Abstract
Bacteria can form biofilms, complex microbial communities protected from environmental stress, on food contact surfaces. Brassicaceae plant has been shown to contain bioactive compounds with antimicrobial activities. The objective of this study was to evaluate the synergistic effects of Brassicaceae species and proteinase K against E. coli O157:H7 biofilm. We determined the minimum biofilm inhibitory concentration, the fractional inhibitory concentration indexes, and the synergistic inhibitory effect of Raphanus sativus var. longipinnatus, R. sativus, and Brassica oleracea var. acephala extracts with proteinase K on E. coli O157:H7. The biofilm showed a 49% reduction with 2 mg/mL R. sativus. The combination of proteinase K 25 µg/mL significantly increased the effect of 2 mg/mL R. sativus var. longipinnatus and the combined treatment yielded up to 2.68 log reduction on stainless steel coupons. The results showed that the combination of R. sativus var. longipinnatus extract and proteinase K could serve as an anti-biofilm agent with synergistic effects for inhibiting E. coli O157:H7 biofilm on stainless steel surfaces.
Subject terms: Antimicrobials, Applied microbiology, Biofilms
Introduction
Escherichia coli O157:H7 is a major foodborne pathogen, which causes abdominal pain, diarrhea and even hemolytic uremic syndrome in humans worldwide1. A major source of E. coli O157:H7 is contaminated ground beef. However, fresh produce has also been recognized as an important source for the transmission of E. coli O157:H7 and has been implicated in an increasing number of foodborne outbreaks2,3. Fresh produce processing facilities can be involved in the transfer of contaminants through wash-water-mediated or direct contact with surfaces contaminated by a biofilm4. Without a sterilization step, it is nearly impossible to control the pathogen even with careful procedures.
Biofilms play an important role in cross-contamination by protecting pathogens from sanitary and sterilizing procedures5. Biofilms can form on any type of abiotic or biotic surface, resulting in serious problems in the food, marine, soil, and biomedical fields6. Many studies have tested the use of disinfectants, essential oils, plant extracts, and other chemicals to prevent biofilm formation or to remove existing biofilms7. Enzymes have been used for the treatment of biofilms formed in food areas8. Also, the application of enzymes on the cleaning of food contact surfaces has been approved by the regulatory agencies9. Proteinase K showed eradication of proteinaceous adhesins during the attachment step and disassembly of the extracellular polymeric substances10. Kim et al. used various enzymes, such as proteinase K and acylase I, for biofilm removal10. In another study, DNase I treatment was used for the removal of extracellular deoxyribonucleic acid, an architectural element of biofilms11. Traditional disinfectants are a health concern to consumers, and the extensive use of antibiotics causes an increase in resistant strains12,13. Plant-derived natural agents have been studied for their potential for biofilm reduction without any resistance or residue of toxic compounds. Recently, antibacterial properties have been studied in several natural compounds such as different plant extracts, essential oils14, and honey15. However, the sole use of natural agents alone has a limited antimicrobial activity, compared to the use of antibiotics and disinfectants. Therefore, in some studies, the combination of synthetic drugs with natural agents was investigated to enhance the antimicrobial effect16,17.
Brassica vegetables are highly nutritive and are recognized as providing antioxidative and antibacterial phytochemicals such as indole phytoalexins phenolics (feruloyl, isoferuloylcholine and hydroxybenzoic), and glucosinolates (glucoiberin, glucoraphanin and glucoalyssin). All of these phytochemicals contribute to the antioxidant, anticarcinogenic, and cardiovascular protective activities of Brassica vegetables18. In the Brassicaceae family, Raphanus sativus var. longipinnatus has been used in traditional medicine for disorders in the respiratory, urinary, and gastrointestinal systems19. Brassicaceae plant extracts, glucosinolate (GLS), and isothiocyanate (ITC) have been intensively investigated for their inhibitory effects on a wide range of microorganisms, including bacteria, fungi, insects, germinating seeds, and nematodes20. A previous study has shown that Brassicaceae plant extracts reduced E. coli O157:H7 during every step of biofilm formation21.
Hurdle technology combines various elements and processes to inhibit microbial growth22. Enterocin AS-48 treatment in combination with other agents such as EDTA, sodium tripolyphosphate, pH, and heat treatment in apple juice increased the permeability of planktonic E. coli O157:H7 cell membranes23. The use of antibiotics in combination with proteinase K showed stronger inhibitory effect on Staphylococcus aureus biofilm than a five-fold amount of gentamycin, streptomycin or ampicillin alone24. Kim et al. showed that combinatorial treatment of acylase I and proteinase K was more efficacious for eradicating Pseudomonas aerugenosa biofilm than either treatment alone10. Therefore, the purpose of this study was to evaluate the combinatorial effect of Brassicaceae plant extracts such as R. sativus var. longipinnatus, R. sativus, and Brassica oleracea var. acephala extracts with proteinase K on E. coli O157:H7 biofilm, and to evaluate their potential application to stainless steel surfaces.
