INTRODUCTION
The world is currently facing a pandemic resulted of a Coronaviridae family virus global spread declared by the World Health Organization (WHO) as public health emergency (1, 2). The first coronaviruses with human infection properties were isolated in 1937, but it was not until 1965 that this agent received its name based on its microscopic crown-shaped structure (3). Due to emergence of this virus and the new wave of infections, worldwide research is focused in better understanding its characteristics in order to outline current and effective ways of fighting against it (1).
Out of the six types of virus from the Coronaviridae Family, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the one responsible for the Coronavirus Disease 2019 (COVID-19) (4); this virus has 80% if its gene structure identical to the SARS-CoV, responsible for the SARS pandemic in 2002 (5).
There is a consensus that the main form of contagion of this disease is from person to person through droplets derived from sneezing or coughing (6) and that the gold standard diagnosis tool is the real-time reverse transcription polymerase chain reaction (RT-PCR) of samples collected by nasopharyngeal and oropharyngeal swab (7). Despite that, the virus has already been isolated in urine (8), feces (8), conjunctiva (9) and saliva (10) from infected patients. Hence, could the virus also be found in the semen of infected males?
There are more than 27 viruses (HIV, mumps, zika, among others) that can be found in semen, which indicates the virus potential to reach organs of the male reproductive system (11–14). Beyond the transmissibility matter, previous studies indicate that, when present in semen, some virus can affect the male fertile potential (15); therefore, it is important to investigate SARS-CoV-2 presence in semen of infected men while also evaluating possible changes on their fertile potential.
In view of the genetic similarity between the etiological agents of SARS and COVID-19, it is possible to infer the probable effects of SARS-CoV-2 on the male reproductive system based on previous studies on SARS-CoV. There are no reports on the presence of SARS-CoV in semen in patients with SARS, however there were descriptions of orchitis and deleterious effects on testicular tissue in autopsies (16, 17) with confirmation of the virus presence in the testicles (18).
Moreover, the mechanism of cellular infection of SARS-CoV-2 is similar to SARS-CoV, due to the link between the viral Spike (S) protein and the Angiotensin converting enzymes 2 (ACE2) cell receptor (19–21). Previous studies have shown the high concentration of these receptors in the germ and somatic cells of the testicular tissue (22). This fact may indicate the testicles tissue vulnerability to contamination by this new virus, reinforcing the importance of monitoring the reproductive function in infected patients.
The purpose of this narrative review is to evaluate published evidence on possible effects of COVID-19 on male reproductive system.
MATERIALS AND METHODS
A narrative review was done with the aim to identify all relevant studies on SARS-CoV-2 and male reproductive system. We performed a search on Pubmed platform using keywords such as “covid 19”, “SARS-CoV-2”, “pandemic”, “infection” and “virus” added to the Boolean operators “AND”, “OR” and combined with others terms such as “cell receptors”, “semen”, “gonadal function” and “testicles”. No temporal limits were set for the database searches as the topic is recent and little published literature is available. Only articles written in English were considered.
Cellular receptors associated with the infectious process
Due to the similarity related to the infection pathogenesis between SARS-CoV and SARS-CoV-2, a recent report has already described the importance of the ACE2 cells receptor for the initial binding between virus and cell, which initiates the cell fusion and invasion process (21). As result, several studies have demonstrated the ACE2 receptor concentrations in different human tissues, predicting the possibility of infection in these systems. For this review, we limited our analysis to studies that evaluated the tissues of the male reproductive system.
