Abstract
The present study investigated biosorption of Pb (II) and Zn (II) using a heavy metal tolerant bacterium Oceanobacillus profundus KBZ 3-2 isolated from a contaminated site. The effects of process parameters such as effect on bacterial growth, pH and initial lead ion concentration were studied. The results showed that the maximum removal percentage for Pb (II) was 97% at an initial concentration of 50 mg/L whereas maximum removal percentage for Zn (II) was at 54% at an initial concentration of 2 mg/L obtained at pH 6 and 30 °C. The isolated bacteria were found to sequester both Pb (II) and Zn (II) in the extracellular polymeric substance (EPS). The EPS facilitates ion exchange and metal chelation-complexation by virtue of the existence of ionizable functional groups such as carboxyl, sulfate, and phosphate present in the protein and polysaccharides. Therefore, the use of indigenous bacteria in the remediation of contaminated water is an eco-friendly way of solving anthropogenic contamination.
Subject terms: Biotechnology, Environmental sciences
Introduction
The contamination of water bodies by heavy metals has resulted in increased research on how to remove such toxic pollutants from the environment. The most investigated heavy metals include chromium (Cr), cadmium (Cd), zinc (Zn), mercury (Hg), lead (Pb), nickel (Ni), and arsenic (As) because of the significant public health and environmental risks that they pose1. These heavy metals are introduced into the environment by human activities such as mining and agriculture2. Reverse osmosis, ion exchange, precipitation, and solvent extraction have been used to decontaminate wastewater before release into the natural environment; however, these technologies are either extremely expensive or not sufficiently effective3. The removal of heavy metals using biological materials has emerged as one of the most promising alternatives, as it does not induce secondary pollution and is cost-effective by avoiding the need for sludge disposal systems4. Biological materials employed for the removal of heavy metals from aqueous solutions include bacteria5, yeast6, algae7, fungi8, agricultural waste4 or wood waste9. The isolation and identification of many indigenously available biological materials are indispensable in sites heavily contaminated with heavy metals.
Heavy metal bioremediation can be classified as either bioaccumulation, a metabolically controlled process that requires the active uptake of heavy metal ions by living biomass, or biosorption, a non-metabolic process that passively binds metal cations onto non-living biomass4. Bioaccumulation requires energy for the uptake of metal ions and typically occurs through the interaction of metal ions and the cell wall. The second step is intracellular uptake, wherein metal ions penetrate the cell membrane and enter the cell to bind on the active sites provided by polysaccharides and proteins. Biosorption relies on the presence of functional groups in the cell wall and/or metabolites exported to the external area. Mechanisms of biosorption include ion exchange, complexation, precipitation, reduction, and chelation10.
Under harsh conditions in the presence of toxic heavy metals and antibiotics, bacteria commonly produce extracellular polymeric substances (EPS) as a protective response. Bacterial EPS are natural polymers of high molecular weight secreted by microorganisms into their environment11. Bacterial EPS typically contains polysaccharides with ionizable functional groups such as carboxyl, sulfate, and phosphate. These functional groups can be deprotonated to anionic species, which interact with cationic metal ions through electrostatic interactions, resulting in the immobilization of heavy metals within the EPS12. Biosorption through EPS has been extensively studied because of its efficient sequestration of toxic metals10,13,14. The metabolism-independent process of biosorption within the EPS is typically more favorable than bioaccumulation within the cell, which is metabolism-dependent12. Biosorption has thus received significant research attention. Despite the isolation of various types of bacteria, the isolation and characterization of indigenous bacteria are indispensable since they are environmentally acceptable and preserve the ecosystem in their locality as evidenced by studies that have used metal tolerant bacteria from industrial effluent15. The local population, soil, water, and food are contaminated with Pb (II) and Zn (II) in the study area at Kabwe Mine, Zambia, Africa, leading Kabwe to be labeled as one of the 10 most polluted places on Earth in 201316.
Therefore, this study aims to assess the heavy metal removal by Oceanobacillus profundus KBZ 3-2, a strain isolated by our research group at the Kabwe Mine site, Zambia, Africa17. This bacterium was isolated when we investigated the biocementation of mine waste by immobilization using ureolytic bacteria by microbially induced calcium carbonate precipitation. The results from the investigation showed that mine waste was stabilized by indigenous bacteria. Additionally, during the characterizing of the bacteria isolated from the site, we found out that O. profundus KBZ 3-2 had a higher biosorption capacity and was worth investigation for heavy metal removal. This study investigated the removal of heavy metals by isolated bacteria in single metal ion batch experiments in a solution prepared to imitate the environment at the abandoned lead–zinc mine at Kabwe, Zambia. The Pb (II) and Zn (II) metal ions were selected for investigation because both pollutants have caused significant health problems to communities near the site18. Moreover, the mechanism of biosorption was discussed and especially focused on the dominant factor affecting biosorption.
