Abstract
Polymer-based nanocapsules have been widely studied as a potential drug delivery system in recent years. Nanocapsules—as one of kind nanoparticle—provide a unique nanostructure, consisting of a liquid/solid core with a polymeric shell. This is of increasing interest in drug delivery applications. In this review, nanocapsules delivery systems studied in last decade are reviewed, along with nanocapsule formulation, characterizations of physical/chemical/biologic properties and applications. Furthermore, the challenges and opportunities of nanocapsules applications are also proposed.
Keywords: polymeric nanocapsule, drug delivery system, encapsulation, nanotechnology
1. Introduction
Over the past century, nanotechnology has increasingly acquired a crucial role in drug delivery [1,2], diagnostic [3,4], biomedical imaging [5,6] and other medicine-related domains [7,8,9]. Employing nanoparticles as delivery system—currently a hot topic of nanotechnology in medical applications—has been widely studied and developed due to their biocompatibility, controlled- and targeted-release abilities [10,11]. Nanoparticles are generally defined as “solid colloidal particles with nano-dimension size (1–1000 nm)” [12,13]. Nevertheless, in the literature, the most common nanoparticle size referred to is between 100–500 nm, in order to avoid fast clearance upon intravenous administration, prolong circulation half-life, and at the same time, increase the probability of crossing various biologic barriers and preventing accumulation in capillaries and/or other organs [14,15]. Polymeric nanoparticles can not only modulate the pharmacokinetic properties of various active substances due to the subcellular size of nanoparticles, but also affect biocompatibility and/or biodegradability of polymers employed to produce the nanoparticles. Depending on their internal structure, polymeric nanoparticles may be further classified as nanospheres or nanocapsules [16]. As suggested by the name, a polymeric nanosphere has usually a regular sphere structure, which is composed of a solid polymeric matrix. In the other hand, a polymeric nanocapsule consists of a liquid/solid core coated with a polymeric shell, which is absent in the nanosphere [17]. In recent years, polymeric nanocapsules have attracted more interest in drug delivery applications, benefitting from their core-shell microstructure. Compared with polymeric nanospheres, the solid/oil core of nanocapsules can effectively increase drug-loading efficiency, while reduce the polymeric matrix content of nanoparticles [18]. In addition, the encapsulated payload can be isolated from tissue environment by the polymeric shell, thereby avoiding the degradation or burst release induced by pH, temperature, enzymes and other factors. Additionally, the polymeric shell can be functionalized by smart molecules able to interact with targeted biomolecules, thus enabling for targeting drug delivery [19,20,21].
Benefitting from above advantages, polymeric nanocapsules have been increased interest and applied in pharmaceutical field as drug delivery carriers. Since several specialized reviews have already discussed in-depth the nanotechnologies and polymeric materials for the formulation of nanocapsules [22,23], in the present review, we focused our attention on the nanocapsules delivery systems developed in last decade. Furthermore, the challenges and opportunities of nanocapsules applications will be heighted and discussed.
2. Current Status of Nanocapsules: Materials and Formulation Techniques
2.1. Common Materials for Preparing Polymeric Nanocapsules
2.1.1. Polymeric Shell
Shell materials play a critical role in the development of polymeric nanocapsules to load, protect and release bioactive substances. The properties of polymers exert significant influence on the stability, encapsulation efficiency, release profile and biodistribution of the nanocapsule as drug delivery system. Biocompatible polymeric materials have been extensively considered as appropriate candidates for nanocapsules development. In most cases, these polymers should be biodegradable for the goal of payload-releasing and elimination of nanoparticles. However, non-biodegradable, but biocompatible polymers such as polyethylene glycol (PEG), polyvinyl alcohol (PVA) have also been widely used to contribute to the fabrication of nanoparticles. They can assist drug release via diffusion, thanks to their hydrophilicity. In addition, they could be cleared from blood via the reticuloendothelial system, eventually—despite not being degraded in smaller molecules [24,25]. To satisfy the different application requirements, various polymers have been employed for the formulation of nanocapsules shell; they can be classified into natural or synthetic polymers according to their source.
Polysaccharides—one of the most important categories of natural polymeric materials—have been broadly used as drug carriers, profiting from biocompatibility, gelation conditions and mucoadhesive properties. In general, polysaccharides are rich in deprotonated amino groups or carboxylic acid groups, resulting in cationic or anionic charges, thereby to form the polymeric shell by electrostatic attractive interactions [26,27,28].
Chitosan, as one of common natural polymers has been broadly used as drug carrier profiting from the biocompatibility, endogenous metabolizable degradable productions, gelation conditions and mucoadhesive properties [29,30]. Nanocapsules formed or covered by chitosan can process a cationic surface charge benefiting from the rich amino groups of chitosan. The cationic surface can improve the interaction between nanoparticles and the bacteria which process negative surface through electrostatic interactions. Poly (lactic -co-glycolic acid) (PLGA) nanocapsules with chitosan shell exhibited a better attachment to the S. aureus and M. abscessus compared to ones without chitosan [31]. Thereby, chitosan-based nanocapsules have been developed as drug delivery system for infectious disease [32]. However, for different applications, the strong cationic surface charge may induce nanoparticle aggregation, protein adsorption, as well as fast removal from the blood circulation [26,27]. Therefore, several anionic polymers have been used in cooperation with chitosan to act as nanocapsules shells in order to solve this problem, such as poly (acrylic acid) (PAA) [33], poly (vinyl alcohol) (PVA) [34] and also anionic polysaccharides [35,36].
Alginate is one of the anionic natural polysaccharides which has been developed as drug carrier, taking advantages of its biocompatibility, low immunogenicity and mild gelation conditions [37,38,39]. In addition to these well-known advantages, alginate is also a pH-responsive polymer, which can provide effective protection for payloads at acidic pH conditions, while achieving drug release at alkaline pH environment [40]. Thereby, nanocapsules fabricated by alginate have been developed as promising strategy for intestinal targeted drug delivery via oral administration [41].
As a biocompatible and biodegradable polyanionic polymer, dextran sulfate has been widely used in pharmaceutical field in drug delivery application [42]. Dextran sulfate is usually associated with chitosan to form multilayer nanocapsules by electrostatic integration [43]. Nanocapsules based on chitosan–dextran sulfate showed a good stability without the need of extra covalent agents [44]. Additionally, the drug release behavior can be modulated by controlling the ratio between chitosan and dextran. Chitosan–dextran nanoparticles formed with higher percentage of carboxymethyl dextran can increase the nanoparticle dissociation, thereby, increasing the gene release in serum or cytoplasm [45,46].
Moreover, common polysaccharides stated above, poly(cyclodextrin) [47], heparin [48] hyaluronan [49] and also other polysaccharides have been used to prepare nanocapsules as drug carrier for different pharmaceutic applications.
Protein-based polymers, as another kind of natural macromolecules, have been processed as polymeric shell for nanocapsules, due to their biocompatibility and tunable properties [50,51]. Albumin is a water soluble and biodegradable protein which shows critical role in the circulating system [52]. Human serum albumin has served as shell for nanocapsules. Besides controlling drug permeation rate, albumin corona can reduce the immunogenicity of nanoparticles and thus assist them to escape from the reorganization of reticuloendothelial system. Due to its biogenic properties, albumin can also serve as targeting ligand for albondin receptors which are overexpressed on endothelial cells of tumor blood vessels, providing an excellent drug targeting system [53]. Additionally, protein can be engineered into a hollow caged nanostructure by self-assemble of a defined number of subunits. These virus-like biomimetic nanocapsules can provide a very narrow size distribution and act as drug delivery system. For example, an HspG41C mutant protein-based nanocapsule formed through self-assembly of 24 individual monomeric proteins was developed as drug carrier for anti-cancer drug doxorubicin. The HspG41C nanocapsule can be easily prepared by self-assemble procedure in water environment and processed a particle size around 12 nm with narrow size distribution. It showed a good biocompatibility and successfully delivered doxorubicin to various cancer cell lines [54]. Another protein-based caged nanostructure as drug delivery system of doxorubicin was developed by Ren et al. [55]. It was fabricated through self-assembling of 60 dihydrolipolyl acyltransferase subunits (E2) and obtained a particle size around 25 nm.
Synthesized materials were indicated advantages over natural materials since they have reproducible quality and purity. In addition, they can be tuned with different chemical ionic, mechanical, solubility and degradability properties, adjusted according to the different pharmaceutical applications [56].
Aliphatic polyesters and relative copolymers are very common synthetic polymers and have been deeply studied and developed as drug delivery systems, due to their biocompatibility and biodegradability. The most common polyesters include poly (lactic acid) (PLA), poly (lactic -co-glycolic acid) (PLGA), poly(ε-caprolactone) (PCL). Compared with PLA and PLGA copolymers, PCLs can provide much longer degradation period. Therefore, PCLs are more suitable for long term drug delivery system or medical applications. In addition, PCLs have lower cost than PLAs and PLGAs, which is also an advantage according to some research work [19,31,57,58,59].
Additionally, Eudragit® polymers are a series of synthetic polymers with different ratios of acrylate and methacrylate portions, and are used as coating materials for various kinds of dosage forms in pharmaceutical applications due to their biocompatibility, biodegradability and flexible ionic properties [56]. Eudragit® polymers have been considered for the formulation of nanocapsules shell. For example, Eudragit® RS100 has been used to form a polymeric shell for the delivery of dihydromyricentin. Ethyl acrylate, methyl methacrylate and a methacrylic acid esters comprise Eudragit® RS100, which can provide 4.5%–6.8% quaternary ammonium groups [60]. The positive charge contributed by ammonium groups of Eudragit® RS100 can neutralize the negative charge of DNA thereby crossing the cell membrane without the molecule being damaged [61].
Moreover, poly (ethylene glycol) (PEG), a biocompatible hydrophilic synthesized polymer, has been widely used as polymeric coating for nanocapsules. PEGylation can be achieved either via self-assemble nanocapsule formulation by amphiphilic PEG copolymer [62], such PLGA-PEG copolymer, or PEG coated after nanocapsules formed [63]. It has been revealed that nanocapsules with PEG exposed on the surface can improve the stability of nanocapsules in biologic media and reduce the immunogenicity [30,64,65].
2.1.2. Liquid/Solid/Hollow Core
The core of nanocapsules can be hollow or consists in a liquid or solid phase, thus making it able to carry different drugs.
Nanocapsules can present an oleic core highly suitable for the encapsulation of lipophilic molecules [30]. Regarding the oily core, vegetable oil (such as soybean oil, palm oil, etc.) or fatty acids (such as medium chain triglycerides) are optimal composition oleic phase for nanocapsules fabrication, due to the capacity to dissolve lipophilic drugs and the safety of oil phase [31,66,67]. Aside from being drug dissolving media, the oil, which can simultaneously provide a therapeutic functionality, is a prompting candidate to form oleic core of nanocapsules. Copaiba oil has been used as oily core of a PCL nanocapsule in order to increase the solubility of imiquimod which is a hydrophobic anti-cancer drug. Meanwhile, copaiba oil benefits for treatments of neoplastic melanoma, micropapillary carcinoma and also has function for anti-inflammatory and analgesia [17,68]. Eudragit® RL 100 nanocapsules loaded with the anti-inflammation desonide drug were developed using açai oil (AO) as oily core. More than be oil core for drug encapsulation, AO also presented anti-inflammatory and anti-proliferative effects contributing to the therapy [67]. Essential oils, such as turmeric oil and lemongrass oil, have been used as oil core due to their antibacterial, antifungal, antioxidant, antimutagenic and anticarcinogenic properties [69].
Polymeric nanocapsules can also be manufactured with aqueous core to act as promoting platform for sustained delivery of hydrophilic molecules [70]. Hydrophilic anti-cancer drugs, such as gemcitabine hydrochloride and doxorubicin, have been successfully encapsulated into polymeric nanocapsules with aqueous core [71,72]. Compared with free drug, drug-loaded nanocapsules presented higher anti-cancer effect. Additionally, the polymeric nanocapsules containing an aqueous core can also effectively encapsulate and protect the hydrophilic bioactive substance. For example, the clinical applications of mono- or oligo-nucleotides presented have been limited due to their low stability and membrane permeability in the biologic environment. The encapsulation into the aqueous core of polymeric nanocapsules can successfully protect them from the degradation in biologic fluids and promote the intracellular penetration, thereby, improve the bioavailability [73,74]. Moreover, the polymeric nanocapsules containing aqueous core can be also considered as potential delivery system for water soluble protein, such as albumin [75].
Moreover, nanocapsules can also be characterized by a hollow internal structure [76,77]. In general, nanocapsules with a hollow core are fabricated by first preparing a solid sphere that is subsequently sacrificed after polymeric shell formation. The use of solid sphere as sacrificial template can specifically provide a strong spherical framework for the assembly of nanocapsules, consisting in multiple polymer shells. Removal of the solid sphere template to provide a hollow core is necessary to modulate the in vivo drug release, and also improve the biocompatibility of the nanocapsules. The materials considered as the template core should be easily removed by mild conditions in order to avoid shell damage. Metallic materials such as iron oxide [78] and gold [33,47] spheres were used as template core and are able to be removed by hydrochloric acid solution or potassium cyanide solution after polysaccharide shell is formed. Calcium carbonate as an inorganic candidate template core of nanocapsules can be removed by adding ethylene diamine tetra acetic acid into a nanocapsule water dispersion at pH 7 [79]. In addition, silica is another inorganic material generally used as template core and removable by complete dissolution in hydrofluoric acid [80]. Additionally, polystyrene could be manufactured into nanospheres by nanoemulsion and treated with trichloromethane that is removed providing a cavity [81].
Finally, the inner core of nanocapsules can also be assembled into solid state polymeric matrix to provide loading capability for different payloads [82]. For example, nanocapsules with pectin gel core have been developed as drug delivery system for the glaucoma treatment via ocular delivery [83]. Benefiting from the porous structure and hydrophilic characteristic of pectin gel, nanocapsules exhibited efficient drug-loading yield and good patient compliance for ocular administration. However, compared with nanocapsules with oleic core or hollow core, the studies for the nanocapsules with solid core are still underdeveloped.
2.2. Polymeric Nanocapsule Formulation
Polymeric nanocapsule formulation techniques were exhaustively reported by Couvreur et al. [84], Mora-Huertas et al. [22] and Kothamasu et al. [23] a decade ago. Three main classical methods were described: interfacial deposition method, nanoemulsion template method and layer-by-layer method. There is no significant technical transformation during the subsequent decade and the existing three strategies have already met the most requirements of nanocapsules fabrication. The innovations in nanocapsule formulation are more focused on creative utilization of materials in order to satisfy different purposes of drug delivery applications. Various new nanocapsules created by using materials with different performances, can provide multiple loading and/or releasing capacities for encapsulated active substances. In this case, as a contribution to updating the state of knowledge without repetition, the latest developments of nanocapsules for drug delivery system in recent are summarized along with brief introduction of the classical formulation techniques.
2.2.1. Interfacial Deposition Method
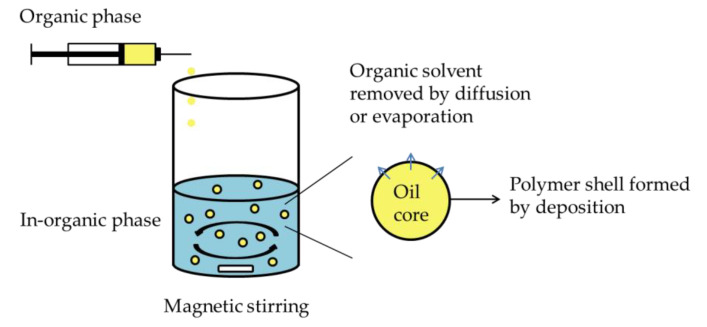
Interfacial deposition method, as well as nanoprecipitation, was extensively used to fabricate nanocapsules. The method was first mentioned by Fessi et al. [85] in 1989. The general nanocapsule formulation by interfacial deposition of performed polymer is described in Figure 1. Using interfacial deposition methods to prepare nanocapsules needs both organic and aqueous phase. In general, organic phase is prepared by dissolving the oil and the payload into appropriate organic solvents and subsequently adding through a thin needle into the aqueous phase. Film formed polymeric substance can be dissolved in organic or water phase according to the property of the polymer. One or more surfactants could be supplemented to increase the nanocapsules stability. Eventually, the water suspension of formed nanocapsules is obtained by removing organic solvent through diffusion or evaporation. The nanocapsules characteristics can be mainly influenced by polymer concentration, injection method of organic phase, volume ratio between organic phase and aqueous phase and also nature of materials [86,87,88]. The interfacial deposition method has been used intensively in the last decade, due to the simple operation without applied external high energy force and extensive applicability for various payloads. For example, Veragten et al. developed an olanzapine-loaded oleic core nanocapsules by interfacial deposition method which successfully presented a great mucin adhesion capacity and potential as a novel strategy for olanzapine oral administration [89]. A series of polymeric nanocapsules were developed by using various antimicrobial essential oils [90]. Interfacial deposition method can also formulate nanocapsules with a hollow core by using organic phase in absence of oleic oil but only organic solvents which can be removed by evaporation once the nanocapsules formed [82]. The studies of polymeric nanocapsule formulation by interfacial deposition methods in recent five years are summarized in Table 1.
Figure 1.
Schematic representation of nanocapsule formulation by interfacial deposition method.
Table 1.
