Abstract
Aims
The COMPARE trial showed a small but significant beneficial effect of 3-year losartan treatment on aortic root dilatation rate in adults with Marfan syndrome (MFS). However, no significant effect was found on clinical endpoints, possibly due to a short follow-up period. The aim of the current study was therefore to investigate the long-term clinical outcomes after losartan treatment.
Methods and results
In the original COMPARE study (inclusion 2008–2009), adult patients with MFS (n = 233) were randomly allocated to either the angiotensin-II receptor blocker losartan® on top of regular treatment (β-blockers in 71% of the patients) or no additional medication. After the COMPARE trial period of 3 years, study subjects chose to continue their losartan medication or not. In a median follow-up period of 8 years, 75 patients continued losartan medication, whereas 78 patients, originally allocated to the control group, never used losartan after inclusion. No differences existed between baseline characteristics of the two groups except for age at inclusion [losartan 34 (interquartile range, IQR 26–43) years, control 41 (IQR 30–52) years; P = 0.031], and β-blocker use (losartan 81%, control 64%; P = 0.022). A pathological FBN1 mutation was present in 76% of patients and 58% of the patients were male. Clinical endpoints, defined as all-cause mortality, aortic dissection/rupture, elective aortic root replacement, reoperation, and vascular graft implantation beyond the aortic root, were compared between the two groups. A per-patient composite endpoint was also analysed. Five deaths, 14 aortic dissections, 23 aortic root replacements, 3 reoperations, and 3 vascular graft implantations beyond the aortic root occurred during follow-up. Except for aortic root replacement, all endpoints occurred in patients with an operated aortic root. Patients who used losartan during the entire follow-up period showed a reduced number of events compared to the control group (death: 0 vs. 5, P = 0.014; aortic dissection: 3 vs. 11, P = 0.013; elective aortic root replacement: 10 vs. 13, P = 0.264; reoperation: 1 vs. 2, P = 0.463; vascular graft implantations beyond the aortic root 0 vs. 3, P = 0.071; and composite endpoint: 14 vs. 26, P = 0.019). These results remained similar when corrected for age and β-blocker use in a multivariate analysis.
Conclusion
These results suggest a clinical benefit of combined losartan and β-blocker treatment in patients with MFS.
Keywords: Marfan syndrome, Angiotensin-II receptor blocker, Losartan, β-blocker
See page 4188 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa418)
Introduction
Adequate diagnosis, as well as improved surgical techniques, have greatly improved life expectancy in Marfan syndrome (MFS) patients over the last decades.1–3 Nevertheless, aortic surgery and complications such as dissections and ruptures have remained as a significant source of morbidity and mortality. Improved pharmaceutical treatments are therefore highly needed.
β-blocker treatment has been considered the main pharmaceutical therapy to retain progressive aortic root dilatation in MFS patients and thus prevent or delay aortic complications, even though evidence was limited, conflicting4,5 and mainly assigned to results from one small dated randomized trial.6 Many researchers have tried to identify new pharmaceutical treatment options to delay aortic dilatation in this patient population. After encouraging experiments in a mouse model of MFS,7 several research groups investigated the effect of angiotensin-II receptor blockers (ARBs)—in particular losartan—on aortic root growth and clinical endpoints in humans.
The first published large randomized controlled trail was the COMPARE trial from the Netherlands, which showed a small but significant 3-year effect of losartan on top of regular treatment (mostly β-blockers) vs. regular treatment (0.77 ± 1.36 vs. 1.35 ± 1.55 mm, P = 0.014) on aortic root dilatation rate in adults with MFS.8 Similar studies on the effect of ARBs on aortic root growth rate in MFS patients showed variable results9–13 (Table 1).
Table 1.
