 (0.60–2.42) | 0.60 (NS) | 1.04 (0.60–1.82) | 0.89 (NS) |
| LDH ≥ 366 U/L | 4.83 (1.76–13.24) | <0.01 | 4.27 (2.01–9.08) | <0.01 | 4.03 (2.28–7.12) | <0.01 |
| D-dimer ≥ 1.18 µg/mL | 2.02 (0.87–4.70) | 0.10 (NS) | 3.59 (1.77–7.27) | <0.01 | 2.47 (1.47–4.16) | <0.01 |
| Ferritin ≥ 592 ng/mL | 2.94 (1.19–7.26) | 0.02 | 2.74 (1.39–5.40) | <0.01 | 2.34 (1.39–3.93) | <0.01 |
| IL-6 ≥ 19 pg/mL | 4.48 (1.59–12.58) | 0.01 | 4.63 (2.05–10.44) | <0.01 | 2.73 (1.48–5.03) | <0.01 |
| Hospital day of peak hs-TnT | 1.32 (1.16–1.51) | <0.01 | 1.40 (1.19–1.65) | <0.01 | 1.61 (1.25–2.07) | <0.01 |
| Male sex | 1.91 (0.83–4.37) | 0.13 (NS) | 1.65 (0.88–3.11) | 0.12 (NS) | 1.45 (0.88–2.39) | 0.15 (NS) |
| Age | 1.03 (1.01–1.06) | 0.01 | 1.01 (1.00–1.03) | 0.15 (NS) | 1.01 (1.00–1.03) | 0.07 (NS) |
| Race (Black vs other) | 1.05 (0.62–1.77) | 0.86 (NS) | 0.94 (0.60–1.46) | 0.78 (NS) | 0.98 (0.70–1.38) | 0.92 (NS) |
| Insurance (private vs other) | 0.31 (0.09–1.07) | 0.06 (NS) | 0.56 (0.26–1.22) | 0.15 (NS) | 0.83 (0.48–1.47) | 0.53 (NS) |
| Smoking history | 0.58 (0.32–1.06) | 0.08 (NS) | 0.64 (0.39–1.03) | 0.07 (NS) | 0.77 (0.52–1.14) | 0.19 (NS) |
| Diabetes history | 1.00 (0.44–2.26) | 0.99 (NS) | 1.69 (0.90–3.18) | 0.10 (NS) | 1.71 (1.03–2.83) | 0.04 |
| Hypertension history | 1.27 (0.49–3.30) | 0.62 (NS) | 1.11 (0.54–2.27) | 0.78 (NS) | 1.75 (0.97–3.18) | 0.06 (NS) |
| Hyperlipidaemia history | 3.40 (1.38–8.38) | 0.01 | 2.85 (1.46–5.56) | <0.01 | 2.15 (1.30–3.57) | <0.01 |
| Renal Impairment history | 1.29 (0.46–3.62) | 0.63 (NS) | 1.31 (0.58–2.95) | 0.51 (NS) | 1.42 (0.73–2.75) | 0.30 (NS) |
| Lung disease history | 1.64 (0.71–3.79) | 0.25 (NS) | 1.07 (0.54–2.13) | 0.85 (NS) | 1.09 (0.63–1.88) | 0.76 (NS) |
| Vascular disease history | 2.78 (1.16–6.67) | 0.02 | 1.78 (0.85–3.74) | 0.13 (NS) | 1.43 (0.76–2.69) | 0.27 (NS) |
| Heart failure history | 2.29 (0.96–5.45) | 0.06 (NS) | 1.89 (0.93–3.84) | 0.08 (NS) | 1.46 (0.80–2.67) | 0.22 (NS) |
OR: odds ratio; CI: confidence interval.
From the above analysis, using the largest set of significant factors that did not correlate with each other, along with age, black race, and sex, multivariable analysis (Table 4) demonstrated independent predictive value of hs-TnT for the primary composite endpoint (OR 2.92; 95% CI 1.27–6.71, p = 0.01). Lactate dehydrogenase (LDH) and C-reactive protein did not significantly correlate with hs-TnT but were also independent predictors of this endpoint. Other biomarkers (ferritin, IL-6, D-dimer) significantly correlated with hs-TnT and were less often measured in the population, so they were not included in the multivariable analysis. Time to peak hs-TnT was also independently associated with increased risk (OR 2.19; 95% CI 1.43-3.35 for each additional day until peak, p < 0.001). When analysed in conjunction with these biomarkers, demographic factors (age, race, sex) were not independent predictors of outcomes.
