Abstract
Background
Placental malaria (PM) has been associated with a higher risk of malaria during infancy. However, it is unclear whether this association is causal, and is modified by infant sex, and whether intermittent preventive treatment in pregnancy (IPTp) can reduce infant malaria by preventing PM.
Methods
Data from a birth cohort of 656 infants born to HIV-uninfected mothers randomised to IPTp with dihydroartemisinin–piperaquine (DP) or Sulfadoxine–pyrimethamine (SP) was analysed. PM was categorized as no PM, active PM (presence of parasites), mild-moderate past PM (> 0–20% high powered fields [HPFs] with pigment), or severe past PM (> 20% HPFs with pigment). The association between PM and incidence of malaria in infants stratified by infant sex was examined. Causal mediation analysis was used to test whether IPTp can impact infant malaria incidence via preventing PM.
Results
There were 1088 malaria episodes diagnosed among infants during 596.6 person years of follow-up. Compared to infants born to mothers with no PM, the incidence of malaria was higher among infants born to mothers with active PM (adjusted incidence rate ratio [aIRR] 1.30, 95% CI 1.00–1.71, p = 0.05) and those born to mothers with severe past PM (aIRR 1.28, 95% CI 0.89–1.83, p = 0.18), but the differences were not statistically significant. However, when stratifying by infant sex, compared to no PM, severe past PM was associated a higher malaria incidence in male (aIRR 2.17, 95% CI 1.45–3.25, p < 0.001), but not female infants (aIRR 0.74, 95% CI 0.46–1.20, p = 0.22). There were no significant associations between active PM or mild-moderate past PM and malaria incidence in male or female infants. Male infants born to mothers given IPTp with DP had significantly less malaria in infancy than males born to mothers given SP, and 89.7% of this effect was mediated through prevention of PM.
Conclusion
PM may have more severe consequences for male infants, and interventions which reduce PM could mitigate these sex-specific adverse outcomes. More research is needed to better understand this sex-bias between PM and infant malaria risk.
Trial registration ClinicalTrials.gov, NCT02793622. Registered 8 June 2016, https://clinicaltrials.gov/ct2/show/NCT02793622
Keywords: Placental malaria, Pregnancy, Infants, Plasmodium falciparum
Background
Plasmodium falciparum remains a major public health problem affecting mainly pregnant women and young children. In pregnant women infected with P. falciparum, parasitized erythrocytes sequester in the placenta, resulting in placental malaria (PM), which is characterized by placental inflammation, parasite infiltration, and deposition of malaria pigment, a product of digestion of haemoglobin by P. falciparum [1]. Placental malaria is associated with adverse effects such as preterm delivery, low birth weight, stillbirth and neonatal mortality [2–4]. Despite the use of preventive measures, including insecticide-treated nets and intermittent preventive therapy during pregnancy (IPTp), the burden of PM among pregnant women living in high malaria transmission settings remains high [5].
There is evidence that PM may impact infants after birth [6–8]. Several observational studies have reported associations between PM and increased risks of malaria, non-malaria febrile illnesses, and anaemia in infancy, possibly due to immune tolerance induced by in utero exposure to malaria antigens [9–13]. However, most of these studies defined PM as the detection of malaria parasites in placental blood by microscopy, which has limited sensitivity and does not account for past placental infections characterized by the presence of malaria pigment [14]. Furthermore, the severity of malaria pigment deposition in the placenta was recently shown to be strongly predictive of adverse birth outcomes including low birth weight and preterm birth [15]. Whether the severity of malaria pigment deposition in the placenta is also associated with the risk of malaria in infancy is unknown.
A recent double-blind randomised controlled trial compared the incidence of malaria during infancy among infants born to mothers who received monthly IPTp with dihydroartemisin-piperaquine (DP) versus Sulfadoxine–pyrimethamine (SP). In this trial, infants born to mothers receiving IPTp-DP had a lower malaria incidence compared to infants born to mothers receiving IPTp-SP. However, this association was observed in male, but not female, infants [16]. To further evaluate the association between PM and the incidence of malaria in infants, a secondary and mediation analysis of these data was carried out to examine how much of the previously observed associations between IPTp and the risk of malaria in infants is mediated through prevention of PM.
Methods
Study design, setting, and participants
Data were collected from a birth cohort of infants born to HIV-uninfected pregnant women enrolled in a randomised controlled trial of monthly IPTp with DP vs SP (Trial registration, ClinicalTrials.gov; NCT02793622) conducted in Busia district, Uganda, an area of perennial high malaria transmission intensity. Details of the study have been previously reported [5, 16, 17]. Pregnant women were enrolled at 12-20 weeks of gestation and followed through delivery. At delivery, placental blood and tissue samples were collected. Following delivery, all live births were followed up to 12 months of age. Mothers were encouraged to bring their infants to a dedicated study clinic open every day for all their medical care. Routine assessments were conducted every 4 weeks for clinical assessment and collection of blood smears for the detection of parasites by microscopy. Infants presenting with a history of fever in the past 24 h or a documented tympanic temperature ≥ 38.0 °C had a thick blood smear collected for detection of malaria parasites and those diagnosed with malaria were treated according to the Uganda Ministry of Health guidelines. Non-malarial illnesses were treated according to the integrated management of childhood illnesses guidelines. At 12, 28, and 52 weeks of age, blood was collected for haemoglobin measurement.
Laboratory methods
Thick blood smears were stained with 2% Giemsa and read by microscopists [5]. Haemoglobin measurements were made using a spectrophotometer (Hemocue, Angelholm, Sweden). Malaria parasites were detected in placental blood by microscopy and loop-mediated isothermal amplification (LAMP) [18]. Placental biopsy specimens were embedded in paraffin wax, sectioned using a rotary microtome, fixed on glass slides, and dehydrated in sequential ethanol baths [19]. Separate slides were stained in 0.1% haematoxylin and 1% eosin for 5 and 1 min, respectively, or in 2% Giemsa for 30 min and examined for presence of intervillous parasite-infected erythrocytes and malaria pigment by two independent readers. The proportion of high-power fields (HPF) with malaria pigment deposition in fibrin was analysed as described [20].
