Abstract
Outcomes for patients with multiple myeloma (MM) have improved substantially in the past decade, with improvements in both progression-free survival and overall survival. Many patients are now achieving a complete response to treatment, and consequently highly sensitive assays are needed for detection of minimal residual disease (MRD) in patients with MM. Results of multicolour flow cytometry and deep-sequencing studies suggest that among patients achieving a complete response, MRD-negative status is associated with significant improvements in progression-free survival and overall survival. Despite the increasing need for MRD testing in patients with MM, considerable heterogeneity in techniques for MRD detection hinders the clinical interpretation of their results. The criteria used to define MRD, strengths and weaknesses of the major types of tests (flow cytometry versus molecular testing), and the optimal sample type (bone marrow aspirate versus peripheral blood) are all unresolved dilemmas in MRD testing. This Review presents an overview of the various techniques for MRD detection in patients with MM. In addition, this article discusses challenges and opportunities for the routine use of MRD testing, possible future directions for clinical trials and implications for drug approval processes.
Introduction
Outcomes of patients with multiple myeloma (MM) have improved substantially in the past decade, in terms of both progression-free survival and overall survival.1–3 As a result of the availability of effective drugs, many patients with MM now achieve good responses to treatment, with approximately 75% achieving a near-complete or complete response (Figure 1).4,5 Complete response to therapy is associated with improved progression-free survival and overall survival, especially if the complete response status is maintained.6 Consequently, the need for highly sensitive assays to detect minimal residual disease (MRD) in patients with MM is increasing. Importantly, the results of studies using cell-based (multicolour flow cytometry) and molecular (gene sequencing) assays suggest that, in patients achieving a complete response to treatment, MRD-negative status is associated with substantial improvements in progression-free, and overall survival. 7–9 MRD status seems, therefore, to be an important prognostic factor in patients with MM. Studies have suggested that early treatment of patients with smouldering myeloma is associated with favourable outcomes.10 MRD testing might also be indicated in patients with high-risk smouldering myeloma receiving treatment for this disease, who are at risk of progression to MM.11
Figure 1 |.
Treatment response rates achieved with commonly used induction regimens in patients with MM. Response rates, including ≥VGPR and overall responses have improved with the introduction of newer induction therapy regimens for the treatment of patients with MM. Abbreviations: CRd, carfilzomib, lenalidomide and dexamethasone; CTD, cyclophosphamide, thalidomide and dexamethasone; Dex, dexamethasone; Len–Dex, lenalidomide and dexamethasone; MM, multiple myeloma; OR, overall response; RVD, revlimid, bortezomib and dexamethasone; Thal–Dex, thalidomide and dexamethosone; VAD, vincristine, doxorubicin and dexamethasone; VCD, bortezomib, cyclophosphamide and dexamethasone; VGPR, very good partial response; VRDC, bortezomib, lenalidomide, dexamethasone and cyclophosphamide; VTD, bortezomib, thalidomide and dexamethasone. Modified with permission from Springer Science+Business Media © Kumar, S. Med. Oncol. 27 (Suppl. 1), S14–S24 (2010).
However, considerable controversy exists regarding the optimal techniques for detection of MRD and the clinical interpretation of test results.12,13 The quality of the specimen tested is an important determinant of the sensitivity of all techniques for assessing MRD. Owing to its broad availability, short turnaround time, high feasibility and relatively low cost per sample, multicolour flow cytometry is increasingly emerging as the standard method of MRD testing in patients with MM. Additional advantages of flow cytometry are that it is quantitative, consistent (the same antibody panel can be used for all patients) and qualitative (the quality of the aspirate can be assessed through measuring the proportion of viable cells and the extent of haemodilution). Furthermore, this technique is particularly suited for routine laboratory testing, and can be used for both diagnosis and predicting the prognosis of symptomatic myeloma and related plasma cell disorders, including smouldering myeloma, plasmacytoma and amyloidosis. The major limitations of flow cytometry as a test for MRD in patients with MM have been the lack of international standardization of the technical elements of the test and of defined criteria to determine MRD positive or negative status, although consensus regarding a standardized protocol was reached in March 2014.14,15
Molecular techniques such as next-generation sequencing (NGS) have also been introduced for MRD testing. Molecular tests could potentially have increased sensitivity, overcome issues of standardization and enable identification of MRD based on analysis of peripheral blood samples rather than bone marrow aspirates. However, the limitations of current molecular assays include low availability, lack of data on success rates and relatively high cost per sample in comparison with cell-based tests.
In 1998, the first guidelines for the standardized assessment of responses to treatment in patients with MM were published.16 The International Myeloma Working Group (IMWG) has since revised and updated these guidelines several times, most recently in 2011.15,17 The current response criteria are based on the assessment of serum and urine monoclonal immunoglobulins, serum free light chains and bone marrow plasma cells (Box 1).17 The latest guidelines include an additional disease response category, ‘stringent complete response’, which requires a complete response to treatment plus a normal serum free light chain ratio, and the absence of clonal plasma cells in bone marrow as determined by immunohistochemistry and/or 2–4 colour flow cytometry (Box 1).17 The sensitivity of immunohistochemistry for the detection of clonal plasma cells is fairly low, however, which might impair the detection of MRD in some patients. Importantly, the 2011 IMWG guidelines17 also include the use of four-colour flow cytometry and allele-specific oligonucleotide (ASO)-PCR in additional criteria to define immunophenotypic and molecular complete responses, respectively. Immunophenotypic complete response is defined as a complete response combined with the absence of abnormal plasma cells in bone marrow aspirate when a minimum of 1 × 106 bone marrow cells are analysed by multiparametric flow cytometry.17 Molecular complete response is defined as a complete response combined with negative ASO-PCR findings.17
Box 1 |. IMWG 2011 treatment response criteria for multiple myeloma.