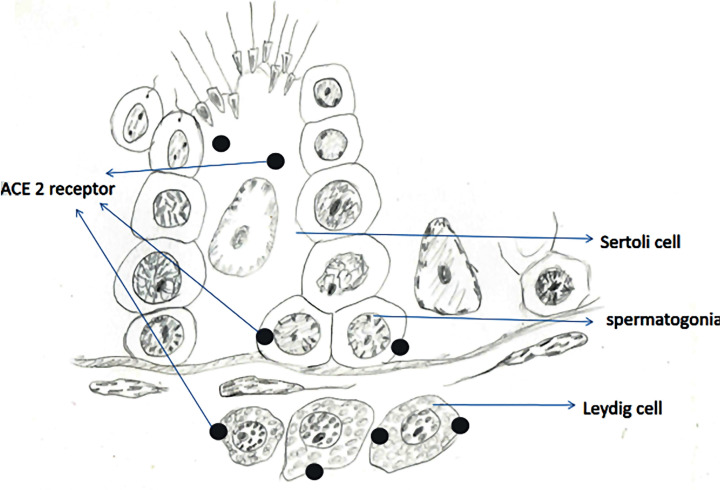
Different methods can be applied to investigate the presence of receptors in a tissue, but most the reviewed studies have done their analysis through bioinformatics associated with gene sequencing of RNA expression (23). The results have demonstrated ACE2 highly expressed in Leydig cells and cells of the seminiferous tubules (24), besides high expression in germ cells (25, 26) (Figure-1). These findings were also confirmed by another study that demonstrated that the testicular tissue has the highest concentration of ACE2 receptors when compared to other human tissues, higher even than the lung tissue, main target of the disease (27). This study still performed immunohistochemistry analysis that showed high ACE2 expression in sperm and Leydig cells, moderate expression in seminiferous vesicle glands and low expression in the prostate and bladder (27).
Figure 1. Scheme depicting the location of ACE2 receptor, a target for SARS-CoV-2 infection, in testicular cells.
These results provide evidences that the testicles are vulnerable to infection by SARS-CoV-2, however, with such a concentration of receptors in the testicular tissue, why is the infection not clinically evident in this system? More recent studies have shown that as important as the presence of ACE2 receptor is the presence of a transmembrane protease named Transmembrane Serine Protease 2 (TMPRSS2). This protease is responsible for assisting the breakdown of the viral S protein favoring its fusion and invasion into the cell (28). When assessing the ACE2 receptors and TMPRSS2 proteases co-expression, a low (29) or extremely rare (30) expression was observed in testicular tissue, in contrast to the high co-expression identified in pneumocytes and nasal epithelial cells (31), which explains the high frequency of respiratory symptoms in COVID-19. This high co-expression was also observed in the ileum, heart and kidney (32), which may be related to the gastrointestinal symptoms described and the high rates of heart and kidney complications associated to the disease (33–35).
According to these findings, the infection by SARS-CoV-2 in the male reproductive system is unlikely to occur. However, it is important to note that virus can find other ways to infect the cell besides ACE2 receptors and TMPRSS2 proteases (36, 37), nevertheless, the RNA sequencing method for ACE2 and TMPRSS2 evaluation is also subject to bias and errors. In that sense, the true effects of the virus on the male reproductive system must be further evaluated through clinical studies.
SARS-CoV-2 presence in semen and other secretions
The initial clinical studies evaluating the presence of SARS-CoV-2 virus in semen of infected patients using RT-PCR tests have not detected virus presence in the samples. These studies, however, evaluated a small number of patients (between 12 and 34 individuals) and most of them were in recovery periods from the disease, on average 30 days after the disease onset (29, 38). Despite this, orchialgia complaints were noted in 19% of the patients (29), which could lead us to infer probable testicular involvement in the disease process but not all patients in the study had a comprehensive genitourinary examination which limits these result interpretations.
A subsequent study analyzed semen from 38 inpatients diagnosed with COVID-19, 15 patients were in the acute phase and 23 were already recovered from the disease. Viruses were found in semen of 6 patients, 4 (15.8%) who were in the acute phase and 2 (8.7%) who were in the recovery phase (2 and 3 days of recovery) (39). This was the first study that demonstrated the presence of the virus in semen.
When considering the nasopharyngeal and oropharyngeal secretion RT-PCR, the peak of sensitivity occurs at the symptoms onset with rare cases maintaining positive results after 21 days of infection, a pattern different from the tracheal secretion that shows the peak of sensitivity at the 11th day of infection and the positivity remains longer (40, 41). These indicates a probable window of virus exposure that can vary according to which secretion that is been evaluated; the study shows that the presence of virus in semen is more evident in the acute phase of disease beginning to identify the window of positivity in this secretion.