Examples of polymeric nanocapsule formulation by interfacial deposition method in recent five years.
| Active Substance | Polymer Film | Core | Surfactant | Organic Phase | In-organic Phase | Particle Size (nm) |
PDI | Zeta Potential (mV) |
Drug Loading (mg/mL) |
Encapsulation Efficiency (%) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dutasteride | Poly-(ε-caprolactone) | Sorbitan monostearate Caprylic/capric triglyceride | Lecithin Polysorbate 80 |
Acetone Ethanol |
Water | 199.0 ± 0.5 | 0.12 | −13.6 ± 0.6 | - | ≥ 96.7 ± 1.8 | [124] |
| Poly-(ε-caprolactone) Chitosan |
224.9 ± 3.4 | 0.23 | +40.2 ± 0.8 | ≥94.7 ± 3.0 | |||||||
| Olanzapine | Poly-(ε-caprolactone) Chitosan |
Sorbitan monostearate Caprylic/capric triglycerides | Lipoid® S75 Polysorbate 80 |
Acetone Ethanol |
Water | 162 ± 12 | 0.24 ± 0.01 | +6.9 ± 0.7 | 1.06 | 42.2 | [89] |
| Clarithromycin | Poly (lactic -co-glycolic acid) | Medium-chain triglycerides | Lipoid® S75 Polysorbate 80 |
Acetone Ethanol |
Water | 94.9 ± 1.3 | 0.21 ± 0.02 | −28.2 ± 0.7 | 0.99 ± 0.02 | 68 ± 1.1 | [31] |
| Poly (lactic -co-glycolic acid) Chitosan | 120.6 ± 2.1 | 0.15 ± 0.01 | +16.5 ± 0.7 | 0.98 ± 0.02 | 67 ± 0.8 | ||||||
| Desonide | Eudragit® RL 100 | Açai oil | Span® 80 Polysorbate 80 |
Acetone | Water | 165 ± 2 | 0.12 ± 0.03 | +13.8 ± 0.3 | 0.26 ± 0.004 | 81.8 ± 1.8 | [67] |
| Medium chain triglyceride | 131 ± 2 | 0.15 ± 0.02 | +6.9 ± 0.7 | 0.26 ± 0.009 | 81.6 ± 0.4 | ||||||
| Imiquimod | Poly (ε-caprolactone) | Copaiba oil | Span® 60 Tween® 80 |
Acetone | Water | 242.1 ± 17 | 0.17 ± 0.1 | −8.9 ± 0.3 | 0.49 ± 0.05 | 98 ± 0.2 | [17,68] |
| 5-fluorouracil | Chitosan Poly(nvinylpyrrolidone- alt-itaconic anhydride) |
Hollow | Span® 80 Tween® 80 |
Dimethyl sulfoxide Acetone |
Water | 118 ± 3.48 | 0.32 ± 0.015 | −12.6 ± 0.15 | 0.45 ± 0.01 (g/g) | 30.0 ± 0.57 | [82] |
| AS1411 aptamer-chitosan Poly(nvinylpyrrolidone- alt-itaconic anhydride) |
133 ± 3.46 | 0.33 ± 0.020 | −19.2 ± 0.12 | 0.34 ± 0.01 (g/g) |
22.5 ± 0.86 | ||||||
| Ketoprofen | Eudragit® S100 | Rose hip oil | Span® 80- Tween® 80 |
Acetone | Water | 186 ± 14 | 0.19 ± 0.03 | −12.9 ± 2.6 | 1.00 ± 0.02 | 99.1 ± 0.5 | [125] |
| Medium chain triglycerides | 193 ± 9 | 0.19 ± 0.01 | −8.7 ± 1.5 | 0.99 ± 0.03 | 99.0 ± 0.2 | ||||||
| Cilostazol | Poly(ε-caprolactone)- Poly(ethylene glycol) |
Capric/caprylic acid triglycerides | Span® 80 Tween® 80 |
Acetone | Water | 130 ± 5.80 | 0.18 ± 0.03 | −37.6 ± 1.50 | 11,984.64 ± 0.10 (μg/mL) |
99.87 | [58] |
| Carvedilol | Poly(ε-caprolactone) | Grape seed oil | Polysorbate 80 | Acetone | Water | 182 ± 7 | -- | −7.6 ± 1 | 0.47 ± 0.002 | -- | [126] |
| Eudragit® S100 | 139 ± 6 | +7.3 ± 3 | 0.48 ± 0.01 | ||||||||
| Bedaquiline | Chitosan Poly(ethylene glycol) | Oleic acid | Span® 85 Tween® 20 |
Ethanol | Water | 455.6 ± 26 | 0.204 ± 0.020 | −9 ± 2 | 25 ± 2 (%) | -- | [30,64] |
| Chitosan | 328 ± 35 | 0.151 ± 0.027 | +26 ± 4 | 28 ± 2 (%) |
70 ± 7 | ||||||
| Indole-3-carbinol | Eudragit® S100 | Rose hip oil | Span® 8 Tween® 80 |
Acetone | Water | 334 ± 1 | < 2.0 | +10.47 ± 0.05 | 0.5 | 44 | [127] |
| Diphenyl diselenide | Poly(ε-caprolactone) | Medium chain triglycerides | Span® 80 Tween® 80 |
Acetone | Water | 240 ± 52 | 0.151 ± 0.06 | −10.9 ± 2.2 | 5 | 98.0 | [128] |
| Cloxacillin benzathine | Poly(ε-caprolactone) | Labrafac® CC | Span®80 Pluronic® F68 |
Methanol Acetone |
Water | 322.4 ± 4.0 | 0.088 ± 0.051 | −28.2 ± 0.6 | 0.183 | 46.73 ± 5.92 | [129] |
| Antimicrobial essential oil | Cellulose acetate | Peppermint | -- | Acetone | Water | ~180 | ~0.30 | ~−40 | 82 (%) | -- | [90] |
| Cinnamon | ~150 | ~0.10 | ~−40 | 14.2 (%) | |||||||
| Lemongrass | ~200 | ~0.20 | ~−35 | 155 (%) | |||||||
| p,p’-methoxyl-diphenyl diselenide | Poly(ε-caprolactone) | Medium chain triglycerides | Span® 80 Tween® 80 |
Acetone | Water | 236 ± 4 | 0.16 ± 0.019 | −5.4 ± 0.057 | 2.5 | 98.78 ± 1.54 | [130,131] |
| Calcitriol | Poly(D,L-lactic acid) | Miglyol® 829 | Montanox® VG80 | Acetone | Water | 183 ± 8 | 0.083 ± 0.028 | −21.3 ± 2.4 | -- | 86 ± 2 | [132] |
| Quercetin | Chitosan | Miglyol® 812 | Lecithin | Ethanol | Water | 190 ± 4 | 0.12 | + 48.4 ± 3.46 | -- | 99 ± 1.2 | [133] |
| Baicalein | 187 ± 2 | 0.12 | +48.1 ± 2.03 | 87 ± 5.1 | |||||||
| Meloxicam | Poly(ε-caprolactone) | Miglyol® 810 | Polysorbate 80 |
Acetone | Water | 247–212 | 0.14 | −36 | 99.9 (%) | -- | [134] |
| Quinine Curcumin |
Poly(ε-caprolactone) | Caprylic/capric triglyceride | Lipoid® S45 | Acetone | Water | 194 ± 1 | 0.119 ± 0.00 | −27.2 ± 0.1 | 98 ± 2.4 (%, Quinine) 96 ± 2.2 (%, Curcumin) |
97 ± 2.1 (Quinine) 90 ± 1.3 (Curcumin) |
[135] |
| Carvedilol | Poly(ε-caprolactone) | Grape oil Sorbitan monostearate | Polysorbate 80 | Acetone | Water | 180 ± 3 | 0.08 ± 0.01 | −6.6 ± 0.6 | -- | 99.1 ± 0.21 | [136] |
| Eudragit® RS 100 | Grape oil | 139 ± 6 | 0.14 ± 0.01 | +9.2 ± 2.4 | 88 ± 1.10 | ||||||
| Doxorubicin | Synthesized poly(ε-caprolactone)- poly [(methyl methacrylate)-co-(2-dimethylamino)ethyl methacrylate)2] | Caprylic triglyceride | Polysorbate 80 | Acetone Ethanol |
Water | 60.6 ± 0.8 | 0.19 ± 0.01 | +13.3 ± 0.9 | 11 μg/mL | 69.7 | [137] |
| Elisidepsin | Polyarginine | Miglyol® 812 Epikuron® 170 |
Poloxamer 188 | Ethanol Acetone | Water | 178 ± 15 | 0.1 | +30 ± 11 | -- | 46 ± 7 | [138] |
| Fluorescent probe DiD-labelled polyarginine | 129 ± 2 | 0.1 | +25 ± 1 | 75 ± 5 | |||||||
| Fluorescein-DHPE-labelled polyarginine | 140 ± 1 | 0.1 | +52 ± 1 | 79 ± 1 | |||||||
| Glycerol monolaurate | Polymeric blende of poly (methyl methacrylate) and poly (ethylene glycol) | Capryc/caprylic triglyceride | Sorbitan monooleate | Acetone | Water | 193.2 ± 4 | 0.044 ± 0.028 | −23.3 ± 3 | -- | -- | [139] |
| Plitidepsin | Poly-aminoacid-poly(ethylene glycol) | Epikuron® 170 Miglyol® |
Poloxamer® 188 | Acetone Ethanol |
Water | 190 ± 15 | 0.1 | −24 ± 5 | -- | 85 ± 4 | [140] |
2.2.2. Nano-emulsion Template Method
As time goes on, high energy instruments were introduced in order to preform nanoemulsion thereby further fabricating into nanocapsules of different ways, usually including organic solvent diffusion/evaporation or monomers coacervation. For nanoemulsion preparation, organic or aqueous phase is emulsified in the aqueous or organic phase in presence of a surfactant, while constant energy support, such as sonication or homogenization. During the nanoemulsion formulation procedure, surfactants are driven to self-assemble at the interface between organic and inorganic phases to reduce the interfacial tension, thereby, achieving a stable state. The shell-formed polymeric materials, active substance, oil and other functional substance may be dissolved or suspended into dispersion phase or continuous phase, according to the requirement of the formulated nanocapsules [91].
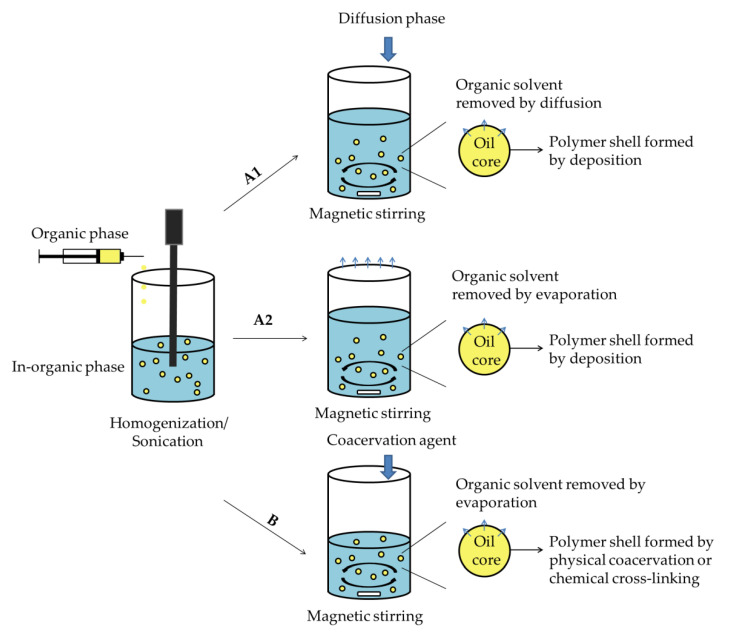
A. Emulsion–diffusion/Evaporation Method
One of the common methods for formulating polymeric nanocapsules via nanoemulsion is the emulsion–diffusion/evaporation method. The general fabrication procedure of nanocapsules via emulsion–diffusion/evaporation method is depicted in Figure 2A. It is based on emulsification of the organic phase into an inorganic phase and subsequent elimination of the organic solvent by diffusion into the external phase or evaporation [91,92]. Nanocapsules are formed by a combination of polymer precipitation and interfacial phenomena during the diffusion—or an evaporation procedure. The polymers that can be used to formulate polymeric nanocapsules by emulsion–diffusion method must possess good solubility in an organic solvent, well miscible with water, such as acetone, ethanol or ethyl acetate, thereby, removing the organic solvent by diffusion into water [93,94]. For instance, Sombra et al. developed propionated Sterculia striata polysaccharide nanocapsules with a Miglyol® L812 oleic core as carrier for amphotericin B [95]. The nanocapsules were formed by using acetone and methanol in organic phase, and subsequently purifying by diffusion into the water phase; the hydrophobic amphotericin B was successfully encapsulated with an excellent encapsulation efficiency of 99.2 ± 1.3%. Moreover, nonpolar solvents such as chloroform or dichloromethane—which are immiscible with water—may be used in the organic phase in the emulsion–evaporation method. A poly(DL-lactide-co-glycolide)-poly(ethylene glycol) nanocapsule was fabricated by dissolving the polymers into dichloromethane as organic phase, and then form nanoemulsion by homogenized organic phase into water phase [96]. The dichloromethane was purified by evaporation. It is also worth noting that the liquid core of this polymeric nanocapsule was composed by perfluorooctyl bromide which can act as cavitation nucleus under ultrasound exposure and used to monitor the nanocapsules collapse in vitro and in vivo. The studies of polymeric nanocapsule formulation by emulsion–diffusion/evaporation methods in recent five years are summarized in Table 2.
Figure 2.
Schematic representation of nanocapsule formulation by nanoemulsion methods: (A). nanoemulsion–diffusion/evaporation method; (B). nanoemulsion–coacervation method.
Table 2.
Examples of polymeric nanocapsule formulation by emulsion–diffusion/evaporation method in recent five years.
| Active Substance | Polymer Film | Core | Surfactant | Organic Phase | In-Organic Phase | Diffusion or Evaporation | Particle Size (nm) |
PDI | Zeta Potential (mv) |
Drug Loading | Encapsulation Efficiency (%) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B | Propionated Sterculia striata polysaccharide | Miglyol® L812 | -- | Acetone Methanol |
Water | Diffusion (Water) |
274.1 ± 8.6 | 0.181 | −39.8 ± 0,2 | -- | 99.2 ± 1.3 | [95,141] |
| Thymoquinone Docetaxel |
Chitosan | Oleic acid- phospholipid | Poloxamer | Ethanol | Water | Diffusion (Water) |
141.7 ± 2.8 | 0.17 ± 0.02 | −8.17 ± 0.1 | 7.7 ± 0.4 (%, Thymoquinone) 4.3 ± 0.4 (%, Docetaxel) |
85.3 ± 3.1 (Thymoquinone) 66.1 ± 3.5 (Docetaxel) |
[142] |
| Essential oil from the L. sidoides leaves | Poly(ε-caprolactone) | Essential oil Ethyl laurate |
Kolliphor® P188 | Ethyl acetate | Water | Diffusion (Water) |
173.6 | 0.2 | −42 | -- | 70.6 | [143] |
| Docetaxel | Poly (D, L-lactide) |
Labrafac® CC | Polyvinyl alcohol | Ethyl acetate | Water | Diffusion (Water) |
115–582 | < 0.05 | −36.5 ± 9 | up to 68.3% | 65–93 | [144] |
| Human neutrophil elastase inhibitor | Pregelatinized modified starch | Capric/caprylic triglycerides | Tween®80 Cetrimide |
Ethanol | water | Evaporation | 100–900 | -- | > 30 | > 0.5 (%) | > 75 | [145,146] |
| Paclitaxel Perfluorooctyl bromide |
Poly(D, L-lactide-co-glycolide)-poly(ethylene glycol) | Perfluorooctyl bromide | Sodium cholate | Dichloromethane | water | Evaporation | 120 | 0.2 | −18 ± 3 | 6 μg/mg | 15 (Paclitaxel) 40 (Perfluorooctyl bromide) |
[96,147] |
| Curcumin | Bovine serum albumin-capped gold nanoclusters | Undecylenic acid | Bovine serum albumin-capped gold nanoclusters (Self assemble) | Dichloromethane | water | Evaporation | 177.54 ± 37.11 | -- | −29.8 ± 4.86 | -- | -- | [13] |
| Catechin rich extract | Eudragit® L 100 | -- | Sodium laureth sulfate | Methanol | Lactose aqueous solution | Evaporation | 100–400 | -- | -- | -- | 40–75 | [94] |
B. Emulsion–coacervation Method
Emulsion–coacervation process is also based on nanoemulsion as template for nanocapsule formulation. However, compared with emulsion–diffusion/evaporation method, the different is the polymeric shell is formed and stabilized by either physical coacervation or chemical cross-linking [97,98,99,100] (Figure 2B). The emulsion–coacervation methods are principally used for polyelectrolyte materials or monomer/polymer possessing cross-linking function groups for nanocapsules fabrication. Electrostatic interaction as a physical coacervation strategy has been used to prepare a polyelectrolyte nanocapsules for encapsulation of brinzolamide to treat glaucoma [83]. Chitosan and pectin which carries positive and negative charges, respectively, have been prepared into nanoemulsion, coacervated with each other by electrostatic attraction, and eventually formed polymeric shell of nanocapsules. Compared with physical coacervation, polymeric shell crosslinked by chemical approach may provide more stable nanocapsule system. A cross-linked starch nanocapsules with aqueous core was developed as delivery system for hydrophilic dye by interfacial polymerization carried out using water in oil (W/O) mini-emulsion method [101]. It indicates that with higher concentration of cross-linker, the nanocapsules can form a thinker polymeric shell to minimize the leakage of hydrophilic payload to the aqueous phase. Additionally, free radical polymerization, such as reversible addition fragmentation chain transfer polymerization (RAFT) or atom transfer radical polymerization (ATRP),has been applied to develop polymeric nanocapsules via emulsion–coacervation method. Typically, an amphiphilic copolymer based on same monomer further used for polymeric shell was synthesized to act as template core and also stabilizer for nanoemulsion preparation. Subsequently, the monomer was added along with cross-linker and initiator and started to form polymeric shell via free radical polymerization. Then the pre-synthesized amphiphilic copolymer was removed by hydrolysis to provide hollow inner structure [102,103,104]. For example, pH sensitive polymeric nanocapsules were formulated by using RAFT/emulsion polymerization. A random copolymer consisting of butyl acrylate and acrylic acid was pre-synthesized as macroRAFT agent [105,106]. N,N-(dimethylamino)ethyl methacrylate or tertiary butyl methacrylate with methyl methacrylate were used to preform nanocapsules. The hollow core was obtained by hydrolysis of trifluoroacetic acid. The nanocapsules showed a rapid drug release at pH 6.5 responding to the pH sensitive property of the polymer. In addition, other chemical reactions were also applied to preform polymeric nanocapsules by emulsion–coacervation method, such sonochemical reaction [66,107] or photopolymerization [108,109]. The studies of polymeric nanocapsules formulated by emulsion–coacervation methods in recent five years are summarized in Table 3.
