Abstract
BRCAness is defined as a phenotypic copy of germline BRCA mutations, which describes presence of homologous recombination defects in sporadic cancers. We detected BRCAness by multiplex ligation-dependent probe amplification (MLPA) and explored whether BRCAness can be used as a predictor of prognosis. BRCAness status was classified for total 121 breast cancer patients. Forty-eight patients (39.7%) were identified as BRCAness positive. Tumors of BRCAness were more likely to be hormone receptors negative (95.8% vs. 50.7%, P < 0.001), nuclear grade III (76.1% vs. 48.4%, P = 0.001) and triple-negative breast cancer subtype (91.6% vs. 42.5%, P < 0.001). Five-year disease free survival (DFS) (54.0% vs. 88.0%, P < 0.001) and overall survival (OS) (76.3% vs. 93.1%, P = 0.002) were significantly lower in BRCAness patients. In neoadjuvant chemotherapy subgroup analysis, clinical response rate for taxane-based regimen was significantly lower in BRCAness patients (58.3% vs. 77.8%, P = 0.041). Cox regression multivariate analysis showed that BRCAness was the independent prognostic factor for DFS (HR 2.962, 95%CI 1.184–7.412, P = 0.020), but not for OS (HR 2.681, 95%CI 0.618–11.630, P = 0.188). BRCAness is associated with specific characteristics and may suggest resistance to taxane-based chemotherapy. BRCAness can be used as a negative prognostic indicator for breast cancer.
Subject terms: Cancer, Molecular biology, Oncology
Introduction
Germline mutations in BRCA1 and BRCA2 have been confirmed to associate with increased risk of developing breast and ovarian cancers since two decades ago1–3. This high risk might be related to the functions of BRCA1 and BRCA2 genes in DNA repair. In cells with germline BRCA1 or BRCA2 mutation, the DNA repair of double-strand DNA breaks (DSB) through homologous recombination (HR) was defective4. The exploration of BRCA1 and BRCA2 as well as homologous recombination deficient (HRD) has driven the development of targeted therapy for HRD, particularly poly (ADP-ribose) polymerase (PARP) inhibitors.
‘BRCAness’ has been reported in sporadic cancers that tumors do not have the germline mutations in BRCA but share phenotypic characteristics with tumors that carry germline BRCA mutations and consequently have defective HR5. Our previous research confirmed the result that same as germline BRCA mutations, BRCAness predicts resistance to taxane-containing regimens in triple negative breast cancer (TNBC) during neoadjuvant chemotherapy (NAC)6,7. Thus, changing regimens for BRCAness TNBC might improve their survival. At the same time, whether germline BRCA mutations can be as a prognostic indicator remains controversial8–12. Some studies have already shown the possibility that BRCAness is essential as a biomarker in TNBC and might be of use for predicting prognosis13,14.
The main purpose of our study was to further investigate whether BRCAness can be used as a prognostic factor for breast cancer with or without NAC.
Results
Characteristics and prognosis of all patients (n = 121)
Of the total 121 patients, 48 (39.7%) were finally identified as BRCAness positive by MLPA. The basic characteristics stratified by BRCAness status were summarized in Table 1. No significant differences were found regarding age at diagnosis, tumor size, lymph nodal status, TNM stage or HER2 status between the two groups. However, BRCAness positive patients showed a significantly higher nuclear grade and lower hormone receptor positive rate compared to the BRCAness negative patients (P < 0.001 and P < 0.001, respectively). Thus, the proportion of TNBC subtype in BRCAness positive group was much higher than that in BRCAness negative group (91.6% vs. 42.5%, P < 0.001).
Table 1.
Basic characteristics stratified by BRCAness status (n = 121).
| BRCAness (n = 48) | Non-BRCAness (n = 73) | P value | |
|---|---|---|---|
| Age at diagnosis | |||
| Range | 27–74 | 31–75 | 0.268 |
| Mean | 48.0 | 49.1 | |
| Tumor size | |||
| T1 | 9 (18.8) | 14 (19.2) | 0.949 |
| T2 | 28 (58.3) | 39 (53.4) | |
| T3 | 6 (12.5) | 11 (15.1) | |
| T4 | 5 (10.4) | 9 (12.3) | |
| Lymph node status | |||
| N0 | 27 (56.2) | 34 (46.6) | 0.065 |
| N1 | 16 (33.3) | 37 (50.7) | |
| N2 | 5 (10.4) | 2 (2.7) | |
| TNM stage | |||
| I | 8 (16.7) | 9 (12.3) | 0.743 |
| II | 28 (58.3) | 47 (64.4) | |
| III | 12 (25.0) | 17 (23.3) | |
| Nuclear grade | |||
| I | 2 (4.3) | 24 (38.7) | < 0.001a |
| II | 9 (19.6) | 8 (12.9) | |
| III | 35 (76.1) | 30 (48.4) | |
| Hormone receptor status | |||
| Positive | 2 (4.2) | 36 (49.3) | < 0.001a |
| Negative | 46 (95.8) | 37 (50.7) | |
| HER2 status | |||
| Positive | 2 (4.2) | 10 (13.7) | 0.122 |
| Negative | 46 (95.8) | 63 (86.3) | |
| Molecular subtype | |||
| Luminal | 2 (4.2) | 36 (49.3) | < 0.001a |
| HER2 | 2 (4.2) | 6 (8.2) | |
| TNBC | 44 (91.6) | 31 (42.5) | |
Data presented as mean and range or n (%).
