Abstract
Oxidative stress is an important event under both physiological and pathological conditions. In this study, we demonstrate how to quantify oxidative stress by measuring total reactive oxygen species (ROS) using 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) staining in colorectal cancer cell lines as an example. This protocol describes detailed steps including preparation of DCFH-DA solution, incubation of cells with DCFH-DA solution, and measurement of normalized intensity. DCFH-DA staining is a simple and cost-effective way to detect ROS in cells. It can be used to measure ROS generation after chemical treatment or genetic modifications. Therefore, it is useful for determining cellular oxidative stress upon environment stress, providing clues to mechanistic studies.
Introduction
Three major reactive oxygen species (ROS) produced by cellular metabolism that are of physiological meaning are superoxide anion, hydroxyl radical, and hydrogen peroxide1. At low concentrations, they participate in physiological cell processes, but at high concentrations they have adverse effects on cell signaling pathways1. Our body has developed antioxidant systems, which are effective against excessive ROS. However, oxidative stress can occur when ROS overwhelm the detoxifying ability of our body, which contributes to many pathological conditions, including inflammation, cancer, and neurodegenerative disease2, 3, 4. The purpose of this method is to determine total cellular ROS in adherent cells using 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) staining. The rationale is that oxidation of DCFH-DA to 2’-7’dichlorofluorescein (DCF) has been used extensively for total ROS detection including hydroxyl radicals (•OH) and nitrogen dioxide (•NO2). Mechanistically, DCFH-DA is taken up by cells where cellular esterase cleaves off the acetyl groups, resulting in DCFH. Oxidation of DCFH by ROS converts the molecule to DCF, which emits green fluorescence at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Compared with detection of fluorescence with flow cytometry and other alternative methods5, advantages of this method using a fluorescence microscope and a plate reader are that it produces clearly visible fluorescent images, and is easy to perform, efficient and cost-effective. This method has been widely used to detect cellular ROS for studying various conditions6, 7, 8. This protocol is used for detecting total ROS in adherent cells. Using this method to detect ROS in suspension cells may need some modifications.
Protocol
1. Cell seeding
Seed 2 × 105 HCT116 colorectal cancer cells per well in a 24-well plate and maintain the cells in Dulbecco’s modified Eagle medium (DMEM) overnight at 37 °C.
Replace the culture medium with or without 100 μM ferrous sulfate (FS) or 10 μM doxorubicin (DOX) containing medium and incubate for 24 h.
2. Preparation of the DCFH-DA solution
Dissolve 4.85 mg of DCFH-DA in 1 mL of dimethyl sulfoxide (DMSO) to make a 10 mM stock solution.
Dilute the stock solution with pre-warmed DMEM into 10 μM working solution right before adding it to the wells.
Vortex the working solution for 10 s.
3. DCFH-DA staining
Remove the drug containing medium and wash once with DMEM.
Add 500 μL of the DCFH-DA working solution into each well and incubate at 37 °C for 30 min.
Remove the DCFH-DA working solution. Wash once with DMEM and 2× with 1× phosphate-buffered saline (PBS).
Add 500 μL of 1× PBS to each well.
4. Imaging acquisition and intensity measurement
Take representative fluorescent images for each well using the green fluorescent protein (GFP) channel on a fluorescence microscope.
After taking images, remove PBS and add 200 μL of radioimmunoprecipitation assay (RIPA) buffer to each well.
Incubate on ice for 5 min, then collect cell lysate into 1.5 mL tubes.
Centrifuge at 21,130 × g for 10 min at 4 °C.
Transfer 100 μL of the supernatant to a black 96 well plate and measure the fluorescence intensity using a fluorescence a microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Transfer 1 μL of the supernatant to a clear 96 well plate containing 100 μL of 1× protein assay solution to measure the protein concentration using the Bradford assay9.
Normalize fluorescence intensities with protein concentrations.
