Abstract
Background
With an increasing number of women delivering in healthcare facilities in Low and Middle Income Countries (LMICs), healthcare workers’ hand hygiene compliance on labour wards is pivotal to preventing infections. Currently there are no estimates of how often birth attendants comply with hand hygiene, or of the factors influencing compliance in healthcare facilities in LMICs.
Methods
We conducted a systematic review to investigate the a) level of compliance, b) determinants of compliance and c) interventions to improve hand hygiene during labour and delivery among birth attendants in healthcare facilities of LMICs. We also aimed to assess the quality of the included studies and to report the intra-cluster correlation for studies conducted in multiple facilities.
Results
We obtained 797 results across four databases and reviewed 71 full texts. Of these, fifteen met our inclusion criteria. Overall, the quality of the included studies was particularly compromised by poorly described sampling methods and definitions. Hand hygiene compliance varied substantially across studies from 0 to 100%; however, the heterogeneity in definitions of hand hygiene did not allow us to combine or compare these meaningfully. The five studies with larger sample sizes and clearer definitions estimated compliance before aseptic procedures opportunities, to be low (range: 1–38%). Three studies described two multi-component interventions, both were shown to be feasible.
Conclusions
Hand hygiene compliance was low for studies with larger sample sizes and clear definitions. This poses a substantial challenge to infection prevention during birth in LMICs facilities. We also found that the quality of many studies was suboptimal. Future studies of hand hygiene compliance on the labour ward should be designed with better sampling frames, assess inter-observer agreement, use measures to improve the quality of data collection, and report their hand hygiene definitions clearly.
Keywords: Hand hygiene, Maternal and newborn health, Labour, Healthcare workers
Background
Globally, infection contributes to at least 9% of maternal deaths [1] and 16% of neonatal deaths [2], the vast majority of this burden concentrates in low and middle income countries (LMICs). Hand hygiene during birth has been long recognised as a key infection prevention opportunity [3, 4]. With an increasing number of women delivering in healthcare facilities in LMICs [5], appropriate hand hygiene compliance of healthcare workers on the labour wards is pivotal to preventing infections.
Several systematic reviews have been published on the compliance, determinants and interventions to improve healthcare workers hand hygiene across the facility environment [6–10]; only two of these reviews include studies from low resource healthcare facilities, none of which provide estimates for the labour ward [7, 8]. Erasmus et al. report a median hand hygiene compliance of 40% for studies from high-income countries [6]; the other, more recent, reviews focus on evaluating existing interventions and do not report summary estimates of compliance, but there is value in collating estimates from observational studies too.
Currently there are no estimates of how often birth attendants comply with hand hygiene, or of the factors influencing their compliance in healthcare facilities in LMICs. Hand hygiene compliance in LMICs may differ in levels and determinants compared to those in high-income countries (HICs), where most published evidence is. For example, there are cultural and contextual elements around the process of labour and delivery that might influence hand hygiene compliance of healthcare workers such as unpredictable workloads, unreliable water supplies, or the concept of pollution and purity around delivery – important among healthcare workers in India and Bangladesh [11, 12]. Finally, detailed estimates on compliance in LMICs and their determinants are useful to inform whether interventions are needed, and how to tailor them.
The aim of this paper is to systematically review the literature from LMICs to:
Estimate birth attendants’ hand hygiene compliance during labour and delivery in healthcare facilities
Assess the quality of the studies reporting these estimates
Investigate what factors influence hand hygiene compliance
Estimate the effectiveness of interventions aimed at increasing hand hygiene compliance
Estimate intra-cluster correlation for hand hygiene compliance comparing variation within and between facilities
Methods
The search was conducted on the 1st of September 2020, updating earlier searches on the 24th of April 2018 and on the 27th of January 2016 over EMBASE, MEDLINE, CINHAL, and the WHO regional databases (the website we used for the latter was not accessible during the last search in spite several attempts). We used a comprehensive set of search terms based on previous systematic reviews [8, 13, 14] and consulted the London School of Hygiene and Tropical Medicine librarian. The search themes included hand hygiene and maternity ward terms with international spelling variations, and it was restricted to LMICs. Additional file 1 details the strategy. Peer reviewed articles were eligible for inclusion, while abstracts and conference proceeding were not. All texts were reviewed using Endnote X7. No protocol was registered for this review.
Duplicates were removed, and titles and abstracts screened for any mention of hand hygiene compliance in labour wards. Two reviewers independently applied the inclusion criteria to the selected full texts. Any discrepancy was resolved through discussion. Once full texts were selected, one author screened references to search for other relevant studies that might be eligible for inclusion. The inclusion criteria were:
- Studies with either of the following estimates for the specific group of healthcare workers attending labour and delivery or working on the labour ward:
- A measure of frequency for hand hygiene compliance (observed or other objective method; self-reports were not included)
- OR an effect size (odds ratio, rate ratio, risk ratio) of factors driving hand hygiene (observed or other objective method; self-reports were not included)
LMICs based studies
Peer-reviewed studies
Intervention or observational studies
Quantitative studies
Studies in any language
Data extraction was done by one author and checked by another. The data extraction form included study type, intervention details, country, urban-rural location, type of healthcare facility, staff cadre, facility ward specification, availability of hand hygiene infrastructure (soap, water, handrub), sample size, sample selection, analysis methods, measurement tools, and the effect size of hand hygiene determinants. We extracted the estimates of hand hygiene compliance by healthcare workers before aseptic procedures (or compliance estimates which were likely to include before aseptic procedure opportunities) for a) types of patient-attendant interactions that could occur during labour and delivery, or b) healthcare workers working in the labour ward. We specifically focused on estimates reflecting hand hygiene opportunities before aseptic procedures these because these are the most pivotal to infection prevention. For each estimate we extracted the hand hygiene definition, the numerator, denominator, the percentage compliance estimates, the number of staff or women observed, the staff cadre, the number of facilities, and the intervention stage details underpinning the individual estimate. We calculated the percentage compliance for each included study where this was possible. We contacted the corresponding author (or if this was not published, the first or senior author whose email we found via their department or on researchgate) when it was not clear from the paper whether a) their observation included procedures around labour and vaginal delivery; or b) when the hand hygiene definition was unclear and the tool used was not available.
Key measures of bias and quality were included in the data extraction. For randomised controlled trials we intended to use the CONSORT guidelines to assess quality. For observational studies, we assessed quality using checklist we developed using eight items adapted from the STROBE guidelines’ [15] methods section (as recommended by Sanderson and colleagues) [16], to the specific context of observing hand hygiene in healthcare settings. Items included assessing 1) sampling methods, 2) quality of data collection, 3) description of the data collectors background, 4) whether inter-observer agreement was estimated, 5) the definition of hand hygiene compliance, 6) details of the tool used for observation, 7) whether study aims were concealed from the study participants and 8) whether the statistical procedures were described. Items were scored positively or negatively, except for items 1, 3 and 6 where we added an extra option of partially met when only one of two criteria was met, and item 7 which could also be scored as unclear.
Intra-cluster correlation (ICC) accounts for the relatedness of data by comparing the variance within clusters with the variance between clusters; it is useful for designing and analysing observational and intervention studies. To obtain the ICC for hand hygiene compliance of the included studies comparing the variation in compliance between and within facilities, we also contacted the authors of studies with multiple facilities (clusters) to ask for:
- Either, the following single measures:
- The standard deviation exhibiting how the cluster means vary from the population mean from cluster to cluster σb (between-cluster variation)
- The standard deviation exhibiting how individual values vary from their cluster mean from individual to individual σw (within-cluster variation). Individuals are birth attendants in our review.
Or, the overall estimated ICC (ρ) = ρ = σb2 / (σb2+ σw2)
We aimed to conduct pooled analysis of the estimates by hand hygiene compliance estimated using similar outcome definitions, measurement tools or investigating similar interventions, unless there are differences in setting or risk of bias; where studies did not use similar outcomes, measurement tools or investigate similar interventions, estimates were described.
