Abstract
Thirty soil samples were taken, and the same number of moss (Dicranum scoparium) and lichen (Pseudevernia furfuracea) bags were exposed to detect environmental pollution in the former mining area Nižná Slaná. Soil and ambient air are influenced by hazardous substances, which leak from old mining bodies due to insufficient or completely missing reclamation. The total content of the risk elements (As, Cd, Co, Cu, Fe, Hg, Mn, Ni, Sb, Se, Pb, Zn) was determined in soil, moss, and lichen samples and in the bodies of Leccinum pseudoscabrum. Biological (soil enzymes—urease, acid phosphatase, alkaline phosphatase, fluorescein diacetate (FDA), ß-glucosidase) and chemical properties (pH) were determined in soil samples. Contamination factor (Cf), degree of contamination (Cd), pollution load index (PLI), and enrichment factor (EF) were used for soil and relative accumulation factor (RAF) for air quality evaluation. Contamination factor values show serious pollution by Cd, Fe, Hg, and Mn. Pollution load index confirmed extremely high pollution almost at all evaluated areas. Soil enzymes reacted to soil pollution mostly by decreasing their activity. Mosses and lichens show differences in the accumulation abilities of individual elements. Regular consumption of L. pseudoscabrum would provide the dose of Cd and Hg below the limit of provisional weekly intake. Based on the bioaccumulation index (BAF) values, L. pseudoscabrum can be characterized as an Hg accumulator.
Keywords: former mining area, L. pseudoscabrum, moss and lichen bag technique, soil contamination, contamination factor, degree of contamination, relative accumulation factor
1. Introduction
The environmental impacts of the spreading of hazardous elements from former mining bodies have been the object of numerous studies all over the world [1,2,3,4]. While some mining bodies represent only a potential risk (become dangerous when disrupted), others are a serious environmental problem causing the degradation of natural ecosystems. The danger of the tailing ponds lies in the dust consistency of sewage sludge, which is easily spreading by the wind at a long distance to the surrounding landscape. Small particles contain hazardous elements such as toxic metals, which are dangerous for the environment and human health. Numerous diseases caused by dust pollution were described in earlier studies [5]. A polluted environment causes defects in both animal and plant bodies [6,7,8]. Effective remediation can reduce the impact of hazardous elements on the environment, but due to the high cost, lack of interest from the government, or unsettled property issues a lot of tailing ponds are left to self-development [9]. A whole range of anthropogenic activities contribute to soil pollution by hazardous substances [10,11,12]. Mining activities and mining-related industries are among the leading ones. Ore mining is inevitably connected with the excavation of huge amounts of dump rocks that disrupt environment balance, cause irreversible changes in landscape structure, and are the source of undesirable substances releasing the soil, water, and air. The state of the soil quality is reflecting on soil conditions, which are responsible for soil fertility and its correct functionality [13].
Moss and lichen transplantation in the polluted sites as an airborne biomonitoring method is widely used [14]. This method has several advantages comparing in situ methods, such as low cost, opportunity to choose exposure time, and monitoring sites [15]. Moss and lichen taxa selection depends on the opportunities in different geographical conditions. Many previous studies deal with the differences between moss and lichen accumulation abilities [16,17]. The ability to accumulate different hazardous elements varies depending on moss/lichen taxa, environmental conditions and time of exposure [18,19].
Soil microbial community plays a key role in soil processes. Soil enzymes catalyze reactions in the soil system that have biochemical significance, participate in nutrient cycles, and transfer energy through organic matter decomposition; nutrients are released to be available for plant growth [20]. Soil enzymes are additionally used as a bioindicator of soil quality [21]; because they are very sensitive to environmental stress, they react quickly to the changes in the soil environment, and their determination is not difficult. The activities of urease (URE), acid phosphatase (ACP), and alkaline phosphatase (ALP), ß-glucosidase (BG), and fluorescein diacetate (FDA) were used in numerous studies to detect the influence of soil pollution to the soil quality [22,23].
The aims of the study are (i) to assess the soil pollution around the former mining bodies in Nižná Slaná mining area using indices (enrichment factor (EF), contamination factor (Cf), degree of contamination (Cd); pollution load index (PLI)), (ii) to determine the influence of soil pollution on the soil quality using soil enzyme activities, (iii) to estimate the ambient air pollution using moss and lichen biomonitoring method through relative accumulation factor (RAF), and (iv) to compare accumulation abilities of the two exposed taxa.
2. Materials and Methods
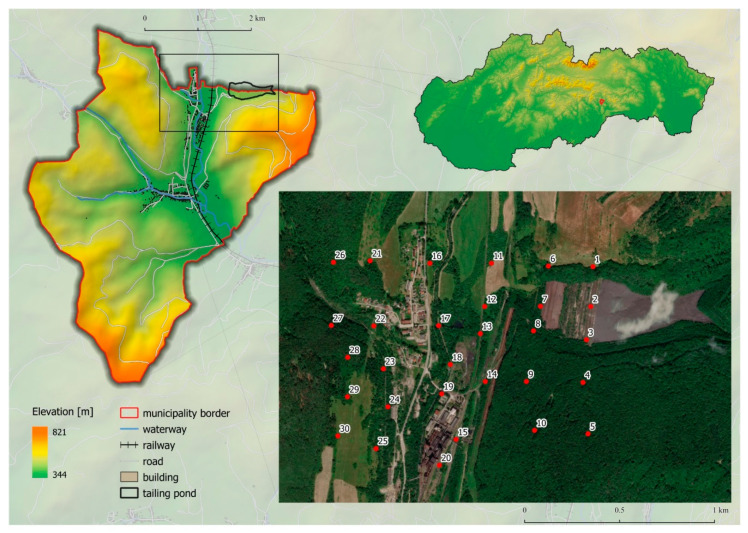
The former mining village Nižná Slaná is situated in the south-eastern part of Slovakia (Figure 1) along the Slaná river valley surrounded by the Slovenské rudohorie hills. Climatological features are typical for a moderately warm to moderately wet climate region, with an average January temperature of −2 °C to −5 °C. According to Köppen climate classification [24], the study area belongs to the warm-summer humid continental climate, with the coldest month average temperature below 0 °C (or −3 °C). All month’s average temperatures were below 22 °C, and at least four months averaging above 10 °C. There were no significant precipitation differences between seasons.
Figure 1.
Study area and sampling points.
The village Nižná Slaná was established solely as the mining municipality in the 14th century, where the iron ores, precious metals, especially silver, pure mercury, and vermilion were mined. The biggest expansion of the mining activities occurred at the beginning of the 19th century what was related to the big iron smelter construction [25]. The mining activities continued until the beginning of the 20th century when all iron ores were exported for processing to the modern ironworks in Hungary. Nowadays, the whole area is included in the list of environmental burdens [9]. The next serious environmental burden in the village cadastre is a tailing pond localized in the northeast direction from the village. The tailing pond [48°44′36.9′’; 25°25′51.24′’] covers an area of 20.6 ha and around 7 million tons of the sludge is stored there. Sludge has the consistency of the fine-grained material that is, during the windy weather, transmitted to the surrounding villages. Due to the unclear ownership relations, the lack of reclamation and the rehabilitation of the dam, security, and stability of the tailing pond is questionable. In past studies, the danger of lung cancer among people under the regular pollution coming from Nižná Slaná mines was confirmed [26].
According to the International Union of the Soil Science recommendation, soils formed by the material of technogenic origins should be defined as the technosol [27]. In our study, sampled material consistency was changing depending on the distance and the direction from the main environmental loads (main mining bodies—tailing pond, iron processing plant), so we used the term “soil” for all the soil/technosol samples.
Soil samples were collected during summer 2018 in the Nižná Slaná village cadastre. Sampling sites were designed as a square network with a distance between two sites of 200 m (due to natural or other obstacles, this distance has not always been fully maintained). The sampling sites network cover the area of the tailing pond, surrounding of the ironwork, and the area which is considered as the most affected by the dust coming from the tailing pond (in the prevailing wind direction) (Figure 1).
