Abstract
IMPORTANCE
Cutaneous leiomyomas can be associated with severe paroxysmal pain in which nerve conduction may have a key role. Medical management of painful cutaneous leiomyomas is generally unsatisfactory.
OBJECTIVE
To assess the efficacy of intralesional botulinum toxin A in the management of pain associated with cutaneous leiomyomas.
DESIGN, SETTING, AND PARTICIPANTS
Randomized, double-blind, placebo-controlled pilot study conducted from January 5, 2009, to March 27, 2014. The setting was a single-center study at the National Institutes of Health among participants 18 years or older with cutaneous leiomyomas characterized by pain at least once weekly and pain of at least 4 on a pain scale ranging from 0 to 10.
INTERVENTIONS
Eighteen participants were randomized to receive intralesional botulinum toxin A (5 U per 1 cm2) or equivalent volumes of intralesional saline placebo.
MAIN OUTCOMES AND MEASURES
The primary outcomes were the differences in average lesional pain assessed by the Brief Pain Inventory and visual analog scale before and after ice provocation over a 4-week period.
RESULTS
No significant difference in average lesional pain was observed between the study arms. Decreased pain was reported in the botulinum toxin vs placebo arms by visual analog scale scores before ice provocation (median, 0.00; range, −3.30 to 0.70 for botulinum toxin and median, 0.40; range, −1.30 to 1.50 for placebo; P = .06); however, this finding was nonsignificant. No significant difference was observed in change in pain after ice provocation. A significant difference was seen between the arms in skin-related quality of life by total Dermatology Life Quality Index (median, −4.00; range, −8.00 to 2.00 for botulinum toxin and median, 0.00; range, −1.00 to 4.00 for placebo; P = .007) and with the specific skin pain–related question on the Dermatology Life Quality Index (median, −1.00; range, −2.00 to 1.00 for botulinum toxin and median, 0.00; range, −1.00 to 0.00 for placebo; P = .048). No significant difference was found in pain as ascertained by Patient Global Impression of Change at week 4. No serious adverse events related to botulinum toxin use were observed.
CONCLUSIONS AND RELEVANCE
The use of botulinum toxin to treat painful cutaneous leiomyomas was associated with improved quality of life and with a trend toward improved pain at rest.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00971620
Cutaneous leiomyomas are smooth muscle tumors, most often arising from the arrector pili muscle, that are associated with pain in 92% of affected individuals.1 Leiomyomas may occur sporadically or in association with a dominantly inherited cancer genodermatosis, termed hereditary leiomyomatosis and renal cell cancer (HLRCC), in which multiple cutaneous lesions are common.2 The intense pain associated with cutaneous leiomyomas in this setting has led affected patients to contemplate suicide, and it often does not respond to systemic pain treatment.3 Local excision may be required for pain relief; however, not all lesions may be amenable to surgery given their location or number. For these patients, current management options are generally unsatisfactory.
The mechanism of pain associated with cutaneous leiomyomas is poorly understood but may be related to neuropeptide release, pressure on nerve fibers within the lesions, or contraction of the arrector pili muscle mediated via α-adrenergic receptors. Studies have demonstrated an increase in nerve density within and around leiomyomas compared with the surrounding dermis,4 with an associated increase in acetylcholinesterase (AchE) staining.5 Furthermore, murine nerve fibers in the arrector pili muscle are immunoreactive for calcitonin gene–related peptide (CGRP), a neuropeptide important in pain conduction.6 These data suggest that nerve conduction pathways could be targeted to treat pain associated with cutaneous leiomyomas.
Botulinum toxin has been used as an analgesic agent for the management of chronic pain syndromes, including chronic migraines and complex regional pain syndrome.7,8 By reducing muscle contraction, botulinum toxin decreases nociceptor sensitization by neuropeptides important in pain transmission, including substance P and CGRP. It has also been hypothesized that botulinum toxin may directly affect non-cholinergic neurons, thereby decreasing substance P, CGRP, and glutamate.9 In this study, we conducted a placebo-controlled trial to determine if pain associated with cutaneous leiomyomas could be reduced with botulinum toxin A.