Materials and methods
Bacterial strain and chemical reagents
The mine waste was exported from the abandoned Kabwe Mine, Zambia under approval No. RCT 7686229, and the import was permitted by Plant Protection Station, Ministry of Agriculture, Forestry and Fisheries, Japan under the approval No. 29-836. The bacterial strain O. profundus KBZ 3-2 used in this study was isolated from the mine waste when screening for ureolytic bacteria for the solidification of sand17. The same methodology described in our previous study was used to screen the bacterium17. Chemical reagents used in this study were obtained from Wako Pure Chemical Industries Ltd., Tokyo, Japan, otherwise mentioned.
Growth of bacteria in the presence of Pb (II)/Zn (II)
After preculturing O. profundus KBZ 3-2, the bacteria were cultured in 100 mL of LB medium containing PbCl2 or ZnCl2 with different concentrations ranging from 0–50 mg/L for 24 h. Cell growth was monitored by checking the turbidity of the culture with a UV–vis spectrophotometer at 600 nm (OD600).
Effect of pH on biosorption of Pb (II)/Zn (II)
The bacteria were inoculated in a 100 mL LB medium containing 20 mg/L of PbCl2 or 2 mg/L of ZnCl2 at different pH ranging from 2 to 9 and adjusted by HNO3 or NaOH. After cultivation at 30 °C at 160 rpm for 24 h, the cell culture was centrifuged at 8000 × g at 4 °C for 10 min to separate the supernatant and precipitate. The heavy metal concentrations of Pb or Zn in the supernatant were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (ICPE-9820, Shimadzu Corporation, Kyoto, Japan). The biosorption efficiency of heavy metals by the bacteria was calculated as (Ci – Cf)/Ci × 100 (%), where Ci is the initial concentration, and Cf is the final concentration of metal ions.
Localization of Pb (II) and Zn (II) in bacterial cells
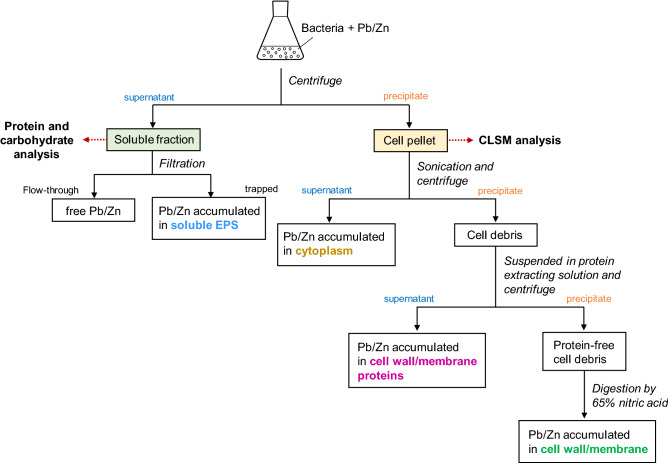
The localization of Pb (II) and Zn (II) in cellular parts was determined according to the methodology reported by Sheng, et al.19 with some modifications and has been illustrated in Scheme 1. Briefly, cells were cultured in 100 mL of LB medium containing 20 mg/L of Pb or 2 mg/L of Zn at 30 °C at 160 rpm for 24 h and were harvested by centrifuge at 8000 × g for 10 min. The supernatant was filtered by a 0.45 μm membrane filter, where EPS and EPS-bound metal ions were trapped and free metal ions passed through. The concentration of Pb (II) or Zn (II) in the filtrate was measured by ICP-AES. The cell pellets were ultrasonicated at 30 kHz for 5 min (Vibra-Cell VCX 130, Sonics & Materials, Inc., Newtown, USA) in 5 mL Tris–HCl (10 mM, pH 8.0), followed by centrifugation at 8000 × g for 10 min to separate the supernatant (cell-free extract: cytoplasm) and precipitate (cell debris: cell wall/membrane). The supernatant was thereafter collected for analysis to determine the cytoplasmic water-soluble Pb (II) or Zn (II). The precipitate, cell debris, was then suspended in protein extracting solution (5% Sodium Dodecyl Sulfate: SDS, 10 mM Tris–HCl, pH 8), followed by centrifugation at 8000 × g for 10 min. The resulting supernatant was used for the determination of metal ions accumulated in cell wall/membrane proteins, and the obtained precipitate was digested by 65% nitric acid to quantify the Pb (II) or Zn (II) accumulated in cell wall/membrane components. The concentration of Pb (II) or Zn (II) accumulated in EPS was calculated by subtracting the metal concentration in the other factions from the initial concentration. Meanwhile, protein and carbohydrate contents in a supernatant (soluble fraction) after 24 h cell cultivation were measured. The protein content in the supernatant was determined by the Bradford protein assay with bovine serum albumin as the standard, and the total carbohydrate content was determined by the phenol sulfuric acid method as previously described20.