Table 3.
Examples of polymeric nanocapsule formulation emulsion–coacervation method in recent five years.
| Active Substance | Polymer Film | Core | Surfactant | Organic Phase | In-organic Phase | Coacervation Method | Particle Size (nm) |
PDI | Zeta Potential (mV) |
Drug Loading (%) |
Encapsulation Efficiency (%) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coumarin 6 | Folic acid decorated-ε-poly-L-lysine-SH | Soybean oil | -- | Soybean oil | Water | Sonochemical method | ~500 | -- | -- | -- | -- | [66] |
| Methotrexate | Human serum albumin Silk fibroin |
n-dodecane | -- | n-dodecane | Potassium phosphate buffer | Sonochemical method | 438–888 | -- | −8.81 - −13.43 | 76.80 ± 17.20 | 98.78 ± 0.08 | [107] |
| Brinzolamide | Chitosan Pectin |
Aqueous core | Tween® 80 | -- | Water | Electrostatic interaction | 240.05 ± 0.08 | 0.32 ± 0.08 | +27.6 ± 0.03 | 18.92 ± 0.25 | 92.20 ± 0.12 | [83] |
| Paclitaxel | Chitosan Poly(isobutyl cyanoacrylate) (Monomer: isobutyl cyanoacrylate) |
Copaiba oil | -- | Ethanol | Water | Interfacial polymerization | 486 ± 3 | 0.17 | +37.1 ± 0.3 | 1.70 ± 0.02 | 74 ± 1 | [148] |
| -- | Monomer: triethylene glycol divinely ether | N-hexadecane | Sodiumdodecyl sulfate | N-hexadecane | Water | Photopolymerization | 218 | 0.18 | −71.5 | -- | -- | [108,109] |
| Polyvinyl pyrrolidone | 293 | 0.27 | −15.1 | |||||||||
| Pluronic® PE 6100 | 308 | 0.12 | −26.8 | |||||||||
| Rhodamine B | Monomer: hydrophilic deprotonated cystamine 2,4-toluene diisocyanate |
Aqueous core | Lubrizol U |
Cyclohexane | Water | Interfacial Polyaddition reaction | 252–444 | ~0.2 | -- | -- | -- | [149] |
| Sulforhodamine 101 | Hydrophilic potato starch Cross-linker: 2,4-toluene diisocyanate |
Aqueous core | Polyglycerol polyricinoleate | Cyclohexane | Sodium chloride aqueous solution | Interfacial polymerization | ~200 | -- | -- | -- | ~70–100 | [101] |
| Coumarin 1 | Monomer: Methyl methacrylate and 2-(diethylamino)ethyl methacrylate Initiator: 2,2-azobis (isobutyronitrile) dextran-based transurf |
Miglyol® 810 | Amphiphilic dextran-based transurf | Methyl methacrylate | Water |
RAFT polymerization | 198 ± 9 | 0.15 | an almost neutral surface | 1.7 | 99 | [103] |
| Bovine serum albumin | Monomer: styrene MacroRAFT agent: Polystyrene-co-polyN-(2-Hydroxypropyl)methacrylamide Crosslinker: divinylbenzene Initiator: 2,2’-azobis(isobutyronitrile) |
Aqueous core | Polystyrene-co-polyN-(2-Hydroxypropyl)methacrylamide | Toluene n-hexane | Sodium chloride aqueous solution | RAFT polymerization | 33–202 | 0.1–0.22 | -- | -- | -- | [104] |
| Capreomycin sulfate | Monomers: N,N-(dimethylamino)ethyl methacrylate or tertiary butyl methacrylate with methyl methacrylate MacroRAFT agent: Poly (butyl acrylate)-co- acrylic acid Crosslinker: Ethylene glycol dimethacrylate Initiator: azo-initiator 4,4‘ - azobis(4-cyanovaleric acid) |
Aqueous core | Dimethyldioctadecyl ammonium bromide | -- | Water | RAFT polymerization | ~130 | -- | -- | 70 | -- | [105,106] |
| Doxorubicin | Monomer: Tert-butyl acrylate Macroinitiator: Folate-poly(ethylene glycol)-b-poly(tert-butyl acrylate) Reducing agent: ascorbic acid Crosslinker: N,N’-bis(acryloyl) cystamine Catalyst: CuBr2 |
Hollow | Folate-poly(ethylene glycol)-b-poly(tert-butyl acrylate) | Cyclohexanone/hexadecane | Water | AGET ATRP polymerization | ~150 | -- | -- | 39.7 | 99.2 | [102] |
C. Double Emulsion Method
Based on the principle of emulsion diffusion/evaporation and emulsion–coacervation method, resulting nanoemulsion can be continually emulsified into a third phase to perform emulsions of emulsions, as well as double emulsion method. Double emulsions are classified into two major types, water in oil in water (W/O/W) and oil in water in oil (O/W/O) according to the phase sequence. The critical principle of double emulsion methods is choosing suitable surfactants to provide good stability between the interfaces of both internal and external emulsion [110,111]. The polymeric shell can be formed by diffusion, evaporation, coacervation or combination of these approaches. A fluorescent polymeric nanocapsule was developed by Campos et al. by double emulsion–evaporation method [112]. Erdmann et al. developed a poly(alkyl cyanoacrylate)-based polymeric nanocapsule by double emulsion method [113]. The polymeric shell was formed by interfacial polymerization of n-butyl cyanoacrylate and propargyl cyanoacrylate. W/O/W double emulsion has been wildly applied for development of nanocapsules containing aqueous core, which is beneficial to encapsulation and sustained release of hydrophilic drugs. Additionally, nanocapsules formed by double emulsion method can provide an optimal platform for encapsulation hydrophobic and hydrophilic substances simultaneously [114]. Two anticancer drugs hydrophilic doxorubicin and hydrophobic paclitaxel were encapsulated into a poly(methacrylic acid)/polyvinyl alcohol-based nanocapsules to improve cancer therapy [115]. The hydrophilic doxorubicin was loaded in the aqueous core by dissolving in the water phase of first emulsion, while the hydrophobic paclitaxel was carried in the polymeric shell by dissolving in the organic phase. Both of the hydrophilic and hydrophobic payloads achieved excellent encapsulation efficiency around 72% and 91%, respectively. Similar result was achieved by Balan et al. that hydrophilic doxorubicin and hydrophobic magnetite were incorporated into the nanocapsules simultaneously by double emulsion methods [116]. The doxorubicin was crosslinked on the chitosan-based shell by the degradable sodium tripolyphosphate, while magnetite was solidified by solvent evaporation. The studies of polymeric nanocapsule formulation by double emulsion methods in recently ten years are summarized in Table 4.
Table 4.
Examples of polymeric nanocapsule formulation by double emulsion method in recent ten years.
| Active Substance | Polymer Film | Core | In-organic Phase 1/Surfactant | Organic Phase/Surfactant | In-organic Phase 2/Surfactant | Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Loading (%) |
Encapsulation Efficiency (%) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gemcitabine hydrochloride | Poly(ethylene glycol) Poly(L, lactide) |
Aqueous core | Water/ Span |
Chloroform Dichloromethane |
Water/ Sodium deoxycholate hydrate |
210 ± 11 | 0.09 ± 0.01 | ~−30 | ~22 | 88.6 | [71] |
| Resiquimod (R848) Muramyl dipeptide (MDP) | Acetalated dextran | Aqueous core | Phosphate buffered saline | Dichloromethane | Phosphate buffered saline/ Polyvinyl alcohol |
~150 | -- | ~−30 | 0.42 (μg/mg, R848) 1.41 (μg/mg, MDP) |
-- | [150] |
| Doxorubicin hydrochloride Magnetic nanoparticles |
N-palmitoyl chitosan | Aqueous core | Water/Tween® 80 | Chloroform | Water/Tween® 80 | 215 ± 23.33 | -- | +15.8 ± 0.42 | 1.54 | 73 | [116] |
| Doxorubicin Paclitaxel Magnetic nanoparticles |
Poly(methacrylic acid) Polyvinyl alcohol |
Aqueous core | Water/ Polyvinyl alcohol |
Chloroform | Water/ Polyvinyl alcohol |
184 ± 10 | -- | ~10 (at pH 4–5) −15 & −30 (at pH 7) |
65.21 (μg/mL, Doxorubicin) 21.74 (μg/mL Paclitaxel) |
72 (Doxorubicin) 91 (Paclitaxel) |
[115] |
| Plasmid DNA PEGylated quantum dots Pyrene Doxorubicin |
Poly(styrene allyl alcohol) |
Aqueous core | Water | Chloroform /Oleic acid |
Water/ Polyvinyl alcohol |
263 ± 42 | -- | -- | -- | ~30–70 (Plasmid DNA) ~40–70 (PEGylated quantum dots) ~80–90 (Pyrene) ~10–60 (Doxorubicin) |
[114] |
| Bovine serum albumin | Poly(lactic acid-co-glycolic acid) |
Aqueous core | Water | Dichloromethane | Water/ Polyvinyl alcohol |
327 | -- | -- | -- | 84.75 ± 1.47 | [151] |
| Poly(3-hydroxybutyrateco- 3-hydroxyvalerate) |
438 | 16.72 ± 1.06 | |||||||||
| Fungicides tebuconazole Carbendazim |
Synthesized poly(phenyleneethynylene) fluorescent polymer Poly(ε-caprolactone) |
Hollow | Acetone (Organic solvent as first phase) |
Chloroform | Water/Polyvinyl alcohol | ~430 | <0.2 | ~−13 | -- | -- | [112] |
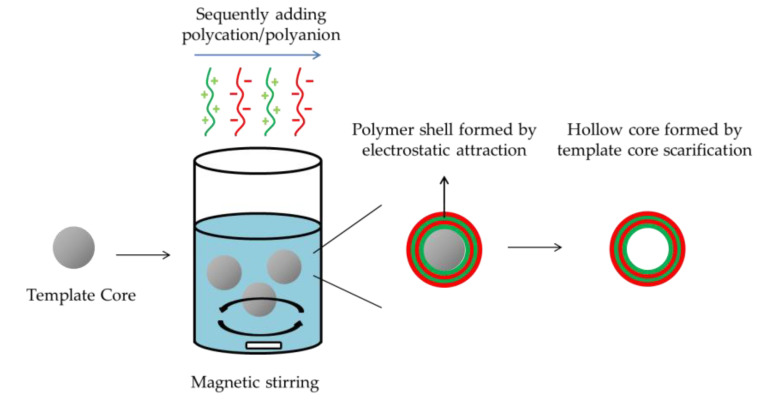
2.2.3. Layer-by-Layer Method
The layer-by-layer (LBL) method has been pointed out as a promising approach for fabrication of nanocapsules comprised of multilayers. The general procedure for the LBL method for preparation of polymeric nanocapsules is described in Figure 3. The LBL method allows the targeting and releasing properties of nanocapsules to be controlled by modulating the composition and thickness of polymeric shells [117]. The mechanism of LBL nanocapsule formulation is generally using electrostatic attraction to achieve sequential deposition of polycations and polyanions on inorganic core, followed by sacrifice of template core. Payload entrapment by sacrificed-core LBL polymeric nanocapsules, commonly, accomplished after nanocapsule formulation by diffusion for hydrophilic payload and electrostatic interaction or hydrophobic effect for hydrophobic substance. Hydrophilic anticancer drug doxorubicin hydrochloride was loaded in a chitosan–pectin-based nanocapsule prepared via LBL method. The loading procedure was simply incubated empty nanocapsules into doxorubicin hydrochloride water solution for 24 hours, resulting a encapsulation efficiency around 76% [118]. Belbekhouch et al. developed multilayer nanocapsules by electrostatic absorption of cationic poly(cyclodextrin) and anionic alginate. The nanoparticle presented a hollow core formed by sacrifice of colloidal gold nanoparticle. Hydrophobic chemicals 4-hydroxy-tamoxifen was loaded by incubated ethanol solution. The results indicated that the 4-hydroxy-tamoxifen was successfully encapsulated retaining its biologic activity [47]. Likewise, an antibiotic amoxicillin was effectively entrapped into a hollow LBL polyelectrolyte nanocapsules by suspended the formulated nanocapsules in the amoxicillin buffer acetate solution. The amoxicillin was interacted with the polymeric shell by electrostatic absorption and achieved encapsulation efficiency around 62–75% [33].
Figure 3.
Schematic representation of nanocapsule formulation by LBL method.
Recently, LBL method has been combined with nanoemulsion method using oil in water nanoemulsion as template core for polyelectrolytes nanocapsule formulation. Lipophilic substance can be effectively loaded by dissolving in the organic phase of nanoemulsion [44]. Positive or negative charged oleic surfactants are commonly used for nanoemulsion preparation to provide a charged interfacial for the deposition of polyelectrolyte layer [16,119]. Taking advantages of the unique multilayer-core nanostructure, the nanocapsules formulated by LBL technique can also carrier different payloads at different position simultaneously. Ledo et al. developed a multilayer polymeric nanocapsule, with the ability to co-encapsulate a chemokine and an RNAi sequence for immunotherapy. The chemokine was entrapped in the lipid core, while, RNAi sequence was covered by subsequent layers of polyarginine and hyaluronic acid [120].
By possible arrangement of the layer order, the nanocapsules formulated by LBL methods can achieve diverse drug release behavior in different pH environment. Therefore, LBL methods are beneficial to formulate nanocapsules as drug delivery system for oral administration to modulating the drug release in different part of digestive tract [121,122,123]. A pH-responsive multilayer prepared by LBL methods was developed which was composed of four polyelectrolytes: poly-L-arginine, sodium alginate, chitosan and Eudragit L100. When using chitosan as outer layer, the nanocapsules showed a desirable delay of drug release at pH 1.2, while, a promising sustained release at pH 7.4 due to the protonated and deprotonated effect of chitosan at pH 1.2 and 7.4, respectively [79]. It has been demonstrated that this pH-sensitive nanocapsule can be considered as potential drug delivery system to achieve intestine targeting via oral administration. Studies of polymeric nanocapsule formulation by LBL methods in recent five years are summarized in Table 5.
Table 5.