Immunophenotypic complete response
Stringent complete response, plus an absence of phenotypically aberrant plasma cells in bone marrow with a minimum of 1 million cells analysed by multicolour flow cytometry (4 or more colours)
Molecular complete response
Stringent complete response, plus a negative ASO-PCR test
Stringent complete response
Meets the criteria for complete response, plus normal FLC ratio and an absence of clonal plasma cells by immunohistochemistry or 2–4-colour flow cytometry
Complete response
Negative immunofixation results in serum and urine
Disappearance of any soft-tissue plasmacytomas
<5% plasma cells in bone marrow
Very good partial response
Serum and urine M-component detectable by immunofixation but not by electrophoresis, or ≥90% reduction in serum M-component plus urine M-component <100 mg per 24 h
Partial response
≥50% reduction of serum M-protein levels and reduction in 24-h urinary M-protein levels by ≥90% or to <200 mg per 24 h
In addition, a ≥50% reduction in the size of soft-tissue plasmacytomas (if present at baseline)
Stable disease
Criteria not met for complete response, very good partial response, partial response or progressive disease
Progressive disease
Increase of 25% from lowest response value in any of the following: serum M-component (absolute increase must be ≥0.5 g/dl); and/or urine M-component (absolute increase must be ≥200 mg per 24 h); and/or definite development of new bone lesions or soft-tissue plasmacytomas, or definite increase in the size of existing bone lesions or soft-tissue plasmacytomas
Abbreviations: FLC, free light chain; IMWG, International Myeloma Working Group. Modified with permission from the American Society of Haematology © Rajkumar, S. V. et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 117, 4691–4695 (2011).13
In this Review, we discuss the various techniques for MRD assessment, potential challenges to the routine use of these tests, and future opportunities for MRD testing in the management of patients with MM.
Techniques for MRD assessment
Multiparametric flow cytometry
Multicolour flow cytometry has been used to differentiate between normal and abnormal plasma cells in patients with MM by enabling detection of aberrant cell-surface marker expression.18 The sensitivity of flow cytometry for detecting MRD is dependent on the quality of the specimen obtained, the number of cells analysed and the capability of the antibody panel to distinguish abnormal from normal plasma cells.14,15 The quality of a bone marrow aspirate becomes a moot point if MRD is demonstrated in a given sample; however, if flow cytometry testing gives a negative result for MRD, the specimen quality must be evaluated. In our opinion, cell viability within a sample should be ≥85% and the presence of bone marrow cell populations (normal precursors) must be demonstrated.14,15 However, previous studies using stored bone marrow aspirates of varying quality and haemodilutions have nonetheless demonstrated the clinical relevance of flow cytometry testing for MRD in patients with MM.8,14,19
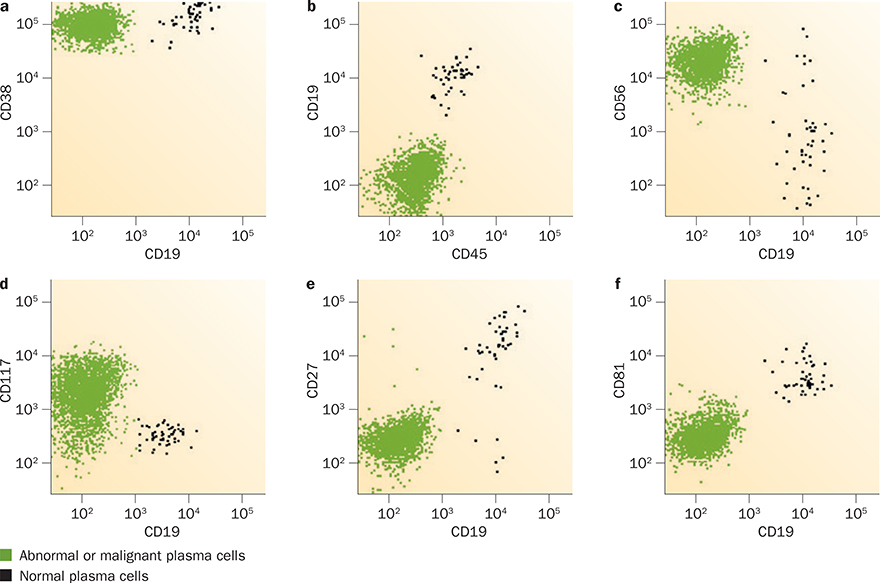
The capability of the selected antibody panel to differentiate between normal and abnormal plasma cells is a key feature in determining the sensitivity of flow-cytometry-based MRD testing. Normal plasma cells characteristically express CD138 (syndecan-1) and strongly express CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1). The features of aberrant plasma cells observed in patients with MRD (Figure 2) include slightly decreased expression of CD38, decreased expression of CD27 and CD81, expression of CD56 (neural cell adhesion molecule 1) and/or CD117 (mast/stem cell growth factor receptor KIT), and the absence of expression of B-lymphocyte antigen CD19 and/or CD45 (receptor-type tyrosine-protein phosphatase C).16 The European Myeloma Network recommends identification of abnormal plasma cells by multicolour flow cytometry testing for CD38, CD45 and CD138 expression simultaneously in all tubes.20 Detection of CD19 and CD56 is also required, and including evaluation of B-lymphocyte antigen CD20, CD27, T-cell-specific surface glycoprotein CD28 and CD117 expression increases the sensitivity of the test.20 Initial studies investigating MRD detection used the now-outdated four-colour flow cytometry; studies using six-colour or eight-colour flow cytometry have reported improved sensitivity (Tables 1–3).8,21 6–10-colour flow cytometry is now the accepted standard in clinical laboratories.14,15,20,22
Figure 2 |.
Flow cytometry testing for minimal residual disease in patients with treated multiple myeloma. This technique relies on differentiation of normal from malignant plasma cells on the basis of their differences in cell-surface marker expression. Key characteristics distinguish malignant plasma cells from normal plasma cells: CD19− (all panels); a | expression of less CD38; b | CD45−; c | CD56+; d | CD117+; e | CD27−; and f | CD81−.
