Abstract
Background
Clinical guidelines recommend a stepped-escalation treatment strategy for type 2 diabetes (T2DM). Across multiple treatment strategies varying in efficacy and costs, no clinical or economic studies directly compared them. This study aims to estimate and compare the cost-effectiveness of 10 commonly used pharmacologic combination strategies for T2DM.
Methods
Based on Chinese guideline and practice, 10 three-stepwise add-on strategies were identified, which start with metformin, then switch to metformin plus one oral drug (i.e., sulfonylurea, thiazolidinedione, α-glucosidase inhibitor, glinide, or DPP-4 inhibitor) as second line, and finally switch to metformin plus one injection (i.e., insulin or GLP-1 receptor agonist) as third line. A cohort of 10,000 Chinese patients with newly diagnosed T2DM was established. From a healthcare system perspective, the Cardiff model was used to estimate the cost-effectiveness of the strategies, with clinical data sourced from a systematic review and indirect treatment comparison of 324 trials, costs from claims data of 1164 T2DM patients, and utilities from an EQ-5D study. Outcome measures include costs, quality-adjusted life-years (QALYs), incremental cost-effectiveness ratios (ICERs), and net monetary benefits (NMBs).
Results
Over 40-year simulation, the costs accumulated for a patient ranged from $7661 with strategy 1 to $14,273 with strategy 10, while the QALY gains ranged from 13.965 with strategy 1 to 14.117 with strategy 8. Strategy 7 was dominant over seven strategies (strategies 2~6, 9~10) with higher QALYs but lower costs. Additionally, at a willingness-to-pay threshold of $30,787/QALY (i.e., 3 times GDP/capita for China), strategy 7 was cost-effective compared with strategy 1 (ICER of strategy 7 vs. 1, $3371/QALY) and strategy 8 (ICER of strategy 8 vs. 7, $132,790/QALY). Ranking the strategies by ICERs and NMBs, strategy 7 provided the best value for money when compared to all other strategies, followed by strategies 5, 9, 8, 1, 3, 6, 10, 2, and 4. Scenario analyses showed that patients insist on pharmacologic treatments increased their QALYs (0.456~0.653) at an acceptable range of cost increase (ICERs, $1450/QALY~$12,360/QALY) or even at cost saving compared with those not receive treatments.
Conclusions
This study provides evidence-based references for diabetes management. Our findings can be used to design the essential drug formulary, infer clinical practice, and help the decision-maker design reimbursement policy.
Supplementary information
The online version contains supplementary material available at 10.1186/s12916-020-01837-x.
Keywords: Type 2 diabetes, Cost-effectiveness, Metformin, Sulfonylurea, Thiazolidinedione, α-Glucosidase inhibitor, Glinide, DPP-4 inhibitor, Insulin, GLP-1 receptor agonist
Background
Type 2 diabetes (T2DM) as a chronic progressive disease imposes a substantial disease burden on patients and the healthcare system [1]. China has the world’s largest diabetes epidemic, with 116.4 million adults with diabetes in 2019 [1]. Total diabetes-related health expenditure reached $109.0 billion, ranking second to the USA globally [1]. How to properly reduce medical expenses along with improving patients’ health has become a common concern of patients, governments, and the society. Chinese clinical guidelines advocate a stepwise failure-driven treatment strategy for blood glucose lowering that leads to the sequential addition of therapies [2]. Metformin is the preferred initial therapy, which is recommended to be maintained throughout the treatment [2]. Once metformin fails to achieve glucose target, multiple oral glucose-lowering drugs were available as add-on therapy to metformin, such as sulfonylurea, thiazolidinedione, α-glucosidase inhibitor, glinide, and dipeptidyl peptidase 4 (DPP-4) inhibitor. If oral dual therapy fails to effectively control glucose, injections like insulin or glucagon-like peptide 1 (GLP-1) receptor agonist can be added onto metformin [2].
A number of choices for add-on glucose-lowering treatment have increased uncertainty regarding the optimal treatment path, while increasing the complexity of treatment choice in clinical practice. A nationwide survey of treatment pattern of oral drugs users in China reported that metformin was used by 53.7% of T2DM patients, followed by sulfonylureas (42.7%), α-glucosidase inhibitors (35.9%), glinides (27.5%), thiazolidinediones (17.2%), and DPP-4 inhibitors (0.8%) [3]. Dual combination therapy was more commonly used than monotherapy, of which metformin plus sulfonylureas was the most common (27.7%), followed by metformin plus glinides [3]. Medication adherence was unsatisfactory in China with 31.9% of the patients altering treatment regimens within 1 year. The main reasons cited for treatment alterations were insufficient efficacy (21.9%), adverse reaction (4.3%), and treatment budget (1.7%) [3]. Poor adherence would in turn impede treatment effect, further to increase the risks of diabetes-related complications and healthcare costs [4–6]. Lack of a comprehensive understanding of the efficacy, safety, and costs of the existing treatments is a potential reason for the failure to choose appropriate treatment.
Informed decisions regarding optimal prescribing and reimbursement of glucose-lowering drugs by healthcare payers require information about efficacy, costs, and cost-effectiveness. It is important to study the costs and benefits of the existing glucose-lowering treatments, to help clinicians and decision-makers choose the most cost-effective treatments. However, clinical evidence of head-to-head studies that directly compared all the glucose-lowering treatments against each other can hardly be fully obtained [7]. Besides, T2DM treatment is a lifetime task that often requires a sequential use of drug monotherapy and a combination of drugs to ensure intensive glycemic control. Thus, it is of value to conduct economic evaluations based on a lifelong treatment strategy, to evaluate the therapies used by a patient from the start of medication to the end of life. This could help us comprehensively understand the impacts of different treatments on disease burden of patients. However, economic evaluations of glucose-lowering treatments in China were mostly short-term cost-effectiveness analyses conducted alongside the clinical trials, and several long-term modeling studies focused on several individual drugs as monotherapy or add-on therapy at a specific treatment stage. There was a lack of clinical or economic studies that directly compared all the existing lifelong treatment strategies against each other within one study.
Therefore, this study aimed to estimate and compare the lifetime cost-effectiveness of 10 commonly used pharmacologic combination strategies for patients with newly diagnosed T2DM in China, so as to provide unified hierarchies of evidence for the holistic management of T2DM.
Methods
From a healthcare system perspective, the cost-effectiveness of the pharmacologic combination strategies was evaluated using an existing stochastic simulation model, the Cardiff diabetes model.
Model overview
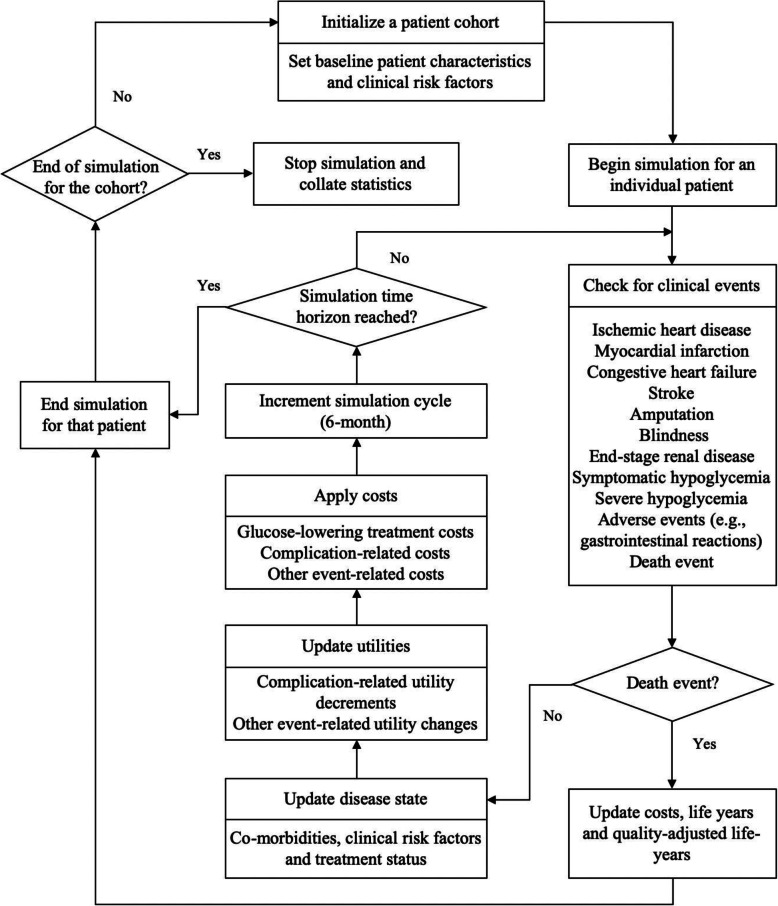
The Cardiff diabetes model is a patient-level fixed-time increment, Monte Carlo micro-simulation model, which is designed to evaluate the cost-effectiveness of comparable treatment strategies in diabetes [8–14]. Each treatment strategy is comprised of three therapy lines. The model simulates multiple disease courses and predicts the occurrences of diabetes-related complications and death based on the United Kingdom Prospective Diabetes Studies (UKPDS) 68 or 82 risk equations, combined with patient characteristics, clinical risk factors, and treatment-induced changes in the clinical risk factors [13, 14]. The risk factors include glycosylated hemoglobin Alc (HbA1c), cholesterol, blood pressure, and weight. The natural progressions of HbA1c, cholesterol, and blood pressure are modeled via the implementation of UKPDS 68 risk equations, and that of weight is modeled linearly based on a weight gain of 0.1 kg per year by default. The logical flow of the model is shown in Fig. 1. Diabetes-related complications include macrovascular events (i.e., ischemic heart disease, myocardial infarction, congestive heart failure, and stroke) and microvascular events (i.e., blindness, end-stage renal disease, and amputation). Model inputs include patient profiles of the initial cohort, treatment effects and pharmacy costs of glucose-lowering treatments, and costs and utility changes associated with diabetes-related events.
Fig. 1.
Logical flow of patient simulation process in the Cardiff diabetes model
The UKPDS 68 risk equations were used in the base-case analysis, and the UKPDS 82 risk equations were tested in the sensitivity analysis. A lifetime horizon of 40 years was simulated with a discount rate of 3% for costs and benefits [15]. Outcome measures include costs, quality-adjusted life-years (QALYs), incremental cost-effectiveness ratios (ICERs), and net monetary benefits (NMBs). The ICER is calculated as incremental costs divided by incremental QALYs of two treatment strategies. As this study had multiple treatment strategies, they were ranked as follows: (i) rank the strategies in order of costs and compare each strategy to its adjacent and lower-cost alternative; (ii) rule out strongly dominated alternatives (i.e., with higher cost but lower QALY compared with its adjacent); (iii) calculate ICERs based on the comparisons of moving to increasingly costly and increasingly effective alternatives; (iv) if the ICER associated with moving to more costly alternative falls, then the lower-cost alternative used to calculate the ICER is extendedly dominated and is ruled out; (v) recalculate ICERs based on comparisons of moving to increasingly costly but increasingly effective alternatives that are neither strongly nor extendedly dominated; and (vi) compare the ICER with willingness-to-pay/QALY—if it is within willingness-to-pay/QALY, the higher-cost alternative used to calculate the ICER is cost-effective; otherwise, the lower-cost one is cost-effective [16]. The NMB is calculated as QALYs times willingness-to-pay/QALY, minus costs of each strategy. It is a rearrangement of the cost-effectiveness decision rule that summarizes the difference between the economic value of health benefits and the costs [16–18]. The larger the NMB value, the more cost-effective the strategy is [16–18]. This study used a willingness-to-pay threshold of ¥212,676 ($30,787)/QALY (3 times gross domestic product per capita of China, 2019) [19] according to the Chinese guideline [20].
Patient cohort and patient profiles
An initial patient cohort of 10,000 Chinese adult patients with newly diagnosed T2DM was established. Patient profiles of the cohort were sourced from a nationwide prospective cohort study [21, 22]. The study recruited 5770 Chinese patients with newly diagnosed T2DM from 79 hospitals across six geographic regions of China, which had comprehensive baseline patient profiles. As it only reported body mass index (BMI) and weight of the patients, height was calculated using BMI and weight (Table 1) [21, 22].
Table 1.
Model inputs: patient profiles, treatment effects, costs, and utility changes
| Patient profiles of the initial cohort | Value | Source | |||||||||
| Patient characteristics | Mean | SE | |||||||||
| Age, year | 55.7 | 0.17 | [21, 22] | ||||||||
| Female proportion, % | 45.8 | 0.70 | |||||||||
| Duration of diabetes, year | 0.50 | – | |||||||||
| Height, metera | 1.65 | 0.02 | |||||||||
| Smokers proportion, % | 22 | 0.50 | |||||||||
| Modifiable risk factors | Mean | SE | |||||||||
| HbA1c, % | 8.40 | 0.03 | [21, 22] | ||||||||
| TC, mg/dlb | 193.05 | 0.66 | |||||||||
| HDL-C, mg/dlb | 46.33 | 0.20 | |||||||||
| LDL-C, mg/dlb | 111.97 | 0.51 | |||||||||
| SBP, mmHg | 129 | 0.18 | |||||||||
| Weight, kgc | 68.20 | 2.42 | |||||||||
| Treatment effects of first therapyd | Metformin | Source | |||||||||
| Mean | SE | ||||||||||
| HbA1c change, % | − 1.57 | 0.22 | Meta-analysis and indirect treatment comparison | ||||||||
| TC change, mg/dlb | − 30.50 | 6.30 | |||||||||
| HDL-C change, mg/dlb | 10.04 | 2.36 | |||||||||
| SBP change, mmHg | − 0.36 | 2.26 | |||||||||
| Weight change, kge | − 7.51 | 1.09 | |||||||||
| Symptomatic hypoglycemia, %f | 1.81 | 1.04 | |||||||||
| Severe hypoglycemia, %f | 0.04 | 0.02 | |||||||||
| Gastrointestinal reactions, % | 2.15 | 1.06 | |||||||||
| Treatment effects of second therapyd | Metformin + sulfonylurea | Metformin + thiazolidinedione | Metformin + α-glucosidase inhibitor | Metformin + glinide | Metformin + DPP-4 inhibitor | Source | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| HbA1c change, % | − 2.61 | 0.18 | − 2.40 | 0.22 | − 2.41 | 0.28 | − 2.64 | 0.25 | − 2.52 | 0.22 | Meta-analysis and indirect treatment comparison |
| TC change, mg/dlb | − 6.56 | 2.36 | − 8.88 | 4.81 | − 31.66 | 12.06 | − 2.70 | 5.10 | − 24.32 | 5.46 | |
| HDL-C change, mg/dlb | − 1.16 | 3.25 | 5.41 | 5.73 | 11.58 | 5.52 | − 4.63 | 6.50 | 2.70 | 4.24 | |
| SBP change, mmHg | − 3.80 | 1.17 | − 4.64 | 3.06 | − 3.68 | 3.02 | – | – | − 6.22 | 2.52 | |
| Weight change, kge | − 0.95 | 1.21 | − 1.42 | 1.69 | − 2.70 | 1.60 | − 8.06 | 1.43 | − 4.14 | 1.36 | |
| Symptomatic hypoglycemia, %f | 4.46 | 0.57 | 1.52 | 0.98 | 0 | 1.37 | 0 | 1.19 | 0.54 | 1.06 | |
| Severe hypoglycemia, %f | 0.09 | 0.01 | 0.03 | 0.02 | 0 | 0.03 | 0 | 0.02 | 0.01 | 0.02 | |
| Gastrointestinal reactions, % | 4.62 | 0.59 | 7.62 | 1.41 | 9.62 | 1.55 | 7.62 | 1.74 | 6.62 | 1.27 | |
| Treatment effects of third therapyd | Metformin + insulin | Metformin + GLP-1 receptor agonist | Source | ||||||||
| Mean | SE | Mean | SE | ||||||||
| HbA1c change, % | − 2.51 | 0.29 | − 2.89 | 0.20 | Meta-analysis and indirect treatment comparison | ||||||
| TC change, mg/dlb | − 69.50 | 12.15 | − 55.60 | 7.88 | |||||||
| HDL-C change, mg/dlb | – | – | 0 | 5.71 | |||||||
| SBP change, mmHg | – | – | − 7.38 | 1.62 | |||||||
| Weight change, kge | − 4.22 | 2.69 | − 7.54 | 0.92 | |||||||
| Symptomatic hypoglycemia, %f | 3.47 | 0.58 | 3.47 | 0.58 | |||||||
| Severe hypoglycemia, %f | 0.07 | 0.01 | 0.07 | 0.01 | |||||||
| Gastrointestinal reactions, % | 11.78 | 2.22 | 6.78 | 0.98 | |||||||
| Costs of glucose-lowering treatments | Cost, ¥ ($)g,i | Individual drug | Cost, ¥ ($)g,h | Source | |||||||
| Metformin | 679.05 (98.30) | Metformin | 679.05 (98.30) | [23] | |||||||
| Sulfonylurea | 632.74 (91.60) | Glyburide | 2.70 (0.39) | ||||||||
| Glimepiride | 1210.95 (175.30) | ||||||||||
| Gliclazide | 414.59 (60.02) | ||||||||||
| Glipizide | 902.72 (130.68) | ||||||||||
| Thiazolidinedione | 1148.06 (166.19) | Rosiglitazone | 938.83 (135.90) | ||||||||
| Pioglitazone | 1357.28 (196.48) | ||||||||||
| α-Glucosidase inhibitor | 1815.60 (262.83) | Acarbose | 1830.98 (265.05) | ||||||||