Materials and methods
Bacteria strains and growth conditions
E. coli O157:H7 ATCC43894 was used from the culture collection of the Food Safety Laboratory at Gyeongsang National University. The bacterium was inoculated in a tryptic soy broth (TSB, Becton Dickinson Co., Franklin Lakes, NJ, USA) and incubated for 16–18 h at 37 °C in a shaking incubator. The cultures were maintained in 15% glycerol at − 80 °C until use.
Preparation of plant extracts
All vegetables were purchased from grocery stores in Jinju-si (Republic of Korea). R. sativus var. longipinnatus, R. sativus, and B. oleracea var. acephala were freeze-dried in a freeze dryer (PVTFD50A, Ilsin Lab. Co., Gyeonggi-do, Republic of Korea). Next, a 25 g sample was suspended in 500 mL of 80% methanol and extracted at 30 °C in a shaking water bath at 100 rpm. The methanol extracts were evaporated at 30 °C. The extraction yield of each sample was from 9.04 to 47.5% (w/w).
Biofilm formation assessment and crystal violet assay
Biofilm formation was assessed using crystal violet assay based on Lim et al. (2017)25. To initiate the attachment of bacteria, the culture medium was inoculated at approximately 107 CFU/mL in polystyrene 96-well plates and incubated at 37 °C for 2 h under aerobic conditions. After the attachment step, the medium was carefully removed, and the 96-well plates were washed with PBS (phosphate-buffered saline, pH 7.0) to remove unattached cells. Fresh medium and the plant extracts and/or proteinase K were then added to evaluate the inhibitory effect on biofilm formation. Fresh media with and without E. coli O157:H7 were used as a positive and negative controls. After incubation at 37 °C for 18 h, a crystal violet (CV) assay was performed. The cultured well plate was washed with PBS to remove unattached cells. Then, 1% CV solution (bioWORLD, Dublin, OH, USA) was added, and the well plate was incubated for 30 min at room temperature. After the incubation, the well plate was washed three times with PBS and absolute ethanol was added and incubated for 15 min. After transferring the stained solution to a new well plate, the absorbance was measured at 595 nm using a SpectraMax M2 (Molecular Devices, Sunnyvale, CA, USA).
Minimum biofilm inhibitory concentration (MBIC) assay
The MBIC was determined with three Brassicaceae plant extracts (R. sativus var. longipinnatus, R. sativus, and B. oleracea var. acephala) and proteinase K (P2308, Sigma-Aldrich, St. Louis, MO, USA). All assays were carried out in triplicate. This assay consisted of initial attachment at 37 °C for 2 h followed by the addition of one of the three plant extracts or proteinase K. After the initial attachment step, fresh media with and without E. coli O157:H7 were used as positive and negative controls. MBIC was determined as the lowest concentration of the extracts and proteinase K that resulted in complete inhibition of visible attachment in the CV assay.
Checkerboard assay against E. coli O157:H7 biofilm
The synergistic antimicrobial effects of one of the three plant extracts (R. sativus var. longipinnatus, R. sativus, and B. oleracea var. acephala) in combination with proteinase K were assessed by a checkerboard assay based on Doern (2014)26. The results were analyzed using the fractional inhibitory concentration index (FICI). Briefly, the calculation of fractional inhibitory concentration (FIC) was compared with the value of MBIC of each agent alone and combined24. The sum of the FIC of each sample and the FIC of proteinase K was the FICI, as given in the following formula.
MBICA; the MBIC of treatment A, MBICB; the MBIC of treatment B.
The FICI was determined using the following definition. An FICI of ≤ 0.5 was defined as synergistic, and an FICI in the range of 0.5–1 was defined as additive. An FICI of 1–4 was defined as indifferent, and an FICI of > 4 was defined as antagonism27.
Biofilm formation assessment on stainless steel coupon
Stainless steel coupons (#304, 2 cm × 2 cm × 0.5 cm) were selected to examine the anti-biofilm effect, since most popular food-contact surfaces are made of stainless steel. The coupons were prepared as follows: immersion in alkaline detergent for 5 min, rinsing with distilled water (DW), and sonication for 10 min. After washing, the coupons were autoclaved for sterilization. The stainless steel coupons were placed in a 6-well plate, and 5 mL of the inoculum with approximately 107 CFU/mL was added and incubated at 37 °C for 2 h to initiate attachment. After attachment, the culture medium was carefully removed, and the coupons were washed with PBS. Fresh medium with one of the Brassicaceae plant extracts and/or proteinase K was added and incubated for another 18 h under aerobic conditions to allow biofilm formation. The treatment concentrations were 1 mg/mL R. sativus var. longipinnatus extract and 100 µg/mL proteinase K, 2 mg/mL R. sativus extract and 100 µg/mL proteinase K, and 4 mg/mL B. oleracea var. acephala extract and 200 µg/mL proteinase K. After incubation, the coupons were washed with PBS and placed in a 50 mL tube containing 15 mL of PBS and 3 g of sterilized glass beads (5-mm diameter). Tubes were vortexed for 1 min and the suspended cells were serially diluted in TSB and spread on trypticase soy agar (TSA, Becton Dickinson Co.) plates for enumeration.