Table 4.
Multivariable analysis of composite endpoints.
| Variable | In-hospital mortality, intubation, or cardiac arrest |
In-hospital mortality, intubation, need for critical care, or cardiac arrest |
||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Hs-TnT ≥ 17 ng/L | 2.35 (0.81–6.79) | 0.12 (NS) | 2.92 (1.27–6.71) | 0.01 |
| CRP ≥ 79 mg/L | 1.71 (0.71–4.15) | 0.23 (NS) | 3.22 (1.56–6.65) | <0.01 |
| LDH ≥ 366 U/L | 4.35 (1.63–11.63) | <0.01 | 3.18 (1.52–6.64) | <0.01 |
| Hospital day of peak hs-TnT (continuous) | 1.42 (1.15–1.77) | <0.01 | 2.19 (1.43–3.35) | <0.01 |
| Male sex | 1.20 (0.53–2.71) | 0.66 (NS) | 1.20 (0.61–2.38) | 0.60 (NS) |
| Age (continuous) | 1.00 (0.97–1.03) | 0.78 (NS) | 0.99 (0.96–1.01) | 0.23 (NS) |
| Race (Black vs other) | 1.32 (0.77–2.25) | 0.31 (NS) | 1.10 (0.70–1.72) | 0.68 (NS) |
| History of hyperlipidaemia | 2.78 (1.11–6.98) | 0.03 | 1.63 (0.76–3.47) | 0.21 (NS) |
| History of diabetes | N/A | N/A | 1.43 (0.72–2.87) | 0.31 (NS) |
OR: odds ratio; CI: confidence interval.
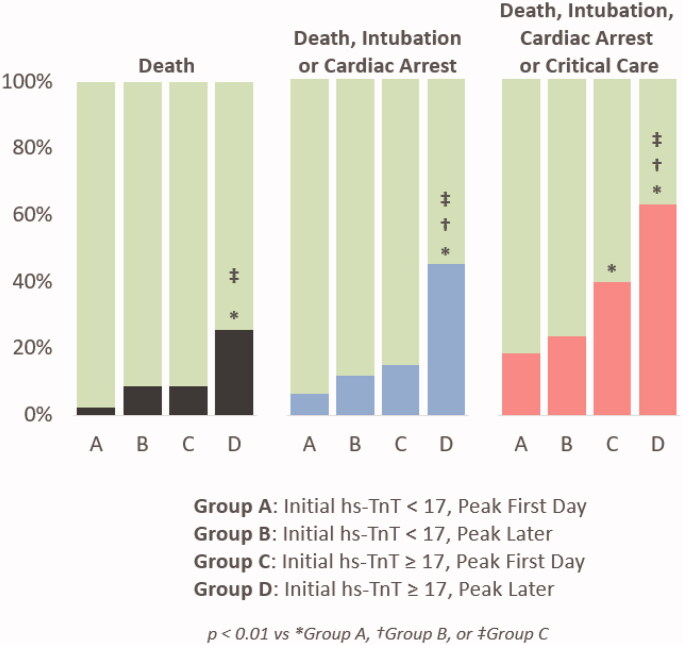
Because both initial hs-TnT and time to peak troponin were independently associated with the composite outcomes on multivariable analysis, the study population was further stratified and analysed by time to peak troponin (Figure 1). Patients who had low initial hs-TnT that peaked during the first day of admission (Group A) had lower event rates than those (Group D) with initially high hs-TnT that continued to uptrend beyond the first day (18% vs 63%, respectively, for the primary endpoint, p < 0.0001). Even in patients with high initial hs-TnT, the event rates were lower if it peaked within the first day (Group C) than if it continued to rise (Figure 1, p < 0.01 for Group C vs Group D in all panels).
Figure 1.