| Voglibose | 1356.07 (196.30) | ||||||||||
| Miglitol | 2259.77 (327.12) | ||||||||||
| Glinide | 1124.13 (162.73) | Repaglinide | 1259.80 (182.37) | ||||||||
| Nateglinide | 988.46 (143.09) | ||||||||||
| DPP-4 inhibitor | 3069.95 (444.41) | Sitagliptin | 2814.34 (407.40) | ||||||||
| Saxagliptin | 3017.02 (436.74) | ||||||||||
| Vildagliptin | 3192.47 (462.14) | ||||||||||
| Linagliptin | 3137.21 (454.14) | ||||||||||
| Alogliptin | 3188.72 (461.60) | ||||||||||
| GLP-1 receptor agonist | 14,578.94 (2110.44) | Exenatide | 18,990.33 (2749.03) | ||||||||
| Liraglutide | 10,167.55 (1471.85) | ||||||||||
| Insulin cost per kg weight per day | 0.137 (0.020) | – | – | ||||||||
| Costs and utility changes associated with diabetes-related events | Fatal costs, ¥ ($)g | Non-fatal costs, ¥ ($)g | Maintenance costs, ¥ ($)g | Source | Utility change | Source | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||||
| Ischemic heart disease | 21,574.18 (3123.07) | 6359.88 (920.65) | 23,860.32 (3454.01) | 1576.61 (228.23) | 3293.02 (476.70) | 435.07 (62.98) | Claims data | − 0.028 | 0.005 | [24] | |
| Myocardial infarction | 39,463.58 (5712.74) | 8360.32 (1210.24) | 53,131.50 (7691.30) | 3774.74 (546.43) | 6543.01 (947.16) | – | − 0.028 | 0.005 | |||
| Congestive heart failure | 35,521.34 (5142.06) | 13,196.13 (1910.27) | 32,469.77 (4700.31) | 6315.33 (914.21) | 3113.24 (450.67) | 1797.6 (260.22) | − 0.028 | 0.005 | |||
| Stroke | 69,427.06 (10,050.24) | 18,792.10 (2720.34) | 25,465.96 (3686.44) | 2418.01 (350.03) | 4274.23 (618.74) | 735.06 (106.41) | − 0.101 | 0.006 | |||
| Amputation | – | – | 22,281.86 (3225.52) | – | 3542.36 (512.79) | – | − 0.118 | 0.009 | |||
| Blindness | – | – | 15,846.21 (2293.89) | 617.89 (89.45) | 5227.01 (756.66) | 484.87 (70.19) | − 0.022 | 0.005 | |||
| End-stage renal disease | 15,531.40 (2248.32) | 3715.52 (537.86) | 16,002.73 (2316.55) | 554.07 (80.21) | 5595.21 (809.96) | 543.32 (78.65) | − 0.058 | 0.006 | |||
| Symptomatic hypoglycemia | – | 0 | – | Assumed | − 0.007 | 0.002 | |||||
| Severe hypoglycemia | – | 4116.10 (595.85) | – | [25] | − 0.008 | 0.004 | |||||
| Gastrointestinal reactions | – | 0 | – | Assumed | − 0.034 | 0 | [26] | ||||
| BMI per unit increase | – | – | – | − 0.0061 | 0.001 | [27] | |||||
| BMI per unit decrease | – | – | – | + 0.0061 | 0.001 | ||||||
| BMI-related costs | Costs, ¥ ($)g | BMI | Costs, ¥ ($)g | BMI | Costs, ¥ ($)g | Source | |||||
| ≤ 23 | 0 | 29 | 14,230.2 (2059.96) | 35 | 29,252.9 (4234.64) | [28] | |||||
| 24 | 1711.3 (247.73) | 30 | 16,734.0 (2422.41) | 36 | 31,756.7 (4597.09) | ||||||
| 25 | 4215.1 (610.18) | 31 | 19,237.8 (2784.86) | 37 | 34,260.5 (4959.54) | ||||||
| 26 | 6718.9 (972.63) | 32 | 21,741.6 (3147.31) | 38 | 36,764.3 (5321.99) | ||||||
| 27 | 9222.7 (1335.08) | 33 | 24,245.4 (3509.76) | 39 | 39,268.0 (5684.42) | ||||||
| 28 | 11,726.4 (1697.51) | 34 | 26,749.1 (3872.19) | ≥ 40 | 41,771.8 (6046.87) | ||||||
Sulfonylurea includes glyburide, glimepiride, gliclazide, and glipizide. Thiazolidinedione includes rosiglitazone and pioglitazone. α-Glucosidase inhibitor includes acarbose, voglibose, and miglitol. Glinide includes repaglinide and nateglinide. DPP-4 inhibitor includes sitagliptin, saxagliptin, vildagliptin, linagliptin, and alogliptin. Insulin includes various kinds of insulin and insulin analogs. GLP-1 receptor agonist includes exenatide and liraglutide
BMI body mass index, DPP-4 dipeptidyl peptidase 4, GLP-1 glucagon-like peptide 1, HbA1c glycosylated hemoglobin Alc, HDL-C high-density lipoprotein-cholesterol, LDL-C low-density lipoprotein-cholesterol, RCT randomized controlled trial, SBP systolic blood pressure, SE standard error, TC total cholesterol
aAs only BMI and weight of the patients were reported, height was calculated by: sqrt (weight/BMI)
bThe unit of cholesterol in the included 324 RCTs was mmol/l, while the Cardiff model requires mg/dl, which was converted by: 1 mg/dl = 0.0259 mmol/l [29]
cThe “criteria of weight for adults” published by the National Health Commission of China defines weight categories as underweight (BMI < 18.5 kg/m2), normal weight (18.5 ≤ BMI < 24 kg/m2), overweight (24 ≤ BMI < 28 kg/m2), and obese (BMI ≥ 28 kg/m2) [30]. As the BMI of patients in our initial cohort was 25 kg/m2, they were overweight
dThe treatment effect of each glucose-lowering treatment was with or without a background of lifestyle interventions
eOnly BMI change was reported in the 324 RCTs, while the Cardiff model requires weight change; thus, it was calculated by: weight = BMI × height2
fHypoglycemia is differentiated as symptomatic and severe ones in the Cardiff model, but most of the 324 RCTs did not clearly differentiate between symptomatic and severe episodes; thus, we estimated that a rate of 2% represented the proportion of severe cases out of all hypoglycemia events [25]
gFor the costs, data are 2019 Chinese yuan, ¥ (2019 US dollar, $). One US dollar was equal to ¥6.908 in 2019 [31]
hAnnual pharmacy cost of a drug was calculated as its retail price times its annual dose. The retail price was sourced from government medicine purchase platform, and the drug dose was obtained from the 324 RCTs. One year was counted as 365 days. For individual drugs, only the drugs reported in the 324 RCTs were included
iAnnual pharmacy costs of each drug class were calculated based on a simple arithmetic average of the costs of individual drugs
Treatment strategy
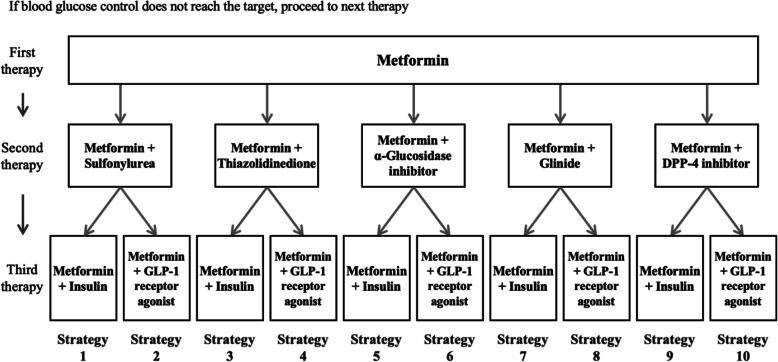
A treatment strategy was defined to consist of a three-stepwise escalation treatment path in this study based on the Chinese clinical guideline [2] and the setting of Cardiff diabetes model. Ten pharmacologic combination strategies were identified based on the Chinese clinical guideline and clinical practice [2]. Patients started the simulation by receiving metformin monotherapy (first step). When patients’ glucose control did not reach the target of HbA1c level < 8%, the first therapy escalation occurred: they switched to an oral dual therapy of one of the five classes of oral drugs (i.e., sulfonylurea, thiazolidinedione, α-glucosidase inhibitor, glinide, or DPP-4 inhibitor) added onto metformin (second step). When the HbA1c level continues to exceed 9%, the second therapy escalation commenced: patients switched to another dual therapy of one injection (i.e., insulin or GLP-1 receptor agonist) added onto metformin, and they stayed on this treatment for the remaining time-horizon or until death (third step). The strategies were labeled as strategy 1 to strategy 10 (Fig. 2). This study assumed that all patients persisted in the allocated three-stepwise strategy for the whole time-horizon, and the criterion for therapy switch was therapy escalation when patient’s HbA1c crosses the specified thresholds. The HbA1c thresholds for therapy escalation were set based on consultation with the doctors. The analyses were conducted based on the whole class of drugs (e.g., sulfonylurea) rather than individual drugs (e.g., glyburide). Sulfonylurea includes glyburide, glimepiride, gliclazide, glipizide, and gliquidone. Thiazolidinedione includes rosiglitazone and pioglitazone. α-Glucosidase inhibitor includes acarbose, voglibose, and miglitol. Glinide includes repaglinide, nateglinide, and mitiglinide. DPP-4 inhibitor includes sitagliptin, saxagliptin, vildagliptin, linagliptin, and alogliptin. Insulin includes various kinds of insulin and insulin analogs. GLP-1 receptor agonist includes exenatide and liraglutide [2].
Fig. 2.
Treatment algorithm for blood glucose control in type 2 diabetes and treatment paths of the 10 pharmacologic combination strategies. Sulfonylurea includes glyburide, glimepiride, gliclazide, glipizide, and gliquidone. Thiazolidinedione includes rosiglitazone and pioglitazone. α-Glucosidase inhibitor includes acarbose, voglibose, and miglitol. Glinide includes repaglinide, nateglinide, and mitiglinide. DPP-4 inhibitor includes sitagliptin, saxagliptin, vildagliptin, linagliptin, and alogliptin. Insulin includes various kinds of insulin and insulin analogs. GLP-1 receptor agonist includes exenatide and liraglutide
Treatment effects
There were no head-to-head studies that directly compared all the glucose-lowering treatments against each other. Thus, treatment effects of the glucose-lowering treatments were sourced from a systematic review and estimated by meta-analysis and indirect treatment comparison (Table 1). Systematic review was conducted by searching Chinese databases (China National Knowledge Infrastructure, WanFang Data, Chongqing VIP) and English databases (PubMed, Embase, Web of Science, ScienceDirect, Cochrane Library). Randomized controlled trials (RCTs) (≥ 12 weeks) published during 1990 to 2016, which estimated the treatment effect of “a glucose-lowering drug added onto metformin vs. metformin” or “metformin vs. placebo/lifestyle intervention” in Chinese T2DM patients (≥ 18 years) with and without a background of lifestyle interventions, were identified. Detailed eligibility criteria were shown in Additional file 1: Table S1. Search terms were type 2 diabetes and the targeted glucose-lowering drugs (Additional file 1: Table S2). The flow diagram of study selection was shown in Additional file 1: Fig. S1. Finally, 324 RCTs were identified [32–355]. Baseline characteristics and risk of bias of these RCTs were shown in Additional file 1: Table S3 and Fig. S2. A series of a priori random-effects meta-analyses based on the assumption of substantial variability in treatment effect size across studies [356–358] and then adjusted indirect treatment comparisons based on the Bucher method [359–361] were conducted to synthesize the absolute treatment effects of glucose-lowering treatments with and without a background of lifestyle interventions from the 324 RCTs. The framework of meta-analysis and indirect treatment comparison was shown in Additional file 1: Table S4. Meta-analysis was done in Stata/SE 15.1, and indirect treatment comparison was done in the indirect treatment comparison calculator developed by the Canadian Agency for Drugs and Technologies in Health [362, 363].
Costs
Direct medical costs for treating T2DM and its related events were estimated. All costs were expressed in both 2019 Chinese yuan (¥) and US dollar ($). One US dollar was equal to ¥6.908 in 2019 [31].
Annual pharmacy costs of each drug class were calculated based on a simple arithmetic average of the costs of individual drugs. The cost of a drug was calculated as its retail price times its annual dose. The retail price was sourced from government medicine purchase platform [23], and the drug dose was obtained from the 324 RCTs (Table 1). A total of 19 drugs were searched in the platform, and 168 products were retrieved as a drug would have multiple products in different specifications, dose forms, or manufacturers (Additional file 1: Table S5). Insulin cost per kilogram weight per day was assumed to be ¥0.137 ($0.020) based on the inherent profile of Cardiff model.
Treatment costs of diabetes-related complications were collected from Jinan municipal claims database. The database was linked with the hospital information system of Shandong Provincial Hospital, and the claims data of adult T2DM patients who were hospitalized and had regular follow-ups for their complications at the hospital between 2013 and 2016 were extracted. The complications include ischemic heart disease, myocardial infarction, congestive heart failure, stroke, blindness, end-stage renal disease, and amputation. Two medical doctors confirmed the patients’ diagnoses of complications by checking their medical records. A total of 1164 T2DM patients with complications were retrieved. The costs associated with complications were split into fatal, non-fatal, and maintenance costs. Cost for severe hypoglycemia was sourced from an observational study in China [25]. Because symptomatic hypoglycemia and gastrointestinal reactions are usually not treated with medication and relevant published evidence was not available, their costs were assumed to be 0. BMI-related costs indicating increased prescribing costs per unit increase in BMI values were estimated from an observational study in China [28] (Table 1).
Utilities
Utility changes associated with diabetes-related events were estimated mainly based on an EQ-5D study, which included 7081 T2DM patients who were enrolled from 75 hospitals in nine cities in China and investigated the utility values of T2DM with and without complications or comorbidities [24]. Utility changes associated with gastrointestinal reactions and BMI-related changes were not reported in this study and thus were retrieved from other sources [26, 27] (Table 1).
Sensitivity analysis
The impact of uncertainty around model inputs was assessed by a series of univariate and probabilistic sensitivity analyses. Univariate sensitivity analyses were carried out by changing discount rate, simulation time, HbA1c thresholds for therapy escalations, risk equations, and BMI-related utilities. Besides, we also conducted a series of scenario analyses to estimate the cost-effectiveness of the 10 pharmacologic combination strategies after removing the effect of nonpharmacologic treatments. Nonpharmacologic treatments were defined as receiving lifestyle interventions and/or placebo, rather than glucose-lowering drugs. Treatment effects of the glucose-lowering treatments and nonpharmacologic treatments were also abstracted from the 324 RCTs by using the method of meta-analysis and indirect treatment comparison (Additional file 1: Table S6). The costs of the glucose-lowering treatments were the same as in the base-case analysis, and those of nonpharmacologic treatments were assumed to be 0.
Results
Base-case results
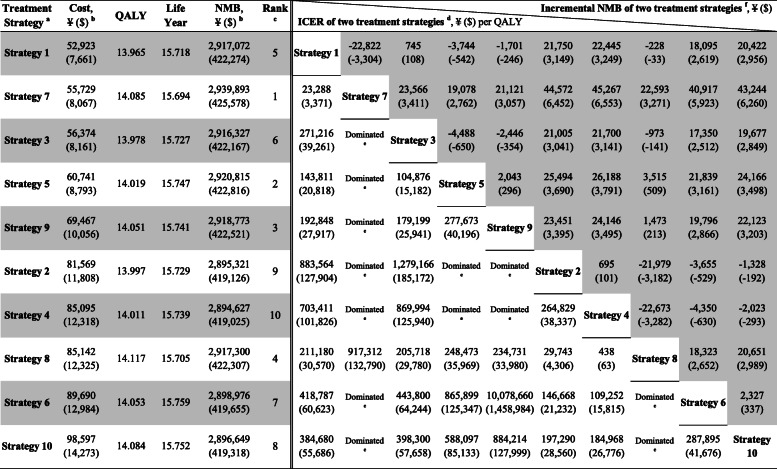
All the 10 pharmacologic combination strategies showed positive effects in controlling HbA1c, cholesterol, weight, and blood pressure levels for Chinese T2DM patients (Additional file 1: Fig. S3-S6). Total costs accumulated over the lifetime ranged from ¥52,923 ($7661) to ¥98,597 ($14,273) for an individual patient under different strategies, the minimum being with strategy 1, resulting in a cost of ¥52,923 ($7661), followed by strategy 7 (¥55,729/$8067) and strategy 3 (¥56,374/ $8161), while strategy 10 resulted in the maximum costs. In terms of health benefits, overall discounted QALYs with different strategies ranged from 13.965 to 14.117 for a patient across his lifetime. Strategy 8 gained the highest QALYs of 14.117, followed by strategy 7 (14.085) and strategy 10 (14.084), while strategy 1 gained the lowest QALYs. In general, strategy 1 was associated with the lowest costs and lowest QALY gains when compared with other nine strategies, whereas strategy 10 resulted in the highest costs but incrementally less QALY gains when compared with strategy 7 and strategy 8 (Table 2, Fig. 3).
Table 2.