Field emission-scanning electron microscopy (FE-SEM)
Field emission scanning electron microscopy was performed based on Lim and Kim (2017)28. The stainless steel was prepared with the treatment of R. sativus var. longipinnatus extract 1 mg/mL and proteinase K 100 µg/mL. After treatment, the samples were fixed with Karnovsky's glutaraldehyde solution containing 0.05 M sodium cacodylate buffer (Sigma-Aldrich), 2% paraformaldehyde (T&I, Gangwon, Republic of Korea), and 2% glutaraldehyde (Georgia Chem, Norcross, GA, USA) at 4 °C for 2 h. After washing twice with 0.05 M sodium cacodylate buffer twice, the samples were incubated in 1% osmium tetroxide (Sigma-Aldrich) with 0.05 M cacodylate buffer at 4 °C for 2 h. The samples were then washed in distilled water, and dehydrated with increasing alcohol concentrations (30%, 50%, 70%, 80%, 90% and 100%). The samples were dried with hexamethyldisilazane (Sigma-Aldrich) for 18–24 h in a biosafety cabinet, and then the samples were sputter-coated with gold (JFC 1100E ion sputtering device, EG&G, USA). The gold-coated samples were examined with a FE-SEM (Philips XL30S FEG, Philips, Eindhoven, Netherlands).
Statistical analysis
Statistical significance was determined by Duncan's multiple range test and a Student-t test procedure of SPSS 12.0 (SPSS Inc., Chicago, IL, USA). The level of statistical significance was p < 0.05.
Results and discussion
Minimum biofilm inhibitory concentration of plant extracts and proteinase K
Brassicaceae plant extracts and proteinase K were tested for their inhibitory effect on E. coli O157:H7 biofilm. In this study, E. coli O157:H7 ATCC43894 (CDC EDL 932) was selected to represent the contamination of pathogenic E. coli on surfaces. This strain is one of the most important foodborne pathogens worldwide, and showed the ability to attach and form biofilms on a variety of food surfaces. This strain has been used as a representative strain for numerous biofilm formation related studies21,25,29,30. Accordingly, this strain can be a practical significance to study E. coli O157:H7 and other EHEC serogroups.
Anti-biofilm effect was tested after the 2 h of initial attachment step. This condition was selected from the previous study with the evaluation of inhibition effect on each step of biofilm formation process; treatment during initial attachment step (anti-attachment), treatment during biofilm development (anti-biofilm), and treatment after biofilm establishment (post anti-biofilm)21. Since the extracts were the most effective during the biofilm development, the minimum biofilm inhibitory concentration (MBIC) was evaluated by treatment after the initial attachment stage of biofilm formation. MBIC values of R. sativus var. longipinnatus, R. sativus, and B. oleracea var. acephala extracts were compared (Table 1). When testing proteinase K, the MBIC value was 1000 µg/mL. E. coli O157:H7 biofilm was sensitive to R. sativus var. longipinnatus, R. sativus, and B. oleracea var. acephala extracts with MBIC of 4, 4, and 8 mg/mL respectively.
Table 1.
Minimum biofilm inhibitory concentration (MBIC) and fractional inhibitory concentration (FIC) of R. sativus var. longipinnatus, R. sativus, and B. oleracea var. acephala extracts with proteinase K against E. coli O157:H7.
| Concentration of plant extract (mg/mL) | Concentration of proteinase K (µg/mL) | FICI§ | |||||
|---|---|---|---|---|---|---|---|
| MBIC of plant extract only |
MBIC of plant extract with proteinase K |
FIC* of plant extract |
MBIC of proteinase K only |
MBIC of proteinase K with plant extract |
FIC proteinase K |
||
| R. sativus var. longipinnatus | 4 | 1 | 0.25 | 1000 | 100 | 0.1 | 0.35 |
| R. sativus | 4 | 2 | 0.5 | 1000 | 100 | 0.1 | 0.6 |
| B. oleracea var. acephala | 8 | 4 | 0.5 | 1000 | 200 | 0.2 | 0.7 |
*Fractional inhibitory concentration (FIC) was calculated from the MBIC of the combined agents divided by the MBIC of each agent alone.
§Fractional inhibitory concentration index (FICI) is the sum of the FIC of each extract and the FIC of proteinase K. The calculation was based on Odds (2003)25.
In a previous study, Brassicaceae plant extracts required an average concentration of 4 mg/mL or more to control E. coli O157:H7 biofilm formation21. The mechanism of reduction by the Brassicaceae plant extracts is unknown but some active compounds, such as caffeic acid, gallic acid and isothiocyanates (ITC), reduced the E. coli O157:H7 biofilm21. Lu et al. (2016) showed that Wasabia japonica (Japanese horseradish) extract containing 59 µg/mL of allyl ITC inhibited E. coli growth31. Studies on 2-thioxo-3-pyrrolidinecarbaldehyde in R. sativus var. longipinnatus documented antimicrobial activity, with the minimum inhibitory concentration against bacteria ranging from 50 to 400 µg/mL32,33. The results showed the removal of bacteria on biofilm formation rather than the bactericidal activity with the natural vegetable extracts.