A higher and longer level of viral load is observed in severe cases when compared to patients with milder symptoms (41). Thus, another point to be considered is that hospitalized patients with potentially severe cases and greater viremia were selected for the study that identified virus presence in the semen, differently from previous studies with negative results that sole evaluated recovered individuals.
In order to validate these results, a prospective follow-up of those patients would be important to understand for how long the virus remains in the semen. Moreover, specific studies to analyze the possibility of viral transmissibility by this secretion could enhance the impact of the infection in the male reproductive system.
Another study yet analyzed prostate secretion in the urine after prostate massage. Viral research was negative in all 23 evaluated patients, even with 75% of them in the acute phase of the disease (42).
Gonadal function of patients with COVID-19
Only one of the reviewed studies evaluated gonadal function in COVID-19 patients using a hormonal profile. When compared to healthy individuals, infected patients showed increased LH levels and decreased Testosterone: LH ratio, indicating a probable initial gonadotoxic effect (43). The study evaluated 81 patients classified with moderate or severe disease, defined as presence of fever and cough associated with radiological changes, which could have biased the comparison with healthy individuals. Feverish conditions are known to potentially alter gonadal function (44, 45) and thus, the changes observed in the study could be related to the fever symptoms and not specifically to the COVID-19 infection.
There are no data in the literature regarding the fertile potential of men with COVID-19 as none of the studies performed a seminal analysis. Different viral infections can have a direct effect on gonadal function, such as mumps infection (46) and other viruses (15). Thus, it is important to prospectively analyze COVID-19 patients in order to investigate gonadal dysfunction associated with the condition.
Future perspectives
SARS-CoV-2 has already been found in semen of infected patients, but several questions remain unanswered: Can SARS-CoV2 virus be transmitted through semen?
Can SARS-CoV-2 infection lead to gonadal dysfunction or fertile potential loss?
Are those changes reversible after disease recovery? Further prospective studies are needed to specifically cover these points.
CONCLUSIONS
As any emergent disease, there are more suspicions and hypotheses than certainties in terms of COVID-19 effects on male reproductive system. Numerous studies have been carried out to better understand the disease and its short and long-term repercussions on health status. As demonstrated in other viral diseases, involvement of the male reproductive system is a possibility and it may reveal a new route of transmission and/or repercussions on its functions. The virus has already been found in the semen of infected patients but its impacts on male reproductive health have yet to be further investigated.
ABBREVIATIONS
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- COVID-19
Coronavirus Disease 2019
- SARS-CoV
Severe Acute Respiratory Syndrome Coronavirus
- SARS
Severe Acute Respiratory Syndrome
- RT-PCR
Real-Time reverse transcription Polymerase Chain Reaction
- HIV
Human Immunodeficiency Virus
- S
Spike protein
- ACE2
Angiotensin converting enzymes 2
- RNA
Ribonucleic acid
- TMPRSS2
Transmembrane Serine Protease 2
- LH
Luteinizing Hormone
REFERENCES
- 1.Coronavirus disease (COVID-19) pandemic [Internet] [[cited 2020 May 5]];World Health Organization. 2020 [No authors] [Internet]. Available at.< https://www.who.int/emergencies/diseases/novel-coronavirus-2019>. [Google Scholar]; 1. [No authors]. Coronavirus disease (COVID-19) pandemic [Internet]. World Health Organization .2020 [cited 2020 May 5]. [Internet]. Available at.<https://www.who.int/emergencies/diseases/novel-coronavirus-2019>
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019nCoV and naming it SARS-CoV-2. Version 2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; 2. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019nCoV and naming it SARS-CoV-2. Version 2. Nat Microbiol. 2020; 5:536-44. [DOI] [PMC free article] [PubMed]