Study outcomes
The primary outcome was the incidence of malaria from birth to 12 months of age. An incident episode of malaria was defined as the presence of fever (history of fever in the past 24 h or a tympanic temperature ≥ 38·0 °C) with a positive thick blood smear not preceded by another malaria episode in the last 14 days. Secondary outcomes included time to first episode of malaria; incidence of complicated malaria (malaria with danger signs or meeting standardized criteria for severe malaria), all-cause hospitalizations; and non-malarial febrile illnesses; prevalence of malaria parasitaemia during routine visits and anaemia (haemoglobin < 10 g/dL); and infant mortality.
Statistical methods
Data were double entered and verified in Microsoft Access, and statistical analyses conducted using Stata (14.2) including all live births with placental histology results. Follow-up began at birth and ended at 12 months of age or premature study withdrawal. The primary exposure variable, PM, was categorized as follows: no PM (absence of parasites or pigment); active PM (parasites detected in placental blood or tissue by microscopy LAMP or histology, with or without pigment [9, 21]); or past PM (presence of pigment, without parasites). Relationships between past PM and infant malaria incidence were initially evaluated by considering the proportion of HPF with pigment as a continuous variable. Given the non-linear nature of this relationship, past PM was further characterized into 2 groups based on best fit associations with the outcome variable: mild-moderate past PM (> 0–20% HPFs with pigment without parasites); or severe past PM (> 20% HPFs with pigment without parasites). Analyses were stratified by infant sex a priori. Associations between PM and the incidence of malaria were performed using negative binomial regression and adjusted for maternal parasitaemia status at enrollment, IPTp arm, gravidity, housing construction type, and clustering for twin gestation. The cumulative risk of any first episode of malaria was compared using a Cox proportional hazards model. For secondary outcomes, incident and repeated prevalence measures were compared using negative binomial regression model and generalized estimating equations with robust standard errors, respectively. Mediation analysis, using inverse odds weighting (IOW) [22], was used to estimate what proportion of the reported effect between maternal IPTp regimen and malaria incidence in infants [16] was mediated through preventing PM (Additional file 1). In brief, three models were used to conduct IOW mediation analyses. The first model used logistic regression to model treatment given mediator (PM) and mediator-outcome confounders. Predicted probabilities obtained from this model were then used to calculate treatment IOWs for each mother-infant pair. The second and third models used negative binomial regression to model the outcome given treatment with and without weights, respectively. The treatment coefficient from the model with weights estimated the direct effect, which was then subtracted from the treatment coefficient of the model without weights (total effect) to estimate the mediated effect. Bias-corrected 95% confidence intervals (CIs) were computed using bootstrapping. The proportion mediated by PM was calculated by dividing the mediated effect by the total effect. In all analyses, p-values of < 0·05 were considered statistically significant.
Results
Study profile and characteristics of study participants
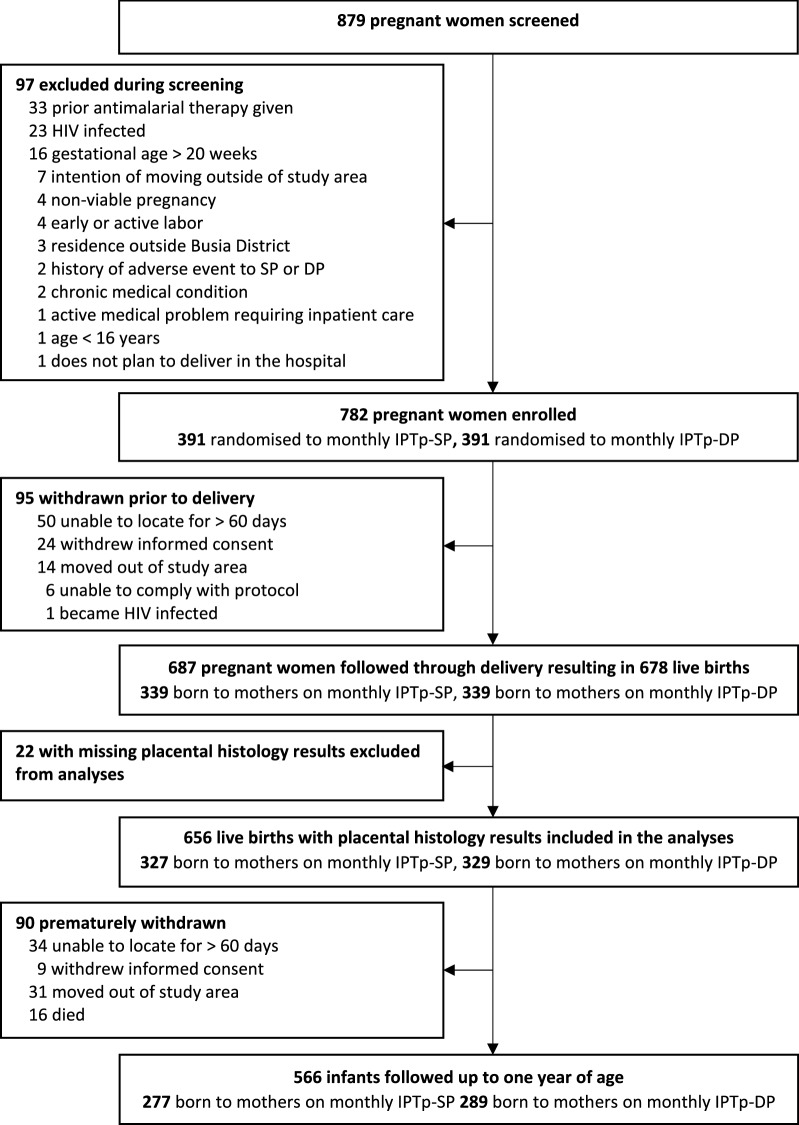
Between September 2016 and May 2017, 782 HIV-uninfected pregnant women were enrolled and randomised to receive either IPTp-SP or IPTp-DP, and 687 (87.9%) followed through delivery resulting in 678 live births (Fig. 1) [16]. A total of 656 infants with placental histology results were included in the analyses. Mean maternal age at enrollment was 24 years and 23.5% infants were born to primigravida mothers. During pregnancy, the incidence of malaria and prevalence of malaria parasitaemia were significantly lower among women randomised to IPTp-DP compared to those randomised to IPTp-SP. At delivery, women randomised to IPTp-DP had a significantly lower prevalence of active PM (2.1% versus 21.7% p < 0.001) and severe past PM (1.8% versus 12.2%, p < 0.001) compared to those randomised to IPTp-SP (Table 1).