Synergistic inhibition by plant extracts and proteinase K
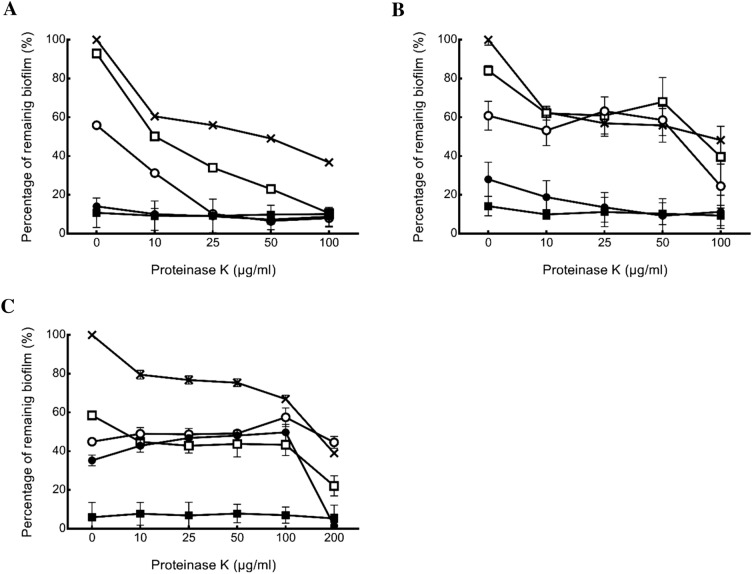
The inhibitory effect of the combination of Brassicaceae plant extracts and proteinase K on E. coli O157:H7 biofilm was analyzed using a checkerboard assay. In the combinatorial treatments, Brassicaceae plant extracts at 1–8 mg/mL and proteinase K at 10–200 µg/mL were used, and the results are expressed as dose–response curves (Fig. 1). Treatment with 1 mg/mL R. sativus var. longipinnatus extract showed the reduction of the biofilm from 7% (with R. sativus var. longipinnatus extract alone) to 89% (R. sativus var. longipinnatus extract combination with proteinase K of 100 µg/mL) (Fig. 1A). At the same concentration, R. sativus extract resulted biofilm reduction from 17% with R. sativus extract alone to 60% in combination with proteinase K (Fig. 1B). When the B. oleracea var. acephala extract was used, the biofilm reduction ranged from 41 to 65% at concentrations of 1 to 4 mg/mL. Biofilm was significantly reduced from 65 to 98%, when 4 mg/mL B. oleracea var. acephala extract and 200 µg/mL proteinase K were treated together (Fig. 1C). The FIC index is presented in Table 1. When R. sativus var. longipinnatus extract and proteinase K were provided together, the MBIC of R. sativus var. longipinnatus extract decreased from 4 to 1 mg/mL. In addition, the combined treatment reduced the treatment concentration of R. sativus and B. oleracea var. acephala extracts from 4 to 2 mg/mL and 8 to 4 mg/mL, respectively. Furthermore, the MBIC value of proteinase K showed a decrease from 1000 to 100 µg/mL when combined with R. sativus var. longipinnatus extract. R. sativus var. longipinnatus had a synergistic effect (FIC index < 0.5) with proteinase K with an FICI value of 0.25. This method can enhance the efficiency of biofilm formation inhibition. R. sativus var. longipinnatus extracts have been studied as a potentially effective anti-biofilm agent in the biofilm development stage21.
Figure 1.
The effect of Brassicaceae plant extracts combined with proteinase K on E. coli O157:H7 biofilms. Percentage of biofilm remaining compared to the biofilm without any treatment after proteinase K treatment with R. sativus var. longipinnatus (A), R. sativus (B) and B. oleracea var. acephala (C) extracts. Concentration of extracts were 8 mg/mL (■), 4 mg/mL (●), 2 mg/mL (○), 1 mg/mL (□) and 0 mg/mL (×). Error bars represent standard error.
Proteinase K is a serine protease, which cleaves peptide bonds C-terminal to breaks down the aliphatic and aromatic amino acids for protein digestion34. The major components of E. coil biofilms are the extracellular polymeric substances (EPS) including nucleic acids, lipids, proteins and exopolysaccharides, which take up to 90% of the dry weight of the biofilm35. Curli, an extracellular protein fiber, is present in the EPS of E. coli and it was degraded by proteinase K to reduce the biofilm formation36. However, proteinase K did not reduce the growth rate of E. coli O157:H737. Former researchers used proteinase K against a wide range of biofilm forming bacteria for food processing facilities and directly on food. Proteinase K were effective to inhibit Listeria monocytogenes and Staphylococcus aureus biofilms on polystyrene and E. coli O157 biofilm on cucumber7,24,38. Properly rinsed surfaces has no possibility of food contamination nor the risk for any enzyme to be considered pollutants39. Based on aforementioned studies and our study, the proteinase K was able to reduce biofilm and to show the prospective applications in food industry. When proteinase K and R. sativus var. longipinnatus extract are combined, proteinase K may decompose related proteins at the time of initial attachment, thereby readily causing the exposure of the sessile cells to the R. sativus var. longipinnatus extract and broadening the antimicrobial range of R. sativus var. longipinnatus extract. Proteinase K and natural substances (thyme oil liposomes) have also shown synergistic effects on inhibiting biofilm formation38. Therefore, the combined treatment using proteinase K and R. sativus var. longipinnatus extract appears to be synergistic by affecting biofilm attachment and development.