We followed the PRISMA guidelines for systematic reviews to report our methods and findings (see Additional file 2) [17].
Results
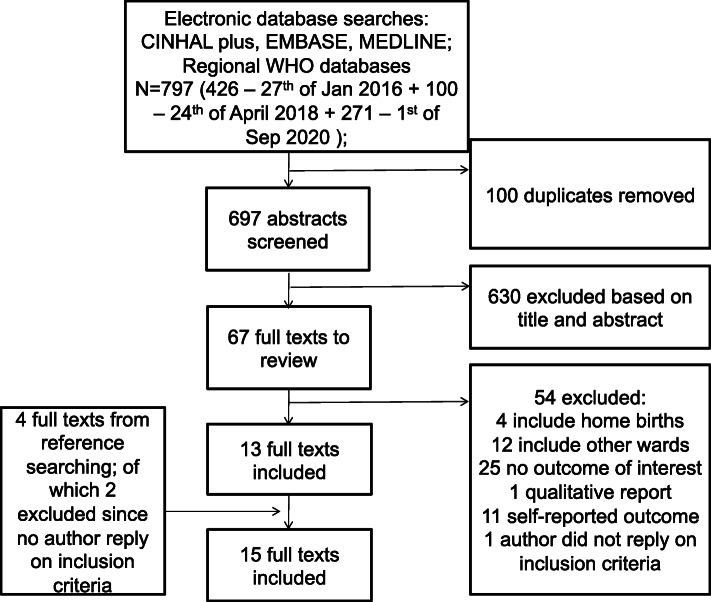
After removing duplicates (100), we obtained 697 results across the four databases and reviewed 71 full texts of which 4 are from reference searching (Fig. 1). We ultimately included fifteen that met our inclusion criteria. The reasons for excluding the fifty-seven studies are in Fig. 1, with the most common being that the study did not report on the outcome of interest, i.e. hand hygiene of healthcare workers during labour or delivery, or in the labour ward. In two articles which were identified via reference searching, it was unclear whether labour and delivery were being studied, and the author of the paper did not reply to enquiry, so these papers were not included.
Fig. 1.
Systematic search flow diagram
Of the fifteen included studies, seven were in Sub-Saharan Africa (Zanzibar-Tanzania, Zimbabwe, two in Ghana, and three in Nigeria), two were in Iran, the rest were located in in South-East Asia: three in India, one in Vietnam, one in the Thai-Myammar border, and one spanned several countries (Cambodia, Lao People’s Democratic Republic, Mongolia, Papua New Guinea, Philippines, Solomon Island, and Vietnam) – see Table 1. The studies were published between 1993 and 2020, with only one study being published prior to 2008. Four studies were conducted in a single facility. Six of the nine studies did not report any information on hand hygiene infrastructure (Table 1); one study discussed how inconvenient the sink location was; one study selected the hospital based on it generally having supplies to provide good quality of maternal care; three studies reported on the general availability of supplies (two positively and one negatively), but it is unclear what elements of hand hygiene infrastructure were surveyed if any. Only four studies reported specifically on the availability of hand hygiene infrastructure. Two of these studies reported that needed supplies were present, except for handrub in the first study [32], and disposable towel in the second [19]; one reported that not all the facilities had needed supplies, but the percentage refers to a wider set of facilities compared to the one observed for hand hygiene [27]; and one reported the availability of 24-h running water (52% of facilities) and soap (65% of facilities) (Table 1) [24].
Table 1.
Study characteristics
| Asp (2011) [18] | Buxton (2019) [19] | Changaee (2014) [20] | Cronin (1993) [21] | Danda (2015) [22] | Delaney (2017) [23] | Friday (2012) [24] | Gon (2018) [25] | Hoogenboom (2015) [26] | Mannava (2019) [27] | Phan (2018) [28] | Simbar (2008) [29] | Spector (2012) [30] | Tyagi (2018) [31] | Yawson (2013) [32] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country; site | Nigeria; Lagos | Nigeria; Ebonyi and Kogi | Iran; Lorestan | Ghana; North & South Birim Districts | Zimbabwe | India; Uttar Pradesh | Nigeria; Edo State | Zanzibar, Tanzania | Thai-Myanmar border; Mae La refugee camp | Cambodia, Lao PDR, Mongolia, Papua New Guinea, Philippines, Solomon Islands, Viet Nam | Vietnam; Ho Chi Minh City | Iran; Kurdistan | India; Karnataka |
India; Telangana, Andhra Pradesh |
Ghana; Accra |
| Study design | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional | Repeated Cross-sectional (nested in randomised trial) | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional | Pre-post multi-component intervention | Cross-sectional | Pre-post multi-component intervention | Cross-sectional | Cross-sectional |
| Facility type | 1 secondary and 1 tertiary maternity care facility |
2 Primary healthcare facilities 2 secondary healthcare facilities 2 Tertiary healthcare facilities |
9 public hospitals |
1 public hospital, 6 public health posts, 5 private maternity homes |
2 University of Zimbabwe Central Hospitals i.e. National referral hospitals | 15 healthcare facilities of the 60 selected for the intervention. The 60 facilities varied between primary and community health centres and first level referral units. | 63 healthcare facilities including primary health centers, private clinics, two secondary/district hospital, 2 tertiary/teaching hospitals |
1 referral hospital 1 maternity Hospital 3 Cottage Hospitals 1 private Hospital 3 district Hospitals 1 Primary healthcare Unit |
Shoklo Malaria Research Unit Clinic |
76 first level referred hospital 25 tertiary hospitals |
Hung Vuong University Hospital | Be-Sat Hospital of Sanandaj and Hafte-Teer Hospital of Beejar | Sub-district level hospital (basic emergency obstetric care and C-sections) |
26 Public secondary healthcare facilities 4 public tertiary healthcare facilities 5 private tertiary healthcare facilities |
Korle-Bu Teaching Hospital (tertiary healthcare facility) |
| Unit/ward | Maternity ward | Labour ward | Unclear. Presumably labour ward | Unclear. Presumably labour ward | Labour & postnatal ward | Labour wards | Delivery wards | Labour wards | Birth centre | Delivery rooms | Delivery suite | Labour & delivery wards | Unclear. Presumably labour ward | Labour ward | Emergency Room and Labour ward |
| Effect size | None | None | None | None | None | None | None | None | None | None | None | None | None | None | None |
| Intervention | None | None | None | None | None | Introduction of Safe Childbirth Checklist with peer coaching | None | None | None | None | Yes; educational intervention | None | Yes; testing checklist | The study is part of a baseline evaluation of a quality improvem. Intervention | None |
| Health professionals involved | Midwives | Doctors, midwives, auxiliary staff | Unclear. Midwives are mentioned in the discussion | Midwives, midwives’ assistants and lay women trained by midwives | Midwives | Any birth attendants | Attending midwives | All staff involved in assisting deliveries | Literate skilled birth attendants resident in the camp and trained by the clinic (not previously trained in midwifery) | Unclear. Any birth attendants | All healthcare workers in the delivery suite. Across all departments in the study they capture doctors, nurses, midwives and techniciansa | Unclear | Any healthcare workera (nurses & obstetricians) who cared for women and newborns from admission for childbirth to discharge | Health care providers working in the labour ward | Doctors and nurses |
| Type of patient-attendant interactions | 52 women during delivery and immediate postpartum | 31 women in active labour (cervical dilation> 3) and admitted to delivery | 200 (low risk) pregnant womenb | 18 vaginal deliveries and 22 neonatal cord-care events | 20 observations in the labour and 17 in the postnatal wards |
1277 deliveries. Specifically before pushing and soon after birth. |
Unclear. Mentions examination and procedures requiring gloves | 781 aseptic procedures during labour and delivery | 20 births | 371 deliveries | All types of hand hygiene opportunity in the delivery suite | 96 women with low risk pregnanciesb | 405 vaginal examinations at admission and 388 deliveries | 242 pre-vaginal examination and 235 deliveries | Unclear |
| Observation period | May 2010 | 4 weeks in July 2017 | Unclear | 2 Months. August–September 1991 | May to June 2014 | 6 to 12 weeks during the intervention from Dec 2014 to Sep 2016 | January to May 2011 | November 2015–April 2017 | 6 weeks. Nov-Dec 2008 |
Unclear 2016–2017 |
August 2014–May 2015 | Throughout 2006 | Baseline: Jul-Sept 2010; Endline: Sept.-Dec 2010 | May 2016–August 2016 | 3 weeks. September 2011 |
| Data collectors | Unclear | Qualified midwifes | Unclear | Project director and co-director (a Ghanaian nurse) | 3 midwives researchers. 2 working at the study institution. 1 just left the study institution | Trained nurses | Unclear. Trained staff | 3 Trained midwifes for each labour ward | 2 Dutch midwifery students (4th year) | Trained doctors, nurses, midwives or public health professionals | 6 infection control staff trained in direct observation. Unclear if worked in study institute |