Event rates stratified by initial hs-TnT and time to peak hs-TnT.
Discussion
This study investigated the relationship between elevated troponin and adverse in-hospital events for patients hospitalized with COVID-19, consistent with prior retrospective studies (Bikdeli et al. 2020). Elevations in troponin had predictive value outside of cardiac events in our study population, as only one patient received a clinical diagnosis of acute coronary syndrome and underwent coronary revascularization. Compared to traditional inflammatory markers, such as ESR and C-reactive protein (CRP), we demonstrated better predictive value for multiple outcomes with hs-TnT and LDH – two biomarkers that are less frequently utilized in risk stratification for inflammatory or infectious disease. Other biomarkers that could be mechanistically linked to cardiovascular events such as IL-6 (through inflammatory pathways) and D-dimer (as a marker of thrombosis) were less often measured as compared to troponin, but were significantly correlated with hs-TnT, indicating that these biomarkers tend to be high as a group in higher-risk patients and that hs-TnT may capture some of the risk inherent in these other pathways. Lastly, our study found significant association between time to peak troponin and adverse clinical outcomes, suggesting that trending this parameter over just the first day of admission could provide useful prognostic information for patients and physicians.
The Task Force of the 4th Universal Definition of Acute Myocardial Infarction recognizes variability in cTn values between sexes in normal populations (Thygesen et al. 2018). In our study population, median hsTnT values for men was 21 ng/L and for women was 14 ng/L. However, there was no independent contribution of sex in our multivariable analysis, and the pattern of outcomes was similar when using a common cut-off rather than sex-specific cut-off values. For this reason, we chose to use the overall median value for our population and report both sexes together.
Our results are in line with prior smaller studies in China demonstrating correlation between troponin and COVID-19 disease severity (Wei et al. 2020). A multi-centre retrospective cohort study from Wuhan, China studied 191 patients hospitalized with COVID-19 (Zhou et al. 2020). Those with higher in-hospital mortality were of older age, elevated sequential organ failure assessment (SOFA) score, and elevated D-dimer >1 µg/mL on admission. Elevated high sensitivity troponin I (hs-TnI) and LDH were also associated with in-hospital mortality. Meta-analysis of similar studies from China show consistent results when utilizing hs-TnI in patients with COVID-19 (Lippi et al. 2020). When comparing hs-TnI values of 341 patients across four studies, significant elevations in hs-TnI values were found in those with severe disease compared to those without. A larger retrospective study from New York not utilizing high-sensitivity assays showed that elevated troponin-I was associated with increased risk of death in a large US population (Lala et al. 2020).
Other studies have found that while inflammatory markers demonstrated correlation with ICU transfer or death, only CRP was found to be independently associated with these outcomes (Cecconi et al. 2020). Our study population was larger and had much higher rates of cardiovascular risk factors and obesity. These factors may account for the differences noted in our results. Based on our results, since almost all patients with COVID-19 had very high inflammatory markers including CRP, ESR, LDH, ferritin, and D-dimer (median values for the study population were all well above the normal reference range), hs-TnT—which was normal in the majority of patients who survived— appears to serve as a better prognostic discriminator when relying on standard laboratory reference ranges.
The aetiology of elevated hs-TnT in patients with COVID-19 is likely multifactorial. Elevations of hs-TnT can reflect a spectrum of myocardial injury ranging from reversible ischaemia to irreversible cellular injury and apoptosis (Hammarsten et al. 2018, Mair et al. 2018). Our study found that only one patient (<1%) with elevated hs-TnT had clinical evidence of acute coronary syndrome. Given this, the elevated hs-TnT in most of our patients was likely a reflection of inflammatory response, demand ischaemia, and microangiopathic disease rather than true myocardial infarction (Tersalvi et al. 2020).