Methods
We conducted a single-center, 12-week, randomized, double-blind, placebo-controlled pilot study from January 5, 2009, to March 27, 2014. The study protocol can be found in Supplement 1. This study was approved by the National Cancer Institute Institutional Review Board. Written informed consent was obtained from participants at the time of enrollment.
Eligible participants were adults 18 years or older with histopathologic confirmation of cutaneous leiomyoma, at least 1 symptomatic leiomyoma with pain of at least 4 on a pain scale ranging from 0 to 10, and pain occurring at least once weekly. Exclusion criteria included the following: metastatic disease; known allergy to botulinum toxin; botulinum toxin therapy in the previous 6 months; neuromuscular junction disorder or peripheral motor neuropathic disorder; pain from other diseases requiring narcotics or causing severe acute debilitating pain; use of pain medications, neuroactive agents, or other therapy directed toward treatment of cutaneous leiomyomas concurrently or within 5 days or 5 half-lives of study enrollment; positive pregnancy test result, breastfeeding, intent to become pregnant or initiate breastfeeding during the study period, or inability to use a reliable form of contraception during the study period. The use of rescue pain medications, specifically oral ibuprofen or acetaminophen, was allowed. Participants receiving chronic nonsteroidal anti-inflammatory drug therapy for pain unrelated to cutaneous leiomyomas were permitted to continue these medications.
Eligible participants underwent masked, blocked randomization (block size of 2) and were randomized to receive intralesional botulinum toxin A (Allergan) or intralesional saline placebo. Masked study drug was administered at week 0. The primary outcomes were assessed at week 4, and the secondary outcomes were assessed at weeks 4 and 12. After the completion of questionnaires and a pain assessment at week 12, study drug was unmasked to both patient and physician. Participants who had received placebo at baseline were offered the option to undergo open-label administration of intralesional botulinum toxin to the treated lesions at week 12 and were followed up as part of an extension arm until week 24. Participants who had received botulinum toxin at week 0 did not receive additional therapy at week 12 and were also followed up until week 24.
The volume of botulinum toxin administered was normalized to surface area of the treated leiomyomas at a concentration of 5 U/cm2. Participants randomized to placebo received intralesional saline of an equivalent volume into the treatment site. A maximum total dose of up to 300 U of botulinum toxin per participant was permitted. The most symptomatic cutaneous leiomyomas were selected as treatment sites. Because of heterogeneity in the size and number of lesions among participants, it was not feasible to treat all lesions in every participant; however, all lesions in the designated treatment sites were injected with a volume equivalent to 5 U/cm2 of botulinum toxin. All pain scores were recorded in reference to the treatment site only.
Ice provocation testing was used to assess cold-induced cutaneous leiomyoma pain in a standardized manner.10 Validated clinical assessment tools were used to evaluate pain, including a pain visual analog scale (VAS) before and after ice provocation testing, the Brief Pain Inventory (BPI), and Patient Global Impression of Change in pain (PGIC). Quality of life was assessed using the Dermatology Life Quality Index (DLQI). Participants were instructed to maintain a pain diary in which daily pain VAS scores and rescue pain medication use were recorded (eFigure in Supplement 2).
This study was initially designed to investigate the differences in the worst lesional pain using the BPI long form (week 0) and short form (weeks 4, 12, and 24). Because the recall period for the worst lesional pain items differs slightly between the BPI long and short forms, before the completion of enrollment and before data analysis, the difference in average lesional pain was selected as a revised primary outcome measure. Therefore, the primary outcomes of the study were (1) determination of the differences in average pain as assessed by the BPI between the 2 study arms from weeks 0 to 4 and (2) assessment of the differences in pain VAS scores before and after ice provocation between the 2 study arms from weeks 0 to 4.