Base-case results: cost-effectiveness of 10 pharmacologic combination strategies and strategy ranking (per patient)
Strategy 1: metformin → metformin + sulfonylurea → metformin + insulin. Strategy 2: metformin → metformin + sulfonylurea → metformin + GLP-1 receptor agonist. Strategy 3: metformin → metformin + thiazolidinedione → metformin + insulin. Strategy 4: metformin → metformin + thiazolidinedione → metformin + GLP-1 receptor agonist. Strategy 5: metformin → metformin + α-glucosidase inhibitor → metformin + insulin. Strategy 6: metformin → metformin + α-glucosidase inhibitor → metformin + GLP-1 receptor agonist. Strategy 7: metformin → metformin + glinide → metformin + insulin. Strategy 8: metformin → metformin + glinide → metformin + GLP-1 receptor agonist. Strategy 9: metformin → metformin + DPP-4 inhibitor → metformin + insulin. Strategy 10: metformin → metformin + DPP-4 inhibitor → metformin + GLP-1 receptor agonist
DPP-4 dipeptidyl peptidase 4, GLP-1 glucagon-like peptide 1, ICER incremental cost-effectiveness ratio, NMB net monetary benefit, QALY quality-adjusted life-year
aThe treatment strategies were presented in the order of ascending costs in this table. Detailed results of the 10 pharmacologic combination strategies were shown in Additional file 1: Table S8
bFor the costs, data are 2019 Chinese yuan, ¥ (2019 US dollar, $). One US dollar was equal to ¥6.908 in 2019 [31]
cThe strategies were ranked based on both the ICERs and the NMBs. The strategy ranking process based on the ICERs was presented in Additional file 1: Table S7. The larger the NMB value, the more cost-effective the strategy is. The ranking results based on the ICERs were equal to that based on the NMBs
dResults of the ICER of two treatment strategies are presented in the triangle area in the lower left corner of the table. The data indicate row-to-column differences; for example, the ICER of strategy 7 vs. strategy 1 is ¥23,288 ($3371)/QALY
e“Dominated” indicates a strategy (row) that is more costly and less effective than its comparator (column); for example, strategy 3 is dominated by strategy 7
fResults of the incremental NMB of two treatment strategies are presented in the triangle area in the upper right corner of the table (gray area). The data indicate row-to-column differences; for example, the incremental NMB of strategy 1 vs. strategy 7 is − ¥22,822 (− $3304)
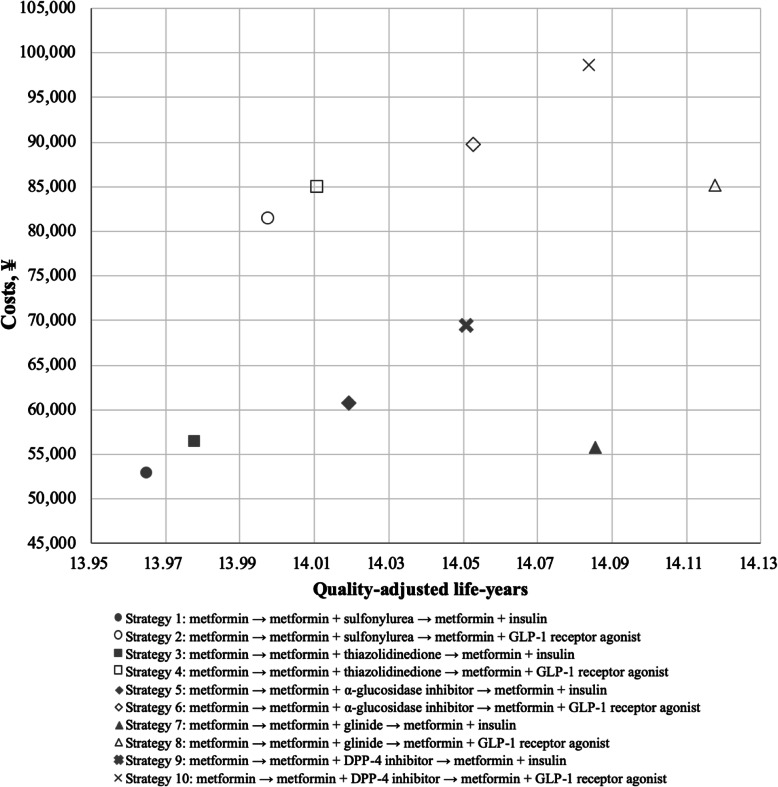
Fig. 3.
Scatter plot of incremental cost-effectiveness ratios for 10 pharmacologic combination strategies. DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1
Overall, strategy 7 was dominant over seven strategies (strategies 2~6, 9~10) with higher QALYs but lower costs, reporting ICERs of − ¥24,215,636 (− $3,505,448)/QALY to − ¥5983 (− $866)/QALY. Because the ICERs were ¥23,288 ($3371)/QALY when moving from strategy 1 to strategy 7 and ¥917,312 ($132,790)/QALY when moving from strategy 7 to strategy 8, strategy 7 was cost-effective compared with strategy 1 and strategy 8 at a willingness-to-pay threshold of ¥212,676 ($30,787)/QALY (Table 2). Ranking the strategies based on the ICERs, strategy 7 provided the best value for money when compared to all other strategies evaluated, followed by strategy 5, strategy 9, strategy 8, strategy 1, strategy 3, strategy 6, strategy 10, strategy 2, and strategy 4 (Additional file 1: Table S7). The results based on the NMBs confirmed the above rankings. Strategy 7 would generate additional ¥19,078 ($2762) in NMB compared to the next best alternative (strategy 5), and ¥45,267 ($6553) in NMB compared to the least cost-effective scenario (strategy 4) (Table 2).
Sensitivity analysis results
Across all the sensitivity analyses, strategy 7 remained the most cost-effective strategy when compared to other strategies. HbA1c thresholds for therapy escalations and risk equations were influential variables for the results. In the univariate sensitivity analyses, either when the discount rate used was 3.5% [364] or when the simulation time used was 30 years, all the strategies were associated with reductions in the costs (the former, 6.7~8.6%; the latter, 4.8~10%) and the QALY gains (the former, 4.8~4.9%; the latter, 2.5~2.6%) compared with that in base case. All the strategies remained base-case ranks in the former scenario, and only the ranks of strategy 8 and 9 swapped in the latter scenario. Besides, either when applying HbA1c thresholds of 7% and 8% for second- and third-line therapy escalation or when using UKPDS 82 risk equations, all the strategies increased the QALYs (the former, 1~2%; the latter, 2~4.1%), yet also increased the costs (the former, 14.6~87.1%; the latter, 0.6~11.9%) compared with that in base case. Overall, three strategies (strategies 2, 4, 7) in the former scenario and two strategies (strategies 7, 9) in the latter scenario remained base-case ranks. When alternative BMI-related utilities were used (i.e., utility impact was 0.017 for per unit decrease in BMI and − 0.047 for per unit increase in BMI) [365], the strategies had an increase of 3.4 to 5.5% in the QALYs compared to that in base case, and four strategies (strategies 3, 6, 7, 9) kept base-case ranks. In the probabilistic sensitivity analyses, compared with that in base case, all strategies had a slight decrease of 0.4 to 0.5% in the QALYs, among which four strategies had an increase of 0.6 to 1.3% and six strategies had a decrease of 0.1 to 1% in the costs. All the strategies remained base-case ranks, which verified the base-case findings (Table 3).
Table 3.
Sensitivity analysis results: cost-effectiveness of 10 pharmacologic combination strategies and strategy ranking (per patient)
| Scenario | Item | Strategy 1 | Strategy 2 | Strategy 3 | Strategy 4 | Strategy 5 | Strategy 6 | Strategy 7 | Strategy 8 | Strategy 9 | Strategy 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Discount rate (costs and benefits) 3.5% | Cost, ¥ ($)a | 49,164 (7117) | 74,539 (10,790) | 52,418 (7588) | 77,860 (11,271) | 56,539 (8185) | 82,183 (11,897) | 51,865 (7508) | 77,920 (11,280) | 64,782 (9378) | 90,586 (13,113) |
| QALY | 13.289 | 13.317 | 13.300 | 13.330 | 13.339 | 13.368 | 13.401 | 13.429 | 13.368 | 13.397 | |
| Rankb | 5 | 9 | 6 | 10 | 2 | 7 | 1 | 4 | 3 | 8 | |
| Simulation time 30 years | Cost, ¥ ($)a | 49,520 (7169) | 73,411 (10,627) | 52,981 (7669) | 76,934 (11,137) | 57,369 (8305) | 81,512 (11,800) | 52,475 (7596) | 77,009 (11,148) | 66,122 (9572) | 90,417 (13,089) |
| QALY | 13.615 | 13.639 | 13.627 | 13.652 | 13.666 | 13.692 | 13.730 | 13.755 | 13.696 | 13.721 | |
| Rankb | 5 | 9 | 6 | 10 | 2 | 7 | 1 | 3 | 4 | 8 | |
| HbA1c thresholds of 7% and 8% for two therapy escalations | Cost, ¥ ($)a | 65,349 (9460) | 152,597 (22,090) | 68,402 (9902) | 155,817 (22,556) | 72,036 (10,428) | 160,022 (23,165) | 66,064 (9563) | 155,819 (22,556) | 79,592 (11,522) | 168,147 (24,341) |
| QALY | 14.110 | 14.202 | 14.120 | 14.214 | 14.168 | 14.263 | 14.307 | 14.399 | 14.219 | 14.313 | |
| Rankb | 4 | 9 | 5 | 10 | 3 | 8 | 1 | 6 | 2 | 7 | |
| Use UKPDS 82 risk equations to run model | Cost, ¥ ($)a | 57,572 (8334) | 91,305 (13,217) | 61,250 (8867) | 95,044 (13,759) | 65,866 (9535) | 99,854 (14,455) | 58,536 (8474) | 85,614 (12,393) | 74,606 (10,800) | 108,863 (15,759) |
| QALY | 14.529 | 14.567 | 14.532 | 14.570 | 14.557 | 14.595 | 14.587 | 14.397 | 14.606 | 14.643 | |
| Rankb | 2 | 6 | 5 | 9 | 4 | 8 | 1 | 10 | 3 | 7 | |
| Utility impact is + 0.017 and − 0.047 for per unit decrease and increase in BMI [365] | Cost, ¥ ($)a | 52,923 (7661) | 81,569 (11,808) | 56,374 (8161) | 85,095 (12,318) | 60,741 (8793) | 89,690 (12,984) | 55,729 (8067) | 85,142 (12,325) | 69,467 (10,056) | 98,597 (14,273) |
| QALY | 14.446 | 14.510 | 14.476 | 14.541 | 14.566 | 14.632 | 14.830 | 14.894 | 14.651 | 14.717 | |
| Rankb | 8 | 10 | 6 | 9 | 4 | 7 | 1 | 2 | 3 | 5 | |
| Probabilistic sensitivity analysis | Cost, ¥ ($)a | 52,563 (7609) | 82,077 (11,881) | 56,194 (8135) | 86,234 (12,483) | 60,440 (8749) | 90,647 (13,122) | 55,150 (7983) | 85,090 (12,318) | 69,411 (10,048) | 99,640 (14,424) |
| QALY | 13.897 | 13.930 | 13.911 | 13.944 | 13.953 | 13.988 | 14.022 | 14.056 | 13.986 | 14.019 | |
| Rankb | 5 | 9 | 6 | 10 | 2 | 7 | 1 | 4 | 3 | 8 | |
| Scenario analysisc | Δ Cost, ¥ ($)a | − 14,581 (− 2111) | 6277 (909) | 9495 (1375) | 31,801 (4604) | 6607 (956) | 26,841 (3885) | − 14,805 (− 2143) | 6542 (947) | 25,963 (3758) | 49,142 (7114) |
| Δ QALY | 0.469 | 0.515 | 0.456 | 0.509 | 0.480 | 0.532 | 0.608 | 0.653 | 0.525 | 0.576 | |
| ICER, ¥ ($)/QALY | Dominantd | 12,189 (1765) | 20,836 (3016) | 62,473 (9044) | 13,754 (1991) | 50,417 (7298) | Dominantd | 10,018 (1450) | 49,490 (7164) | 85,380 (12,360) |
Strategy 1: metformin → metformin + sulfonylurea → metformin + insulin. Strategy 2: metformin → metformin + sulfonylurea → metformin + GLP-1 receptor agonist. Strategy 3: metformin → metformin + thiazolidinedione → metformin + insulin. Strategy 4: metformin → metformin + thiazolidinedione → metformin + GLP-1 receptor agonist. Strategy 5: metformin → metformin + α-glucosidase inhibitor → metformin + insulin. Strategy 6: metformin → metformin + α-glucosidase inhibitor → metformin + GLP-1 receptor agonist. Strategy 7: metformin → metformin + glinide → metformin + insulin. Strategy 8: metformin → metformin + glinide → metformin + GLP-1 receptor agonist. Strategy 9: metformin → metformin + DPP-4 inhibitor → metformin + insulin. Strategy 10: metformin → metformin + DPP-4 inhibitor → metformin + GLP-1 receptor agonist
BMI body mass index, DPP-4 dipeptidyl peptidase 4, GLP-1 glucagon-like peptide 1, HbA1c glycosylated hemoglobin Alc, ICER incremental cost-effectiveness ratio, NMB net monetary benefit, QALY quality-adjusted life-year
aFor the costs, data are 2019 Chinese yuan, ¥ (2019 US dollar, $). One US dollar was equal to ¥6.908 in 2019 [31]
bThe strategies were ranked based on both the ICERs and the NMBs, as in the base-case analysis. The ICER is calculated as incremental costs divided by incremental QALYs of two strategies. The NMB is calculated as QALYs times willingness-to-pay/QALY, minus costs of each strategy. The larger the NMB value, the more cost-effective the strategy is. The ranking results based on the ICERs were equal to that based on the NMBs
cIn the scenario analysis, the strategies were compared with nonpharmacologic treatment (only receiving lifestyle interventions and/or placebo, rather than glucose-lowering drugs)
d“Dominant” indicates a strategy that is less costly and more effective than nonpharmacologic treatment; for example, strategy 7 is dominant over nonpharmacologic treatment
In the scenario analyses, compared with nonpharmacologic treatments, all the strategies predicted fewer incidences of diabetes-related complications and mortality, thus resulting in a mean incremental QALY of 0.532 for a patient over 40 years, by a range of 0.456 QALYs with strategy 3 to 0.653 QALYs with strategy 8. Two strategies reduced costs by ¥14,581 ($2111) with strategy 1 and ¥14,805 ($2143) with strategy 7, and eight strategies increased costs by ¥6277 ($909) with strategy 2 to ¥49,142 ($7114) with strategy 10. Consequently, two strategies (strategies 1, 7) were cost-saving with higher QALY gains but lower costs, and eight strategies were cost-effective with an ICER of ¥10,018 ($1450)/QALY for strategy 8 to ¥85,380 ($12,360)/QALY for strategy 10, when compared to nonpharmacologic treatments (Table 3).
Discussion
There are multiple glucose-lowering treatments in China; however, their effectiveness and especially the costs vary from each other. Choosing high efficacious, safe, and affordable treatments is the key for most T2DM patients to insist on long-term medication. This was the first study to estimate and compare the lifetime cost-effectiveness of 10 commonly used pharmacologic combination strategies for T2DM patients in China.
Results showed that based on current clinical practice, strategy 7—involving the sequential addition of glinide and insulin to first-line metformin therapy—was the most cost-effective strategy, while strategy 4—involving the sequential addition of thiazolidinedione and GLP-1 receptor agonist to first-line metformin therapy—was the least cost-effective strategy when compared with all other strategies evaluated. All five strategies that use metformin plus GLP-1 receptor agonist as third line were associated with higher costs compared to those that use metformin plus insulin as third line. When keeping the second-line therapy the same, a strategy using metformin plus GLP-1 receptor agonist as third line resulted in both higher QALYs and higher costs compared with a strategy using metformin plus insulin. When keeping the third-line therapy the same, addition of glinide to metformin as second line was associated with the highest QALY gains, followed by addition of DPP-4 inhibitor, α-glucosidase inhibitor, thiazolidinedione, and sulfonylurea; conversely, addition of sulfonylurea to metformin resulted in the lowest costs, followed by addition of glinide or thiazolidinedione, and DPP-4 inhibitor was related to the highest costs. Previous study reported that the most commonly used dual therapy was metformin plus sulfonylureas, followed by metformin plus glinides in a real-world setting in China [3]. Our findings that metformin plus sulfonylureas costs less than other dual therapies may somewhat account for this phenomenon. However, if patients want more health benefits, it is of value to try metformin plus glinide.
Sensitivity analyses somewhat confirmed the base-case results. All the strategies remained base-case ranks in the probabilistic sensitivity analyses and in the univariate sensitivity analyses where the discount rate used was 3.5%. Although the ranks of some strategies changed in other sensitivity analyses, strategy 7 remained the most cost-effective strategy when compared to other strategies. This implied that the ranking results should be used cautiously to avoid misleading conclusions instead of denying their actual value for decision-makers. Besides, prescribers should additionally take into consideration patients’ individual needs, preferences, and values, because a patient-centered approach is recommended in choosing pharmacologic treatments for T2DM [366, 367]. Whatever, our results may have a practical importance, as healthcare services are mainly provided by public medical institutions, and value-based price negotiations are increasingly used for reimbursement approvals in China. Because there are many patients not aware of the importance of persistent pharmacologic treatments for T2DM in China [368], we additionally quantified the long-term impact of pharmacologic treatments on disease burden of T2DM after removing the effect of nonpharmacologic treatments. A patient persisted in pharmacologic combination treatments over 40 years was projected to obtain an incremental benefit of 0.532 QALYs at an acceptable range of cost increase or even at cost saving, compared with those who did not receive pharmacologic treatments.