Fig. 1.
Study profile. IPTp intermittent preventive treatment in pregnancy, DP dihydroartemisinin–piperaquine, SP sulfadoxine–pyrimethamine
Table 1.
Characteristics of study participants
| Characteristic | Maternal IPTp arm | |
|---|---|---|
| Monthly SP (N = 327) | Monthly DP (N = 329) | |
| Maternal characteristics at enrolment | ||
| Age in years, mean (SD) | 24.0 (5.9) | 24.0 (5.7) |
| Gravidity, n (%) | ||
| Primigravida/secundigravida | 152 (46.5%) | 156 (47.4%) |
| Multigravida | 175 (53.5%) | 173 (52.6) |
| House-hold type, n (%) | ||
| Modern House | 77 (23.6%) | 71 (21.6%) |
| Traditional House | 250 (76.5%) | 258 (78.4%) |
| Parasite prevalence by microscopy or qPCR, n (%) | ||
| No parasites | 53 (16.2%) | 63 (19.2%) |
| Sub-microscopic parasitaemia | 111 (33.9%) | 88 (26.8%) |
| Microscopic parasitaemia | 163 (49.9%) | 178 (54.1%) |
| Maternal characteristics during pregnancy | ||
| Parasite prevalence by microscopy, n/N (%)a | 797/2212 (36.0%) | 355/2260 (15.7%) |
| Incidence of malaria (episodes/ppy) | 0.59 | 0.09 |
| Placental malaria status | ||
| Placental malaria status, n (%) | ||
| No PM | 119 (36.4%) | 232 (70.5%) |
| Active PM | 71 (21.7%) | 7 (2.1%) |
| Past PM (Mild-moderate pigment) | 97 (29.7%) | 84 (25.5%) |
| Past PM (Severe pigment) | 40 (12.2%) | 6 (1.8%) |
| Characteristics of infants at birth | ||
| Preterm birth, n (%) | 25 (7.7%) | 17 (5.2%) |
| Gestation age in weeks, mean (SD) | 39.4 (1.9) | 39.6 (1.6) |
| Low birth weight, n (%) | 33 (10.1%) | 25 (7.6%) |
| Birth weight in grams, mean (SD) | 3055 (505) | 3024 (409) |
| Female sex, n (%) | 161 (49.2%) | 175 (53.2%) |
SP Sulfadoxine–pyrimethamine, DP dihydroartemisinin–piperaquine, SD standard deviation, ppy per person year, qPCR quantitative polymerase chain reaction
aDefined as number of routine positive blood smears divided by total number of routine blood smears
No PM no parasites or pigment detected, active PM parasites detected with or without pigment, Past PM (Mild-moderate) > 0–20% of high-power fields with pigment but no parasites, Past PM (severe) > 20%–60% of high-power fields with pigment but no parasites
Association between placental malaria and the incidence of malaria in infants
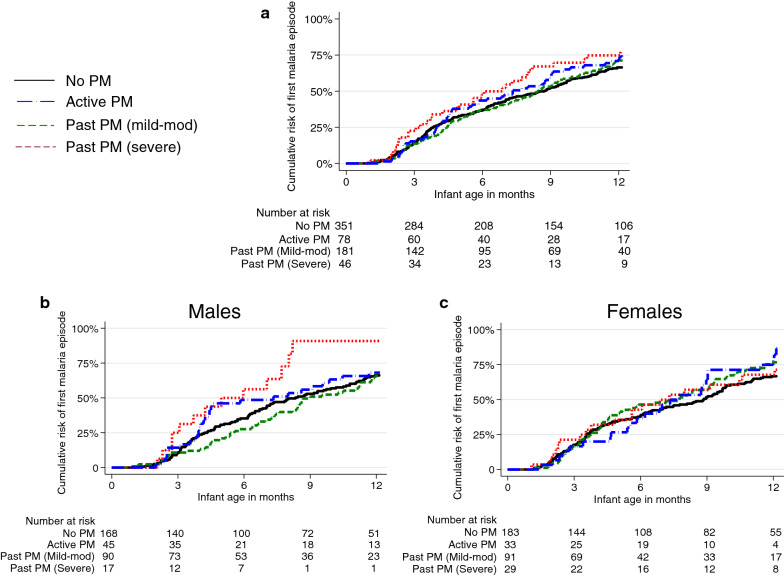
Overall, 1088 incident episodes of malaria were diagnosed over 596.6 person years of follow-up (1.82 episodes per person year). Each 1% increase in the proportion of HPF with pigment deposition in fibrin was associated with a higher incidence of malaria in infants but the difference was not statistically significant (adjusted incidence rate ratio [aIRR] 1.98, 95% CI 0.76–5.20, p = 0.16). However, on stratifying by infant sex, significant interaction was observed (P-interaction [pint] = 0.03). Among male infants, a 1% increase in HPF with pigment deposition in fibrin was associated with over 5 times higher incidence of malaria (aIRR 5.20, 95% CI 1.70–15.94, p = 0.004). No significant difference in the incidence of malaria was observed in female infants (aIRR 0.53, 95% CI 0.13–2.11 p = 0.37). Considering PM as a categorical variable, compared to infants born to mothers with no PM, the incidence of malaria was higher among infants born to mothers with active PM (aIRR 1.30, 95% CI 1.00–1.71, p = 0.05) and those born to mothers with severe past PM (aIRR 1.28, 95% CI 0.89–1.83, p = 0.18, Table 2), but the differences were not statistically significant. However, effect modification by infant sex was also observed in the association between categories of PM and the incidence of malaria in infants (pint = 0.02). Compared to no PM, severe past PM was associated with a higher incidence of malaria among male infants (aIRR 2.17, 95% CI 1.45–3.25, p < 0.001), but not among female infants (Table 2) .There were no significant associations between active PM or mild-moderate past PM and the incidence of malaria in male or female infants. There was no significant difference in the overall rate of first malaria episode among infants born to mothers with active PM (adjusted hazard ratio [aHR] 1.03, 95% CI 0.72–1.47, p = 0.88), mild-moderate past PM (aHR 0.92, 95% CI 0.71–1.18, p = 0.51), or severe past PM (aHR 1.18, 95% CI 0.75–1.86, p = 0.47), compared to those born to mothers with no PM. However, the association between PM and the rate of first malaria episode was also modified by infant sex. Compared to no PM, severe past PM was associated with a significantly higher rate of first malaria episode among male infants (aHR 1.99, 95% CI 1.04–3.81, p = 0.04; Fig. 2); no significant association was observed in female infants.