aStatistically significant difference.
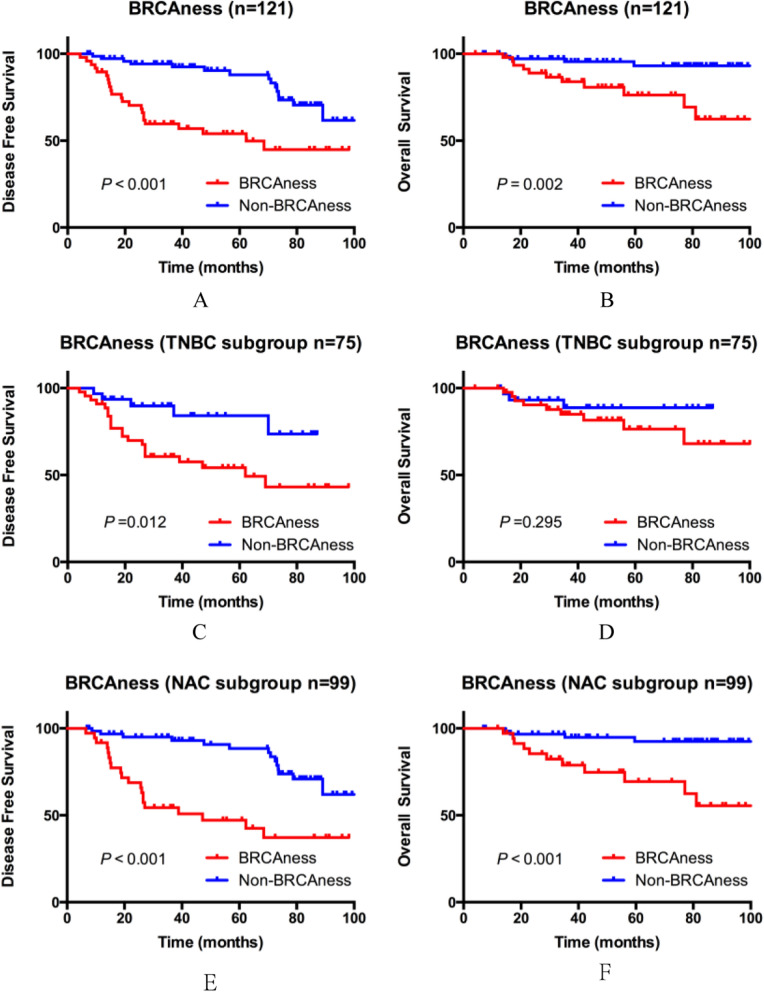
The median follow-up of all patients was 57.7 months (range, 4.2–102.5 months). Kaplan–Meier analysis demonstrated that BRCAness positive patients showed significantly worse 5-year DFS (54.0% vs. 88.0%, P < 0.001) and OS (76.3% vs. 93.1%, P = 0.002) compared with BRCAness negative patients in whole population (Fig. 1A,B). In TNBC subgroup, BRCAness tumors also show significantly worse DFS (54.2% vs. 84.2%, P = 0.012) but slightly worse OS (76.5% vs. 88.8%, P = 0.295) (Fig. 1C,D).
Figure 1.
Kaplan–Meier survival curves by BRCAness.
Analysis of prognostic factors in NAC subgroup (n = 99)
In NAC subgroup, the overall pCR rate was 17.2% (17/99), as well as cRR was 70.7% (70/99). Thirty-six patients were identified as BRCAness positive in this subgroup. As described in our previous research7, BRCAness was indicated significantly associated with lower cRR (58.3% vs. 77.8%, P = 0.041) after taxane-based regimens, but not with overall pCR rate (13.9% vs. 19.0%, P = 0.513).