Overview of randomized ARB trials in MFS patients
| Drugs tested | FU in years | Primary outcome | Results | ||
|---|---|---|---|---|---|
|
COMPARE,8 The Netherlands (n = 233) |
Losartan | Add-on therapy | 3.0 | Change in absolute root diameter | P = 0.014 |
| No additional drug | |||||
|
Taiwan9 (n = 29) |
Losartan | Add-on therapy | 2.9 | Change in absolute diameter and dilatation rate of root per year | P = 0.020 |
| Atenolol or propranolol | |||||
|
Marfan Sartan,10 France (n = 299) |
Losartan | Add-on therapy | 3.5 | Rate of change in root Z-score per year | P = 0.36 |
| Placebo | |||||
|
LOAT,11 Spain (n= 140) |
Losartan | Head to head trial | 3.0 | Change in absolute diameter or Z-score of root and ascending aorta | P = 0.193 |
| Atenolol | |||||
|
Pediatric Heart Network,12 USA (n = 608) |
Losartan | Head to head trial | 3.0 | Rate of change in root Z-score per year | P = 0.080 |
| Atenolol | |||||
|
AIMS,13 England (n = 192) |
Irbesartan | Add on therapy | 5.0 | Absolute change in aortic root diameter per year | P = 0.030 |
| Placebo | |||||
None of the trials thus far could demonstrate significant effects on clinical endpoints, probably due to the relatively short follow-up period, with a relatively low event rate. The aim of the current study was therefore to assess the long-term effects of losartan on clinical endpoints in patients originally enrolled in the COMPARE trial.
Methods
This study complies with the Declaration of Helsinki and was written in accordance with the Strengthening the Reporting of Observation Studies in Epidemiology (STROBE) statement.14
Study background
The design and conduct of the multicentre randomized open-label COMPARE (COzaar in Marfan Patients Reduces aortic Enlargement) trial (NTR1423) have been described in detail elsewhere.8,15 In brief, the COMPARE trial included patients from four Dutch academic hospitals with a specialized MFS clinic (Academic Medical Center, Amsterdam; Radboud University Medical Center, Nijmegen; University Medical Center Groningen, Groningen; and Leiden University Medical Center, Leiden) in 2008 and 2009. Patients were eligible for inclusion if they were ≥18 years old and diagnosed with MFS according to the Ghent criteria of 1996.
The major exclusion criteria were intolerance for, or present use of, angiotensin-converting enzyme inhibitors and/or ARBs, aortic root diameter >50 mm, history of aortic dissection, or the presence of more than one vascular prosthesis.
Patients were randomly 1:1 assigned to losartan on top of regular treatment (β-blockers in 71% of the patients) vs. regular treatment alone. Patients in the losartan group started on 50 mg daily, the dosage was doubled after 14 days. After that, if tolerated, patients continued with 100 mg losartan daily. Twenty-five patients returned to 50 mg, in two of the cases patients reduced dosage to 25 mg. The original follow-up period of the trial was 3 years.
Current study design
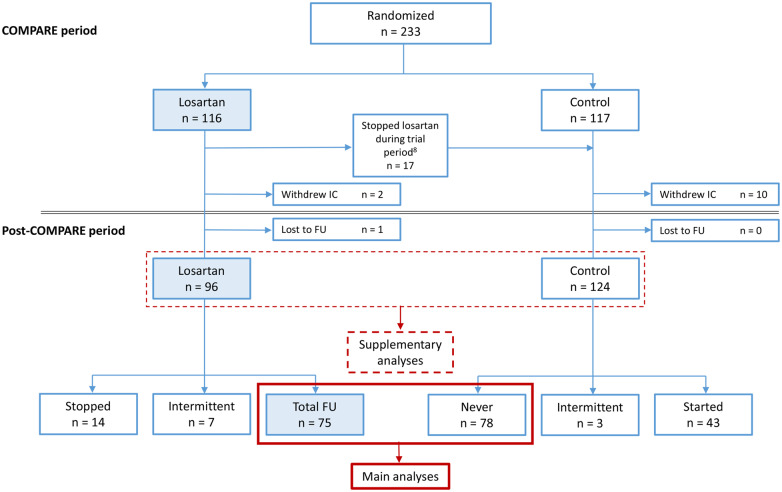
Due to the retrospective study design, formal ethical approval was waived by the local medical ethics committee of the Amsterdam UMC. This was in accordance with the Dutch law as specified in the Medical Research Involving Human Subjects Act. After the 3-year COMPARE trial period, use of medication was left to the discretion of the patient and the treating physician. From the 233 originally randomized patients, 220 could be traced and analysed after a median follow-up period of 8 years (Figure 1). Baseline patient characteristics were extracted from the original trial database. Data concerning mutation profiles and clinical endpoints were derived from electronic patient files. All available data regarding medication status, medication changes, compliance, and side effects were also obtained from electronic patient files and prescription notes. We compared the occurrence of all-cause mortality, aortic dissection or rupture, elective aortic root replacement, reoperation, and implantation of vascular grafts beyond the aortic root as primary endpoints between the patients that used losartan (n = 75) and those patients that never used losartan (n = 78) during the entire follow-up period (median 8 years). Supplementary analyses were performed on the group of patients that were initially allocated to losartan (n = 96) and used this for 3 years vs. the control group (n = 124). See Figure 1 for the flowchart.