The secondary outcomes included (1) persistence of pain control at week 12 based on the BPI and VAS scores, (2) change in quality of life as measured by total DLQI, (3) change in participant pain based on the PGIC, (4) change in magnitude and frequency of painful episodes based on the daily pain diary, and (5) change in immunohistochemical staining of nerve fibers and neuropeptides from weeks 0 to 12. Pain severity and pain interference were also assessed by the BPI. Pain severity was defined by the mean of the worst, least, average, and current pain scores, and pain interference was defined as the mean of the 7 BPI interference items based on consensus recommendations for BPI use in chronic pain clinical trials.11
Lesional skin samples were collected at weeks 0 and 12 in both study arms and submitted for routine diagnostic evaluation in the Laboratory of Pathology, Center for Cancer Research, National Cancer Institute. At the completion of the trial, immunohistochemical evaluation for c-fos (a marker of neuronal activation after pain stimulation) and AchE was performed using formalin-fixed, paraffin-embedded tissue. Four-micrometer sections were deparaffinized in xylene and graded alcohols and were subjected to antigen retrieval in citrate buffer (pH 9) for 20 minutes in a pressure cooker. Anti–c-fos (ab27436, rabbit polyclonal at 1:4 dilution; Abcam) and anti-AchE (ab2802, mouse monoclonal clone ZR3 at 1:100 dilution; Abcam) primary antibodies were applied to tissues for 60 minutes at room temperature. Antigen-antibody complexes were visualized using a 3,3′-diaminobenzidine chromogen (Dako North America). Slides were counterstained with hematoxylin. Positive and negative controls were used to ensure the specificity of the immunohistochemical reactions. Immunohistochemistry was reviewed and scored by an observer (S.M.H.) blinded with respect to time point and treatment. The c-fos staining was scored as 0 (none), 1 (scattered), 2 (<66% of tumor cells), or 3 (≥66% of tumor cells). The AchE staining was scored as 0 (none), 1 (rare), 2 (scattered), or 3 (focal or greater).
The sample size for this pilot study was determined to provide an adequate number of patients to identify a difference between the 2 study arms using a 1-sided, 2-sample t test with α = .10. A planned accrual of 18 participants (9 patients per study arm) permitted the trial to have 78% power to assess a difference in the primary end points equal to 1 SD of the difference between time points. Given the small sample size, the differences in the primary and secondary end points were compared between the study arms using the Wilcoxon rank sum test, and P < .05 was considered statistically significant for the primary end points. For the set of exploratory analyses performed, P < .01 was considered statistically significant. The difference in the medians between the study arms for the primary end points was determined using a Hodges-Lehmann estimator. Daily pain diary VAS scores were evaluated by averaging the values for each sequential 7-day period for the first 12 weeks and assessing for the differences in the values between the 2 study arms with an exact 2-tailed Wilcoxon rank sum test.
Analyses of immunohistochemical studies were performed by comparing the difference in staining scores between the study arms from weeks 0 to 12 using the exact Wilcoxon rank sum test. Spearman rank correlation analyses were performed to determine the correlation between changes in pain score from baseline to week 4 and changes in immunohistochemical score, focusing on results obtained separately by study arm. For these correlations, |r| exceeding 0.70 would be considered a strong correlation, while |r| between 0.50 and 0.70 would be considered a moderately strong correlation.
Results
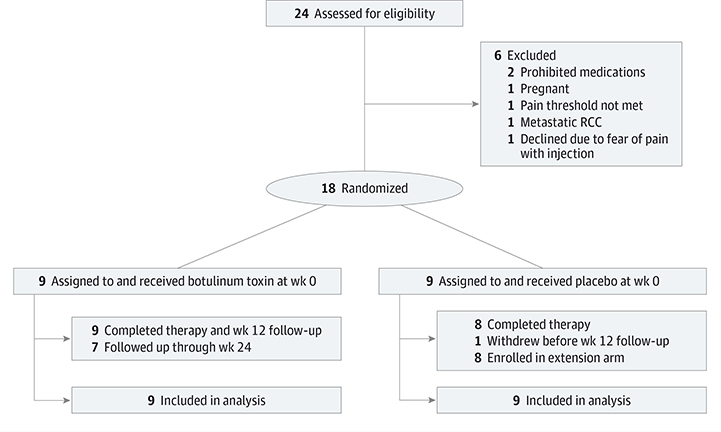
Twenty-four patients with cutaneous leiomyomas were screened for participation. Eighteen participants were randomized and equally distributed into 2 study arms, with 9 participants in each arm (Figure 1).
Figure 1. Flow Diagram of Patient Enrollment and Progress Through the Study.
RCC indicates renal cell carcinoma.