With this proposed mechanism, it would be of interest to know if targeted anti-inflammatory therapies, such as IL-6 inhibitors, decrease troponin elevation and if this response is predictive of improvement in outcomes. While we can speculate that use of IL-6 inhibitor therapy would decrease inflammation, as has been reported in other clinical scenarios, so far, no clear benefit for directed anti-IL-6 therapy has been found in COVID-19 patients with regards to our outcomes of interest (Kleveland et al. 2016, Cortegiani et al. 2020). Additionally, although we have identified the prognostic value of a cardiac biomarker, the outcomes we are reporting on are not limited to cardiovascular events. Our findings suggest that elevated hs-TnT serves as a marker of risk of future adverse events based on the current severity of the infectious disease and inflammatory process, but not necessarily as a marker of hard cardiovascular outcomes. Therefore, we are unable to comment on potential benefit, and no clear evidence currently exists, for cardiac-specific therapies in these patients (Sandoval et al. 2020).
To the best of our knowledge, our study is the first to investigate the prognostic utility of hs-TnT in patients with COVID-19 in the US. Furthermore, our study was done in a vulnerable population with high rates of cardiovascular comorbidities. Elevations of TnT have been associated with increased risk of cardiovascular events, similar to TnI; however, TnT has also been associated with increased all-cause mortality, which may make it a more useful tool when attempting to stratify patients presenting with primarily non-cardiac pathology (Aldous et al. 2011, Rusnak et al. 2017, Welsh et al. 2019). Furthermore, while hs-TnI and hs-TnT are comparable in detecting myocardial infarction, the correlation at lower concentrations between the two measures is weak and may be due to differences in how the assays, even though they are cardio-specific, interact with skeletal muscle troponin (Jaffe et al. 2011, Rittoo et al. 2014, Welsh et al. 2018). Thus, because many institutions use hs-TnT as their primary troponin assay, it is important to demonstrate its prognostic utility in COVID-19.
Additionally, we found that late versus early peaking of hs-TnT serves as a useful metric to provide additional prognostic information for mortality and our composite endpoint. This suggests that it may be useful for providers to not only check initial levels, but to trend hs-TnT through the first day of presentation. Our findings suggest that hs-TnT levels that peak within the first day, even if elevated, are associated with significantly lower rates of mortality, ICU stay, and cardiac arrest. This time-dependent troponin relationship is a new finding for patients with COVID-19.
Of note, our study specifically focused on a predominantly black population in a lower socio-economic region of the city of Chicago. Our observed in-hospital mortality rate of 9.4% is within the range of in-hospital mortality rates for COVID-19 in the US. However, black patients have been observed to have disproportionately high mortality rates from COVID-19 throughout the country (Johns Hopkins Coronavirus Resource Center 2020). In Chicago, black patients have the highest mortality rates (City of Chicago: COVID-19 Reports 2020). In other cities, black patients have represented up to 70% of COVID-19 deaths despite comprising just under one-third of the population (Price-Haywood et al. 2020). Although not the primary focus of our study, and while our mortality rate was not disproportionately high, it is important to know that disparities of health outcomes for black patients are well documented for a range of diseases, attributable to limited access to health care, socioeconomic factors, and long-standing structural inequities.
Our rates of admission for those who presented to the ED and were found to be COVID-19 positive are high. This is likely a reflection of the fact that our institution implemented, at the start of the pandemic, a system for testing most patients at drive-through centres or at COVID-specific outpatient clinics. Minimally symptomatic patients who tested positive were sent home; only those who were found to have abnormal vital signs or significant symptoms were sent to the ED for further evaluation. Therefore, this outpatient triage meant that most patients who presented to the ED and were COVID-19 positive were already determined to likely require admission.
Our study has several limitations. Due to its retrospective design, data collection relied on chart review and clinical documentation for assessment of comorbid conditions and medication use. As patients were not prospectively enrolled, not all patients presenting with COVID-19 had every laboratory biomarker measured. Still, 95% of the patients did have hs-TnT measured on presentation, so this biomarker was well-represented. The effect of viral load on biomarker elevation and outcomes was unavailable, as cycle threshold values were not reported for patients with positive COVID-19 tests. Future studies investigating this would be of interest. Additionally, those patients still hospitalized at the time of chart review were excluded from analysis. These patients were all hospitalized for at least 12 days by the time of initial data entry, which is one standard deviation above the average LOS for our studied population, and this may be indicative of a sicker patient population.