Table 1 |.
Techniques for assessment of MRD in patients with multiple myeloma
| Parameter | ASO-PCR | VDJ sequencing | Exome or genome sequencing | Flow cytometry |
|---|---|---|---|---|
| Universal assay | No (patient-specific primers) | Yes | Yes | Yes |
| Sensitivity* | 1 in 105 | 1 in 105 | Unknown | 1 in 104 to 105‡ |
| Sample source | Bone marrow aspirate | Bone marrow aspirate or peripheral blood | Bone marrow aspirate or peripheral blood | Bone marrow aspirate |
| Sample quality assessment | No | No | No | Yes |
| Sampling error | Likely | Can be overcome by using peripheral blood | Can be overcome by using peripheral blood | Likely |
| Clonal evolution | Not detected | Limited detection | Detectable | Not detected |
| Turnaround time | Days | 1 week | Days to weeks | Hours |
| Interobserver variation | Likely | Unknown | Unknown | Substantial |
| Clinical benefit associated with MRD-negative status | Improvements in PFS and OS | Improvements in PFS and OS | Unknown | Improvements in PFS and OS |
Expressed as the minimum cell sample size required for detection of one clonal cell.
The sensitivity of eight-colour or 10-colour flow cytometry (1 in 105) is higher than four-colour or six-colour flow cytometry (1 in 104).
Abbreviations: ASO, allele-specific oligonucleotide; MRD, minimal residual disease; OS, overall survival; PFS, progression-free survival; VDJ, variable diversity joining.
Table 3 |.
Recommended eight-colour flow cytometry panel22
| Fluorophore | Target cell-surface marker in tube 1 | Target cell-surface marker in tube 2* |
|---|---|---|
| FITC | CD38 | CD38 |
| PE | CD56 | CD56 |
| PerCP-Cy5.5 | CD45 | CD229 |
| PC7 | CD19 | CD19 |
| APC | CD117 | cIgκ |
| APC-C750 | CD81 | cIgλ |
| V450 | CD138 | CD138 |
| BV510 | CD27 | CD27 |
Tube 2 is complementary if further demonstration of clonality is needed among cells with phenotypic deviation are identified in Tube 1.
Abbreviations: APC, allophycocyanin; APC-C750, allophycocyanin-cyanine 750; BV510, brilliant violet 510; cIgκ, cytoplasmic immunoglobulin κ type; cIgλ, cytoplasmic immunoglobulin λ type; FITC, fluorescein isothiocyanate; PC7, phycoerythrin-cyanine 7; PerCP-Cy5.5, peridinin chlorophyll protein-cyanine 5.5; PE, phycoerythrin; V450, violet 450.
Antibody panel design is a crucial and difficult part of flow cytometry testing for MRD in patients with MM. In addition to enabling the identification of appropriate antigens, the panel must be tested extensively to determine optimal monoclonal antibody and fluorochrome combinations, which must then be validated for use in clinical trials. Several six-colour and eight-colour panels have demonstrated good sensitivity, interlaboratory concordance and prognostic utility in clinical trials.8,22 These validated panels evaluate the expression of CD27, CD56, CD81 and CD117 in various combinations with CD19, CD38, CD45 and CD138, and the European Myeloma Network and International Clinical Cytometry Society and European Society for Clinical Cell Analysis Myeloma MRD Consensus Committee recommended that laboratories should adopt one of these validated panels for use in MRD testing (Tables 2 and 3).14,15,22
Table 2 |.
Recommended six-colour flow cytometry panel8
| Fluorophore | Target cell-surface marker in tube 1 | Target cell-surface marker in tube 2* |
|---|---|---|
| FITC | CD27 | CD56 |
| PE | CD81 | CD117 |
| PerCP-Cy5.5 | CD19 | CD19 |
| PE-Cy7 | CD38 | CD38 |
| APC | CD138 | CD138 |
| APC-Cy7 | CD45 | CD45 |
Tube 2 is complementary if further demonstration of clonality is needed among cells with phenotypic deviation are identified in Tube 1.
Abbreviations: APC, allophycocyanin; APC-Cy7, allophycocyanin-cyanine 7; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP-Cy5.5, peridinin chlorophyll protein-cyanine 5.5; PE-Cy7, phycoerythrin-cyanine 7.
Plasma cells are present in low numbers in bone marrow aspirates. Consequently, a minimum of 20–30 malignant cells must be detected to identify MRD using flow cytometry, because the lower limit of detection for this technique is dependent on the total number of cells analysed. Although only 500,000 cellular detection events have been analysed in some studies,8 acquisition of a higher number of detection events is considered the current best practice; 2 × 106 events is the acceptable minimum, and 3–5 × 106 events is thought to be optimal.14,15 With appropriate antibody panels and acquisition numbers, a lower limit of detection of 1 in 104 can be attained, and some laboratories report a lower limit of detection of 1 in 105.13
The prognostic utility of flow cytometry has been demonstrated in the management of patients with MM treated using both transplant and nontransplant approaches (Table 4). The Spanish PETHEMA group7 has shown that patients who attain MRD-negative status after induction with six alternating cycles of two different drug combinations—vincristine, carmustine, melphalan, cyclophosphamide and prednisone, and vincristine, carmustine, adriamycin and dexamethasone—followed by high-dose chemo therapy and autologous stem-cell transplantation (HDT–ASCT) have markedly better progression-free survival (71 months versus 37 months) and overall survival (not reached versus 89 months). In this study, MRD-negative status was associated with improved progression-free survival and overall survival in patients with both standard-risk and high-risk cytogenetic features. 7 High-risk cytogenetic features include t(4;14)(p16.3;q32), t(14;16)(q32;q23) and 17p deletion.