Same as overall prognostic analysis, BRCAness positive patients still experienced significantly shorter 5-year DFS (47.2% vs. 88.4%, P < 0.001) and OS (69.4% vs. 92.5%, P < 0.001) in NAC subgroup (Fig. 1E,F). Univariate analysis of the clinicopathologic characteristics revealed that hormone receptor negative, TNBC, non-pCR and BRCAness positive were significantly associated with worse DFS, while only TNBC and BRCAness positive were associated with poorer OS (Table 2). Multivariate analysis revealed that TNBC, non-pCR and BRCAness positive were the independent and negative prognostic factor for DFS, while no factor can be as an independent factor for OS (Table 2). However, what should be noted is that because all the 15 patients who died of breast cancer were in non-pCR group, pCR could not be included as a factor in this Cox regression analysis for OS (Table 2).
Table 2.
Cox regression analysis of DFS and OS in NAC subgroup (n = 99).
| Factor | Disease free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Univariate analysis | ||||||
|
Tumor size (> 2 cm vs. ≤ 2 cm) |
2.912 | 0.697–12.173 | 0.143 | 0.997 | 0.225–4.422 | 0.966 |
|
Lymph node status (positive vs. negative) |
1.884 | 0.900–3.944 | 0.093 | 0.832 | 0.296–2.341 | 0.728 |
|
TNM stage (III vs. I–II) |
1.328 | 0.646–2.729 | 0.441 | 0.686 | 0.194–2.434 | 0.560 |
|
Nuclear grade (III vs. I–II) |
1.484 | 0.705–3.124 | 0.298 | 1.937 | 0.630–5.954 | 0.248 |
| Hormone receptor (positive vs. negative) | 0.272 | 0.121–0.610 | 0.002 a | 0.016 | 0.001–1.085 | 0.055 |
|
Molecular subtype (TNBC vs. non-TNBC) |
2.676 | 1.304–5.492 | 0.007 a | 5.207 | 1.449–18.706 | 0.011 a |
|
Pathological response (pCR vs. non-pCR) |
0.113 | 0.015–0.827 | 0.032 a | N/A | N/A | N/A |
|
Clinical response (CR + PR vs. SD + PD) |
0.862 | 0.412–1.805 | 0.694 | 0.523 | 0.185–1.477 | 0.221 |
|
BRCAness status (positive vs. negative) |
3.966 | 1.978–7.954 | < 0.001 a | 6.145 | 1.943–19.441 | 0.002 a |
| Multivariate analysis | ||||||
|
Molecular subtype (TNBC vs. non-TNBC) |
2.758 | 1.037–7.340 | 0.042 a | 4.126 | 0.817–20.841 | 0.086 |
|
Pathological response (pCR vs. non-pCR) |
0.054 | 0.007–0.422 | 0.005 a | N/A | N/A | N/A |
|
Clinical response (CR + PR vs. SD + PD) |
2.034 | 0.910–4.547 | 0.083 | 1.215 | 0.411–3.597 | 0.724 |
|
BRCAness status (positive vs. negative) |
2.962 | 1.184–7.412 | 0.020 a | 2.681 | 0.618–11.630 | 0.188 |
aStatistically significant difference.
In NAC subgroup, part of the patients did not have enough archival tumor tissues of CNB specimen or surgical specimen to analyze BRCAness by MLPA. Except for this part, total 42 non-pCR patients received BRCAness detection by both specimens. Twelve BRCAness patients changed to be Non-BRCAness status after NAC. These patients showed trend toward better clinical response than the other 7 patients with persistent BRCAness (75.0% vs. 28.6%, P = 0.074). BRCAness status remained to be stable in all the 22 Non-BRCAness patients after NAC. And this group still showed the best prognosis (Table 3). Only one Non-BRCAness patient changed to be BRCAness after NAC, so we did not include this case in Table 3. This was a TNBC patient who was resistant to taxane , and then died within 3 years.
Table 3.
Basic characteristics and prognosis stratified by changes in BRCAness status (n = 41).