- 3.Entenda a diferença entre Coronavírus, Covid-19 e Novo Coronavírus. Os primeiros casos desse agente foram registrados na cidade de Wuhan, na China. [[cited 2020 march 11]];Governo do Brasil. 2020 [No authors] [Internet]. Available at. < https://www.gov.br/pt-br/noticias/saude-evigilancia-sanitaria/2020/03/entenda-a-diferenca-entrecoronavirus-covid-19-e-novo-coronavirus>. [Google Scholar]; 3. [No authors]. Entenda a diferença entre Coronavírus, Covid-19 e Novo Coronavírus. Os primeiros casos desse agente foram registrados na cidade de Wuhan, na China. Governo do Brasil. 2020. [cited 2020 march 11]. [Internet]. Available at. <https://www.gov.br/pt-br/noticias/saude-evigilancia-sanitaria/2020/03/entenda-a-diferenca-entrecoronavirus-covid-19-e-novo-coronavirus>
- 4.CDC COVID Data Tracker. Coronavirus Disease 2019 (COVID-19) CDC Center For Disease Control And Prevention. 2020 [No authors] [Internet]. Available at. < https://covid.cdc.gov/covid-data-tracker/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcasesupdates%2Fcases-in-us.html#cases>. [Google Scholar]; 4. [No authors]. CDC COVID Data Tracker. Coronavirus Disease 2019 (COVID-19). CDC Center For Disease Control And Prevention. 2020 [Internet]. Available at. < https://covid.cdc.gov/covid-data-tracker/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcasesupdates%2Fcases-in-us.html#cases >
- 5.Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]; 5. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020; 525:135–40. [DOI] [PMC free article] [PubMed]
- 6.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11–11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; 6. Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020; 7:11. [DOI] [PMC free article] [PubMed]
- 7.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045–2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]; 7. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020; 25:2000045. [DOI] [PMC free article] [PubMed]
- 8.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]; 8. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020; 382:1708-20. [DOI] [PMC free article] [PubMed]
- 9.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARSCoV-2 infection. J Med Virol. 2020 Jun;92(6):589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]; 9. Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARSCoV-2 infection. J Med Virol. 2020 Jun;92(6):589-594. [DOI] [PMC free article] [PubMed]
- 10.Chen L, Zhao K, Peng J, Li X, Deng X, Geng Z, et al. Detection of 2019-nCoV in Saliva and Characterization of Oral Symptoms in COVID-19 Patients. SSRN. 2020 doi: 10.1111/cpr.12923. Available at. < https://ssrn.com/abstract=3557140>. [DOI] [PMC free article] [PubMed] [Google Scholar]; 10. Chen L, Zhao K, Peng J, Li X, Deng X, Geng Z, et al., Detection of 2019-nCoV in Saliva and Characterization of Oral Symptoms in COVID-19 Patients. SSRN. 2020. Available at. <https://ssrn.com/abstract=3557140>. [DOI] [PMC free article] [PubMed]
- 11.Zea-Mazo JW, Negrette-Mejía YA, Cardona-Maya W. Virus de transmisión sexual: relación semen y virus [Virus of sexual transmission: semen and virus relationship] Actas Urol Esp. 2010;34:845–853. [PubMed] [Google Scholar]; 11. Zea-Mazo JW, Negrette-Mejía YA, Cardona-Maya W. Virus de transmisión sexual: relación semen y virus [Virus of sexual transmission: semen and virus relationship]. Actas Urol Esp. 2010; 34:845-53. [PubMed]
- 12.Gornet ME, Bracero NJ, Segars JH. Zika Virus in Semen: What We Know and What We Need to Know. Semin Reprod Med. 2016;34:285–292. doi: 10.1055/s-0036-1592312. [DOI] [PubMed] [Google Scholar]; 12. Gornet ME, Bracero NJ, Segars JH. Zika Virus in Semen: What We Know and What We Need to Know. Semin Reprod Med. 2016; 34:285-92. [DOI] [PubMed]