Table 4 |.
Selected studies of MRD testing in patients with multiple myeloma
| Study | Treatment arms | Test method | Outcomes (MRD-negative versus MRD-positive) |
|---|---|---|---|
| Paiva et al. (2008)7 | 6 alternating cycles of VBMCP and VBAD, followed by HDT-ASCT (n = 577) | 4-colour flow cytometry | Median PFS 71 months vs 37 months (P <0.001) Median OS not reached vs 89 months (P = 0.02) |
| Paiva et al. (2011)21 | 6 cycles of VMP or VTP (n = 102) | 4-colour flow cytometry | Median PFS not reached vs 35 months (P = 0.02) Median OS not significantly different |
| Korthals et al. (2012)28 | Idarubicin or dexamethasone plus HDT–ASCT (n = 53) | ASO-PCR | Median EFS 35 months vs 20 months (P = 0.001) Median OS 70 months vs 45 months (P = 0.04) |
| Rawstron et al. (2013)8 | CVAD or CTD plus HDT–ASCT (n = 378) | 6-colour flow cytometry | Median PFS 28.6 months vs 15.5 months (P <0.001) Median OS 80.6 months vs 59 months (P = 0.018) |
| Puig et al. (2014)27 | VBMCP or VBAD induction therapy plus HDT–ASCT or 6 cycles of VMP or VTP (n = 170) | ASO-PCR | VBMCP or VBAD induction therapy plus HDT–ASCT: median PFS 54 months vs 27 months (P = 0.001); OS not significantly different 6 cycles of VMP or VTP: median PFS not reached versus 31 months (P = 0.029); OS not significantly different |
| Martinez-Lopez et al. (2014)9 | VBMCP or VBAD induction therapy plus HDT–ASCT or 6 cycles of VMP or VTP (n = 133) | Next-generation VDJ sequencing | Median time to progression 80 months vs 31 months (P <0.0001) Median OS not reached versus 81 months (P = 0.02) |
Abbreviations: ASO-PCR, allele-specific oligonucleotide PCR; CTD, cyclophosphamide, thalidomide and dexamethasone; CVAD, cyclophosphamide, vincristine, adriamycin and dexamethasone; EFS, event-free survival; HDT–ASCT, high-dose (chemo)therapy and autologous stem-cell transplantation; MRD, minimal residual disease; OS, overall survival; PFS, progression-free survival; VBAD, vincristine, carmustine, adriamycin and dexamethasone; VBMCP vincristine, carmustine, melphalan, cyclophosphamide and prednisone; VDJ, variable-diversity-joining; VMP, bortezomib, melphalan and prednisone; vs, versus; VTP bortezomib, thalidomide and prednisone.
In an analysis of 241 patients treated with induction chemotherapy and HDT–ASCT, the presence of high-risk cytogenetic features at baseline and persistence of MRD-positive status at day 100 after HDT–ASCT were associated with the loss of complete response within 1 year of HDT–ASCT and poor outcome (median overall survival 39 months).23 In this study, 147 patients achieved a complete response, as defined by the standard IMWG criteria, and MRD status was predictive of prognosis even in this subset of patients:23 53 of these 147 patients were MRD-positive, and 5-year progression-free survival (30% versus 62%, P <0.001 ) and overall survival (59% versus 87%, P = 0.009) rates were significantly improved in the MRD-negative subgroup.23
In patients who were not eligible for transplantation, the same investigators showed that patients who were MRD-negative after six cycles of either bortezomib, melphalan and prednisone (VMP), or bortezomib, thalidomide and prednisone (VTP) had significantly improved progression-free survival (not reached versus 35 months, P = 0.02).21 In these patients, the category of response correlated with its duration; those achieving MRD-negative status, a stringent complete response or complete response had markedly better outcomes than patients with a partial response (3-year progression-free survival 90%, 69%, 60% and 35%, respectively). In an update of this study,24 the prognostic utility of flow-cytometry-based MRD testing was assessed in a subset of 153 patients with long-term follow up (median of 72 months). 34 patients (22%) achieved MRD-negative status, which translated to significantly improved progression-free survival (59 months versus 29 months, P <0.001) and overall survival (not reached versus 57 months, P = 0.013).24 When the analysis was restricted to patients who achieved a complete response, 70% of those treated with VMP achieved MRD-negative status, versus only 45% of those treated with VTP.24
Similar results were also seen in the large Myeloma IX study.8 The investigators used multicolour flow cytometry to detect MRD in 378 patients, 100 days after induction therapy with either cyclophosphamide, vincristine, doxorubicin and dexamethasone or cyclophosphamide, thalidomide and dexamethasone followed by HDT–ASCT.8 MRD-negative status was associated with significantly improved progression-free survival (28.6 months versus 15.5 months, P <0.001) and overall survival (80.6 versus 59 months, P = 0.019). Furthermore, 31 of the 214 patients who achieved a complete response (as defined by conventional IMWG criteria) had evidence of MRD by flow cytometry testing.8 These patients had significantly shorter progression-free survival than MRD-negative patients with a complete response (14.1 months versus 34.3 months, P = 0.0068) and a non-significant trend towards shorter overall survival (61.9 months versus not reached, P = 0.093).8 Furthermore, the proportion of patients achieving MRD-negative status was similar in patients with standard-risk and high-risk cytogenetic features (61.5% and 59.8%, respectively);8 MRD-negative status was also associated with statistically significant improvements in progression-free survival in both groups.8 In this study, the best outcomes were noted in patients with standard-risk cytogenetic features and MRD-negative status, whereas the worst outcomes were seen in those with high-risk cytogenetic features and MRD-positive status.8
Allele-specific oligonucleotide PCR
ASO-PCR of variable diverse joining (VDJ) heavy chain rearrangements is used for assessment of MRD status in patients with MM.25–27 Several small, early studies used ASO-PCR probes derived from baseline samples of the complementarity determining region 3 of the immunoglobulin heavy chain (IgH) in these analyses.25 However, this approach is time-consuming and expensive, owing to the high cost of producing patient-specific probes. Additionally, ASO-PCR is a qualitative but not quantitative method of MRD assessment. An alternative approach is to use real-time quantitative allele-specific oligonucleotide PCR (ASO-qPCR), which provides an accurate measurement of MRD. This method can also be used with a consensus antibody panel for detection of myeloma-specific IgH rearrangements in association with ASO primers to obtain selective amplification of clonal IgH rearrangements (Tables 1 and 4).26