| BRCAness +/− (n = 12) |
BRCAness +/+ (n = 7) |
BRCAness −/− (n = 22) |
P value 1* | P value 2* | |
|---|---|---|---|---|---|
| Tumor size | |||||
| T1 | 1 (8.3) | 1 (14.3) | 2 (9.1) | 0.914 | 0.974 |
| T2 | 7 (58.3) | 3 (42.9) | 14 (63.6) | ||
| T3 | 3 (25.0) | 2 (28.6) | 4 (18.2) | ||
| T4 | 1 (8.3) | 1 (14.3) | 2 (9.1) | ||
| Lymph node status | |||||
| N0 | 6 (50.0) | 3 (42.9) | 10 (45.5) | 0.405 | 0.748 |
| N1 | 6 (50.0) | 3 (42.9) | 11 (50.0) | ||
| N2 | 0 (0) | 1 (14.3) | 1 (4.5) | ||
| TNM stage | |||||
| I | 0 (0) | 1 (14.3) | 1 (4.5) | 0.324 | 0.724 |
| II | 8 (66.7) | 3 (42.9) | 15 (68.2) | ||
| III | 4 (33.3) | 3 (42.9) | 6 (27.3) | ||
| Nuclear grade | |||||
| I | 1 (10.0) | 0 (0) | 8 (44.4) | 0.638 | 0.166 |
| II | 3 (30.0) | 3 (42.9) | 4 (22.2) | ||
| III | 6 (60.0) | 4 (57.1) | 6 (33.3) | ||
| Molecular subtype | |||||
| Luminal | 2 (16.7) | 0 (0) | 16 (72.7) | 0.354 | 0.001a |
| HER2 | 1 (8.3) | 0 (0) | 3 (13.6) | ||
| TNBC | 9 (75.0) | 7 (100.0) | 3 (13.6) | ||
| Clinical response | |||||
| CR + PR | 9 (75.0) | 2 (28.6) | 18 (81.8) | 0.074 | 0.677 |
| PD + SD | 3 (25.0) | 5 (71.4) | 4 (18.2) | ||
| Prognosis | |||||
| 5 years-DFS | 40.0% | 28.6% | 100.0% | 0.611 | < 0.001a |
| 5 years-OS | 78.6% | 83.3% | 100.0% | 0.757 | 0.001a |
Data presented as mean and range or n (%).
*P Value 1: BRCAness +/− (n = 12) versus BRCAness +/+ (n = 7).
*P Value 2: BRCAness +/− (n = 12) versus BRCAness −/− (n = 22).
aStatistically significant difference.
Correlation between BRCAness and germline BRCA mutaions (n = 63)
Total 63 patients who were suspected of having germline BRCA mutations by physicians or genetic counsellors had received genetic test, in which 15 patients were identified as carrier of germline BRCA1 mutation, 5 patients carried germline BRCA2 mutation, and 43 were BRCA wild type. Nine patients with germline BRCA1 mutation (60.0%), 2 patients with germline BRCA2 mutation (40.0%) and 25 patients with BRCA wild type (58.1%) were identified as BRCAness positive. The difference in proportion of BRCAness was not statistically significant between patients with and without germline BRCA mutations. (55.0% vs. 58.1%, P = 0.815).
Discussion
Since BRCA genes were identified, it has been confirmed that germline BRCA mutation breast cancer has some distinctive clinicopathological features compared to sporadic breast cancer. BRCA1 mutation breast cancers usually express as TNBC subtype, and associate with higher tumor grade, poorer differentiated, higher proportion of medullary and atypical medullary carcinomas6,8,15–18. BRCA2-mutation cancers always show a slightly increasing trend of the incidence to be lobular or tubulolobular carcinomas19,20.
Several preclinical and clinical studies indicated that germline BRCA mutation tumors tended to be resistant to taxane21,22. Similarly, correlation between BRCAness and chemotherapy efficacy have been observed in some studies. Mori and colleagues indicated that BRCAness might be a predictive factor for anthracycline-based adjuvant chemotherapy in TNBC13. Noguchi and colleagues and our previous research also reported that BRCAness predicted resistance to taxane-containing regimens in TNBC6,7. Furthermore, the current study showed that BRCAness status predicted resistance to taxane-based treatment regardless of molecular subtypes, suggesting the similar correlations of the genetic entities and sensitivity to chemotherapy in germline BRCA mutation and BRCAness. Meanwhile, similar as the characteristics of germline BRCA1 mutation, BRCAness was also associated with higher tumor grade, hormone receptor negativity, and higher proportion of TNBC subtype according to our results. Therefore, we can preliminarily conclude that there are similarities between BRCAness and germline BRCA1 mutation in clinicopathological characteristics of breast cancer.
Although according to the results of existing researches, whether germline BRCA mutations can predict the prognosis of breast cancer patients is still controversial, BRCAness showed a possibility to be a negative prognostic indicator for TNBC subtype based on the results of some previous studies13,14. Our result further confirmed a correlation between BRCAness and poor prognosis of all breast cancer patients regardless of molecular subtype. Furthermore, in NAC subgroup analysis, BRCAness was an independent prognostic factor for DFS. However, the reason why BRCAness could not be as an independent factor for OS might relate to the small number of involved patients and low mortality rate. Therefore, a bigger sample size is necessary for our further research to establish this trend and effect on OS.