Examples of polymeric nanocapsule formulation by layer-by-layer methods in recent five years.
| Active Substance | Cationic Layer | Anionic Layer | Core | Phase | Particle Size (nm) |
PDI | Zeta Potential (mV) |
Drug Loading (%) |
Encapsulation Efficiency (%) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Curcumin | Poly-L-arginine (ARG) Chitosan (CS) |
Sodium alginate (ALG) Eudragit® L100 (EUD) |
CaCO3 (Sacrificial template) |
0.05 M NaCl aqueous solution | 400 ± 50 | ~0.2 | −43 ± 1.75 | 5.1 | 70 | [79] |
| Composition sequence: ARG-ALG-ARG-ALG-CS-EUD | ||||||||||
| Pyrene 4-hydroxy-tamoxifen | Poly(cyclodextrin) (PCD) |
Sodium alginate (ALG) |
Gold (Sacrificial template) |
Water | ~60 | -- | ~+20 | -- | -- | [47] |
| Composition sequence: (PCD/ALG)×4-PCD | ||||||||||
| RNAi Chemokines CCL2 |
Polyarginine (ARG) |
Hyaluronic acid (HA) |
Glyceryl-monolete cubic gel |
Water | ~ 150 | ~0.2 | −40 | -- | 46.0 ± 1.0 (CCL2) 68.2 ± 3.2 (RNAi) |
[120] |
| Composition sequence: ARG-RNAi-ARG-HA | ||||||||||
| Amoxicillin | Chitosan (CS) |
Poly(acrylic acid) (PAA) |
Gold (Sacrificial template) |
-- | <100 | -- | ~+40 (CS out layer) ~-20 (PAA out layer) |
3.5–5.5 | 62–75 | [33] |
| Composition sequence: (CS/PAA)×4 or 8 | ||||||||||
| Camptothecin | Poly-L-lysine (PLL) |
Poly-L-glutamic acid sodium salt (PGA) |
Hollow (Aionic surfactant docusate sodium salt) |
0.015 M NaCl aqueous solution | ~120 | <0.2 | ~−5 | 3.2 (μg/ml) | 98 | [65] |
| Composition sequence: (PLL/PGA)×2-PLL-PEG | ||||||||||
| IutA antigen | Chitosan (CS) |
Dextran sulfate (DEX) |
Vitamin E (Oleic core) |
Water | ~200 | ~0.2 | ~−40 | -- | ~70 | [44] |
| CS-DEX | ||||||||||
| Indomethacin | Chitosan orPoly(ethylene imine) | Poly(acrylic acid) | Cetyltrimethylammonium bromide or hexamethylene-1,6-bis(dimethylhexadecylammoniumdibromide (Oleic core) |
Water | ~90–200 | -- | −50 – −55 | ~9–18 | ~50–60 | [119] |
| (PAA/PEI)×3 or 5 or 7 | ||||||||||
| Cyclosporine A | Poly-L-lysine (PLL) |
Poly(L-glutamic acid) sodium salt (PGA) |
Hollow (Aionic surfactant docusate sodium salt) |
0.015 M NaCl aqueous solution | 157.5 (PLL) 160 (PLL/PGA) |
0.224 (PLL) 0.117 (PLL-PGA) |
+43 −41 |
-- | -- | [152] |
| PLL PLL-PGA | ||||||||||
| Doxorubicin hydrochloride | Chitosan (CS) |
Pectin (Pec) |
SiO2 (Sacrificial template) |
Water | 473.10 ± 3.03 | 0.169 | −28.33 ± 3.01 | 20.32 ± 0.33 | 76.51 ± 1.53 | [118] |
| (Pec/CS)×2-Pec | ||||||||||
| Bovine serum albumin | Cationic quaternary ammonium starch (QAS) |
Anionic carboxymethyl starch (CMS) |
Bovine serum albumin particles | Water | 20–300 | -- | 0 – −10 | -- | up to 45.52 | [121] |
| CMS/QAS/CMS | ||||||||||
| Clozapine | Poly-L-lysine (PLL) |
Poly-L-glutamic acid (PGA) |
Hollow (Aionic surfactant docusate sodium salt) |
Water | ~100 | <0.2 | −4 ± 6 | -- | -- | [16] |
| (PLL/PGA)×4-PGA-PEG | ||||||||||
3. Highlighted Applications of Nanocapsules as Drug Delivery System
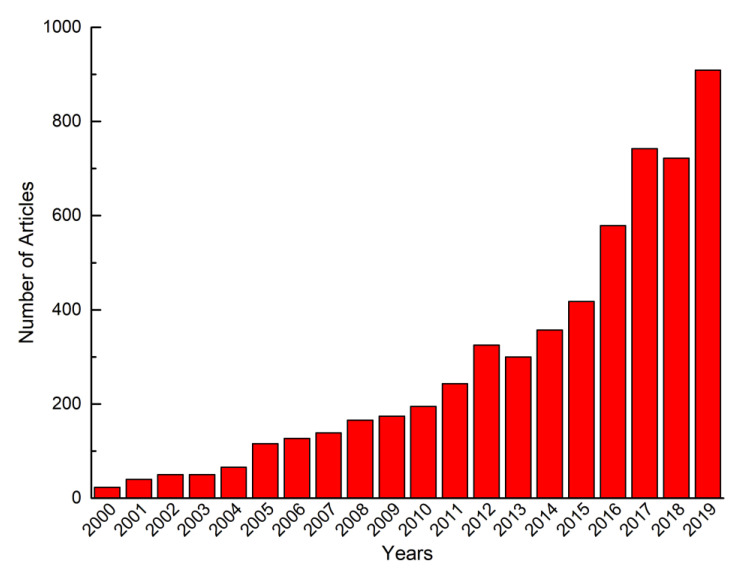
Nanocapsules are very widely applied as delivery systems for the protection and encapsulation of proteins, peptides, enzymes, hormones, metabolites, genes and other drugs for various biomedical applications such as anti-inflammation therapy, anti-cancer therapy and immunotherapy [115,153,154,155]. Research focused on polymeric nanocapsules for pharmaceutical applications presents a dramatic increase in the past two decades (Figure 4). Applications of nanocapsules as drug delivery and targeting systems over recent decades are summarized in Table 6.
Figure 4.
Number of articles focused on polymeric nanocapsules for medical applications (based on statistics of Science Direct).
Table 6.
Examples of polymeric nanocapsules applications as drug delivery system in recent years.
| Active Substance | Therapy | Main Achieved Advantage | Polymer Membrane | Core | Formulation Method | Ref. |
|---|---|---|---|---|---|---|
| Olanzapine | Inhibition of startle response (PPI) in rats following oral administration |
Chitosan coated increase the mucoadhesion and the brain delivery | Poly (ε-caprolactone) Chitosan |
Sorbitan monostearate and caprylic/capric triglycerides | Interfacial deposition | [89] |
| Clarithromycin | Aerosol delivery | Improved bioavailability of poorly soluble drugs | Poly (lactic-co-glycolic acid) | Medium-chain triglycerides | Interfacial deposition | [31] |
| Desonide | Anti-inflammatory | Protecting active substance from hash environment (Photosensitive) | Eudragit® RL 100 | Açai oil or medium chain triglyceride | Interfacial deposition | [67] |
| Imiquimod | Cervical cancer | Improved bioavailability of poorly soluble drugs | Poly (ε-caprolactone) | Copaiba oil | Interfacial deposition | [17,68] |
| 5-fluorouracil | Anti-cancer therapy | Toxicity moderation Targeting delivery (AS1411 aptamer) Improved bioavailability of poorly soluble drugs |
Chitosan Poly (Nvinylpyrrolidone- alt-itaconic anhydride) |
Hollow | Interfacial deposition | [82] |
| Ketoprofen | Anti-inflammatory | Protecting active substance from hash environment (Photo) Toxicity moderation |
Eudragit® S100 | Rose hip oil | Interfacial deposition | [125] |
| Cilostazol | Peripheral arterial disease | Sustained delivery | Poly(ε-caprolactone)- poly (ethylene glycol) |
Capric/caprylic acid triglycerides | Interfacial deposition | [58] |
| Carvedilol | Sublingual administration Heart failure, hypertension and coronary artery diseases |
Improved bioavailability of poorly soluble drugs | Eudragit® S100 Poly(ε-caprolactone) |
Grape seed oil | Interfacial deposition | [126] |
| Bedaquiline | Mycobacterium tuberculosis | Toxicity moderation Improved bioavailability of poorly soluble drugs |
Chitosan–poly (ethylene glycol) | Oleic acid | Interfacial deposition | [30,64] |
| Indole-3-carbinol | Antinocieptive | Protecting active substance from hash environment (Photo, temperature and pH) | Eudragit® S100 | Rose hip oil | Interfacial deposition | [127] |
| Diphenyl diselenide | Malignant cutaneous melanoma | Improved bioavailability of poorly soluble drugs Protecting active substance from hash environment (Photosensitive) |
Poly(ε-caprolactone) | Medium chain triglycerides | Interfacial deposition | [128] |
| Cloxacillin benzathine | Mastitis | Improved bioavailability of poorly soluble drugs | Poly(ε-caprolactone) | Labrafac® CC | Interfacial deposition | [129] |
| Curcumin | Breast cancer therapy | Improved bioavailability of poorly soluble drugs Sustained delivery |
Human serum albumin | Olive oil | Interfacial deposition | [172,173] |
| Amphotericin B | Systemic fungal infection and leishmania | Toxicity moderation Improved bioavailability of poorly soluble drugs |
Synthesized propionated Sterculia striata polysaccharide | Miglyol® L812 | Emulsion–diffusion/evaporation | [95,141] |
| Thymoquinone Docetaxel |
Anti-cancer therapy | Toxicity moderation Improved bioavailability of poorly soluble drugs |
Chitosan | Oleic acid-phospholipid | Emulsion–diffusion/evaporation | [142] |
| Brinzolamide | Glaucoma treatment Ocular delivery |
Sustained delivery Improved bioavailability of poorly soluble drugs |
Chitosan Pectin |
Aqueous core | Emulsion–coacervation | [83] |
| Coumarin 6 | Cancer | Targeting delivery (folic acid as ligand) | Folic acid decorated reductive-responsive ε-poly-L-lysine | Soybean oil | Emulsion–coacervation | [66] |
| Paclitaxel | Anti-cancer drug delivery via oral administration | Chitosan coated nanocapsules for oral delivery | Chitosan Poly(isobutyl cyanoacrylate) | Copaiba oil | Emulsion–coacervation | [148] |
| Copaiba oil | Potential mucoadhesive nanoparticles | Improved bioavailability of poorly soluble drugs | Chitosan–poly(isobutylcyanoacrylate) | Copaiba oil | Emulsion–coacervation | [174] |
| Curcumin | Oral administration Selective drug delivery in the colon |
Protecting active substance from hash environment | Poly-L-arginine Sodium alginate Chitosan Eudragit® L100 |
Hollow (Template CaCO3) |
Layer-by-layer | [79] |
| Pyrene 4-hydroxy-tamoxifen |
Anti-estrogenic delivery | Improved bioavailability of poorly soluble drugs | Poly(cyclodextrin) Sodium alginate |
Hollow (Template Gold) |
Layer-by-layer | [47] |
| RNAi Chemokines CCL2 |
Cancer immunotherapy | Improved bioavailability of poorly soluble drugs | Polyarginine and hyaluronic acid | Glyceryl-monooleate cubic gel | Layer-by-layer | [120] |
| Amoxicillin | Antibiotic therapy | Protecting active substance from hash environment (Photosensitive) | Chitosan Poly(acrylic acid) |
Hollow (Template Gold) |
Layer-by-layer | [33] |
| Camptothecin | Anti-cancer therapy | Improved bioavailability of poorly soluble drugs | Poly-L-lysine Poly-L-glutamic acid sodium salt | Hollow | Layer-by-layer | [65] |
| IutA antigen | — | Protecting active substance from hash environment (biochemical degradation) Improved bioavailability of poorly soluble drugs |
Chitosan Dextran sulfate |
Vitamin E | Layer-by-layer | [44] |
3.1. Improved Bioavailability of Poorly Soluble Drugs
One of the most prompting reasons to use polymeric nanocapsules as a drug delivery system is the enhancement of permeability, as well as solubility of drugs. By modulating the interaction between cells and drug, nanocapsules can effectively improve the bioavailability of drugs, as compared to unloaded free drugs. In particular, hydrophobic drugs may be prepared in water-dispersion dosage form—assisted by encapsulation of nanohydrogels—thereby to achieving specific administration methods. For example, clarithromycin is one of the FDA-approved antibiotics against respiratory disease, such as cystic fibrosis. Aerosolized administration is an effective method for clarithromycin to reach infected organs, i.e., respiratory system. However, efficacy is limited due to poor aqueous solubility. It has been found that clarithromycin-loaded PLGA/chitosan nanocapsules are significantly more effective than the same drug in free form by aerosolized administration [31].
Additionally, since most anti-cancer drugs have hydrophobic properties, various nanocapsules have been developed as delivery systems to overcome this limitation and enhance efficacy of anti-cancer therapy. The hydrophobic cancer drug imiquimod has been loaded into the copaiba oil core of poly (ε-caprolactone) nanocapsules. After encapsulation, imiquimod performed a good dispersion in water assisted by nanocapsules for cervical cancer treatment [17].
3.2. Sustained Delivery
For treatments of some diseases, it is necessary to maintain long-term therapeutic levels of drug concentration. However, frequent, repeated intakes of medicines by various administration methods are not only inconvenient for patients, but also limited by the side effects of drug toxicity. The core-shell structure of polymeric nanocapsules has been investigated the potential to provide a considerable inner space for maintaining relative high dosages of drugs, as the polymeric shell can possess controlled-release profiles for sustained delivery [83,156,157,158]. For example, to limit several side effects, the common oral dosage of cilostazol is 100-mg, twice per day—which represents a disadvantage in treatment compliance. Long-term release of cilostazol for treatment of peripheral arterial disease has been achieved by loading it into nanocapsules assembled by a poly(ε-caprolactone)-poly (ethylene glycol) shell and oil core [58]. Prolonged and sustained release of the drug over a period of for 6 days has been achieved without burst effects by both mechanisms of polymeric shell degradation and diffusion. The nanocapsules provided an effective method to ensure long-term, therapeutic-sufficient concentrations, while better for patients’ convenience.
3.3. Targeted Delivery
The polymeric shell of nanocapsules has been indicated as an ideal subject to be covered with targeting ligands that lead nanocapsules to cancer cells, immune cells or other critical cells on contact with selective destruction [159,160]. Functionalization of nanocapsules by specific bioactive ligands has emerged as an intense therapeutic strategy for cancer therapy for achieving drug accumulation at tumor-targeted regions, thereby either enhancing therapeutic effects, or reducing side effects on healthy tissue [161,162].
Common ligands used for active targeting include antibodies, peptides, nucleic aptamers, vitamins and carbohydrates [163,164,165,166]. One potent method for ligand decoration onto nanoparticles is chemical conjugation on the polymer—prioritized, and then exposed on the surface of nanocapsules by assembling the ligand-conjugated polymer into shell. In general, bioactive ligands and polymeric materials are rich in amino, carboxyl, hydroxyl and thiol groups, which act as conjugation linkages. Shi et al. [66] formulated a ε-poly-L-lysine (PLL)-based nanocapsules as a tumor targeting delivery system by using folic acid as a ligand. Folic acid was successfully grafted onto the main chain of PLL polymers by applying the reaction between carboxyl groups of folic acid to N-hydroxysuccinimide group of PLL.
As another example, amino groups of AS1411 aptamers were linked onto carboxymethyl chitosan via an esterification reaction, which is utilized as a tumor-cell targeting drug delivery system [82].
3.4. Stabilization under Harsh Environment
Nanocapsules have been investigated to provide a shielding environment of payloads such as peptides, proteins, enzymes or other bioactive substance to maintain stability against degradation induced by pH, temperature, chemical or light during delivery or fabrication procedures.
A kind of multilayer nanocapsule—fabricated by a layer-by-layer method—has been developed and has achieved intestine release via oral administration [79]. The payload was successfully protected against degradation in the first part of digestive system via nanocapsule encapsulation. The permeability of nanocapsules were modulated by a stimuli-responsive, multilayered polymeric shell made of four polyelectrolytes, including Eudragit® L100, chitosan, sodium alginate, and poly-L-arginine, thereby presenting a delayed release in the acidic pH environment of the gastrointestinal tract, while a fast release at the pH found in the intestines.
Additionally, nanocapsules can also provide stable fabrication procedures for light-sensitive drugs. Photosensitive drugs such as desonide and ketoprofen have been encapsulated into the oil core of Eudragit® RL 100 nanocapsules, which was confirmed to enable avoiding drug photodegradation under UV radiation [67,125].
3.5. Toxicity Moderation
For active substances, high toxicity and serious side reactions for host systems and healthy tissue is a major limitation for therapeutic treatments—especially for cancer chemotherapy and pharmacology [167,168,169]. Nanocapsules have been used as a strategy to reduce toxicity and side effects, while maintaining the pharmacological activity associated with the drug. The payload may be adequately separated from the external environment with a polymeric shell [170,171]. For example, amphotericin B is an effective drug against fungal infection, but its use is associated with a series of side effects such as fever, cardiac arrhythmia and nefrotoxicity, due to the aggregation pattern in solution. The drug has been encapsulated in the oil core of propionate Sterculia striata polysaccharide nanocapsules with a high loading yield, which successful reduced hepatotoxicity through a good distribution of amphotericin B-loaded nanocapsules [95].
4. Challenge of Nanocapsules as Drug Delivery System
According to a fair amount of theoretical and experimental work, polymeric nanocapsules have demonstrated enormous potentials and wide spaces to be applied as drug delivery systems for various biomedical applications. Nanocapsules are being implemented to overcome limitations of conventional drug delivery approaches by achieving high drug-content loading for both hydrophilic and hydrophobic drugs, as well as targeted and sustained delivery. However, there are still several technical challenges with polymeric nanocapsules that need to be further developed before industrial application.
4.1. Thickness Characterization of Polymeric Shell
Compared with nanospheres, the core-shell morphologic structure is one of the particular advantages of nanocapsules as drug delivery system. The thickness of the polymer shell plays a critical role in nanocapsule formulation, active substance protection as well as release profiles [66,175]. However, there is little discussion regarding the quantitative measurement of the polymeric shell of nanocapsules in most of studies; only few examples can be here reported. The main characterization method of the thickness is observation via transmission electron microscopy (TEM) [47,148]. For example, a 10-nm polymeric shell has been qualitatively estimated by TEM from argon oil/oleoyl polyoxylglycerides/Eudragit® RS nanocapsules [176].
To obtain more specific values for shell thickness, mathematical calculation has also been carried out. The shell thickness of the nanocapsules formulated based on the scarified core template can be calculated as the different value between the particle radius of the completed nanocapsules and the scarified template. The thickness of polymeric shell of polydopamine/Au hollow nanocapsules was calculated as 70 nm [81].