In the past decade, several studies in China have introduced economic simulation models to predict and compare the long-term costs and benefits of several glucose-lowering therapies for T2DM. Palmer et al., Wu et al., Li et al., and Chen et al. evaluated the cost-effectiveness of different types of insulins using CORE diabetes model [369–373]. Gu et al. and Shao et al. estimated the cost-effectiveness of saxagliptin (or dapagliflozin) vs. acarbose (or glimepiride), and exenatide vs. insulin glargine using the Cardiff diabetes model [374–378]. Zhu and Chen assessed the cost-effectiveness of vildagliptin vs. pioglitazone vs. glimepiride, and sitagliptin vs. glimepiride vs. acarbose as add-on therapy to metformin using UKPDS model [379, 380]. However, all previous studies focused on comparing several individual drugs as monotherapy or add-on therapy at a specific disease stage and keeping therapies at other disease stages the same. Besides, they all used utility data from foreign populations to calculate QALYs. Unlike them, our study first included almost all individual drugs available in China to achieve comparisons between different drug classes; second, we targeted at holistic treatment strategies over lifelong disease stages; third, we used utilities of Chinese patients for the first time. Therefore, our study was poorly comparable with previous studies. Only one study was found to compare the short-term cost-effectiveness of six classes of oral glucose-lowering drugs in China, based on daily pharmacy costs and blood glucose reductions. Result showed that thiazolidinedione was most cost-effective in reducing fasting and 2 h postprandial blood glucose, followed by biguanide, glinide, sulfonylurea, α-glucosidase inhibitor, and Chinese traditional medicine [7]. However, this study was also not comparable with our study.
There were several limitations in this study. First, systematic review and meta-analysis have some inherent methodological challenges, such as low study quality, potential publication bias, and unexplainable heterogeneity, that might affect the quality of clinical evidence. Besides, because there was no direct clinical evidence for comparing different dual therapies against each other, indirect treatment comparison was used, which might introduce more uncertainties around synthesized estimates compared with a direct head-to-head estimate. Second, since there are no published risk equations based on the Chinese population, the UKPDS risk equations were used in our study. However, our study population and the UKPDS population somewhat differed. On the one hand, the UKPDS population consists of 82% white, 10% Asian Indian, and 8% Afro-Caribbean patients with newly diagnosed T2DM [13, 381], while our population were Chinese patients with newly diagnosed T2DM. On the other hand, the baseline risk factors, such as HbA1c level (8.4% in our population vs. 7.1% in UKPDS population), between the two populations were not fully identical. Thus, it is uncertain whether the UKPDS risk equations may fit the Chinese patients very well. Future studies should pay attention to this issue. Third, our analyses were conducted based on the whole class of drugs. However, not all drugs within a class have the same efficacy, especially individual insulins and GLP-1 receptor agonists. Thus, the efficacy used in our study might be biased, depending on which individual drugs and RCTs have been included in the meta-analysis. Besides, patients are prescribed an individual drug rather than an average drug within a class in the real world, which might limit the generalizability of our results. Fourth, because the Cardiff model is not able to model more than three escalation steps, treatment strategies in our study are only composed of a three-stepwise treatment path, so that other relevant and longer treatment paths were not evaluated which may alter the findings of the study. For the same reason, although the use of insulins is stepwise (e.g., usually basal insulin is initiated first, followed by bolus insulin), we simply included all types of insulins into one insulin class. Fifth, evidence showed that insufficient efficacy was the main reason for treatment alteration in China, followed by adverse reaction [3]. Our study assumed that therapy escalation occurs when patient’s HbA1c fails to be controlled. However, as there is a lack of China-specific evidence on treatment alteration induced by adverse reaction of every study therapy, we did not include this in our analysis. Sixth, patients starting injectable therapy in the model are at a much later stage of the disease, while the patients in the RCTs informing this treatment step might be at an earlier stage of the disease; thus, they are likely at a different risk level. Seventh, since there is a lack of China-specific disutility values for blindness and end-stage renal disease, we conservatively used that of retinopathy and nephropathy as alternatives. This may lead to an underestimation of both complications on health. Lastly, because sodium-glucose cotransporter 2 inhibitors were not included in the Chinese clinical guideline at the time of our study, we did not include this drug class.
Conclusions
This study provided comprehensive evidence on costs and benefits of 10 commonly used pharmacologic combination strategies for patients, clinicians, and healthcare decision-makers within a unified hierarchy. This could improve their understanding of the cost-effectiveness of the existing treatment strategies in China, and help them choose high cost-effective treatments based on individual patient’s preference and need in clinical practice. In general, it is found that a strategy starting with metformin, then transitioning to metformin plus glinide as second line and finally using metformin plus insulin as third line, provided the best value for money when compared to all other strategies evaluated. Besides, this study highlighted the importance of promoting timely, rational, and sustained pharmacologic treatments in reducing patients’ disease burden. Our findings can be used to improve clinical guidelines, design the essential drug formulary, infer clinical practice, and help the decision-maker to design reimbursement policy.
Supplementary Information
Additional file 1: Table S1. Eligibility criteria. Table S2. Full electronic search strategy for PubMed. Table S3. Baseline characteristics of studies included in the meta-analysis and indirect treatment comparison. Table S4. Framework for estimation of absolute treatment effects of glucose-lowering treatments based on meta-analysis and indirect treatment comparison. Table S5. Retail prices of the glucose-lowering drugs collected from government medicine purchase platform. Table S6. Treatment effects of glucose-lowering treatments and nonpharmacologic treatment in the scenario analyses. Table S7. Base-case results: cost-effectiveness of ten pharmacologic combination strategies and strategy ranking process based on the ICERs (per patient). Table S8. Detailed results for the ten pharmacologic combination strategies: base-case analysis. Fig. S1. PRISMA flow diagram of study selection. Fig. S2. Risk of bias graph. Fig. S3. The trajectories of HbA1c in ten pharmacologic combination strategies over time: base-case analysis. Fig. S4. The trajectories of cholesterol in ten pharmacologic combination strategies over time: base-case analysis. Fig. S5. The trajectories of weight in ten pharmacologic combination strategies over time: base-case analysis. Fig. S6. The trajectories of SBP in ten pharmacologic combination strategies over time: base-case analysis.
Acknowledgements
Not applicable.
Abbreviations
- BMI
Body mass index
- DPP-4
Dipeptidyl peptidase 4
- GLP-1
Glucagon-like peptide 1
- HbA1c
Glycosylated hemoglobin Alc
- HDL-C
High-density lipoprotein-cholesterol
- ICER
Incremental cost-effectiveness ratio
- LDL-C
Low-density lipoprotein-cholesterol
- NMB
Net monetary benefit
- QALY
Quality-adjusted life-year
- SBP
Systolic blood pressure
- SE
Standard error
- TC
Total cholesterol
- T2DM
Type 2 diabetes
- UKPDS
United Kingdom Prospective Diabetes Studies
Authors’ contributions
SG contributed to the study design, data collection, statistical analysis, model analysis, data interpretation, and drafting and review of the manuscript. HD contributed to the study design, model analysis, data interpretation, and review of the manuscript. LS and HS contributed to the study design, data interpretation, and review of the manuscript. XW, XH, and YG contributed to data collection and statistical analysis. SG and HD are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors read and approved the final manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Diabetes Federation . IDF diabetes atlas ninth edition. 2019. [Google Scholar]
- 2.Weng J, Ji L, Jia W, Lu J, Zhou Z, Zou D, et al. Standards of care for type 2 diabetes in China. Diab Metab Res Rev. 2016;32:442–458. doi: 10.1002/dmrr.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji L, Lu J, Weng J, Jia W, Tian H, Zhu D, Xing X, Guo L. China type 2 diabetes treatment status survey of treatment pattern of oral drugs users. J Diabetes. 2015;7:166–173. doi: 10.1111/1753-0407.12165. [DOI] [PubMed] [Google Scholar]
- 4.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27:2800–2805. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo YF, Raji MA, Markides KS, Ray LA, Espino DV, Goodwin JS. Inconsistent use of diabetes medications, diabetes complications, and mortality in older Mexican Americans over a 7-year period: data from the Hispanic established population for the epidemiologic study of the elderly. Diabetes Care. 2003;26:3054–3060. doi: 10.2337/diacare.26.11.3054. [DOI] [PubMed] [Google Scholar]
- 6.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 7.Hao G. Cost-effectiveness of oral hypoglycemic drugs in the treatment of type 2 diabetes based on Bayesian mixed treatment comparison. Beijing: Beijing University of Chinese Medicine; 2014. [Google Scholar]
- 8.McEwan P, Peters JR, Bergenheim K, Currie CJ. Evaluation of the costs and outcomes from changes in risk factors in type 2 diabetes using the Cardiff stochastic simulation cost-utility model (DiabForecaster) Curr Med Res Opin. 2006;22:121–129. doi: 10.1185/030079906X80350. [DOI] [PubMed] [Google Scholar]
- 9.McEwan P, Evans M, Bergenheim K. A population model evaluating the costs and benefits associated with different oral treatment strategies in people with type 2 diabetes. Diab Obes Metab. 2010;12:623–630. doi: 10.1111/j.1463-1326.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 10.The Mount Hood 4 Modeling Group Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diab Care. 2007;30:1638–1646. doi: 10.2337/dc07-9919. [DOI] [PubMed] [Google Scholar]
- 11.McEwan P, Evans M, Kan H, Bergenheim K. Understanding the inter-relationship between improved glycaemic control, hypoglycaemia and weight change within a long-term economic model. Diab Obe Metab. 2010;12:431–436. doi: 10.1111/j.1463-1326.2009.01184.x. [DOI] [PubMed] [Google Scholar]
- 12.McEwan P, Bergenheim K, Yuan Y, Tetlow A, Gordon J. Assessing the relationship between computational speed and precision. Pharmacoeconomics. 2010;28:665–674. doi: 10.2165/11535350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68) Diabetologia. 2004;47:1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 14.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS Outcomes Model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56:1925–1933. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. The world health report 2002 - Chapter 5. http://www.who.int/whr/2002/en/Chapter5.pdf?ua=1. Accessed 1 Jan 2018.
- 16.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW: Methods for the economic evaluation of health care programmes (fourth edition). Oxford: Oxford University Press; 2015.
- 17.Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ. 2002;11:415–430. doi: 10.1002/hec.678. [DOI] [PubMed] [Google Scholar]
- 18.Trippoli S. Incremental cost-effectiveness ratio and net monetary benefit: current use in pharmacoeconomics and future perspectives. Eur J Int Med. 2017;43:e36. doi: 10.1016/j.ejim.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 19.National Bureau of Statistics of China. Gross Domestic Product (GDP) per capita. http://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0201&sj=2019. Accessed 15 Apr 2020.
- 20.Chinese Group for Pharmacoeconomic Evaluations Guidelines . China guidelines for pharmacoeconomic evaluations (2020 Chinese-English version) 1. Beijing: China Market Press; 2020. [Google Scholar]
- 21.Cai X, Hu D, Pan C, Li G, Lu J, Ji Q, et al. Evaluation of effectiveness of treatment paradigm for newly diagnosed type 2 diabetes patients in Chin: a nationwide prospective cohort study. J Diab Investig. 2020;11:151–161. doi: 10.1111/jdi.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai X, Hu D, Pan C, Li G, Lu J, Ji Q, et al. The risk factors of glycemic control, blood pressure control, lipid control in Chinese patients with newly diagnosed type 2 diabetes _ a nationwide prospective cohort study. Sci Rep. 2019;9:7709. doi: 10.1038/s41598-019-44169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Integrated Management Platform of Beijing Medicine Sunshine Purchase. http://210.73.89.76/ServiceSelect/GetServiceSelectList. Accessed 5 May 2018.
- 24.Zhang Y, Wu J, Chen Y, Shi L. EQ-5D-3L decrements by diabetes complications and comorbidities in China. Diabetes Ther. 2020;11:939–950. doi: 10.1007/s13300-020-00788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng YM, Wu J, Xie K. Incidence and cost of hypoglycemia episode in patients with type 2 diabetes mellitus (T2DM) Chinese Rural Health Serv Adm. 2012;32:1195–1198. [Google Scholar]
- 26.Rajan N, Boye KS, Gibbs M, Lee YJ, Davey P, Ball M, Babineaux SM. Utilities for type 2 diabetes treatment-related attributes in a South Korean and Taiwanese population. Value Health Reg Issues. 2016;9:67–71. doi: 10.1016/j.vhri.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14:217–230. doi: 10.1002/hec.910. [DOI] [PubMed] [Google Scholar]
- 28.Guo H, Li J, Jiang Z. Follow-up effects of the increased physical activity on the glucolipid metabolic factors and medical costs in type 2 diabetic patients. Chinese J Rehabil Med. 2007;22:395–398. [Google Scholar]
- 29.Joint committee on revision of standards of medical care for adult dyslipidemia in China. Standards of medical care for adult dyslipidemia in China (2016 version). Chinese Circ J. 2016;31:937–53.
- 30.National Health Commission of the People’s Republic of China. Criteria of weight for adults. http://www.nhc.gov.cn/wjw/yingyang/201308/a233d450fdbc47c5ad4f08b7e394d1e8.shtml. Accessed 1 Sept 2020.
- 31.The Organisation for Economic Co-operation and Development (OECD). Exchange rates. https://data.oecd.org/conversion/exchange-rates.htm. Accessed 15 Apr 2020.
- 32.Bai N. The efficacy and side effects of metformin in the treatment of type 2 diabetes mellitus complicated with non-alcoholic fatty liver disease. China Health Standard Manage. 2016;7:78–80. [Google Scholar]
- 33.Ruan Y, Tan QY, Ruan Y, Wang XJ, Yao J, Wang HL, Wang J. Clinical observation of metformin in the treatment of type 2 diabetes with non-alcoholic fatty liver disease. China Pharm. 2015;26:4659–4661. [Google Scholar]
- 34.Guo W, Gao MS, Ye ZH, Li Y, Tu JJ, Lei WM. Effects of metformin on the serum nesfatin-1 and liver steatosis in type 2 diabetic patients with nonalcoholic fatty liver disease. Chinese J Difficult Complicated Cases. 2014;13:374–377. [Google Scholar]
- 35.Li L, Zhu K, Qu J, Xia A, Zhang W. Clinical effect of liraglutide and metformin hydrochloride on overweight diabetic patients with poor glycemic control. Clin Med China. 2014;30:67–69. [Google Scholar]
- 36.Guo DL. Analysis of the efficacy of metformin in the treatment of type 2 diabetes. Diab New World. 2014;34:1–2.
- 37.Yang WC, Dang Y, Qiao L, Wang H, Li CQ, Dang XY. Effects of exercise therapy and metformin in newly diagnosed type 2 diabetes. J Henan Normal Univ. 2013;41:122–126. [Google Scholar]
- 38.Liu YT, Hu B, Jian L, Li JW, Huang L. Influence of metformin on the level of serum vaspin in T2DM patients. Chinese J Diab. 2013;21:705–708. [Google Scholar]
- 39.Xi Y. Efficacy of liraglutide in patients with newly diagnosed type 2 diabetes and obesity and its influence on their micro-inflammation. Chinese Gen Pract. 2013;16:3339–3340+3345. [Google Scholar]
- 40.Chen Y. The level of visfatin in adipo-patients with type 2 diabetes mellitus and the influence of pioglitazone on it. Master. Guangdong: Guangdong Medical University; 2008.
- 41.Deng HO, Lin K, Li DF, Li YL. Effect of metformin on serum testosterone in male patients with type 2 diabetes. Guangdong Med J. 2007;28:601–602. [Google Scholar]
- 42.Li HZ, Zhang C, Wang M, Liu XH. Effect of metformin and Avandia on lipid metabolism in patients with type 2 diabetes. J Clin Res. 2007;24:319–321. [Google Scholar]
- 43.Mei Q. Therapeutic effect of metformin on type 2 diabetes mellitus complicated with nonalcoholic fatty liver. Pract Pharm Clin Remedies. 2006;9:346–347. [Google Scholar]
- 44.Wang L, Ning J. Effects of metformin on C-reactive protein and complement factor C3 in obese patients with type 2 diabetes. Shanxi Med J. 2006;35:888–889. [Google Scholar]
- 45.Wu GT, Han YQ, Wang JY, Li WJ, Gu Y, Shi Y. Effects of metformin on insulin resistance in type 2 diabetes mellitus patients. J Tongji Univ. 2001;22:31–32+35. [Google Scholar]
- 46.Qu JC, Zhu KS, Wang T, Li L, Zhao L. Effect of rosiglitazone and metformin on lipid metabolism in patients with type 2 diabetes. China Med. 2011;6:286–287. [Google Scholar]
- 47.Guo M, Mi J, Jiang Q, Xu J, Tang Y, Tian G, Wang B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2014;41:650–656. doi: 10.1111/1440-1681.12265. [DOI] [PubMed] [Google Scholar]
- 48.Wang JP. Clinical observation of metformin combined with acarbose in the treatment of type 2 diabetes. Guide China Med. 2016;14:141–142. [Google Scholar]
- 49.Han JK, Pang WY, Yang XZ, Xue L. The clinical observation of metformin combined with acarbose in patients with type 2 diabetes. China Health Standard Manag. 2016;7:116–118. [Google Scholar]
- 50.Wang HY. Clinical efficacy of acarbose in the adjuvant treatment of type 2 diabetes. Chinese J Convalescent Med. 2016;25:71–72. [Google Scholar]
- 51.Kong LL. Clinical efficacy of metformin combined with acarbose in the treatment of type 2 diabetes. Med Hygiene. 2016;2:163.