Figure 1.
Flowchart of losartan use during COMPARE and post-COMPARE period. FU, follow-up; IC, informed consent.
All mentioned primary clinical endpoints were also combined to one patient-specific composite endpoint, where each individual patient with multiple primary endpoints could reach only one composite endpoint.
Statistical analysis
Categorical data, reported in numbers and percentages were compared between groups using the Fisher’s exact test. Continuous data were interpreted as median and interquartile range (IQR) or as mean and standard deviation, depending on the distribution of the data. Distribution of the data was tested with the Shapiro–Wilk test. Comparison of continuous variables between groups were executed with either parametric (Student’s t-test) or non-parametric (Mann–Whitney U) tests, depending on the distribution.
Time-to-event outcomes were evaluated by Kaplan–Meier analyses and log-rank test. Treatment effects were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs) using Cox regressions. Where applicable, corrections were made using multivariable Cox regressions. Sensitivity analyses were performed in the total cohort with a time-dependent Cox regression.
All analyses were carried out in SPSS Statistics (version 25, IBM Corp, Armonk, NY, USA). All statistical tests were considered significant if the P-value was < 0.05.
Results
The COMPARE trial randomized 233 patients to either losartan on top of regular treatment, or to regular treatment. Twelve patients withdrew consent at a later stage, resulting in a total of 221 patients who were eligible for the current study. One patient was lost to follow-up, resulting in available follow-up data of 220 patients. Of these 220 patients, a total of 96 patients (44%) used losartan for 3 years, the other group of 124 (56%) patients was composed of the original control group and 17 patients who originally dropped out of the losartan group shortly after inclusion due to side effects (mostly dizziness or low blood pressure). The analyses concerning these two groups are displayed in the Supplementary material online.
After the COMPARE trial period, 14 patients stopped losartan treatment and 43 patients of the control group started losartan treatment at any time after trial close-out according to the discretion of the patient and the treating physician. The main analyses were performed on the group of patients that used losartan during the initial trial period plus the entire follow-up period (n = 75) vs. the group that never used losartan (n = 78) (Figure 1). Patients from both groups did not use other types of ARBs during the follow-up period.
Baseline characteristics
Baseline characteristics are shown in Table 2. The median follow-up period from randomization to the last hospital visit was 7.9 years (IQR 6.4–8.8). The average age was 37 (IQR 27–47) and differed significantly between the two groups [losartan 34 (IQR 26–43), control 41 (IQR 30–52)]. Approximately half of the patients were male [losartan n = 45 (60%), control n = 44 (56%)] and roughly 76% of the patients had a pathological FBN1 mutation [losartan n = 55 (73%), control n = 61 (78%)]. Further genetic analysis revealed 1 MYH11 mutation and 1 TGFB2 mutation in the losartan group and 1 MYH11, 2 TGFB2, 1 TGFBR1, and 1 TGFBR2 mutation in the control group. Presence of a native aortic root at inclusion was 68% in the losartan group vs. 59% in the control group. Furthermore, 77% of the patients in the losartan group and 64% in the control group used β-blockers (in most patients atenolol or metoprolol, and in three patients sotalol) at randomization. In the post-trial period, 81% of the patients in the losartan group and 64% in the control group used β-blockers at some point, either intermittently or continuously. In the patients using atenolol or metoprolol, most were dosed between 50 and 100 mg per day (87%), with only 12% <50 mg and 1% of the patients >100 mg. The three patients using sotalol were dosed 40 mg twice per day.
Table 2.