Scheme 1.
Flowchart for the analytical procedure for the determination of the distribution of heavy metal in different cellar parts.
Confocal laser scanning microscopy (CLSM) analysis
The bacteria cells were cultured in 100 mL LB medium for 24 h in the presence of 20 mg/L of Pb (II) and collected by centrifuge at 8000 × g for 10 min, followed by suspension in saline buffer. Three different staining dyes were sequentially added to the cell suspension to stain the DNA (DAPI Nucleic Acid Stain, Molecular probes, Invitrogen), EPS (Wheat Germ Agglutinin, Alexa Fluor 633 Conjugate, Molecular Probes, Invitrogen), and Pb (II) (Leadmium Green AM Dye, Molecular Probes, Invitrogen). The samples were analyzed with a CLSM (Nikon A1 and Ti-E) equipped with a Plan Apo VC × 60 objective lens (NA 1.40, Nikon).
Results and discussion
Effect of Pb (II) and Zn (II) on bacterial growth
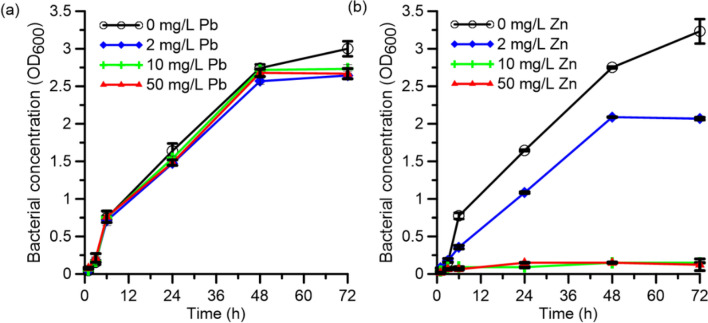
Bacterial growth under exposure to the heavy metal ions of interest was assessed to gauge the applicability of the strain for bioremediation. Figure 1 shows the effect of Pb (II) and Zn (II) on the growth of O. profundus KBZ 3-2 in different concentrations. The bacterial cells grew well in all the tested concentrations of Pb (II), demonstrating resistance to Pb (II) contamination up to 50 mg/L. However, the bacteria were less tolerant of Zn (II), displaying growth only in 2 mg/L Zn (II). This result indicates that the bacteria can be used for bioremediation of Pb (II) and Zn (II), albeit at a lower concentration for the latter. Inefficient biosorption of Zn (II) at higher Zn(II) concentrations occurs because Zn (II) is a trace element required for the growth of heterotrophic bacteria21, such as O. profundus. However, at higher concentrations, Zn (II) hinders the survival of the bacteria; this is consistent with previous results that Zn (II) is a highly reactive divalent metal ion and that it would readily displace metal ions required for bacterial growth22. Moreover, Zn (II) is a known antibacterial agent because it is a strong oxidative agent that causes cell membrane disruption, leading to cell death22.
Figure 1.
Microbial growth of O. profundus KBZ 3-2 in the presence of (a) Pb (II) and (b) Zn (II) with different concentrations.