- 52.Zeng BF. Efficacy and prognosis of acarbose combined with metformin in patients with type 2 diabetes complicated with coronary heart disease. Harbin Med J. 2016;36:159–160. [Google Scholar]
- 53.Liu J. Clinical observation of acarbose combined with metformin in the treatment of type 2 diabetes. Med Forum. 2015;19:5047–5049. [Google Scholar]
- 54.Wang XQ. Efficacy of acarbose combined with metformin in the treatment of patients with newly diagnosed type 2 diabetes and hyperlipidemia. J Pract Gynecol Endocrinol. 2015;2:172–173. [Google Scholar]
- 55.Li QD. Clinical analysis of acarbose combined with metformin in the treatment of type 2 diabetes. Diab New World. 2015;35:59–60.
- 56.Liu P. Clinical observation of glucobay combined with metformin in the treatment of type 2 diabetes. Guide China Med. 2015;13:71–72. [Google Scholar]
- 57.Huang LY, Liao B, Yang M, Liu JH, Quan HM, Qin AP. Efficacy of acarbose combined with metformin in the treatment of newly diagnosed type 2 diabetes with hyperlipidemia. Med Aesthetics Beauty. 2015;24:211.
- 58.Su ZL. Clinical effect of metformin and acarbose in the treatment of obesity and diabetes. Med Inf. 2015;28:23. [Google Scholar]
- 59.A YC. The effects of acarbose combined with metformin on blood glucose and blood fat of patients with type 2 diabetes. Diab New World. 2015;35:25–7.
- 60.Tan YL, Huang YN. Effect of acarbose combined with metformin on blood glucose control and islet function in elderly patients with refractory type 2 diabetes. Clin Med Eng. 2014;21:1422–6.
- 61.Zheng Q, Huangfu YH. Clinical observation of acarbose combined with metformin in the treatment of type 2 diabetes. China Pharm. 2014;25:2251–2253. [Google Scholar]
- 62.Zeng XY. Effect of acarbose combined with metformin in the treatment of elderly patients with type 2 diabetes. Jilin Med J. 2014;35:2159–2160. [Google Scholar]
- 63.Zhou XH, Zhong J, Ye D. The efficacy of acarbose combined with metformin in treatment of type 2 diabetes. China Modern Doctor. 2013;51:68–70. [Google Scholar]
- 64.Liang JH, Chen LZ, Gong ZZ. Efficacy of metformin sustained-release tablets combined with acarbose in the treatment of obese type 2 diabetes. J Guangdong Med Coll. 2012;30:192–193. [Google Scholar]
- 65.Zhang MH. Effects of metformin and acarbose on insulin resistance in patients with type 2 diabetes. Jilin Med J. 2011;32:3682–3683. [Google Scholar]
- 66.Zhu ZL, Qiu XC, Zhu HP, Ding ZJ. Application of acarbose combined with metformin in treatment of newly diagnosed type 2 diabetes patients. Heilongjiang Med J. 2011;24:223–225. [Google Scholar]
- 67.Zheng YB, Zhou HY, Wu XB. Observation of voglibose combined with metformin in the treatment of newly diagnosed obese type 2 diabetes. Strait Pharmaceutical J. 2010;22:117–118. [Google Scholar]
- 68.He K, Liu LH, Ji SM. Efficacy observation of miglitol combined with metformin in the treatment of type 2 diabetes mellitus. Chinese J Clin Ration Drug Use. 2016;9:1–2. [Google Scholar]
- 69.Huang Y. Efficacy of miglitol combined with metformin in the treatment of type 2 diabetes. Chinese Baby. 2015;(4):206–7.
- 70.He K, Liu LH, Shi ZN, Yuan YH. Efficacy of miglitol combined with metformin in the treatment of type 2 diabetes. People’s Military Surg. 2014;57:1216–1217. [Google Scholar]
- 71.Shao HM. Comparison of the effect of glyburide and metformin in the treatment of type 2 diabetes. For all Health. 2015;9:118. [Google Scholar]
- 72.Mao ZH, Tai SEGL. The effect of glimepiride combined with metformin on insulin resistance in patient with type 2 diabetes. Chinese Foreign Med Res. 2016;14:134–135. [Google Scholar]
- 73.Xu YX. Efficacy of glimepiride combined with metformin in the treatment of type 2 diabetes. Modern Diagn Treat. 2016;27:4046–4047. [Google Scholar]
- 74.Ma CH. Influence of liraglutide on insulin resistance in patients with type 2 diabetes mellitus. Master. Xinjiang: Xinjiang Medical University; 2016. [Google Scholar]
- 75.Yang GM, Wang WN. Clinical observation of metformin combined with glimepiride in the treatment of type 2 diabetes. China Med Pharm. 2016;6:145–147. [Google Scholar]
- 76.Yang QF, Nie FR, Xu WL. The clinical efficacy and safety of metformin combined with liraglutide in the treatment of type 2 diabetes mellitus. Lingnan J Emerg Med. 2016;21:40–42. [Google Scholar]
- 77.Xu YJ. Clinical observation of glimepiride combined with metformin in the treatment of 39 cases of type 2 diabetes. Chinese J Ethnomed Ethnopharm. 2015;24:85. [Google Scholar]
- 78.Sun SJ. Clinical observation of glimepiride and metformin in the treatment of type 2 diabetes. Jilin Med J. 2014;35:948–949. [Google Scholar]
- 79.Li X. Efficacy of glimepiride combined with metformin in the treatment of type 2 diabetes. Clin Med. 2012;32:57–58. [Google Scholar]
- 80.Wang Y. Efficacy and safety of metformin combined with glimepiride in the treatment of new-onset type 2 diabetes. China Pract Med. 2012;7:186–187. [Google Scholar]
- 81.Zhao HY. Efficacy of different drugs combined with metformin in the treatment of new-onset type 2 diabetes. Modern J Integr Tradit Chinese Western Med. 2012;21:2785–2787. [Google Scholar]
- 82.Ning J, Tang WL, Zhou ZG, Feng Q, Liu YH, Zhou WD. Effect of glimepiride and metformin on insulin resistance in type 2 diabetes. China J Modern Med. 2006;16:2224–7.
- 83.Wang J. Clinical efficacy of glimepiride combined with metformin in the treatment of type 2 diabetes. Med J Liaoning. 2015;29:177–178. [Google Scholar]
- 84.Su YS. Efficacy of metformin combined with gliclazide in the treatment of type 2 diabetes. Shenzhen J Integr Tradit Chinese Western Med. 2016;26:126–127. [Google Scholar]
- 85.Wei J. Clinical effect of gliclazide combined with metformin in the treatment of type 2 diabetes. J Clin Med Lit. 2015;2:322. [Google Scholar]
- 86.Guo YP. Clinical effect of gliclazide combined with metformin in the treatment of type 2 diabetes. World Latest Med Information. 2015;15:105+108. [Google Scholar]
- 87.Qin SL, Zhu B, Xu X, Tang YH, Zhu Z. Effect of gliclazide sustained-release tablets on the glucose and PAI-1 of patients with new-onset type 2 diabetes mellitus. J Clin Med Pract. 2014;18:11–13. [Google Scholar]
- 88.Luo JY, Wu K. Clinical efficacy of gliclazide combined with metformin in the treatment of new-onset type 2 diabetes. Jilin Med J. 2014;35:8051–8052. [Google Scholar]
- 89.Wei J. Efficacy and safety of metformin combined with gliclazide sustained-release tablets in the treatment of type 2 diabetes. J Front Med. 2013;3:207.
- 90.Zhang JF. Clinical experience of gliclazide sustained-release tablets combined with metformin in the treatment of type 2 diabetes. Contemp Med. 2013;19:90–91. [Google Scholar]
- 91.He F. Clinical efficacy of metformin combined with gliclazide sustained-release tablets in the treatment of type 2 diabetes. Gems of Health. 2013;(7):420.
- 92.Teng YP, Zhang Q, Ma YF. Gliclazide sustained-release tablets in the treatment of 86 cases of new-onset type 2 diabetes. China Med Pharm. 2012;2:34–35. [Google Scholar]
- 93.Luo ZZ, Wang YL, He SX. Clinical efficacy of metformin combined with gliclazide sustained release capsule in the treatment of type 2 diabetes. China Med Pharm. 2012;2:100–101. [Google Scholar]
- 94.Zhong XX. Efficacy of gliclazide combined with metformin in the treatment of type 2 diabetes mellitus. J Qiqihar Univ Med. 2012;33:2787–2788. [Google Scholar]
- 95.Xu QL, Huang CX. Clinical observation of gliclazide combined with metformin in the treatment of obese type 2 diabetes. China Pract Med. 2007;2:99–100. [Google Scholar]
- 96.Xu H. Efficacy of metformin combined with gliclazide in the treatment of type 2 diabetes. Contemp Med Forum. 2015;13:281–282. [Google Scholar]
- 97.Mei Z, Cheng Y. Clinical efficacy of glipizide (Disha Tablets) combined with metformin in the treatment of type 2 diabetes. China Health Care Nutr. 2014;24:2390. [Google Scholar]
- 98.Zhao Y. Efficacy of glipizide combined with metformin in the treatment of new-onset type 2 diabetes. Chinese J Med. 2014;49:69–71. [Google Scholar]
- 99.Sun R, Pan BB, Su XF, Ma JH. Observation of the effects of metformin and/or glipizide on 8-isoprostaglandin F2α and blood glucose levels in patients with type 2 diabetes. Chinese J Diab. 2013;21:60–62. [Google Scholar]
- 100.Guo SB, Gao SX. Clinical efficacy of metformin plus glipizide on the patients with type 2 diabetes mellitus. Med Recapitulate. 2012;18:1606–1607. [Google Scholar]
- 101.Li P, Chen W, Li L, Liu C, Shan ZY, Su BL, Ge HQ. Glipizide/metformin therapy for patients with type 2 diabetes: a multi-center, randomized double-masked study. Chinese Pharm J. 2011;46:1362–1365. [Google Scholar]
- 102.Ji Z. Clinical observation on the efficacy and safety of the compound of metformin and glipizide in the treatment of type 2 diabetes with poor glycemic control. Master. Liaoning: China Medical University; 2011. [Google Scholar]
- 103.Yao L, Lv XF, Ji X, Zhang JL. Clinical study on efficacy and safety of fixed-dose metformin/glipizide on type 2 diabetes mellitus. Chinese J Clin Pharmacol Ther. 2010;15:803–808. [Google Scholar]
- 104.Yao J, Lu GZ, Yang JK, Liu JJ, Zhu LY, Lv XF, et al. Multi-center, randomized, double-masked, double-mimic active-controlled clinical trial of glipizide/metformin hydrochloride tablets for patients with type 2 diabetes. Chinese J Clin Pharmacol. 2008;24:387–391. [Google Scholar]
- 105.Cui HM. Observation on the treatment of type 2 diabetes patients with secondary sulfonylurea failure. J Pract Med Tech. 2007;14:1280–1281. [Google Scholar]
- 106.Kan LBNE, Mai MTM, Dong JJ. Effect of metformin combined with repaglinide in the treatment of newly diagnosed type 2 diabetes. Med Hygiene. 2016;2:183.
- 107.Yang QY. Effect of metformin combined with repaglinide in the treatment of type 2 diabetes. Contemp Med Symp. 2016;14:19–20. [Google Scholar]
- 108.Lei JJ, Chen YJ. Clinical observation of repaglinide in the treatment of elderly patients with type 2 diabetes. Chinese Commun Doctors. 2016;32:38–40.
- 109.Yang J. Efficacy of repaglinide combined with metformin in the treatment of young and middle-aged patients with type 2 diabetes. Diab New World. 2016;19:29–30.
- 110.Wang K. Preliminary observation on the effect of repaglinide combined with metformin in type 2 diabetes. Psychol Doctor. 2016;22:53–54. [Google Scholar]
- 111.Li J. Efficacy of metformin combined with NovoNorm in the treatment of type 2 diabetes. Psychol Doctor. 2016;22:52–53. doi: 10.4236/psych.2016.71007. [DOI] [Google Scholar]
- 112.Hu ZY. Effect of repaglinide combined with metformin in the treatment of newly diagnosed type 2 diabetes. Med Inf. 2016;29:64–65. [Google Scholar]
- 113.Sun ZC. Efficacy of repaglinide combined with metformin in the treatment of type 2 diabetes. Diab New World. 2015;35:29–30.
- 114.Yu YY. Effect of repaglinide combined with metformin in the treatment of obese patients with type 2 diabetes. J North Pharm. 2015;12:23–24. [Google Scholar]
- 115.Liang F. Clinical study of repaglinide combined with metformin in the treatment of patients with type 2 diabetes. Med Inf. 2015;28:96. [Google Scholar]
- 116.Sun X. Application value of metformin combined with repaglinide in the treatment of newly diagnosed type 2 diabetes. Guide China Med. 2015;13:80–81. [Google Scholar]
- 117.Li B. Effect of repaglinide combined with metformin in the treatment of type 2 diabetes. China Health Care Nutr. 2015;25:271.
- 118.Tan LL. Clinical efficacy of metformin combined with NovoNorm in the treatment of type 2 diabetes. Diab New World. 2014;34:11–2.
- 119.Jiang GJ. Efficacy of metformin combined with repaglinide in the treatment of type 2 diabetes. J Front Med. 2014;4:183–4.
- 120.Han XF. Efficacy of repaglinide combined with metformin in the treatment of type 2 diabetes. J China Prescription Drug. 2014;12:67–68. [Google Scholar]
- 121.Huang JQ. Clinical observation of repaglinide and metformin in treatment of type 2 diabetes mellitus. Chinese Community Doctors. 2014;30:52+54. [Google Scholar]
- 122.He M. Clinical observation of repaglinide combined with metformin in the treatment of type 2 diabetes. China Pract Med. 2013;8:144–145. [Google Scholar]
- 123.He M. Clinical observation of repaglinide in the treatment of 61 cases of newly diagnosed type 2 diabetes. Chinese J Modern Drug Appl. 2013;7:149–150. [Google Scholar]
- 124.Li L, Han CD. The clinical efficacy of repaglinide and metformin in the treatment of newly diagnosed type 2 diabetes. Chinese J Med Guide. 2013;15:2071+2073. [Google Scholar]
- 125.Xu SL. Efficacy of repaglinide combined with metformin in the treatment of newly diagnosed type 2 diabetes. China Pract Med. 2013;8:144–146. [Google Scholar]
- 126.Jin Y, Fan R, Cai JG. Clinical efficacy of metformin combined with NovoNorm in the treatment of type 2 diabetes. Guide China Med. 2012;10:185+188. [Google Scholar]
- 127.Xue F. Efficacy of repaglinide combined with metformin in the treatment of new-onset type 2 diabetes. J Front Med. 2012;2:156–7.
- 128.Zhou YX. Efficacy of NovoNorm combined with metformin in the treatment of type 2 diabetes. J Front Med. 2012;2:152. [Google Scholar]
- 129.Chen EF. Effect of NovoNorm combined with metformin on glucose control of type 2 diabetes. Strait Pharma J. 2012;24:231–233. [Google Scholar]
- 130.Zhu AQ. Clinical experience of repaglinide combined with metformin in the treatment of type 2 diabetes. Chinese Commun Doctors. 2012;14:63. [Google Scholar]
- 131.Jin SL. Efficacy observation of repaglinide and metformin in the treatment of newly diagnosed obese type 2 diabetes mellitus. China Pharmacy. 2011;22:1876–1877. [Google Scholar]
- 132.Chen YP, He YX. The efficacy of repaglinide in combination with metformin in newly diagnosed type 2 diabetes. National Med Front China. 2010;5:25–26+61. [Google Scholar]
- 133.Yan GQ. Effect of repaglinide combined with metformin in the treatment of patients with newly diagnosed type 2 diabetes. Contemp Med Forum. 2015;13:128–129. [Google Scholar]
- 134.Yan XM. Efficacy of combined use of metformin and repaglinide in the treatment of 47 cases of type 2 diabetes. Contemp Med Forum. 2015;13:188–189. [Google Scholar]
- 135.Shao JZ. Clinical efficacy of nateglinide in the treatment of type 2 diabetes. Contemp Med. 2013;19:142–144. [Google Scholar]
- 136.Yao L, Wu YT, Zhang W, Xia CQ, Tian GX, Wang XB. Clinical effect of sitagliptin phosphate combined with metformin in treatment of type 2 diabetes mellitus. Chinese J Clin Healthcare. 2016;19:352–354. [Google Scholar]
- 137.Pang XX. Efficacy of sitagliptin combined with metformin in the treatment of type 2 diabetes. J Pract Diabetol. 2016;12:42–43. doi: 10.1007/s11428-015-0055-4. [DOI] [Google Scholar]
- 138.Han M. Efficacy of sitagliptin combined with metformin in the treatment of type 2 diabetes. World Latest Med Inf. 2016;16:79–80. [Google Scholar]
- 139.Yu DY, Guan LX. Effect analysis of the incipient type 2 diabetes by metformin in combination with sitagliptin. China Health Standard Manage. 2016;7:104–105. [Google Scholar]
- 140.Wang XL. Efficacy analysis of sitagliptin in the treatment of diabetes. Clin Res Pract. 2016;1:41. [Google Scholar]
- 141.Qiao YC. Clinical study of sitagliptin phosphate combined with metformin in the treatment of newly diagnosed type 2 diabetes. Clin Res. 2016;24:71–72. [Google Scholar]
- 142.Yang QM. Efficacy and safety of sitagliptin combined with metformin in the treatment of newly diagnosed type 2 diabetes. J Front Med. 2016;6:207–208. [Google Scholar]
- 143.Wu XH. Effect of sitagliptin combined with metformin in the treatment of type 2 diabetes. Chinese J Crit Care Med. 2016;36:99–100. [Google Scholar]
- 144.Xu YL. Efficacy and safety of sitagliptin combined with metformin in the treatment of type 2 diabetes. J Clin Med Lit. 2016;3:970–971. [Google Scholar]
- 145.Xia CW. Application value of metformin combined with sitagliptin in patients with type 2 diabetes. Med Hygiene. 2016;2:221.