A female predominance was present in both study arms (8 of 9 for botulinum toxin and 6 of 9 for placebo). Because of the small number of patients, we observed variation in location and distribution of lesions between the study arms. All participants who underwent HLRCC genetic testing were found to have a fumarate hydratase mutation. One participant elected not to undergo testing. The volume of injected study drug did not differ significantly between the 2 study arms (mean [SD], 2.41 [0.70] mL for botulinum toxin and mean [SD], 1.12 [0.38] mL for placebo; P = .17) (Table and eTable 1 in Supplement 2). Most participants characterized their pain as sharp (83% [15 of 18]), tender (81% [13 of 16]), stabbing (75% [12 of 16]), shooting (72% [13 of 18]), penetrating (71% [12 of 17]), or miserable (71% [12 of 17]).
Table.
Characteristics of Study Participants
| Study Arm | ||
|---|---|---|
| Characteristic | Botulinum Toxin (n = 9) |
Placebo (n = 9) |
| Age, median (interquartile range), y | 55 (24–71) | 43 (25–60) |
| Ratio of men to women | 1:8 | 3:6 |
| Leiomyoma distribution | ||
| Single | 1 | 3 |
| Segmental | 7 | 6 |
| Disseminated | 1 | 0 |
| Location of lesions | ||
| Neck | 1 | 0 |
| Torso | 6 | 4 |
| Extremity | 2 | 5 |
| Volume of study drug administered, mean (SD), mLa | 2.41 (0.70) | 1.12 (0.38) |
P = .17.
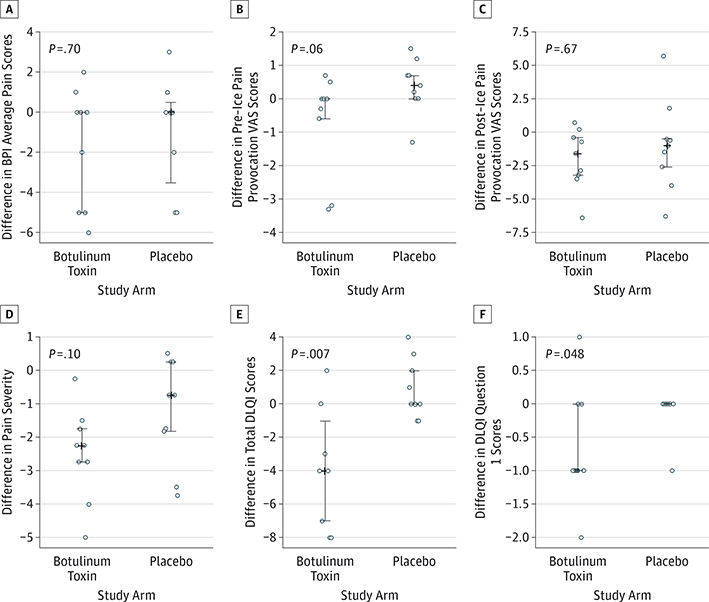
Ice provocation testing resulted in increased lesional pain at baseline (mean [SD], 1.13 [0.40] before provocation and mean [SD], 5.64 [0.67] after provocation; P < .001). No significant difference in change in average pain was observed between the 2 study arms from weeks 0 to 4 (median, 0.00; range, −6.00 to 2.00 for botulinum toxin and median, 0.00; range, −5.00 to 3.00 for placebo; P = .70) (Hodges-Lehmann estimator of the median difference, 0.00; 95% CI, −2.00 to 4.00) (Figure 2A). However, 44% (4 of 9) of participants in the botulinum toxin arm reported greater than 50% reduction in average pain compared with 22% (2 of 9) of participants in the placebo arm (eTable 2 in Supplement 2). Reduced pre–ice pain provocation from weeks 0 to 4 was observed in the botulinum toxin arm compared with the placebo arm (median, 0.00; range, −3.30 to 0.70 for botulinum toxin and median, 0.40; range, −1.30 to 1.50 for placebo; P = .06) (Hodges-Lehmann estimator of the median difference, −0.70; 95% CI, −1.90 to 0.00); however, this finding was statistically nonsignificant (Figure 2B). We observed that 56% (5 of 9) of participants who received botulinum toxin demonstrated reduced pre–ice pain provocation VAS scores compared with 11% (1 of 9) of participants who received placebo. Five participants who received placebo compared with 1 participant who received botulinum toxin reported increased pre–ice pain provocation from weeks 0 to 4 (eTable 2 in Supplement 2). No significant difference was seen in change in perceived pain after ice provocation between the study arms from weeks 0 to 4 (median, −1.60; range, −6.40 to 0.70 for botulinum toxin and median, −1.00; range, −6.30 to 5.70 for placebo; P = .67) (Hodges-Lehmann estimator of the median difference, −0.60; 95% CI, −3.80 to 1.90) (Figure 2C).