Unclear. “Researcher” |
Student nurses previously unknown to hospital staff with no clinical responsibility | 12 nursing graduates trained for direct observation | 6 nurses specifically trained in infection control |
| Tool used for observation | Checklist developed for study. Based on protocol by Christensson et al. (2001) [33] | Standardised direct observation tool developed for study. Based on a previous tool developed for a qualitative care study. But it references a report [34]; unclear | Checklist developed for study. Content validity assessed | Checklist created for study using criteria from e.g. the WHO Global Programme on AIDS, 1989 | Checklist for labour ward developed for study | The WHO Safe Childbirth Checklist | Adapted tool from a previous study based in India. Unclear reference | Observation tool developed for study based on WHO guidelines on hand hygiene in healthcare, 2009 and WHO hand hygiene technical reference manual, 2009 | Checklist developed for study, drawing on WHO Safe Motherhood Needs Assessment v1.1 2001 | Standard checklist based on EENC Module 1: Annual Implementation Review and Planning Guide | Checklist using the WHO Guidelines on Hand Hygiene in Health Care, 2009. Observation checklist content validity reviewed by MoH and University staff | Tool developed for study based mainly on WHO’s protocol of normal birth, 1997& 2006 | WHO Safe Childbirth Checklist presumed to have been used |
Checklist adopted from the WHO concept of five moments of hand hygiene, 2010 |
Modified version of the WHO form for hand hygiene direct observation, 2010 |
| Study aim disclosed to participants | Unclear. Non participant observation | Study aims were explained to the participants and to the whole staff | Unclear | Participants not told when observation would take place or what practices were observed | Observers were “inside participants” assisting midwives in their work. Checklist was filled after procedures in private | Birth attendants were aware of the observation –observation included many aspects of quality of care; not hand hygiene only. | Unclear. Birth attendants were not previously informed of the walk-in visits but they were not blinded. | Healthcare workers were aware of the observation but were told that the observation was about overall quality of care (not specifically hand hygiene) | Unclear | Unclear | Unclear. Healthcare workers were aware of the observation period | Study aims were explained to the participants midwives | Nature of the intervention, which included awareness practices included in the WHO Safe Birth Checklist (e.g. hand hygiene), presumably clear to participants | Unclear | Unclear. Health workers in these service centres were not aware of being observed |
| Sampling | Unclear | The facilities with the highest number of deliveries were selected for each state (one primary, one secondary and one tertiary). Any woman who met inclusion criteria and gave consent was invited to participate up to 5 woman per facility. Unclear how the timing of facility visits was scheduled | Non-random quota sampling used to recruit 200 women. 10–30 selected in different stages of labour in each hospital. Sample size calculations justified. Unclear how different stages of labour or women and timing of visits were selected | Observation took place when the project staff visited a facility at a time when a woman was in labour. All midwives on duty when observation took place were included. Occasionally called by facility when delivery expected. Not clear how the timing of facility visits for observation were scheduled | All midwives at the time of the observation were included in the study. Not clear how the timing for facility visits were scheduled |
15 facilities for independent observation were based on pragmatic sample. Independent observer visited facilities during non-intervention days for a period of 6 to 12 weeks with the goal to reach 240 pose points in each facility. A mother was observed for as many pause points as possible. Not clear how the timing for facility visits were scheduled |
Public and private health facilities with high caseloads of pregnant women were selected from eight Local government areas. Presumably one walk-in visit for each hospital. Not clear how visits were scheduled and how many deliveries were observed |
Observation occured in the 10 highest-volume labour wards for a mode of 6 days each (5–14 days range) for 24 h a day. All attendants. Involved in assisting deliveries during observation. Not very clear how they selected which healthcare worker to observe. Sample size calculations justified. |
Unclear |
Random selection of 3 national or regional tertiary hospital, 4–12 provincial hospital and 2–4 district hospitals in each country. Deliveries were observed over 1–2 days in each hospital selected. Un clear the selection of delivery observation period; but it mentioned observation was limited to the time of the assessments. Not clear how the timing for facility visits were scheduled |
Unclear | Women’s selection – quota sampling (1 in 3 women) proportionally divided between morning, evening and night shifts. Not clear if all women received the full set of observations | Observation took place 24-h for a minimum of 6 days weekly; unobserved days were random. Observation was carried out at admission, from start of pushing to 1 h after birth, discharge. Unclear how women were selected each stage | In the labour room observer spent 6 h a day (either morning or evening shift) for 6 days observing 2–3 mothers a day. No details on how visits or shifts were scheduled. Only one woman was observed at the time | Observation in times & locations with high care density. Each centre was observed at a different time of day for 2 days between 8 AM-5 PM. Not clear how they selected which healthcare worker to observe |
| Water/Soap/handrub availability | Unclear. Sinks were not located in convenient locations | All facilities had soap and water in the delivery unit but during 1 observation there was no soap. All delivery units had a sink with connected tap available but 2 used veronica buckets. No disposable towerls | None | Unclear. Only reported missing items. Water, soap, handrub were not mentioned as missing | Unclear. They report broadly that basic supplies were often unavailable (not clear is specific to hand hygiene supplies) | Unclear | 24-h running water was present in 52% of the observed facilities. Soap in 65% of facilities. | Unclear | Unclear. All essential equipment for standard antenatal care, and essential care of obstetric complications was present. | 147 hospitals assessed for WASH services. 72% of hospitals had clean sinks with running water, soap or handrub in the delivery rooms. Data is not specific for the 101 hospital where deliveries were observed | None | None | Unclear. Hospitals selected based on general availability of supplies | unclear | Resources observed once. Water, soap and single-use towel for drying available on labour ward. Handrub not available |
aUnclear if all mentioned cadres were observed during labour and delivery
bUnclear whether hand hygiene was observed for all of these
Quality of primary studies
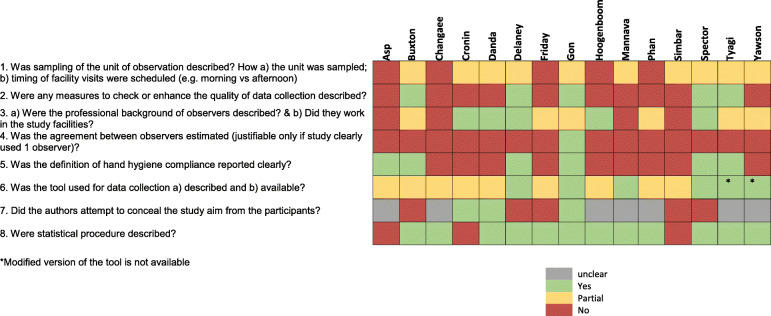
All studies used observation as their primary method of data collection. The methods were described in most articles only partially. The lowest ranked quality indicators were 1) sampling, 2) methods to enhance data quality during data collection, 3) measurement of inter-observer agreement, and 4) the level of description of the hand hygiene compliance definition – see Fig. 2.
Fig. 2.
