Abstract
Background
B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor (CAR)-T-cell therapy is an emerging treatment option for multiple myeloma. The aim of this systematic review and meta-analysis was to determine its safety and clinical activity and to identify factors influencing these outcomes.
Methods
We performed a database search using the terms “BCMA,” “CAR,” and “multiple myeloma” for clinical studies published between 01/01/2015 and 01/01/2020. The methodology is further detailed in PROSPERO (CRD42020125332).
Results
Twenty-three different CAR-T-cell products have been used so far in 640 patients. Cytokine release syndrome was observed in 80.3% (69.0–88.2); 10.5% (6.8–16.0) had neurotoxicity. A higher neurotoxicity rate was reported in studies that included more heavily pretreated patients: 19.1% (13.3–26.7; I2 = 45%) versus 2.8% (1.3–6.1; I2 = 0%) (p < 0.0001). The pooled overall response rate was 80.5% (73.5–85.9); complete responses (CR) were observed in 44.8% (35.3–54.6). A pooled CR rate of 71.9% (62.8–79.6; I2 = 0%) was noted in studies using alpaca/llama-based constructs, whereas it was only 18.0% (6.5–41.1; I2 = 67%) in studies that used retroviral vectors for CAR transduction. Median progression-free survival (PFS) was 12.2 (11.4–17.4) months, which compared favorably to the expected PFS of 1.9 (1.5–3.7) months (HR 0.14; p < 0.0001).
Conclusions
Although considerable toxicity was observed, BCMA-targeted CAR-T-cell therapy is highly efficacious even in advanced multiple myeloma. Subgroup analysis confirmed the anticipated inter-study heterogeneity and identified potential factors contributing to safety and efficacy. The results of this meta-analysis may assist the future design of CAR-T-cell studies and lead to optimized BCMA CAR-T-cell products.
Keywords: BCMA, CAR-T, Multiple myeloma
Introduction
Multiple myeloma (MM) is defined by a malignant proliferation of plasma cells in the bone marrow (BM) [1, 2]. As the second most common hematological malignancy after lymphomas, it accounts for 1% of all cancers [3]. Recent epidemiological studies have indicated a steady increase in the incidence and prevalence of MM, mainly attributable to the aging population and therapeutic advances improving survival [4]. Indeed, over the past two decades, the landscape of myeloma treatment has dramatically changed with the advent of several novel therapies, including monoclonal antibodies (mAbs) [5].
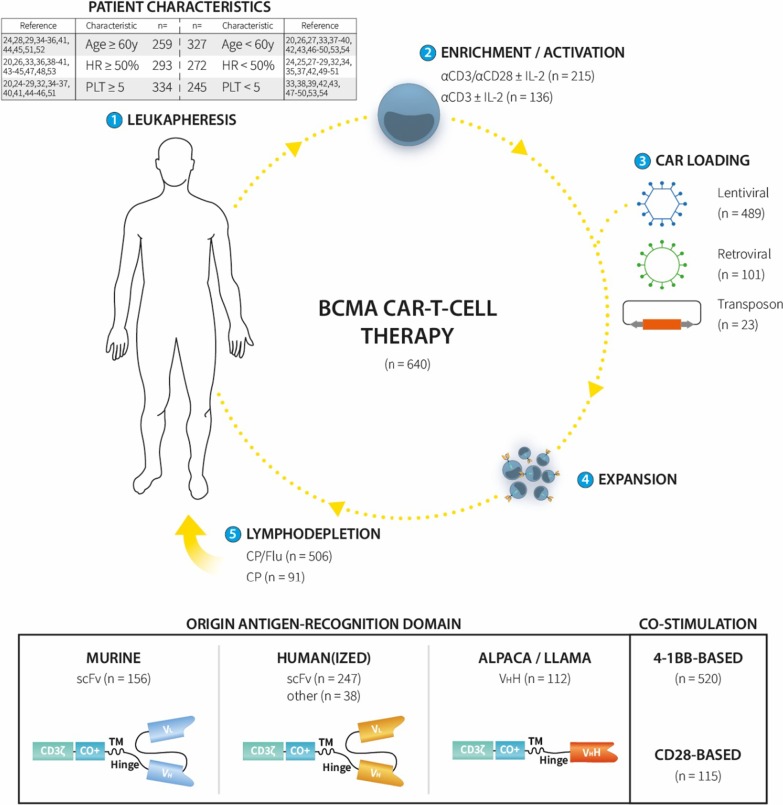
Recently, chimeric antigen receptor (CAR)-T-cell immunotherapy has entered the clinical trial arena [6, 7]. CAR-T cells are autologous lymphocytes collected by leukapheresis and genetically modified (most often by lentiviral or retroviral transduction) to express a CAR. Following ex vivo expansion, the cells are then reinfused to the patient who is usually first conditioned with lymphodepleting chemotherapy (Fig. 1) [8]. CARs are synthetic receptors that bear characteristics of a mAb and a T-cell receptor (TCR); they contain an antigen-recognition domain from a mAb (usually in single-chain variable fragment [scFv] format) and CD3ζ [9]. The mAb part is responsible for HLA-independent binding of the CAR-T cell to a target expressed on the tumor cell surface, whereas the CD3ζ chain triggers T-cell activation by mimicking TCR signaling. Most CAR constructs also contain one (2nd generation) or more (3rd generation) co-stimulatory domains, such as 4-1BB or CD28 (Fig. 1) [8].
Fig. 1.
Overview of BCMA CAR-T-cell therapies used to date in multiple myeloma (MM) patients. Twenty-three different BCMA CAR-T-cell products involving 640 patients were identified. All products were derived from autologous T cells collected by apheresis (1), and enriched and activated ex vivo by anti-CD3/CD28 stimulation ± interleukin (IL)-2 or by single anti-CD3 stimulation ± IL-2 (2). The CAR gene was introduced in the T cells by lentiviral or retroviral transduction, or using a transposon (3). The resultant CAR-T cells were then further expanded (4) and administered to the patient by intravenous infusion, usually after lymphodepleting conditioning with cyclophosphamide (CP) ± fludarabine (Flu) (5). The BCMA CAR-T-cell products used to date can be divided into three groups based on the origin of the extracellular antigen-recognition domain: murine, human(ized), or alpaca/llama. The murine and human(ized) CAR constructs are usually based on the antigen-binding domain of a monoclonal antibody (mAb) in single-chain fragment variable (scFv) format with the variable regions of the heavy (VH) and light chains (VL) linked together in a single chain. Alpaca/llama BCMA CAR constructs are based on the structure of a camelid nanobody containing one or more VHH domains. In addition, the intracellular co-stimulatory domain allows a further subdivision in 4-1BB-based and CD28-based BCMA CAR-T-cell products. Age = studies in whom the median patient age was ≥ or < 60 years. CO+ = co-stimulatory domain. HR = studies with a median of ≥ or < 50% high-risk myeloma patients (based on cytogenetics and/or International Staging System [ISS] score). n = number of patients. PLT = studies in which the median number of prior lines of therapy was ≥ or < 5. TM = transmembrane domain.
Although several antigens are undergoing clinical evaluation, B-cell maturation antigen (BCMA) has been the most popular myeloma target antigen so far [10–12]. BCMA is involved in cell survival and is expressed exclusively on the surface of B-cell lineage cells, including malignant plasma cells [10, 11]. The impressive clinical results of CD19-targeted CAR-T cells in CD19+ hematological malignancies [6, 13, 14] have created high expectations for CAR-T-cell therapy in other cancers [15]. However, it remains unclear whether these expectations are justified in the context of MM since doubts have recently been raised about the durability of therapeutic activity [16]. Moreover, CAR-T-cell therapy can produce potentially life-threatening toxicities, such as cytokine release syndrome (CRS) and neurotoxicity [17].
Current evidence on BCMA-targeted CAR-T-cell therapy in MM is restricted to relatively small, non-randomized early phase clinical trials. Hence, at this stage, it is difficult to obtain a clear sight on the toxicity and efficacy that can be expected from this novel therapeutic approach in relapsed/refractory MM patients. To the best of our knowledge, there has been only one attempt so far to systematically aggregate the outcome data of BCMA CAR-T-cell clinical studies [18]. In that report, Gagelmann et al. included 15 studies comprising a total of 285 patients. Here, we were able to identify 27 studies involving 23 different BCMA CAR-T-cell products and a total of 640 patients, making it the most comprehensive systematic review and meta-analysis to date of the safety and clinical efficacy of BCMA-targeted CAR-T-cell therapy in MM. Moreover, this study is also the first to identify potential patient- and treatment-related factors influencing toxicity and efficacy, which helps us to understand the different outcomes between bb2121 (idecabtagene vicleucel) and LCAR-B38M (ciltacabtagene autoleucel), the two most advanced, late-stage BCMA CAR-T-cell products which are likely to receive regulatory approval in the years to come. Furthermore, controlled trials are lacking, making it challenging to assess the true progression-free survival (PFS) benefit that is reported in individual clinical studies. In this meta-analysis, we incorporated a surrogate control arm, composed of patients treated with inactive doses of BCMA CAR-T cells. PFS data from this control population were used to determine the expected outcome in order to more accurately assess the therapeutic benefit of BCMA CAR-T-cell therapy in relapsed/refractory MM patients.