In total, 30 soil samples of 500 g of the topsoil (0–10 cm), were sampled from each sampling site, stored in the plastic bags and transported to the laboratory. One part of each sample was frozen (−18°C) and later used for enzyme activity analysis. The second part was dried at room temperature, handy crushed, cleaned from the roots and dead plant parts, and finally sieved through a mesh sieve (2 mm).
The total content of the hazardous elements As, Cd, Co, Cu, Fe, Hg, Mn, Ni, Sb, Se, Pb, and Zn was determined in the soil also in moss and lichen samples. Elemental analysis was carried out on an Agilent ICP-OES spectrometer 720 (Agilent Technologies Inc., Santa Clara, CA, USA) with axial plasma configuration and with an auto-sampler SPS-3 (Agilent Technologies, GmbH, Waldbronn, Germany). Assessment of the hazardous elements in the soil, moss, and lichen samples was described several times in detail in earlier studies [17,23,28,29]. This procedure was maintained for this study.
Exchangeable soil pH was measured by pH meter inoLab pH 720 in a solution of 20 g of soil and 50 mL of CaCl2 (c = 0.01 mol L−1) (Sigma-Aldrich, Ltd., Bratislava, Slovakia). The activity of urease was determined by a spectrometer as an ammonium release (at 410 nm) after 24 h incubation (at 37 °C) of soil samples with a urea solution [30]. The activity of acid and alkaline phosphatase was measured by a spectrometer as a phenol release (at 510 nm) after 3h of incubation (at 37 °C) of soil samples with a phenyl phosphate solution and acetate buffer (pH = 5) for acid phosphatase and borate buffer (pH = 10) for alkaline phosphatase [31]. Fluorescein diacetate activity (FDA) was measured by the spectrometer at 490 nm after the 1h of incubation at 30 °C using fluorescein diacetate as a soil after soil hydrolysis [32]. The activity of ß-glucosidase (BG) was spectrophotometrically determined (464 nm) as a p-nitrophenol release after the 1h incubation at 37 °C of the soil samples with 4-nitrophenyl glucopyranoside [33].
Contamination factor (Cf) described by [34] was used to express the level of soil pollution by individual hazardous elements. The calculation was conducted as follows Equation (1):
| (1) |
where: is the total concentration of the hazardous element in soil and is the background level of the hazardous element. Background levels for As, Cd, Co, Cu, Fe, Hg, Mn, Ni, Pb, Sb, Se, and Zn obtained from [35,36] were are 7.20, 0.30, 60.0, 19.0, 530, 0.08, 20.0, 25.0, 25.0, 1.04, 0.10, and 65.0 mg kg-1, respectively. Hakanson (1981) [34] divided contamination factor values into 4 classes: low contamination factor (if < 1), (ii) moderate contamination factor (if 1< 3); (iii) considerable contamination factor (if 3< 6) and (iv) very high contamination factor (if 6). Overall contamination of the study area by individual elements was expressed by the degree of contamination (Cd) which was used to express the overall contamination of the study area by individual elements, and was calculated as follows Equation (2):
| (2) |
According to Hakanson [29], four classes of the degree of contamination Cd are intended: (i) low degree of contamination (if Cd < 8), (ii) moderate degree of contamination (if 8 ≤ Cd < 16), (iii) considerable degree of contamination (if 16 ≤ Cd < 32), and (iv) very high degree of contamination (if Cd ≥ 32). The comprehensive level of soil pollution at each sampling site was expressed by the pollution load index (PLI) calculated according to [32] as follows Equation (3):
| PLI = (Cf1 × Cf2 × Cf3 × …. × Cfn)1/n | (3) |
where: n is a number of assessed elements, Cf is a contamination factor of individual pollutants. Wang [37] divided the PLI values into four classes as follows: (i) no pollution (PLI < 1), (ii) moderate pollution (1 ≤ PLI < 2), (iii) heavy pollution (2 ≤ PLI < 3), (iv) extreme pollution (PLI ≥ 3).
Enrichment factor (EF) is an indicator used to assess the presence and intensity of anthropogenic contamination on the surface soil. Enrichment factor for each of the risk elements in the substrate was calculated as follows Equation (4):
| (4) |
where: Cx/Cref is the ratio of the concentration of the hazardous element in the substrate sample to the concentration of the reference element. In this study, the reference metal was selected based on the correlation coefficient analysis from risk elements that are neither likely to be affected by anthropogenic activities not correlated with heavy metal pollutants. The background values of the elements were obtained from studies of [35,36]. Background values of As, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Sb, Se, Sr, Zn are 7.2, 0.30, 0.30, 60, 19, 520, 0.08, 20, 25, 25, 1.04, 0.10, 130, and 65, respectively. Enrichment factor values are divided into 5 classes (i) EF < 2 deficiency to minimal enrichment; (ii) 2 ≤ EF < 5 moderate enrichment; (iii) 5 ≤ EF < 20 significant enrichment; (iv) 20 ≤ EF < 40 very high enrichment; (v) EF ≥ 40 extremely high enrichment.
One moss (Dicranum scoparium) and one lichen (Pseudevernia furfuracea) taxa were collected during May 2018 in Čergov Mts (East Slovakia). Increased conservation attention of Čergov Mts. because of specific plant and animal species occurrence is a prerequisite for low environmental contamination. Additionally, no significant source of pollution (industrial factories, etc.) is localized in the surrounding area. The sites of moss and lichen sampling were localized at least 1000 m from the main and at least 500 m from the forest roads. Approximately 500 g of each taxon was collected, stored in a paper bag, and transported to the laboratory where the samples were manually cleaned from the dead parts, needles, and soil particles. Moss and lichen were washed three times (immersed to the water) in deionized water, lasting 5, 10, and 20 min (approximately 10 L water per 100 g of moss and lichen dry weight). After the washing, samples were hand-squeezed and air-dried in an over at 40 °C for 24 h (Venticell 111, BMT, a.s., Czech Republic).
Nylon net (2 mm) was cut at pieces (10 × 10 cm) where approximately 5 g of each taxon was wrapped and tied. One moss and one lichen bag were stored in the laboratory as a control sample (for the initial concentration determination). Each taxon, in two replicates was exposed in 30 sampling sites (in total 120 samples—two taxa, in two replicates in 30 sampling sites). Moss and lichen exposition sites were identical to the soil sampling sites. Bags were exposed on the trees or scrubs, approximately 2 m high, hanging outside the tree (at the end of the branch). After 8 weeks of the exposition, the samples were collected and analyzed for the risk elements concentration.
For elemental analysis, an Agilent ICP-OES spectrometer 720 (Agilent Technologies Inc., Santa Clara, CA, USA), with axial plasma configuration and with an autosampler SPS-3 (Agilent Technologies, GmbH, Waldbronn, Germany), was used. As mentioned above, the methodology of hazardous element determination was described in detail in earlier studies [17,23,28,29].
Relative accumulation factor (RAF) was used to assess the content of each hazardous element in exposed mosses and lichens. RAF was calculated as follows Equation (5):
| RAF = (Cexposed − Cinitial)/Cinitial | (5) |
where Cexposed is the content of the hazardous element after exposure and Cinitial represent the content of the hazardous element before exposure (not exposed sample—control).
The content of toxic elements was studied also in the mushroom species Leccinum pseudoscabrum (Kallenb.) Šutara (n = 27). Mushrooms were sampled during summer 2018, in parallel with the soil sampling and moss exposition. The occurrence of mushrooms was accidental in the evaluated area. The health risk from the mushroom consumption was evaluated based on the exposure to the Cd and Hg that was compared with the provisional tolerable weekly intake (PTWI). The PTWI set for Cd and Hg are 0.0062 and 0.004 mg kg−1 body weight (BW), respectively [38,39]. The exposure to the Cd and Hg was calculated as follows Equation (6):
| (6) |
where E is exposure, is a concentration of the element i, intake stands for the consumption of the mushrooms per week (based on the [40] that is 0.23 kg of fresh matter per week), and BW is body weight (70 kg for an average adult). The amount of fresh weight was calculated based on the assumption that dry matter represents 10% in mushrooms [41].