- 13.Zafer M, Horvath H, Mmeje O, van der Poel S, Semprini AE, Rutherford G, et al. Effectiveness of semen washing to prevent human immunodeficiency virus (HIV) transmission and assist pregnancy in HIV-discordant couples: a systematic review and meta-analysis. Fertil Steril. 2016;105:645–655.:e2. doi: 10.1016/j.fertnstert.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; 13. Zafer M, Horvath H, Mmeje O, van der Poel S, Semprini AE, Rutherford G, et al. Effectiveness of semen washing to prevent human immunodeficiency virus (HIV) transmission and assist pregnancy in HIV-discordant couples: a systematic review and meta-analysis. Fertil Steril. 2016; 105:645-55.e2. [DOI] [PMC free article] [PubMed]
- 14.Ternavasio-de la Vega HG, Boronat M, Ojeda A, García-Delgado Y, Angel-Moreno A, Carranza-Rodríguez C, et al. Mumps orchitis in the post-vaccine era (1967-2009): a single-center series of 67 patients and review of clinical outcome and trends. Medicine (Baltimore) 2010;89:96–116. doi: 10.1097/MD.0b013e3181d63191. [DOI] [PubMed] [Google Scholar]; 14. Ternavasio-de la Vega HG, Boronat M, Ojeda A, García-Delgado Y, Angel-Moreno A, Carranza-Rodríguez C, et al. Mumps orchitis in the post-vaccine era (1967-2009): a single-center series of 67 patients and review of clinical outcome and trends. Medicine (Baltimore). 2010; 89:96-116. [DOI] [PubMed]
- 15.Garolla A, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Foresta C. Sperm viral infection and male infertility: focus on HBV, HCV, HIV HPV, HSV, HCMV, and AAV. J Reprod Immunol. 2013;100:20–29. doi: 10.1016/j.jri.2013.03.004. [DOI] [PubMed] [Google Scholar]; 15. Garolla A, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Foresta C. Sperm viral infection and male infertility: focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J Reprod Immunol. 2013; 100:20-9. [DOI] [PubMed]
- 16.Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol Reprod. 2006;74:410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]; 16. Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006; 74:410-6. [DOI] [PMC free article] [PubMed]
- 17.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]; 17. Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005; 202:415-24. [DOI] [PMC free article] [PubMed]
- 18.Zhao JM, Zhou GD, Sun YL, Wang SS, Yang JF, Meng EH, et al. [Clinical pathology and pathogenesis of severe acute respiratory syndrome] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2003;17:217–221. [PubMed] [Google Scholar]; 18. Zhao JM, Zhou GD, Sun YL, Wang SS, Yang JF, Meng EH, et al. [Clinical pathology and pathogenesis of severe acute respiratory syndrome]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2003; 17:217-21. [PubMed]
- 19.Millet JK, Kien F, Cheung CY, Siu YL, Chan WL, Li H, et al. Ezrin interacts with the SARS coronavirus Spike protein and restrains infection at the entry stage. PLoS One. 2012;7:e49566. doi: 10.1371/journal.pone.0049566. [DOI] [PMC free article] [PubMed] [Google Scholar]; 19. Millet JK, Kien F, Cheung CY, Siu YL, Chan WL, Li H, et al. Ezrin interacts with the SARS coronavirus Spike protein and restrains infection at the entry stage. PLoS One. 2012; 7:e49566. [DOI] [PMC free article] [PubMed]
- 20.Song W, Gui M, Wang X, Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14:e1007236. doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]; 20. Song W, Gui M, Wang X, Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018; 14:e1007236. [DOI] [PMC free article] [PubMed]
- 21.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; 21. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020; 395:565-74. [DOI] [PMC free article] [PubMed]
- 22.Douglas GC, O'Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI, et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]; 22. Douglas GC, O'Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI, et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004; 145:4703-11. [DOI] [PubMed]
- 23.McDavid A, Finak G, Chattopadyay PK, Dominguez M, Lamoreaux L, Ma SS, et al. Data exploration, quality control and testing in single-cell qPCR-based gene expression experiments. Bioinformatics. 2013;29:461–467. doi: 10.1093/bioinformatics/bts714. [DOI] [PMC free article] [PubMed] [Google Scholar]; 23. McDavid A, Finak G, Chattopadyay PK, Dominguez M, Lamoreaux L, Ma SS, et al. Data exploration, quality control and testing in single-cell qPCR-based gene expression experiments. Bioinformatics. 2013; 29:461-7. [DOI] [PMC free article] [PubMed]