Effect of pH on adsorption
The pH of the solution is among the most important parameters in biosorption because the initial pH of the solution is a key factor that affects the metal speciation, metal solubility, and the dissociation of the functional groups23,24. Figure 2 shows the effect of pH on heavy metal removal efficiency. The removal percentage of metal ions was as low as 18% for Pb (II) and 12% for Zn (II) at pH 2. The maximum removal percentage for Pb (II) was 97% at an initial concentration of 50 mg/L whereas maximum removal percentage for Zn (II) was 54% at an initial concentration of 2 mg/L obtained at pH 6. This low removal percentage at low pH was attributed to the protonation of functional groups, which were responsible for metal ion adsorption22. Similar findings by earlier investigators have also attributed lower biosorption efficiency to protonation or poor ionization of functional groups at low pH, resulting in a weak complex affinity of the metal ions25,26. On the other hand, at higher pH values, the binding efficiency increased because the functional groups were negatively charged, thereby facilitating biosorption of the metal cations through ion exchange and metal chelation complexation. Additionally, the resulting effective pH for biosorption is consistent with the data obtained from bacteria in the Bacillaceae family, which showed an effective pH range of 5–922. A sharp increase in biosorption was observed in Pb and a gradual increase for Zn was probably caused by the gradual exhaustion of active sites for the biosorption of zinc ions. This trend has been observed in previous investigation5. Table 1 represents a comparison between O. profundus KBZ 3-2 with other biosorbent materials. The results show the O. profundus KBZ 3-2 can be used as an effective biosorbent.
Figure 2.
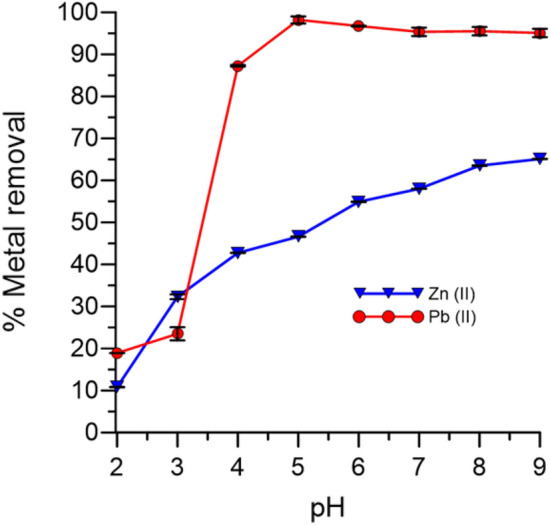
Effect of pH on biosorption by O. profundus KBZ 3-2.
Table 1.
Comparison between O. profundus KBZ 3-2 with other biosorbents.
| Metal ion | Biosorbent | Metal ion conc | Adsorption efficiency (%) | Ref |
|---|---|---|---|---|
| Pb | Pseudomonas sp. | 1 mg/L | 87.9 | 27 |
| S. maltophilia | 39.6 mg/L | 96 | 28 | |
| B. iodium | 100 mg/L | 87 | 29 | |
| O. profundus KBZ 3–2 | 50 mg/L | 97 | This study | |
| Zn | S. maltophilia | 20.6 mg/L | 96 | 28 |
| Pseudomonas sp. | 1 mg/L | 49.8 | 27 | |
| Zinc sequestering bacterium VMSDCM | 0.21 mol/g of biomass | 5 | ||
| O. profundus KBZ 3–2 | 2 mg/L | 54 | This study | |
Localization of Pb (II) and Zn (II) in a cell
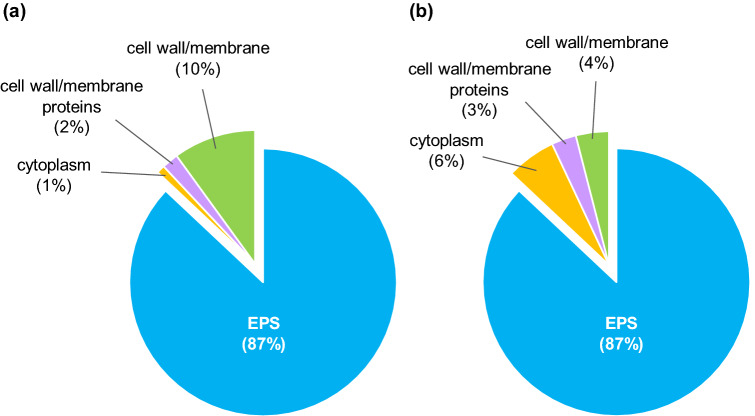
We found there was no Pb/Zn left in the filtrate after filtration of the supernatant of cell culture added with Pb/Zn, which indicates heavy metal ions were totally trapped by the bacteria. The localization of metal ions in the different cellular parts of the bacteria was investigated to understand the possible mechanism of Pb (II) and Zn (II) accumulation in the bacteria cells. The percentages of metal ions in (1) soluble EPS, (2) the cytoplasm, (3) cell wall/membrane proteins, and (4) the cell wall/membrane of O. profundus KBZ 3-2 are shown in Fig. 3. The highest concentration for both elements was in soluble EPS (87%), which is produced in the soluble phase by bacteria as a defense mechanism against heavy metal contamination22. Pb (II) accumulation in other parts was determined in the cytoplasm (1%), cell wall/membrane proteins (2%), and the cell wall/membrane (10%). Zn (II) was found in the cytoplasm (6%), cell membrane/wall proteins (3%), and the cell wall/membrane (4%). For both heavy metals, biosorption would predominantly be metabolism-independent since it primarily occurred on the exterior of the cell, EPS. We can conclude that metabolism-dependent biosorption has a minor role30.