Table 2.
Association between different measures of placental malaria and incidence of malaria during infancy
| Infant category | Placental malaria categories (N) | Malaria episodes | Person years of follow-up | Malaria incidencea | Unadjusted | Adjustedb | ||
|---|---|---|---|---|---|---|---|---|
| IRR (95% CI) | p value | IRR (95% CI) | p-value | |||||
| All | No PM (351) | 574 | 327.4 | 1.75 | Reference group | Reference group | ||
| Active PM (78) | 152 | 68.9 | 2.21 | 1.27 (0.99–1.62) | 0.06 | 1.30 (1.00-1.71) | 0.05 | |
| Past PM (mild-mod) (181) | 271 | 157.8 | 1.72 | 0.97 (0.79–1.18) | 0.74 | 0.94 (0.76-1.16) | 0.55 | |
| Past PM (severe) (46) | 91 | 42.5 | 2.14 | 1.22 (0.91–1.63) | 0.19 | 1.28 (0.89-1.83) | 0.18 | |
| Male | No PM (168) | 255 | 154.5 | 1.65 | Reference group | Reference group | ||
| Active PM (45) | 86 | 39.9 | 2.16 | 1.31 (0.93–1.87) | 0.13 | 1.27 (0.87–1.84) | 0.22 | |
| Past PM (mild-mod) (70) | 129 | 77.7 | 1.66 | 0.98 (0.72–1.35) | 0.92 | 1.02 (0.76–1.36) | 0.90 | |
| Past PM (severe) (17) | 49 | 15.3 | 3.20 | 1.87 (1.29–2.71) | 0.001 | 2.17 (1.45–3.25) | <0.001 | |
| Female | No PM (183) | 319 | 172.9 | 1.84 | Reference group | Reference group | ||
| Active PM (33) | 66 | 29.0 | 2.28 | 1.24 (0.89–1.73) | 0.21 | 1.29 (0.87–1.91) | 0.21 | |
| Past PM (mild-mod) (91) | 142 | 80.1 | 1.77 | 0.96 (0.73–1.24) | 0.74 | 0.81 (0.61–1.08) | 0.15 | |
| Past PM (severe) (29) | 42 | 27.1 | 1.55 | 0.85 (0.58–1.24) | 0.40 | 0.74 (0.46–1.20) | 0.22 | |
CI confidence interval, IRR incidence rate ratio, mild-mod mild-moderate, PM placental malaria
aEpisodes of malaria per person year of follow-up
bAdjusted for gravidity, maternal IPTp, maternal parasitaemia at enrolment, and household type
No PM no parasites or pigment detected, active PM parasites detected with or without pigment, past PM (mild-mod) > 0–20% of high-power fields with pigment but no parasites, past PM(severe) > 20%–60% of high-power fields with pigment but no parasites
Fig. 2.
Time to first episode of malaria stratified by infant sex. a All infants, b male infants c female infants. PM placental malaria, mild-mod mild-moderate, No PM no parasites or pigment detected, active PM parasites detected with or without pigment, past PM (mild-mod) > 0–20% of high-power fields with pigment but no parasites, past PM(severe) > 20%–60% of high-power fields with pigment but no parasites
Association between placental malaria and other malaria outcomes in infants
The incidence of complicated malaria was non-significantly higher among infants born to mothers with active PM (aIRR 1.72, 95% CI 0.81–3.66, p = 0.16), and severe past PM (aIRR 2.44, 95% CI 0.93–6.37, p = 0.07; Table 3). The prevalence of parasitaemia detected during routine visits was also similar among infants born to mothers with active or past PM compared to infants born to mothers with no PM (Table 3). However, effect modification by infant sex was also observed in the association between PM and other malaria outcomes. Among male infants, severe past PM was associated with a nearly four-fold higher incidence of complicated malaria (aIRR 3.88, 95% CI 1.14–13.03, p = 0.03) and a more than two-fold higher prevalence of malaria parasitaemia during routine visits (risk ratio 2.24, 95% CI 1.34–3.74, p = 0.002) compared to no PM. No significant associations were observed among female infants (Table 3).
Table 3.