- 24.Fan C, Li K, Ding Y, Lu W, Wang J. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019nCoV infection. MedRxiv. 2020 doi: 10.3389/fmed.2020.563893. [Internet]. Available at. < https://www.medrxiv.org/content/10.1101/2020.02.12.20022418v1>. [DOI] [PMC free article] [PubMed] [Google Scholar]; 24. Fan C, Li K, Ding Y, Lu W, Wang J. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019nCoV infection. MedRxiv. 2020. [Internet]. Available at. <https://www.medrxiv.org/content/10.1101/2020.02.12.20022418v1> [DOI] [PMC free article] [PubMed]
- 25.Wang Z, Xu X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells. 2020;9:920–920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]; 25. Wang Z, Xu X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells. 2020; 9:920. [DOI] [PMC free article] [PubMed]
- 26.Shen Q, Xiao X, Aierken A, Liao M, Hua J. The ACE2 Expression in Sertoli Cells and Germ Cells May Cause Male Reproductive Disorder After Sars-cov-2 Infection. OSF Preprints. 2020 doi: 10.31219/osf.io/fs5hd. Available at. <. >. [DOI] [PMC free article] [PubMed] [Google Scholar]; 26. Shen Q, Xiao X, Aierken A, Liao M, Hua J. The ACE2 Expression in Sertoli Cells and Germ Cells May Cause Male Reproductive Disorder After Sars-cov-2 Infection. OSF Preprints. 2020. Available at. < 10.31219/osf.io/fs5hd>. [DOI] [PMC free article] [PubMed]
- 27.Zhang J, Wu Y, Wang R, Lu K, Tu M, Guo H, et al. Bioinformatic Analysis Reveals That the Reproductive System is Potentially at Risk from SARS-CoV-2. Preprints. 2020 2020020307. Available at. <10.20944/preprints202002.0307.v>. [Google Scholar]; 27. Zhang J, Wu Y, Wang R, Lu K, Tu M, Guo H, et al. Bioinformatic Analysis Reveals That the Reproductive System is Potentially at Risk from SARS-CoV-2. Preprints. 2020, 2020020307. Available at. <10.20944/preprints202002. 0307.v>.
- 28.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.:e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; 28. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020; 181:271-280.e8. [DOI] [PMC free article] [PubMed]
- 29.Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; 29. Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020; 113:1135-9. [DOI] [PMC free article] [PubMed]
- 30.Stanley KE, Thomas E, Leaver M, Wells D. Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 2020;114:33–43. doi: 10.1016/j.fertnstert.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; 30. Stanley KE, Thomas E, Leaver M, Wells D. Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 2020;114:33-43. [DOI] [PMC free article] [PubMed]
- 31.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; 31. Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681-7. [DOI] [PMC free article] [PubMed]
- 32.Qi J, Zhou Y, Hua J, Zhang L, Bian J, Liu B, et al. The scRNA-seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to COVID-19 infection. BioRxiv. 2020 doi: 10.3390/ijerph18010284. [Internet]. Available at. < https://www.biorxiv.org/content/10.1101/2020.04.16.045690v1>. [DOI] [PMC free article] [PubMed] [Google Scholar]; 32. Qi J, Zhou Y, Hua J, Zhang L, Bian J, Liu B, et al. The scRNA-seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to COVID-19 infection. BioRxiv. 2020. [Internet]. Available at. <https://www.biorxiv.org/content/10.1101/2020.04.16.045690v1> [DOI] [PMC free article] [PubMed]
- 33.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020 Jun 11;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]; 33. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020 Jun 11;382:2372-4. [DOI] [PMC free article] [PubMed]
- 34.Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, et al. ST-Segment Elevation in Patients with Covid-19 -A Case Series. N Engl J Med. 2020 Jun 18;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]; 34. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, et al. ST-Segment Elevation in Patients with Covid-19 -A Case Series. N Engl J Med. 2020 Jun 18;382:2478-80. [DOI] [PMC free article] [PubMed]