Methods
Search strategy and selection criteria
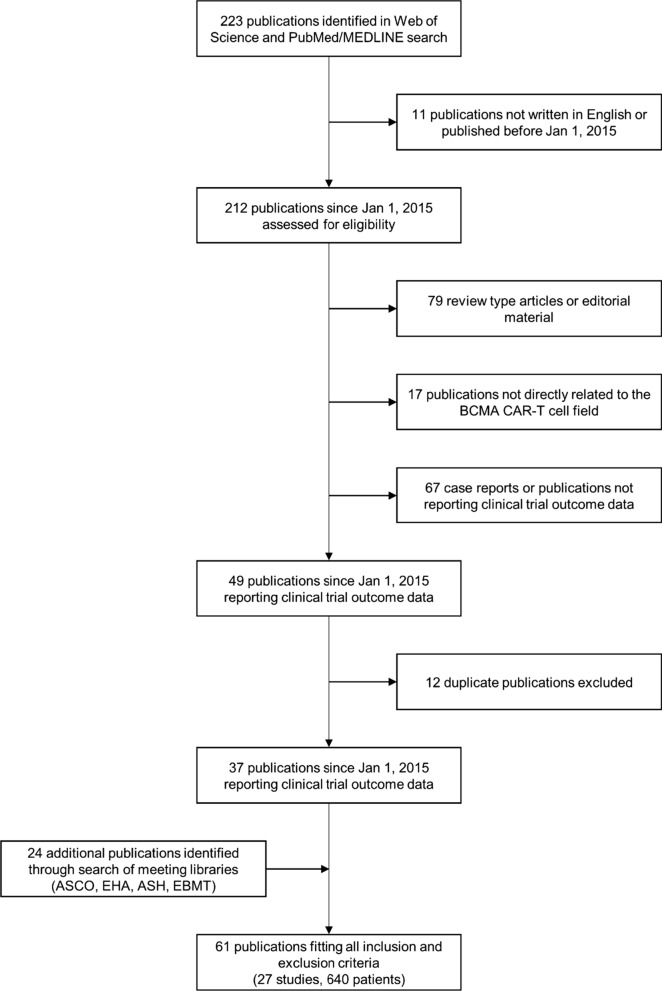
This study involves a systematic review and meta-analysis of the risks and benefits of BCMA CAR-T-cell therapy in MM patients. Relevant clinical studies were identified by a systematic search of Web of Science (Clarivate Analytics) and PubMed/MEDLINE using the following search terms: “B-cell maturation antigen” or “BCMA,” “chimeric antigen receptor” or “CAR,” and “multiple myeloma.” Additional records were retrieved by screening published conference abstracts of American Society of Clinical Oncology (ASCO), American Society of Hematology (ASH), European Group for Blood and Marrow Transplantation (EBMT), and European Hematology Association (EHA). All clinical trial designs (i.e., controlled and uncontrolled studies) were considered. Since the first clinical report of CAR-T-cell therapy in MM was published in 2015 [19], the search was restricted to studies published between January 1, 2015, and January 1, 2020. Only clinical trials registered on Clinicaltrials.gov (NCT-number) or Chinese Clinical Trial Registry (ChiCTR-number) and published in English, either as full scientific article or as abstract during the annual scientific meetings of ASCO, ASH, EBMT, or EHA, were taken into consideration. Patient data were solely extracted from these publications, and no requests for additional original patient data were made to the authors of these studies. Reviews and non-scientific publications were not used for data collection to avoid duplicate data, but were used to ensure accurate and appropriate data selection. Database searches and data collection were conducted independently by three authors (GR, MT, and SA). Data were omitted if no unanimous consensus over their inclusion was found. The PRISMA flow diagram in Fig. 2 depicts the search strategy that was followed to identify the relevant publications (Fig. 2).
Fig. 2.
Search strategy and study selection. ASCO = American Society of Clinical Oncology. ASH =American Society of Hematology. BCMA = B-cell maturation antigen. CAR = chimeric antigen receptor. EBMT = European Group for Blood and Marrow Transplantation. EHA = European Hematology Association
Data analysis
Table S1 (Additional file 1) provides an overview of the 61 publications that were retrieved following the PRISMA flow diagram depicted in Fig. 2. Based on the clinical trial registration number (NCT-number or ChiCTR-number), the CAR-T-cell product name, and the study group (lead author and affiliation), we were able to identify 27 different studies comprising 640 unique patients. For several studies, overlapping publications were identified; to avoid duplicate data, only the most recent and/or the largest (based on the number of included patients) records were considered (Additional file 1: Table S1). As shown in Table S1, there were two exceptions to this rule. For study NCT02546167 (CART-BCMA UPenn), we decided to use the full publication [20] rather than the meeting abstract [21]. For study NCT03661554 (BCMA nanoantibody), the latest publication involving 16 CAR-T-infused patients was not considered because outcome data were incompletely reported (only for 7 patients) [22].
Primary outcome measures were CAR-T-cell-related toxicities (i.e., CRS and neurotoxicity), and objective response rate (ORR). ORR was defined as the sum of (stringent) complete responses ([s]CR) and (very good) partial responses ([VG]PR), according to IMWG criteria [23]. Progression-free survival (PFS) was used as secondary outcome measure. We collected data on the following patient- and disease-related variables: number of patients, median age, myeloma risk (based on cytogenetics and/or International Staging System [ISS] score), and prior lines of therapy. Information on the following treatment-related variables was extracted: origin and type of the CAR antigen-recognition domain, enrichment/activation method, loading strategy, type of co-stimulatory domain, cell dosage, and lymphodepletion regimen.
We conducted a meta-analysis for proportions to estimate the overall proportion of CRS/neurotoxicity and ORR/CR. Because of the diversity between the studies, a random-effects model was used. Heterogeneity was judged by forest plots and I2. Results are reported as proportions with 95% confidence interval (CI). Subgroup analyses were performed to assess differences between groups of studies. P values were calculated based on the between subgroups heterogeneity statistic.
Median PFS with 95% CI was calculated from individual patient data, which were retrieved using computerized analysis of published Swimmer plots and/or Kaplan–Meier survival curves. We verified the correctness of the retrieved data by back-checking that the calculated median PFS was identical to the published median PFS of each study. A comparative analysis was performed between CAR-T cells used at active doses with inactive doses, where an inactive dose was defined as a CAR-T cell dose that failed to produce both CRS and ORR rates of > 50%. This corresponded to the patients included in the lowest dose cohorts of the following four early phase BCMA CAR-T-cell studies with a dose-escalation design: NCT02658929 [24], NCT02546167 [20], NCT02215967 [25], and NCT03070327 [26]. In the absence of randomized controlled trials, the latter served as a surrogate control group to determine the expected PFS. A marginal Cox regression model with clustering per study was used to assess differences in PFS between the subgroups. All statistical analyses were performed using R v3.4.4. (R Foundation for Statistical Computing, Vienna, Austria). This study was registered with PROSPERO (CRD42020125332).