Risk of bias and quality assessment
Sampling
We required two aspects of the sampling methods to be described: a) how the unit of observation (e.g. woman, procedure or healthcare worker) was sampled and b) how the timing of facility visits were scheduled. None described both aspects sufficiently; five articles did not describe them at all. As detailed in Table 1, it was often unclear how different women or healthcare workers were selected for observation.
Quality during data collection
Only four articles directly addressed the procedures adopted to ensure a better quality of data collection. Buxton et al. report that data collection did not start until results were consistent during the training period [19]. Spector et al. included on-site reviews of all observation forms within 72 h by the local study coordinator, and in-built data management checks confirming the data collected were logical [30]. Gon et al. provided tailored feedback to data collectors based on the results of the inter-observer exercise run in the first month [25]. Tyagi et al. incorporated quality checks in their tool as a results of the training [31].
Inter-observer agreement
Gon et al. is the only study that reports the results of interobserver agreement. This was calculated between pairs of data collectors in the first month of the study; the range of kappa statistics results was 0.73–0.93 for three pairs of data collectors [25]. Buxton et al. report that inter-rater reliability was monitored during the training period but do not report their results [19]. Spector et al. [30] attempted to examine agreement between observers – specifically, they reported that periodic assessments were used to confirm that data collectors achieved 100% concordance on a sample of three observations. Yawson and Hesse only report that different pairs of technical personnel visited the unit each day in order to limit intra-observer bias [32].
Definition of outcome
Hand hygiene compliance was not defined clearly in most studies. Each definition is reported in detail in Table 2. Some studies did not report whether soap use or handrub was necessary to achieve adequate hand hygiene and did not refer to guidelines that specifically do [20, 22, 24, 26, 27, 29]; in addition often studies did not report if other aspects of hand hygiene such as the sequence of actions preceding or following hand washing/rubbing, technique or duration were assessed in the summative compliance estimates – except for Gon et al. and Buxton et al. We describe here the studies where definitions presented additional anomalies. Yawson and Hesse, and Phan et al. mentioned that they followed the hand hygiene guidelines by the WHO but it was not clear which aspects of the guidelines they included. Buxton et al. also mentioned that they followed the WHO guidelines but created their own categories of hand hygiene ranging from the least hygienic (category 5) to the most hygienic (category 1) which included hand washing with soap, new gloves applied and no potential recontamination. Cronin et al., Danda et al., Friday et al. and Hoogenboom et al. chose a less informative definition of hand hygiene compliance because their denominator referred to whole individuals, group of individuals or facilities rather than specific patient-healthcare worker interactions (e.g. hand washed at least once or at least one birth attendant washed hands). In Changaee et al., it was not clear how they calculated their estimate of desirable hand washing.
Table 2.
Compliance estimates before aseptic procedures during labour or delivery
| Asp (2011) [18] | Buxton (2019) [19] | Changaee (2014) [20] | Cronin (1993) [21] | Danda (2015) [22] | Delaney (2017) [23] | Friday (2012) [24] | Gon (2018) [25] | Hoogenboom (2015) [26] | Mannava (2019) [27] | Phan (2018) [28] | Simbar (2008) [29] | Spector (2012) [30] | Tyagi (2018) [31] | Yawson (2013) [32] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Estimate | |||||||||||||||
| Outcome definition | Hand washing with soap or hand disinfection | Hand hygiene compliance before aseptic procedures. Hand washing with soap and new gloves applied without potential recontaminationc. | Unclear. Desirable hand washing. Estimated % was compliance with desirable status defined as 68–100% score. Unclear if soap necessary | Number of midwives who hand scrubbed with Dettol or soap and water | Whether each midwife washed her hands at least once. Unclear if with soap | Hand hygiene compliance measured by independent observers during non-intervention days. Hand washing with soap and water or alcohol rub | Unclear. Hand hygiene compliance at the facility level. Unclear if soap necessary and if handrub allowed. | Hand hygiene compliance before aseptic procedures. Steps included hand washing with alcohol-based hand rub or wash hands with soap and water, avoided recontamination and donning glovesc. | Hand washing of at least one of the birth attendant present. Unclear if with soap | Hand hygiene compliance. Unclear if with soap | Hand hygiene compliance is the ratio of the number of performed actions to the number of opportunities. Presumably, soap & water or handrub necessaryc. | Hand washing; Unclear if with soap | Hands washed with clean water and soap, and clean gloves worn for admission vaginal examination. Proportion of each birth practice successfully delivered | Hand hygiene compliance. Hand washing with soap and water or waterless alcohol-based hand rubc. | Hand hygiene compliance (% of times performed hand hygiene of all observed moments when required). Presumably, soap& water or handrub necessaryc. |
| Opportunity type | Before contact with patient during delivery | Before aseptic procedures during labour and delivery (including vaginal examination) | Second stage of labour; unclear if before or after what type of contact | Before delivery | Before procedures in the labour and postnatal ward | Before delivery | Before examining patients | Before aseptic procedures during labour and delivery | Before or after delivery | Before gloving for delivery | 5 types of WHO hand hygiene opportunities in the delivery suite e.g. before patient contact | Second stage of labour; unclear if before or after what type of contact | Before delivery | Before delivery | Before aseptic/clean procedures in the labour and emergency room |
| Numerator | 1 | 7 | Unclear | 0 | 14 | 374 | 30a | 75 | 15 | 300 | 142 | Unclear | 41a | 80a | 31a |
| Denominator | 52 | 201 | Unclear | 18 | 37 | 1027 | 63 | 781 | 20 | 371 | 507 | Unclear | 388 | 235 | 116 |
| Compliance % | 1.9%a | 4.0% | 11.5% | 0%a | 37.8%a | 36% | 48% | 9.6% | 75.0%a | 81.0% | 28.0%a | < 20.0%b | 10.6%a | 34.0% | 27.0% |
| N individuals | 52 women | 31 women | 200 women | 18 women | 37 midwives | 1277 deliveries | Unclear | 104 birth attendants | 20 women | 371 women | Unclear | 96 women | Unclear | 235 deliveries | Unclear |
| N facilities | 2 | 6 | 9 | Unclear | 2 | 15 | 63 | 10 | 1 | 101 | 1 | 2 | 1 | 35 | 1 |
|
Cadre/ intervention |
NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Before the intervention | NA | Before the intervention | NA | Doctors |
| 2nd Estimate | |||||||||||||||
| Outcome definition | As above | Hands were washed; unclear if with soap | As above | As above | As above | As above | As above | ||||||||
| Opportunity type | Before vaginal examination | Before wound care for episiotomy and vaginal tears | Before putting on gloves | Before touching delivery area surfaces and equipment | Before vaginal examination | Before per-vaginal examination | Before aseptic/clean procedures in the labour and emergency room | ||||||||
| Numerator | 6 | 4 | 32a | 289 | 5.3a | 92a | 4a | ||||||||
| Denominator | 121 | 4 | 63 | 371 | 405 | 242 | 18 | ||||||||
| Compliance % | 5.0% | 100%a | 51.0% | 78.0% | 1.3% | 38.0% | 21.2% | ||||||||
| N individuals | 31 women | 4 women | Unclear | 371 women | Unclear | Unclear | Unclear | ||||||||
| N facilities | 6 | Unclear | 63 | 101 | 1 | 35 | 1 | ||||||||
| Cadre/intervention | NA | NA | NA | Before the intervention | NA | Nurses | |||||||||
| 3rd Estimate | |||||||||||||||
| Outcome definition | Hands were washed; unclear if with soap | ||||||||||||||
| Opportunity type | Cord care; unclear if before/after | ||||||||||||||
| Numerator | 9 | ||||||||||||||
| Denominator | 22 | ||||||||||||||
| Compliance % | 40.9%a | ||||||||||||||
| N individuals | 22 newborns | ||||||||||||||
| N facilities | Unclear | ||||||||||||||
| Cadre/intervention | NA | ||||||||||||||
aEstimates imputed by systematic review author
bLess than 20% was considered a level that is “not acceptable”. No exact estimate provided – estimated from Fig. 1 of Simbar et al.