Association between placental malaria and other malaria outcomes during infancy
| Outcome measure | Infant category | Placental malaria categories | Number of cases (incidence PPY) | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|---|---|
| IRR (95% CI) | p-value | IRR (95% CI) | p-value | ||||
| Incidence of complicated malaria | All | No PM (351) | 30 (0.092) | Reference group | Reference group | ||
| Active PM (78) | 12 (0.17) | 1.89 (0.93–3.87) | 0.08 | 1.72 (0.81–3.66) | 0.16 | ||
| Past PM (mild-mod) (181) | 14 (0.09) | 0.97 (0.52–1.81) | 0.91 | 0.99 (0.52–1.92) | 0.98 | ||
| Past PM (severe) (46) | 9 (0.21) | 2.30 (0.97–5.43) | 0.06 | 2.44 (0.93–6.37) | 0.07 | ||
| Male | No PM (168) | 13 (0.08) | Reference group | Reference group | |||
| Active PM (45) | 6 (0.04) | 1.78 (0.63–5.02) | 0.27 | 1.18 (0.39–3.62) | 0.77 | ||
| Past PM (mild-mod) (90) | 11 (0.14) | 1.68 (0.76–3.68) | 0.20 | 1.54 (0.44–3.45) | 0.30 | ||
| Past PM (severe) (17) | 6 (0.39) | 4.61 (1.50–14.22) | 0.008 | 3.88 (1.14–13.03) | 0.03 | ||
| Female | No PM (183) | 17 (0.10) | Reference group | Reference group | |||
| Active PM (33) | 6 (0.21) | 2.09 (0.78–5.64) | 0.15 | 2.60 (0.91–7.44) | 0.14 | ||
| Past PM (mild-mod) (91) | 3 (0.04) | 0.38 (0.11–1.29) | 0.12 | 0.44 (0.13–1.54) | 0.20 | ||
| Past PM (severe) (29) | 3 (0.11) | 1.12 (0.34–3.66) | 0.85 | 1.63 (0.46–5.81) | 0.45 | ||
| Prevalence measures | Infant category | Placental malaria categories | Prevalence n/N (%) | Risk ratio (95% CI) | p-value | Risk ratio (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Prevalence of prasitaemia | All | No PM (351) | 377/4170 (9.0%) | Reference group | Reference group | ||
| Active PM (78) | 82/858 (9.6%) | 1.07 (0.76–1.52) | 0.70 | 1.19 (0.82–1.73) | 0.36 | ||
| Past PM (mild-mod) (181) | 154/1993 (7.7%) | 0.85 (0.65–1.12) | 0.26 | 0.86 (0.65–1.14) | 0.28 | ||
| Past PM (severe) (46) | 61/542 (11.3%) | 1.22 (0.84–1.77) | 0.30 | 1.38 (0.90–2.10) | 0.14 | ||
| Male | No PM (168) | 170/1964 (8.7%) | Reference group | Reference group | |||
| Active PM (45) | 50/498 (10.0%) | 1.17 (0.73–1.86) | 0.52 | 1.19 (0.71–2.00) | 0.51 | ||
| Past PM (mild-mod) (90) | 70/976 (7.2%) | 0.81 (0.55–1.20) | 0.29 | 0.87 (0.58–1.31) | 0.50 | ||
| Past PM (severe) (17) | 31/191 (16.2%) | 1.80 (1.08–2.99) | 0.02 | 2.24 (1.34–3.74) | 0.002 | ||
| Female | No PM (183) | 207/2206 (9.4%) | Reference group | Reference group | |||
| Active PM (33) | 32/360 (8.9%) | 0.97 (0.57–1.66) | 0.91 | 1.05 (0.61–1.83) | 0.85 | ||
| Past PM (mild-mod) (91) | 84/1017 (8.3%) | 0.90 (0.61–1.31) | 0.57 | 0.79 (0.53–1.17) | 0.24 | ||
| Past PM (severe) (29) | 31/351 (8.6%) | 0.90 (0.54–1.51) | 0.70 | 0.86 (0.48–1.52) | 0.60 | ||
CI confidence interval, IRR incidence rate ratio, mild-mod mild-moderate, PM placental malaria, PPY per person year
aAdjusted for gravidity, maternal IPTp, maternal parasitaemia at enrolment, and household type
No PM no parasites or pigment detected, active PM parasites detected with or without pigment, past PM (mild-mod) = > 0–20% of high-power fields with pigment but no parasites; past PM(severe) = > 20%–60% of high-power fields with pigment but no parasites
Association between placental malaria and non-malarial outcomes in infancy
There were 16 deaths (2.4% of infants) and 25 all-cause hospitalisations during follow-up. Severe past PM was associated with a non-significant higher incidence of all-cause hospitalisations among infants (aIRR 2.90, 95% CI 0.59–14.44, p = 0.19; Table 4) compared to no PM. There were no significant differences in the incidence of non-malaria febrile illnesses and prevalence of anaemia during routine visits among infants born to mothers in different PM categories compared to no PM (Table 4).
Table 4.
Association between placental malaria and non-malaria outcomes in infants during the first year of life
| Outcome measure | Placental malaria category (N) | Number of cases (incidence PPY) | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|---|
| IRR (95% CI) | p-value | IRR (95% CI) | p-value | |||
| Incidence of all-cause hospitalisations | No PM (351) | 9 (0.027) | Reference group | Reference group | ||
| Active PM (78) | 3 (0.044) | 1.66 (0.40–7.00) | 0.49 | 1.14 (0.22–5.98) | 0.87 | |
| Past PM (mild-mod) (181) | 7 (0.044) | 1.72 (0.58–5.12) | 0.33 | 1.26 (0.44–3.64) | 0.66 | |
| Past PM (severe (46) | 6 (0.141) | 4.85 (1.38–17.00) | 0.01 | 2.90 (0.59–14.44) | 0.19 | |
| Incidence of non-malarial febrile illnesses | No PM (351) | 1128 (3.45) | Reference group | Reference group | ||
| Active PM (78) | 203 (2.95) | 0.86 (0.72–1.03) | 0.10 | 0.84 (0.64–1.10) | 0.20 | |
| Past PM (mild-mod) (181) | 536 (3.40) | 0.98 (0.86–1.13) | 0.82 | 1.03 (0.84–1.25) | 0.79 | |
| Past PM (severe (46) | 152 (3.58) | 1.04 (0.85–1.26) | 0.72 | 1.06 (0.72–1.57) | 0.76 | |
| Prevalence measures | Categories | Prevalence n/N (%) | Risk ratio (95% CI) | p-value | Risk ratio (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Anaemiac | No PM (351) | 227/934 (24.3%) | Reference group | Reference group | ||
| Active PM (78) | 48/190 (25.3%) | 1.07 (0.76–1.50) | 0.69 | 1.00 (0.69–1.47) | 0.98 | |
| Past PM (mild-mod) (181) | 106/441 (24.0%) | 1.01 (0.80–1.29) | 0.92 | 0.94 (0.73–1.22) | 0.64 | |
| Past PM (severe (46) | 37/118 (31.4%) | 1.29 (0.94–1.77) | 0.12 | 1.16 (0.79–1.70) | 0.44 | |
CI confidence interval, IRR incidence rate ratio, mild-mod mild-moderate, PM placental malaria
aAdjusted for gravidity, maternal IPTp, maternal parasitaemia at enrolment, and household type
b Defined as haemoglobin < 10 g/dL measured routinely at 12, 28, and 52 weeks of age
No PM no parasites or pigment detected, active PM parasites detected with or without pigment, past PM (mild-mod) > 0–20% of high-power fields with pigment but no parasites, past PM (severe) > 20%–60% of high-power fields with pigment but no parasites
Does prevention of PM explain the difference in infant malaria incidence between IPTp-DP and IPTp-SP?