The surface parameter variation before and after polymer coating can be also applied for the estimation of shell thickness. It has been demonstrated that variation versus salinity of the medium—which may be attributed to the nanoparticle surface—can be calculated through the Eversole and Boardman equation [177]. The thickness of dextran’s outer layer of a Miglyol® 810/dextran nanocapsules was estimated as 5 nm by calculating the salinity difference using the Eversole and Boardman equation [103].
4.2. Organic Solvent Free Formation
Organic solvents play a critical role in polymeric nanocapsule formulation. Organic solvents have to be strictly eliminated from the formulation by purification procedure because of their toxicity in humans. Nonetheless, traces of organic solvents that cannot be adequately removed may cause toxic effects in clinical application. Additionally, nanoparticles are usually captured by immune cells—such as macrophages or mononuclear phonotypes—which indicates that the trigger of inflammation and the toxic organic solvent is considered as one of the reason for this inflammatory situation induced by nanoparticles [178]. Despite potential toxic risks, the organic solvent removal step can also influence the stability of nanoparticles [179] Therefore, it is significant and imperative to develop polymeric nanocapsule using solvent-free methods.
There are several studies that report the formulation of polymeric nanocapsules by solvent-free approach. Two formulation strategies are possible: either by using solvent-free organic phase for nanoemulsion preparation or form polymeric shell by polymerization in aqueous environment. A solvent-free protamine nanocapsule was developed as carrier for a model lipophilic anti-inflammatory agent, cyclosporine A. The nanocapsules were prepared by a nanoemulsion method. The organic phase was prepared by mixing Maisine 35–1 and Tween® 80 which acted as oleic compound and surfactant, respectively. Anionic surfactant sodium deoxycholate and polyethylene glycol-40 stearate were added to the water phase. Polyethylene glycol-40 stearate was added to prevent the aggregation of nanocapsules. The organic and inorganic phases were prepared into nanoemulsion and added to protamine aqueous solution [180] along with magnetic stirring to obtain a nanocapsule formulation. Resulted protamine nanoparticles presented a particle size around 160–180 nm with a narrow distribution (PDI = 0.2). This solvent-free method can be considered as a promising strategy for nanocapsule prepared by water-soluble polymers. Another interesting example is that of Steelandt et al. who developed polymeric nanocapsules containing an oleic core and a poly-ε-caprolactone shell [181]. Poly-ε-caprolactone was heated at the glass transition temperature then mixed with oleic compound and surfactant to act as organic phase for emulsion preparation. This method can be considered as potential method for preparation of nanocapsules based on hydrophobic crystalline or amorphous polymer.
The polymeric nanocapsules can also be prepared by monomer polymerization in organic solvent free condition to preform polymeric shell. For example, a nanocapsule was formulated by free radical polymerization of monomers acrylamide and 2-aminoethyl methacrylate hydrochloride in the deoxygenated buffer [182]. However, the polymerization method can also lead new toxic issue by residual monomers, cross linker or other chemical compounds.
4.3. Aggregation and Storage
Based on fabrication procedure, nanocapsules are in general yielded into aqueous dispersion. However, it has been highlighted that nanocapsules tend to be unstable and aggregate in water suspension, thereby inducing leaking of payload. In this case, to store nanocapsules into dried form is generally preferable to improve the stability of nanocapsules and also prolong the store term [183]. Moreover, to adapt preparations of particular pharmaceutical dosage forms, such as oral tablet, it is necessary to manufacture the polymeric nanocapsules suspension into dry state for further preservation and usage [129,148,184]. Spray drying as one of the common drying method has been applied for recovery of nanocapsules into solid dry state. To enhance the stability of nanocapsules by avoiding their aggregation during the drying process, protectants such as inulin or polyvinyl alcohol were used [126,185]. But the application of spray drying method may induce thermal degradation of polymeric nanocapsules and also drug loading [186]. Lyophilization carried out in presence of a lyoprotectant is another strategy for desiccation of nanocapsules [53]. However, thin polymeric shell of nanocapsules may compromise the stability of the whole delivery system because of the low working pressure, especially when nanocapsules are characterized by an oil or a hollow core [187]. After all, lyophilization is considered an expensive technique due to the experimental conditions, time and equipment need [188]. Therefore, optimization for an appropriate desiccation procedure for the stabilization and storage of polymeric nanocapsules is most urgent needed.
4.4. Sterilization
Sterilization of polymeric nanocapsules is also a major challenge in the development of appropriate drug delivery system for clinical trials and commercial manufactures. Several approaches have been studied to sterilize nanoparticles for in vivo experiments. Membrane filtration is a physical method to sterilize nanoparticles by using 0.22 μm filters: this method allows for the removal of microorganisms from nanocapsules water suspension [189]. This method does not need extraneous heat, radiation or chemicals which could induce damage or degradation of polymeric nanocapsules [190,191]. However, membrane filtration presents the limitation for the nanocapsules possessing particle size higher 200 nm. In addition, it could cause decrease of product yield due to filter clogging by nanocapsules. Autoclaving is another effective technique that has been applied for polymer nanoparticles sterilization via controlled temperature and pressure [192,193]. However, aggregation, morphology deformation and also chemical degradation were observed for several polymeric nanoparticles [194,195]. Additionally, size increasing was detected for nanocapsules with an oil core Miglyol®-based. The size increased depended either on swelling of polymeric shell or expansion of oil core under the harsh environment of temperature and pressure [196]. Gamma irradiation can provide homogeneous sterilization to inactive the microorganisms and avoid the risk of high temperature or pressure [197,198]. However, it may cause physical or chemical degeneration of either polymeric nanocapsules or loaded drugs. As a conclusion, at the present, to maintain a contaminant-free fabrication procedure of polymeric nanocapsules is the most optimal strategy for the preparation of sterilized nanocapsules. Additionally, novel and advanced strategies for sterilization of polymeric nanocapsules are imperative.
Furthermore, more novel polymeric materials, surfactants and also chemical ingredients have been used to formulate functional polymeric nanocapsules in order to meet applications in demand which are bringing more opportunities but also challenges for the polymeric nanoparticles as drug delivery systems.
5. Conclusions
Research of polymeric nanoparticles has widely attracted attention recently. This minireview has reported the studies and progress of polymeric nanocapsules as drug delivery system in pharmaceutical field, in last decade. To perform the specific core-shell nanostructure, various materials were used for polymeric nanocapsule formulation via the interfacial deposition method, nano-emulsion template method or lay-by layer method. The selection of polymers and formulation methods mainly depends on the characteristics of pharmaceutical ingredient and application purpose. Polymeric nanocapsules as drug delivery systems can improve the bioavailability of payloads and achieve sustained and targeted delivery. Likewise, they can also effectively reduce the harmful effects between payload and tissue environments. By loading the drug inside polymeric nanocapsules, it can protect the drug from failure or degradation caused by biologic environment. Meanwhile, it can also reduce the side-effect induced by drug to health tissue. To date, many studies have mainly focused on the development and characterization of bioactive substance-loaded polymeric nanocapsules. However, the storage and sterilization methods of formed drug-loaded polymeric nanocapsules are needed attentions and researches. Additionally, future perspectives in polymeric nanocapsules should focus on studies of using new and most performing polymers to develop advanced delivery system, thereby extending the applications of polymeric nanocapsules in pharmaceutical fields.
Author Contributions
Conceptualization, P.D.M. and R.C.; methodology, P.D.M.; investigation, P.D.M., R.C., S.D. and M.R.G.; resources, P.D.M.; writing—original draft preparation, S.D. and P.D.M.; writing—review and editing, S.D., R.C., M.R.G. and P.D.M.; supervision, P.D.M. and R.C.; funding acquisition, P.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge receipt of funding from the European Commission through an H2020-MSCA-ITN-2015 award, as part of the ISPIC project (grant number 675743), an H2020-MSCA-RISE-2016 award through the CHARMED project (grant number 734684) and an H2020-MSCA-RISE-2017 award through the CANCER project (grant number 777682).
Conflicts of Interest
The authors confirm that this article content has no conflicts of interest.
References
- 1.Farokhzad O.C., Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 2.Suri S.S., Fenniri H., Singh B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007;2:16. doi: 10.1186/1745-6673-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng M.M.-C., Cuda G., Bunimovich Y.L., Gaspari M., Heath J.R., Hill H.D., Mirkin C.A., Nijdam A.J., Terracciano R., Thundat T. Nanotechnologies for biomolecular detection and medical diagnostics. Curr. Opin. Chem. Biol. 2006;10:11–19. doi: 10.1016/j.cbpa.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Jain K.K. Nanodiagnostics: Application of nanotechnology in molecular diagnostics. Expert Rev. Mol. Diagn. 2003;3:153–161. doi: 10.1586/14737159.3.2.153. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Yang L., Chen Z.G., Shin D.M. Application of nanotechnology in cancer therapy and imaging. CA A Cancer J. Clin. 2008;58:97–110. doi: 10.3322/CA.2007.0003. [DOI] [PubMed] [Google Scholar]
- 6.Koo O.M., Rubinstein I., Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Gu F., Chan J., Wang A., Langer R., Farokhzad O. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 8.Jurgons R., Seliger C., Hilpert A., Trahms L., Odenbach S., Alexiou C. Drug loaded magnetic nanoparticles for cancer therapy. J. Phys. Condens. Matter. 2006;18:S2893. doi: 10.1088/0953-8984/18/38/S24. [DOI] [Google Scholar]
- 9.Tocco I., Zavan B., Bassetto F., Vindigni V. Nanotechnology-based therapies for skin wound regeneration. J. Nanomater. 2012;2012:714134. doi: 10.1155/2012/714134. [DOI] [Google Scholar]
- 10.Singh R., Lillard J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelperina S., Kisich K., Iseman M.D., Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005;172:1487–1490. doi: 10.1164/rccm.200504-613PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moinard-Checot D., Chevalier Y., Briançon S., Fessi H., Guinebretière S. Nanoparticles for drug delivery: Review of the formulation and process difficulties illustrated by the emulsion-diffusion process. J. Nanosci. Nanotechnol. 2006;6:2664–2681. doi: 10.1166/jnn.2006.479. [DOI] [PubMed] [Google Scholar]
- 13.Fu C., Ding C., Sun X., Fu A. Curcumin nanocapsules stabilized by bovine serum albumin-capped gold nanoclusters (BSA-AuNCs) for drug delivery and theranosis. Mater. Sci. Eng. C. 2018;87:149–154. doi: 10.1016/j.msec.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaumet M., Vargas A., Gurny R., Delie F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Lukasiewicz S., Szczepanowicz K., Podgorna K., Blasiak E., Majeed N., Ogren S.O.O., Nowak W., Warszynski P., Dziedzicka-Wasylewska M. Encapsulation of clozapine in polymeric nanocapsules and its biological effects. Colloids Surf. B Biointerfaces. 2016;140:342–352. doi: 10.1016/j.colsurfb.2015.12.044. [DOI] [PubMed] [Google Scholar]
- 17.Frank L., Gazzi R., De Andrade Mello P., Buffon A., Pohlmann A., Guterres S. Imiquimod-loaded nanocapsules improve cytotoxicity in cervical cancer cell line. Eur. J. Pharm. Biopharm. 2019;136:9–17. doi: 10.1016/j.ejpb.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Pohlmann A.R., Weiss V., Mertins O., Da Silveira N.P., Guterres S.S. Spray-dried indomethacin-loaded polyester nanocapsules and nanospheres: Development, stability evaluation and nanostructure models. Eur. J. Pharm. Sci. 2002;16:305–312. doi: 10.1016/S0928-0987(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 19.Trindade I.C., Pound-Lana G., Pereira D.G.S., De Oliveira L.A.M., Andrade M.S., Vilela J.M.C., Postacchini B.B., Mosqueira V.C.F. Mechanisms of interaction of biodegradable polyester nanocapsules with non-phagocytic cells. Eur. J. Pharm. Sci. 2018;124:89–104. doi: 10.1016/j.ejps.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Teixeira M., Alonso M.J., Pinto M.M.M., Barbosa C.M. Development and characterization of PLGA nanospheres and nanocapsules containing xanthone and 3-methoxyxanthone. Eur. J. Pharm. Biopharm. 2005;59:491–500. doi: 10.1016/j.ejpb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Guterres S.S., Alves M.P., Pohlmann A.R. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147–157. doi: 10.1177/117739280700200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mora-Huertas C.E., Fessi H., Elaissari A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010;385:113–142. doi: 10.1016/j.ijpharm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Kothamasu P., Kanumur H., Ravur N., Maddu C., Parasuramrajam R., Thangavel S. Nanocapsules: The weapons for novel drug delivery systems. Bioimpacts: BI. 2012;2:71–81. doi: 10.5681/bi.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shastri V.P. Non-degradable biocompatible polymers in medicine: Past, present and future. Curr. Pharm. Biotechnol. 2003;4:331–337. doi: 10.2174/1389201033489694. [DOI] [PubMed] [Google Scholar]
- 25.Almeida J.P.M., Chen A.L., Foster A., Drezek R.J.N. In vivo biodistribution of nanoparticles. Nanomedicine. 2011;6:815–835. doi: 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]
- 26.Chen H., Wang L., Yeh J., Wu X., Cao Z., Wang Y.A., Zhang M., Yang L., Mao H. Reducing non-specific binding and uptake of nanoparticles and improving cell targeting with an antifouling PEO-b-PγMPS copolymer coating. Biomaterials. 2010;31:5397–5407. doi: 10.1016/j.biomaterials.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyer C., Whittaker M.R., Bulmus V., Liu J., Davis T.P. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater. 2010;2:23–30. doi: 10.1038/asiamat.2010.6. [DOI] [Google Scholar]
- 28.Li P., Dai Y.-N., Zhang J.-P., Wang A.-Q., Wei Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. IJBS. 2008;4:221–228. [PMC free article] [PubMed] [Google Scholar]
- 29.Younes I., Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015;13:1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Matteis L., Jary D., Lucía A., García-Embid S., Serrano-Sevilla I., Pérez D., Ainsa J.A., Navarro F.P., De la Fuente J.M. New active formulations against M. tuberculosis: Bedaquiline encapsulation in lipid nanoparticles and chitosan nanocapsules. Chem. Eng. J. 2018;340:181–191. doi: 10.1016/j.cej.2017.12.110. [DOI] [Google Scholar]