Results
As shown in Table 1 and Figs. 1 and 2, 27 studies involving 23 different BCMA CAR-T-cell products were identified. Data were available from 640 BCMA CAR-T-cell treated patients. For 11 CAR-T-cell products, the extracellular BCMA-recognition domain of the CAR consisted of a human(ized) mAb in scFv format (Table 1) [55]. In one study (NCT03288493), the antigen-recognition domain was composed of a centyrin, a human fibronectin type III-based antibody mimetic [45, 56], while another (NCT03602612) used a human heavy-chain-only binding domain [44]. All other studies used non-human antibodies, either murine scFV mAb or nanobodies derived from alpaca or llama [46, 57]. Bb2121 and LCAR-B38M, the two most advanced BCMA CAR-T-cell products, used a murine- and llama antibody-based CAR construct, respectively (Table 2). The method used for T-cell enrichment/activation was not reported in the majority of the studies; anti-CD3 and anti-CD28 antibodies (usually coupled to magnetic beads) or an anti-CD3 antibody alone, with or without interleukin (IL)-2, were mostly used [58]. Lentiviral (489/640 patients; 76.4%) and, to a lesser extent, gamma-retroviral transduction (101/640 patients; 15.8%) were the preferred transduction methods (Table 1). NCT03288493 (23/640 patients; 3.6%) was the only clinical trial so far in which a non-viral delivery method was applied (i.e., a transposon). In two trials (ChiCTR-1800018143 and ChiCTR-1900027678), the method of CAR loading was not defined (Table 1) [33, 54]. In 520/640 patients (81.3%), a 4-1BB-based second-generation CAR construct was used; the other patients received BCMA CAR-T cells with a CD28 co-stimulatory domain (either alone or in combination with OX40 or 4-1BB). One study (ChiCTR-1900027678) did not disclose the type of co-stimulatory domain [54]. CAR-T cell dosages varied considerably across the different studies, from 0.07 × 106/kg to > 1000 × 106 cells. This variation is also exemplified in Table 2, comparing bb2121 and LCAR-B38M, showing a tenfold difference between both studies in CAR-T-cell dosage used (Table 2). Cyclophosphamide, usually in combination with fludarabine, was the most frequently used lymphodepleting chemotherapy regimen.
Table 1.
Multiple myeloma CAR-T-cell clinical trials targeting BCMA
| Trial # ref. (product name) | n = | Origin mAb | Expansion | Loading | Co-stimulation | T-cell dosage | Conditioning | Toxicity | Clinical response |
|---|---|---|---|---|---|---|---|---|---|
| ChiCTR-OIC17011272 [27] (CD19 & BCMA CAR-T) | 21 | Murine scFv | aCD3 | Lentiviral | 4-1BB | 1 × 106/kg | CP/Flu |
CRS gr. 1–2 (86%), gr. ≥ 3 (5%) Neurotoxicity (10%) |
sCR/CR (57%)/VGPR (24%) PR (14%) |
| NCT02658929 [24, 28] (bb2121) | 43/39 | Murine scFv | aCD3 + aCD28 | Lentiviral | 4-1BB | 50–800 × 106 | CP/Flu |
CRS gr. 1–2 (58%), gr. ≥ 3 (5%) Neurotoxicity (33%) |
sCR/CR (44%)/VGPR (23%) PR (10%) |
| NCT03274219 [29] (bb21217) | 38 | Murine scFv |
aCD3 + aCD28 + PI3k inhibitor |
Lentiviral | 4-1BB | 150–450 × 106 | CP/Flu |
CRS gr. 1–2 (61%), gr. ≥ 3 (5%) Neurotoxicity (24%) |
sCR/CR (13%)/VGPR (34%) PR (5%) |
| ChiCTR-OPC16009113 [30, 31] (BCMA-CAR T) | 28 | Murine scFv | aCD3 | Lentiviral | CD28/4-1BB | 5.4–25 × 106/kg | CP/Flu | CRS gr. ≥ 3 (14%) |
sCR/CR (61%)/VGPR (4%) PR (21%) |
| NCT02215967 (1) [25, 32] (NCI BCMA CAR-T) | 10 | Murine scFv |
aCD3 + IL-2 |
Retroviral | CD28 | 0.3–3 × 106/kg | CP/Flu | CRS gr. 1–2 (30%) |
VGPR (10%) PR (10%) |
| NCT02215967 (2) [25] (NCI BCMA CAR-T) | 16 | Murine scFv |
aCD3 + IL-2 |
Retroviral | CD28 | 9 × 106/kg | CP/Flu |
CRS gr. 1–2 (56%), gr. ≥ 3 (38%) Neurotoxicity (6%) |
sCR/CR (13%)/VGPR (50%) PR (19%) |
| ChiCTR-1800018143 [33] (BM38 CAR) | 22 | Humanized scFv | ND | ND | 4-1BB | 0.5–4 × 106/kg | CP/Flu | CRS gr. 1–2 (68%), gr. ≥ 3 (23%) |
sCR/CR (55%)/VGPR (9%) PR (24%) |
| NCT02546167 [20] (CART-BCMA UPenn) | 25 | Human scFv | aCD3/CD28 | Lentiviral | 4-1BB | 50–500 × 106 | CP or none |
CRS gr. 1–2 (56%), gr. ≥ 3 (32%) Neurotoxicity (32%) |
sCR/CR (8%)/VGPR (20%) PR (20%) |
| NCT03302403, NCT03380039, NCT03716856 [34, 35] (CT053) | 24 | Human scFv | aCD3/CD28 | Lentiviral | 4-1BB | 50–180 × 106 | CP/Flu |
CRS gr. 1–2 (63%) Neurotoxicity (8%) |
sCR/CR (79%)/VGPR (4%) PR (4%) |
| NCT03430011 [36] (JCARH125) | 44 | Human scFv | ND | Lentiviral | 4-1BB | 50–450 × 106 | CP/Flu |
CRS gr. 1–2 (70%), gr. ≥ 3 (9%) Neurotoxicity (25%) |
sCR/CR (27%)/VGPR (20%) PR (34%) |
| NCT03815383 [37] (C-CAR088) | 5 | Human scFv | ND | Lentiviral | 4-1BB | 1–3 × 106/kg | CP/Flu | CRS gr. 1–2 (80%) |
sCR/CR (20%)/VGPR (60%) PR (20%) |
| ChiCTR-1800018137 [38] (CT103A) | 18 | Human scFv | ND | Lentiviral | 4-1BB | 1–6 × 106/kg | CP/Flu | CRS gr. 1–2 (72%), gr. ≥ 3 (22%) |
sCR/CR (67%)/VGPR (17%) PR (17%) |
| NCT03549442 [39] (CART-BCMA + CTL119) | 16 | Human scFv | ND | Lentiviral | 4-1BB | 500 × 106 | CP/Flu | CRS gr. 1–2 (88%) |
sCR/CR (19%)/VGPR (25%) PR (25%) |
| NCT03338972 [40] (FCARH143) | 11 | Human scFv | aCD3/CD28 | Lentiviral |
4-1BB + EGFRt |
50–150 × 106 | CP/Flu |
CRS gr. 1–2 (91%) Neurotoxicity (9%) |
sCR/CR (55%)/VGPR (36%) PR (9%) |
| NCT03502577 [41] (FCARH143 + GSI) | 10 | Human scFv | ND | Lentiviral |
4-1BB + EGFRt |
50–300 × 106 | CP/Flu |
CRS gr. 1–2 (60%), gr. ≥ 3 (40%) Neurotoxicity (60%) |
sCR/CR (30%)/VGPR (50%) PR (20%) |
| NCT03196414 [42] (SZ-MM-CART01) | 29/28 | Humanized scFv | aCD3 | Lentiviral | CD28/OX40 | 20–82 × 106/kg | CP/Flu |
CRS gr. 1–2 (66%), gr. ≥ 3 (34%) Neurotoxicity (3%) |
sCR/CR (54%)/VGPR (4%) PR (29%) |
| NCT03455972 [43] (SZ-MM-CART02) | 32 | Humanized scFv | aCD3 | Lentiviral | CD28/OX40 | 50 × 106/kg |
BUCY or Mel + autoHSCT |
CRS gr. 1–2 (97%), gr. ≥ 3 (3%) |
sCR/CR (72%)/VGPR (ND) PR (ND) |
| NCT03070327 [26] (MCARH171) | 10/11 | Human scFv | ND | Retroviral |
4-1BB + EGFRt |
1 × 106/kg or 150–450 × 106 |
CP/Flu or CP |
CRS gr. 1–2 (40%), gr. ≥ 3 (20%) Neurotoxicity (10%) |
VGPR (45%) PR (18%) |
| NCT03602612 [44] (FHVH33) | 15 | Human VH | ND | Retroviral | 4-1BB | ND | CP/Flu |