- 146.Li LQ. Clinical effect of metformin combined with sitagliptin phosphate in the treatment of newly diagnosed type 2 diabetes. World Clin Med. 2016;10:104+108. [Google Scholar]
- 147.Hu GH. Clinical effect of metformin combined with sitagliptin in the treatment of newly diagnosed type 2 diabetes. Clin Res. 2016;24:234–235. [Google Scholar]
- 148.Yan YH. Clinical observation of sitagliptin combined with metformin in the treatment of type 2 diabetes. J Med Theory Pract. 2016;29:3074–3075. [Google Scholar]
- 149.Li G. Clinical efficacy and safety of sitagliptin combined with metformin in the treatment of obese patients with type 2 diabetes. World Latest Med Inf. 2016;16:210–211. [Google Scholar]
- 150.Xiong M. Clinical efficacy of metformin combined with sitagliptin in the treatment of patients with newly diagnosed diabetes. Chinese Baby. 2016;(3):16–7.
- 151.Zhang TT. Efficacy of metformin combined with sitagliptin in the treatment of patients with type 2 diabetes and its effect on blood lipid level. Modern Pract Med. 2016;28:1037–1038. [Google Scholar]
- 152.Qi BB, Song WC, Jiang DM, Ji M. Efficacy of dipeptidyl peptidase-4 inhibitor sitagliptin combined with metformin in the treatment of type 2 diabetes. Jiangsu Med J. 2016;42:1728–1729. [Google Scholar]
- 153.Yang F. Clinical efficacy of sitagliptin combined with metformin in the treatment of type 2 diabetes. J Today Health. 2016;15:26. [Google Scholar]
- 154.Liu XL, Hu CH, Liu JH. Efficacy of sitagliptin combined with metformin in the treatment of newly diagnosed type 2 diabetes with non-alcoholic fatty liver disease. Modern Pract Med. 2016;28:894–895. [Google Scholar]
- 155.Jiang X. Clinical effect of sitagliptin combined with metformin in the treatment of type 2 diabetes. Contin Med Educ. 2016;30:156–157. [Google Scholar]
- 156.Fu HY. Efficacy of sitagliptin combined with metformin in the treatment of type 2 diabetes. China Rural Health. 2015;(2):47–8.
- 157.Huang YL. Effect of the new hypoglycemic drug sitagliptin in type 2 diabetes. Chinese Manipulation Rehabil Med. 2015;6:70–71. [Google Scholar]
- 158.Yuan XJ, Ma LF, Yang H. Efficacy evaluation of metformin combined with sitagliptin phosphate in the treatment of newly diagnosed type 2 diabetes. Chinese J Modern Drug Appl. 2015;9:123–124. [Google Scholar]
- 159.Li Z, He XT, Li T, Sun ZH. Clinical analysis of sitagliptin combined with metformin in treatment of type 2 diabetes. China Health Standard Manage. 2015;6:86–87. [Google Scholar]
- 160.Wu QP. Clinical efficacy of sitagliptin in adjuvant treatment of obese patients with type 2 diabetes and its influence on their quality of life. Henan Med Res. 2015;24:11–13. [Google Scholar]
- 161.Zhuo FT, Zhang LT, Gao XH. Effect of sitagliptin on pancreatic B cell function in the treatment of newly diagnosed type 2 diabetes. Pract Pharm Clin Remedies. 2015;18:622–624. [Google Scholar]
- 162.Zhang J. Efficacy of sitagliptin combined with metformin in the treatment of type 2 diabetes. Chinese Pract J Rural Doctor. 2015;22:28–29. [Google Scholar]
- 163.Chen P. Clinical observation of dipeptidyl peptidase IV inhibitor combined with metformin on treatment of patients with type 2 diabetes. J North Pharm. 2015;12:88–89. [Google Scholar]
- 164.Fan SH. Effect of sitagliptin combined with metformin in the treatment of newly diagnosed type 2 diabetes. Modern Pract Med. 2015;27:1632–1634. [Google Scholar]
- 165.Zhang N, Wang Y, Liu YL. Effect of sitagliptin combined with metformin in the treatment of type 2 diabetes. Int Med Health Guid News. 2015;21:3211–3213. [Google Scholar]
- 166.Zhang HT, Wang F, Dong L. Efficacy of sitagliptin combined with metformin in the treatment of patients with newly diagnosed type 2 diabetes. J Pract Diabetol. 2015;11:22–23. doi: 10.1007/s11428-014-1321-6. [DOI] [Google Scholar]
- 167.Wang KL, Sun ZP, Han Y, Li HM. Therapeutic effect of DPP-4 inhibitor in the treatment of newly diagnosed type II diabetes. J Harbin Univ Commerce. 2014;30:659–661. [Google Scholar]
- 168.Yang H, Fang CH. Effect of sitagliptin on serum hs-CRP of patients with newly diagnosed type 2 diabetes. Clin J Tradit Chinese Med. 2014;26:559–560. [Google Scholar]
- 169.Liu SY, Lu XH. Efficacy of sitagliptin combined with metformin in the treatment of newly diagnosed type 2 diabetes. J China Prescription Drug. 2014;12:40–41. [Google Scholar]
- 170.Guo YF, Zhang DQ. Clinical observation of sitagliptin phosphate tablets combined with metformin on treatment of elderly patients with type 2 diabetes. China Modern Med. 2014;21:93–94. [Google Scholar]
- 171.Huang BX. Clinical observation of sitagliptin combined with metformin in the treatment of newly diagnosed diabetes. Chinese Commun Doctors. 2014;30:46–47. [Google Scholar]
- 172.Qiu W. Metformin combined with sitagliptin in treating type 2 diabetes patients complicated by metabolic syndrome. Chinese J Modern Drug Appl. 2014;8:128–129. [Google Scholar]
- 173.Shi AJ. Efficacy of sitagliptin combined with metformin in the treatment of obese patients with type 2 diabetes. Modern Diagn Treat. 2014;25:2743–2745. [Google Scholar]
- 174.Ye X. Effect of sitagliptin on islet β-cell function in type 2 diabetes. Modern Diagn Treat. 2014;25:5728–5729. [Google Scholar]
- 175.Wei ZX, Wu J, Hu HB, Ma WJ. Clinical efficacy of metformin combined with sitagliptin in the treatment of early type 2 diabetes. For all Health. 2014;8:496. [Google Scholar]
- 176.Zhang LL. Clinical efficacy of sitagliptin and metformin in treatment of type 2 diabetes mellitus. China Med Pharm. 2013;3:65–66. [Google Scholar]
- 177.Li WM. Study on the effectiveness and safety of sitagliptin and metformin in patients with type 2 diabetes. J Clin Exp Med. 2013;12:922–923. [Google Scholar]
- 178.Pan YP, Zheng QD, Chen W. Effect of sitagliptin combined with metformin in the treatment of type 2 diabetes. Chinese J Rural Med Pharm. 2013;20:21–22. [Google Scholar]
- 179.Jiao XM, Xu XP, Zhao J, Lv XF. Clinical observation on treatment of newly diagnosed type 2 diabetes with sitagliptin and metformin. Clin Focus. 2013;28:44–45+48. [Google Scholar]
- 180.Zhang HY, Dong L. Clinical efficacy of sitagliptin combined with metformin in the treatment of new-onset type 2 diabetes. China Health Care Nutr. 2013;23:3900–3901. [Google Scholar]
- 181.Nie FR, Xu WL, Yang QF. The effect observation of metformin combined with sitagliptin in treatment of early type 2 diabetes mellitus. Pract J Cardiac Cereb Pneumal Vascular Dis. 2013;21:81–82. [Google Scholar]
- 182.Zhao XH. Efficacy of sitagliptin combined with metformin in the treatment of type 2 diabetes. China Foreign Med Treat. 2012;31:77+79. [Google Scholar]
- 183.Huang BX. Clinical observation of sitagliptin combined with metformin in the treatment of newly diagnosed diabetes. National Medi Front China. 2011;6:58. [Google Scholar]
- 184.Yang W, Guan Y, Shentu Y, Li Z, Johnson-Levonas AO, Engel SS, Kaufman KD, Goldstein BJ, Alba M. The addition of sitagliptin to ongoing metformin therapy significantly improves glycemic control in Chinese patients with type 2 diabetes. J Diab. 2012;4:227–237. doi: 10.1111/j.1753-0407.2012.00213.x. [DOI] [PubMed] [Google Scholar]
- 185.Su DY, Xue L, Pang WY. Efficacy of metformin combined with sitagliptin in the treatment of type 2 diabetes. China's Naturopathy. 2016;24:80–81. [Google Scholar]
- 186.Yang YP, Ma LN. Efficacy of saxagliptin in patients with type 2 diabetes and its effects on hemorheology and vascular elasticity. Women's Health Res. 2016;(21):21–2.
- 187.Zhao JY, Chen B, Peng XD. Effect of saxagliptin therapy on pancreatic β-cell function and micro-inflammatory state of elderly patients with type 2 diabetes. J Hainan Med Univ. 2016;22:1254–1257. [Google Scholar]
- 188.Ding R, Ding GC, Cai RH. Efficacy and safety of dipeptidyl peptidase-4 inhibitor combined with metformin in the treatment of type 2 diabetes. Chinese J Biochem Pharm. 2016;36:154–156+159. [Google Scholar]
- 189.Li M, Zhao W. Clinical efficacy of DPP-4 inhibitor combined with metformin in the treatment of type 2 diabetes. Guangzhou Med J. 2015;46:65–67. [Google Scholar]
- 190.Wang SY, Li J, Cao GL, Zhou T. Effect of metformin alone or combined with saxagliptin on islet B cell functions in elderly patients with newly diagnosed type 2 diabetes. Pract Geriatr. 2015;29:232–234. [Google Scholar]
- 191.Gao F. Clinical efficacy of saxagliptin in newly diagnosed type 2 diabetic patients. Int Med Health Guid News. 2015;21:2441–2442. [Google Scholar]
- 192.Li J. Experience in the application of saxagliptin combined with metformin in the treatment of type 2 diabetes. Modern Diagn Treat. 2015;26:516–517. [Google Scholar]
- 193.Liang M. A randomized controlled study of saxagliptin combined with metformin in the treatment of patients with newly diagnosed type 2 diabetes. Cap Food Med. 2015;22:75–6.
- 194.Feng J, Shi P. Efficacy evaluation of saxagliptin combined with metformin for treating 48 cases of elderly type 2 diabetes. China Pharmaceuticals. 2015;24:90–92. [Google Scholar]
- 195.Sun L. The effect of saxagliptin in treatment of type 2 diabetes. China Modern Doctor. 2015;53:27–29. [Google Scholar]
- 196.Geng XH. Efficacy of saxagliptin combined with metformin in the treatment of newly diagnosed type 2 diabetes. Chinese J Pract Med. 2015;42:72–73. [Google Scholar]
- 197.Zhao SY. Effect of saxagliptin combined with metformin in the treatment of elderly patients with type 2 diabetes. Chinese J New Clin Med. 2015;8:642–644. [Google Scholar]
- 198.Li XY, Liu MJ. Clinical efficacy and safety of saxagliptin combined with metformin on patients with type 2 diabetes. Chinese J Clin Pharmacol. 2015;31:169–171+192. [Google Scholar]
- 199.Xie WS, Ou B. Efficacy of saxagliptin combined with metformin in the treatment of type 2 diabetes. Modern Diagn Treat. 2015;26:1784–1785. [Google Scholar]
- 200.Diao YL, Yu JH, Wang J. Clinical observation of saxagliptin combined with metformin in the treatment of type 2 diabetes. China Pharm. 2015;26:3761–3763. [Google Scholar]
- 201.Wan J, Li JL, Luo QR, Ceng JE. Efficacy of metformin or saxagliptin alone and in combination for treating type 2 diabetes. J Yangtze Univ. 2015;12:1–2+37. [Google Scholar]
- 202.Sun Y, Li Y, Wang MZ. Efficacy of saxagliptin combined with metformin in the treatment of patients with newly diagnosed type 2 diabetes. Chinese J Gerontol. 2014;34:4172–4173. [Google Scholar]
- 203.Lu JE, Pang LR, Gong WK. Efficacy of saxagliptin combined with metformin in the treatment of type 2 diabetes. Modern Pract Med. 2014;26:191–192. [Google Scholar]
- 204.Dong L. Clinical efficacy and safety of saxagliptin combined with metformin in the treatment of type 2 diabetes. Guide China Med. 2014;12:117–118. [Google Scholar]
- 205.Sun Y, Jin J, Wang MZ. A preliminary study on the efficacy of saxagliptin combined with metformin in the treatment of new-onset type 2 diabetes. Chinese J Control Endemic Dis. 2014;29:232–233. [Google Scholar]
- 206.Fang Y, Zhang Y, Lin X. Clinical observation of saxagliptin and metformin in the treatment of type 2 diabetes. Modern Diagn Treat. 2013;24:3196–3197. [Google Scholar]
- 207.Li JJ, Liu CM, Dong XY, Zhang L. Saxagliptin add-on to metformin improves β cell functions in newly diagnosed patients with type 2 diabetes. China Med Pharm. 2013;3:16–18. [Google Scholar]
- 208.Wen Q. Efficacy and safety of saxagliptin combined with metformin in the treatment of type 2 diabetes. Chinese-foreign Women's Health. 2013;21:156–7.
- 209.Li R, Wang HC, Liu MJ. Effect of vildagliptin combined with metformin on improving islet β-cell function and insulin resistance of patients with newly diagnosed type 2 diabetes. Modern J Integr Tradit Chinese Western Med. 2016;25:2575–2577. [Google Scholar]
- 210.Zhao XW, Tian J, Shen JX, Yang LX, Zhou YH. The effect of vildagliptin on pulmonary function in obese patients with type 2 diabetes treated with metformin. Anhui Med Pharm J. 2016;20:369–373. [Google Scholar]
- 211.Xin CH, Liu F, Kuang JS. Clinical study of vildagliptin combined with metformin in the treatment of type 2 diabetes. Chinese J Modern Drug Appl. 2016;10:137–138. [Google Scholar]
- 212.Meng ZL, Chu XQ. Efficacy of vildagliptin combined with metformin in the treatment of type 2 diabetes. J Clin Med Lit. 2016;3:49+52. [Google Scholar]
- 213.Zheng ZP, Tian F, Zheng ZG, Yu M. Effects of vildagliptin combined with metformin on carotid intima-media thickness and serum adiponectin and homocysteine levels in type 2 diabetes mellitus patients with cardio-cerebral vascular disease. J Nanchang Univ. 2015;55:62–65+69. [Google Scholar]
- 214.Shangguan HY, Xin B, Li J, Guo W, Luo Q. Clinical observation of vildagliptin combined with metformin in the treatment of type 2 diabetes. Chinese J Clin Res. 2015;28:1605–1607. [Google Scholar]
- 215.Xin CH, Li Z, Luo WG, Wang N, Kuang JS, Xue XF. Intervention effect of vildagliptin combined with metformin on risk factors of cardiovascular disease in overweight patients with type 2 diabetes mellitus. Med Pharm J Chinese People's Liberation Army. 2015;27:83–87. [Google Scholar]
- 216.Xie LK, Qi CR, Sun YD. Clinical observation of vildagliptin combined with metformin in the treatment of 160 cases of type 2 diabetes with obesity. China Foreign Med Treat. 2015;34:108–9.
- 217.Liang CY, Tan SY, Wang YL. Clinical efficacy of vildagliptin (DPP-4 inhibitor) combined with oral hypoglycemic drugs in the treatment of 84 cases of type 2 diabetes. J Pract Diabetol. 2015;11:49–50. [Google Scholar]
- 218.Gao XH. Clinical efficacy of metformin combined with vildagliptin in the treatment of type 2 diabetes. Chinese J Modern Drug Appl. 2015;9:105–106. [Google Scholar]
- 219.Yan GJ. Clinical study of DPP-4 inhibitor combined with metformin in the treatment of type 2 diabetes. Modern Health. 2015;(6):38.