- 31.Anversa Dimer F., De Souza Carvalho-Wodarz C., Goes A., Cirnski K., Herrmann J., Schmitt V., Patzold L., Abed N., De Rossi C., Bischoff M., et al. PLGA nanocapsules improve the delivery of clarithromycin to kill intracellular Staphylococcus aureus and Mycobacterium abscessus. Nanomedicine. 2019;24:102125. doi: 10.1016/j.nano.2019.102125. [DOI] [PubMed] [Google Scholar]
- 32.Jeon S.J., Oh M., Yeo W.-S., Galvao K.N., Jeong K.C. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS ONE. 2014;9:e92723. doi: 10.1371/journal.pone.0092723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belbekhouche S., Mansour O., Carbonnier B. Promising sub-100 nm tailor made hollow chitosan/poly(acrylic acid) nanocapsules for antibiotic therapy. J. Colloid Interface Sci. 2018;522:183–190. doi: 10.1016/j.jcis.2018.03.061. [DOI] [PubMed] [Google Scholar]
- 34.Shagholani H., Ghoreishi S.M., Mousazadeh M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015;78:130–136. doi: 10.1016/j.ijbiomac.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 35.Cafaggi S., Russo E., Stefani R., Leardi R., Caviglioli G., Parodi B., Bignardi G., De Totero D., Aiello C., Viale M. Preparation and evaluation of nanoparticles made of chitosan or N-trimethyl chitosan and a cisplatin–alginate complex. J. Control. Release. 2007;121:110–123. doi: 10.1016/j.jconrel.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 36.Douglas K.L., Tabrizian M. Effect of experimental parameters on the formation of alginate–chitosan nanoparticles and evaluation of their potential application as DNA carrier. J. Biomater. Sci. Polym. Ed. 2005;16:43–56. doi: 10.1163/1568562052843339. [DOI] [PubMed] [Google Scholar]
- 37.Wang T., Feng Z., He N., Wang Z., Li S., Guo Y., Xu L. A novel preparation of nanocapsules from alginate-oligochitosan. J. Nanosci. Nanotechnol. 2007;7:4571–4574. doi: 10.1166/jnn.2007.882. [DOI] [PubMed] [Google Scholar]
- 38.Sarmento B., Ferreira D., Veiga F., Ribeiro A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006;66:1–7. doi: 10.1016/j.carbpol.2006.02.008. [DOI] [Google Scholar]
- 39.George M., Abraham T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan-a review. J. Control. Release. 2006;114:1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Yang J., Chen J., Pan D., Wan Y., Wang Z. pH-sensitive interpenetrating network hydrogels based on chitosan derivatives and alginate for oral drug delivery. Carbohydr. Polym. 2013;92:719–725. doi: 10.1016/j.carbpol.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay P., Chakraborty S., Bhattacharya S., Mishra R., Kundu P. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int. J. Biol. Macromol. 2015;72:640–648. doi: 10.1016/j.ijbiomac.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 42.Dhaneshwar S.S., Mini K., Gairola N., Kadam S. Dextran: A promising macromolecular drug carrier. Indian J. Pharm. Sci. 2006;68:705–714. doi: 10.4103/0250-474X.31000. [DOI] [Google Scholar]
- 43.Tiyaboonchai W., Limpeanchob N. Formulation and characterization of amphotericin B–chitosan–dextran sulfate nanoparticles. Int. J. Pharm. 2007;329:142–149. doi: 10.1016/j.ijpharm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Crecente-Campo J., Lorenzo-Abalde S., Mora A., Marzoa J., Csaba N., Blanco J., Gonzalez-Fernandez A., Alonso M.J. Bilayer polymeric nanocapsules: A formulation approach for a thermostable and adjuvanted E. coli antigen vaccine. J. Control. Release. 2018;286:20–32. doi: 10.1016/j.jconrel.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Anitha A., Deepagan V., Rani V.D., Menon D., Nair S., Jayakumar R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate–chitosan nanoparticles. Carbohydr. Polym. 2011;84:1158–1164. doi: 10.1016/j.carbpol.2011.01.005. [DOI] [Google Scholar]
- 46.Tekie F.S.M., Kiani M., Zakerian A., Pilevarian F., Assali A., Soleimani M., Dinarvand R., Arefian E., Atashi A., Amini M. Nano polyelectrolyte complexes of carboxymethyl dextran and chitosan to improve chitosan-mediated delivery of miR-145. Carbohydr. Polym. 2017;159:66–75. doi: 10.1016/j.carbpol.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 47.Belbekhouche S., Oniszczuk J., Pawlak A., El Joukhar I., Goffin A., Varrault G., Sahali D., Carbonnier B. Cationic poly(cyclodextrin)/alginate nanocapsules: From design to application as efficient delivery vehicle of 4-hydroxy tamoxifen to podocyte in vitro. Colloids Surf. B Biointerfaces. 2019;179:128–135. doi: 10.1016/j.colsurfb.2019.03.060. [DOI] [PubMed] [Google Scholar]
- 48.Ismail M., Du Y., Ling L., Li X. Artesunate-heparin conjugate based nanocapsules with improved pharmacokinetics to combat malaria. Int. J. Pharm. 2019;562:162–171. doi: 10.1016/j.ijpharm.2019.03.031. [DOI] [PubMed] [Google Scholar]
- 49.Abellan-Pose R., Teijeiro-Valino C., Santander-Ortega M.J., Borrajo E., Vidal A., Garcia-Fuentes M., Csaba N., Alonso M.J. Polyaminoacid nanocapsules for drug delivery to the lymphatic system: Effect of the particle size. Int. J. Pharm. 2016;509:107–117. doi: 10.1016/j.ijpharm.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 50.Mahmoudi M., Lynch I., Ejtehadi M.R., Monopoli M.P., Bombelli F.B., Laurent S. Protein−nanoparticle interactions: Opportunities and challenges. Chem. Rev. 2011;111:5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 51.DeFrates K., Markiewicz T., Gallo P., Rack A., Weyhmiller A., Jarmusik B., Hu X. Protein polymer-based nanoparticles: Fabrication and medical applications. Int. J. Mol. Sci. 2018;19:1717. doi: 10.3390/ijms19061717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karimi M., Bahrami S., Ravari S.B., Zangabad P.S., Mirshekari H., Bozorgomid M., Shahreza S., Sori M., Hamblin M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016;13:1609–1623. doi: 10.1080/17425247.2016.1193149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaber M., Hany M., Mokhtar S., Helmy M.W., Elkodairy K.A., Elzoghby A.O. Boronic-targeted albumin-shell oily-core nanocapsules for synergistic aromatase inhibitor/herbal breast cancer therapy. Mater. Sci. Eng. C. 2019;105:110099. doi: 10.1016/j.msec.2019.110099. [DOI] [PubMed] [Google Scholar]
- 54.Toita R., Murata M., Abe K., Narahara S., Piao J.S., Kang J.-H., Ohuchida K., Hashizume M. Biological evaluation of protein nanocapsules containing doxorubicin. Int. J. Nanomed. 2013;8:1989–1999. doi: 10.2147/IJN.S40239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren D., Kratz F., Wang S.W. Protein nanocapsules containing doxorubicin as a pH-responsive delivery system. Small. 2011;7:1051–1060. doi: 10.1002/smll.201002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Da Silva Júnior W.F., De Oliveira Pinheiro J.G., Moreira C.D., De Souza F.J., De Lima Á.A. Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics. Elsevier; Amsterdam, The Netherlands: 2017. Alternative technologies to improve solubility and stability of poorly water-soluble drugs; pp. 281–305. [DOI] [Google Scholar]
- 57.Yao M.H., Ma M., Chen Y., Jia X.Q., Xu G., Xu H.X., Chen H.R., Wu R. Multifunctional Bi2S3/PLGA nanocapsule for combined HIFU/radiation therapy. Biomaterials. 2014;35:8197–8205. doi: 10.1016/j.biomaterials.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Gomes M.L.S., Da Silva Nascimento N., Borsato D.M., Pretes A.P., Nadal J.M., Novatski A., Gomes R.Z., Fernandes D., Farago P.V., Zanin S.M.W. Long-lasting anti-platelet activity of cilostazol from poly(epsilon-caprolactone)-poly(ethylene glycol) blend nanocapsules. Mater. Sci. Eng. C. 2019;94:694–702. doi: 10.1016/j.msec.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 59.Fustier C., Chang T.M. PEG-PLA nanocapsules containing a nanobiotechnological complex of polyhemoglobin-tyrosinase for the depletion of tyrosine in melanoma: Preparation and in vitro characterisation. J. Nanomed. Biother. Discov. 2012;2:1–9. doi: 10.4172/2155-983X.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haznedar S., Dortunc B. Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int. J. Pharm. 2004;269:131–140. doi: 10.1016/j.ijpharm.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 61.De Gomes M.G., Pando Pereira M., Guerra Teixeira F.E., Carvalho F., Pinto Savall A.S., Ferreira Bicca D., Monteiro Fidelis E., Botura P.E., Weber Cibin F., Pinton S., et al. Assessment of unloaded polymeric nanocapsules with different coatings in female rats: Influence on toxicological and behavioral parameters. Biomed. Pharm. 2020;121:109575. doi: 10.1016/j.biopha.2019.109575. [DOI] [PubMed] [Google Scholar]
- 62.Diou O., Fattal E., Delplace V., Mackiewicz N., Nicolas J., Mériaux S., Valette J., Robic C., Tsapis N. RGD decoration of PEGylated polyester nanocapsules of perfluorooctyl bromide for tumor imaging: Influence of pre or post-functionalization on capsule morphology. Eur. J. Pharm. Biopharm. 2014;87:170–177. doi: 10.1016/j.ejpb.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Bahmani B., Gupta S., Vullev V.I., Anvari B., Upadhyayula S. Effect of polyethylene glycol coatings on uptake of indocyanine green loaded nanocapsules by human spleen macrophages in vitro. J. Biomed. Opt. 2011;16:051303. doi: 10.1117/1.3574761. [DOI] [PubMed] [Google Scholar]
- 64.De Matteis L., Alleva M., Serrano-Sevilla I., Garcia-Embid S., Stepien G., Moros M., De la Fuente J.M. Controlling properties and cytotoxicity of chitosan nanocapsules by chemical grafting. Mar. Drugs. 2016;14:175. doi: 10.3390/md14100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bzowska M., Karabasz A., Szczepanowicz K. Encapsulation of camptothecin into pegylated polyelectrolyte nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2018;557:36–42. doi: 10.1016/j.colsurfa.2018.05.070. [DOI] [Google Scholar]
- 66.Shi C., Zhong S., Sun Y., Xu L., He S., Dou Y., Zhao S., Gao Y., Cui X. Sonochemical preparation of folic acid-decorated reductive-responsive epsilon-poly-L-lysine-based microcapsules for targeted drug delivery and reductive-triggered release. Mater. Sci. Eng. C. 2020;106:110251. doi: 10.1016/j.msec.2019.110251. [DOI] [PubMed] [Google Scholar]
- 67.Rosa P., Friedrich M.L., Dos Santos J., Librelotto D.R.N., Maurer L.H., Emanuelli T., Da Silva C.B., Adams A.I.H. Desonide nanoencapsulation with acai oil as oil core: Physicochemical characterization, photostability study and in vitro phototoxicity evaluation. J. Photochem. Photobiol. B Biol. 2019;199:111606. doi: 10.1016/j.jphotobiol.2019.111606. [DOI] [PubMed] [Google Scholar]
- 68.Venturini C.G., Bruinsmann F.A., Contri R.V., Fonseca F.N., Frank L.A., D’Amore C.M., Raffin R.P., Buffon A., Pohlmann A.R., Guterres S.S. Co-encapsulation of imiquimod and copaiba oil in novel nanostructured systems: Promising formulations against skin carcinoma. Eur. J. Pharm. Sci. 2015;79:36–43. doi: 10.1016/j.ejps.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 69.Natrajan D., Srinivasan S., Sundar K., Ravindran A. Formulation of essential oil-loaded chitosan–alginate nanocapsules. J. Food Drug Anal. 2015;23:560–568. doi: 10.1016/j.jfda.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abulateefeh S.R., Alkawareek M.Y., Alkilany A.M. Tunable sustained release drug delivery system based on mononuclear aqueous core-polymer shell microcapsules. Int. J. Pharm. 2019;558:291–298. doi: 10.1016/j.ijpharm.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 71.Cosco D., Paolino D., De Angelis F., Cilurzo F., Celia C., Di Marzio L., Russo D., Tsapis N., Fattal E., Fresta M. Aqueous-core PEG-coated PLA nanocapsules for an efficient entrapment of water soluble anticancer drugs and a smart therapeutic response. Eur. J. Pharm. Biopharm. 2015;89:30–39. doi: 10.1016/j.ejpb.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 72.Vrignaud S., Anton N., Passirani C., Benoit J.P., Saulnier P. Aqueous core nanocapsules: A new solution for encapsulating doxorubicin hydrochloride. Drug Dev. Ind. Pharm. 2013;39:1706–1711. doi: 10.3109/03639045.2012.730526. [DOI] [PubMed] [Google Scholar]
- 73.Lambert G., Fattal E., Pinto-Alphandary H., Gulik A., Couvreur P. Polyisobutylcyanoacrylate nanocapsules containing an aqueous core for the delivery of oligonucleotides. Int. J. Pharm. 2001;214:13–16. doi: 10.1016/S0378-5173(00)00624-4. [DOI] [PubMed] [Google Scholar]
- 74.Hillaireau H., Le Doan T., Chacun H., Janin J., Couvreur P. Encapsulation of mono-and oligo-nucleotides into aqueous-core nanocapsules in presence of various water-soluble polymers. Int. J. Pharm. 2007;331:148–152. doi: 10.1016/j.ijpharm.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 75.Li S., He Y., Li C., Liu X. In vitro release of protein from poly(butylcyanoacrylate) nanocapsules with an aqueous core. Colloid Polym. Sci. 2004;283:480–485. doi: 10.1007/s00396-004-1173-5. [DOI] [Google Scholar]
- 76.Gil P.R., Loretta L., Muñoz_Javier A., Parak W.J. Nanoparticle-modified polyelectrolyte capsules. Nano Today. 2008;3:12–21. doi: 10.1016/S1748-0132(08)70040-9. [DOI] [Google Scholar]
- 77.Johnston A.P., Cortez C., Angelatos A.S., Caruso F. Layer-by-layer engineered capsules and their applications. Curr. Opin. Colloid Interface Sci. 2006;11:203–209. doi: 10.1016/j.cocis.2006.05.001. [DOI] [Google Scholar]
- 78.Shagholani H., Ghorbani M., Nikpay A., Soltani M. Chitosan nanocapsule-mounted cellulose nanofibrils as nanoships for smart drug delivery systems and treatment of avian trichomoniasis. J. Taiwan Inst. Chem. Eng. 2019;95:290–299. doi: 10.1016/j.jtice.2018.07.014. [DOI] [Google Scholar]
- 79.Elbaz N.M., Owen A., Rannard S., McDonald T.O. Controlled synthesis of calcium carbonate nanoparticles and stimuli-responsivemulti-layered nanocapsules for oral drug delivery. Int. J. Pharm. 2019;574 doi: 10.1016/j.ijpharm.2019.118866. [DOI] [PubMed] [Google Scholar]
- 80.Menard M., Meyer F., Parkhomenko K., Leuvrey C., Francius G., Begin-Colin S., Mertz D. Mesoporous silica templated-albumin nanoparticles with high doxorubicin payload for drug delivery assessed with a 3-D tumor cell model. Biochim. Biophys. Acta Gen. Subj. 2019;1863:332–341. doi: 10.1016/j.bbagen.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 81.Shang B., Zhang X., Ji R., Wang Y., Hu H., Peng B., Deng Z. Preparation of colloidal polydopamine/Au hollow spheres for enhanced ultrasound contrast imaging and photothermal therapy. Mater. Sci. Eng. C. 2020;106:110174. doi: 10.1016/j.msec.2019.110174. [DOI] [PubMed] [Google Scholar]
- 82.Rata D.M., Cadinoiu A.N., Atanase L.I., Bacaita S.E., Mihalache C., Daraba O.M., Gherghel D., Popa M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C. 2019;103:109828. doi: 10.1016/j.msec.2019.109828. [DOI] [PubMed] [Google Scholar]
- 83.Dubey V., Mohan P., Dangi J.S., Kesavan K. Brinzolamide loaded chitosan-pectin mucoadhesive nanocapsules for management of glaucoma: Formulation, characterization and pharmacodynamic study. Int. J. Biol. Macromol. 2019 doi: 10.1016/j.ijbiomac.2019.10.219. [DOI] [PubMed] [Google Scholar]
- 84.Couvreur P., Barratt G., Fattal E., Vauthier C. Nanocapsule technology: A review. Crit. Rev. Ther. Drug Carr. Syst. 2002;19 doi: 10.1615/CritRevTherDrugCarrierSyst.v19.i2.10. [DOI] [PubMed] [Google Scholar]
- 85.Fessi H., Puisieux F., Devissaguet J.P., Ammoury N., Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989;55:R1–R4. doi: 10.1016/0378-5173(89)90281-0. [DOI] [Google Scholar]
- 86.Lu Z., Bei J., Wang S. A method for the preparation of polymeric nanocapsules without stabilizer. J. Control. Release. 1999;61:107–112. doi: 10.1016/S0168-3659(99)00112-1. [DOI] [PubMed] [Google Scholar]
- 87.Ferranti V., Marchais H., Chabenat C., Orecchioni A., Lafont O. Primidone-loaded poly-ε-caprolactone nanocapsules: Incorporation efficiency and in vitro release profiles. Int. J. Pharm. 1999;193:107–111. doi: 10.1016/S0378-5173(99)00325-7. [DOI] [PubMed] [Google Scholar]
- 88.Lertsutthiwong P., Noomun K., Jongaroonngamsang N., Rojsitthisak P., Nimmannit U. Preparation of alginate nanocapsules containing turmeric oil. Carbohydr. Polym. 2008;74:209–214. doi: 10.1016/j.carbpol.2008.02.009. [DOI] [Google Scholar]