Baseline characteristics of 153 patients included in the main analyses
| Total (n = 153) | Losartan (n = 75) | Control (n = 78) | SMD | P-value | |
|---|---|---|---|---|---|
| Clinical follow-up (years) | 7.9 (6.4–8.8) | 8.2 (7.1–9.4) | 7.6 (5.5–8.6) | 0.470 | 0.005 |
| Age at inclusion (years) | 37 (27–47) | 34 (26–43) | 41 (30–52) | 0.371 | 0.031 |
| Male | 89 (58%) | 45 (60%) | 44 (56%) | 0.073 | 0.743 |
| FBN1 mutation | 116 (76%) | 55 (73%) | 61 (78%) | 0.114 | 0.181 |
| Dominant negative | 71 (61%) | 32 (58%) | 39 (66%) | 0.164 | 0.441 |
| Haploinsufficient | 43 (37%) | 23 (42%) | 20 (34%) | 0.164 | 0.441 |
| β-blocker use | |||||
| At randomization | 108 (71%) | 58 (77%) | 50 (64%) | 0.208 | 0.079 |
| At any point | 111 (73%) | 61 (81%) | 50 (64%) | 0.383 | 0.022 |
| Native aortic root | |||||
| At randomization | 97 (63%) | 51 (68%) | 46 (59%) | 0.188 | 0.314 |
| Aortic dimension by MRI | n = 97 | n = 51 | n = 46 | ||
| Native aortic root (mm) | 45 (40–48) | 46 (40–48) | 44 (40–49) | 0.022 | 0.831 |
| Ascending aorta (mm) | 28 (25–30) | 27 (25–31) | 28 (26–30) | 0.061 | 0.616 |
| n = 153 | n = 75 | n = 78 | |||
| Aortic arch (mm) | 24 (22–26) | 24 (22–26) | 25 (23–27) | 0.282 | 0.082 |
| Descending aorta (mm) | 21 (19–23) | 21 (18–23) | 22 (20–23) | 0.173 | 0.197 |
The mean arterial pressure 3 years after inclusion was 86 mmHg in the losartan group and 87 mmHg in the control group. No significant differences in baseline characteristics were shown, except for age (P = 0.031) and β-blocker use (P = 0.022).
To ensure generalizability of the original randomization in the COMPARE study (n = 233) to the current main analysis (n = 153) we compared baseline characteristics of the current losartan group vs. the losartan patients not eligible for our current analysis within the COMPARE cohort. Similarly, the current control group was compared to the control patients not eligible for our current analysis. In both comparisons the number of patients with a native aortic root at randomization was lower in patients included in the current main analysis than in the patients excluded from our current analysis (native aortic root in current study losartan group 68% vs. excluded patients losartan group 92%, current study control group 59% vs. excluded patients control group 89%). So presumably, patients included in our current main analysis were more at risk for complications (because more patients operated), however at similar risk in both treatment groups (losartan vs. control, P = 0.314). Further baseline characteristics were not significantly different for both analyses.
Clinical endpoints
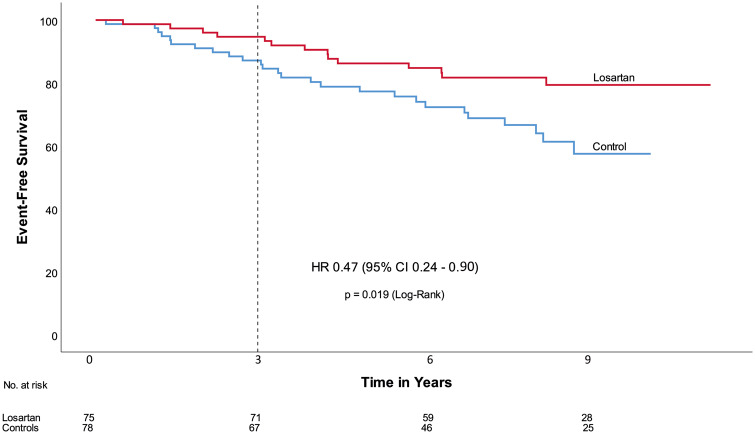
Among the 153 patients, five deaths (four with a cardiovascular cause), and 14 aortic dissections (13 Type B dissections, and one dissection of unknown origin) occurred during follow-up. Causes of death were aortic dissection/rupture (n = 3), bowel ischaemia (n = 1), and cancer (n = 1). All-cause mortality occurred in none of the patients in the losartan group, vs. five patients in the control group (6%) (HR 0.01, 95% CI 0.00–17.4, P = 0.014) (Table 3). Cardiovascular mortality (n = 4) differed also significantly between the two groups (unadjusted, HR 0.09, 95% CI 0.00–0.86, P = 0.033). Aortic dissection/rupture occurred in three patients of the losartan group (4%), vs. 11 patients in the control group (14%) (HR 0.23, 95% CI 0.06–0.81, P = 0.013). Among the 97 patients who had a native aortic root at randomization, 23 (24%) underwent aortic root replacement during follow-up, 10 (20%) in the losartan group vs. 13(28%) in the control group (HR 0.63, 95% CI 0.28–1.4, P = 0.264). Reoperation (n = 3) was more frequently encountered in the control group (n = 2) than in the losartan group (n = 1) (HR 0.42, 95% CI 0.04–4.60, P = 0.463). The same was shown for vascular grafts beyond the aortic root (losartan n = 0, control n = 3, HR 0.01, 95% CI 0.0–153.2). The one patient in the losartan group that underwent reoperation actually had a MYH11 mutation and underwent a transcatheter aortic valve implantation procedure in a severely calcified aortic homograft. In the control group, from the thirteen patients who underwent elective aortic root surgery, one patient had a MYH11 mutation and one patient a TGFB2 mutation. Other patients with TGFβ-related mutations did not reach endpoints. Finally, losartan treatment resulted in a significant reduction in the composite endpoint (HR 0.47, 95% CI 0.24–0.90, P = 0.019) (Take home figure). The same was observed when corrected for age, sex, mean arterial pressure, β-blocker use, and presence of a native aortic root at randomization, except for cardiovascular mortality (P = 0.070) (Table 4). Univariate analyses of age, as well as β-blocker use on clinical endpoints did not show any significant effect.