- 35.Rabb H. Kidney diseases in the time of COVID-19: major challenges to patient care. J Clin Invest. 2020;130:2749–2751. doi: 10.1172/JCI138871. [DOI] [PMC free article] [PubMed] [Google Scholar]; 35. Rabb H. Kidney diseases in the time of COVID-19: major challenges to patient care. J Clin Invest. 2020; 130:2749-51. [DOI] [PMC free article] [PubMed]
- 36.Ke Wang, Wei Chen, Yu-Sen Zhou, Jian-Qi Lian, Zheng Zhang, Peng Du, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. BioRxiv. 2020 doi: 10.1038/s41392-020-00426-x. [Internet]. Available at. < https://www.biorxiv.org/content/10.1101/2020.03.14.988345v1>. [DOI] [PMC free article] [PubMed] [Google Scholar]; 36. Ke Wang, Wei Chen, Yu-Sen Zhou, Jian-Qi Lian, Zheng Zhang, Peng Du, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. BioRxiv 2020. [Internet]. Available at. <https://www.biorxiv.org/content/10.1101/2020.03.14.988345v1> [DOI] [PMC free article] [PubMed]
- 37.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV Version 2. Nat Commun. 2020;11:1620–1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; 37. Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Version 2. Nat Commun. 2020; 11:1620. [DOI] [PMC free article] [PubMed]
- 38.Song C, Wang Y, Li W, Hu B, Chen G, Xia P, et al. Absence of 2019 Novel Coronavirus in Semen and Testes of COVID-19 Patients. Biol Reprod. 103:4–6. doi: 10.1093/biolre/ioaa050. 202023. [DOI] [PMC free article] [PubMed] [Google Scholar]; 38. Song C, Wang Y, Li W, Hu B, Chen G, Xia P, et al. Absence of 2019 Novel Coronavirus in Semen and Testes of COVID-19 Patients. Biol Reprod. 202023;103:4-6 [DOI] [PMC free article] [PubMed]
- 39.Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw Open. 2020;3:e208292. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]; 39. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw Open. 2020; 3:e208292. [DOI] [PMC free article] [PubMed]
- 40.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]; 40. Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020; 382:1177-9. [DOI] [PMC free article] [PubMed]
- 41.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; 41. To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020; 20:565-74. [DOI] [PMC free article] [PubMed]
- 42.Weihe quan, qingyou zheng, jinfei tian, jun chen, zhigang liu, xiangqiu chen, et al. No SARS-CoV-2 in expressed prostatic secretion of patients with coronavirus disease 2019: a descriptive multicentre study in China. medRxiv. 2020 [Google Scholar]; 42. Weihe quan, qingyou zheng, jinfei tian, jun chen, zhigang liu, xiangqiu chen, et al. No SARS-CoV-2 in expressed prostatic secretion of patients with coronavirus disease 2019: a descriptive multicentre study in China. medRxiv 2020.
- 43.Ling Ma, Wen Xie, Danyang Li, Lei Shi, Yanhong Mao, Yao Xiong, et al. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. MedRxiv. 2020 [Internet]. Available at. < https://www.medrxiv.org/content/10.1101/2020.03.21.20037267v2>. [Google Scholar]; 43. Ling Ma, Wen Xie, Danyang Li, Lei Shi, Yanhong Mao, Yao Xiong, et al. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. MedRxiv 2020. [Internet]. Available at. <https://www.medrxiv.org/content/10.1101/2020.03.21.20037267v2>
- 44.Carlsen E, Andersson AM, Petersen JH, Skakkebaek NE. History of febrile illness and variation in semen quality. Hum Reprod. 2003;18:2089–2092. doi: 10.1093/humrep/deg412. [DOI] [PubMed] [Google Scholar]; 44. Carlsen E, Andersson AM, Petersen JH, Skakkebaek NE. History of febrile illness and variation in semen quality. Hum Reprod. 2003; 18:2089-92. [DOI] [PubMed]
- 45.Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia. 2007;39:203–215. doi: 10.1111/j.1439-0272.2007.00794.x. [DOI] [PubMed] [Google Scholar]; 45. Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia. 2007; 39:203-15. [DOI] [PubMed]
- 46.Zhang S, An Y, Li J, Guo J, Zhou G, Li J, et al. Relation between the testicular sperm assay and sex hormone level in patients with azoospermia induced by mumps. Int J Clin Exp Med. 2015;8:21669–21673. [PMC free article] [PubMed] [Google Scholar]; 46. Zhang S, An Y, Li J, Guo J, Zhou G, Li J, et al. Relation between the testicular sperm assay and sex hormone level in patients with azoospermia induced by mumps. Int J Clin Exp Med. 2015; 8:21669-73. [PMC free article] [PubMed]