CRS gr. 1–2 (87%), gr. ≥ 3 (7%) Neurotoxicity (27%) |
sCR/CR (20%)/VGPR (7%) PR (53%) |
| NCT03288493 [45] (P-BCMA-101) | 23/19 | Human centyrin | None | Transposon |
4-1BB + rimiducid SS |
51–1143 × 106 | CP/Flu |
CRS gr. 1–2 (9%) Neurotoxicity (4%) |
sCR/CR + VGPR (26%) PR (42%) |
| NCT03661554 [46] (BCMA nanoantibody) | 9 | Alpaca VHH | ND | Lentiviral | 4-1BB | 250–900 × 106 | CP/Flu |
CRS gr. 1–2 (67%), gr. ≥ 3 (22%) Neurotoxicity (11%) |
sCR/CR (56%)/VGPR (33%) PR (11%) |
| NCT03090659 (1) [47, 48] (LCAR-B38M) | 17 | Llama VHH |
aCD3/CD28 + IL-2 |
Lentiviral | 4-1BB | 0.21–1,52 × 106/kg | CP/Flu or CP | CRS gr. 1–2 (59%), gr. ≥ 3 (41%) | sCR/CR (82%)/VGPR (6%) |
| NCT03090659 (2) [49, 50] (LCAR-B38M) | 57 | Llama VHH |
aCD3/CD28 + IL-2 |
Lentiviral | 4-1BB | 0.07–2,1 × 106/kg | CP |
CRS gr. 1–2 (82%), gr. ≥ 3 (7%) Neurotoxicity (2%) |
sCR/CR (73%)/VGPR (4%) PR (11%) |
| NCT03548207 [51] (LCAR-B38M) | 29 | Llama VHH | ND | Lentiviral | 4-1BB | 0.5–0.9 × 106/kg | CP/Flu |
CRS gr. 1–2 (86%), gr. ≥ 3 (7%) Neurotoxicity (10%) |
sCR/CR (69%)/VGPR (17%) PR (14%) |
| ChiCTR-1800017404 [52] (BCMA CAR-T) | 33/32 | ND | ND | Lentiviral | 4-1BB | 1–6 × 106/kg | CP/Flu | CRS gr. 1–2 (52%), gr. ≥ 3 (48%) |
sCR/CR (66%)/VGPR (22%) PR (13%) |
| NCT03093168 [53] (HRAIN BCMA-CART) | 49 | ND | ND | Retroviral |
4-1BB + EGFRt |
9 × 106/kg | CP/Flu | CRS gr. 1–2 (12%), gr. ≥ 3 (6%) |
sCR/CR (45%)/VGPR (18%) PR (14%) |
| ChiCTR-1900027678 [54] (GC012F) | 5 | ND | ND | ND | ND | 1–2 × 106/kg | CP/Flu or none | CRS gr. 1–2 (80%) | sCR/CR (20%)/VGPR (80%) |
| Pooled studies | 639/630 | CRS gr. 1–4 (80.3%) | ORR (80.5%) | ||||||
| (95% CI 69.0–88.2; I2 = 83%) | (95% CI 73.5–85.9; I2 = 61%) | ||||||||
| Neurotoxicity (10.5%) | |||||||||
| (95% CI 6.8–16.0; I2 = 58%) |
aCD3 + aCD28 = anti-CD3 and anti-CD28 antibodies. aCD3/CD28 + IL-2 = anti-CD3 and anti-CD28-coated beads plus interleukin-2. AutoHSCT = autologous hematopoietic stem cell transplant. BCMA = B-cell maturation antigen. BUCY = busulfan and cyclophosphamide. CAR = chimeric antigen receptor. CP = cyclophosphamide. CR = complete response. CRS = cytokine release syndrome. EGFRt = truncated epidermal growth factor receptor. Flu = fludarabine. Gr. = grade. GSI = gamma-secretase inhibitor. IL-2 = interleukin-2. Mel = melphalan. n = number of patients evaluable for toxicity/clinical response. ND = not disclosed. PI3k = phosphoinositide 3-kinase. PR = partial response. scFv = single-chain fragment variable. SS = safety switch. sCR = stringent complete response. Trial # = study registration number in Clinicaltrials.gov (NCT#) or Chinese Clinical Trial Registry (ChiCTR-#). VGPR = very good partial response. VHH = nanobody
Table 2.
Comparison of KarMMa (bb2121) and LEGEND-2 (LCAR-B38M) clinical studies
| bb2121 / KarMMa [59] | LCAR-B38M / LEGEND-2 (Xi’an site) [49, 50] | |
|---|---|---|
| Alternative product name | ide-cel | cilta-cel |
| Trial # (study phase) | NCT03361748 (phase II) | NCT03090659 (phase I) |
| n of patients | 128 (54 at RD of 450 × 106) | 57 |
| Expansion method | aCD3 + aCD28 | aCD3/CD28 + IL-2 |
| Loading method | Lentiviral | Lentiviral |
| CAR-T structure | Murine scFv | Llama 2xVHH |

|

|
|
| Lymphodepletion | CP/Flu | CP |
| CAR-T cell dosage(s) | 150–300 to 450 × 106 | 32.3 × 106 (3.3 to 126.2 × 106) |
| Patient characteristics | ||
| Age (range), y | 61 (33–78) | 54 (27–72) |
| Median n PLT (range) | 6 (3–16) | 3 (1–9) |
| High-risk featuresa | 51% | 37% |
| CRS | 96.3%b | 89.5% |
| Gr. 1–2 | 90.7% | 82.5% |
| Gr. ≥ 3 | 5.6% | 7.0% |
| Median onset (range) | 1d (1–10) | 9d (1–19) |
| Median duration (range) | 7d (1–63) | 9d (3–57) |
| Tocilizumab use | 67% | 46% |
| Neurotoxicity | 20.4%b | 1.8% |
| ORR | 82%b | 88% |
| MRD− CR | 28% | 68% |
| CR | 11% | 5% |
| VGPR | 26% | 4% |
| PR | 17% | 11% |
| Median PFS (95% CI) | 12.1m (8.8–12.3)b | 19.9m (9.6–31) |
aCD3 + aCD28 = anti-CD3 and anti-CD28 antibodies. aCD3/CD28 + IL-2 = anti-CD3 and anti-CD28-coated beads plus interleukin-2. cilta-cel = ciltacabtagene autoleucel. CP = cyclophosphamide. CP/Flu = cyclophosphamide plus fludarabine. CR = complete response. CRS = cytokine release syndrome. d = days. Gr. = grade. ide-cel = idecabtagene vicleucel. m = months. MRD = minimal residual disease. n = number. ORR = objective response rate. PFS = progression-free survival. PLT = prior lines of treatment. RD = recommended dose. scFv = single-chain variable fragment. (VG)PR = (very good) partial response. VHH = heavy-chain variable region. Trial # = study registration number in Clinicaltrials.gov (NCT#). y = years
aHigh-risk defined as R-ISS stage 3 and/or high-risk genetics (del(17p), t(4;14), t(14;16))
bData shown for the 450 × 106 dose cohort only
Among 639 patients evaluable for safety, 80.3% (69.0–88.2) experienced CRS (Table 1). CRS is graded on a scale from 1 to 4 [17]; severe CRS (i.e., grade ≥ 3) occurred in 14.1% of patients (9.6–20.4). As shown in Table 2, detailing the key differences between the two most advanced BCMA CAR-T products bb2121 and LCAR-B38M, the median time of CRS onset varied greatly between 1 and 9 days. The median duration was between 7 and 9 days for bb2121 and LCAR-B38M, respectively; CRS could last to up to 2 months (Table 2). The pooled CRS rate was 61.0% (35.3–81.8; I2 = 84%), 83.8% (70.9–91.7; I2 = 71%), and 91.0% (83.8–95.2; I2 = 0%) in studies using CAR constructs with murine-based, human(ized), and alpaca/llama-derived antigen-binding domains, respectively (Additional file 2: Table S2 and Fig. S1). Despite the apparently lower CRS rate in studies using murine scFv-based CAR constructs, individual studies revealed a clear “dose-toxicity” relation. For example, with the bb2121 CAR-T product, which contains a murine anti-BCMA scFv, a CRS rate of 96.3% was noted at the recommended phase II dose of 450 × 106 cells (Table 2), whereas it was only 75.7% and 50.0% at the 300 × 106 and 150 × 106 dose levels, respectively [59].