Male infants born to mothers who received IPTp-DP have been previously reported to have a lower incidence of malaria in infancy compared to male infants whose mothers received IPTp-SP [16]. Consistent with this prior analysis, male infants with placental histology results born to mothers who received IPTp-DP had 23% less malaria than male infants born to mothers who received IPTp-SP (IRR 0.77, 95% CI 0.61–0.99, p = 0.049), but this association was not observed in female infants (Table 5). Mediation analysis showed that among all infants, 43% of IPTp-DP’s greater effect on preventing malaria during infancy than IPTp-SP was attributed to preventing PM (IRRmediated 0.95, versus IRRtotal 0.89, Table 5). In males, this proportion was 89.7% (IRRmediated 0.79 versus IRRtotal 0.77). Among female infants, the proportion of mediated effect was not calculated because the direct and mediated effects were in opposing directions.
Table 5.
Effect of IPTp on malaria incidence in infants that is mediated by preventing placental malaria
| Infant category | Total effect | Direct effect | Mediated effect | % of mediated effecta | |||
|---|---|---|---|---|---|---|---|
| IRR (95% CI) | p-value | IRR (95% CI) | p-value | IRR (95% CI) | p-value | ||
| All sexes | 0.89 (0.75–1.04) | 0.16 | 0.94 (0.73–1.17) | 0.58 | 0.95 (0.77–1.16) | 0.61 | 43.5% |
| Male | 0.77 (0.61–0.99) | 0.05 | 0.97 (0.67–1.36) | 0.89 | 0.79 (0.56–1.09) | 0.17 | 89.7% |
| Females | 1.01 (0.81–1.26) | 0.96 | 0.92 (0.71–1.22) | 0.56 | 1.09 (0.87–1.38) | 0.45 | – |
CI confidence intervals, IRR incidence rate ratio
IRRs represent the effect of IPTp DP versus SP on the incidence of malaria in infants. Confidence intervals reported here were obtained by bootstrapping and may differ from those reported in the primary analysis which used the delta method specifying robust standard errors
aProportion of mediated effect was calculated using [ln(IRRmediated effect)/ln(IRRtotal effect)]*100
Discussion
In this secondary analysis of data from a birth cohort of infants born to women randomised to receive monthly IPTp with SP vs DP, infants born to mothers with active PM and severe past PM had a non-significant higher incidence of malaria and complicated malaria during the first year of life compared to infants born to mothers without PM. However, the association between severe past PM and infant malaria was sex-specific. In male, but not female, infants, severe past PM was associated with a significantly higher incidence of malaria, a higher rate of first malaria episode, a higher incidence of complicated malaria, and a higher prevalence of parasitaemia during routine visits, compared to no PM. No sex-specific differences were observed between active PM and the incidence of malaria in infancy. Importantly, male infants born to mothers given IPTp-DP had significantly less malaria in infancy than males born to mothers given IPTp-SP, and 89.7% of this effect was mediated through prevention of PM.
Several prior studies have reported associations between active PM defined as detection of parasites in placental blood or tissue and an increased risk of malaria during infancy [9–11, 23–25]. Active PM detected by microscopy was associated with a higher risk of malaria infection in Ugandan infants [23], an increased rate of first parasitaemia in Beninese infants [11], and a higher risk of first episode of malaria in Gabonese infants [24]. In Mozambique, active PM detected by histology was also associated with higher odds of malaria compared to no PM [9]. The results from the current study, although not statistically significant, are consistent with these prior studies. The exact mechanisms through which PM might impact on the incidence of malaria in infancy are not well understood, but may be due to the effects of PM on the fetal immune system, including modulation of innate and adaptive cellular immune responses, as well as altered maternal–fetal transfer of antibodies to P. falciparum [26]. Alternatively, these associations may represent confounding secondary to shared levels of exposure to malaria parasites between mothers and their infants. To limit the effect of confounding by malaria exposure in the current study, IPTp arm and markers of exposure, including housing structure and maternal parasitaemia at enrolment were adjusted for, but the possibility of residual confounding persists.
Importantly, in this study, there was an association between the severity of past PM, defined as the proportion of HPF with malaria pigment deposition, with malaria risk, but only in male infants. Severity of malaria pigment deposition in the placenta has been previously reported as a strong predictor of adverse birth outcomes [15]. To our knowledge, this is the first report to suggest that severity of past PM is also associated with increased risk of malaria in infancy, and that infant sex may modify these associations. Although the precise mechanism by which infant sex modifies the relationship between PM and infant malaria risk remains uncertain, there is a growing body of evidence of sex-based differences in susceptibility to infectious diseases in infants [27, 28]. While one study in southern Sudan suggested that pregnant women who bore female infants were more likely to have PM than those who bore male infants [29], several adverse pregnancy outcomes, including stillbirths, have been shown to be more common in males than in females [30]. This suggests that in utero fetal exposures may have more severe consequences for male infants than female infants [31]. Furthermore, male infants exposed to malaria in utero have been shown to have higher frequencies of regulatory T cells in cord blood compared to female infants with similar exposure [32], suggesting that in utero malaria exposure may differentially induce tolerance to malaria antigens in male, but not female, infants. Alternatively, other sex-based differences, including malaria-induced responses to toll-like receptor ligands [27, 32], expression of x-chromosome encoded genes [33], and/or glucocorticoid receptor expression [34] may be responsible for these findings. Future studies are needed to better understand how PM may result in different sex-specific outcomes in infants.
IPTp with highly effective drugs such as DP can reduce the severity of PM, and in this study, IPTp-DP was associated with a statistically significant lower incidence of malaria among male infants, and a non-significant lower incidence of malaria among all infants, compared with IPTp-SP. In the mediation analysis, 89.7% of the effect of IPTp-DP vs SP on the incidence of malaria among male infants was mediated through prevention of PM. Although this result was not statistically significant, possibly due to limited sample size to conduct this stratified mediation analysis, these results suggest that in addition to preventing adverse birth outcomes in all infants, effective interventions in pregnancy that reduce severe PM may also result in a lower risk of malaria in male infants.