Figure 3.
Distribution of (a) Pb(II) and (b) Zn(II) in different cellular parts of O. profundus KBZ 3-2.
The amount of cytoplasmic Zn (II) in Fig. 3b was higher than that of Pb (II) in Fig. 3a, probably because Zn (II) is used for microbial metabolic processes in trace amounts. The formation of EPS-Pb could have prevented the entry of Pb into the cell; hence a small amount of Pb (II) was detected inside the cell. EPS contains different carbohydrates and their derivatives31, which are usually polyanionic because of the functional groups. This allows biosorption through mechanisms such as ion exchange and metal complexation32. Thus, we propose that the dominant mechanism of Pb (II) and Zn (II) biosorption by O. profundus KBZ 3-2 occurs by soluble EPS being actively secreted by a bacterium to defend itself from toxic ions, thereby binding Pb (II) and Zn (II) ions from solutions through metal chelation-complexation and consequently removing Pb (II) and Zn (II) through metabolism-independent process biosorption. Bioaccumulation also occurred, albeit at a significantly lesser degree, as evidenced by the presence of heavy metals within the cell.
Composition of EPS released by the bacteria
Figure 4a shows the photo of a centrifuged bacterial suspension-cultured for 24 h in the presence of 20 mg/L Pb. We observed the supernatant containing soluble EPS and the precipitates containing bacterial cells. The composition of soluble EPS was analyzed over time in terms of proteins and carbohydrates (Fig. 4b), because it is the main component responsible for metal sequestration. Typically, EPS consists of water, protein, polysaccharides, nucleic acid, uronic acid, and humic acid33–35. The EPS had 105 µg/L of protein and 679 µg/L of carbohydrates; these components are primarily responsible for the biosorption of Pb (II) and Zn (II) through metal chelation-complexation because they contribute to the negatively charged carboxyl group (–COO–), sulfate group (–SO3–), and phosphate group (–PO3–). This result is consistent with the characteristic of a bacterium having defensive mechanisms for Pb (II). Previous studies have also shown that bacterium can survive in the conditions with high heavy metal exposure by developing resistance and could be isolated and used for the bioremediation of Zn (II), Cd (II), Cu (II), and Pb (II)27,31,32. A representative diagram for the bacterial biosorption mechanism is depicted in Fig. 5.
Figure 4.
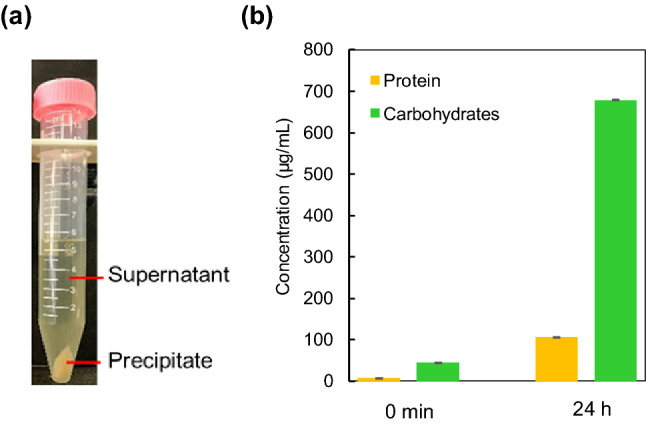
Analysis of O. profundus KBZ 3-2 cultured in 20 mg/L Pb for 24 h; (a) image of centrifuged culture, (b) quantitative determination of proteins and carbohydrates in soluble EPS.
Figure 5.