Table 3.
Clinical endpoints of 153 patients included in the main analyses
| Total (n = 153) | Losartan (n = 75) | Control (n = 78) | Unadjusted Cox Regression |
||
|---|---|---|---|---|---|
| HR (95% CI) | P-valuea | ||||
| Clinical endpoints | |||||
| Root replacement (n = 97) | 23 (24%) | 10 (20%) | 13 (28%) | 0.63 (0.28–1.43) | 0.264 |
| Reoperation | 3 (2%) | 1 (1%) | 2 (3%) | 0.42 (0.04–4.60) | 0.463 |
| Operation beyond the aortic root | 3 (2%) | 0 | 3 (4%) | 0.01 (0.0–153.2) | 0.071 |
| Aortic dissection | 14 (9%) | 3 (4%) | 11 (14%) | 0.23 (0.06–0.81) | 0.013 |
| All-cause mortality | 5 (3%) | 0 | 5 (6%) | 0.01 (0.00–17.4) | 0.014 |
| Composite endpoint | 40 (26%) | 14 (19%) | 26 (33%) | 0.47 (0.24–0.90) | 0.019 |
P-value of the Score-test of the Cox regression model.
Take home figure.
Event free survival. Time = 0 refers to the date of randomization. The dotted line indicates the end of the initial COMPARE trial period. CI, confidence interval; HR, hazard ratio.
Table 4.
Clinical endpoints of 153 patients included in the main analyses, adjusted for age, sex, mean arterial pressure, β-blocker use, and native aortic root at randomization
| Total (n = 153) | Losartan (n = 75) | Control (n = 78) | Adjusted Cox regression |
||
|---|---|---|---|---|---|
| HR (95% CI) | P-valuea | ||||
| Clinical endpoints | |||||
| Root replacement (n = 97) | 23 (24%) | 10 (20%) | 13 (28%) | 0.50 (0.21–1.22) | 0.058 |
| Reoperation | 3 (2%) | 1 (1%) | 2 (3%) | 0.41 (0.03–5.55) | 0.522 |
| Operation beyond the aortic root | 3 (2%) | 0 | 3 (4%) | 0 (0–1.67E+181) | 0.015 |
| Aortic dissection | 14 (9%) | 3 (4%) | 11 (14%) | 0.26 (0.07–0.94) | 0.001 |
| All-cause mortality | 5 (3%) | 0 | 5 (6%) | 0 (0–3.13E+162) | 0.018 |
| Composite endpoint | 40 (26%) | 14 (19%) | 26 (33%) | 0.43 (0.22–0.84) | 0.064 |
P-value of the Score-test of the Cox regression model.
Supplementary analyses on the original cohort revealed similar results (Supplementary material online, Tables S1 and S2, Figure S1). In a sensitivity analysis, we performed a time-dependent Cox regression analysis in the total cohort, with losartan use as time-varying covariate. Similar results were seen for all endpoints, with an HR of 0.59 (95% CI 0.36–0.99) for the composite endpoint (Supplementary material online, Table S3).
Discussion
This study, with a median follow-up period of 8 years, is the first to show a clinical benefit associated with losartan treatment in MFS patients, after our previous report on the possible protective effect of losartan on the occurrence of Type B dissections in an earlier study.16 Although losartan treatment in MFS patients has not fulfilled the expectations based on the impressive results in the MFS mouse experiments, our study provides arguments supporting the assumption that there is a place for ARB treatment in addition to β-blocker treatment in MFS patients.