- 89.Veragten A., Contri R.V., Betti A.H., Herzfeldt V., Frank L.A., Pohlmann A.R., Rates S.M.K., Guterres S.S. Chitosan-coated nanocapsules ameliorates the effect of olanzapine in prepulse inhibition of startle response (PPI) in rats following oral administration. React. Funct. Polym. 2020;148 doi: 10.1016/j.reactfunctpolym.2020.104493. [DOI] [Google Scholar]
- 90.Liakos I.L., Iordache F., Carzino R., Scarpellini A., Oneto M., Bianchini P., Grumezescu A.M., Holban A.M. Cellulose acetate—Essential oil nanocapsules with antimicrobial activity for biomedical applications. Colloids Surf. B Biointerfaces. 2018;172:471–479. doi: 10.1016/j.colsurfb.2018.08.069. [DOI] [PubMed] [Google Scholar]
- 91.Anton N., Benoit J.-P., Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release. 2008;128:185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 92.Quintanar-Guerrero D., Allémann E., Doelker E., Fessi H. Preparation and characterization of nanocapsules from preformed polymers by a new process based on emulsification-diffusion technique. Pharm. Res. 1998;15:1056–1062. doi: 10.1023/A:1011934328471. [DOI] [PubMed] [Google Scholar]
- 93.Esmaeili A., Niknam S. Preparation of polyamide nanocapsules of Elaeagnus angustifolia L. delivery with in vivo studies. Ind. Crop. Prod. 2014;55:49–55. doi: 10.1016/j.indcrop.2014.01.053. [DOI] [Google Scholar]
- 94.Monika P., Basavaraj B., Murthy K., Ahalya N., Gurudev K. Nanocapsules of catechin rich extract for enhanced antioxidant potential and in vitro bioavailability. J. Appl. Pharm. Sci. 2017;7:184–188. doi: 10.7324/JAPS.2017.70126. [DOI] [Google Scholar]
- 95.Sombra F.M., Rosa Richter A., Rodrigues de Araujo A., De Oliveira Silva Ribeiro F., De Fatima Souza Mendes J., Oliveira Dos Santos Fontenelle R., Alves da Silva D., Paula H.C.B., Feitosa J.P.A., Goycoolea F.M., et al. Development of amphotericin B-loaded propionate sterculia striata polysaccharide nanocarrier. Int. J. Biol. Macromol. 2019 doi: 10.1016/j.ijbiomac.2019.10.053. [DOI] [PubMed] [Google Scholar]
- 96.Boissenot T., Bordat A., Larrat B., Varna M., Chacun H., Paci A., Poinsignon V., Fattal E., Tsapis N. Ultrasound-induced mild hyperthermia improves the anticancer efficacy of both Taxol(R) and paclitaxel-loaded nanocapsules. J. Control. Release. 2017;264:219–227. doi: 10.1016/j.jconrel.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 97.Ma J., Feng P., Ye C., Wang Y., Fan Y. An improved interfacial coacervation technique to fabricate biodegradable nanocapsules of an aqueous peptide solution from polylactide and its block copolymers with poly (ethylene glycol) Colloid Polym. Sci. 2001;279:387–392. doi: 10.1007/s003960000467. [DOI] [Google Scholar]
- 98.Xiao Z., Li W., Zhu G., Zhou R., Niu Y. Study of production and the stability of styrallyl acetate nanocapsules using complex coacervation. Flavour Fragr. J. 2016;31:283–289. doi: 10.1002/ffj.3306. [DOI] [Google Scholar]
- 99.Bae K.H., Lee Y., Park T.G. Oil-encapsulating PEO−PPO−PEO/PEG shell cross-linked nanocapsules for target-specific delivery of paclitaxel. Biomacromolecules. 2007;8:650–656. doi: 10.1021/bm0608939. [DOI] [PubMed] [Google Scholar]
- 100.Lee K., Bae K.H., Lee Y., Lee S.H., Ahn C.H., Park T.G. Pluronic/polyethylenimine shell crosslinked nanocapsules with embedded magnetite nanocrystals for magnetically triggered delivery of siRNA. Macromol. Biosci. 2010;10:239–245. doi: 10.1002/mabi.200900291. [DOI] [PubMed] [Google Scholar]
- 101.Steinmacher F., Baier G., Musyanovych A., Landfester K., Araújo P., Sayer C. Design of cross-linked starch nanocapsules for enzyme-triggered release of hydrophilic compounds. Processes. 2017;5:25. doi: 10.3390/pr5020025. [DOI] [Google Scholar]
- 102.Tian K., Zeng J., Zhao X., Liu L., Jia X., Liu P. Synthesis of multi-functional nanocapsules via interfacial AGET ATRP in miniemulsion for tumor micro-environment responsive drug delivery. Colloids Surf. B Biointerfaces. 2015;134:188–195. doi: 10.1016/j.colsurfb.2015.06.057. [DOI] [PubMed] [Google Scholar]
- 103.Forero Ramirez L.M., Boudier A., Gaucher C., Babin J., Durand A., Six J.L., Nouvel C. Dextran-covered pH-sensitive oily core nanocapsules produced by interfacial Reversible Addition-Fragmentation chain transfer miniemulsion polymerization. J. Colloid Interface Sci. 2020;569:57–67. doi: 10.1016/j.jcis.2020.02.066. [DOI] [PubMed] [Google Scholar]
- 104.Ishizuka F., Utama R.H., Kim S., Stenzel M.H., Zetterlund P.B. RAFT inverse miniemulsion periphery polymerization in binary solvent mixtures for synthesis of nanocapsules. Eur. Polym. J. 2015;73:324–334. doi: 10.1016/j.eurpolymj.2015.10.020. [DOI] [Google Scholar]
- 105.Loiko O.P., Van Herk A.M., Ali S.I., Burkeev M.Z., Tazhbayev Y.M., Zhaparova L.Z. Controlled release of Capreomycin sulfate from pH-responsive nanocapsules. e-Polymers. 2013;2013:189–202. doi: 10.1515/epoly.2013.13.1.189. [DOI] [Google Scholar]
- 106.Ali S.I., Heuts J.P., Van Herk A.M. Vesicle-templated pH-responsive polymeric nanocapsules. Soft Matter. 2011;7:5382–5390. doi: 10.1039/c1sm05266g. [DOI] [Google Scholar]
- 107.Tallian C., Herrero-Rollett A., Stadler K., Vielnascher R., Wieland K., Weihs A.M., Pellis A., Teuschl A.H., Lendl B., Amenitsch H., et al. Structural insights into pH-responsive drug release of self-assembling human serum albumin-silk fibroin nanocapsules. Eur. J. Pharm. Biopharm. 2018;133:176–187. doi: 10.1016/j.ejpb.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 108.Artusio F., Bazzano M., Pisano R., Coulon P.-E., Rizza G., Schiller T., Sangermano M. Polymeric nanocapsules via interfacial cationic photopolymerization in miniemulsion. Polymer. 2018;139:155–162. doi: 10.1016/j.polymer.2018.02.019. [DOI] [Google Scholar]
- 109.Benedetti M., Congdon T.R., Bassett S.P., Alauhdin M., Howdle S.M., Haddleton D.M., Pisano R., Sangermano M., Schiller T.L. Synthesis of polymeric microcapsules by interfacial-suspension cationic photopolymerisation of divinyl ether monomer in aqueous suspension. Polym. Chem. 2017;8:972–975. doi: 10.1039/C6PY01782G. [DOI] [Google Scholar]
- 110.Bilati U., Allémann E., Doelker E. Sonication parameters for the preparation of biodegradable nanocapsulesof controlled size by the double emulsion method. Pharm. Dev. Technol. 2003;8:1–9. doi: 10.1081/PDT-120017517. [DOI] [PubMed] [Google Scholar]
- 111.Ashjari M., Khoee S., Mahdavian A.R. Controlling the morphology and surface property of magnetic/cisplatin-loaded nanocapsules via W/O/W double emulsion method. Colloids Surf. A Physicochem. Eng. Asp. 2012;408:87–96. doi: 10.1016/j.colsurfa.2012.05.035. [DOI] [Google Scholar]
- 112.Campos E.V.R., Oliveira J.L., Zavala-Betancourt S.A., Ledezma A.S., Arias E., Moggio I., Romero J., Fraceto L.F. Development of stained polymeric nanocapsules loaded with model drugs: Use of a fluorescent poly(phenyleneethynylene) Colloids Surf. B Biointerfaces. 2016;147:442–449. doi: 10.1016/j.colsurfb.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 113.Erdmann C., Mayer C. Permeability profile of poly(alkyl cyanoacrylate) nanocapsules. J. Colloid Interface Sci. 2016;478:394–401. doi: 10.1016/j.jcis.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 114.Hu S.H., Chen S.Y., Gao X. Multifunctional nanocapsules for simultaneous encapsulation of hydrophilic and hydrophobic compounds and on-demand release. ACS Nano. 2012;6:2558–2565. doi: 10.1021/nn205023w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chiang C.S., Hu S.H., Liao B.J., Chang Y.C., Chen S.Y. Enhancement of cancer therapy efficacy by trastuzumab-conjugated and pH-sensitive nanocapsules with the simultaneous encapsulation of hydrophilic and hydrophobic compounds. Nanomedicine. 2014;10:99–107. doi: 10.1016/j.nano.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 116.Balan V., Dodi G., Tudorachi N., Ponta O., Simon V., Butnaru M., Verestiuc L. Doxorubicin-loaded magnetic nanocapsules based on N-palmitoyl chitosan and magnetite: Synthesis and characterization. Chem. Eng. J. 2015;279:188–197. doi: 10.1016/j.cej.2015.04.152. [DOI] [Google Scholar]
- 117.Cuomo F., Lopez F., Piludu M., Miguel M.G., Lindman B., Ceglie A. Release of small hydrophilic molecules from polyelectrolyte capsules: Effect of the wall thickness. J. Colloid Interface Sci. 2015;447:211–216. doi: 10.1016/j.jcis.2014.10.060. [DOI] [PubMed] [Google Scholar]
- 118.Ji F., Li J., Qin Z., Yang B., Zhang E., Dong D., Wang J., Wen Y., Tian L., Yao F. Engineering pectin-based hollow nanocapsules for delivery of anticancer drug. Carbohydr Polym. 2017;177:86–96. doi: 10.1016/j.carbpol.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 119.Mirgorodskaya A.B., Kushnazarova R.A., Nikitina A.V., Semina I.I., Nizameev I.R., Kadirov M.K., Khutoryanskiy V.V., Zakharova L.Y., Sinyashin O.G. Polyelectrolyte nanocontainers: Controlled binding and release of indomethacin. J. Mol. Liq. 2018;272:982–989. doi: 10.1016/j.molliq.2018.10.115. [DOI] [Google Scholar]
- 120.Ledo A.M., Sasso M.S., Bronte V., Marigo I., Boyd B.J., Garcia-Fuentes M., Alonso M.J. Co-delivery of RNAi and chemokine by polyarginine nanocapsules enables the modulation of myeloid-derived suppressor cells. J. Control. Release. 2019;295:60–73. doi: 10.1016/j.jconrel.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Y., Chi C., Huang X., Zou Q., Li X., Chen L. Starch-based nanocapsules fabricated through layer-by-layer assembly for oral delivery of protein to lower gastrointestinal tract. Carbohydr. Polym. 2017;171:242–251. doi: 10.1016/j.carbpol.2017.04.090. [DOI] [PubMed] [Google Scholar]
- 122.Cook M.T., Tzortzis G., Khutoryanskiy V.V., Charalampopoulos D. Layer-by-layer coating of alginate matrices with chitosan–alginate for the improved survival and targeted delivery of probiotic bacteria after oral administration. J. Mater. Chem. B. 2013;1:52–60. doi: 10.1039/C2TB00126H. [DOI] [PubMed] [Google Scholar]
- 123.Rivera M.C., Pinheiro A.C., Bourbon A.I., Cerqueira M.A., Vicente A.A. Hollow chitosan/alginate nanocapsules for bioactive compound delivery. Int. J. Biol. Macromol. 2015;79:95–102. doi: 10.1016/j.ijbiomac.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 124.Ushirobira C.Y., Afiune L.A.F., Pereira M.N., Cunha-Filho M., Gelfuso G.M., Gratieri T. Dutasteride nanocapsules for hair follicle targeting: Effect of chitosan-coating and physical stimulus. Int. J. Biol. Macromol. 2020;151:56–61. doi: 10.1016/j.ijbiomac.2020.02.143. [DOI] [PubMed] [Google Scholar]
- 125.Ramos P.T., Pedra N.S., Soares M.S.P., Da Silveira E.F., Oliveira P.S., Grecco F.B., Da Silva L.M.C., Ferreira L.M., Ribas D.A., Gehrcke M., et al. Ketoprofen-loaded rose hip oil nanocapsules attenuate chronic inflammatory response in a pre-clinical trial in mice. Mater. Sci. Eng. C. 2019;103:109742. doi: 10.1016/j.msec.2019.109742. [DOI] [PubMed] [Google Scholar]
- 126.Dos Santos Chaves P., Frank L.A., Torge A., Schneider M., Pohlmann A.R., Guterres S.S., Beck R.C.R. Spray-dried carvedilol-loaded nanocapsules for sublingual administration: Mucoadhesive properties and drug permeability. Powder Technol. 2019;354:348–357. doi: 10.1016/j.powtec.2019.06.012. [DOI] [Google Scholar]
- 127.Gehrcke M., Sari M.H.M., Ferreira L.M., Barbieri A.V., Giuliani L.M., Prado V.C., Nadal J.M., Farago P.V., Nogueira C.W., Cruz L. Nanocapsules improve indole-3-carbinol photostability and prolong its antinociceptive action in acute pain animal models. Eur. J. Pharm. Sci. 2018;111:133–141. doi: 10.1016/j.ejps.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 128.Ferreira L.M., Cervi V.F., Sari M.H.M., Barbieri A.V., Ramos A.P., Copetti P.M., De Brum G.F., Nascimento K., Nadal J.M., Farago P.V., et al. Diphenyl diselenide loaded poly(epsilon-caprolactone) nanocapsules with selective antimelanoma activity: Development and cytotoxic evaluation. Mater. Sci. Eng. C. 2018;91:1–9. doi: 10.1016/j.msec.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 129.Araújo R.S., Garcia G.M., Vilela J.M.C., Andrade M.S., Oliveira L.A.M., Kano E.K., Lange C.C., e Brito M.A.V.P., De Mello Brandão H., Mosqueira V.C.F. Cloxacillin benzathine-loaded polymeric nanocapsules: Physicochemical characterization, cell uptake, and intramammary antimicrobial effect. Mater. Sci. Eng. C. 2019;104:110006. doi: 10.1016/j.msec.2019.110006. [DOI] [PubMed] [Google Scholar]
- 130.Marcondes Sari M.H., Zborowski V.A., Ferreira L.M., Jardim N.D.S., Araujo P.C.O., Bruning C.A., Cruz L., Nogueira C.W. Enhanced pharmacological actions of p,p′-methoxyl-diphenyl diselenide-loaded polymeric nanocapsules in a mouse model of neuropathic pain: Behavioral and molecular insights. J. Trace Elem. Med. Biol. 2018;46:17–25. doi: 10.1016/j.jtemb.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 131.Sari M.H.M., Ferreira L.M., Zborowski V.A., Araujo P.C.O., Cervi V.F., Brüning C.A., Cruz L., Nogueira C.W. p,p′-Methoxyl-diphenyl diselenide-loaded polymeric nanocapsules are chemically stable and do not induce toxicity in mice. Eur. J. Pharm. Biopharm. 2017;117:39–48. doi: 10.1016/j.ejpb.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 132.Nicolas S., Bolzinger M.A., Jordheim L.P., Chevalier Y., Fessi H., Almouazen E. Polymeric nanocapsules as drug carriers for sustained anticancer activity of calcitriol in breast cancer cells. Int. J. Pharm. 2018;550:170–179. doi: 10.1016/j.ijpharm.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 133.Omwenga E.O., Hensel A., Shitandi A., Goycoolea F.M. Chitosan nanoencapsulation of flavonoids enhances their quorum sensing and biofilm formation inhibitory activities against an E.coli Top 10 biosensor. Colloids Surf. B Biointerfaces. 2018;164:125–133. doi: 10.1016/j.colsurfb.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 134.Weber D.M., Voss G.T., De Oliveira R.L., Da Fonseca C.A.R., Paltian J., Rodrigues K.C., Ianiski F.R., Vaucher R.A., Luchese C., Wilhelm E.A. Topic application of meloxicam-loaded polymeric nanocapsules as a technological alternative for treatment of the atopic dermatitis in mice. J. Appl. Biomed. 2018;16:337–343. doi: 10.1016/j.jab.2018.03.003. [DOI] [Google Scholar]
- 135.Velasques K., Maciel T.R., De Castro Dal Forno A.H., Teixeira F.E.G., Da Fonseca A.L., Varotti F.P., Fajardo A.R., Avila D.S., Haas S.E. Co-nanoencapsulation of antimalarial drugs increases their in vitro efficacy against Plasmodium falciparum and decreases their toxicity to Caenorhabditis elegans. Eur. J. Pharm. Sci. 2018;118:1–12. doi: 10.1016/j.ejps.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 136.Chaves P.D., Ourique A.F., Frank L.A., Pohlmann A.R., Guterres S.S., Beck R.C. Carvedilol-loaded nanocapsules: Mucoadhesive properties and permeability across the sublingual mucosa. Eur. J. Pharm. Biopharm. 2017;114:88–95. doi: 10.1016/j.ejpb.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 137.Franco C., Antonow M.B., Beckenkamp A., Buffon A., Ceolin T., Tebaldi M.L., Silveira G.P., Guterres S.S., Pohlmann A.R. PCL-b-P(MMA-co-DMAEMA)2 new triblock copolymer for novel pH-sensitive nanocapsules intended for drug delivery to tumors. React. Funct. Polym. 2017;119:116–124. doi: 10.1016/j.reactfunctpolym.2017.08.010. [DOI] [Google Scholar]