Bioaccumulation factor (BAF) indicates the ability of mushrooms to uptake elements from soil/substrate to their above-ground parts. BAF is calculated as follows Equation (7) (mg kg-1 of dry matter):
| (7) |
where is the measured content of the element i. Based on Barker [42] the mushrooms can be divided into three groups: accumulators (BAF > 1), indicators (BAF = 1), and excluders (BAF < 1).
The map outputs were processed using the open-source Geographic Information System (GIS) using software QGIS (version 2.18) and open data from OpenStreetMap contributors [43]. All statistical analyses were performed in R studio [44]. Data were log-transformed prior to analysis. The non-parametrical Mann–Whitney U test was used to compare significant differences in accumulation abilities between taxa.
3. Results
3.1. Hazardous Element Content in Soil Samples
The values of hazardous elements in soils determined at 30 sampling sites expressed by descriptive statistics are listed in Table 1. Data were subsequently used for the indices calculation to express the state of pollution, the spreading of pollution, and the most contaminated sites in the study area. From the Table 1 it is clear, that As, Cd, Fe, Hg, Mn, and Sb exceeded the limit values at all 30 sampling sites, while Co, Cu, Ni, Pb, and Se exceeded the limit values only on some of them. The limit value for Cr and Zn was not exceeded in the study area. The area was a long time known as Fe, Hg, and precious metals deposit [45]. Dangerous pollution by As was confirmed in the study area by earlier studies [46].
Table 1.
Descriptive statistics for hazardous element values in soils (LV—limit values for Slovak soils, set by Act. No 220/2004 Coll of Laws [47] and background values (BV) according to Čurlik and Šefčík [35] and Kabata-Pendias [36].
| Elements | As | Cd | Co | Cr | Cu | Fe | Hg | Mn | Ni | Pb | Sb | Se | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg kg−1 DW (dry weight)] | |||||||||||||
| min | 26.2 | 3.17 | 1.35 | 4.58 | 14.2 | 16,330 | 0.71 | 675 | 3.33 | 12.9 | 2.62 | 0.01 | 16.3 |
| max | 1834 | 53.3 | 56.5 | 52.6 | 301 | 133,196 | 20.6 | 11,510 | 177 | 223 | 109 | 7.38 | 95.9 |
| avg | 203 | 14.8 | 15.7 | 21.2 | 76.4 | 47,352 | 4.73 | 3797 | 37.8 | 62.5 | 15.9 | 1.00 | 57.2 |
| med | 85.5 | 9.23 | 13.2 | 22.3 | 56.9 | 35,999 | 2.73 | 2986 | 24.8 | 42.9 | 10.1 | 0.21 | 55.3 |
| stdev | 329 | 12.1 | 11.4 | 10.5 | 57.8 | 26,913 | 4.91 | 2836 | 36.1 | 57.0 | 19.8 | 1.52 | 20.7 |
| LV | 25.0 | 0.70 | 15.0 | 70.0 | 60.0 | 550 | 0.50 | 550 | 50.0 | 70.0 | 0.70 | 0.40 | 150 |
| BV | 7.20 | 0.30 | 0.30 | 60.0 | 19.0 | 530 | 0.08 | 20.0 | 25.0 | 25.0 | 1.04 | 0.10 | 65.0 |
3.2. Contamination Factor, Degree of Contamination and Pollution Load Index
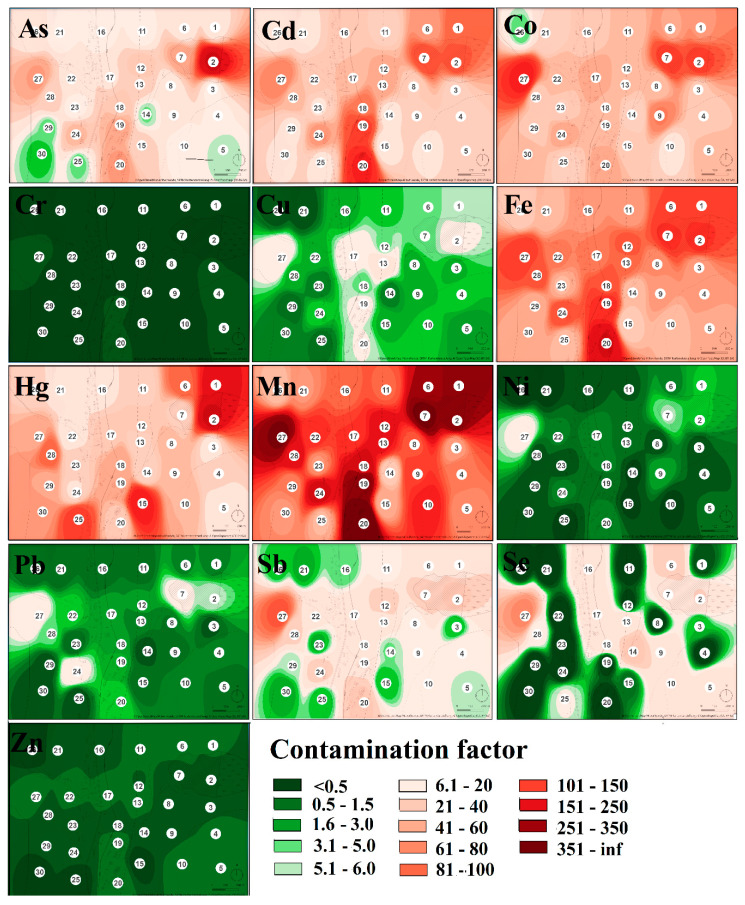
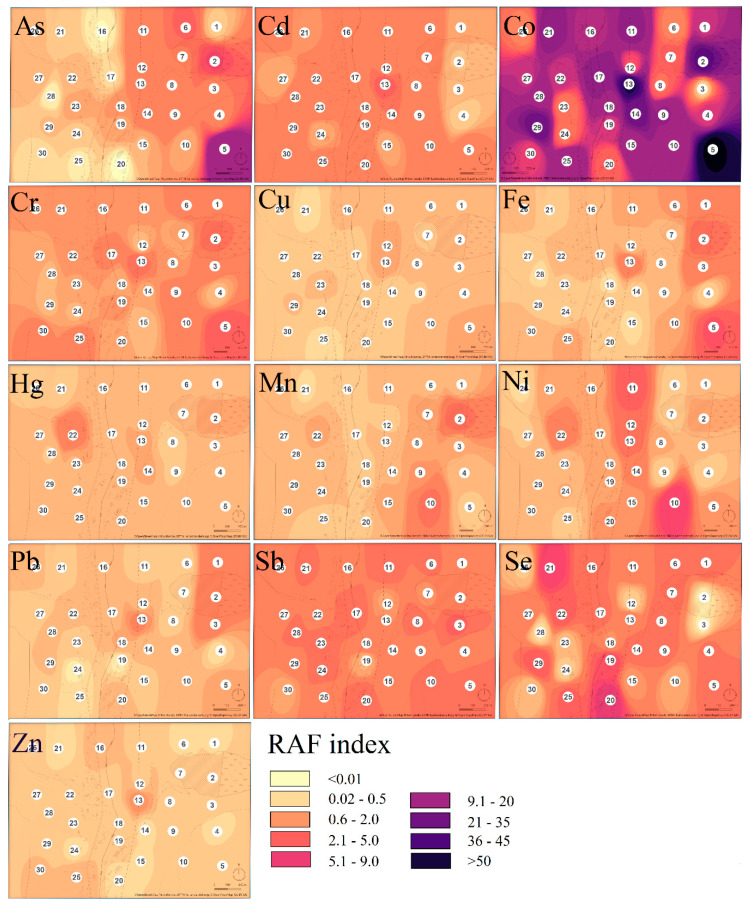
The contamination factor (Cf) which was calculated for each sampling site and each hazardous element confirmed that sampling sites are low in their contamination by Cr, low to moderately contaminated by Zn, considerably to very highly contaminated by Co, moderately to very highly contaminated by Sb, low to very highly contaminated by As, Cu, Ni, Pb, Se, and very highly contaminated by Cd, Fe, Mn, and Hg (Table 2). The level of contamination by individual elements in the study area expressed by the contamination factor was displayed by heatmaps (Figure 2).
Table 2.