Outcomes following discharge are beyond the scope of this analysis. While we were able to record ED presentations and re-admissions to our institution and those within the Epic CareEverywhere system, institutions outside this network could not be queried. For mortality, the study was limited to in-hospital deaths within our hospital system, and we did not query outside databases. Finally, decreased incidence of acute myocardial infarction during the COVID-19 era has been observed at multiple centres (Solomon et al. 2020). The reason for this finding is likely multifactorial, perhaps including altered social habits (resulting in a true reduction), and unwillingness to come to a hospital when symptoms strike (which would be a false reduction). The vast majority of patients in these studies did not have COVID-19, so the finding is not directly related to the viral infection, and our study focuses on patients who have been admitted to the hospital for COVID-19.
Conclusions
Although multiple pathologies can result in elevated hs-TnT values, there is a positive correlation between elevated values and adverse events in patients hospitalized with COVID-19, supporting its role in risk stratification. Utilization of hs-TnT in combination with LDH levels may further help risk-stratify patients at initial presentation, while other biomarkers correlated with these two biomarkers and thus were redundant. Our study also provides evidence for trending hs-TnT values through the first day of admission to provide prognostic information regarding in-hospital outcomes. Compared to other biomarkers such as CRP and D-dimer, which were more uniformly elevated regardless of eventual outcome, the majority of survivors had normal levels of initial hs-TnT that did not trend upward beyond the first day, so hs-TnT served as a better discriminating factor of overall prognosis. Further prospective studies are warranted to better understand the mechanism of biomarker elevations and their ability to predict adverse events in patients with COVID-19 beyond their index hospitalization.
Supplementary Material
Disclosure statement
The authors report no conflicts of interest.
Author contributions
Dr. Alenghat had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Singh, Nguyen, Alenghat. Acquisition, analysis or interpretation of data: All authors. Drafting of the manuscript: Singh, Alenghat. Critical revision of the manuscripts for important intellectual content: All authors. Statistical analysis: Singh, Besser, and Alenghat. Supervision: Alenghat.
References
- Aikawa, T., et al. , 2020. Myocardial injury characterized by elevated cardiac troponin and in-hospital mortality of COVID-19: an insight from a meta-analysis. Journal of medical virology.doi: 10.1002/jmv.26108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldous, S., et al. , 2011. Comparison of high sensitivity and contemporary troponin assays for the early detection of acute myocardial infarction in the emergency department. Annals of clinical biochemistry, 48 (3), 241–248. [DOI] [PubMed] [Google Scholar]
- Bagai, A., et al. , 2017. Use of troponin assay 99th percentile as the decision level for myocardial infarction diagnosis. American heart journal, 190, 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch, S., et al. , 2020. The potential health care costs and resource use associated with COVID-19 in the United States. Health affairs (project hope), 39 (6), 927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli, B., et al. , 2020. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. Journal of the American college of cardiology, 75 (23), 2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi, M., et al. , 2020. Early predictors of clinical deterioration in a cohort of 239 patients hospitalized for COVID-19 infection in Lombardy, Italy. Journal of clinical medicine, 9 (5), 1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- City of Chicago: COVID-19 Reports , 2020. Latest Data. Available from: https://www.chicago.gov/city/en/sites/covid-19/home/latest-data/2020-05-17.html [Accessed 5 June 2020].
- Cortegiani, A., et al. , 2020. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarsten, O., et al. , 2018. Possible mechanisms behind cardiac troponin elevations. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals, 23 (8), 725–734. [DOI] [PubMed] [Google Scholar]
- Harris, P., et al. , 2019. REDCap Consortium. The REDCap consortium: building an international community of software platform partners. Journal of biomedical informatics, 95, 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, P.A., et al. , 2009. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics, 42 (2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendren, N., et al. , 2020. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation, 141 (23), 1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazio, M., et al. , 2020. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation, or myocarditis. Heart (British Cardiac Society), 106 (15), 1127–1131. [DOI] [PubMed] [Google Scholar]
- Jaffe, A., et al. , 2011. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. Journal of the American college of cardiology, 58 (17), 1819–1824. [DOI] [PubMed] [Google Scholar]
- Januzzi, J., 18 Mar 2020. Troponin and BNP Use in COVID-19. American College of Cardiology. Available from: www.acc.org/latest-in-cardiology/articles/2020/03/18/15/25/troponin-andbnp-use-in-covid19/ [Accessed 5 June 2020].