In this study, there was no significant association between PM and the incidence of non-malaria febrile illnesses in infancy, contrary to other reports that suggest that in utero exposure may also influence immune responses to non-malarial infections [7, 35]. Furthermore, effective prevention of PM with IPTp-DP was not associated with a lower incidence of non-malarial febrile illnesses in infancy compared with IPTp-SP [16]. These data suggest that PM may specifically impact infant malaria risk, although the possibility that it may impact other non-malaria infections cannot be excluded.
This study had some limitations. This secondary analysis was exploratory in nature and did not use categories of PM based on malaria pigment deposition in fibrin used by previous studies [15, 20]. However, prior studies assessed associations between the severity of placental pigment and adverse birth outcomes. This is the first study to assess associations between the severity of placental pigment deposition and infant malaria risk. It would be desirable to come up with a standardized classification system relating the severity of placental pigment deposition to infant malaria risk using data from multiple independent studies from different epidemiological settings. There were relatively small number of infants in the severe past and active PM categories, and these findings should, therefore, be interpreted with caution. The study was conducted in a very high malaria transmission setting and therefore its findings cannot be generalized to other malaria transmission settings. Finally, exclusion of infants with missing placental histology results (3.2%) and losses to follow-up may have reduced the power of the study and introduced bias if the infants excluded and those lost to follow-up were different from those who completed follow-up. However, there were no significant differences between infants excluded from the analysis, those lost to follow-up, and the infants who completed the study.
Conclusions
Overall, this study suggests that severe malaria pigment deposition in placental tissue is associated with a higher incidence of malaria during the first year of life among infants residing in a setting of high malaria transmission intensity; however, this association was seen in only male infants. Highly effective interventions which reduce both severe past and active PM could be protective in male infants. Future research is needed to evaluate the association between PM and the risk of malaria in infancy in larger studies conducted in moderate and high malaria transmission settings and to evaluate mechanistic pathways between PM and infant malaria risk.
Supplementary information
Additional file 1. Detailed description of mediation analysis using the inverse odds ratio weighting (IORW) approach.
Acknowledgements
We are grateful to the pregnant women and their infants who participated in this study, the administration of Masafu General Hospital, Busia for the support, and staff members of Infectious Diseases Research Collaboration for running the study. This manuscript was part of the first author’s PhD studies which were funded by the Fogarty International Center. We are also grateful to Dr Emily Webb for providing statistical advice.
Abbreviations
- aIRR
Adjusted incidence rate ratio
- CI
Confidence interval
- DP
Dihydroartemisinin–piperaquine
- HPF
High-power fields
- IOW
Inverse odds weighting
- IPTp
Intermittent preventive therapy during pregnancy
- IRR
Incidence rate ratio
- LAMP
Loop mediated isothermal amplification
- PM
Placental malaria
- SP
Sulfadoxine–pyrimethamine
Authors’ contributions
DH, MK, and GD conceived the study with input from AK, PJ, MN, and TC. RK, TO, AK, TC, and GD developed the procedures and wrote the protocol. RK and TO coordinated the fieldwork with input from AK, MN and TR. PJ and HO coordinated the laboratory work. AK conducted the data analysis with support from PJ and MR. SS and DC participated in the analysis, manuscript writing and revision. All authors reviewed the final manuscript and gave permission for publication. All authors read and approved the final manuscript.
Funding
This work was supported by grants received from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant Number: P01 HD059454), the Fogarty International Centers training grant (D43TW7375), the March of Dimes Foundation (Basil O’Connor Award to PJ), and the Bill and Melinda Gates Foundation (OPP1141549). The funders did not play a role in the study design, data collection, analysis, and interpretation, and writing the manuscript.
Availability of data and materials
All data generated or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Makerere University School of Biomedical Sciences Ethics Committee, Uganda National Council of Science and Technology, University of California San Francisco Research Ethics Committee, Stanford University Institutional Review Board, and London School of Hygiene and Tropical Medicine Ethics Committee. Parents/guardians of all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abel Kakuru, Email: akakuru@idrc-uganda.org.
Prasanna Jagannathan, Email: prasj@stanford.edu.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12936-020-03522-z.