The pooled neurotoxicity rate was 10.5% (6.8–16.0), with a considerable variation between the different studies. For example, in the bb2121 study, 20.4% of the patient experienced some sort of neurological symptoms, whereas only 1.8% of the LCAR-B38M-treated patients had neurotoxicity (Table 2). The origin of the antigen-recognition domain (murine, human(ized), or alpaca/llama) had no impact on neurotoxicity (Additional file 3: Table S3). Lymphodepletion with cyclophosphamide and fludarabine, a known neurotoxic agent, did not lead to more neurological events as compared to cyclophosphamide alone or no lymphodepletion. A lower rate of neurotoxicity was observed in studies that used anti-CD3 mAbs alone instead of anti-CD3/CD28 mAbs for T-cell enrichment/activation (4.9% [2.1–10.9; I2 = 0%] versus 15.9% [8.1–28.9; I2 = 66%]; p = 0.028). A similar observation was made for studies that used CD28 instead of 4-1BB as co-stimulatory backbone (3.4% [1.2–9.3; I2 = 0%] versus 12.9% [8.2–19.6; I2 = 59%]; p = 0.018). A higher rate of neurotoxicity was observed in studies in which the median patient age was ≥ 60 years (20.5% [12.5–31.9; I2 = 63%] versus 6.4% [3.3–12.0; I2 = 38%]; p = 0.0043), and in studies in which the median number of prior lines of therapy was ≥ 5 (19.1% [13.3–26.7; I2 = 45%] versus 2.8% [1.3–6.1; I2 = 0%]; p < 0.0001; Additional file 3: Fig. S2).
A total of 630 patients were evaluable for clinical response (Table 1). The pooled ORR was 80.5% (73.5–85.9) with (s)CR in 44.8% (35.3–54.6) of patients. Responses occurred rapidly, usually within the first month after CAR-T-cell infusion. Despite the higher likelihood to achieve a deep response in studies that included less pretreated patients (CR: 57.6% [45.2–69.0; I2 = 63%]; p = 0.011), a (s)CR rate of 32.9% (21.1–47.4; I2 = 77%) was still achieved in studies with a median of ≥ 5 prior lines of therapy. Concerning the treatment-related variables, a superior CR rate of 71.9% (62.8–79.6; I2 = 0) was noted in studies with an alpaca/llama-derived BCMA-recognition domain (p < 0.0001 compared to their human and murine counterparts; Additional file 4: Fig. S3). Responses were usually deeper in studies that used an alpaca/llama-based anti-BCMA CAR construct, as exemplified by LCAR-B38M in Table 2. Finally, the CR rate was only 18.0% (6.5–41.1; I2 = 67%) in studies that used a retroviral instead of a lentiviral vector (50.6% [39.8–61.4; I2 = 77%]) for CAR-T-cell transduction (p = 0.015; Additional file 4: Table S4).
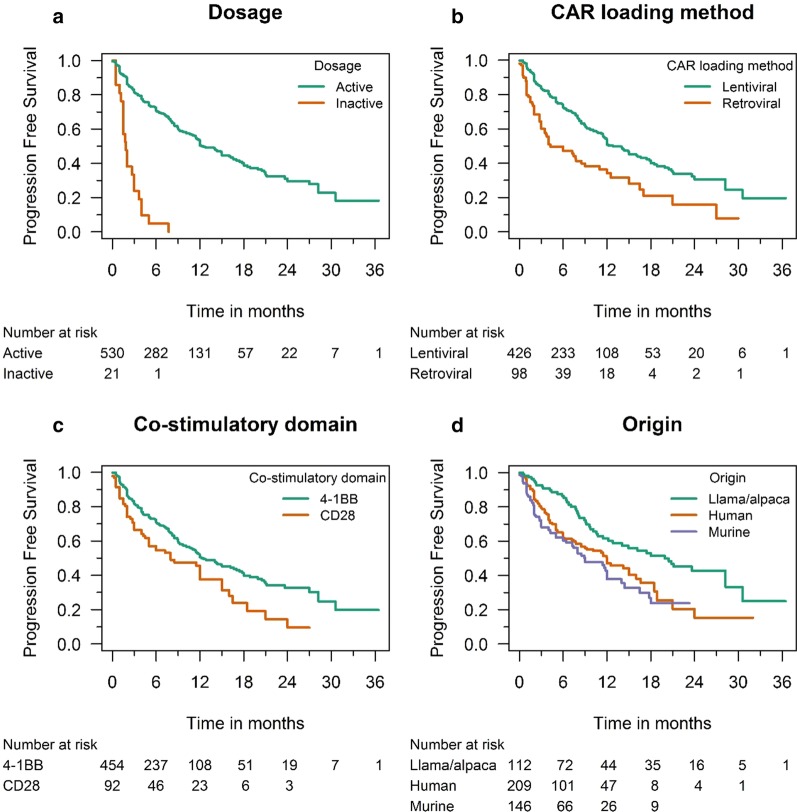
PFS data were available for 551 patients; the median PFS of patients treated with active BCMA CAR-T-cell doses was 12.2 months (11.4–17.4), comparing favorably to the 1.9-month PFS (1.5–3.7) observed in patients treated with inactive doses in the dose-escalation studies NCT02658929, NCT02546167, NCT02215967, and NCT03070327 (hazard ratio [HR] 0.14; p < 0.0001; Fig. 3a). In line with the superior clinical response rate, patients treated with lentivirally transduced CAR-T cells had a significantly longer PFS than those treated with retroviral constructs (12.8 months [11.4–19.9] versus 4.3 months [3.0–15.0]; HR 0.48; p = 0.0065; Fig. 3b; Additional file 4: Table S4). Although no difference was seen in terms of ORR, we observed a shorter PFS among patients treated with BCMA CAR-T-cells containing a CD28-based co-stimulatory backbone (8.0 months [4.0–15.0] versus 12.2 months [10.8–17.4] with a 4-1BB-based co-stimulatory domain); however, this difference was not statistically significant (HR 0.63, p = 0.061; Fig. 3c). The median PFS in the bb2121 study was 12.1 months (8.8–12.3); in the LCAR-B38M study, a median PFS of 19.9 months (9.6–31) was reported (Table 2). The longest PFS rates were observed in studies that used alpaca/llama constructs (p = 0.0005; Fig. 3d; Additional file 2: Table S2).
Fig. 3.
Kaplan–Meier progression-free survival curves
Discussion
This meta-analysis provides insights into the risk and benefits of BCMA CAR-T-cell therapy in MM, into the diversity of the patient populations included and BCMA CAR-T-cell products used, and into the various factors that potentially contribute to toxicity and efficacy. As of January 1, 2020, 27 registered clinical studies have been published involving 23 different CAR-T-cell products and 640 patients. A high response rate was observed, with demonstrable responses in 8/10 patients (nearly half of whom had a CR). Toxicity was equally high, with CRS occurring in 8/10 patients and neurotoxicity in 1/10 patients. Despite the high initial response rate, responses were usually temporary and relapses were frequently observed, resulting in a median PFS of 12.2 months in patients receiving active doses of BCMA CAR-T cells. The two most advanced BCMA CAR-T products are bb2121 or idecabtagene vicleucel (which contains a murine anti-BCMA scFv as antigen-recognition domain) and LCAR-B38M or ciltacabtagene autoleucel (which contains two llama anti-BCMA heavy chain variable regions or VHH) [24, 48, 50].
At the recommended phase II dose level of 450 × 106 cells, the murine BCMA CAR-T product bb2121 yielded comparable CRS rates as its human(ized) or alpaca/llama-based counterparts (Table 2) [59], indicating that not the species origin of the CAR antigen-recognition domain but the CAR-T cell dosage is a major determinant of CRS. Albeit mostly low grade, neurotoxicity occurred in up to 1 out of 5 patients treated with bb2121; in LCAR-B38M-treated patients, the neurotoxicity rate was tenfold lower (Table 2). Since the origin of the antigen-recognition domain (murine, human, or alpaca/llama) was not found to be a risk factor for neurotoxicity in this meta-analysis, other factors should have contributed to the observed difference in neurotoxicity rate between bb2121 and LCAR-B38M. In the LEGEND-2 study conducted at the Xi’An site in China (NCT03090659) [49, 50], the largest study with LCAR-B38M published to date, the lymphodepleting regimen consisted of cyclophosphamide alone, whereas cyclophosphamide and fludarabine were used in the KarMMa pivotal phase II study with bb2121 (NCT03361748) (Table 2). The use of fludarabine for lymphodepletion, which by itself can cause neurotoxicity, was shown to increase the risk of neurologic adverse events in the CD19 CAR-T-cell field [60]. Our meta-analysis, however, failed to demonstrate a role for fludarabine in the higher rate of neurotoxicity in the KarMMa study of bb2121. Dual anti-CD3/CD28 stimulation during CAR-T-cell culture and 4-1BB as co-stimulatory domain were identified as potential risk factors for neurotoxicity in this study. As indicated in Table 2, both KarMMa (bb2121) and LEGEND-2 (LCAR-B38M) used anti-CD3/CD28-stimulated 4-1BB-based CAR-T cells. We, therefore, believe that these factors are not major drivers of neurotoxicity and, at least, do not explain the difference in neurotoxicity rates between both studies. Although this is in sharp contrast with what has been observed in studies with CD19 CAR-T cells [60, 61], this meta-analysis pointed to a higher risk of neurotoxicity in BCMA CAR-T-cell studies in which the median patient age was ≥ 60 years and/or in which the median number of prior lines of anti-myeloma treatments was ≥ 5. As shown in Table 2, LEGEND-2 (LCAR-B38M) tended to include younger and less pretreated patients, possibly explaining the lower frequency of neurological events as compared to KarMMa (bb2121).