Inhibition of biofilm formation on stainless steel surfaces
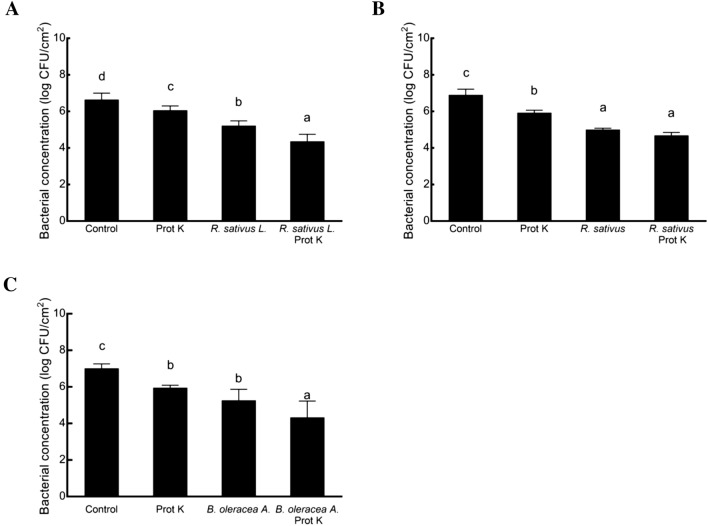
To examine the combination effect on food-contact surfaces, we compared the survival rates of E. coli O157:H7 on stainless steel surfaces. In this study, the results were determined by assessing the viable cell count (Fig. 2). The E. coli O157:H7 living cells in biofilm decreased from 6.66 log CFU/cm2 to 6.07, 5.23, and 4.37 log CFU/cm2 with proteinase K, R. sativus var. longipinnatus extract, and a combination thereof, respectively. The reduction of the combined proteinase K and R. sativus var. longipinnatus extract was 2.29 log CFU/cm2, which was greater than the sum of proteinase K alone and R. sativus var. longipinnatus extract alone. In this experiment, the combined treatments with proteinase K had significantly decreases in biofilm formation compared to the Brassicaceae plant extracts alone, with reductions of 2.29, 2.22, and 2.68 log CFU/cm2 for R. sativus var. longipinnatus, R. sativus, and B. oleracea var. acephala extracts, respectively. Overall, significant reduction was showed in all treatment group compared to the control. While the natural plant extract has certain antibacterial activity, it is hard to expect complete or close to complete removal as antibiotics. Our research aim was to discover and evaluate the combination of biofilm inhibitors on the cleaning and protection of surfaces to provide an efficiently greenery alternative to substitute the harmful and ineffective chemical biocides. Studies on the removal of bacteria from stainless steel surfaces using disinfectants are important for solving hygiene problems in the food industry. It has been reported that the biofilm of E. coli O157:H7 is more resistant to disinfectants than planktonic cells on food-contact surfaces40. While essential oils can be an alternative to disinfectants, they require high concentrations with a strong organoleptic flavor to affect the taste of food products41. Therefore, the combination of proteinase K and Brassicaceae plant extracts may be a possible alternative antimicrobial agent on stainless steel surfaces.
Figure 2.
Inhibition of E. coli O157:H7 biofilm formation by proteinase K (prot K) and R. sativus var. longipinnatus (A), R. sativus (B) and B. oleracea var. acephala (C) extracts on stainless steel coupons. The treated concentrations of the plant extracts and proteinase K, were 1 mg/mL R. sativus var. longipinnatus extract and 100 µg/mL proteinase K, 2 mg/mL R. sativus extract and 100 µg/mL proteinase K, and 4 mg/mL B. oleracea var. acephala extract and 200 µg/mL proteinase K. The control is biofilm formation of E. coli O157:H7 on stainless steel without any treatment. Error bars represent standard error. Different lowercase letters are significantly different (P ˂ 0.05).
Field emission-scanning electron microscopy (FE-SEM)
The treated bacteria were observed using FE-SEM to investigate the morphology of bacteria in response to the combined treatment on the stainless steel surface (Fig. 3I and J). R. sativus var. longipinnatus extract at 1 mg/mL and proteinase K at 100 µg/mL were used based on the FICI index (Table 1). Bacterial cells were embedded in a biofilm layer with the production of an extracellular matrix (Fig. 3A and B). Both proteinase K (Fig. 3C and D) and R. sativus var. longipinnatus extract (Fig. 3E and F) decreased the number of bacterial cells and reduced the development of extracellular polymeric substances. Interestingly, R. sativus var. longipinnatus extract hindered the bacterial cell division, producing elongated E. coli O157:H7, while proteinase K only reduced the number of attached cells. Bacterial cells treated with the combination of R. sativus var. longipinnatus extract and proteinase K were markedly decreased with only elongated cells remaining, and most of the outermost layer of the bacterial cells disappeared (Fig. 3G and H). Further, they showed the synergistic effect of proteinase K treatment when used with antibiotics.
Figure 3.