The fact that patients in the control group were significantly older than patients in the losartan group could have influenced our results. However, no significant effect of age on the defined endpoints could be found and correction for age did only affect the endpoint ‘cardiovascular mortality’ by multivariate analysis (although all these four cardiovascular deaths occurred in the control group). Moreover, our supplementary analyses on the original cohort, where no difference in age existed, showed the same results as in our primary analyses. Therefore, we feel assured that the influence of age on our results has been minimal and not significant as a whole. The same counts for β-blocker use at any time, which was significantly more frequent in the losartan group. Although dosages of β-blockers were highly variable and in no case at the level of those applied in the Pediatric Heart study12 a beneficial effect of β-blockers on clinical endpoints cannot be ruled out on the basis of our results. As such, it only supports our assumption that β-blockers and losartan should be combined for the optimal protective effect on clinical endpoints in MFS patients.
It has been shown previously that MFS patients with prior prophylactic aortic surgery are more at risk for Type B dissection, even when the descending aorta is only slightly dilated.16 We could replicate these findings as all aortic complications occurred in operated patients. In addition, we have shown that almost all dissections (which were mostly Type B dissections) occurred in patients without losartan, suggesting losartan could be protective for Type B dissections without a previously dilated descending aorta. The fact that more patients with an operated aortic root were present in the control cohort could have influenced our results. However, again our findings on the effect of losartan treatment are strengthened by multivariate analysis where the presence of an operated aortic root did not change the results, and also by our secondary analyses, where there was no difference between groups in the presence of an operated aortic root.
The original COMPARE study showed a small but significant effect of losartan treatment on aortic root dilatation rate in adults with MFS during a follow-up period of 3 years. However, this effect was not universally replicated by other randomized controlled trials, thereby casting doubt over the efficacy of losartan or other ARBs for treatment of aortic disease in MFS patients. The lack of a superior effect on aortic dilatation rate of ARBs over β-blockers in these studies could be due to the relatively short follow-up periods and low aortic dilatation rates, suggesting mildly affected individuals.10,11 Aortic imaging by echocardiography [and not by magnetic resonance imaging (MRI) as in the COMPARE study] may have been insufficient to detect these very low aortic root dilatation rates. The Spanish LOAT trial however, where patients were randomized to either losartan or atenolol, also found no differences in aortic root dilatation rates by MRI.11 The authors of the LOAT trial recently published an extension of the trial (with a mean follow-up of 6.7 ± 1.5 years) which did not show a difference with regard to clinical events or aortic root growth between the losartan and atenolol group, yet there was a trend towards a beneficial effect of losartan over atenolol.17 These findings may imply that a combined use of a β-blocker and ARB is more beneficial than β-blocker or ARB alone. Although β-blocker use did not show a significant difference with regard to clinical events in this study, it showed a trend towards a beneficial effect. This strengthens the hypothesis that a combination of losartan and β-blocker treatment could be advantageous for MFS patients. This theory is further supported by the results from the recently published double-blinded randomized AIMS trial, where 56% of the patients received a β-blocker, and a beneficial effect of irbesartan was demonstrated. During 5-year follow-up, the author showed a slower aortic root dilatation rate in the irbesartan group compared to the placebo group with a statistically significant reduction of 0.22 mm per year (P = 0.030).13
In our cohort, FBN1 mutations were detected in 73% in the losartan group and in 78% in the control group, thus equally distributed over both groups. Tuning in on the two FBN1 mutation subtypes (haploinsufficient, HI or dominant negative, DN mutations), there was no significant difference in the distribution between treatment groups (Table 2). Due to the relative limited number of patients reaching an endpoint it was not possible to assess medication effects on the genetic HI and DN subgroups of FBN1 mutations.
Despite our encouraging results, the modest number of events in relatively small selected populations may compel us to await further evidence on ARBs and clinical events in MFS. For example, the upcoming randomized controlled trial by Gambarin et al.,18 which randomizes a planned 291 patients between three treatment arms: (i) losartan, (ii) nebivolol, and (iii) losartan plus nebivolol, with a follow-up of 4 years. Furthermore, interesting data has yet to come from the Marfan Treatment Trialists’ Collaboration, which will perform a prospective, meta-analysis based on individual patient data from all randomized trials in MFS of (i) ARBs vs. placebo (or open-label control) and (ii) ARBs vs. β-blockers.19 The results of these two studies are of paramount importance, possibly generating sufficient evidence to come to a conclusive advice with regard to ARB therapy for MFS patients. In the meantime, losartan could already be considered an acceptable treatment option for MFS patients, preferably in combination with β-blockers, or when β-blockers are not well tolerated.