Although ASO-qPCR can detect one clonal MM plasma cell in 105 normal plasma cells, making this technique slightly more sensitive and specific than multiparameter flow cytometry, an important clinical aspect of any assay is the failure rate. In this regard, ASO-PCR is only feasible in up to 80% of patients with MM, owing to a lack of known clonal targets for amplification in the IgH locus in the remaining patients.26,27 Multicolour flow cytometry and ASO-qPCR are currently applicable only in bone marrow specimens and are, therefore, both limited by their reliance on blind bone marrow biopsy sampling, which is invasive and can miss patchy disease lesions. As with multicolour flow cytometry, the clinical utility of ASO-qPCR for MRD detection in patients with MM has been established in a range of studies.27–29 For instance, the Spanish PETHEMA group27 reported results of MRD testing in 170 patients who achieved at least a partial response to treatment. In this study, ASO-qPCR for MRD detection was possible in only 42% of patients (n = 71).27 Lack of clonality detection (n = 31), unsuccessful sequencing (n = 17), and suboptimal ASO performance (n = 51) precluded successful MRD detection in the other patients. Among the patients in whom MRD testing was feasible, the findings of ASO-qPCR and multicolour flow cytometry showed a strong, significant correlation (r = 0.881, P <0.001).27 MRD-negative status on ASO-qPCR testing was associated with significantly improved progression-free survival in both transplant-eligible (54 months versus 27 months, P = 0.001) and transplant-ineligible patients (not reached versus 31 months, P = 0.029).27 When analysis was limited to the 62 patients who attained a complete response as defined by IMWG criteria, MRD-negative status on ASO-qPCR testing was associated with significantly improved progression-free survival (49 months versus 26 months, P = 0.001) and overall survival (not reached versus 60 months, P = 0.008).27 In a separate study of 62 patients with MM treated with idarubicin plus dexamethasone and ASCT, event-free survival was significantly improved (35 months versus 20 months, P = 0.001) in the patients who attained MRD-negative status on ASO-PCR testing.28
Fluorescent PCR detection of clonal immunoglobulin heavy and light chain rearrangements has been used as a qualitative MRD test in 130 patients with newly diagnosed MM who achieved a very good partial response or better after induction therapy.29 MRD-negative status was associated with significantly improved progression-free survival (61 months versus 36 months, P = 0.006) and overall survival (not reached versus 66 months, P = 0.03).29 Although fluorescent PCR has a lower sensitivity of detection than ASO-PCR (in the order of 1 in 103), the technique was feasible in 91.5% of patients.
Next-generation molecular techniques
VDJ gene sequencing
Several studies have described the use of consensus primers and high-throughput sequencing to detect clonal immunoglobulin VDJ gene rearrangements. This approach is very sensitive, and could detect one clonal cell in 106 normal cells in diagnostic samples from patients with acute lymphoblastic leukaemia and chronic lymphocytic leukaemia.30,31 Furthermore, this technique can be used to assess MRD in both bone marrow aspirates and cell-free DNA (cfDNA) in plasma.30,31
The clinical significance of next-generation VDJ sequencing has been reported in a study of 133 patients who attained at least a very good partial response after induction therapy (Table 2).9 VDJ sequencing was feasible in 91% of these patients (n = 121), and MRD-negative status by VDJ sequencing was associated with significantly prolonged time to progression (80 months versus 31 months, P <0.0001) and overall survival (not reached versus 81 months, P = 0.02).9 When the analysis was limited to 62 patients with a complete response as defined by IMWG criteria, the 36 (58%) patients with MRD-positive status had a significantly shorter time to progression (35 months versus 131 months, P = 0.0009) than the 26 (42%) patients with MRD-negative status.9 The effect of VDJ-sequencing-based MRD assessment in patients with different cytogenetic risk profiles could not be investigated in this study, as the cohort only included eight patients with high-risk cytogenetic features.9 VDJ sequencing had a fairly high concordance with ASO-PCR (r = 0.74) and multicolour flow cytometry (r = 0.76)9 in the 99 patients who underwent MRD testing using both techniques. Specifically, 82 patients had concordant results and 12 were MRD-negative on flow cytometry but MRD-positive by VDJ sequencing. The remaining five patients were MRD-negative by VDJ sequencing but MRD-positive on flow cytometry (only one of these patients had disease progression at the time of reporting).9 The time to progression for the 12 patients with MRD-negative flow cytometry and MRD-positive VDJ sequencing results was shorter than that of patients with MRD-negative results for both techniques (50 months versus not reached).9 ASO-PCR was available in 41 patients, and demonstrated a similar level of concordance with flow cytometry findings as that of VDJ sequencing. These results suggest that next generation sequencing (NGS) based assays could be more sensitive and specific for MRD in MM than flow cytometry testing. However this possibility will need to be validated in future studies that prospectively compare the two techniques (Table 1).
Whole-exome or whole-genome sequencing
Whole-exome or whole-genome sequencing to identify tumour-specific mutations in diagnostic samples from patients with treated MM is technically feasible, and could be used to detect MRD. Whole-exome or whole-genome sequencing is potentially a more accurate method than VDJ sequencing for identifying clonal evolution. However, to date, this technique has not been evaluated for MRD detection in patients with MM.