cFollowed WHO guidelines 2009 “WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care is Safer Care” or “Hand Hygiene Technical Reference Manual”
Another aspect of the definition is the type of hand hygiene opportunity (when hand hygiene should occur). The WHO hand hygiene guidelines refer to five key hand hygiene opportunities: before clean/clean procedures, after exposure to body fluids, before touching the patient, after touching the patient, after touching the patient’s surrounding. Studies did not always report what the type of contact (before vs. after; contact with intact skin i.e. “touching a patient” or non-intact/mucous membrane i.e. clean/aseptic procedures). Indeed, Changaee et al., and Simbar et al. were contacted for further information on their hand hygiene definition as it was unclear if it was before after the procedure/contact, but did not reply [29, 32]. Further enquires were also made to Yawson and Hesse, and Friday et al. on their definitions but with no reply [24, 32]. Another unclear area is what procedures during labour or delivery were captured. Studies that clearly outline this are Gon et al. and Buxton et al. [19, 25]
Hand hygiene compliance estimates during labour and delivery
We extracted estimates that were clearly for aseptic procedures, and estimates for which this was not clear or where aseptic procedures were not the exclusive focus. Definitions across the studies were extremely heterogeneous and hence we did not combine their estimates; compliance estimates varied from 0 to 100%. Spector et al. reported a baseline compliance of 1.3% before vaginal examinations during admission and 10.6% before deliveries [30]. A follow up study of the same intervention by Delaney et al. reported compliance before delivery at 36% after 2 months of intervention measured by independent observers during non-intervention days [23]. Buxton et al. found an overall compliance of 4% before aseptic procedures during labour and delivery, and a compliance of 5% before vaginal examination [19]. Gon et al. reported overall compliance with hand rubbing/washing, glove use and avoiding recontamination in 9.6% of opportunities before aseptic procedures during labour and delivery [25]. Yawson and Hesse reported hand hygiene compliance before aseptic procedures across both the labour and emergency room (we assumed that the emergency room was primarily dedicated to pregnant women); among doctors, compliance was 27.0%, whereas among nurses it was 21.2% [32]. Phan et al. reported the baseline compliance to be 28% across five types of WHO hand hygiene opportunities (before patient contact, before aseptic task etc.) observed in the delivery suite [28]. Mannava et al. reported a compliance 81% before gloving for delivery [27]. Simbar et al. [29] and Changaee et al. [20] reported on compliance during second stage of labour, although it was unclear whether compliance was before or after interaction with the patient or which type of interaction i.e. aseptic procedure, touching the patient. Simbar et al. reported a compliance level below 20.0%, which they describe as unacceptable [29]. We could not interpret the estimate by Chanagaee et al. because the definition of compliance was ambiguous [20]. Asp et al. report a compliance of 1.9% before contact with patient during delivery or immediate postpartum; it is unclear if this includes aseptic procedures or not [18]. Hoogenboom et al. found that in 75.0% of deliveries, either before or after the delivery, at least one birth attendant present hand washed [26]. Danda et al. reported compliance before procedures (not clear what type) across the labour and postnatal wards – here, 37.8% of midwives washed their hands at least once [22]. Friday et al. measured compliance before examining patients in the labour ward (48%) and before putting on gloves (51%). However, the compliance represents the percentage of facilities, rather than opportunities or individuals, that comply [24]. Finally, Cronin et al. reported that the midwives scrub hands in none of the 18 deliveries they observed (currently this practice is not necessary before delivery); however, all used either water and soap, or Dettol to perform hand hygiene [21]. All the four observations of wound care in this study were preceded by hand washing (100%) but only 40.9% of the cord-care observations (not clear if before or after cord care).
Table 3 describes the estimates extracted related to “before aseptic procedures” opportunities, from the smallest to the largest, as well as whether we considered their sample size adequate, their definition sufficiently good and whether the authors provided isolated estimates specifically for opportunities before aseptic procedures during labour and delivery. Five studies presented better definitions and larger sample sizes, and were specific to aseptic procedures during labour and birth: Spector et al. [30]; Gon et al. [25]; Buxton et al. [19]; Tyagi et al. [31]; Delaney et al. [23].
Table 3.
Selected compliance estimates summarised
| % Compliance | Author | Type of opportunity | Sample size | Definition | Specific estimate before aseptic proc. during labour and delivery |
|---|---|---|---|---|---|
| 0 | Cronin [21] | Before delivery | Small | Suboptimal | No |
| 1.3 | Spector [30] | Before vaginal exam. | Adequate | Good | Yes |
| 1.9 | Asp [18] | Before contact | Adequate | Suboptimal | No |
| 4.0 | Buxton [19] | Before aseptic procedures | Adequate | Good | Yes |
| 5.0 | Buxton [19] | Before vaginal examination | Adequate | Good | Yes |
| 9.6 | Gon [25] | Before aseptic procedures | Adequate | Good | Yes |
| 10.6 | Spector [30] | Before delivery | Adequate | Good | Yes |
| 11.5 | Changaee [20] | II stage of labour | Adequate | Suboptimal | No |
| < 20 | Simbar [29] | II stage of labour | Adequate | Suboptimal | No |
| 21.2 | Yawson [32] | Before aseptic (doct.) | Adequate | Satisfactory | Uncleara |
| 27.0 | Yawson [32] | Before aseptic (nurs.) | Adequate | Satisfactory | Uncleara |
| 28.0 | Phan [28] | All 5 types of opp. | Adequate | Satisfactory | No |
| 34.0 | Tyagi [31] | Before delivery | Adequate | Good | Yes |
| 36.0 | Delaney [23] | Before delivery (independent observers) | Adequate | Good | Yes |
| 37.8 | Danda [22] | Before procedures | Small | Suboptimal | No |
| 38.0 | Tyagi [31] | Before vaginal examination | Adequate | Good | Yes |
| 40.9 | Cronin [21] | During cord care | Small | Suboptimal | Yes |
| 48.0 | Friday [24] | Before examining patients | Unclear | Suboptimal | Unclear |
| 51.0 | Friday [24] | Before putting on gloves | Unclear | Suboptimal | Unclear |
| 75.0 | Hoogenboom [26] | During delivery | Small | Suboptimal | No |
| 78.0 | Mannava [27] | Before touching any delivery areas or surface | Adequate | Satisfactory | Yes |
| 81.0 | Mannava [27] | Before gloving for delivery | Adequate | Satisfactory | Yes |
| 100 | Cronin [21] | Before wound care | Small | Satisfactory | Yes |
aEmergency room may not only cater for labouring women
Technique and duration of hand hygiene, and avoiding recontamination
Only three studies [21, 25, 32] reported on aspects of hand hygiene quality such as technique and duration. Cronin et al. reported qualitatively that hand washings were generally not timed (not within the expected duration). Yawson and Hesse reported that on the labour ward, 50% or more of staff used soap and running water for hand washing, and dried hands with clean single use towels. Less than 50% washed hands for 40–60 s, or cleaned hands with alcohol handrub, or performed the appropriate handwashing technique [32]. Gon et al. reported the level of adequate rubbing/washing technique at 30.7% [25] defined as one of the hand gesture required by the WHO technical reference manual [35] i.e. “right palm over left dorsum with interlaced fingers and vice versa”; adequate duration was at 14.6% defined as ≥10 s based on the local guidelines for infection prevention [25].
Cronin et al. discuss qualitatively the concept of avoiding hand or glove recontamination before a procedure. This is a quote from their article.
“frequent breaks in technique included … the midwife’s gloved hands touching the patient’s bed, leg, abdomen, and perineal pad before the delivery.” [37].
Gon et al. defined recontamination of hands or gloves as any touch on potentially contaminated surfaces within the workflow after glove donning or hand rubbing/washing when preparing for a an aseptic procedure e.g. touching an unclean delivery surface, unclean hand-drying material, the woman and newborn outside the defined patient zone, the woman’s bed, trolley, unclean objects used during hand hygiene, and other unclean surfaces, unless classified as outside the workflow and provide an exhaustive list of these actions and that of patient zone within which touching surfaces is allowed [25]. They report that birth attendants risked recontaminating their hands or gloves in 45.3% of the opportunities when rubbing/washing or glove donning occurred [25].