Limitations
The limitations of the study include its retrospective design and the fact that information about medication use was obtained from patient files, which resulted in a lack of granular information about the patterns of drug use and reasoning for interrupted or stopped treatment among study participants.
There was no a priori hypothesis for long-term follow-up defined. Therefore, potential effects with respect to the original allocation have diluted over time and patient-crossover has occurred, for which no additional analyses have been performed.
In previous studies, it has been shown that the risk of cardiovascular complications increases with age in MFS patients. Therefore, uniformity in age between the two groups in the main analyses would have strengthened our results even more.
Furthermore, in the original trial, patients were eligible for inclusion if they were diagnosed with MFS according to the Ghent criteria of 1996. These guidelines have been revised in 2010 and more elaborate genetic testing has become increasingly available since then. Therefore, our study population included seven patients with another mutation than in the FBN1 gene, which would qualify for a different diagnosis under current guidelines: MYH11 mutations [familial thoracic aortic aneurysm disease (FTAAD)]; TGFB2, TGFBR1, and TGFBR2 mutations (Loeys–Dietz syndrome).20 However, omitting these patients from our study population did not influence our results. Thereby, many of the TGF-β related aortopathies are treated in concordance with Marfan guidelines.
Conclusion
Treatment with losartan is associated with an overall improved clinical outcome in patients with MFS after >8 years follow-up. Until more prospective long-term data become available, losartan treatment, preferably in combination with β-blockers, should be considered as a suitable treatment option for MFS patients.
Data availability
The datasets analyzed during the current study will become available from the corresponding author on reasonable non-commercial request.
Funding
This work was supported by the AMC Foundation.
Conflict of interest: R.B. and V.d.W. obtained grants from the AMC Foundation, supporting M.M.v.A., R.I., and H.J. during the conduct of the study. All other authors have declared no conflict of interest.
Supplementary Material
References
- 1. Murdoch JL, Walker BA, Halpern BL, Kuzma JW, McKusick VA.. Life expectancy and causes of death in the Marfan syndrome. N Engl J Med 1972;286:804–808. [DOI] [PubMed] [Google Scholar]
- 2. Finkbohner R, Johnston D, Crawford ES, Coselli J, Milewicz DM.. Marfan syndrome. Long-term survival and complications after aortic aneurysm repair. Circulation 1995;91:728–733. [DOI] [PubMed] [Google Scholar]
- 3. Silverman DI, Burton KJ, Gray J, Bosner MS, Kouchoukos NT, Roman MJ, Boxer M, Devereux RB, Tsipouras P.. Life expectancy in the Marfan syndrome. Am J Cardiol 1995;75:157–160. [DOI] [PubMed] [Google Scholar]
- 4. Selamet Tierney ES, Feingold B, Printz BF, Park SC, Graham D, Kleinman CS, Mahnke CB, Timchak DM, Neches WH, Gersony WM.. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with Marfan syndrome. J Pediatr 2007;150:77–82. [DOI] [PubMed] [Google Scholar]
- 5. Ladouceur M, Fermanian C, Lupoglazoff JM, Edouard T, Dulac Y, Acar P, Magnier S, Jondeau G.. Effect of beta-blockade on ascending aortic dilatation in children with the Marfan syndrome. Am J Cardiol 2007;99:406–409. [DOI] [PubMed] [Google Scholar]
- 6. Shores J, Berger KR, Murphy EA, Pyeritz RE.. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med 1994;330:1335–1341. [DOI] [PubMed] [Google Scholar]
- 7. Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC.. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science (New York, NY) 2006;312:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Groenink M, den Hartog AW, Franken R, Radonic T, de Waard V, Timmermans J, Scholte AJ, van den Berg MP, Spijkerboer AM, Marquering HA, Zwinderman AH, Mulder BJ.. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013;34:3491–3500. [DOI] [PubMed] [Google Scholar]