Descriptive statistics expressing the values of the hazardous elements contamination factor (Cf) and the degree of contamination (Cd).
| Cf | As | Cd | Co | Cr | Cu | Fe | Hg | Mn | Ni | Pb | Sb | Se | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| min | 3.64 | 10.6 | 4.49 | 0.08 | 0.75 | 30.8 | 8.87 | 33.8 | 0.13 | 0.52 | 2.52 | 0.01 | 0.25 |
| max | 25.0 | 178 | 188 | 0.88 | 15.9 | 251 | 257 | 575 | 7.09 | 8.93 | 105 | 73.8 | 1.48 |
| avg | 28.3 | 496 | 52.4 | 0.35 | 4.02 | 89.3 | 59.1 | 189 | 1.51 | 2.49 | 15.3 | 9.95 | 0.88 |
| med | 11.9 | 30.8 | 43.9 | 0.37 | 2.99 | 67.9 | 34.1 | 149 | 0.99 | 1.72 | 9.71 | 2.12 | 0.85 |
| stdev | 45.8 | 40.5 | 38.1 | 0.18 | 3.04 | 50.8 | 61.4 | 141 | 1.44 | 2.28 | 19.0 | 15.2 | 0.32 |
| Cd | 847 | 1488 | 1571 | 10.6 | 120 | 2680 | 26.4 | 1774 | 5696 | 45.3 | 74.9 | 457 | 8.49 |
Figure 2.
The heatmap of the contamination state in the study area expressed by contamination factor values.
As mentioned above, the area was the most seriously contaminated by Fe, Mn, and Hg with the centers of pollution localized in the body of the tailing pond and in the area of the former ore processing plant (sampling site no. 19 and 20). Serious levels of Cd were also confirmed around the processing plant. Regarding the heatmap, serious soil pollution appeared on sampling site no. 27, localized at the same altitude as the tailing pond but on the other side of the valley. According to Zobeck and Van Pelt [48], windborne transport and atmospheric deposition may play a significant role in spreading contaminants. Prevailing winds in the smelting operation areas control the transport and could take away the hazardous elements over a long distance [49].
Degree of contamination (Cd) is used for the overall assessment of the contamination in the study area. According to the degree of contamination values (Cd), the study area is very highly contaminated by As, Cd, Co, Cu, Fe, Hg, Mn, Ni, Pb, Sb, and Se; considerably contaminated by Zn; and moderately contaminated by chromium.
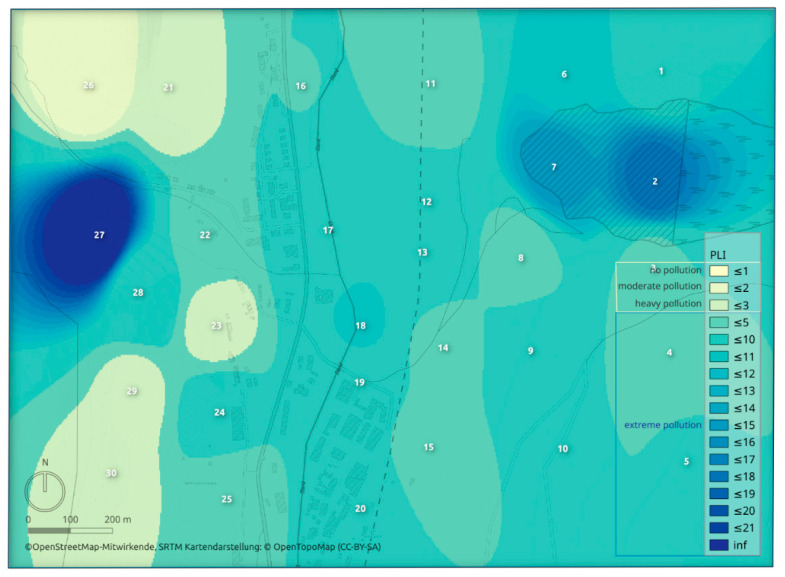
The pollution load index (PLI) was used to express the level of pollution at each sampling site considering individual elements’ contamination factor values. The state of the pollution in the study area expressed by PLI is visualized by heatmap (Figure 3). Almost all study area was, based on the PLI index values, evaluated as extremely polluted. The most serious pollution was determined (the same as in the case of contamination factor values) in the area of the tailing pond and around the sampling site no. 27.
Figure 3.
The pollution state in the study area expressed by the pollution load index (PLI).
3.3. Enrichment Factor Values in the Soil Samples
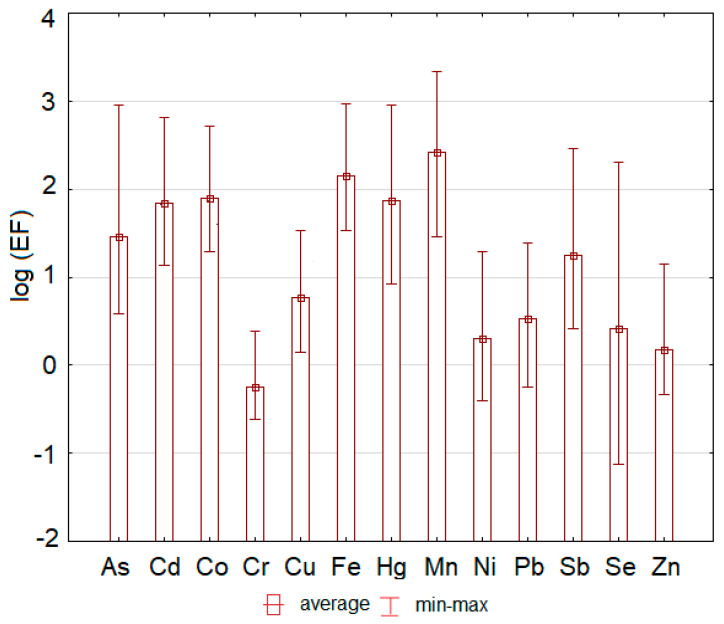
Values of EF lower than 2 (0.3 on a log scale) suggest that the trace element concentration comes from natural sources [50], but higher values of EF suggest an anthropogenic origin of the elements [51]. The most serious situation was determined for Fe (min–max: 33.9–934) and Mn (28.6–2140), where enrichment factor reached very high to extreme values at all sampling sites (Figure 4).
Figure 4.
The hazardous elements enrichment factor values (expressed as the logarithm of the enrichment factor for better visualization) determined at each sampling site of the study area.
Because the area is well known as the former iron mining area, high values of iron were expected. In the case of As (3.79–899), Co (19.5–516), and Sb (2.59–289), moderate to extreme enrichment was determined. The enrichment factor of Cd (13.5–661) and Hg (8.47–908) reached significant to extreme values. The values of Cr (0.24–2.40), Se (0.08–202), Zn (0.47–13.9), and Ni (0.39–19.4) ranged between minimal and moderate or minimal and significant values. Enrichment factors for Cu (1.42–33.8) and Pb (0.56–24.5) ranged between minimal and very high values.
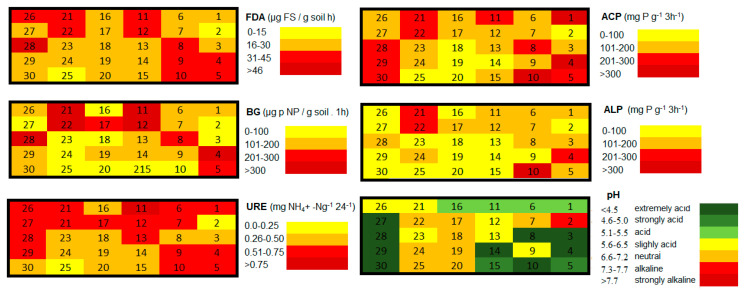
The activity of soil enzymes varied depending on the distance from the tailing pond and the prevailing wind orientation which brings toxic substances from the tailing pond. According to the meteorological measurements [52], the wind is heading from northwest to southeast, which is reflected in more significant pollution in the south-eastern part of the monitored area (Figure 5). Soil enzyme activities are greatly influenced by soil properties and could be significant indicators of heavy metal toxicity [22]. It has been repeatedly shown that risk elements’ toxicity could negatively affect soil enzymes which is manifested mainly by their reduced activity [53]. In previous studies, soil enzymes have been found to react differently to individual metal contamination. According to Acosta Martinez and Tabatabai [54], lead and cadmium have the greatest inhibitory effect on the activity of soil enzymes. Urease activity is significantly reduced by high lead content [55]. Copper has a significant inhibitory effect on β-glucosidase activity and arsenic on both urease and phosphatase activity [56]. Numerous studies have focused on others soil parameters that influence the activity of soil enzymes [20,57]. In addition to the content of risk elements in the soil, the activity of soil enzymes is also affected by temperature, humidity, soil pH, organic matter, nutrient availability, and chemical properties of the litter [58].