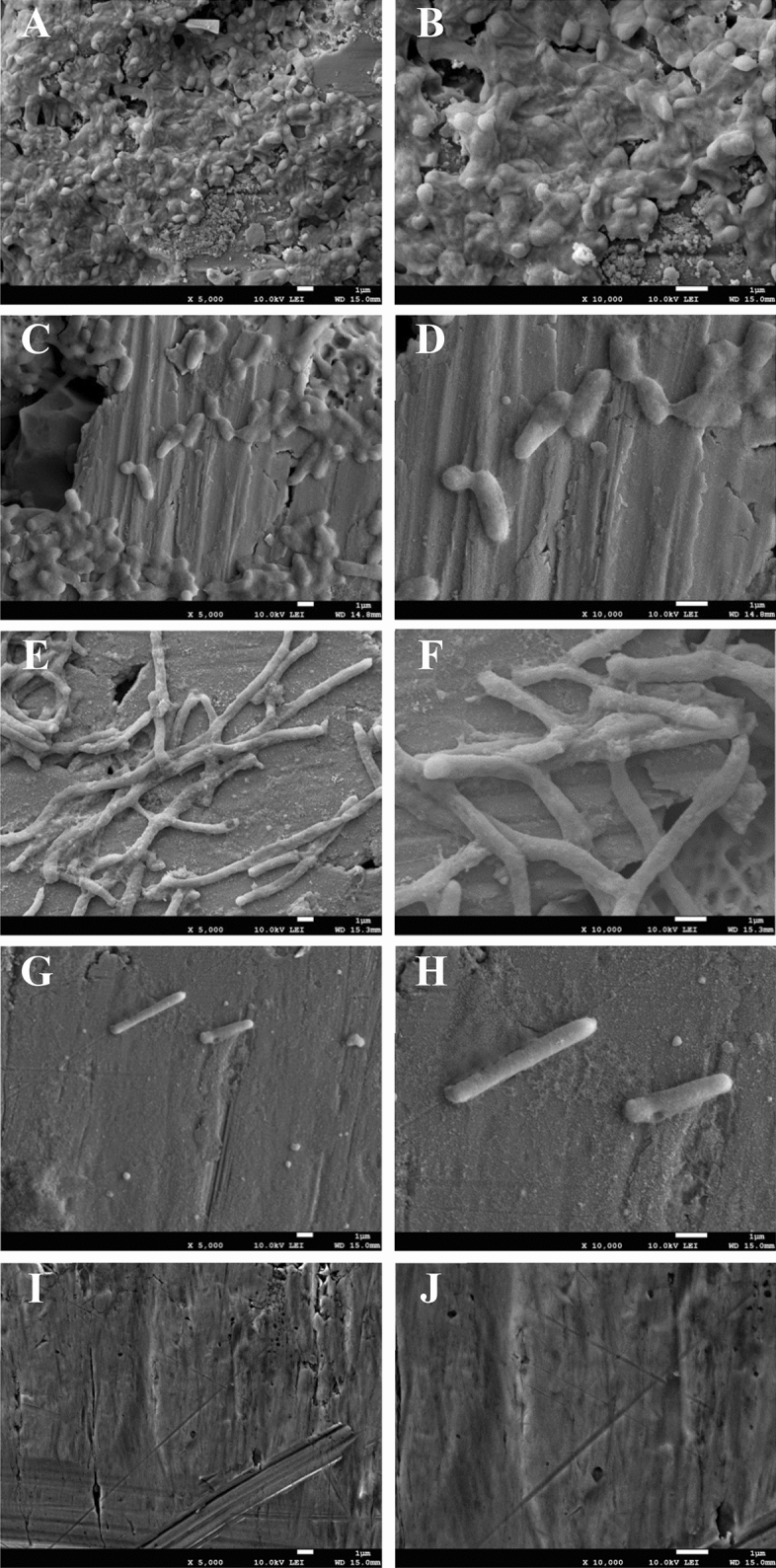
Field-emission scanning electron microscopy analysis of biofilm eradication on stainless steel coupon. Biofilms formed by E. coli O157:H7 were exposed to 100 µg/mL proteinase K (C,D) and, 1 mg/mL R. sativus var. longipinnatus extract (E,F) alone or the combination thereof (G,H) and compared with a positive control (A,B). Negative controls (I,J) are an untreated stainless steel coupon. The magnification of A,C,E,G and, I is 5000×, and B,D,F,H and, J is 10,000x.
Inhibition of bacteria by R. sativus var. longipinnatus extract was mediated through cell division inhibition, of which can be caused by targeting the related genes. Several research groups studied the inhibitory effect of berberine and curcumin on the cell division related genes, with the disruption of the biofilms of E. coli, and S. aureus, S. epidermidis, and Enterococcus, respectively, by inhibiting the assembly of FtsZ in the Z-ring42,43. The bacterial cell division and septum formation play a vital role in the process of the formation and development of biofilm. This type of treatment can lead to bacteriostatic control during treatment. While further study on the inhibition mechanism of the extracts is needed, our study shows a potential action on cell division.
In conclusion, the combination of R. sativus var. longipinnatus extract and proteinase K has synergistic activity against E. coli O157:H7 biofilms. The synergistic effect of R. sativus var. longipinnatus extract and proteinase K may result from the disruption of the biofilm structure and inhibition of biofilm persistence by interrupting cell division with the assembly of cell division proteins. The results from this study support the potential use of R. sativus var. longipinnatus extract to control E. coli O157:H7 in combination with other methods.