Figure 2. Primary and Secondary Outcomes Assessed at Week 4.
In A through F, vertical lines ending in small horizontal line segments represent the 25th to 75th percentiles; the medians are shown as a plus sign when the data are adequately spread to distinguish these from the first or third quartiles. BPI indicates brief pain inventory; DLQI, Dermatology Life Quality Index; VAS, visual analog scale.
Although reduced pain severity was reported in all participants who received botulinum toxin from weeks 0 to 4, the difference between the study arms did not reach statistical significance (median, −2.25; range, −5.00 to −0.25 for botulinum toxin and median, −0.75; range, −3.75 to 0.50 for placebo; P = .10) (Figure 2D). No significant difference in interference of pain in daily activities was noted from weeks 0 to 4 between the study arms.
Skin-related quality of life significantly improved in patients who received botulinum toxin compared with patients who received placebo (median, −4.00; range, −8.00 to 2.00 for botulinum toxin and median, 0.00; range, −1.00 to 4.00 for placebo; P = .007) (Figure 2E). We further observed significantly reduced skin pain in the botulinum toxin arm from weeks 0 to 4 as ascertained by question 1 on the DLQI (“Over the last week, how itchy, sore, painful or stinging has your skin been?”) (median, −1.00; range, −2.00 to 1.00 for botulinum toxin and median, 0.00; range, −1.00 to 0.00 for placebo; P = .048) (Figure 2F).
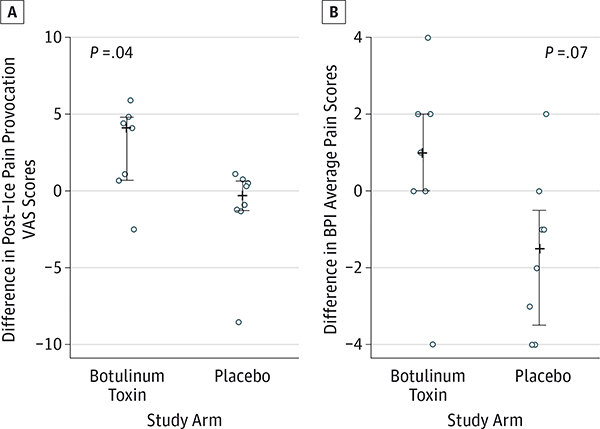
All participants randomized to placebo elected to receive open-label botulinum toxin at week 12 and demonstrated superior improvement in post–ice pain provocation VAS scores (median, 4.10; range, −2.50 to 5.90 for botulinum toxin and median, −0.30; range, −8.50 to 1.10 for placebo; P = .04) compared with participants from weeks 12 to 24 treated with botulinum toxin at week 0 (Figure 3A). Average pain scores from weeks 12 to 24 were also decreased in the botulinum toxin arm vs the placebo arm; however, this difference did not reach statistical significance (median, 1.00; range, −4.00 to 4.00 for botulinum toxin and median, −1.50; range, −4.00 to 2.00 for placebo; P = .07) (Figure 3B).
Figure 3. Secondary Outcomes Assessed at Week 24.
In A and B, the plus sign refers to the median value. The vertical lines ending in small horizontal line segments represent the 25th to 75th percentiles. BPI indicates brief pain inventory; VAS, visual analog scale.
No significant differences in pre–ice pain provocation, post–ice pain provocation, average pain, pain severity, pain interference, or skin-related quality of life were seen between the study arms from weeks 0 to 12. In addition, no significant difference in the median change in PGIC scores was observed between the study arms from weeks 4 to 12. Pain diary scores at time points throughout the 12-week clinical trial did not differ significantly between the study arms. The use of rescue pain medications was infrequent in both study arms. Eight study participants recorded the use of over-the-counter rescue pain medications; of these, 6 of 8 reported 5 or fewer instances of pain medication use for cutaneous leiomyomas.