Figure 5.
The values of soil enzymes (URE—urease; ALP—alkaline phosphatase, ACP—acid phosphatase, BG—ß-glucosidase, FDA—fluorescein diacetate) and soil pH determined at each sampling site of a study area.
The lowest values of all evaluated soil enzymes were determined at sampling site 2, which is the body of the tailing pond. With the increasing distance from the tailing pond activity of the soil enzymes increase, with the exception of the most polluted area (regularly polluted by sewage sludge of dust consistency transmitted by the wind). The values of soil reaction varied between extremely acid and alkaline [35]. It has been documented in several studies all over the world that sewage sludge pH values used to reach strongly alkaline [59] to neutral values [60]. In accordance with the studies, strongly alkaline soil reaction was determined in the body of the tailing pond, and slightly acid to neutral values were assessed in the most polluted part of the study area.
3.4. Hazardous Element in the Ambient Air of Nižná Slaná
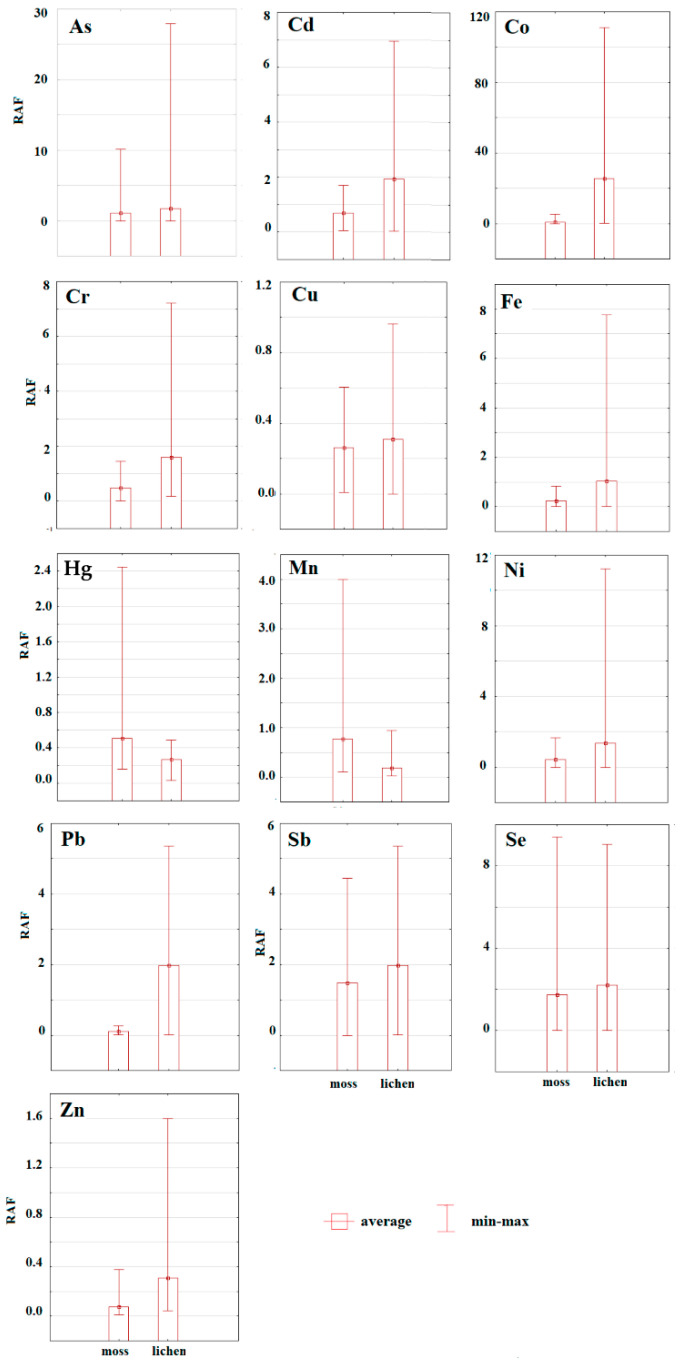
The contents of the hazardous elements in moss and lichen expressed by RAF values are listed in Figure 6. Based on the results we can conclude that lichen is a better accumulator of all evaluated hazardous elements, except Hg. Cecconi et al. [61] have confirmed excellent accumulation abilities of Pseudevernia furfuracea, which is why it is often used in bioaccumulation studies. Adamo [62] confirmed that lichens often accumulate higher concentrations of the hazardous element than mosses. However, because of easier accessibility, mosses are still more popular in bioaccumulation studies [61]. As mentioned above, only Hg values reached higher contents in moss compared to lichen. In the study of Shao et al. [63], who analyzed the content of Hg in mosses and lichens, higher values were found in lichen tissues. Additionally, the correlation between the altitude and the Hg content in the moss samples was also confirmed in this study.
Figure 6.
Differences between hazardous elements determined in moss and lichen taxa expressed by relative accumulation factor (RAF) values.
Comparing average RAF values (regardless of the taxa) of individual elements we can conclude that pollution by hazardous elements decreased in the following order: Co>Se>Sb>As>Cd>Cr>Ni>Fe>Pb>Hg>Cu>Zn (Figure 7).
Figure 7.
RAF values regardless of the taxa (average value) in the study area.
A non-parametrical Mann–Whitney U test was used to verify significant differences between taxa in their accumulation abilities (Table 3).
Table 3.
Results of the Mann–Whitney U test to compare significant differences in accumulation abilities between taxa.
| Element | U | Z | p-Value | |
|---|---|---|---|---|
| As | Between taxa | 408 | −0.61 | 0.53 |
| Cd | 148 | −4.46 | 0.001 ** | |
| Co | 276 | −2.55 | 0.011 * | |
| Cr | 158 | −4.33 | 0.001 ** | |
| Cu | 449 | −0.01 | 0.99 | |
| Fe | 300 | −2.21 | 0.03 * | |
| Hg | 204 | −3.62 | 0.001 ** | |
| Ni | 389 | −0.89 | 0.37 | |
| Mn | 82 | −5.43 | 0.001 ** | |
| Pb | 138 | −4.60 | 0.001 ** | |
| Sb | 337 | −1.66 | 0.09 | |
| Se | 403 | −0.68 | 0.49 | |
| Zn | 224 | −3.34 | 0.001 ** |
* p < 0.05; ** p < 0.01.
Significantly higher ability to accumulate Hg was found for moss (Dicranum scoparium). Lichen (Pseudevernia furfuracea) was found as a significantly better accumulator of Cd, Co, Cr, Fe, Mn, Pb, and Zn. For As, Cu, Ni, Sb, and Se, no significant differences between evaluated taxa were confirmed. Bargagli et al. [64] have found a higher concentration of lithophile elements (Al, Cr, Fe, Mn, Ni, and Ti) in moss and atmophile elements (Hg, Cd, Pb, Cu, V, and Zn) in lichens. On the other hand, Loppi and Bonini [65] confirmed higher values of Hg and Zn in moss samples comparing mosses. Because the mosses and lichens exhibit different accumulation abilities of individual elements, in many studies mosses and lichens are used together to reach comprehensive results [66].
3.5. BAF Evaluation
The genus Leccinum is characterized by the high variability of BAF values (Table 4). Jarzyńska and Falandysz [67] and Medyk et al. [68] reported that the genus Leccinum have BAF > 1 for Ag, Cd, Cu, Hg, K, Mg, Na, P, Rb, and Zn and BAF < 1 for Al, Ba, Ca, Cr, Fe, Mn, Ni, Pb, and Sr. The BAF values indicate that L. pseudoscabrum accumulates only Hg (Table 2). In general, the genus Leccinum is reported to have BAF values of Hg more than 1 [69].