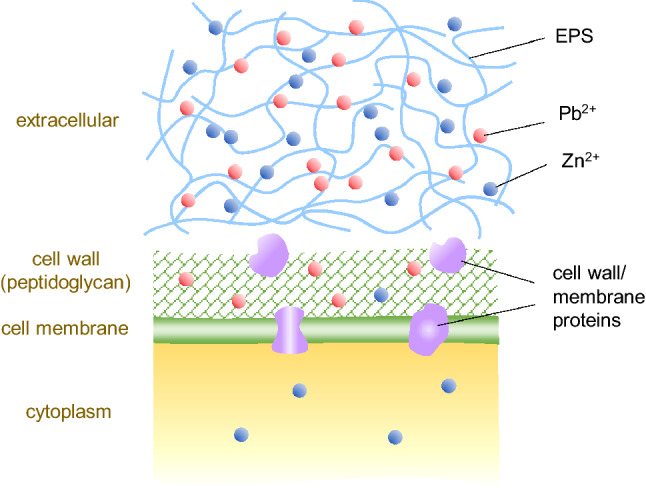
Representative diagram for biosorption mechanism for Pb(II) and Zn(II).
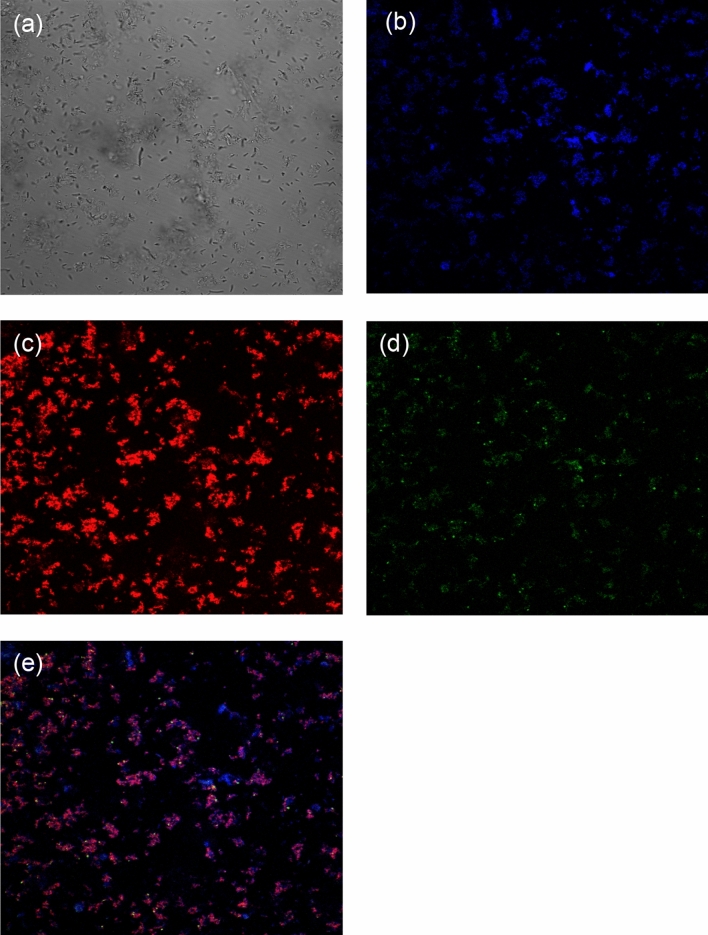
CLSM analysis
As indicated in the study methods, we conducted a CLSM analysis of grown cells in the presence of 20 mg/L Pb (II) to further confirm the native associations of cell-EPS-metal ion aggregates. Most of the EPS was found in the supernatant after centrifugation, some EPS would be staying around the cells. Figure 6 shows the CSLM images of the bacterial suspension, showing the bright-field image (a), the bacterial cells (b, in blue), EPS (c, in red), and Pb (II) (d, in green). The correlation and visualization of each component (bacterial cells, EPS, and Pb (II)) confirm the adsorption of Pb ions onto the cells and their surroundings. Since the cells were surrounded by the remaining EPS, which was secreted by cells, the protein and carbohydrates in EPS would be responsible for the heavy metal detoxification via complexation with metal ions. Consequently, EPS has an important role in the adsorption of different toxic heavy metals, which is consistent with the previous report13.
Figure 6.
Confocal images of O. profundus KBZ 3-2 cultured in 20 mg/L Pb for 24 h; (a) bright-field image, (b) bacterial cells stained by DAPI (blue), (c) EPS stained by Alexa Fluor 633-conjugated agglutinin (red), (d) Pb (II) stained by Leadmium Green AM Dye (green), (e) overlay image of (b–d).
Conclusion
The study demonstrated that O. profundus KBZ 3-2 isolated from Pb–Zn contaminated soil at the Kabwe Mine site in Zambia was capable of removing Pb (II) and Zn (II) from water. The proposed mechanism of biosorption was through chelation-complexation on the present functional groups of EPS excreted by the bacteria. O. profundus KBZ 3-2 was a highly efficient biosorbent and had high potential in upscaling its biosorption purpose for bioremediation at Kabwe Mine site as an eco-friendly solution to contaminated site restoration. This is recommended for the cleaning of contaminated water from the mine site.