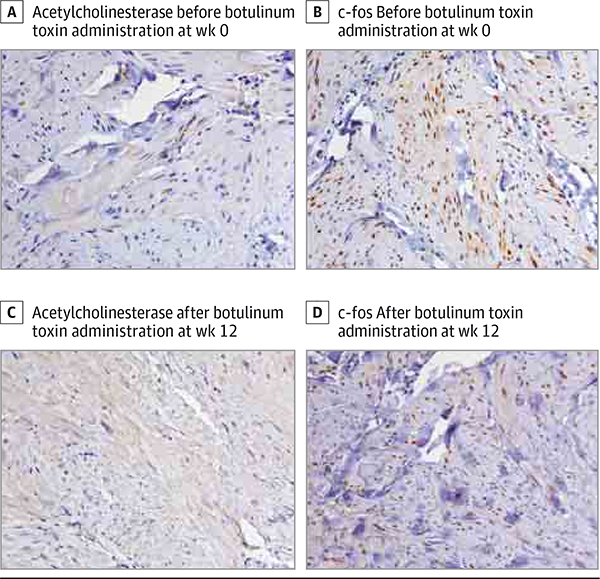
The differences in immunohistochemical staining of AchE (median, 1.00; range, 0.00 to 2.00 [n = 5] for botulinum toxin and median, 0.00; range, −1.00 to 0.00 [n = 3] for placebo; P = .07) and c-fos (median, −1.00; range, −1.00 to 0.00 [n = 5] for botulinum toxin and median, 0.00; range, 0.00 to 1.00 [n = 3] for placebo; P = .07) between the 2 study arms from weeks 0 to 12 did not reach statistical significance but suggested increased spread of AchE and decreased neuronal activation, respectively, in participants treated with botulinum toxin. Although based on limited data, these findings were further supported by a negative correlation between AchE staining from weeks0 to 12 (Figure 4) and changes in pre–ice pain provocation (Spearman r = −0.92, P = .03 [n = 5]) and post–ice pain provocation (Spearman r = −0.72, P = .17 [n = 5]) from weeks 0 to 4 in participants treated with botulinum toxin.
Figure 4. Immunohistochemical Staining of Cutaneous Leiomyomas for Acetylcholinesterase and c-fos Before and After Botulinum Toxin Administration.
All images are original magnification ×320. Comparing A and C, increased acetylcholinesterase expression in nerve fibers is observed after botulinum toxin administration, consistent with increased spread of acetylcholinesterase. Comparing B and D, decreased c-fos expression in the leiomyoma tumor cells is observed after botulinum toxin administration, consistent with decreased neuronal activation.
No significant adverse events were observed during this study. However, participants reported pain associated with intralesional injection of both botulinum toxin and placebo.
Discussion
In this study, we demonstrated that the use of intralesional botulinum toxin is associated with improvement in skin-related quality of life. We also showed that intralesional botulinum toxin is associated with improved pain at rest and reduced pain severity associated with cutaneous leiomyomas; however, these findings were not statistically significant.
The use of botulinum toxin has demonstrated efficacy for the management of other chronic pain conditions, including chronic migraines, complex regional pain syndrome, and neuropathic pain.7,8 Improvement following botulinum toxin injection has also been described for cutaneous leiomyoma pain in 2 case reports.12,13 To our knowledge, our study is the first controlled trial that systematically examines the efficacy of botulinum toxin for chronic pain associated with a skin condition.
The inherent subjectivity associated with pain perception presents unique challenges for the study of pain. What may be considered moderate pain by one individual might be perceived as intense pain by another. As a result, the differences in pain perception make it difficult to compare absolute pain scores from one individual or study arm with those of another. Therefore, to standardize pain assessment in this study, we chose to compare the differences in individual pain scores between time points in both study arms.