- 138.Lollo Lollo G., Gonzalez-Paredes A., Garcia-Fuentes M., Calvo P., Torres D., Alonso M.J. Polyarginine Nanocapsules as a Potential Oral Peptide Delivery Carrier. J. Pharm. Sci. 2017;106:611–618. doi: 10.1016/j.xphs.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 139.Lopes L.Q., Santos C.G., Vaucher Rde A., Raffin R.P., Santos R.C. Nanocapsules with glycerol monolaurate: Effects on Candida albicans biofilms. Microb. Pathog. 2016;97:119–124. doi: 10.1016/j.micpath.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 140.Lollo G., Hervella P., Calvo P., Aviles P., Guillen M.J., Garcia-Fuentes M., Alonso M.J., Torres D. Enhanced in vivo therapeutic efficacy of plitidepsin-loaded nanocapsules decorated with a new poly-aminoacid-PEG derivative. Int. J. Pharm. 2015;483:212–219. doi: 10.1016/j.ijpharm.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 141.Matoso Sombra F., Richter A.R., De Araujo A.R., De Oliveira Silva Ribeiro F., De Fatima Souza Mendes J., Dos Santos Fontenelle R.O., Da Silva D.A., Beserra de Paula H.C., Pessoa de Andrade Feitosa J., Martin Goycoolea F., et al. Nanocapsules of Sterculia striata acetylated polysaccharide as a potential monomeric amphotericin B delivery matrix. Int. J. Biol. Macromol. 2019;130:655–663. doi: 10.1016/j.ijbiomac.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 142.Zafar S., Akhter S., Ahmad I., Hafeez Z., Alam Rizvi M.M., Jain G.K., Ahmad F.J. Improved chemotherapeutic efficacy against resistant human breast cancer cells with co-delivery of Docetaxel and Thymoquinone by Chitosan Grafted Lipid Nanocapsules: Formulation optimization, in vitro and in vivo studies. Colloids Surf. B Biointerfaces. 2019 doi: 10.1016/j.colsurfb.2019.110603. [DOI] [PubMed] [Google Scholar]
- 143.Pinto N.D.O.F., Rodrigues T.H.S., Pereira R.D.C.A., Silva L.M.A.E., Cáceres C.A., Azeredo H.M.C.D., Muniz C.R., Brito E.S.D., Canuto K.M. Production and physico-chemical characterization of nanocapsules of the essential oil from Lippia sidoides Cham. Ind. Crop. Prod. 2016;86:279–288. doi: 10.1016/j.indcrop.2016.04.013. [DOI] [Google Scholar]
- 144.Youm I., Yang X., Murowchick J.B., Youan B.-B.C. Encapsulation of docetaxel in oily core polyester nanocapsules intended for breast cancer therapy. Nanoscale Res. Lett. 2011;6:630. doi: 10.1186/1556-276X-6-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marto J., Pinto P., Fitas M., Goncalves L.M., Almeida A.J., Ribeiro H.M. Safety assessment of starch-based personal care products: Nanocapsules and pickering emulsions. Toxicol. Appl. Pharm. 2018;342:14–21. doi: 10.1016/j.taap.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 146.Marto J., Ruivo E., Lucas S.D., Goncalves L.M., Simoes S., Gouveia L.F., Felix R., Moreira R., Ribeiro H.M., Almeida A.J. Starch nanocapsules containing a novel neutrophil elastase inhibitor with improved pharmaceutical performance. Eur. J. Pharm. Biopharm. 2018;127:1–11. doi: 10.1016/j.ejpb.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 147.Boissenot T., Fattal E., Bordat A., Houvenagel S., Valette J., Chacun H., Gueutin C., Tsapis N. Paclitaxel-loaded PEGylated nanocapsules of perfluorooctyl bromide as theranostic agents. Eur. J. Pharm. Biopharm. 2016;108:136–144. doi: 10.1016/j.ejpb.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 148.Xavier-Jr F.H., Gueutin C., Chacun H., Vauthier C., Egito E.S.T. Mucoadhesive paclitaxel-loaded chitosan-poly (isobutyl cyanoacrylate) core-shell nanocapsules containing copaiba oil designed for oral drug delivery. J. Drug Deliv. Sci. Technol. 2019;53 doi: 10.1016/j.jddst.2019.101194. [DOI] [Google Scholar]
- 149.Behzadi S., Stadler J., Hosseinpour S., Crespy D., Landfester K. Suppressing non-controlled leakage of hydrophilic payloads from redox-responsive nanocapsules. Colloids Surf. A Physicochem. Eng. Asp. 2017;532:2–7. doi: 10.1016/j.colsurfa.2017.07.077. [DOI] [Google Scholar]
- 150.Passlick D., Piradashvili K., Bamberger D., Li M., Jiang S., Strand D., Wich P.R., Landfester K., Bros M., Grabbe S., et al. Delivering all in one: Antigen-nanocapsule loaded with dual adjuvant yields superadditive effects by DC-directed T cell stimulation. J. Control. Release. 2018;289:23–34. doi: 10.1016/j.jconrel.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 151.Yilgor P., Hasirci N., Hasirci V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J. Biomed. Mater. Res. Part A. 2010;93:528–536. doi: 10.1002/jbm.a.32520. [DOI] [PubMed] [Google Scholar]
- 152.Piotrowski M., Jantas D., Leśkiewicz M., Szczepanowicz K., Warszyński P., Lasoń W. Polyelectrolyte-coated nanocapsules containing cyclosporine A protect neuronal-like cells against oxidative stress-induced cell damage. Colloids Surf. A Physicochem. Eng. Asp. 2018;555:264–269. doi: 10.1016/j.colsurfa.2018.07.005. [DOI] [Google Scholar]
- 153.Baran E.T., Özer N., Hasirci V. Poly (hydroxybutyrate-co-hydroxyvalerate) nanocapsules as enzyme carriers for cancer therapy: An in vitro study. J. Microencapsul. 2002;19:363–376. doi: 10.1080/02652040110105355. [DOI] [PubMed] [Google Scholar]
- 154.Kim C.S., Mout R., Zhao Y., Yeh Y.-C., Tang R., Jeong Y., Duncan B., Hardy J.A., Rotello V.M. Co-delivery of protein and small molecule therapeutics using nanoparticle-stabilized nanocapsules. Bioconjugate Chem. 2015;26:950–954. doi: 10.1021/acs.bioconjchem.5b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Prasetyanto E.A., Bertucci A., Septiadi D., Corradini R., Castro-Hartmann P., De Cola L. Breakable hybrid organosilica nanocapsules for protein delivery. Angew. Chem. Int. Ed. 2016;55:3323–3327. doi: 10.1002/anie.201508288. [DOI] [PubMed] [Google Scholar]
- 156.Huu V.A.N., Luo J., Zhu J., Zhu J., Patel S., Boone A., Mahmoud E., McFearin C., Olejniczak J., De Gracia Lux C. Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye. J. Control. Release. 2015;200:71–77. doi: 10.1016/j.jconrel.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yuan Q., Shah J., Hein S., Misra R. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010;6:1140–1148. doi: 10.1016/j.actbio.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 158.Xia X., Hu Z., Marquez M. Physically bonded nanoparticle networks: A novel drug delivery system. J. Control. Release. 2005;103:21–30. doi: 10.1016/j.jconrel.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 159.Richards D.A., Maruani A., Chudasama V. Antibody fragments as nanoparticle targeting ligands: A step in the right direction. Chem. Sci. 2017;8:63–77. doi: 10.1039/C6SC02403C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Walters R., Kraig R.P., Medintz I., Delehanty J.B., Stewart M.H., Susumu K., Huston A.L., Dawson P.E., Dawson G. Nanoparticle targeting to neurons in a rat hippocampal slice culture model. Asn Neuro. 2012;4:383–392. doi: 10.1042/AN20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yameen B., Choi W.I., Vilos C., Swami A., Shi J., Farokhzad O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release. 2014;190:485–499. doi: 10.1016/j.jconrel.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Davis M.E., Chen Z., Shin D.M. Nanoscience and Technology: A Collection of Reviews from Nature Journals. World Scientific; Singapore: 2010. Nanoparticle therapeutics: An emerging treatment modality for cancer; pp. 239–250. [Google Scholar]
- 163.Kansal S., Tandon R., Dwivedi P., Misra P., Verma P., Dube A., Mishra P.R. Development of nanocapsules bearing doxorubicin for macrophage targeting through the phosphatidylserine ligand: A system for intervention in visceral leishmaniasis. J. Antimicrob. Chemother. 2012;67:2650–2660. doi: 10.1093/jac/dks286. [DOI] [PubMed] [Google Scholar]
- 164.Xiao Z., Farokhzad O.C. Aptamer-functionalized nanoparticles for medical applications: Challenges and opportunities. ACS Nano. 2012;6:3670–3676. doi: 10.1021/nn301869z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Farokhzad O.C., Jon S., Khademhosseini A., Tran T.-N.T., LaVan D.A., Langer R. Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004;64:7668–7672. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 166.Bogdan N., Vetrone F., Roy R., Capobianco J.A. Carbohydrate-coated lanthanide-doped upconverting nanoparticles for lectin recognition. J. Mater. Chem. 2010;20:7543–7550. doi: 10.1039/c0jm01617a. [DOI] [Google Scholar]
- 167.Williams S.A., Schreier A.M. The effect of education in managing side effects in women receiving chemotherapy for treatment of breast cancer. Oncol. Nurs. Forum. 2007;31:E16–E23. doi: 10.1188/04.ONF.E16-E23. [DOI] [PubMed] [Google Scholar]
- 168.Sun C.C., Bodurka D.C., Weaver C.B., Rasu R., Wolf J.K., Bevers M.W., Smith J.A., Wharton J.T., Rubenstein E.B. Rankings and symptom assessments of side effects from chemotherapy: Insights from experienced patients with ovarian cancer. Supportive Care Cancer. 2005;13:219–227. doi: 10.1007/s00520-004-0710-6. [DOI] [PubMed] [Google Scholar]
- 169.Sitzia J., Huggins L. Side effects of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) chemotherapy for breast cancer. Cancer Pract. 1998;6:13–21. doi: 10.1046/j.1523-5394.1998.1998006013.x. [DOI] [PubMed] [Google Scholar]
- 170.Wang J., Sun X., Mao W., Sun W., Tang J., Sui M., Shen Y., Gu Z. Tumor redox heterogeneity-responsive prodrug nanocapsules for cancer chemotherapy. Adv. Mater. 2013;25:3670–3676. doi: 10.1002/adma.201300929. [DOI] [PubMed] [Google Scholar]
- 171.Lu S., Xu L., Kang E.T., Mahendran R., Chiong E., Neoh K.G. Co-delivery of peptide-modified cisplatin and doxorubicin via mucoadhesive nanocapsules for potential synergistic intravesical chemotherapy of non-muscle-invasive bladder cancer. Eur. J. Pharm. Sci. 2016;84:103–115. doi: 10.1016/j.ejps.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 172.Galisteo-Gonzalez F., Molina-Bolivar J.A., Navarro S.A., Boulaiz H., Aguilera-Garrido A., Ramirez A., Marchal J.A. Albumin-covered lipid nanocapsules exhibit enhanced uptake performance by breast-tumor cells. Colloids Surf. B Biointerfaces. 2018;165:103–110. doi: 10.1016/j.colsurfb.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 173.Molina-Bolívar J.A., Galisteo-González F. Olive-oil nanocapsules stabilized by HSA: Influence of processing variables on particle properties. J. Nanoparticle Res. 2015;17 doi: 10.1007/s11051-015-3192-1. [DOI] [Google Scholar]
- 174.Xavier-Junior F.H., Egito E.S.T.D., Morais A.R.D.V., Alencar E.D.N., Maciuk A., Vauthier C. Experimental design approach applied to the development of chitosan coated poly(isobutylcyanoacrylate) nanocapsules encapsulating copaiba oil. Colloids Surf. A Physicochem. Eng. Asp. 2018;536:251–258. doi: 10.1016/j.colsurfa.2017.02.055. [DOI] [Google Scholar]
- 175.Bouchemal K., Couenne F., Briançon S., Fessi H., Tayakout M. Polyamides nanocapsules: Modeling and wall thickness estimation. AIChE J. 2006;52:2161–2170. doi: 10.1002/aic.10828. [DOI] [Google Scholar]
- 176.Nassar T., Rom A., Nyska A., Benita S. Novel double coated nanocapsules for intestinal delivery and enhanced oral bioavailability of tacrolimus, a P-gp substrate drug. J. Control. Release. 2009;133:77–84. doi: 10.1016/j.jconrel.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 177.Rouzes C., Gref R., Leonard M., De Sousa Delgado A., Dellacherie E. Surface modification of poly (lactic acid) nanospheres using hydrophobically modified dextrans as stabilizers in an o/w emulsion/evaporation technique. J. Biomed. Mater. Res. 2000;50:557–565. doi: 10.1002/(SICI)1097-4636(20000615)50:4<557::AID-JBM11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 178.Sahana D.K., Mittal G., Bhardwaj V., Kumar M.N. PLGA nanoparticles for oral delivery of hydrophobic drugs: Influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drug. J. Pharm. Sci. 2008;97:1530–1542. doi: 10.1002/jps.21158. [DOI] [PubMed] [Google Scholar]
- 179.Kumar V., Prud’homme R.K. Nanoparticle stability: Processing pathways for solvent removal. Chem. Eng. Sci. 2009;64:1358–1361. doi: 10.1016/j.ces.2008.11.017. [DOI] [Google Scholar]
- 180.Jakubiak P., Thwala L.N., Cadete A., Préat V., Alonso M.J., Beloqui A., Csaba N. Solvent-free protamine nanocapsules as carriers for mucosal delivery of therapeutics. Eur. Polym. J. 2017;93:695–705. doi: 10.1016/j.eurpolymj.2017.03.049. [DOI] [Google Scholar]
- 181.Steelandt J., Salmon D., Gilbert E., Almouazen E., Renaud F.N., Roussel L., Haftek M., Pirot F. Antimicrobial nanocapsules: From new solvent-free process to in vitro efficiency. Int. J. Nanomed. 2014;9:4467–4474. doi: 10.2147/IJN.S64746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Villegas M.R., Baeza A., Usategui A., Ortiz-Romero P.L., Pablos J.L., Vallet-Regi M. Collagenase nanocapsules: An approach to fibrosis treatment. Acta Biomater. 2018;74:430–438. doi: 10.1016/j.actbio.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 183.Ezhilarasi P., Karthik P., Chhanwal N., Anandharamakrishnan C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioprocess Technol. 2013;6:628–647. doi: 10.1007/s11947-012-0944-0. [DOI] [Google Scholar]
- 184.Wang Y., Kho K., Cheow W.S., Hadinoto K. A comparison between spray drying and spray freeze drying for dry powder inhaler formulation of drug-loaded lipid–polymer hybrid nanoparticles. Int. J. Pharm. 2012;424:98–106. doi: 10.1016/j.ijpharm.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 185.Wang Y., Zheng Z., Wang K., Tang C., Liu Y., Li J. Prebiotic carbohydrates: Effect on physicochemical stability and solubility of algal oil nanoparticles. Carbohydr. Polym. 2020;228:115372. doi: 10.1016/j.carbpol.2019.115372. [DOI] [PubMed] [Google Scholar]
- 186.Patel R., Patel M., Suthar A. Spray drying technology: An overview. Indian J. Sci. Technol. 2009;2:44–47. doi: 10.17485/ijst/2009/v2i10/30719. [DOI] [Google Scholar]
- 187.Moretton M.A., Chiappetta D.A., Sosnik A. Cryoprotection–lyophilization and physical stabilization of rifampicin-loaded flower-like polymeric micelles. J. R. Soc. Interface. 2011;9:487–502. doi: 10.1098/rsif.2011.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Assegehegn G., Brito-de la Fuente E., Franco J.M., Gallegos C. The importance of understanding the freezing step and its impact on freeze drying process performance. J. Pharm. Sci. 2018 doi: 10.1016/j.xphs.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 189.Verdun C., Couvreur P., Vranckx H., Lenaerts V., Roland M. Development of a nanoparticle controlled-release formulation for human use. J. Control. Release. 1986;3:205–210. doi: 10.1016/0168-3659(86)90081-7. [DOI] [Google Scholar]
- 190.Konan Y.N., Cerny R., Favet J., Berton M., Gurny R., Allémann E. Preparation and characterization of sterile sub-200 nm meso-tetra (4-hydroxylphenyl) porphyrin-loaded nanoparticles for photodynamic therapy. Eur. J. Pharm. Biopharm. 2003;55:115–124. doi: 10.1016/S0939-6411(02)00128-5. [DOI] [PubMed] [Google Scholar]
- 191.Allemann E., Konan Y., Gurny R., Boch R.E. Compositions and Methods for Delivery of Photosensitive Drugs. 7,455,858B2. U.S. Patent. 2008 Nov 25;
- 192.Heiati H., Tawashi R., Phillips N. Drug retention and stability of solid lipid nanoparticles containing azidothymidine palmitate after autoclaving, storage and lyophilization. J. Microencapsul. 1998;15:173–184. doi: 10.3109/02652049809006847. [DOI] [PubMed] [Google Scholar]
- 193.Seyfoddin A., Shaw J., Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010;17:467–489. doi: 10.3109/10717544.2010.483257. [DOI] [PubMed] [Google Scholar]
- 194.Masson V., Maurin F., Fessi H., Devissaguet J. Influence of sterilization processes on poly (ε-caprolactone) nanospheres. Biomaterials. 1997;18:327–335. doi: 10.1016/S0142-9612(96)00144-5. [DOI] [PubMed] [Google Scholar]
- 195.Özcan I., Bouchemal K., Segura-Sánchez F., Abaci Ö., Özer Ö., Güneri T., Ponchel G. Effects of sterilization techniques on the PEGylated poly (γ-benzyl-l-glutamate)(PBLG) nanoparticles. Acta Pharm. Sci. 2009;51 doi: 10.2147/IJN.S15493. [DOI] [Google Scholar]
- 196.Rollot J., Couvreur P., Roblot-Treupel L., Puisieux F. Physicochemical and morphological characterization of polyisobutyl cyanoacrylate nanocapsules. J. Pharm. Sci. 1986;75:361–364. doi: 10.1002/jps.2600750408. [DOI] [PubMed] [Google Scholar]
- 197.Long D., Wu G., Chen S. Preparation of oligochitosan stabilized silver nanoparticles by gamma irradiation. Radiat. Phys. Chem. 2007;76:1126–1131. doi: 10.1016/j.radphyschem.2006.11.001. [DOI] [Google Scholar]
- 198.Lamanna M., Morales N.J., García N.L., Goyanes S. Development and characterization of starch nanoparticles by gamma radiation: Potential application as starch matrix filler. Carbohydr. Polym. 2013;97:90–97. doi: 10.1016/j.carbpol.2013.04.081. [DOI] [PubMed] [Google Scholar]