Acknowledgements
This work was supported by JSPS KAKENHI under Grant Number JP18H03395, JST/JICA SATREPS (Science and Technology Research Partnership for Sustainable Development; No. JPMJSA1501), and JST aXis (Accelerating Social Implementation for SDGs Achievement; No. JPMJAS2001). We are grateful to Dr. K. Kobayashi and the Nikon Imaging Center at Hokkaido University for being very helpful with confocal microscopy, image acquisition, and analysis.
Author contributions
W.M., K.N. and S.K. designed the research. K.B., I.N. and M.C. provided the samples that made the study possible. W.M. and A.A. performed the experiments and analyzed the data. W.M. and K.N. wrote the manuscript, and K.B., I.N., and T.I. carefully read to edit the manuscript. M.It., T.S., H.N., S.N., and M.Is. participated in discussions of this work. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp. Suppl. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jena S, Dey SK. Heavy metals. Am. J. Environ. Stud. 2016;1:48–60. [Google Scholar]
- 3.Ayangbenro AS, Babalola OO. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health. 2017;14:1–16. doi: 10.3390/ijerph14010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdi O, Kazemi M. A review study of biosorption of heavy metals and comparison between different biosorbents. J. Mater. Environ. Sci. 2015;6:1386–1399. [Google Scholar]
- 5.Vishal M, Shail D, Chandrajit B. Optimization of physical parameters for batch mode Zn (II) ion removal from liquid phase: A potential biosorption study. Environ. Prog. Sustain. Energy. 2013;32:213–222. doi: 10.1002/ep.10637. [DOI] [Google Scholar]
- 6.Rodríguez, I. A. et al. Biosorption of heavy metals by Candida albicans. Adv. Bioremediat. Phytoremediat. 43–63, 10.5772/intechopen.72454 (2018).
- 7.Anastopoulos I, Kyzas GZ. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015;209:77–86. doi: 10.1016/j.molliq.2015.05.023. [DOI] [Google Scholar]
- 8.Prasad ASA, Varatharaju G, Anushri C, Dhivyasree S. Biosorption of lead by Pleurotus florida and Trichoderma viride. Br. Biotechnol. J. 2013;3:66–78. doi: 10.9734/BBJ/2013/2348. [DOI] [Google Scholar]
- 9.Mishra V, Balomajumder C, Agarwal VK. Design and optimization of simultaneous biosorption and bioaccumulation (SBB) system: A potential method for removal of Zn(II) ion from liquid phase. Desalin. Water Treat. 2013;51:3179–3188. doi: 10.1080/19443994.2012.749027. [DOI] [Google Scholar]
- 10.Vishan I, Laha A, Kalamdhad A. Biosorption of Pb(II) by Bacillus badius AK strain originating from rotary drum compost of water hyacinth. Water Sci. Technol. 2017;75:1071–1083. doi: 10.2166/wst.2016.590. [DOI] [PubMed] [Google Scholar]
- 11.Caruso, C. et al. Extracellular polymeric substances with metal adsorption capacity produced by Pseudoalteromonas sp. MER144 from Antarctic seawater. Environ. Sci. Pollut. Res. 25, 4667–4677 (2018). [DOI] [PubMed]
- 12.Saba, Rehman, Y., Ahmed, M. & Sabri, A. N. Potential role of bacterial extracellular polymeric substances as biosorbent material for arsenic bioremediation. Bioremediat. J.23, 72–81 (2019).
- 13.More TT, Yadav JSS, Yan S, Tyagi RD, Surampalli RY. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014;144:1–25. doi: 10.1016/j.jenvman.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Shameer, S. Biosorption of lead , copper and cadmium using the extracellular polysaccharides ( EPS ) of Bacillus sp ., from solar salterns. 3 Biotech6, 1–10 (2016). [DOI] [PMC free article] [PubMed]
- 15.Mishra V, Balomajumder C, Agarwal VK. Biological removal of heavy metal zinc from industrial effluent by zinc sequestering bacterium VMSDCM. Clean Technol. Environ. Policy. 2013;16:555–568. doi: 10.1007/s10098-013-0655-x. [DOI] [Google Scholar]
- 16.Blacksmith, I. The worlds worst 2013: The top ten toxic threats. 10.1017/CBO9781107415324.004 (2013).