Challenges in MRD testing
Clinical perspectives
Several unresolved questions preclude the widespread clinical implementation of emerging techniques such as multicolour flow cytometry and NGS techniques for MRD assessment in patients with MM. The various techniques have inherent limitations and advantages that might differentially affect their use and interpretation of the findings.
Multicolour flow cytometry is the most extensively investigated method of MRD testing in patients with MM. The European Myeloma Network has provided recommendations for selecting the antibody panels used to identify abnormal plasma cells.20,22 Despite these recommendations, a 2014 survey of major medical institutions in the USA on the use of multicolour flow cytometry for MRD testing in patients with MM revealed a 100-fold difference in sensitivity between tests used in different centres.12 Consequently, the results of multicolour flow cytometry MRD testing could be interpreted as positive or negative in the same patient, depending on the test parameters and the criteria used to define MRD positivity. One reason for this variation is that the optimal number of cells that must be analysed and the number of abnormal plasma cells that must be detected to establish MRD positivity remain to be determined (Figure 3). Currently, flow cytometry technology is widely available, but the quality of testing and expertise in this technique are not uniform. Furthermore, although the prognostic utility of MRD testing using flow cytometry and/or next-generation molecular techniques has been demonstrated in multiple studies, the implications for patient management are not well established. At the time of writing, no published studies have demonstrated any benefit from adjusting therapy based on results of MRD testing.
Figure 3 |.
Effect of sample size on flow cytometry testing for minimal residual disease in treated patients with multiple myeloma. The total number of acquired events (that is, the number of cells assessed in bone marrow aspirate) analysed by multicolour flow cytometry has a substantial effect on the reliability of the test results. All panels depict healthy (black) and malignant (green) plasma cells in a bone marrow sample obtained from the same patient. a | 84 malignant plasma cells were detected by analysing 3,000,000 events. b | 30 cells were detected in 1,000,000 events. c | 14 malignant cells were detected in 500,000 events. d | Only 6 malignant cells were detected in 100,000 events.
The presence of other clonal processes (such as monoclonal B-cell lymphocytosis) that can lead to false-positive MRD test results is another challenge to the routine clinical use of molecular techniques, including NGS. Indeed, 7.1% of healthy blood donors over the age of 45 years have evidence of monoclonal B-cell lymphocytosis, and the prevalence of this condition increases with age.32
Reliance on blind bone marrow aspiration for obtaining samples for use in MRD testing is another inherent limitation of both flow cytometry and molecular techniques. This issue is especially important in patients with an apparently complete response, as a patchy distribution of malignant cells within the bone marrow might be missed owing to sampling errors.33 Currently, the clinical relevance of potential bone marrow sampling errors is poorly understood. Image-guided biopsy at the time of MRD assessment might help overcome these sampling errors; although, the feasibility and clinical significance of such approaches will need to be demonstrated in well-designed clinical studies. The presence of extra medullary disease can also impair the utility of MRD testing. Molecular imaging studies indicate that residual disease might persist in the bone marrow and/or at extra medullary sites after completion of therapy.31 For example, nonsecreting plasma cells or precursor cells could be missed by conventional M-protein and free-light-chain assays. As mentioned above, currently, no systematic assessments using imaging-guided biopsy sampling integrated with advanced molecular assays have been conducted to address these challenges.
VDJ sequencing and whole-genome or whole-exome sequencing have the potential to overcome some of these issues by avoiding the need for analysis of bone marrow biopsy or aspirate samples. Circulating DNA fragments carrying tumour-specific mutations (circulating tumour DNA [ctDNA]) constitute a small fraction of the cfDNA in patients with cancer.34 Tagged amplicon sequencing and/or whole-genome sequencing of DNA from breast-cancer specimens and matched normal tissue samples have been used to successfully detect cancer-specific somatic mutations.35 These mutations were used to design personalized assays for the monitoring of ctDNA by PCR or tagged amplicon deep sequencing of plasma cfDNA obtained from peripheral blood samples. Detection of ctDNA had increased sensitivity compared with detection of other circulating biomarkers, such as mucin-1 and circulating tumour cells, and provided the earliest measure of treatment responses in 10 of 19 women with metastatic breast cancer (53%).35 However, this approach has not been studied in patients with MM, and a key unresolved question is whether the amount of ctDNA present in patients with this cancer is sufficient for detection. Preliminary studies suggest that, in a newly diagnosed patient with MM, the concentration of circulating MM-specific cfDNA in peripheral blood is approximately 100-fold lower than the tumour-specific DNA concentration in DNA extracted from sorted CD138+ cells obtained by bone marrow aspiration.36 The very low concentration of circulating tumour-specific cfDNA might pose a challenge for MRD testing of plasma or serum samples, even with highly sensitive sequencing methods. Whether NGS can be used to detect MRD using peripheral blood samples is currently only speculation and requires further investigation. As a result, the question of optimal sample type (that is, bone marrow aspirate versus peripheral blood) for MRD testing also remains unanswered.
Laboratory perspectives
Laboratory issues that influence MRD testing and the standardized reporting of results are equally important considerations. For instance, the quality of bone marrow aspirates greatly affects flow cytometry results and probably also affects the results of other MRD tests. As described previously, the optimal sample type for use in MRD testing remains unresolved, although bone marrow aspiration is probably the most-sensitive sampling method at present. Analysis of ctDNA (from plasma or serum) might give results that are not affected by a non-uniform disease distribution, but this sampling technique is likely to be less sensitive than bone marrow aspiration owing to the very limited amount of ctDNA in patients with MM.36
Several large studies in patients with MM and other malignancies have identified clonal diversity of the neoplasm at baseline and genomic evolution of the malignant cells with treatment and progression of disease.37–39 Neither multicolour flow cytometry nor ASO-PCR can identify clonal heterogeneity, which might limit the reliability of MRD detection, especially if the recurrent disease is driven by a distinct subclone. VDJ sequencing has a limited capability to detect clonal heterogeneity in patients with MM. The results of a study published only in abstract form to date revealed tumour oligoclonality, detected using VDJ sequencing in approximately 12% of patients with MM.40 Clonal diversity and genomic evolution might be most-accurately identified by whole-exome or whole-genome sequencing.39 However, these techniques have not been validated for MRD testing in patients with MM, and further studies are needed before the routine use of such tests can be implemented.