Buxton et al. reported avoiding recontamination as part of hand hygiene compliance in the most hygienic category but did not specify the definition of what behaviours are included in recontamination [19].
Interventions, effect size for hand hygiene determinants and ICC
Three studies report interventions aimed at increasing hand hygiene compliance. Two studies relied on a pre-post intervention design, without randomization or control wards; one only reported the intervention period without baseline. The three studies reported on interventions including several components – two of these studies discuss the same intervention. Phan et al. [28] tested an educational program on hand hygiene provided to healthcare workers over two 3 h sessions. The educational model used experiential learning and incorporated novel techniques of learning that allowed for consideration of past hand hygiene experiences. Fifty two out of 53 healthcare staff in the delivery suite participated in the intervention. The intervention improved hand hygiene overall in the selected wards, but the effect was largest in the delivery suite increasing from 28 to 61.8% across all five types of WHO hand hygiene opportunities [28]. The improvement was sustained over a period of 6 months of post intervention follow-up. Given the nature of the intervention, we assumed that participants were not blinded to the aim of the intervention.
Spector et al. tested a four-components childbirth safety program based on the WHO Safe Childbirth Checklist [30]. After the intervention, hand hygiene compliance increased respectively from 1.3 to 97.8% before vaginal examination during admission and from 10.6 to 99.5% before delivery. The checklist included prompts on elements of hand hygiene; therefore, the healthcare workers were not blinded to the aim of the intervention. Delaney et al. [23] also describes the introduction of the WHO’s Safe Childbirth Checklist. This was part of a large randomised control trial, but the article included here focuses on the 60 facilities that received the intervention. There is no control or baseline group for comparing hand hygiene without the intervention. The main comparison is between the first month of intervention and the latter 7–8 months carried out by the same peer-coaches who run the intervention – compliance before delivery was respectively 76% and 94%. The independent assessment of hand hygiene described above showed compliance at 36% between 2 and 5 months of the intervention period. Given the presence of peer coaching, participants were not blinded to the aim of the intervention.
A few studies looked quantitatively at the association between potential determinants and hand hygiene compliance (measured via observation or other objective method) – but none of these were individual level determinants except for cadre. These appear to be all unadjusted associations. Mannava et al. reported that hand hygiene compliance before touching any delivery surfaces was lower in tertiary hospitals at 71%, vs 83% for first-level referral hospitals (p-value < 0.001), and higher in hospitals where all delivery rooms had soap and a sink with water compared to hospitals where needed supplies was not available in all rooms (50% vs 39%, p-value = 0.29) [27]. Buxton et al. tested the association between hand hygiene compliance and cadre, national state, and facility type - these were not found to be associated; they do find an association with shift – with the morning shift having higher compliance compared to the afternoon (p-value = 0.0034) and night (p-value = 0.008) [19]. Tyagi et al. described hand hygiene compliance by facility type, reporting a compliance of 100% in private facilities compared to 27% in public facilities (p-value = 0.011) [31]. They do not find an association with facility level and facility load [31]. Gon et al. report that hand hygiene compliance did not vary much by observer or by shift, indeed the confidence intervals overlapped across the of these categories [25].
With regards to the ICC, we present here the results we gathered from studies with the larger sample size and clearer definitions, involving more than two facilities, and where authors replied to our request. Estimates of rho in Buxton et al. [19] and Gon et al. [25], are both closer to 0 than 1 indicating that variance within facilities appear higher than between facilities (Table 4).
Table 4.
ICC results
ICC for the variation between and within individuals is also provided by Gon et al. and reports higher variance within than between individuals [25].
Discussion
We performed a systematic review of published studies reporting estimates of birth attendants’ hand hygiene compliance conducted in healthcare facilities in LMICs. We found fifteen studies that met our inclusion criteria. Hand hygiene compliance estimates were extremely diverse, ranging from 0 to 100%; the heterogeneity in definitions of hand hygiene did not allow us to combine or compare these meaningfully. Four studies (Cronin et al., Hoogenboom et al., Friday et al., and Mannava et al.) reported higher compliance. Except for Mannava et al., these with higher compliance also had a very small or unclear sample, and used an individual level or group level definition for the denominator rather than the number of patient-attendant interactions (hand hygiene opportunities) as recommended by the WHO hand hygiene guidelines [21, 22, 26]. The studies [19, 23, 25, 28, 30–32] with larger sample sizes and clearer definitions suggest compliance to hand hygiene before aseptic procedures to be low, between 1.3 and 38.0%. We have three estimates for hand hygiene before vaginal examination which spans between 1.3% [30] and 38% [31]; and we have five estimates for hand hygiene before labour/delivery-related procedures spans between 4 and 36% [23]. Overall, the quality of the included studies was particularly compromised by poorly described sampling methods and definitions.
The studies included were published in the last 18 years and spanned 14 countries between Sub-Saharan Africa, South East Asia and the Middle East. Four studies only included one facility, limiting their generalizability. The supplies of key hand hygiene infrastructure were poorly described, except in four studies. The quality of the studies included was generally poor with a high risk of bias with a few exceptions. The weakest aspect of the studies was their description of the sampling strategy, as most studies did not describe how the unit of observation was sampled (whether women, healthcare workers or specific procedures). Also, the reported definitions of hand hygiene were often incomplete. For most studies it was unclear whether the use of soap was a necessary condition to achieve hand washing compliance. In addition, the type of hand hygiene opportunity was often poorly described i.e. before or after the interaction with the patient; aseptic procedures vs. contact with the patient intact skin. Finally, in four studies the denominator did not rely on patient-worker interactions but on the overall performance of an individual or a group, or on the number facilities were hand hygiene was observed. This finding, of poor methods in conducting and reporting of observational studies on hand hygiene and more broadly of healthcare workers, was reported elsewhere [6, 36].
Beyond the basic aspects of quality required for any observational study and described by the STROBE guidelines [15], future studies focusing on hand hygiene during labour and delivery should design and report the following more clearly:
what sampling strategy was used to observe either workers, women, or patient-worker interactions; and how facilities visits were scheduled;
the methods used to ensure the quality of data collection in the study e.g. data monitoring
the inter-observer agreement where multiple observers are employed;
the definition of hand hygiene using the WHO hand hygiene guidelines [37] (i.e. soap necessary for hand washing; which type of hand hygiene opportunity e.g. before vs. after, touching intact skin vs. aseptic procedure; denominator based on patient-worker interactions rather than individual or group level performance; types of procedures involved in the aseptic procedure; sequence of actions required to comply to hand hygiene);
Our findings of low birth attendants’ hand hygiene compliance are consistent with other systematic reviews or multi-country studies in LMICs of hand hygiene among healthcare workers more generally, which report compliance estimates ranging from 22 to 35% during non-intervention periods [38, 39]. Similarly to these studies, our estimates point to a slight lower compliance in LMICs compared to high-income settings. With approximately 140 million women delivering worldwide, most of which are in LMICs and at least half of which occur in healthcare facilities where quality of care is suboptimal, these low estimates of hand hygiene compliance during labour/delivery are worrisome [5, 40, 41]. If correct, these estimates pose a substantial risk to infection prevention during birth in LMICs where both mothers and newborns are still largely affected by infection [1, 2, 42].
None of the included studies specifically investigated the wide range of individual determinants of hand hygiene compliance – except for cadre examined in one study. Four however report compliance estimates by study or facility characteristics. Three studies [30, 32] investigated the effect of two different interventions on hand hygiene, a checklist on quality of care at birth and an education program. Both were successful in increasing substantially the hand hygiene compliance during labour/delivery. Given the nature of their study design – pre-post intervention without a control ward, or without baseline, and with study participants who are no blinded – these interventions tell us more about the feasibility of these interventions in these specific contexts compared to anything conclusive about their scope for improving hand hygiene more widely in LMICs. With regards to ICC, from 2 studies we find that variation is greater within than between facilities.