Acknowledgements
This research was supported by the Main Research program (E0162104-05) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science and ICT and the National Research Foundation of Korea (NRF-2019R1C1C100242712).
Author contributions
O.K. and J.K. conceived the experiments. D.N. and W.H. conducted the experiments. D.N., W.H., J.K. and O.K. analyzed the results. D.N., W.H. and O.K. wrote the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beuchat LR. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 2002;4:413–423. doi: 10.1016/S1286-4579(02)01555-1. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (2013).
- 3.Centers for Disease Control and Prevention. Multistate outbreak of shiga toxin-producing Escherichia coli O157:H7 infections linked to leafy greens (final update). https://www.cdc.gov/ecoli/2017/o157h7-12-17/index.html (2018).
- 4.Delaquis P, Bach S, Dinu LD. Behavior of Escherichia coli O157:H7 in leafy vegetables. J. Food Protect. 2007;70:1966–1974. doi: 10.4315/0362-028X-70.8.1966. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Rodríguez F, Valero A, Carrasco E, García RM, Zurer G. Understanding and modelling bacterial transfer to foods: A review. Trends Food Sci. Technol. 2008;19:131–144. doi: 10.1016/j.tifs.2007.08.003. [DOI] [Google Scholar]
- 6.Sadekuzzaman M, Yang S, Mizan MFR, Ha SD. Current and recent advanced strategies for combating biofilms. Compr. Rev. Food Sci. Food. 2015;14:491–509. doi: 10.1111/1541-4337.12144. [DOI] [Google Scholar]
- 7.Nguyen UT, Burrows LL. DNase I and proteinase K impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilms. Int. J. Food Microbiol. 2014;187:26–32. doi: 10.1016/j.ijfoodmicro.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Meireles A, Borges A, Giaouris E, Simões M. The current knowledge on the application of anti-biofilm enzymes in the food industry. Food Res. Int. 2016;86:140–146. doi: 10.1016/j.foodres.2016.06.006. [DOI] [Google Scholar]
- 9.Schmidt RH. Basic elements of equipment cleaning and sanitizing in food processing and handling operations: University of Florida Cooperative Extension Service. Florida: Institute of Food and Agriculture Sciences, EDIS; 1997. [Google Scholar]
- 10.Kim LH, et al. Effects of enzymatic treatment on the reduction of extracellular polymeric substances (EPS) from biofouled membranes. Desalin. Water Treat. 2013;51:6355–6361. doi: 10.1080/19443994.2013.780996. [DOI] [Google Scholar]
- 11.Kim SH, et al. Biofilm formation of Campylobacter strains isolated from raw chickens and its reduction with DNase I treatment. Food Control. 2017;71:94–100. doi: 10.1016/j.foodcont.2016.06.038. [DOI] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Multistate outbreak of shiga toxin-producing Escherichia coli O157:H7 infections linked to ready-to-eat salads. https://www.cdc.gov/ecoli/2013/o157h7-11-13/index.html (2013).
- 13.Kim BS, et al. The effect of antiseptics on adipose-derived stem cells. Plast. Reconstr. Surg. 2017;139:625–637. doi: 10.1097/PRS.0000000000003125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno T, et al. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter. 2003;8:207–215. doi: 10.1046/j.1523-5378.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 15.Maddocks SE, Lopez MS, Rowlands RS, Cooper RA. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology. 2012;158:781–790. doi: 10.1099/mic.0.053959-0. [DOI] [PubMed] [Google Scholar]
- 16.Palaniappan K, Holley RA. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol. 2010;140:164–168. doi: 10.1016/j.ijfoodmicro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Rosato A, Vitali C, De Laurentis N, Armenise D, Milillo MA. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine. 2007;14:727–732. doi: 10.1016/j.phymed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Jahangir M, Kim HK, Choi YH, Verpoorte R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009;8:31–43. doi: 10.1111/j.1541-4337.2008.00065.x. [DOI] [Google Scholar]
- 19.Jadoun J, Yazbak A, Rushrush S, Rudy AA, H. Identification of a new antibacterial sulfur compound from Raphanus sativus seeds. Evid. Complement. Altern. 2016;2016:1–7. doi: 10.1155/2016/9271285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avato P, D’Addabbo T, Leonetti P, Argentieri MP. Nematicidal potential of Brassicaceae. Phytochem. Rev. 2013;12:791–802. doi: 10.1007/s11101-013-9303-7. [DOI] [Google Scholar]
- 21.Hu WS, Nam DM, Choi JY, Kim JS, Koo OK. Anti-attachment, anti-biofilm, and antioxidant properties of Brassicaceae extracts on Escherichia coli O157:H7. Food Sci. Biotechnol. 2019;28:1881–1890. doi: 10.1007/s10068-019-00621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reis JA, Paula AT, Casarotti SN, Penna ALB. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012;4:124–140. doi: 10.1007/s12393-012-9051-2. [DOI] [Google Scholar]