Although a previous clinical trial of CD19 CAR-T-cell therapy in CLL failed to demonstrate such correlation [62], we observed a lower rate of deep responses ([s]CR) in studies that included more heavily pretreated patients (≥ 5 prior lines of treatment). This explains why in the LEGEND-2 study a higher proportion of LCAR-B38M-treated patients achieved an (MRD-negative) CR status as compared to the bb2121-treated patients in KarMMA. The rationale behind this is that apheresis products of less pretreated MM patients contain “fitter” T cells [63], resulting in better clinical responses. Autologous BCMA CAR-T-cell therapies are now being positioned earlier in the course of the disease (NCT03549442, NCT03455972) in an attempt to produce deeper and more durable clinical responses. The fact that KarMMa included more high-risk MM patients as compared to LEGEND-2 (Table 2) likely played no role in the lower deep response rate. Indeed, in this meta-analysis, myeloma risk was not associated with reduced activity, indicating that BCMA CAR-T-cell therapy is also highly efficacious in the high need subgroup of high-risk MM patients. Another factor possibly contributing to the superior therapeutic activity of LCAR-B38M is related to the use of (two) llama VHHs as antigen-binding domain in contrast to the murine scFv-based CAR construct of bb2121. It is known that CARs based on heavy-chain-only antibodies (such as alpaca or llama-derived VHH) have superior BCMA-binding capability of VHH compared to traditional scFv-based domains [64, 65]. This is also reflected by the fact that tenfold lower CAR-T cell dosages were required in LEGEND-2 (LCAR-B38M) as compared to KarMMa (bb2121). To summarize, although head-to-head trials between bb2121 and LCAR-B38M have not been conducted, the results of this meta-analysis indicate that the differences in terms of MRD-negativity, depth of response, and, consequently, PFS, between both products are in large part attributable to the different patient populations included and possibly also to the type of antigen-recognition domain used.
Although there was no statistically significant difference in terms of ORR, PFS was markedly longer in the 4-1BB subgroup. This corroborates recent research showing longer CAR-T-cell persistence and improved response durability with 4-1BB-based as compared to CD28-based CD19 CAR-T cells [66]. Although this should still be confirmed in a randomized controlled trial, our results also seem to favor the use of lentiviral over retroviral vectors for CAR-T-cell transduction given their superior clinical activity without increasing toxicity. Non-viral CAR loading methods, such as DNA transposons, are gaining popularity but how these compare to lentiviral or retroviral transduction in terms of toxicity and activity remains to be established.
We observed a sixfold increase in median PFS in the treatment group compared to the control group, which received an inactive CAR-T-cell dose. The low PFS (~ 2 months) in the control group is congruent with previous literature [67] and illustrates the grim prognosis of the patients included so far in BCMA CAR-T-cell studies. In contrast to what is observed in the field of CD19-directed CAR-T-cell therapy for diffuse large B-cell lymphoma, the tail of the PFS curve did not reveal a plateau. This indicates that the majority of the patients will eventually relapse. Possible explanations are lack of CAR-T cell persistence, antigen escape, the hostile tumor microenvironment, and exhaustion. Persistence can be improved by altering the CD4/CD8 composition of the infusion product [21, 40], or by enriching the product with stem cell memory T cells [45, 68]. BCMA downregulation or loss was observed in several trials [20, 25, 32, 40]; this can be mediated by shedding of BCMA from the cell surface [8] or by CAR-T cell-induced trogocytosis. The latter not only leads to reduced tumor cell recognition, but also to CAR-T-cell fratricide [69]. In order to prevent BCMA shedding, γ-secretase inhibitors are being combined with BCMA CAR-T cells (NCT03502577) [41]. Another approach to circumvent antigen escape is co-targeting of BCMA and another antigen, such as CD19 (NCT03196414, NCT03455972) [27, 43], or simultaneous targeting of two BCMA epitopes as in LCAR-B38M [47–51]. Relapse can also occur despite CAR-T-cell persistence and maintained BCMA expression. The hypoxic niche in the BM, where MM cells reside, impairs cytokine secretion and granzyme B release from BCMA CAR-T cells [70]. In addition, upregulation of immune checkpoint molecules, such as programmed death-1 (PD-1), results in BCMA CAR-T-cell exhaustion which can be restored by PD-1 blockade [71]. Tonic signaling in the absence of antigen can induce CAR-T-cell exhaustion as well; proper selection of the antigen-recognition domain [45] and the co-stimulatory domain [69] can help to minimize CAR tonic signaling.
In conclusion, this meta-analysis provides robust evidence for the high clinical activity of BCMA CAR-T-cell therapies in MM and shows that several patient- and treatment-related factors might contribute to their toxicity and efficacy. These findings may inform the design of future CAR-T-cell studies in MM.
Supplementary information
Additional file 1. Overview of the 61 publications identified following the PRISMA flow diagram.
Additional file 2. Subgroup comparison for antigen-recognition domain origin and forest plot for CRS (grouped by antigen-recognition domain).
Additional file 3. Subgroup comparison for neurotoxicity and forest plot for neurotoxicity (grouped by lines of prior therapy).
Additional file 4. Subgroup comparison for CAR loading method and forest plot for (s)CR (grouped by antigen-recognition domain).
Acknowledgements
Part of this work was presented during the 4th French International Symposium on CAR T Cells (CAR T Day), which took place in Lille, France, from 15/01/2020 until 16/01/2020, and which was organized by Prof Suman Mitra and Prof Ibrahim Yakoub-Agha (Lille, France).
Authors’ contributions
SA conceived the study design; SA, MT, and GR did the literature search and contributed to writing of the report. KW performed the statistical analysis. SA and GR designed the figures. DCD, DF, WS, YC, ZNB, EL, and FL revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by Grants G.0535.18N and 1524919N from the Research Fund (FWO) Flanders (Belgium), by a grant of the Baillet-Latour Fund, by a grant of the Belgian Foundation against Cancer (Stichting tegen Kanker) [2016-138 (FAF-C/2016/764)], by a grant of the Foundation Horlait-Dapsens, and by the Shanghai Rising-Star Program (18QA1401000). We also received support from the Belgian non-profit organisation Organisation for Medical Education & Research vzw, from a Methusalem Fund from the University of Antwerp, from the Kaushik Bhansali Fund, and from Gilead Sciences and Janssen Pharmaceuticals. GR is supported by a Doctoral Grant Strategic Basic Research of the FWO (Grant 1S72821N), an Emmanuel van der Schueren fellowship from Kom op tegen Kanker (Stand up to Cancer, Belgium), and the public utility foundation MeToYou (Belgium). DCD is supported by a DOC-PRO PhD grant of the Special Research Fund (BOF) of the University of Antwerp and by Grant G053518N from the FWO. SA is a senior clinical investigator of the FWO and holder of the 2020 Gilead BeLux fellowship.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gils Roex and Marijke Timmers share first authorship
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13045-020-01001-1.