Cutaneous leiomyomas may be associated with pain at rest that may wax and wane over several minutes to hours. Using pain VAS scores to assess pain at rest, we observed a trend toward reduced pain in participants who received botulinum toxin compared with those who received placebo; however, this change did not reach statistical significance, possibly because of the limited sample size.
Based on the BPI, we observed reduced pain severity in patients treated with botulinum toxin, although this result did not reach statistical significance. Nevertheless, these data suggest that botulinum toxin may reduce the intensity of pain associated with these lesions. We also found a marked improvement in skin-related quality of life and skin pain in the botulinum toxin arm compared with the placebo arm. These findings indicate that amelioration of chronic skin pain may improve quality of life by reducing skin pain and improving social, emotional, and functional status.
The exact mechanism of pain reduction by botulinum toxin is not well understood. However, our immunohistochemical data suggest 2 possible mechanisms. First, botulinum toxin may reduce cutaneous leiomyoma pain through blockade of cholinergic pathways, which may lead to reduction in arrector pili muscle contraction as demonstrated by increased spread of AchE14 from weeks 0 to 12. Second, botulinum toxin may reduce neuronal activation as demonstrated by decreased c-fos staining from weeks 0 to 12. In various settings, c-fos is known to be a marker of neuronal activation.
Given the rarity of HLRCC and sporadic skin leiomyomas, our study was limited by a small sample size, which may have impaired our ability to detect small differences between the study arms. Furthermore, the inherent subjectivity in how pain is experienced between patients may have biased our results toward accepting the null hypothesis. Although ice provocation has been used to assess provoked pain in the setting of cutaneous leiomyoma previously, this measure is not validated as yet, and the degree of pain provoked by ice can vary among patients. To date, no reliable objective measure of pain has been devised to quantify the degree of provoked pain. Given these limitations, we used several validated measures of pain to corroborate our primary outcome assessments. Unfortunately, repeated provoked measurements at each time point were not possible because of escalation in pain following multiple provocations, as well as ethical considerations regarding repeated pain induction. Nevertheless, the double-blind nature of the study and the use of validated pain scales provide preliminary evidence that botulinum toxin has potential usefulness for sensory pain associated with skin disease, including skin leiomyomas.
All but one patient in this study underwent fumarate hydratase testing, confirming a diagnosis of HLRCC. Therefore, based on our cohort, we cannot draw conclusions about the differences in pain mechanism or severity in sporadically occurring cutaneous leiomyomas. Intralesional administration of botulinum toxin and placebo led to temporary worsening of pain symptoms. Nonetheless, all participants who received intralesional placebo at week 0 chose to enroll in the extension arm and receive open-label botulinum toxin, suggesting that pain associated with intralesional drug administration is tolerable. The development of a topically administered formulation of botulinum toxin may circumvent this practical limitation.15–17
Conclusions
In conclusion, we demonstrated in this study that botulinum toxin is associated with improvement in skin-related quality of life in individuals with cutaneous leiomyomas. We further showed that botulinum toxin may reduce pain at rest associated with cutaneous leiomyomas by specifically reducing pain intensity. Botulinum toxin may reduce pain associated with cutaneous leiomyomas through cholinergic mechanisms or through reduction in neuronal activation. To our knowledge, this is the first systematic study to demonstrate the benefit of botulinum toxin for pain amelioration associated with cutaneous leiomyomas. Given the rarity of cutaneous leiomyomas, a multicenter trial would likely be required to obtain a sample size sufficient to further determine the efficacy of skin pain reduction by botulinum toxin. Ideally, future studies would use a topical formulation of botulinum toxin to improve tolerability and adherence to therapy.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. Botulinum toxin A was provided by Allergan through a material transfer agreement with the National Institutes of Health.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Additional Contributions: Effie Nomicos, RN, BSN, MSN, Sharon Osgood, BSN, RN, CMSRN, and Amanda Johnson, BA (Dermatology Branch, Center for Cancer Research, National Cancer Institute), assisted with this study. Sandra Mitchell, PhD, CRNP (Outcomes Research Branch, National Center Institute), provided helpful discussions. Jennifer Martinez, BA, and Kris Ylaya, BA (Laboratory of Pathology, Center for Cancer Research, National Cancer Institute), helped with histology and immunohistochemistry.
Contributor Information
Haley B. Naik, Dermatology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland.