- 220.Jia XW, Jia HT. Short-term effects and safety analysis of metformin combined with vildagliptin in treatment of type 2 diabetes mellitus. China J Emerg Resuscitation Disaster Med. 2015;10:460–463. [Google Scholar]
- 221.Li Y, He YQ, Wang GT. Efficacy of vildagliptin combined with metformin in the treatment of type 2 diabetes. China Rural Health. 2014;504.
- 222.Hao YH, Hao YJ. Effectiveness study of vildagliptin combined with metformin in the treatment of newly diagnosed type 2 diabetes. Shanxi Med J. 2014;43:1192–1193. [Google Scholar]
- 223.Peng L, Ma JH, You W, Xi Q. The influence of vildagliptin tablets on amylase levels of urine and serum in patients with type 2 diabetes. Hebei Med. 2014;20:723–726. [Google Scholar]
- 224.Lu SM. Efficacy of DPP-4 inhibitor combined with metformin in the treatment of type 2 diabetes and its influence on cardiovascular risk factors. Clinical Educ Gen Pract. 2014;12:537–539. [Google Scholar]
- 225.Zou Y, Huang SY, Yan YM, Wu M. Efficacy and safety of vildagliptin combined with metformin in the treatment of type 2 diabetes. J Pract Diabetol. 2014;10:37–38. [Google Scholar]
- 226.Yan ZD, Hu JP, Tao DQ, Zhou HY. The efficacy and safety of vildagliptin combined with metformin in the treatment of type 2 diabetes. Chinese J Difficult Complicated Cases. 2014;13:156–158. [Google Scholar]
- 227.Lai KB, Shi ZZ, Huang ZC, Guo ZQ, Liao QH, Chen YH. Effects of vildagliptin combined with metformin on IL-6, TNF-a, CRP in patients with type 2 diabetes. Modern Diagn Treat. 2014;25:5544–5.
- 228.Cheng LY. DPP-4 inhibitor combined with metformin in the treatment of 75 cases of type 2 diabetes. J Yangtze Univ. 2014;11:66–68. [Google Scholar]
- 229.Chen XS, Li GY, Wu JY. Clinical efficacy of vildagliptin combined with metformin in the treatment of elderly patients with type 2 diabetes. Cap Med. 2014;21:73–4.
- 230.Liu XH, Zeng CH, Rao YL. Clinical application value of metformin combined with vildagliptin in the treatment of type 2 diabetes. Chinese J Difficult Complicated Cases. 2013;12:137–138. [Google Scholar]
- 231.Yan J. Clinical observation on the combined effect of vildagliptin and metformin in patients with type 2 diabetes mellitus. Master. Jilin: Jilin University; 2013. [Google Scholar]
- 232.Zhao TQ, Wang LM. Therapeutic comparison between pure metformin and DPP-4 inhibitor as add-on therapy to metformin in T2DM treatment. Chinese J Diab. 2012;20:367–369. [Google Scholar]
- 233.Ji LN, Pan CY, Lu JM, Li H, Zhu DL, Li Q, et al. Efficacy and safety of combination therapy with vildagliptin and metformin versus metformin uptitration in Chinese patients with type 2 diabetes inadequately controlled with metformin monotherapy: a randomized, open-label, prospective study (VISION) Diab Obes Metab. 2016;18:775–782. doi: 10.1111/dom.12667. [DOI] [PubMed] [Google Scholar]
- 234.Su Y, Su YL, Lv LF, Wang LM, Li QZ, Zhao ZG. A randomized controlled clinical trial of vildagliptin plus metformin combination therapy in patients with type II diabetes mellitus. Exp Ther Med. 2014;7:799–803. doi: 10.3892/etm.2014.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pan C, Xing X, Han P, Zheng S, Ma J, Liu J, et al. Efficacy and tolerability of vildagliptin as add-on therapy to metformin in Chinese patients with type 2 diabetes mellitus. Diab Obes Metab. 2012;14:737–744. doi: 10.1111/j.1463-1326.2012.01593.x. [DOI] [PubMed] [Google Scholar]
- 236.Zhao XW, Tian J, Shen JX, Yang LX, Zhou YH. Clinical observation of the effect of linagliptin on cardiovascular risk factors in obese type 2 diabetic patients treated with metformin. Guizhou Med J. 2016;40:40–42. [Google Scholar]
- 237.Zheng FP. The effects of dipeptidyl peptidase-4 inhibitor alogliptin on lipid profile in patients with newly diagnosed type 2 diabetes mellitus. Master. Liaoning: China Medical University; 2016. [Google Scholar]
- 238.Weng Y, Wang JK, Song YL, Tang YS, Li Y, Zheng FP, Li CL, Zhou YJ. Efficacy and safety of alogliptin combined with metformin in the treatment of type 2 diabetes mellitus and nonalcoholic fatty liver disease. Pract Pharm Clin Remedies. 2016;19:713–715. [Google Scholar]
- 239.Zhao YP, Liu J, Tai H, Dong QY, Kuang JS. Effect of alogliptin combined with metformin on hemodynamic changes of retrobulbar arterytype and oxidative stress indexes in type 2 diabetes patients. Modern J Integr Tradit Chinese Western Med. 2016;25:1163–1165+1182. [Google Scholar]
- 240.Dong QY, Lin L, Kuang JS. Effect of alogliptin on lung function in obese patients with type 2 diabetes treated with metformin. J Clin Pulm Med. 2016;21:392–396. [Google Scholar]
- 241.Dong QY, Kuang JS. The effect of alogliptin on content of oxidative related substances in obese type 2 diabetes patients treated by metformin. Lab Med Clin. 2016;13:328–331. [Google Scholar]
- 242.Wang L. Efficacy and safety of alogliptin combined with metformin in the treatment of patients with newly diagnosed type 2 diabetes. Med Forum. 2016;20:545–546. [Google Scholar]
- 243.Tai H, Wang MY, Zhao YP, Li LB, Dong QY, Liu XG, Kuang JS. The effect of alogliptin on pulmonary function in obese patients with type 2 diabetes inadequately controlled by metformin monotherapy. Medicine. 2016;95:1–11. doi: 10.1097/MD.0000000000004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Su Y, Lv LF, Li QZ, Zhao ZG, Su YL. The clinical research on the treatment of type 2 diabetes mellitus by DPP-4 inhibitor. Chinese J Diab. 2014;22:886–889. [Google Scholar]
- 245.Li CY. Efficacy of rosiglitazone combined with metformin capsule in the treatment of type 2 diabetes. J Pract Med Tech. 2016;23:94–95. [Google Scholar]
- 246.Xie LH. Clinical observation of metformin combined with pioglitazone in the treatment of incipient type 2 diabetes. China Pharm. 2016;27:1629–1631. [Google Scholar]
- 247.Li JY. Prognostic observation of rosiglitazone combined with metformin in the treatment of type 2 diabetes. Diab New World. 2014;34:11+13.
- 248.Lei CY, Liang J. Clinical observation of metformin combination therapy for treating type 2 diabetes. Med Inf. 2014;27:80.
- 249.Yang XD, Li WQ. Rosiglitazone hydrochloride combined with metformin in the treatment of 75 cases of type 2 diabetes. J Commun Med. 2013;11:51–52. [Google Scholar]
- 250.Wang SQ, Wang JY, Chen YQ, Man J. Effects of rosiglitazone on serum adiponectin and visfatin levels in patients with type 2 diabetes mellitus. Int Med Health Guid News. 2013;19:1904–1907. [Google Scholar]
- 251.Yu CY. Clinical study of metformin combined with rosiglitazone in the treatment of type 2 diabetes. Chinese Foreign Med Res. 2013;11:21–22. doi: 10.1186/1749-8546-8-21. [DOI] [Google Scholar]
- 252.Gao Y. Clinical study of metformin combined with rosiglitazone in the treatment of type 2 diabetes. Chinese J Pract Med. 2013;40:68–69. [Google Scholar]
- 253.Liu M. Efficacy of rosiglitazone tartrate combined with metformin in the treatment of type 2 diabetes. Guide China Med. 2012;10:578–579. [Google Scholar]
- 254.Luo YJ. Clinical curative effect analysis of rosiglitazone combined with metformin in treatment of type 2 diabetes. China Modern Med. 2012;19:82–83. [Google Scholar]
- 255.Wang SY, Shen TT. Observation of curative effect of rosiglitazone combined with metformin on type 2 diabetes mellitus. Modern J Integr Tradit Chinese Western Med. 2012;21:1046–1047+1050. [Google Scholar]
- 256.Si JL. Evaluation on the efficacy and safety of metformin combined with rosiglitazone in treatment of type II diabetes. Med J West China. 2012;24:2326–2327+2330. [Google Scholar]
- 257.Liu WJ, Luo Y, Guo L. The clinical observation of rosiglitazone combined with metformin in treatment of type 2 diabetes mellitus. Med J West China. 2011;23:2184–2185+2187. [Google Scholar]
- 258.Li YG, Zeng JE, Ning SX. Clinical observation of rosiglitazone combined with metformin sustained-release tablets in the treatment of newly diagnosed type 2 diabetes. J Clin Exp Med. 2011;10:128–129. [Google Scholar]
- 259.Xie YQ. Clinical observation of rosiglitazone combined with metformin in the treatment of new-onset type 2 diabetes. Chinese J Modern Drug Appl. 2010;4:53. [Google Scholar]
- 260.Qi MP, Wei CY, Wu SM. Rosiglitazone combined with metformin rapidly improves insulin resistance. J Baotou Med Coll. 2010;26:64–65. [Google Scholar]
- 261.Li GH. The evaluation of therapeutic effect and safety of rosiglitazone combined with metformin on incipient type 2 diabetes. Hebei Med J. 2010;32:1995–1997. [Google Scholar]
- 262.Zhong FD, Guo XZ, Sha YC, Jiao GW, Xi YN, Mo LL. Effect of rosiglitazone combined with metformin sustained-release tablets in the treatment of newly diagnosed type 2 diabetes. Med J Commun. 2009;23:48–49. [Google Scholar]
- 263.Xia ZX, Guo YX, Zhou M, Yan J. Efficacy of metformin sustained-release tablets combined with rosiglitazone maleate in the treatment of type 2 diabetes. J Clin Exp Med. 2009;8:27–28. [Google Scholar]
- 264.Li Y, Pang J, Li SL, Xu J, Bai XG. Relationship between insulin resistance and high-sensitivity C-reactive protein in obese patients with type 2 diabetes and the treatment effects of insulin sensitizer. J Pract Med. 2009;25:4158–4160. [Google Scholar]
- 265.Han SH, Hu HK, Zhang YF, Huang Z, Chen YX. Efficacy of rosiglitazone combined with metformin in the treatment of type 2 diabetes. Sichuan Med J. 2007;28:51–52. [Google Scholar]
- 266.Wang SF, Qu XH, Zhao Y. Comparison of the efficacy of rosiglitazone and metformin in the treatment of new-onset type 2 diabetes. Chinese J Diabetes. 2006;14:415–416. [Google Scholar]
- 267.Zhang GQ. Clinical efficacy of Avandia combined with Glucophage in the treatment of type 2 diabetes. J Nongken Med. 2004;26:275–276. [Google Scholar]
- 268.Chen HM, Li HB, Qiu G, Dai SR, Tang YX. Effect of pioglitazone combined with metformin on serum retinol-binding protein 4 (RBP4) in patients with newly diagnosed type 2 diabetes. Chinese J Clin Healthcare. 2016;19:366–368. [Google Scholar]
- 269.Zhang CR. To observe the intervention effect of pioglitazone on blood lipid of type 2 diabetes mellitus. Diab New World. 2016;19:39–51.
- 270.Li SQ. Clinical efficacy evaluation on hypoglycemic effect of metformin hydrochloride combined with pioglitazone hydrochloride in patients with type 2 diabetes. Anti-Infect Pharm. 2016;13:464–466. [Google Scholar]
- 271.Jin HM. Effects of pioglitazone and metformin on blood lipids and vascular endothelial cell function in patients with type 2 diabetes. World Latest Med Inf. 2016;16:118+120. [Google Scholar]
- 272.Zhu WH. Efficacy of metformin combined with pioglitazone in the treatment of type 2 diabetes. J North Pharm. 2015;12:64–65. [Google Scholar]
- 273.Song RS, Liu FY, Liu FZ. A comparison study on glycemic control effect and safety of pioglitazone/metformin fixed dose combination and metformin monotherapy in the treatment of type 2 diabetes. Health Guide. 2015;20:81. [Google Scholar]
- 274.Wen L, Zhang JW, Li BY, Chen N. Efficacy of pioglitazone combined with metformin in the treatment of obese type 2 diabetes. Med Inf. 2015;28:279. [Google Scholar]
- 275.Liu SH. Effect of metformin combined with pioglitazone in the treatment of type 2 diabetes. Clin Res. 2015;23:59–60. [Google Scholar]
- 276.Chang C. Clinical effect of pioglitazone combined with metformin in the treatment of newly diagnosed type 2 diabetes. Med Inf. 2015;28:194–195. [Google Scholar]
- 277.Zhang CH. Clinical efficacy of metformin combined with pioglitazone in the treatment of type 2 diabetes. World Latest Med Inf. 2015;15:112–1.
- 278.Lu S. Efficacy of pioglitazone in the treatment of type 2 diabetes patients with obesity. Med Aesthetics Cosmetol. 2015;24:298.
- 279.Zhou YQ, Jin F, Zhang M, Hu JS. Effect of metformin combined with pioglitazone in the treatment of elderly patients with type 2 diabetes. J Front Med. 2015;5:60–61. [Google Scholar]
- 280.Ren YL. Effect of pioglitazone and metformin in the treatment of type 2 diabetes. China Health Standard Manage. 2015;6:103–104. [Google Scholar]
- 281.Wang T. Effect analysis of metformin combined with pioglitazone in the treatment of type 2 diabetes. Chinese J Clin Ration Drug Use. 2015;8:40–41. [Google Scholar]
- 282.Zhu XX. Efficacy of metformin combined with pioglitazone in the treatment of type 2 diabetes. Diab New World. 2015;35:28+30.
- 283.Yang HM, Qin QH. The curative effect of pioglitazone joint metformin in treatment of type 2 diabetes patients. J Front Med. 2015;5:146–148. [Google Scholar]
- 284.Yang XL. Clinical observation of pioglitazone hydrochloride combined with metformin sustained-release tablets in the treatment of 180 cases of type 2 diabetes. Contemp Med. 2015;21:138–139. [Google Scholar]
- 285.Yang QM. Analysis of effectiveness of pioglitazone hydrochloride combined with metformin sustained-release tablets in the treatment of type 2 diabetes mellitus. Chinese Commun Doctors. 2015;31:23–24. [Google Scholar]
- 286.Ma ZX. Efficacy of pioglitazone hydrochloride combined with metformin sustained-release tablets in the treatment of type 2 diabetes. Diab New World. 2015;35:52–3.
- 287.Liu HY. Efficacy of metformin combined with pioglitazone in treatment of type 2 diabetes. China Modern Doctor. 2014;52:49–50+53. [Google Scholar]
- 288.Deng MX. Clinical observation on the effect of metformin combined with pioglitazone on newly diagnosed type 2 diabetes patients. China Health Care Nutr. 2014;24:1798–1799. [Google Scholar]
- 289.Shen CM. Clinical value analysis of metformin combined with pioglitazone in the treatment of refractory type 2 diabetes. Shaanxi Med J. 2014;43:1077–1079. [Google Scholar]
- 290.Li HJ, Zhang DH, Liu HJ. Effect of pioglitazone combined with metformin on insulin resistance and adipocytokine levels in patients with type 2 diabetes. Chinese J Gerontol. 2014;34:2416–2418. [Google Scholar]
- 291.Du YJ. Efficacy of metformin combined with pioglitazone in the treatment of type 2 diabetes. Health Res. 2014;34:212–213. [Google Scholar]
- 292.Zhou JJ. Efficacy of pioglitazone combined with metformin in the treatment of type 2 diabetes. Med Inf. 2014;27:74. [Google Scholar]
- 293.Wang H, Ye D. Effects of pioglitazone joint metformin on vascular endothelial cell function and insulin resistance in type 2 diabetes patients. China Modern Doctor. 2013;51:25–27. [Google Scholar]
- 294.Zhong J. Efficacy of metformin hydrochloride combined with pioglitazone in the treatment of type 2 diabetes. Seek Med Ask Med. 2013;11:303–304. [Google Scholar]
- 295.Li X. Clinical efficacy of pioglitazone combined with metformin in the treatment of 59 cases of type 2 diabetes. Chinese J Clin Ration Drug Use. 2013;6:42–43. [Google Scholar]
- 296.Xiao XY. Efficacy of metformin combined with pioglitazone in the treatment of 48 cases of newly diagnosed type 2 diabetes. Med Innov China. 2013;10:37–38. [Google Scholar]
- 297.Yan T, Yang R, Yu DF, Wang J, Sun J, Cheng YZ, Yang XY, He L, Cai DH. Effects of pioglitazone/metformin fixed-dose combination therapy and metformin monotherapy on serum vaspin, adiponectin, and interleukin-6 levels in patients with type 2 diabetes. Chinese J Endocrinol Metab. 2013;29:509–511. [Google Scholar]
- 298.Bi XS. Therapeutic efficacy of metformin combined with pioglitazone in treatment of type 2 diabetes. Prev Treat Cardiovasc Dis. 2013;(1):49–51.