Table 4.
Content of the studied elements (mg kg−1 DW) and their BAF values.
| Element Content | As | Cd | Co | Cr | Cu | Fe | Hg | Mn | Ni | Pb | Sb | Se | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmushroom | 2.77 | 1.66 | 0.02 | 0.12 | 19.2 | 46.6 | 5.77 | 17.4 | 1.32 | 0.25 | 0.84 | 2.66 | 73.3 |
| Ctopsoil | 107 | 4.88 | 11.7 | 22.6 | 44.9 | 49,517 | 4.35 | 3811 | 27.4 | 48.8 | 5.73 | 2.91 | 79.1 |
| BAF | 0.03 | 0.34 | 0.00 | 0.01 | 0.43 | 0.00 | 1.33 | 0.00 | 0.05 | 0.01 | 0.15 | 0.92 | 0.93 |
Cmushroom: average content of the element in the mushroom, Ctopsoil: average content of the element in the topsoil.
3.6. Health Risk Assessment
The genus Leccinum is a popular and valuable wild-grown edible mushroom used traditionally as a gourmet food in Central and Eastern Europe and Scandinavia [60,67,70,71]. If we assume the consumption of 23 g fresh matter of L. pseudoscabrum per week, the intake of Cd and Hg would represent 5.9% and 47.4%, respectively, of the PTWI set for these toxic elements. The loss of Hg when the mushrooms are cooked is relatively low (around 10% when blanching for 10 min.) [72]. There is an increased risk of potentially harmful exposure to Hg, due to the fact, that almost 50% of the PTWI for Hg is reached by the consumption of a very small amount (23 g per week) of the mushrooms. Other foods that are consumed in much higher quantities would considerably increase the health risk. The average consumption of mushrooms would not represent a high health risk related to the Cd intake and thus can be considered safe.
4. Conclusions
Spreading of the hazardous elements from non-reclaimed mining bodies at the former mining area Nižná Slaná (Slovakia) was evaluated in soils and airborne air using biomonitoring methods. The hazardous elements’ values were expressed by factors and indices. The results of the contamination factor pointed out very high contamination of soils by Cd, Fe, Mn, and Hg and the pollution load index confirmed that almost the whole evaluated area is extremely polluted. Biological (soil enzymes) and chemical (soil reaction) soil properties react significantly to the environmental stress. The tailing pond and area of ore processing plants were found as the main sources of undesirable substances and the spreading of the pollution predominantly by wind caused serious pollution in the whole study area. The moss and lichen bag technique was used as the airborne biomonitoring method. While moss (Dicranum scoparium) showed significantly better ability to accumulate Hg, lichen (Pseudevernia furfuracea) was found as significantly suitable for Cd, Co, Cr, Fe, Mn, Pb, and Zn accumulation. Results of the BAF values indicate that L. pseudoscabrum is an accumulator of Hg. The average consumption of mushrooms can be considered as safe. However, whereas the study area also includes residential areas and pastures under the influence of dust emissions and agricultural lands with polluted soil, where agricultural crops are grown, we still consider the study area very dangerous in terms of health risk.
Author Contributions
L.D.: Writing—original draft, Methodology, Investigation. J.Á.: Writing, Methodology, Investigation. L.B.: Investigation, Analysis, Review. M.H.: GIS processing, Writing, Review. M.M.: Investigation, GIS processing. J.M.: Review, Editing and Revising. I.J.: Review, Editing, Investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic VEGA No. 1/0326/18 and VEGA No. 1/0591/18.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bech J., Roca N., Tume P., Ramos-Miras J., Gil C., Boluda R. Screening for new accumulator plants in potential hazards elements polluted soil surrounding Peruvian mine tailings. Catena. 2016;136:66–73. doi: 10.1016/j.catena.2015.07.009. [DOI] [Google Scholar]
- 2.Singovszká E., Bálintová M., Holub M. Heavy metal contamination and its indexing approach for sediment in Smolnik creek (Slovakia) Clean Technol. Environ. 2016;18:305–313. doi: 10.1007/s10098-015-0991-0. [DOI] [Google Scholar]
- 3.Zhang L., Wang Y. Mercury Contamination from Historic Gold Mining to Water Bodies and Soils in Zhaoyuan, Shandong, Eastern China. Nat. Environ. Pollut. Technol. 2016;15:559–564. doi: 10.1016/j.envpol.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Dudek M., Tajduś K., Misa R., Sroka A. Predicting of land surface uplift caused by the flooding of underground coal mines—A case study. Int. J. Rock Mech. Min. Sci. 2020;132:104377. doi: 10.1016/j.ijrmms.2020.104377. [DOI] [Google Scholar]
- 5.Combes A., Franchineau G. Fine particle environmental pollution and cardiovascular diseases. Metabolism. 2019;100:153944. doi: 10.1016/j.metabol.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Pošiváková T., Hromada R., Laktičová K.V., Vargová M., Korytár Ľ., Svajlenka J., Húska M., Hatalová E., Pošivák L., Klein R. Concentrations of selected toxic elements in ewe living near an environmentally loaded area of eastern Slovakia. Ann. Agric. Environ. Med. 2017;24:667–670. doi: 10.26444/aaem/75639. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadi Jahromi N.S., Jonoubi P., Majd A., Dehghani M. Root structural changes of two remediator plants as the first defective barrier against industrial pollution, and their hyperaccumulation ability. Environ. Monit. Assess. 2019;191:148. doi: 10.1007/s10661-019-7240-7. [DOI] [PubMed] [Google Scholar]
- 8.Xiong L., Xu Z., Wang H., Liu Z., Xie D., Wang A., Kong F. The association between ambient air pollution and birth defects in four cities in Hunan province, China, from 2014 to 2016. Medicine. 2019;98:e14253. doi: 10.1097/MD.0000000000014253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enviroportal Environmental Burden Information System of Slovak Republic. [(accessed on 12 October 2020)];2019 Available online: https://envirozataze.enviroportal.sk/
- 10.Tajduś K. The nature of mining-induced horizontal displacement of surface on the example of several coal mines. Arch. Min. Sci. 2014;59:971–986. doi: 10.2478/amsc-2014-0067. [DOI] [Google Scholar]
- 11.Pandey B., Agrawal M., Singh S.J. Ecological risk assessment of soil contamination by trace elements around coal mining area. Soils Sediments. 2016;16:159. doi: 10.1007/s11368-015-1173-8. [DOI] [Google Scholar]
- 12.Jiang Y., Misa R., Tajduś K., Sroka A., Jiang Y. A new prediction model of surface subsidence with Cauchy distribution in the coal mine of thick topsoil condition. Arch. Min. Sci. 2020;65:147–158. [Google Scholar]
- 13.Dai S., Li Y., Zhou T., Zhao Y. Reclamation of heavy metals from contaminated soil using organic acid liquid generated from food waste: Removal of Cd, Cu, and Zn, and soil fertility improvement. Environ. Sci. Pollut. Res. 2017;24:15260. doi: 10.1007/s11356-017-9139-6. [DOI] [PubMed] [Google Scholar]
- 14.Aničić-Urošević M., Milićević T. Moss Bag Biomonitoring of Airborne Pollutants as an Ecosustainable Tool for Air Protection Management: Urban and Agricultural Scenario. In: Shukla V., Kumar N., editors. Environmental Concerns and Sustainable Development. Springer; Singapore: 2020. pp. 29–62. [Google Scholar]
- 15.Zhao L., Zhang C., Jia S., Liu Q., Chen Q., Li X., Liu X., Wu Q., Zhao L., Liu H. Element bioaccumulation in lichens transplanted along two roads: The source and integration time of elements. Ecol. Indic. 2019;99:101–107. doi: 10.1016/j.ecolind.2018.12.020. [DOI] [Google Scholar]