Representative Results
HCT116 colorectal cancer cells were treated with 100 μM FS or 10 μM DOX to induce oxidative stress7. As shown in Figure 1, green fluorescence was dramatically increased by both FS and DOX as expected. To quantify the relative intensity change, the cells were lysed after taking images and normalized with protein concentrations. The quantified fluorescence intensity was significantly increased by FS or DOX in HCT116 cells.
Figure 1: Iron treatment increases ROS in colorectal cancer cells.
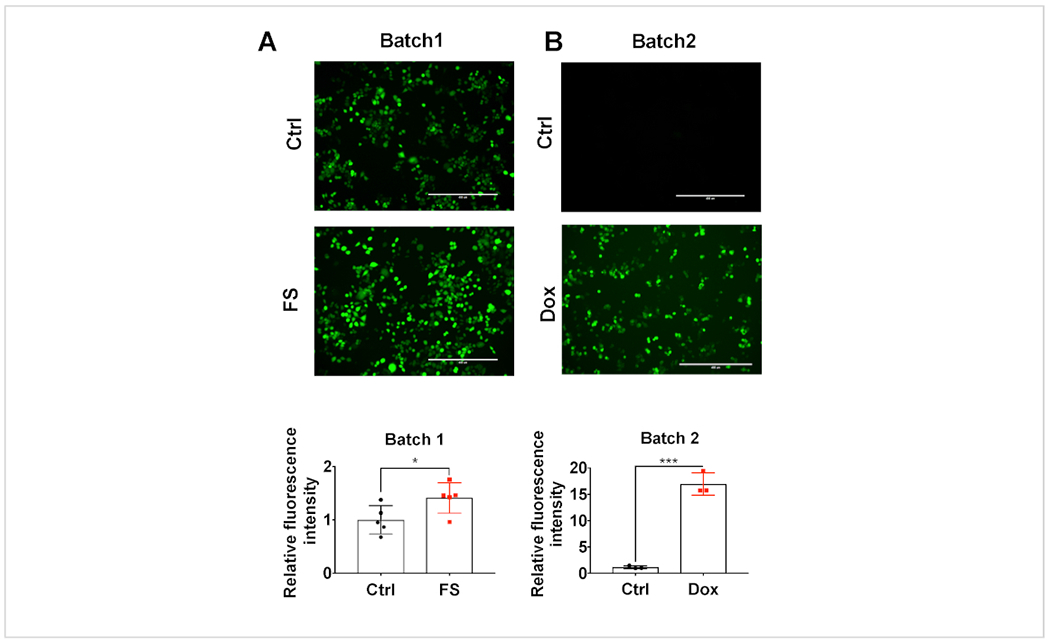
Representative fluorescent images taken by a fluorescence microscope and intensity quantification by a fluorescence microplate reader for 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) staining in HCT116 cells after untreated control (Ctrl), (A) 100 μM ferrous sulfate (FS) or (B) 10 μM doxorubicin (DOX) treatment for 24 h. Scale bar = 400 μm. * = p < 0.05; *** = p < 0.001.
Discussion
The experimental protocol described here is easily reproducible to measure cellular total ROS. The critical steps include making DCFH-DA solution fresh and avoiding light exposure, minimizing cell status disturbance and extensive PBS washing right before taking images. For the preparation of DCFH-DA working solution, the stock solution should be added into pre-warmed DMEM right before adding into the 24 well plate. The reason is that old solutions that generate high background fluorescence or light exposure will lead to photobleaching. Most studies use 1× PBS or 1× Hanks’ balanced salt solution (HBSS) to dilute DCFH-DA and use it as reaction buffer10. However, when using HCT116 and RKO, dilution of DCFH-DA stock solution with PBS and fetal bovine serum free DMEM generated high background signal even in untreated cell. This may be due to cell status disturbance. In addition, the DCFH-DA working solution should be added slowly along the well wall. Disturbance of cell status will generate high fluorescence signal compared to the undisturbed nearby area. It is also critical to wash at least twice with PBS before taking images to reduce auto-fluorescence of the phenol containing DMEM. Phenol-free DMEM may be a better choice but we show here that PBS washing was sufficient to minimize auto-fluorescence. As shown in Figure 1, even in untreated control groups two different batches of experiments could result in different representative images. To control experimental variations, we recommend treating cells with diluted DCFH-DA working solution (as in the protocol) instead of adding stock solution directly onto the cells. Also, images should be taken in fields with similar cell densities and the same exposure time. Finally, it is important to perform experiments on all comparison groups at the same time.