All laboratories that currently undertake MRD testing in patients with MM have developed institutional standards and benchmarks for testing and reporting of results. Nonetheless, considerable interobserver (that is, interlaboratory) variability in test sensitivity and interpretation remains a key challenge in MRD testing. The large variation in sensitivity12 and the lack of global standards for implementation of MRD testing creates a major challenge for clinicians interpreting the results of tests from different laboratories. For instance, a patient with treated MM who has attained a complete response might be described as MRD-positive by one laboratory and as MRD-negative by another (Figure 3).
Interlaboratory differences in test sensitivity can also be associated with NGS-based methodologies, depending on the depth (coverage) of sequencing and the level of bioinformatic support for identification of tumour-specific mutations. Furthermore, several molecular techniques (including VDJ sequencing and whole-exome sequencing) are not widely available and the reproducibility of these tests across laboratories is not well established. Further progress in MRD testing urgently requires the threshold values associated with clinical outcomes such as progression-free survival and overall survival to be defined in patients with MM.
Finally, the phenotype of the residual malignant cells is an inherent conceptual problem with MRD testing using flow cytometry. Controversial data based on small numbers of patients suggest that tumour-propagating or tumour-initiating cells might have a phenotype that more closely resembles B cells than that of plasma cells.41 NGS techniques might be the most-sensitive approach to detect these cells. However, we currently lack sufficient data to make definite conclusions in this regard.42,43
Conclusions
An increasing body of research shows that MRD-negative status translates into improved PFS and overall survival in patients with MM.7–9,21,23–29 The majority of these data rely on MRD testing using multicolour flow cytometry.7,8,21,23,24 This technique offers many clinical advantages, including its broad availability across institutions and the fact that final results can be generated in local laboratories within hours of sample collection. However, the lack of standardization of MRD testing using multicolour flow cytometry is an important limitation, especially regarding how to define positive and negative test results.12,14,15 Indeed, processing, analysis and interpretation of MRD testing based on multicolour flow cytometry in patients with MM varies greatly between laboratories in the USA.12 To overcome these problems, some groups have proposed that the uniform response criteria for management of patients with MM should be amended to include accurate definitions of MRD-positive and MRD-negative status. Furthermore, a consensus exists among IMWG members that MRD-negative status should be incorporated into new response criteria for use in clinical trials. Once the definitions of MRD in patients with MM are standardized, the next generation of clinical trials should include MRD assessment by cell-based and molecular techniques as a key end point linked to traditional end points, including progression-free survival and overall survival. Consequently, MRD could potentially be considered for regulatory purposes, including drug approval, in the field of MM.
In addition to these advances in multicolour flow cytometry, parallel efforts are underway to develop molecular tests (including PCR and sequencing-based assays) to determine MRD status in patients with MM. Although the clinical relevance remains to be proven, molecular techniques (such as NGS assays) offer advantages in terms of monitoring the biology of MRD cell populations, and a higher sensitivity of detection than other test methods.
Beyond the assay methods themselves, it is possible that the type of sample analysed to determine MRD status might change in the future. Current MRD assays are typically based on bone marrow aspirates. Given the nature of modern assays (such as sequencing), it is possible that future MRD assays in patients with MM will instead be based on peripheral blood samples. Before such assays can be implemented in clinical care, they will have to be further developed and validated in prospective studies. Lastly, extrapolating from reports showing that progression-free survival and overall survival are improved in patients with MM who achieve a sustained (rather than transient) stringent complete response,6 we can conjecture that sustained MRD-negative status will be associated with a better outcome than transient MRD-negative status. Future studies are needed to provide data on these aspects.
On a clinical note, increased use of MRD testing could create several new clinical research opportunities in MM. A major area of interest would be to use MRD testing to monitor the effectiveness of response-adapted strategies in the management of patients with this disease. For example, patients who remain MRD-positive during initial treatment might require dose intensification or a change of induction therapy. MRD testing might also help to identify patients who could benefit from consolidative HDT–ASCT and/or prolonged (maintenance) therapy. In addition, MRD testing could even provide guidance on the identification of patients who might be eligible for cessation of maintenance therapy.
From a regulatory standpoint, despite the absence of an established cure for MM, ongoing improvements in drug treatment are leading to improved survival across several age groups,1 and an increasing proportion of patients with the disease achieving the most-stringent categories of response. Consequently, a need is emerging for validation of reliable surrogate end points that can be used to facilitate drug approval, and contribute to a healthy pipeline of therapeutics for MM. Thus, establishing standardized definitions of MRD linked to traditional clinical end points might be a key step in unlocking the future treatment of MM.
Key points.
Outcomes of patients with multiple myeloma have improved substantially in the past decade
A large proportion of patients achieve a complete response to current therapies
More-sensitive assays for the detection of MRD are required
Standardization and guidelines on the use of MRD testing in clinical trials and in the clinic are required
Acknowledgements
S.M. and M.S.-S. gratefully acknowledge support from the Intramural Research Program of the National Cancer Institute, Bethesda, MD, USA.
Footnotes
Competing interests
O.L. declares that he has acted as a consultant for BMJ Publishing, Celgene, Medscape, Millennium and Onyx. The other authors declare no competing interests.
Contributor Information
Sham Mailankody, Multiple Myeloma Section, Lymphoid Malignancies Branch, Centre for Cancer Research, National Institutes of Health, National Cancer Institute, 9000 Rockville Pike, Bethesda, MD 20892, USA..