- 23.Ananou S, Gálvez A, Martínez-Bueno M, Maqueda M, Valdivia E. Synergistic effect of enterocin AS-48 in combination with outer membrane permeabilizing treatments against Escherichia coli O157:H7. J. Appl. Microbiol. 2005;99:1364–1372. doi: 10.1111/j.1365-2672.2005.02733.x. [DOI] [PubMed] [Google Scholar]
- 24.Shukla S, Rao TS. Dispersal of Bap-mediated Staphylococcus aureus biofilm by proteinase K. J. Antibiot. 2013;66:55–60. doi: 10.1038/ja.2012.98. [DOI] [PubMed] [Google Scholar]
- 25.Lim ES, Lee JE, Kim JS, Koo OK. Isolation of indigenous bacteria from a cafeteria kitchen and their biofilm formation and disinfectant susceptibility. LWT Food Sci. Technol. 2017;77:376–382. doi: 10.1016/j.lwt.2016.11.060. [DOI] [Google Scholar]
- 26.Doern CD. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014;52:4124–4128. doi: 10.1128/JCM.01121-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odds FC. Synergy, antagonism, and what the checkerboard puts between them. J. Antimicrob. Chemoth. 2003;52:1–1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 28.Lim ES, Kim JS. Role of eptC in biofilm formation by Campylobacter jejuni NCTC11168 on polystyrene and glass surfaces. J. Microbiol. Biotechnol. 2017;27:1609–1616. doi: 10.4014/jmb.1610.10046. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Oh S, Park S, Kim SH. Interactive transcriptome analysis of enterohemorrhagic Escherichia coli (EHEC) O157:H7 and intestinal epithelial HT-29 cells after bacterial attachment. Int. J. Food Microbiol. 2009;131:224–232. doi: 10.1016/j.ijfoodmicro.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Sheng H, Xue Y, Zhao W, Hovde CJ, Minnich SA. Escherichia coli O157:H7 curli fimbriae promotes biofilm formation, epithelial cell invasion, and persistence in cattle. Microorganisms. 2020;8:580–597. doi: 10.3390/microorganisms8040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Z, et al. Antibacterial activities of wasabi against Escherichia coli O157:H7 and Staphylococcus aureus. Front. Microbiol. 2016;7:1403–1409. doi: 10.3389/fmicb.2016.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friis P, Kjaer A. 4-Methylthio-3-butenyl isothiocyanate, the pungent principle of radish root. Acta Chem. Scand. 1966;20:698–705. doi: 10.3891/acta.chem.scand.20-0698. [DOI] [Google Scholar]
- 33.Matsuoka H, Takahashi A, Yanagi K, Uda Y. Antimicrobial action of 2-Thioxo-3-pyrrolidinecarbaldehyde, a major thiolactam compound generated from the pungent principle of radish in an aqueous medium. Food Sci. Technol. Int. Tokyo. 1997;3:353–356. doi: 10.3136/fsti9596t9798.3.353. [DOI] [Google Scholar]
- 34.Morris GE, Frost LC, Head LP. Monoclonal-antibody studies of creatine kinase. The proteinase K-cleavage site. Biochem. J. 1985;228:375–381. doi: 10.1042/bj2280375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birjiniuk A, et al. Single particle tracking reveals spatial and dynamic organization of the Escherichia coli biofilm matrix. New J. Phys. 2014;16:085014. doi: 10.1088/1367-2630/16/8/085014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vacheva A, Ivanova R, Paunova-Krasteva T, Stoitsova S. Released products of pathogenic bacteria stimulate biofilm formation by Escherichia coli K-12 strains. A. Van. Leeuw. J. Microb. 2012;102:105–119. doi: 10.1007/s10482-012-9718-y. [DOI] [PubMed] [Google Scholar]
- 37.Lim ES, Koo OK, Kim MJ, Kim JS. Bio-enzymes for inhibition and elimination of Escherichia coli O157:H7 biofilm and their synergistic effect with sodium hypochlorite. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui H, Ma C, Lin L. Co-loaded proteinase K/thyme oil liposomes for inactivation of Escherichia coli O157:H7 biofilms on cucumber. Food Funct. 2016;7:4030–4040. doi: 10.1039/C6FO01201A. [DOI] [PubMed] [Google Scholar]
- 39.Troller JA. Sanitation in Food Processing. Cambridge: Academic Press; 1993. pp. 30–51. [Google Scholar]
- 40.Ryu JH, Beuchat LR. Biofilm formation by Escherichia coli O157:H7 on stainless steel: Effect of exopolysaccharide and curli production on its resistance to chlorine. Appl. Environ. Microbiol. 2005;71:247–254. doi: 10.1128/AEM.71.1.247-254.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abd-AllA MA, Abd-El-Kader MM, Abd-El-Kareem F, El-Mohamedy RSR. Evaluation of lemongrass, thyme and peracetic acid against gray mold of strawberry fruits. J. Agric. Technol. 2011;7:1775–1787. [Google Scholar]
- 42.Boberek JM, Stach J, Good L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS ONE. 2010;5:1–9. doi: 10.1371/journal.pone.0013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rai D, Singh JK, Roy N, Panda D. Curcumin inhibits FtsZ assembly: An attractive mechanism for its antibacterial activity. Biochem. J. 2008;410:147–155. doi: 10.1042/BJ20070891. [DOI] [PubMed] [Google Scholar]