- 299.Zhu YF, Li B. Clinical analysis of metformin combined with pioglitazone in the treatment of 134 cases of type 2 diabetes. China Health Industry. 2012;9:57–58. [Google Scholar]
- 300.Yang YB. Clinical observation of pioglitazone hydrochloride combined with metformin in the treatment of type 2 diabetes. Chinese Foreign Med Res. 2012;10:23–24. [Google Scholar]
- 301.Yang F. Clinical efficacy and safety of pioglitazone combined with metformin in the treatment of type 2 diabetes. Modern Diagn Treat. 2012;23:1360–1362. [Google Scholar]
- 302.Jiang H. Efficacy of metformin combined with pioglitazone in the treatment of type 2 diabetes. China Pract Med. 2012;7:153–155. [Google Scholar]
- 303.Shao P, Shen M. Efficacy of metformin combined with pioglitazone in the treatment of new-onset type 2 diabetes. Chinese Foreign Med Res. 2012;10:22. [Google Scholar]
- 304.Yuan ML. Clinical observation of the combination of metformin and pioglitazone in treating obese patients with newly diagnosed type 2 diabetes mellitus. Chinese J Med Guide. 2012;14:433–434. [Google Scholar]
- 305.Teng YP. A controlled study of pioglitazone combined with metformin in treating type 2 diabetes. China Health Ind. 2012;9:5–6. [Google Scholar]
- 306.Du SZ. Clinical efficacy of pioglitazone combined with metformin for the treatment of type 2 diabetes mellitus. J Clin Res. 2011;28:1941–1943. [Google Scholar]
- 307.Yang Y, Qi Y, Xie J, Ye YQ, Liu ZP, Xing XL, Yao CH, Bai RJ, Qiu YZ. Effect of pioglitazone on blood lipid level of patients with newly diagnosed type 2 diabetes and its clinical significance. Chinese J Microcirc. 2011;21:30–31+34. [Google Scholar]
- 308.Guo HF. Efficacy of pioglitazone and metformin in the treatment of type 2 diabetes. Chinese J Misdiagnostics. 2011;11:3136–3137. [Google Scholar]
- 309.Wang GH. Pioglitazone and metformin in the treatment of new-onset type 2 diabetes. J Jianghan Univ. 2010;38:84–85. [Google Scholar]
- 310.Hong YJ, Guo WY. Analysis on clinical efficacy and safety of pioglitazone combined with metformin in the treatment of type 2 diabetes. China Pharm. 2010;21:2258–2259. [Google Scholar]
- 311.Wang XP, Chen YS, Song PY, Wang JH, Wen AM. Efficacy of pioglitazone and metformin in the treatment of type 2 diabetes. Heilongjiang Med J. 2009;22:30–31. [Google Scholar]
- 312.Deng JH. Efficacy of pioglitazone combined with metformin in the treatment of type 2 diabetes. Proc Clin Med. 2009;18:809–811. [Google Scholar]
- 313.Cao XJ. Pioglitazone hydrochloride combined with metformin in the treatment of newly diagnosed type 2 diabetes. Modern J Integr Tradit Chinese Western Med. 2009;18:1360–1361. [Google Scholar]
- 314.Wang QK, Weng XY. Observation on the effect of pioglitazone hydrochloride combined with metformin in type 2 diabetes. China Trop Med. 2007;7:1118+1164. [Google Scholar]
- 315.Wang LH. Efficacy of pioglitazone and metformin in the treatment of type 2 diabetes. Heilongjiang Med J. 2010;23:623. [Google Scholar]
- 316.Zhang YM, Hu CH, Liu JH. Effect of pioglitazone combined with metformin on homocysteine in patients with type 2 diabetes and fatty liver. Prev Treat Cardio Cereb Vasc Dis. 2013;13:410–411. [Google Scholar]
- 317.Liu SJ. Efficacy of insulin glargine combined with metformin in the treatment of type 2 diabetes. China Pract Med. 2015;10:157–158. [Google Scholar]
- 318.Zhang WH, Hu HX. Efficacy and safety of insulin glargine combined with metformin in intensive treatment of new-onset type 2 diabetes. J North Pharm. 2015;12:144–145. [Google Scholar]
- 319.Ma L. Efficacy of insulin glargine combined with metformin in the treatment of type 2 diabetes. Jilin Med J. 2015;36:714–715. [Google Scholar]
- 320.He RY. Clinical efficacy and safety of insulin glargine injection combined with metformin in the treatment of type 2 diabetes. Diab New World. 2015;35:36.
- 321.Li M. Clinical study of insulin glargine combined with metformin in the treatment of type 2 diabetes. Med Hygiene. 2015;1:149.
- 322.He YF, Zhao Q. A randomized controlled study of metformin monotherapy and different combination regimens in the treatment of type 2 diabetes. Chinese Manipulation Rehabil Med. 2014;5:122–123. [Google Scholar]
- 323.Lv CY. Clinical observation of the effect of insulin glargine combined with metformin on fasting blood glucose. J Pract Diabetol. 2008;4:28–29. [Google Scholar]
- 324.Yao JY. Efficacy of insulin glargine combined with metformin in the treatment of type 2 diabetes. China Pharmaceuticals. 2013;22:4–5. [Google Scholar]
- 325.Ma YM, Huang Y. Clinical observation of insulin glargine combined with metformin in treatment of type 2 diabetes. China J Pharm Econ. 2015;10:83–4.
- 326.Li RL. Effects of Novolin N combined with metformin on blood glucose, C-peptide and CRP in patients with type 2 diabetes. Modern J Integr Tradit Chinese Western Med. 2014;23:1207–1209. [Google Scholar]
- 327.Wang JQ. Clinical study of insulin aspart 30 combined with metformin in the treatment of type 2 diabetes. Chinese-foreign Women’s Health. 2014;22:173.
- 328.Wang DY. Clinical efficacy of insulin detemir combined with metformin in the treatment of type 2 diabetes. Strait Pharm J. 2011;23:124–125. [Google Scholar]
- 329.Sun B. Clinical observation of insulin combined with metformin in the treatment of newly diagnosed type 2 diabetes. Chinese J Misdiagnostics. 2009;9:2839. [Google Scholar]
- 330.Cao YQ, Fan HB, Lu QH, Dong Y, Gu YP. Effect observation on metformin combined with Humulin in treatment of obese type 2 diabetes with onset diabetic foot. Drugs Clin. 2014;29:615–618. [Google Scholar]
- 331.Wang GF, Bai YX, Lou DL, Liu HX, Wang B, Guo Y, Liu Y. Effect of intensive insulin therapy on β-cell function in overweight patients with newly diagnosed type 2 diabetes. Guide China Med. 2013;11:186–187. [Google Scholar]
- 332.Wu ZY. Effect of insulin aspart 30 combined with metformin in the treatment of elderly patients with newly diagnosed type 2 diabetes. Chinese Med J Metallurgical Ind. 2016;33:392. [Google Scholar]
- 333.Jiang LY. Clinical observation of exenatide combined with metformin in the treatment of obese type 2 diabetes. Chinese J Trauma Disabil Med. 2015;23:53–55. [Google Scholar]
- 334.Hu H. Efficacy of exenatide combined with metformin in the treatment of type 2 diabetes. Diab New World. 2015;35:26–7.
- 335.Li BW, Yu JL, Zhang H, Jiang L, Li YL, Li N, Zhang P. Effect of GLP-1 receptor agonist on early insulin secretion and body weight in type 2 diabetes. Med Inf. 2015;28:142–143. [Google Scholar]
- 336.Pan NN. Clinical observation of exenatide combined with metformin in the treatment of elderly type 2 diabetes. Chinese J Clin Ration Drug Use. 2014;7:76–77. [Google Scholar]
- 337.Niu L, Hong T, Zhao S. Clinical efficacy of exenatide combined with metformin in treatment of obese type 2 diabetes. Chinese J Hosp Pharm. 2013;33:1418–1420. [Google Scholar]
- 338.Wang F, Ren HQ, Shen XB. Effect of liraglutide combined with metformin on clinical outcomes and HOMA-β, HOMA-IR index in obese patients with type 2 diabetes. Guizhou Med J. 2016;40:1159–1161. [Google Scholar]
- 339.Wang XC. The role of liraglutide on the body fat composition of obese patients with type 2 diabetes among the Uygur nationality in Xinjiang. Master. Xinjiang: Xinjiang Medical University; 2016. [Google Scholar]
- 340.Song WR. Observation of clinical effect of liraglutide combined with metformin in the treatment of obese patients with type 2 diabetes. Drug Eval. 2016;13:23–26. [Google Scholar]
- 341.Wang CX, Gao H, Wang YL. Effect of liraglutide combined with metformin on vascular endothelial function in the Uygur and the Han nationality obese patients with type 2 diabetes in Xinjiang. J Xinjiang Med Univ. 2016;39:968–971+974. [Google Scholar]
- 342.Fang ZD. Follow up study of liraglutide long-term hypoglycemic treatment on delaying disease progression in patients with type 2 diabetes. Syst Med. 2016;1:49–51. [Google Scholar]
- 343.Zhao YL, Chen JB, Zhang FJ, Chu YN, Zhang XK. Effect of liraglutide combined with metformin on glycolipid metabolism in early diabetic patients. Chinese J Pract Med. 2016;43:104–105. [Google Scholar]
- 344.Wei DH. Clinical analysis of metformin combined with liraglutide in the treatment of type 2 diabetes. Med Hygiene. 2016;2:217.
- 345.Gao HM, Shang Y. Clinical curative effect of liraglutide combined with metformin in treatment of type 2 diabetes. China Foreign Med Treat. 2016;35:137–9.
- 346.Sun F. Clinical effect of liraglutide combined with metformin in the treatment of type 2 diabetes. Clin Res Pract. 2016;1:33–34. [Google Scholar]
- 347.Xue GF, Yang XR. Clinical efficacy of liraglutide combined with metformin in the treatment of type 2 diabetes. J Today Health. 2016;15:160. [Google Scholar]
- 348.Yao YN, Zhu ZL, Wang YL. Clinical efficacy of liraglutide combined with metformin hydrochloride in the treatment of Uygur and Han nationality obese patients with type 2 diabetes. J Xinjiang Med Univ. 2015;38:1527–1530+1535. [Google Scholar]
- 349.Liu CL. Influence of liraglutide on main physiological indices of type 2 diabetes patients. China Pharmaceuticals. 2015;24:30–31. [Google Scholar]
- 350.Zhao FL, Qi M, Liu H. Effect of liraglutide combined with metformin on type 2 diabetes patients with obesity and its impact on micro-inflammatory state. China J Modern Med. 2015;25:43–46. [Google Scholar]
- 351.Wang HQ, Tang GW, Lin SY. Effects of Victoza combined with metformin on insulin resistance in patient with type 2 diabetes. Jilin Med J. 2015;36:3859–3861. [Google Scholar]
- 352.Yu YZ. Clinical effect of liraglutide (Victoza) in the treatment of diabetes. World Latest Med Inf. 2015;15:117. [Google Scholar]
- 353.He J, Jiang Q. Clinical effects of liraglutide combined with metformin in the treatment of type 2 diabetes mellitus. China Modern Med. 2014;21:80–82. [Google Scholar]
- 354.Tian SJ, Li D. Effect of metformin combined with liraglutide in the treatment of newly diagnosed type 2 diabetes. Clin Med China. 2013;29:939–941. [Google Scholar]
- 355.Liang C, Zhou L. Clinical efficacy of liraglutide combined with metformin in the treatment of type 2 diabetes mellitus. Chinese J Biochem Pharm. 2012;33:656–658. [Google Scholar]
- 356.Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A, Hooper L, Group P Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;366:l4697. doi: 10.1136/bmj.l4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.McKenzie JE, Herbison GP, Deeks JJ. Impact of analysing continuous outcomes using final values, change scores and analysis of covariance on the performance of meta-analytic methods: a simulation study. Res Synth Methods. 2016;7:371–386. doi: 10.1002/jrsm.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Musso G, Gambino R, Cassader M, Paschetta E. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. 2019;365:l1328. doi: 10.1136/bmj.l1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. doi: 10.1016/S0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 360.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14:417–428. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 362.Wells GA, Sultan SA, Chen L, Khan M, Coyle D. Indirect treatment comparison [computer program]. Version 1.0. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 363.Wells GA, Sultan SA, Chen L, Khan M, Coyle D. Indirect evidence: indirect treatment comparisons in meta-analysis. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 364.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. https://www.nice.org.uk/process/pmg9/chapter/the-reference-case. Accessed 1 Aug 2019. [PubMed]
- 365.Lane S, Levy AR, Mukherjee J, Sambrook J, Tildesley H. The impact on utilities of differences in body weight among Canadian patients with type 2 diabetes. Curr Med Res Opin. 2014;30:1267–1273. doi: 10.1185/03007995.2014.899207. [DOI] [PubMed] [Google Scholar]
- 366.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diab Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diab Care. 2019;42:S90–S102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 368.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 369.Palmer JL, Gibbs M, Scheijbeler HW, Kotchie RW, Nielsen S, White J, Valentine WJ. Cost-effectiveness of switching to biphasic insulin aspart in poorly-controlled type 2 diabetes patients in China. Adv Ther. 2008;25:752–774. doi: 10.1007/s12325-008-0080-4. [DOI] [PubMed] [Google Scholar]
- 370.Palmer JL, Beaudet A, White J, Plun-Favreau J, Smith-Palmer J. Cost-effectiveness of biphasic insulin aspart versus insulin glargine in patients with type 2 diabetes in China. Adv Ther. 2010;27:814–827. doi: 10.1007/s12325-010-0078-6. [DOI] [PubMed] [Google Scholar]
- 371.Wu J, Zheng Y. Cost-utility analysis of biphasic insulin aspart 30 versus premixed human insulin in Chinese patients with type 2 diabetes. Chinese Pharm J. 2010;45:1116–1120. [Google Scholar]
- 372.Li H, Xu F, Wang F. Cost-effectiveness of biphasic insulin aspart 30 combined with metformin in patients with type 2 diabetes mellitus. Chinese J New Drugs. 2011;20:2163–2170. [Google Scholar]
- 373.Chen X, Wei Y, Li W, Yang W, Guan H. Cost-effectiveness analysis of switch from premix human insulin to biphasic insulin aspart 30 in Chinese patients with type 2 diabetes mellitus. Chinese J Diab Mellitus. 2015;7:741–747. [Google Scholar]
- 374.Gu S, Wang X, Qiao Q, Gao W, Wang J, Dong H. Cost-effectiveness of exenatide twice daily vs insulin glargine as add-on therapy to oral antidiabetic agents in patients with type 2 diabetes in China. Diab Obes Metab. 2017;19:1688–1697. doi: 10.1111/dom.12991. [DOI] [PubMed] [Google Scholar]
- 375.Gu S, Zeng Y, Yu D, Hu X, Dong H. Cost-effectiveness of saxagliptin versus acarbose as second-line therapy in type 2 diabetes in China. PLoS One. 2016;11:e0167190. doi: 10.1371/journal.pone.0167190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Gu S, Mu Y, Zhai S, Zeng Y, Zhen X, Dong H. Cost-effectiveness of dapagliflozin versus acarbose as a monotherapy in type 2 diabetes in China. PLoS One. 2016;11:e0165629. doi: 10.1371/journal.pone.0165629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Gu S, Deng J, Shi L, Mu Y, Dong H. Cost-effectiveness of saxagliptin vs glimepiride as a second-line therapy added to metformin in type 2 diabetes in China. J Med Econ. 2015;18:808–820. doi: 10.3111/13696998.2015.1049542. [DOI] [PubMed] [Google Scholar]
- 378.Shao H, Zhai S, Zou D, Mir MU, Zawadzki NK, Shi Q, Liu S, Shi L. Cost-effectiveness analysis of dapagliflozin versus glimepiride as monotherapy in a Chinese population with type 2 diabetes mellitus. Curr Med Res Opin. 2017;33:359–369. doi: 10.1080/03007995.2016.1257978. [DOI] [PubMed] [Google Scholar]
- 379.Zhu J, Chen W. Economic evaluation of vildagliptin as add-on therapy to metformin in diabetes mellitus treatment in China. China J Pharm Econ. 2013;8:11–6.
- 380.Zhu J, Chen W. Pharmacoeconomic evaluation of sitagliptin in the treatment of type 2 diabetes mellitus. China Pharm. 2016;27:157–162. [Google Scholar]
- 381.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Eligibility criteria. Table S2. Full electronic search strategy for PubMed. Table S3. Baseline characteristics of studies included in the meta-analysis and indirect treatment comparison. Table S4. Framework for estimation of absolute treatment effects of glucose-lowering treatments based on meta-analysis and indirect treatment comparison. Table S5. Retail prices of the glucose-lowering drugs collected from government medicine purchase platform. Table S6. Treatment effects of glucose-lowering treatments and nonpharmacologic treatment in the scenario analyses. Table S7. Base-case results: cost-effectiveness of ten pharmacologic combination strategies and strategy ranking process based on the ICERs (per patient). Table S8. Detailed results for the ten pharmacologic combination strategies: base-case analysis. Fig. S1. PRISMA flow diagram of study selection. Fig. S2. Risk of bias graph. Fig. S3. The trajectories of HbA1c in ten pharmacologic combination strategies over time: base-case analysis. Fig. S4. The trajectories of cholesterol in ten pharmacologic combination strategies over time: base-case analysis. Fig. S5. The trajectories of weight in ten pharmacologic combination strategies over time: base-case analysis. Fig. S6. The trajectories of SBP in ten pharmacologic combination strategies over time: base-case analysis.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.