Neha Korde, Myeloma Service, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Alexander M. Lesokhin, Myeloma Service, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA
Nikoletta Lendvai, Myeloma Service, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Hani Hassoun, Myeloma Service, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Maryalice Stetler-Stevenson, Flow Cytometry Laboratory, Laboratory of Pathology, National Institutes of Health, National Cancer Institute, 9000 Rockville Pike, Bethesda, MD 20892, USA..
Ola Landgren, Myeloma Service, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
References
- 1.Kristinsson SY, Anderson WF & Landgren O Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia 28, 1346–1348 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Turesson I, Velez R, Kristinsson SY & Landgren O Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J. Clin. Oncol. 28, 830–834 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 28, 1122–1128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakubowiak AJ et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood 120, 1801–1809 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korde N et al. Phase II clinical and correlative study of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed multiple myeloma (MM) patients. Blood 122, 538 (2013). [Google Scholar]
- 6.Kapoor P et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J. Clin. Oncol. 31, 4529–4535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paiva B et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood 112, 4017–4023 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawstron AC et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J. Clin. Oncol. 31, 2540–2547 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Lopez J et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood 123, 3073–3079 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mateos MV et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N. Engl. J. Med. 369, 438–447 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Ahn IE, Mailankody S, Korde N & Landgren O Dilemmas in treating smoldering multiple myeloma. J. Clin. Oncol 10.1200/JCO.2014.56.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanders A, Stetler-Stevenson M & Landgren O Minimal residual disease testing in multiple myeloma by flow cytometry: major heterogeneity. Blood 122, 1088–1089 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roschewski M et al. Minimal residual disease: what are the minimum requirements? J. Clin. Oncol. 32, 475–476 (2014). [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. FDA-NCI roundtable: symposium on flow cytometry detection of minimal residual disease in multiple myeloma [online], http://www.fda.gov/MedicalDevices/NewsEvents/WorkshopsConferences/ucm402038.htm (2014). [DOI] [PubMed]
- 15.Landgren O et al. Flow cytometry detection of minimal residual disease in multiple myeloma: lessons learned at FDA-NCI roundtable symposium. Am. J. Hematol. 89, 1159–1160 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Blade J et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT European Group for Blood and Marrow Transplant. Br. J. Haematol. 102, 1115–1123 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar SV et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 117, 4691–4695 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mailankody S et al. Molecular and biologic markers of progression in monoclonal gammopathy of undetermined significance to multiple myeloma. Leuk. Lymphoma 51, 2159–2170 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawstron AC et al. Flow cytometric disease monitoring in multiple myeloma: the relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood 100, 3095–3100 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Rawstron AC et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica 93, 431–438 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Paiva B et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J. Clin. Oncol. 29, 1627–1633 (2011). [DOI] [PubMed] [Google Scholar]
- 22.VanDongen JJ et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 26, 1908–1975 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paiva B et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood 119, 687–691 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Mateos MV. et al. Update of the GEM2005 trial comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood. doi: 10.1182/blood-2014-05-573733. . [DOI] [PubMed] [Google Scholar]
- 25.Corradini P et al. High-dose sequential chemoradiotherapy in multiple myeloma: residual tumor cells are detectable in bone marrow and peripheral blood cell harvests and after autografting. Blood 85, 1596–1602 (1995). [PubMed] [Google Scholar]
- 26.Ladetto M et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia 28, 1299–1307 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Puig N et al. Critical evaluation of ASO RQ-PCR for minimal residual disease evaluation in multiple myeloma. A comparative analysis with flow cytometry. Leukemia 28, 391–397 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Korthals M et al. The level of minimal residual disease in the bone marrow of patients with multiple myeloma before high-dose therapy and autologous blood stem cell transplantation is an independent predictive parameter. Biol. Blood Marrow Transplant. 18, 423–431 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Lopez J et al. Clinical applicability and prognostic significance of molecular response assessed by fluorescent-PCR of immunoglobulin genes in multiple myeloma. Results from a GEM/PETHEMA study. Br. J. Haematol. 163, 581–589 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Faham M et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood 120, 5173–5180 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logan AC et al. High-throughput VDJ sequencing for quantification of minimal residual disease in chronic lymphocytic leukemia and immune reconstitution assessment. Proc. Natl Acad. Sci. USA 108, 21194–21199 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shim YK et al. Monoclonal B-cell lymphocytosis in healthy blood donors: an unexpectedly common finding. Blood 123, 1319–1326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamagni E et al. Prognostic relevance of 18F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 118, 5989–5995 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Schwarzenbach H, Hoon DS & Pantel K Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 11, 426–437 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Dawson SJ et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 368, 1199–1209 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Vij R et al. Deep sequencing reveals myeloma cells in peripheral blood in majority of multiple myeloma patients. Clin. Lymphoma Myeloma Leuk. 14, 131–139 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Chapman MA et al. Initial genome sequencing and analysis of multiple myeloma. Nature 471, 467–472 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolli N et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat. Commun. 5, 2997 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lohr JG et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell 25, 91–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Lopez J et al. Deep sequencing reveals oligoclonality at the immunoglobulin locus in multiple myeloma patients. Blood 122, 401 (2013). [Google Scholar]
- 41.Huff CA & Matsui W Multiple myeloma cancer stem cells. J. Clin. Oncol. 26, 2895–2900 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trepel M et al. Phenotypic detection of clonotypic B cells in multiple myeloma by specific immunoglobulin ligands reveals their rarity in multiple myeloma. PLoS ONE 7, e31998 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiele B. et al. Next-generation sequencing of peripheral B-lineage cells pinpoints the circulating clonotypic cell pool in multiple myeloma. Blood 123, 3618–3621 (2014). [DOI] [PubMed] [Google Scholar]