- 17.Mwandira W, et al. Solidification of sand by Pb(II)-tolerant bacteria for capping mine waste to control metallic dust: Case of the abandoned Kabwe Mine, Zambia. Chemosphere. 2019;228:17–25. doi: 10.1016/j.chemosphere.2019.04.107. [DOI] [PubMed] [Google Scholar]
- 18.Yabe J, et al. Lead poisoning in children from townships in the vicinity of a lead-zinc mine in Kabwe, Zambia. Chemosphere. 2015;119:941–947. doi: 10.1016/j.chemosphere.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Sheng, Y. et al. Cadmium tolerant characteristic of a newly isolated Lactococcus lactis subsp. lactis. Environ. Toxicol. Pharmacol. 48, 183–190 (2016). [DOI] [PubMed]
- 20.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 21.Bong, C. W., Alfatti, F. M., Zam, F. A., Obayashi, Y. & Suzuki, S. The effect of zinc exposure on the bacteria abundance and proteolytic activity in seawater. Interdiscip. Stud. Environ. Chem. Responses Contam. 57–63 (2010).
- 22.Capdevila DA, Wang J, Giedroc DP. Bacterial strategies to maintain zinc metallostasis at the host-pathogen interface. J. Biol. Chem. 2016;291:20858–20868. doi: 10.1074/jbc.R116.742023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu, W. Bin, Shi, J. J., Wang, C. H. & Chang, J. S. Biosorption of lead, copper and cadmium by an indigenous isolate Enterobacter sp. J1 possessing high heavy-metal resistance. J. Hazard. Mater. 134, 80–86 (2006). [DOI] [PubMed]
- 24.Kalita D, Joshi SR. Study on bioremediation of lead by exopolysaccharide producing metallophilic bacterium isolated from extreme habitat. Biotechnol. Rep. 2017;16:48–57. doi: 10.1016/j.btre.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jnr MH, Harcourt P. Studies on the effect of pH on the sorption of Pb2+ and Cd2+ ions from aqueous solutions by Caladium bicolor (Wild Cocoyam) biomass. Electron. J. Biotechnol. 2004;7:313–323. [Google Scholar]
- 26.Pehlivan E, Altun T, Parlayici S. Modified barley straw as a potential biosorbent for removal of copper ions from aqueous solution. Food Chem. 2012;135:2229–2234. doi: 10.1016/j.foodchem.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Sundaramanickam, A. A comparative study of physico-chemical investigation along Parangipettai and Cuddalore coast. J. Environ. Sci. Technol. 1–10, 10.3923/jest.2008.1.10 (2008).
- 28.Wierzba S. Biosorption of lead(II), zinc(II) and nickel(II) from industrial wastewater by Stenotrophomonas maltophilia and Bacillus subtilis. Polish J. Chem. Technol. 2015;17:79–87. doi: 10.1515/pjct-2015-0012. [DOI] [Google Scholar]
- 29.De J, Ramaiah N, Vardanyan L. Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar. Biotechnol. 2008;10:471–477. doi: 10.1007/s10126-008-9083-z. [DOI] [PubMed] [Google Scholar]
- 30.Muthu M, Wu HF, Gopal J, Sivanesan I, Chun S. Exploiting microbial polysaccharides for biosorption of trace elements in aqueous environments—Scope for expansion via nanomaterial intervention. Polymers (Basel). 2017;9:1–12. doi: 10.3390/polym9120721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shameer, S. Biosorption of lead, copper and cadmium using the extracellular polysaccharides (EPS) of Bacillus sp., from solar salterns. 3 Biotech6, 1–10 (2016). [DOI] [PMC free article] [PubMed]
- 32.Igiri BE, et al. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018;2018:1–16. doi: 10.1155/2018/2568038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tourney J, Ngwenya BT. The role of bacterial extracellular polymeric substances in geomicrobiology. Chem. Geol. 2014;386:115–132. doi: 10.1016/j.chemgeo.2014.08.011. [DOI] [Google Scholar]
- 34.Shi Y, et al. Exploiting extracellular polymeric substances (EPS) controlling strategies for performance enhancement of biological wastewater treatments: An overview. Chemosphere. 2017;180:396–411. doi: 10.1016/j.chemosphere.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Nouha K, Kumar RS, Balasubramanian S, Tyagi RD. Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. (China) 2018;66:225–245. doi: 10.1016/j.jes.2017.05.020. [DOI] [PubMed] [Google Scholar]