Seth M. Steinberg, Biostatistics and Data Management Section, Office of the Clinical Director, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland.
Lindsay A. Middelton, Urologic Surgery and the Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland.
Stephen M. Hewitt, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland.
Rena C. Zuo, Dermatology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland.
W. Marston Linehan, Urologic Surgery and the Urologic Oncology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland.
Heidi H. Kong, Dermatology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland.
Edward W. Cowen, Dermatology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland.
REFERENCES
- 1.Toro JR, Nickerson ML, Wei MH, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73(1):95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98(6): 3387–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batchelor RJ, Lyon CC, Highet AS. Successful treatment of pain in two patients with cutaneous leiomyomata with the oral alpha-1 adrenoceptor antagonist, doxazosin. Br J Dermatol. 2004;150(4): 775–776. [DOI] [PubMed] [Google Scholar]
- 4.Raj S, Calonje E, Kraus M, Kavanagh G, Newman PL, Fletcher CD. Cutaneous pilar leiomyoma: clinicopathologic analysis of 53 lesions in 45 patients. Am J Dermatopathol. 1997;19(1):2–9. [DOI] [PubMed] [Google Scholar]
- 5.Mustakallio KK, Levonen E, Niemi M. Histochemical studies on cutaneous leiomyomatosis, I: innervation of normal arrector pili muscles and pilomyomas. Br J Dermatol. 1963;75:60–70. [DOI] [PubMed] [Google Scholar]
- 6.Peters EM, Botchkarev VA, Botchkareva NV, Tobin DJ, Paus R. Hair-cycle–associated remodeling of the peptidergic innervation of murine skin, and hair growth modulation by neuropeptides. J Invest Dermatol. 2001;116(2):236–245. [DOI] [PubMed] [Google Scholar]
- 7.Arezzo JC. Possible mechanisms for the effects of botulinum toxin on pain. Clin J Pain. 2002;18(6) (suppl):S125–S132. [DOI] [PubMed] [Google Scholar]
- 8.Argoff CE. A focused review on the use of botulinum toxins for neuropathic pain. Clin J Pain. 2002;18(6)(suppl):S177–S181. [DOI] [PubMed] [Google Scholar]
- 9.Capek P, Dickerson TJ. Sensing the deadliest toxin: technologies for botulinum neurotoxin detection. Toxins (Basel). 2010;2(1): 24–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archer CB, Whittaker S, Greaves MW. Pharmacological modulation of cold-induced pain in cutaneous leiomyomata. Br J Dermatol 1988;118 (2):255–260. [DOI] [PubMed] [Google Scholar]
- 11.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3): 337–345. [DOI] [PubMed] [Google Scholar]
- 12.Onder M, Adişen E. A new indication of botulinum toxin: leiomyoma-related pain. J Am Acad Dermatol. 2009;60(2):325–328. [DOI] [PubMed] [Google Scholar]
- 13.Sifaki MK, Krueger-Krasagakis S, Koutsopoulos A, Evangelou GI, Tosca AD. Botulinum toxin type A: treatment of a patient with multiple cutaneous piloleiomyomas. Dermatology. 2009;218(1):44–47. [DOI] [PubMed] [Google Scholar]
- 14.Borodic GE, Ferrante R, Pearce LB, Smith K. Histologic assessment of dose-related diffusion and muscle fiber response after therapeutic botulinum A toxin injections. Mov Disord. 1994;9(1):31–39. [DOI] [PubMed] [Google Scholar]
- 15.Brandt F. Efficacy and safety evaluation of a novel botulinum toxin topical gel for the treatment of moderate to severe lateral canthal lines. Dermatol Surg. 2011;37(7):1060. [DOI] [PubMed] [Google Scholar]
- 16.Collins A, Nasir A. Topical botulinum toxin. J Clin Aesthet Dermatol. 2010;3(3):35–39. [PMC free article] [PubMed] [Google Scholar]
- 17.Glogau R, Blitzer A, Brandt F, Kane M, Monheit GD, Waugh JM. Results of a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of a botulinum toxin type A topical gel for the treatment of moderate-to-severe lateral canthal lines. J Drugs Dermatol 2012;11(1):38–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.