- 16.Vingiani S., De Nicola F., Purvis W.O., Cocha-Grana E., Muniatequi-Lorenzo S., López-Mahía P., Giordano S., Adamo P. Active Biomonitoring of Heavy Metals and PAHs with Mosses and Lichens: A Case Study in the Cities of Naples and London. Water Air Soil Pollut. 2015;226:240. doi: 10.1007/s11270-015-2504-5. [DOI] [Google Scholar]
- 17.Demková L., Árvay J., Bobuľská L., Hauptvogl M., Michalko M. Activity of the soil enzymes and moss and lichen biomonitoring method used for the evaluation of soil and air pollution from tailing pond in Nižná Slaná (Slovakia) J. Environ. Sci. Health A. 2019;54:485–497. doi: 10.1080/10934529.2019.1567158. [DOI] [PubMed] [Google Scholar]
- 18.Tretiach M., Adamo P., Bargagli R., Baruffo L., Carletti L., Crisafulli P., Giordano S., Modenesi P., Orlando S., Pittao E. Lichen and moss bags as monitoring devices in urban areas. Part I: Influence of exposure on vitality. Environ. Pollut. 2007;146:380–391. doi: 10.1016/j.envpol.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 19.Aničić M., Tasić M., Frontasyeva M.V., Tomašević M., Rajšić S., Strelkova L.P., Popović A., Steinnes E. Active biomonitoring with wet and dry moss: A case study in an urban area. Environ. Chem. Lett. 2009;7:55–60. doi: 10.1007/s10311-008-0135-4. [DOI] [Google Scholar]
- 20.Bobuľská L., Demková L., Čerevková M., Renčo M. Invasive Goldenrod (Solidago gigantea) influences soil microbial activities in forest and grassland ecosystems in central Europe. Diversity. 2020;11:134. doi: 10.3390/d11080134. [DOI] [Google Scholar]
- 21.Bobuľská L., Kožej J., Demková L. Determination of soil enzymatic activity by risk elements in environmentally loaded areas in Slovakia. Studia Oecologica. 2018;12:37–44. doi: 10.21062/ujep/231.2019/a/1802-212X/SO/12/1/37. [DOI] [Google Scholar]
- 22.Xian Y., Wang M., Chen W. Quantitative assessment on soil enzyme activities of heavy metal contaminated soils with various soil properties. Chemosphere. 2015;139:604–608. doi: 10.1016/j.chemosphere.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 23.Demková L., Oboňa J., Árvay J., Michalková J., Lošák T. Biomonitoring of road dust pollution along the streets with various traffic density. Pol. J. Environ. Stud. 2019;28:3687–3696. doi: 10.15244/pjoes/97354. [DOI] [Google Scholar]
- 24.Kottek M., Greiser J., Beck C., Rudolf B., Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006;15:529–563. doi: 10.1127/0941-2948/2006/0130. [DOI] [Google Scholar]
- 25.Brehuv J., Špaldon T., Šestinová O., Slančo P., Hančul’ák J., Bobro M. Contamination of the Water and Sediment Load from the Drainage Basin of the Slaná River by Influence of Former and Present Mining Activities. Acta Fac. Ecol. 2007;16:91–100. [Google Scholar]
- 26.Icsó J., Szöllösová M., Sorahan T. Lung cancer among iron ore miners in east Slovakia: A case-control study. Occup. Environ. Med. 1994;51:642–643. doi: 10.1136/oem.51.9.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.IUSS Working Group WRB . World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. FAO; Italy, Rome: 2015. World Soil Resources Reports No. 106. [Google Scholar]
- 28.Demková L., Árvay J., Bobuľská L., Tomáš J., Stanovič R., Lošák T., Harangozo Ľ., Vollmannová A., Bystrická J., Musilová J., et al. Accumulation and environmental risk assessment of heavy metals in soil and plants of four different ecosystems in a former polymetallic ore mining and smelting area (Slovakia) J. Environ. Sci. Health A. 2017;52:479–490. doi: 10.1080/10934529.2016.1274169. [DOI] [PubMed] [Google Scholar]
- 29.Demková L., Árvay J., Bobuľská L., Hauptvogl M., Hrstková M. Open mining pits and heaps of waste material as the source of undesirable substances: Biomonitoring of air and soil pollution in former mining area (Dubnik, Slovakia) Environ. Sci. Pollut. Res. 2019;26:35227–35239. doi: 10.1007/s11356-019-06582-0. [DOI] [PubMed] [Google Scholar]
- 30.Khaziev F.K. Soil Enzyme Activity. Nauka; Moscow, Russia: 1976. [Google Scholar]
- 31.Grejtovsky A. Effects of Improvement Practices on Enzymatic Activities of Heavy-textured Alluvial Soil. Rostl. Výroba. 1991;1:299–307. [Google Scholar]
- 32.Green V.S., Stott D.E., Diack M. Assay for Fluorescein Diacetate Hydrolytic Activity: Optimization for Soil Samples. Soil Biol. Biochem. 2006;38:693–701. doi: 10.1016/j.soilbio.2005.06.020. [DOI] [Google Scholar]
- 33.Eivazi F., Tabatabai M.A. Glucosidases and Galactosidases in Soils. Soil Biol. Biochem. 1988;20:601–606. doi: 10.1016/0038-0717(88)90141-1. [DOI] [Google Scholar]
- 34.Hakanson L. An Ecological Risk Index for Aquatic Pollution Control. A Sedimentol. Approach Water Res. 1980;14:975–1001. [Google Scholar]
- 35.Čurlík J., Šefčík P. Geochemical Atlas of the Slovak Republic. Part. V: Soils. Ministry of the Environment; Bratislava, Slovakia: 1991. [Google Scholar]
- 36.Kabata-Pendias A. Trace Elements in Soils and Plants. CRC Press Taylor & Francis Group; New York, NY, USA: 2011. [Google Scholar]
- 37.Wang X., He M., Xie J., Xi J., Lu X. Heavy metal pollution of the world largest antimony mine-affected agricultural soils in Hunan province (China) J. Soils Sediments. 2010;10:827–837. doi: 10.1007/s11368-010-0196-4. [DOI] [Google Scholar]
- 38.WHO Evaluation of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives. [(accessed on 27 October 2020)]; 2011 (WHO Technical Report Series; No. 959) Available online: https://apps.who.int/iris/bitstream/handle/10665/44514/WHO_TRS_959_eng.pdf;jsessionid=414A27E824AF85BD65BAB51130C680DF?sequence=1.
- 39.WHO Evaluation of Certain Food Additives and Contaminants: Seventy-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives. [(accessed on 27 October 2020)]; 2013 (WHO Technical Report Series; No. 983) Available online: https://apps.who.int/iris/bitstream/handle/10665/98388/9789241209830_eng.pdf?sequence=1.
- 40.Statistical Organization of Slovak Republic Food Consumption in the SR in 2018. [(accessed on 27 October 2020)];2019 Available online: www.statistics.sk.
- 41.Kalač P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009;113:9–16. doi: 10.1016/j.foodchem.2008.07.077. [DOI] [Google Scholar]
- 42.OSM (OpenStreetMap) [(accessed on 10 December 2019)];2016 Available online: https://www.openstreetmap.org/#map=4/50.18/-1.41.
- 43.R Core Team . A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- 44.Musilová J., Árvay J., Vollmannová A., Tóth T., Tomáš J. Environmental Contamination by heavy metals in region with previous mining activity. Bull. Environ. Contam. Toxicol. 2016;97:569. doi: 10.1007/s00128-016-1907-3. [DOI] [PubMed] [Google Scholar]
- 45.Baker A.J.M. Accumulators and Excluders Strategies in Response of Plants to Heavy Metals. J. Plant. Nutr. 1981;3:643–654. doi: 10.1080/01904168109362867. [DOI] [Google Scholar]
- 46.Hronec O., Vilček J., Tomáš J., Adamišin P., Huttmanová E. Environmental Components Quality in Problem Areas in Slovaki. Mendel University in Brno; Brno, Czech Republic: 2010. [Google Scholar]
- 47.Act of the National Council of the Slovak Republic No. 220/2004 Coll. [(accessed on 27 October 2020)]; Available online: http://www.podnemapy.sk/portal/verejnost/konsolidacia/z_220_2004.pdf.