References
- 1.Bianchi G, Anderson KC. Understanding biology to tackle the disease: multiple myeloma from bench to bedside, and back. CA Cancer J Clin. 2014;64(6):422–444. doi: 10.3322/caac.21252. [DOI] [PubMed] [Google Scholar]
- 2.Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385(9983):2197–2208. doi: 10.1016/S0140-6736(14)60493-1. [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Terpos E. Multiple myeloma. Ann Oncol. 2010;21:vii143–vii150. doi: 10.1093/annonc/mdq370. [DOI] [PubMed] [Google Scholar]
- 4.Turesson I, Bjorkholm M, Blimark CH, Kristinsson S, Velez R, Landgren O. Rapidly changing myeloma epidemiology in the general population: increased incidence, older patients, and longer survival. Eur J Haematol. 2018;101:237–244. doi: 10.1111/ejh.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abramson HN. The multiple myeloma drug pipeline-2018: a review of small molecules and their therapeutic targets. Clin Lymphoma Myeloma Leuk. 2018;18(9):611–627. doi: 10.1016/j.clml.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Roex G, Feys T, Beguin Y, Kerre T, Poiré X, Lewalle P, et al. Chimeric antigen receptor-T-cell therapy for B-cell hematological malignancies: an update of the pivotal clinical trial data. Pharmaceutics. 2020;12(2):194. doi: 10.3390/pharmaceutics12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Zhang L, Brockman QR, Zhan F, Chen L. Chimeric antigen receptor T cell therapies for multiple myeloma. J Hematol Oncol. 2019;12(1):120. doi: 10.1186/s13045-019-0823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen AD. CAR-T cells and other cellular therapies for multiple myeloma: 2018 update. Am Soc Clin Oncol Educ Book. 2018;38:e6–e15. doi: 10.1200/EDBK_200889. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann J, Schüßler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9(9):1183–1197. doi: 10.15252/emmm.201607485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danhof S, Hudecek M, Smith EL. CARs and other T cell therapies for MM: the clinical experience. Best Pract Res Clin Haematol. 2018;31(2):147–157. doi: 10.1016/j.beha.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timmers M, Roex G, Wang Y, Campillo-Davo D, Van Tendeloo VFI, Chu Y, et al. Chimeric antigen receptor-modified T cell therapy in multiple myeloma: beyond B cell maturation antigen. Front Immunol. 2019;10:1613. doi: 10.3389/fimmu.2019.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu B, Jiang T, Liu D. BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol. 2020;13(1):125. doi: 10.1186/s13045-020-00962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Luo F, Yang J, Zhao C, Chu Y. New chimeric antigen receptor design for solid tumors. Front Immunol. 2017;8:1934. doi: 10.3389/fimmu.2017.01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Agostino M, Raje N. Anti-BCMA CAR T-cell therapy in multiple myeloma: can we do better? Leukemia. 2020;34(1):21–34. doi: 10.1038/s41375-019-0669-4. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Zhao J. Cytokine release syndrome: grading, modeling, and new therapy. J Hematol Oncol. 2018;11(1):121. doi: 10.1186/s13045-018-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagelmann N, Ayuk F, Atanackovic D, Kröger N. B cell maturation antigen-specific chimeric antigen receptor T cells for relapsed or refractory multiple myeloma: a meta-analysis. Eur J Haematol. 2020;104(4):318–327. doi: 10.1111/ejh.13380. [DOI] [PubMed] [Google Scholar]
- 19.Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373(11):1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129(6):2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen AD, Melenhorst JJ, Garfall AL, Lacey SF, Davis M, Vogl DT, et al. Predictors of T cell expansion and clinical responses following B-cell maturation antigen-specific chimeric antigen receptor T cell therapy (CART-BCMA) for relapsed/refractory multiple myeloma (MM) Blood. 2018;132:1974. doi: 10.1182/blood-2018-99-119665. [DOI] [Google Scholar]
- 22.Han L, Gao Q, Zhou K, Zhou J, Fang B, Zhang J, et al. The phase I clinical study of CART targeting BCMA with humanized alpaca-derived single-domain antibody as antigen recognition domain. J Clin Oncol. 2019;37:2535. doi: 10.1200/JCO.2019.37.15_suppl.2535. [DOI] [Google Scholar]
- 23.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 24.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36(22):2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mailankody S, Ghosh A, Staehr M, Purdon TJ, Roshal M, Halton E, et al. Clinical responses and pharmacokinetics of MCARH171, a human-derived bcma targeted CAR T cell therapy in relapsed/refractory multiple myeloma: final results of a phase I clinical trial. Blood. 2018;132:959. doi: 10.1182/blood-2018-99-119717. [DOI] [Google Scholar]
- 27.Yan Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol. 2019;6(10):e521–e529. doi: 10.1016/S2352-3026(19)30115-2. [DOI] [PubMed] [Google Scholar]
- 28.Raje N, Berdeja J, Lin Y, Munshi N, Siegel DSD, Liedtke M, et al. bb2121 anti-BCMA CAR T-cell therapy in patients with relapsed/refractory multiple myeloma: updated results from a multicenter phase I study. J Clin Oncol. 2018;36(15):8007. doi: 10.1200/JCO.2018.36.15_suppl.8007. [DOI] [Google Scholar]
- 29.Berdeja JG, Alsina M, Shah ND, Siegel DS, Jagannath S, Madduri D, et al. Updated results from an ongoing phase 1 clinical study of bb21217 anti-bcma CAR-T-cell therapy. Blood. 2019;134:927. doi: 10.1182/blood-2019-126660. [DOI] [Google Scholar]
- 30.Xu J, Wang Q, Xu H, Gu C, Jiang L, Wang J, et al. Anti-BCMA CAR-T cells for treatment of plasma cell dyscrasia: case report on POEMS syndrome and multiple myeloma. J Hematol Oncol. 2018;11(1):128. doi: 10.1186/s13045-018-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Wang Q, Zhu H, Mao X, Wang Y, Zhang Y, et al. T cells expressing anti B-cell maturation antigen chimeric antigen receptors for plasma cell malignancies. Blood. 2018;132:1013. doi: 10.1182/blood-2018-99-116898. [DOI] [Google Scholar]
- 32.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Mei H, Hu Y, Guo T, Liu L, Jiang H, et al. A bispecific CAR-T cell therapy targeting bcma and CD38 for relapsed/refractory multiple myeloma: updated results from a phase 1 dose-climbing trial. Blood. 2019;134:930. doi: 10.1182/blood-2019-130340. [DOI] [Google Scholar]
- 34.Jie J, Hao S, Jiang S, Li Z, Yang M, Zhang W, et al. Phase 1 trial of the safety and efficacy of fully human anti-bcma CAR T cells in relapsed/refractory multiple myeloma. Blood. 2019;134:4435. doi: 10.1182/blood-2019-126104. [DOI] [Google Scholar]
- 35.Jiang S, Jin J, Hao S, Yang M, Chen L, Ruan H, et al. Low dose of human scFv-derived BCMA-targeted CAR-T cells achieved fast response and high complete remission in patients with relapsed/refractory multiple myeloma. Blood. 2018;132:960. doi: 10.1182/blood-2018-99-113220. [DOI] [Google Scholar]
- 36.Mailankody S, Htut M, Lee KP, Bensinger W, Devries T, Piasecki J, et al. JCARH125, anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: initial proof of concept results from a phase 1/2 multicenter study (EVOLVE) Blood. 2018;132:957. doi: 10.1182/blood-2018-99-113548. [DOI] [Google Scholar]
- 37.Yao X, Zhu S, Huang J, Qu X, Zhu J, Wei Y, et al. Developing a novel anti-bcma CAR-T for relapsed or refractory multiple myeloma. Blood. 2019;134:50. doi: 10.1182/blood-2019-125372. [DOI] [Google Scholar]
- 38.Li C, Wang J, Wang D, Hu G, Yang Y, Zhou X, et al. Efficacy and safety of fully human BCMA targeting CAR T cell therapy in relapsed/refractory multiple myeloma. Blood. 2019;134:929. doi: 10.1182/blood-2019-128468. [DOI] [Google Scholar]
- 39.Garfall AL, Cohen AD, Lacey SF, Tian L, Hwang W-T, Vogl DT, et al. Combination anti-BCMA and anti-CD19 CAR T cells as consolidation of response to prior therapy in multiple myeloma. Blood. 2019;134:1863. doi: 10.1182/blood-2019-131515. [DOI] [Google Scholar]
- 40.Green DJ, Pont M, Sather BD, Cowan AJ, Turtle CJ, Till BG, et al. Fully human BCMA targeted chimeric antigen receptor T cells administered in a defined composition demonstrate potency at low doses in advanced stage high risk multiple myeloma. Blood. 2018;132:1011. doi: 10.1182/blood-2018-99-117729. [DOI] [Google Scholar]