References
- 1.Sharma L, Shukla G. Placental malaria: a new insight into the pathophysiology. Front Med (Lausanne). 2017;4:117. doi: 10.3389/fmed.2017.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker PG, ter Kuile FO, Garske T, Menendez C, Ghani AC. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health. 2014;2:e460–e467. doi: 10.1016/S2214-109X(14)70256-6. [DOI] [PubMed] [Google Scholar]
- 3.Moore KA, Simpson JA, Scoullar MJL, McGready R, Fowkes FJI. Quantification of the association between malaria in pregnancy and stillbirth: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1101–e1112. doi: 10.1016/S2214-109X(17)30340-6. [DOI] [PubMed] [Google Scholar]
- 4.Kapisi J, Kakuru A, Jagannathan P, Muhindo MK, Natureeba P, Awori P, et al. Relationships between infection with Plasmodium falciparum during pregnancy, measures of placental malaria, and adverse birth outcomes. Malar J. 2017;16:400. doi: 10.1186/s12936-017-2040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kajubi R, Ochieng T, Kakuru A, Jagannathan P, Nakalembe M, Ruel T, et al. Monthly Sulfadoxine–pyrimethamine versus dihydroartemisinin–piperaquine for intermittent preventive treatment of malaria in pregnancy: a double-blind, randomised, controlled, superiority trial. Lancet. 2019;393:1428–1439. doi: 10.1016/S0140-6736(18)32224-4. [DOI] [PubMed] [Google Scholar]
- 6.Laufer MK. Beyond birthweight: benefits and risks of preventing malaria in pregnancy. Lancet. 2019;393:1388–1390. doi: 10.1016/S0140-6736(18)32615-1. [DOI] [PubMed] [Google Scholar]
- 7.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12:330–340. doi: 10.1016/S1473-3099(11)70341-3. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Nixon CE, Miller O, Choi NK, Kurtis JD, Friedman JF, et al. Impact of malaria in pregnancy on risk of malaria in young children: systematic review and meta-analyses. J Infect Dis. 2020;222:538–550. doi: 10.1093/infdis/jiaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardaji A, Sigauque B, Sanz S, Maixenchs M, Ordi J, Aponte JJ, et al. Impact of malaria at the end of pregnancy on infant mortality and morbidity. J Infect Dis. 2011;203:691–699. doi: 10.1093/infdis/jiq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudova S, Divala T, Mungwira R, Mawindo P, Tomoka T, Laufer MK. Placental but not peripheral Plasmodium falciparum infection during pregnancy is associated with increased risk of malaria in infancy. J Infect Dis. 2017;216:732–735. doi: 10.1093/infdis/jix372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Port A, Watier L, Cottrell G, Ouedraogo S, Dechavanne C, Pierrat C, et al. Infections in infants during the first 12 months of life: role of placental malaria and environmental factors. PLoS One. 2011;6:e27516. doi: 10.1371/journal.pone.0027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutabingwa TK, Bolla MC, Li JL, Domingo GJ, Li X, Fried M, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2005;2:e407. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakuru A, Staedke SG, Dorsey G, Rogerson S, Chandramohan D. Impact of Plasmodium falciparum malaria and intermittent preventive treatment of malaria in pregnancy on the risk of malaria in infants: a systematic review. Malar J. 2019;18:304. doi: 10.1186/s12936-019-2943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: comparison of blood film preparation methods and of blood films with histology. J Clin Microbiol. 2003;41:1370–1374. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ategeka J, Kakuru A, Kajubi R, Wasswa R, Ochokoru H, Arinaitwe E, et al. Relationships between measures of malaria at delivery and adverse birth outcomes in a high-transmission area of Uganda. J Infect Dis. 2020;222:863–870. doi: 10.1093/infdis/jiaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakuru A, Jagannathan P, Kajubi R, Ochieng T, Ochokoru H, Nakalembe M, et al. Impact of intermittent preventive treatment of malaria in pregnancy with dihydroartemisinin–piperaquine versus Sulfadoxine–pyrimethamine on the incidence of malaria in infancy: a randomized controlled trial. BMC Med. 2020;18:207. doi: 10.1186/s12916-020-01675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okiring J, Olwoch P, Kakuru A, Okou J, Ochokoru H, Ochieng TA, et al. Household and maternal risk factors for malaria in pregnancy in a highly endemic area of Uganda: a prospective cohort study. Malar J. 2019;18:144. doi: 10.1186/s12936-019-2779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins H, Gonzalez IJ, Polley SD, Angutoko P, Ategeka J, Asiimwe C, et al. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis. 2013;208:645–652. doi: 10.1093/infdis/jit184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natureeba P, Ades V, Luwedde F, Mwesigwa J, Plenty A, Okong P, et al. Lopinavir/ritonavir-based antiretroviral treatment (ART) versus efavirenz-based ART for the prevention of malaria among HIV-infected pregnant women. J Infect Dis. 2014;210:1938–1945. doi: 10.1093/infdis/jiu346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muehlenbachs A, Fried M, McGready R, Harrington Whitney E, Mutabingwa Theonest K, Nosten F, et al. A novel histological grading scheme for placental malaria applied in areas of high and low malaria transmission. J Infect Dis. 2010;202:1608–1616. doi: 10.1086/656723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31:85–93. doi: 10.1016/S0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen Tchetgen EJ. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am J Epidemiol. 2015;181:349–356. doi: 10.1093/aje/kwu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Beaudrap P, Turyakira E, Nabasumba C, Tumwebaze B, Piola P, Boum Ii Y, et al. Timing of malaria in pregnancy and impact on infant growth and morbidity: a cohort study in Uganda. Malar J. 2016;15:92. doi: 10.1186/s12936-016-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz NG, Adegnika AA, Breitling LP, Gabor J, Agnandji ST, Newman RD, et al. Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis. 2008;47:1017–1025. doi: 10.1086/591968. [DOI] [PubMed] [Google Scholar]
- 25.Sylvester B, Gasarasi DB, Aboud S, Tarimo D, Massawe S, Mpembeni R, et al. Prenatal exposure to Plasmodium falciparum increases frequency and shortens time from birth to first clinical malaria episodes during the first two years of life: prospective birth cohort study. Malar J. 2016;15:379. doi: 10.1186/s12936-016-1417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington WE, Kakuru A, Jagannathan P. Malaria in pregnancy shapes the development of fetal and infant immunity. Parasite Immunol. 2018;12:e12573. doi: 10.1111/pim.12573. [DOI] [PubMed] [Google Scholar]
- 27.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 28.Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis. 2014;209(Suppl 3):S120–S126. doi: 10.1093/infdis/jiu232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adam I, Salih MM, Mohmmed AA, Rayis DA, Elbashir MI. Pregnant women carrying female fetuses are at higher risk of placental malaria infection. PLoS One. 2017;12:e0182394. doi: 10.1371/journal.pone.0182394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mondal D, Galloway TS, Bailey TC, Mathews F. Elevated risk of stillbirth in males: systematic review and meta-analysis of more than 30 million births. BMC Med. 2014;12:220. doi: 10.1186/s12916-014-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198(43):e41–e45. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prahl M, Jagannathan P, McIntyre TI, Auma A, Wamala S, Nalubega M, et al. Sex disparity in cord blood FoxP3(+) CD4 T regulatory cells in infants exposed to malaria in utero. Open Forum Infect Dis. 2017;4:ofx022. doi: 10.1093/ofid/ofx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clifton VL. Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Rachas A, Le Port A, Cottrell G, Guerra J, Choudat I, Bouscaillou J, et al. Placental malaria is associated with increased risk of nonmalaria infection during the first 18 months of life in a Beninese population. Clin Infect Dis. 2012;55:672–678. doi: 10.1093/cid/cis490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Detailed description of mediation analysis using the inverse odds ratio weighting (IORW) approach.
Data Availability Statement
All data generated or analysed during the current study are available from the corresponding author on reasonable request.