- Johns Hopkins Coronavirus Resource Center , 2020. Mortality Analyses. Available from: coronavirus.jhu.edu/data/mortality/ [Accessed 5 June 2020].
- Kleveland, O., et al. , 2016. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. European heart journal, 37 (30), 2406–2413. [DOI] [PubMed] [Google Scholar]
- Lala, A., et al. , on behalf of the Mount Sinai Covid Informatics Center, 2020. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. Journal of the American college of cardiology, 76 (5), 533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey, A., et al. , for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration), 2009. A new equation to estimate glomerular filtration rate. Annals of internal medicine, 150 (9), 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi, G., Lavie, C., and Sanchis-Gomar, F.. 2020. Cardiac troponin in COVID-19. Progress in cardiovascular diseases, 63 (3), 390–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair, J., et al. , 2018. How is cardiac troponin released from injured myocardium? European heart journal. Acute cardiovascular care, 7 (6), 553–560. [DOI] [PubMed] [Google Scholar]
- Matsushita, K., et al. , for the Chronic Kidney Disease Prognosis Consortium, 2012. Comparison of risk prediction using the CKD-EPI equation and the MDRD Study equation for estimated glomerular filtration rate. JAMA, 307 (18), 1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneret, D., Gellerstedt, M., and Bonnefont-Rousselot, D., 2018. Determination of age- and sex-specific 99th percentiles for high-sensitive troponin T from patients: an analytical imprecision- and partitioning-based approach. Clinical chemistry and laboratory medicine (CCLM), 56 (5), 685–696. [DOI] [PubMed] [Google Scholar]
- Price-Haywood, E., et al. , 2020. Hospitalization and mortality among Black patients and White patients with Covid-19. The New England journal of medicine, 382 (26), 2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittoo, D., et al. , 2014. Elevation of cardiac troponin T, but not cardiac troponin I, in patients with neuromuscular diseases: implications for the diagnosis of myocardial infarction. Journal of the American college of cardiology, 63 (22), 2411–2420. [DOI] [PubMed] [Google Scholar]
- Rusnak, J., et al. , 2017. High sensitivity troponins discriminate different morphologies of coronary artery plaques being assessed by coronary computed tomography angiography. Disease markers, 2017, 9306409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval, Y., Januzzi, J., and Jaffe, A., 2020. Cardiac troponin for assessment of myocardial injury in COVID-19. Journal of the American college of cardiology, 76 (10), 1244–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, S., et al. , 2020. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. European heart journal, 41 (22), 2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, M., et al. , 2020. The Covid-19 pandemic and the incidence of acute myocardial infarction. The New England journal of medicine, 383 (7), 691–693. [DOI] [PubMed] [Google Scholar]
- Tersalvi, G., et al. , 2020. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. Journal of cardiac failure, 26 (6), 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen, K., et al. , Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction, 2018. Fourth Universal Definition of Myocardial Infarction (2018). Journal of the American college of cardiology, 72 (18), 2231–2264. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration , 2020. Medical Device Databases. Available from: www.accessdata.fda.gov/cdrh_docs/reviews/K162895.pdf/ [Accessed 5 June 2020].
- U.S. Food and Drug Administration , Department of Health and Human Services, 18 Jan 2017. Section 501(k) Premarket Notification – Elecsys Troponin T Gen 5 STAT.
- Wei, J., et al. , 2020. Acute myocardial injury is common in patients with covid-19 and impairs their prognosis. Heart, 106 (15), 1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh, P., et al. , 2018. Comparison between high-sensitivity cardiac troponin T and cardiac troponin I in a large general population cohort. Clinical chemistry, 64 (11), 1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh, P., et al. , 2019. Cardiac troponin T and troponin I in the general population. Circulation, 139 (24), 2754–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization , 2020. Coronavirus Disease (COVID-19) Dashboard. Available from: https://covid19.who.it [Accessed 6 August 2020].
- Zhou, F., et al. , 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet, 395, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.