- 9. Chiu HH, Wu MH, Wang JK, Lu CW, Chiu SN, Chen CA, Lin MT, Hu FC.. Losartan added to beta-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013;88:271–276. [DOI] [PubMed] [Google Scholar]
- 10. Milleron O, Arnoult F, Ropers J, Aegerter P, Detaint D, Delorme G, Attias D, Tubach F, Dupuis-Girod S, Plauchu H, Barthelet M, Sassolas F, Pangaud N, Naudion S, Thomas-Chabaneix J, Dulac Y, Edouard T, Wolf JE, Faivre L, Odent S, Basquin A, Habib G, Collignon P, Boileau C, Jondeau G.. Marfan Sartan: a randomized, double-blind, placebo-controlled trial. Eur Heart J 2015;36:2160–2166. [DOI] [PubMed] [Google Scholar]
- 11. Forteza A, Evangelista A, Sánchez V, Teixidó-Turà G, Sanz P, Gutiérrez L, Gracia T, Centeno J, Rodríguez-Palomares J, Rufilanchas JJ, Cortina J, Ferreira-González I, García-Dorado D.. Efficacy of losartan vs. atenolol for the prevention of aortic dilation in Marfan syndrome: a randomized clinical trial. Eur Heart J 2016;37:978–985. [DOI] [PubMed] [Google Scholar]
- 12. Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD, Pearson GD, Selamet Tierney ES, Levine JC, Atz AM, Benson DW, Braverman AC, Chen S, De Backer J, Gelb BD, Grossfeld PD, Klein GL, Lai WW, Liou A, Loeys BL, Markham LW, Olson AK, Paridon SM, Pemberton VL, Pierpont ME, Pyeritz RE, Radojewski E, Roman MJ, Sharkey AM, Stylianou MP, Wechsler SB, Young LT, Mahony L, Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014;371:2061–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mullen M, Jin XY, Child A, Stuart AG, Dodd M, Aragon-Martin JA, Gaze D, Kiotsekoglou A, Yuan L, Hu J, Foley C, Van Dyck L, Knight R, Clayton T, Swan L, Thomson JDR, Erdem G, Crossman D, Flather M; AIMS Investigators. Irbesartan in Marfan syndrome (AIMS): a double-blind, placebo-controlled randomised trial. Lancet (London, England) 2019;394:2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England) 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 15. Radonic T, de Witte P, Baars MJ, Zwinderman AH, Mulder BJ, Groenink M; COMPARE study group. Losartan therapy in adults with Marfan syndrome: study protocol of the multi-center randomized controlled COMPARE trial. Trials 2010;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. den Hartog AW, Franken R, Zwinderman AH, Timmermans J, Scholte AJ, van den Berg MP, de Waard V, Pals G, Mulder BJ, Groenink M.. The risk for type B aortic dissection in Marfan syndrome. J Am Coll Cardiol 2015;65:246–254. [DOI] [PubMed] [Google Scholar]
- 17. Teixido-Tura G, Forteza A, Rodríguez-Palomares J, González Mirelis J, Gutiérrez L, Sánchez V, Ibáñez B, García-Dorado D, Evangelista A.. Losartan versus atenolol for prevention of aortic dilation in patients with Marfan syndrome. J Am Coll Cardiol 2018;72:1613–1618. [DOI] [PubMed] [Google Scholar]
- 18. Gambarin FI, Favalli V, Serio A, Regazzi M, Pasotti M, Klersy C, Dore R, Mannarino S, Vigano M, Odero A, Amato S, Tavazzi L, Arbustini E.. Rationale and design of a trial evaluating the effects of losartan vs. nebivolol vs. the association of both on the progression of aortic root dilation in Marfan syndrome with FBN1 gene mutations. J Cardiovasc Med (Hagerstown) 2009;10:354–362. [DOI] [PubMed] [Google Scholar]
- 19. Pitcher A, Emberson J, Lacro RV, Sleeper LA, Stylianou M, Mahony L, Pearson GD, Groenink M, Mulder BJ, Zwinderman AH, De Backer J, De Paepe AM, Arbustini E, Erdem G, Jin XY, Flather MD, Mullen MJ, Child AH, Forteza A, Evangelista A, Chiu HH, Wu MH, Sandor G, Bhatt AB, Creager MA, Devereux RB, Loeys B, Forfar JC, Neubauer S, Watkins H, Boileau C, Jondeau G, Dietz HC, Baigent C.. Design and rationale of a prospective, collaborative meta-analysis of all randomized controlled trials of angiotensin receptor antagonists in Marfan syndrome, based on individual patient data: a report from the Marfan Treatment Trialists’ Collaboration. Am Heart J 2015;169:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MacCarrick G, Black JH 3rd, Bowdin S, El-Hamamsy I, Frischmeyer-Guerrerio PA, Guerrerio AL, Sponseller PD, Loeys B, Dietz HC 3rd.. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med 2014;16:576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study will become available from the corresponding author on reasonable non-commercial request.