Our systematic review covered four separate databases, has a clearly reported search strategy adapted from previous systematic reviews on the topic, did not pose any restrictions based on language, and used independent double full text screening and article extraction. A potential weakness is that our search might have missed articles which included hand hygiene in the broader framework of quality of care during birth or infection prevention and control and which did not mention hand hygiene in their title or abstract. We did not assess publication bias, but this would be more of an issue for intervention studies that found negative results for example than for observational studies reporting on compliance estimates. Finally, the set of health care facilities included in this systematic review is unlikely to represent health care facilities across LMICs. Without random sampling from the reference population of health care facilities, estimates of hand hygiene may be subject to selection bias stemming from researchers non-random decisions about which facilities to study. For example, researchers may be more likely to sample from higher volume facilities where deliveries are frequent than to sample from lower volume facilities. Studies suggest that higher volume facilities are better equipped for attending deliveries, but they maybe more prone to crowding which in turn makes hand hygiene more challenging [43]. Only Gon et al., Mannava et al., Tyagi et al. [25, 27, 31] can be regarded representative of the reference population which they targeted, respectively: high-volume labour wards in Zanzibar, hospitals implementing EENC in the countries included from South East Asia, hospitals with a newborn unit in Andhra Pradesh and Telengana regions of India who did not receive a quality improvement intervention. It is hard to make this inference for Friday et al. because of their group level definition of hand hygiene. [24]
Conclusions
In conclusion, we found fifteen articles reporting the hand hygiene compliance of healthcare workers during labour and delivery in LMICs. Compliance including before aseptic procedures opportunities for studies with larger sample sizes and clear definitions was low, ranging between 1 and 38%. This is an opportunity for infection prevention reduction during birth in LMICs facilities since effective interventions in this area are likely to reduce infection rate among mothers and newborns. We also found that the quality of many studies was suboptimal. In particular, future studies of hand hygiene compliance during the labour ward should be designed with better sampling frame, assess inter-observer agreement, use measures to improve quality of data collection and report their hand hygiene definitions clearly.
Supplementary Information
Additional file 1. “Systematic review search strategy” – it includes the search strategy for each database used in our review.
Additional file 2. “PRISMA 2009 Checklist” – It includes the details of our manuscript against the PRISMA checklist.
Acknowledgements
We thank Kerry Wong, Sophie Sarassat, and Andreia Leite for their help in translating articles from Chinese, French and Portuguese respectively. We also thank the librarian, Jane Falconer, at the London School of Hygiene and Tropical Medicine for her suggestions on the search strategy. Finally, we thank Sandra Virgo for running the updated search strategy in April 2018.
Abbreviations
- CONSORT
Consolidated Standards of Reporting Trials
- HICs
High Income Countries
- ICC
Intra-cluster correlation LMICs
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- STROBE
Strengthening the Reporting of Observational studies in Epidemiology
- WHO
World Health Organisation
Authors’ contributions
Conceived and designed the study: GG OC. Analysed the data: GG first reviewer, MDB and LD second reviewers. Wrote the paper: GG MDB SN OC LD. Data interpretation: GG MDB SN OC LD. The authors have read and approved the manuscript.
Funding
The Soapbox Collaborative contributed by funding the corresponding author, GG.
The writing of this paper provided part of the background.
needed for the HANDS study funded by the Medical Research Council−PHIND scheme, award number MR/N015975/1, and the CLEAN Study, funded by the United Kingdom Joint Global Health Trials (Wellcome, MRC, DFID, and DOH), award number MR/R019274/1. SN is supported by an award jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement which is also part of the EDCTP2 programme supported by the European Union. Grant Reference MR/K012126/1.
None of the funding bodies had any role in the design of the study or the collection, analysis, and interpretation of the data, or in writing the manuscript.
All authors have read and approved the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giorgia Gon, Email: giorgia.gon@lshtm.ac.uk.
Mícheál de Barra, Email: micheal.debarra@brunel.ac.uk.
Lucia Dansero, Email: lucia.dansero@gmail.com.
Stephen Nash, Email: stephen.nash@lshtm.ac.uk.
Oona M. R. Campbell, Email: oona.campbell@lshtm.ac.uk
Supplementary Information
The online version contains supplementary material available at 10.1186/s12913-020-05925-9.
References
- 1.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oza S, Lawn JE, Hogan DR, Mathers C, Cousens SN. Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000-2013. Bull World Health Organ. 2015;93:19–28. doi: 10.2471/BLT.14.139790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould IM. Alexander Gordon, puerperal sepsis, and modern theories of infection control--Semmelweis in perspective. Lancet Infect Dis. 2010;10:275–278. doi: 10.1016/S1473-3099(09)70304-4. [DOI] [PubMed] [Google Scholar]
- 4.Semmelweis I. The etiology, concept and prophylaxis of childbed fever. Madison: The University of Wisconsin Press; 1983. [Google Scholar]
- 5.Campbell OMR, Calvert C, Testa A, Strehlow M, Benova L, Keyes E, et al. The scale, scope, coverage, and capability of childbirth care. Lancet. 2016;388:2193–2208. doi: 10.1016/S0140-6736(16)31528-8. [DOI] [PubMed] [Google Scholar]
- 6.Erasmus V, Daha TJ, Brug H, Richardus JH, Behrendt MD, Vos MC, et al. Systematic review of studies on compliance with hand hygiene guidelines in hospital care. Infect Control Hosp Epidemiol. 2010;31:283–294. doi: 10.1086/650451. [DOI] [PubMed] [Google Scholar]
- 7.Vindigni SM, Riley PL, Jhung M. Systematic review: handwashing behaviour in low- to middle-income countries: outcome measures and behaviour maintenance. Tropical Med Int Health. 2011;16:466–477. doi: 10.1111/j.1365-3156.2010.02720.x. [DOI] [PubMed] [Google Scholar]
- 8.Luangasanatip N, Hongsuwan M, Limmathurotsakul D, Lubell Y, Lee AS, Harbarth S, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta-analysis. The BMJ. 2015;351:h3728. doi: 10.1136/bmj.h3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould DJ, Moralejo D, Drey N, Chudleigh JH, Taljaard M. Interventions to improve hand hygiene compliance in patient care. Cochrane Database Syst Rev. 2017;9:CD005186. doi: 10.1002/14651858.CD005186.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schweizer ML, Reisinger HS, Ohl M, Formanek MB, Blevins A, Ward MA, et al. Searching for an optimal hand hygiene bundle: a meta-analysis. Clin Infect Dis. 2014;58:248–259. doi: 10.1093/cid/cit670. [DOI] [PubMed] [Google Scholar]
- 11.Cross S, Afsana K, Banu M, Mavalankar D, Morrison E, Rahman A, et al. Hygiene on maternity units: lessons from a needs assessment in Bangladesh and India. Glob Health Action. 2016;9. 10.3402/gha.v9.32541. [DOI] [PMC free article] [PubMed]
- 12.Gon G, Restrepo-Méndez MC, Campbell OMR, Barros AJD, Woodd S, Benova L, et al. Who delivers without water? A multi country analysis of water and sanitation in the childbirth environment. PLoS One. 2016;11:e0160572. doi: 10.1371/journal.pone.0160572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huis A, van Achterberg T, de Bruin M, Grol R, Schoonhoven L, Hulscher M. A systematic review of hand hygiene improvement strategies: a behavioural approach. Implement Sci. 2012;7:92. doi: 10.1186/1748-5908-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould DJ, Moralejo D, Drey N, Chudleigh JH, Taljaard M. Interventions to improve hand hygiene compliance in patient care. Cochrane Database Syst Rev. 2017;9:CD005186. 10.1002/14651858.CD005186.pub4. [DOI] [PMC free article] [PubMed]
- 15.STROBE Initiative Group . STROBE checklist for cohort, case-control, and cross-sectional studies (combined) 2007. [Google Scholar]
- 16.Sanderson S, Tatt ID, Higgins JPT. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 17.PRISMA. http://www.prisma-statement.org/. Accessed 24 Apr 2017.