Due to the significance of ROS, specific ROS detection, in addition to total ROS detection, has also been developed. For example, cellular production of superoxide can be detected by dihydroethidium, which upon oxidation results in hydroxylation at the 2-position to form 2-hydroxyethidium. As 2-hydroxyethidium intercalates into cellular DNA, red fluorescence with excitation and emission wavelengths of 535 nm and 635 nm, respectively, can be observed. Mitochondrial superoxide can be visualized with the MitoSOX reagent, a cationic derivative of dihydroethidium that enters live cells and specifically targets mitochondria. The oxidation product of Mitosox which generates red fluorescence can intercalates into mitochondrial DNA. Chemoselective fluorescent naphthylimide peroxide probe was developed for H2O2 detection11. In addition, detection of hydroxyl radicals using fluorescence spectrophotometry was also reported12.
In summary, here we described a simple and optimized protocol for detecting cellular total ROS using cost-effective DCFH-DA staining.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health (K01DK114390), a Research Scholar Grant from the American Cancer Society (RSG-18-050-01-NEC), a Research Pilot Project Grant from University of New Mexico Environmental Health Signature Program and Superfund (P42 ES025589), a Shared Resources Pilot Project Award and a Research Program Support Pilot Project Award from UNM comprehensive cancer center (P30CA118100), and a new investigator award from the Dedicated Health Research Funds at the University of New Mexico School of Medicine.
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.Birben E et al. Oxidative stress and antioxidant defense. World Allergy Organization Journal. 5 (1), 9–19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim GH et al. The Role of Oxidative Stress in Neurodegenerative Diseases. Experimental Neurobiology. 24 (4), 325–40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan LB, Chandel NS Mitochondrial reactive oxygen species and cancer. Cancer & Metabolism. 2, 17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Formentini L et al. Mitochondrial ROS Production Protects the Intestine from Inflammation through Functional M2 Macrophage Polarization. Cell Reports. 19 (6), 1202–1213 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Rakotoarisoa M et al. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega. 4 (2), 3061–3073 (2019). [Google Scholar]
- 6.Mateen S et al. Increased Reactive Oxygen Species Formation and Oxidative Stress in Rheumatoid Arthritis. PLoS One. 11 (4), (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H et al. The interaction of Hemin and Sestrin2 modulates oxidative stress and colon tumor growth. Toxicology and Applied Pharmacology. 374, 77–85 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SH et al. Sotetsuflavone inhibits proliferation and induces apoptosis of A549 cells through ROS-mediated mitochondrial-dependent pathway. BMC Complementary and Alternative Medicine. 18, 235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruger NJ The Bradford Method For Protein Quantitation In The Protein Protocols Handbook. Edited by Walker JM, 17–24, Humana Press; Totowa, NJ: (2009). [Google Scholar]
- 10.Tetz LM et al. Troubleshooting the dichlorofluorescein assay to avoid artifacts in measurement of toxicant-stimulated cellular production of reactive oxidant species. Journal of Pharmacological and Toxicological Methods. 67 (2), 56–60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong L et al. Hydrogen peroxide detection with high specificity in living cells and inflamed tissues. Regenerative Biomaterials. 3 (4), 217–22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu LZ et al. Quantitative detection of hydroxyl radical generated in quartz powder/phosphate buffer solution system by fluorescence spectrophotometry. Guang Pu Xue Yu Guang Pu Fen Xi. 34 (7), 1886–9 (2014). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