- 48.Zobeck T.M., Van Pelt R.S. Wind-induced dust generation and transport mechanics on a bare agricultural field. J. Hazard. Mater. 2006;132:26–38. doi: 10.1016/j.jhazmat.2005.11.090. [DOI] [PubMed] [Google Scholar]
- 49.Csavina J., Landázuri A., Wonaschutz A., Rine K., Rheinheimer P., Barbaris B., Conant W., Sáez A.E., Betterton E.A. Metal and metalloid contaminants in atmospheric aerosols from mining operations. Water Air Soil Pollut. 2011;221:145–157. doi: 10.1007/s11270-011-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J., Liu C.L. Riverine composition and estuarine geochemistry of particulate metals in China-weathering features, anthropogenic impact and chemical fluxes. Estuar. Coast. Shelf Sci. 2002;54:1051–1070. doi: 10.1006/ecss.2001.0879. [DOI] [Google Scholar]
- 51.Sutherland R.A., Tolosa C.A., Tack F.M.G., Verloo M.G. Characterization of selected element concentrations and enrichment ratios in background and anthropogenically impacted roadside areas. Arch. Environ. Contam. Toxicol. 2000;38:428–438. doi: 10.1007/s002440010057. [DOI] [PubMed] [Google Scholar]
- 52.ECMWF—European Center for Medium Range Weather Forecast Advancing Global NWP through International Collaboration. [(accessed on 27 October 2020)]; Available online: http://www.ecmwf.int/
- 53.Duan C., Fang L., Yang C., Chen W., Cui Y., Li S. Reveal the response of enzyme activities to heavy metals through in situ zymography. Ecotoxicol. Environ. Saf. 2018;158:106–111. doi: 10.1016/j.ecoenv.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 54.Acosta-Martinez F., Cuevas C., Walter T., Iglesias I. Urban organic wastes effects on soil chemical properties in degraded semiarid ecosystem; Proceedings of the Seventeenth WCSS; Bangkok, Thailand. 14–21 August 2002; pp. 1–9. [Google Scholar]
- 55.Karaca A., Cetin S.C., Turgay O.C., Kizilkaya R. Effects of Heavy Metals on Soil Enzyme Activities. In: Sherameti I., Varma A., editors. Soil Heavy Metals. Soil Biology. Springer; Berlin/Heidelberg, Germany: 2010. [Google Scholar]
- 56.Speir T.W., Kettles H.A., Parshotam A., Searle P.L., Vlaar L.N.C. Simple kinetic approach to determine the toxicity of As[V] to soil biological properties. Soil Biol. Biochem. 1999;31:705–713. doi: 10.1016/S0038-0717(98)00169-2. [DOI] [Google Scholar]
- 57.Dou F., Wright A.L., Mylavarapu E.S., Jiang X., Matocha J.E. Soil enzyme activities and organic matter composition affected by 26 years of continuous cropping. Pedosphere. 2016;26:618–628. doi: 10.1016/S1002-0160(15)60070-4. [DOI] [Google Scholar]
- 58.Baldrian P., Snajdr V., Mehautová V., Dobiášová P., Cajthaml T., Valášková V. Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol. Biochem. 2012;56:60–68. doi: 10.1016/j.soilbio.2012.01.020. [DOI] [Google Scholar]
- 59.Acosta-Martinez V., Tabatabai M.A. Arylamidase activity in soils: Effect of trace elements and relationships to soil properties and activities of amidohyrolases. Soil Biol. Biochem. 2001;33:17–23. doi: 10.1016/S0038-0717(00)00109-7. [DOI] [Google Scholar]
- 60.Parkpain S., Sirisukhodom A.A. Carbonell-Barrachina Heavy metals and nutrients chemistry in sewage sludge amended Thai soils. J. Environ. Sci. Health A. 1998;33:573–597. doi: 10.1080/10934529809376749. [DOI] [Google Scholar]
- 61.Cecconi E., Incerti G., Capozzi F., Adamo P., Bargagli R., Benesperi R., Carniel F.C., Favero-Longo S.E., Giordano S., Puntillo D., et al. Background element content of the lichen Pseudevernia furfuracea: A supra-national state of art implemented by novel field data from Italy. Sci. Total Environ. 2018;622–623:282–292. doi: 10.1016/j.scitotenv.2017.11.276. [DOI] [PubMed] [Google Scholar]
- 62.Adamo P., Bargagli R., Giordano S., Modenesi P., Monaci F., Pittao E., Spagnuolo V., Tretiach M. Natural and pre-treatments induced variability in the chemical composition and morphology of lichens and mosses selected for active monitoring of airborne elements. Environ. Pollut. 2008;152:11–19. doi: 10.1016/j.envpol.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Shao J., Liu C., Zhang Q., Fu J., Yang R., Shi J., Cai Y. Characterization and speciation of mercury in mosses and lichens from the high-altitude Tibetan Plateau. Jiang, G. Environ. Geochem Health. 2017;39:475. doi: 10.1007/s10653-016-9828-y. [DOI] [PubMed] [Google Scholar]
- 64.Bargagli R., Monaci F., Borghini F., Agnorelli C. Mosses and lichens as biomonitors of trace metals. A comparison study on Hypnum cupressiforme and Parmelia caperata in a former mining district in Italy. Environ. Pollut. 2002;116:279–287. doi: 10.1016/S0269-7491(01)00125-7. [DOI] [PubMed] [Google Scholar]
- 65.Loppi S., Bonini I. Lichens and mosses as biomonitors of trace elements in area with thermal springs and fumarole activity (Mt. Amiata central Italy) Chemosphere. 2000;41:1333–1336. doi: 10.1016/S0045-6535(00)00026-6. [DOI] [PubMed] [Google Scholar]
- 66.Kłos A., Ziembik Z., Rajfur M., Dołhańczuk-Śródka A., Bochenek Z., Bjerke J.W., Tømmervik H., Zagajewski B., Ziółkowski D., Jerz D., et al. Using moss and lichens in biomonitoring of heavy-metal contamination of forest areas in southern and north-eastern Poland. Sci. Total Environ. 2018;627:438–449. doi: 10.1016/j.scitotenv.2018.01.211. [DOI] [PubMed] [Google Scholar]
- 67.Jarzyńska G., Falandysz J. Trace elements profile of Slate Bolete (Leccinum duriusculum) mushroom and associated upper soil horizon. J. Geochem. Explor. 2012;121:69–75. doi: 10.1016/j.gexplo.2012.07.001. [DOI] [Google Scholar]
- 68.Mędyk M., Loganathan B., Bielawski L., Falandysz J. Inorganic elemental concentrations in birch bolete mushroom (Leccinum scabrum) and topsoil: Contamination profiles, bioconcentation and annual variations. J. Environ. Sci. Health Part B. 2018;53:831–839. doi: 10.1080/03601234.2018.1505087. [DOI] [PubMed] [Google Scholar]
- 69.Falandysz J., Drewnowska M. Distribution of mercury in Amanita fulva (Schaeff.) Secr. mushrooms: Accumulation, loss in cooking and dietary intake. Ecotoxicol. Environ. Saf. 2015;115:9–54. doi: 10.1016/j.ecoenv.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Falandysz J., Kunito T., Kubota R., Bielawski L., Mazur A., Falandysz J.J., Tanabe S. Selected elements in Brown Birch Scaber Stalk Leccinum scabrum. J. Environ. Sci. Health A. 2007;42:2081–2088. doi: 10.1080/10934520701626993. [DOI] [PubMed] [Google Scholar]
- 71.Falandysz J. Mineral constituents in Leccinum scabrum from lowland locations in the central Europe and their relation to concentration in forest topsoil. J. Environ. Sci. Health B. 2018;53:546–560. doi: 10.1080/03601234.2018.1462914. [DOI] [PubMed] [Google Scholar]
- 72.Krasińska G., Falandysz J. Mercury in Orange Birch Bolete Leccinum versipelle and soil substratum: Bioconcentration by mushroom and probable dietary intake by consumers. Environ. Sci. Pollut. Res. 2016;23:860–869. doi: 10.1007/s11356-015-5331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]