- 18.Asp G, Sandberg J, Ezechi O, Pettersson KO. Challenges of immediate newborn care in maternity units in Lagos, Nigeria: an observational study. J Obstet Gynaecol. 2011;31:612–616. doi: 10.3109/01443615.2011.593652. [DOI] [PubMed] [Google Scholar]
- 19.Buxton H, Flynn E, Oluyinka O, Cumming O, Esteves Mills J, Shiras T, et al. Hygiene during childbirth: an observational study to understand infection risk in healthcare facilities in Kogi and Ebonyi states, Nigeria. Int J Environ Res Public Health. 2019;16:1301. doi: 10.3390/ijerph16071301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Changaee F, Simbar M, Irajpour A, Akbari S. Quality assessment of Peripartum care. Iran Red Crescent Med J. 2014;16. 10.5812/ircmj.9069. [DOI] [PMC free article] [PubMed]
- 21.Cronin WA, Quansah MG, Larson E. Obstetric infection control in a developing country. J Obstet Gynecol Neonatal Nurs. 1993;22:137–144. doi: 10.1111/j.1552-6909.1993.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 22.Danda G, Dube K, Dube P, Mudokwenyu-Rawdon C, Bedwell C. An observational study of midwives’ practices to prevent peripartum sepsis in Zimbabwe. Afr J Midwifery Womens Health. 2015;9:17–21. doi: 10.12968/ajmw.2015.9.1.17. [DOI] [Google Scholar]
- 23.Marx Delaney M, Maji P, Kalita T, Kara N, Rana D, Kumar K, et al. Improving adherence to essential birth practices using the WHO safe childbirth checklist with peer coaching: experience from 60 public health facilities in Uttar Pradesh, India. Glob Health Sci Pract. 2017;5:217–231. doi: 10.9745/GHSP-D-16-00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friday O, Edoja O, Osasu A, Chinenye N, Cyril M, Lovney K, et al. Assessment of infection control practices in maternity units in southern Nigeria. Int J Qual Health Care. 2012;24:634–640. doi: 10.1093/intqhc/mzs057. [DOI] [PubMed] [Google Scholar]
- 25.Gon G, de Bruin M, de Barra M, Ali SM, Campbell OM, Graham WJ, et al. Hands washing, glove use, and avoiding recontamination before aseptic procedures at birth: a multicenter time-and-motion study conducted in Zanzibar. Am J Infect Control. 2019;47:149–156. doi: 10.1016/j.ajic.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoogenboom G, Thwin MM, Velink K, Baaijens M, Charrunwatthana P, Nosten F, et al. Quality of intrapartum care by skilled birth attendants in a refugee clinic on the Thai-Myanmar border: a survey using WHO safe motherhood needs assessment. BMC Pregnancy and Childbirth. 2015;15:17. doi: 10.1186/s12884-015-0444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mannava P, Murray JC, Kim R, Sobel HL. Status of water, sanitation and hygiene services for childbirth and newborn care in seven countries in East Asia and the Pacific. J Glob Health. 9. 10.7189/jogh.09.020430. [DOI] [PMC free article] [PubMed]
- 28.Phan HT, Tran HTT, Tran HTM, Dinh APP, Ngo HT, Theorell-Haglow J, et al. An educational intervention to improve hand hygiene compliance in Vietnam. BMC Infect Dis. 2018;18. 10.1186/s12879-018-3029-5. [DOI] [PMC free article] [PubMed]
- 29.Simbar M, Ghafari F, Zahrani ST, Majd HA. Assessment of quality of midwifery care in labour and delivery wards of selected Kordestan medical Science University hospitals. Int J Health Care Qual Assur. 2009;22:266–277. doi: 10.1108/09526860910953539. [DOI] [PubMed] [Google Scholar]
- 30.Spector JM, Agrawal P, Kodkany B, Lipsitz S, Lashoher A, Dziekan G, et al. Improving quality of Care for Maternal and Newborn Health: prospective pilot study of the WHO safe childbirth checklist program. PLoS One. 2012;7:e35151. doi: 10.1371/journal.pone.0035151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyagi M, Hanson C, Schellenberg J, Chamarty S, Singh S. Hand hygiene in hospitals: an observational study in hospitals from two southern states of India. BMC Public Health. 2018;18. 10.1186/s12889-018-6219-6. [DOI] [PMC free article] [PubMed]
- 32.Yawson AE, Hesse AAJ. Hand hygiene practices and resources in a teaching hospital in Ghana. J Infect Dev Ctries. 2013;7:338–347. doi: 10.3855/jidc.2422. [DOI] [PubMed] [Google Scholar]
- 33.Christensson K, Pettersson KO, Bugalho A, null C, Manuela M, Dgedge C, et al. The challenge of improving perinatal care in settings with limited resources. Observations of midwifery practices in Mozambique. Afr J Reprod Health. 2006;10:47–61. doi: 10.2307/30032443. [DOI] [PubMed] [Google Scholar]
- 34.MCHIP . Quality of antenatal and delivery care services in six countries in sub-Saharan Africa. Washington, DC: MCHIP; 2013. [Google Scholar]
- 35.WHO. Hand hygiene technical reference manual: WHO; 2009. http://apps.who.int/iris/bitstream/handle/10665/44196/9789241598606_eng.pdf;jsessionid=C23CC7AC25035E11B1F80D3012E835E4?sequence=1. Accessed 27 Nov 2017.
- 36.Jeanes A, Coen PG, Gould DJ, Drey NS. Validity of hand hygiene compliance measurement by observation: a systematic review. Am J Infect Control 2018;0. doi:10.1016/j.ajic.2018.08.004. [DOI] [PubMed]
- 37.World Health Organization . WHO guidelines on hand hygiene in health care: first global patient safety challenge clean care is safer care. Geneva: WHO; 2009. [PubMed] [Google Scholar]
- 38.Allegranzi B, Gayet-Ageron A, Damani N, Bengaly L, McLaws M-L, Moro M-L, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13:843–851. doi: 10.1016/S1473-3099(13)70163-4. [DOI] [PubMed] [Google Scholar]
- 39.Amazian K, Abdelmoumène T, Sekkat S, Terzaki S, Njah M, Dhidah L, et al. Multicentre study on hand hygiene facilities and practice in the Mediterranean area: results from the NosoMed network. J Hosp Infect. 2006;62:311–318. doi: 10.1016/j.jhin.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Graham W, Woodd S, Byass P, Filippi V, Gon G, Virgo S, et al. Diversity and divergence: the dynamic burden of poor maternal health. Lancet. 10.1016/S0140-6736(16)31533-1. [DOI] [PubMed]
- 41.Montagu D, Yamey G, Visconti A, Harding A, Yoong J. Where do poor women in developing countries give birth? A multi-country analysis of demographic and health survey data. PLoS One. 2011;6:e17155. doi: 10.1371/journal.pone.0017155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seale AC, Mwaniki M, Newton CRJC, Berkley JA. Maternal and early onset neonatal bacterial sepsis: burden and strategies for prevention in sub-Saharan Africa. Lancet Infect Dis. 2009;9:428–438. doi: 10.1016/S1473-3099(09)70172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruk ME, Leslie HH, Verguet S, Mbaruku GM, Adanu RMK, Langer A. Quality of basic maternal care functions in health facilities of five African countries: an analysis of national health system surveys. Lancet Glob Health. 2016;4:e845–e855. doi: 10.1016/S2214-109X(16)30180-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. “Systematic review search strategy” – it includes the search strategy for each database used in our review.
Additional file 2. “PRISMA 2009 Checklist” – It includes the details of our manuscript against the PRISMA checklist.
Data Availability Statement
All data generated or analysed during this study are included in this published article.