- 41.Cowan AJ, Pont M, Sather BD, Turtle CJ, Till BG, Nagengast AM, et al. Efficacy and safety of fully human BCMA CAR T cells in combination with a gamma secretase inhibitor to increase BCMA surface expression in patients with relapsed or refractory multiple myeloma. Blood. 2019;134:204. doi: 10.1182/blood-2019-129405. [DOI] [Google Scholar]
- 42.Yan L, Yan Z, Shang J, Shi X, Jin S, Kang L, et al. Sequential CD19- and BCMA-specific chimeric antigen receptor T cell treatment for RRMM: report from a single center study. Blood. 2019;134:578. doi: 10.1182/blood-2019-129740. [DOI] [Google Scholar]
- 43.Shi X, Yan L, Shang J, Kang L, Jin S, Kang H, et al. Combined infusion of anti-CD19 and anti-BCMA CART cells after early or later transplantation in the front line was superior to salvage therapy for high risk MM. Blood. 2019;134:1949. doi: 10.1182/blood-2019-131546. [DOI] [Google Scholar]
- 44.Mikkilineni L, Manasanch EE, Lam N, Vanasse D, Brudno JN, Maric I, et al. T cells expressing an anti-B-cell maturation antigen (BCMA) chimeric antigen receptor with a fully-human heavy-chain-only antigen recognition domain induce remissions in patients with relapsed multiple myeloma. Blood. 2019;134:3230. doi: 10.1182/blood-2019-129088. [DOI] [Google Scholar]
- 45.Gregory T, Cohen AD, Costello CL, Ali SA, Berdeja JG, Ostertag EM, et al. Efficacy and safety of p-BCMA-101 CAR-T cells in patients with relapsed/refractory (r/r) multiple myeloma (MM) Blood. 2018;132:1012. doi: 10.1182/blood-2018-99-111419. [DOI] [Google Scholar]
- 46.Han L, Gao Q, Zhou K, Yin Q, Fang B, Zhou J, et al. Development and evaluation of CART targeting bcma with humanized alpaca-derived single-domain antibody as antigen recognition domain. Blood. 2018;132:1976. doi: 10.1182/blood-2018-99-114980. [DOI] [Google Scholar]
- 47.Xu J, Chen L-J, Yang S-S, Sun Y, Wu W, Liu Y-F, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci U S A. 2019;116(19):9543–9551. doi: 10.1073/pnas.1819745116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Xu J, Fu W, Sr, Jin S, Yang S, Yan S, et al. Updated phase 1 results of a first-in-human open-label study of LCAR-B38M, a structurally differentiated chimeric antigen receptor T (CAR-T) cell therapy targeting B-cell maturation antigen (BCMA) Blood. 2019;134:1858. doi: 10.1182/blood-2019-130008. [DOI] [Google Scholar]
- 49.Zhao W-H, Liu J, Wang B-Y, Chen Y-X, Cao X-M, Yang Y, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11(1):141. doi: 10.1186/s13045-018-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B-Y, Zhao W-H, Liu J, Chen Y-X, Cao X-M, Yang Y, et al. Long-term follow-up of a phase 1, first-in-human open-label study of LCAR-B38M, a structurally differentiated chimeric antigen receptor T (CAR-T) cell therapy targeting B-cell maturation antigen (BCMA), in patients (pts) with relapsed/refractory multiple myeloma (RRMM) Blood. 2019;134:579. doi: 10.1182/blood-2019-124953. [DOI] [Google Scholar]
- 51.Madduri D, Usmani SZ, Jagannath S, Singh I, Zudaire E, Yeh T-M, et al. Results from CARTITUDE-1: a phase 1b/2 study of JNJ-4528, a CAR-T cell therapy directed against B-cell maturation antigen (BCMA), in patients with relapsed and/or refractory multiple myeloma (R/R MM) Blood. 2019;134:577. doi: 10.1182/blood-2019-121731. [DOI] [Google Scholar]
- 52.Hu Y, Yanlei Z, Wei G, Alex Hong C, Huang H. Potent anti-tumor activity of BCMA CAR-T therapy against heavily treated multiple myeloma and dynamics of immune cell subsets using single-cell mass cytometry. Blood. 2019;134:1859. doi: 10.1182/blood-2019-130341. [DOI] [Google Scholar]
- 53.Fu W, Sr, Du J, Jiang H, Cheng Z, Wei R, Yu K, et al. Efficacy and safety of CAR-T therapy with safety switch targeting bcma for patients with relapsed/refractory multiple myeloma in a phase 1 clinical study. Blood. 2019;134:3154. doi: 10.1182/blood-2019-127608. [DOI] [Google Scholar]
- 54.Zhang H, Gao L, Liu L, Wang J, Wang S, Gao L, et al. A BCMA and CD19 bispecific CAR-T for relapsed and refractory multiple myeloma. Blood. 2019;134:3147. doi: 10.1182/blood-2019-131056. [DOI] [Google Scholar]
- 55.Cho SF, Anderson KC, Tai YT. Targeting B cell maturation antigen (BCMA) in multiple myeloma: potential uses of BCMA-based immunotherapy. Front Immunol. 2018;9:1821. doi: 10.3389/fimmu.2018.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hermanson DL, Barnett BE, Rengarajan S, Codde R, Wang X, Tan Y, et al. A novel BCMA-specific, centyrin-based CAR-T product for the treatment of multiple myeloma. Blood. 2016;128(22):2127. doi: 10.1182/blood.V128.22.2127.2127. [DOI] [Google Scholar]
- 57.Zhao W-H, Liu J, Wang B-Y, Chen Y-X, Cao X-M, Yang Y, et al. Updated analysis of a phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B-cell maturation antigen, in patients with relapsed/refractory multiple myeloma. Blood. 2018;132:955. doi: 10.1182/blood-2018-99-110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34(4):985–1005. doi: 10.1038/s41375-020-0734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munshi NC, Anderson JLD, Shah N, Jagannath S, Berdeja JG, Lonial S, et al. Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma (RRMM): initial KarMMa results. J Clin Oncol. 2020;38:8503. doi: 10.1200/JCO.2020.38.15_suppl.8503. [DOI] [Google Scholar]
- 60.Gust J, Hay KA, Hanafi L-A, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–971. doi: 10.1158/2159-8290.CD-17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dancy E, Garfall AL, Cohen AD, Fraietta JA, Davis M, Levine BL, et al. Clinical predictors of T cell fitness for CAR T cell manufacturing and efficacy in multiple myeloma. Blood. 2018;132:1886. doi: 10.1182/blood-2018-99-115319. [DOI] [Google Scholar]
- 64.Strohl WR, Naso M. Bispecific T-cell redirection versus chimeric antigen receptor (CAR)-T cells as approaches to kill cancer cells. Antibodies. 2019;8(3):41. doi: 10.3390/antib8030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lam N, Trinklein ND, Buelow B, Patterson GH, Ojha N, Kochenderfer JN. Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains. Nat Commun. 2020;11(1):283. doi: 10.1038/s41467-019-14119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ying Z, He T, Wang X, Zheng W, Lin N, Tu M, et al. Parallel comparison of 4–1BB or CD28 co-stimulated CD19-targeted CAR-T cells for B cell non-Hodgkin's lymphoma. Mol Ther Oncolytics. 2019;15:60–68. doi: 10.1016/j.omto.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jagannath S, Lin Y, Goldschmidt H, Reece DE, Nooka AK, Otero PR, et al. KarMMa-RW: a study of real-world treatment patterns in heavily pretreated patients with relapsed and refractory multiple myeloma (RRMM) and comparison of outcomes to KarMMa. J Clin Oncol. 2020;38:8525. doi: 10.1200/JCO.2020.38.15_suppl.8525. [DOI] [Google Scholar]
- 68.Shah N, Alsina M, Siegel DS, Jagannath S, Madduri D, Kaufman JL, et al. Initial results from a phase 1 clinical study of bb21217, a next-generation anti bcma CAR T therapy. Blood. 2018;132:488. doi: 10.1182/blood-2018-99-116953. [DOI] [Google Scholar]
- 69.Hamieh M, Dobrin A, Cabriolu A, van der Stegen SJC, Giavridis T, Mansilla-Soto J, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568(7750):112–116. doi: 10.1038/s41586-019-1054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berahovich R, Liu X, Zhou H, Tsadik E, Xu S, Golubovskaya V, et al. Hypoxia selectively impairs CAR-T cells in vitro. Cancers. 2019;11(5):602. doi: 10.3390/cancers11050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernabei L, Garfall AL, Melenhorst JJ, Lacey SF, Stadtmauer EA, Vogl DT, et al. PD-1 inhibitor combinations as salvage therapy for relapsed/refractory multiple myeloma (MM) patients progressing after BCMA-directed CAR-T Cells. Blood. 2018;132:1973. doi: 10.1182/blood-2018-99-119514. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Overview of the 61 publications identified following the PRISMA flow diagram.
Additional file 2. Subgroup comparison for antigen-recognition domain origin and forest plot for CRS (grouped by antigen-recognition domain).
Additional file 3. Subgroup comparison for neurotoxicity and forest plot for neurotoxicity (grouped by lines of prior therapy).
Additional file 4. Subgroup comparison for CAR loading method and forest plot for (s)CR (grouped by antigen-recognition domain).
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].