In NAC subgroup, part of the patients changed their BRCAness status after receiving NAC. Although we highlighted this change in our result part, we still believe that it cannot be as a key point because of the small number of patients. In the analysis of relevance between the change and characteristics, BRCAness patients who changed to be Non-BRCAness status after NAC showed better clinical response than the persistent BRCAness patients. We considered it might be because of the reason that more normal-cell contamination after NAC affected BRCAness results. But this change might also suggest that HR function could be restored during NAC, thus the sensitivity of taxane improved in this subgroup. However, the prognosis was more related to initial BRCAness status.
In our research preparation period, we anticipated a higher percentage of BRCAness positive in germline BRCA mutation breast cancer compared to sporadic breast cancer. However, our final result confirmed that the difference in proportion of BRCAness was not statistically significant between patients with and without germline BRCA mutations. It might suggest that germline BRCA mutations and BRCAness detected by somatic cell were quite different. As described in the conclusion of TNT trail23, defects in HR might be revertible, while mutational signatures as a permanent ‘scar’ of prior would not be expected to disappear. BRCA genes and HRD were now known as the principle of PARP inhibitor targeted therapy4. Although our research did not cover this part, we still believe that PARP inhibitors may also be beneficial for BRCAness patients, which requires new clinical trials to further confirm.
BRCAness is associated with specific characteristics of breast cancer and may suggest resistance to taxane-based chemotherapy. BRCAness can also be used as a negative prognostic indicator for all breast cancer patients regardless of molecular subtype.
Patients and methods
Patients
This study was approved by the ethical committee of Showa University. All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects. One hundred and twenty one patients who were diagnosed of early invasive breast cancer from July 2005 to July 2017 at Showa University Hospital Breast Center were involved in this study. All the patients were at TNM stage I-III, which we defined it as early breast cancer due to the opportunity for surgery. Patients were involved from two different parts. Seventy-three patients were enrolled according to the principle that all the patients had received NAC with taxane and/or anthracycline for primary breast cancer from October 2010 to March 2013 at Showa University Hospital Breast Center. Most of these patients had been involved in a randomized controlled trial in which we compared the efficacy of docetaxel and albumin-bound paclitaxel as NAC. The other 48 patients were randomly selected from TNBC patients with confirmed germline BRCA mutation status. Excluding 22 patients without NAC or NAC without taxane, a total of 99 patients had received taxane-based NAC (Fig. 2).
Figure 2.
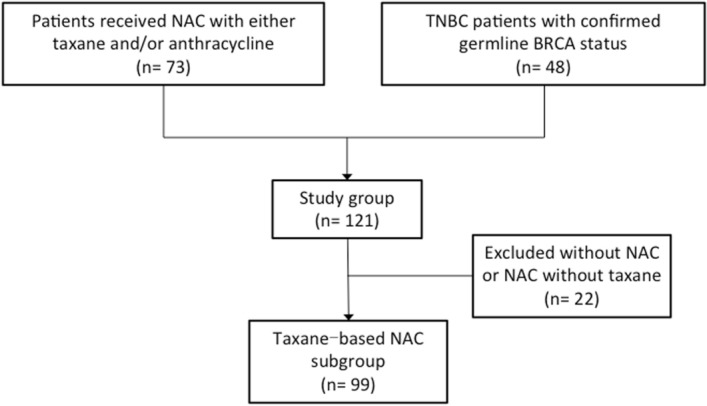
Consort diagram.
Ultrasonography and magnetic resonance imaging (MRI) were performed before treatment, and at the end of first and second cycle of NAC. For patients whose tumor was determined to progress after two cycles of NAC, tanaxe-based regimen would be changed to a second-line regimen, or surgery would be performed immediately. Clinical response for taxane was based on imaging before NAC and after the last cycle of taxane-based NAC, using the Response Evaluation Criteria in Solid Tumors24. Clinical response rate (cRR) was summarized the rate of clinical complete response (cCR) and clinical partial response (cPR).
Survival endpoints of this study were disease-free survival (DFS) and overall survival (OS). DFS was defined as the interval from first treatment to recurrence or metastasis. OS was defined as the interval from first treatment to death of any cause.
Pathology
In this study, the pathological reports of the routine surgical specimens were used. Tumor, node, and metastasis staging of breast cancer was performed following the Cancer Staging Manual (8th edition) of the American Joint Committee on Cancer (2016). Tumors were graded using the modified Black’s nuclear grading system.
Molecular classification was based on the expression of estrogen receptor (ER), progesterone receptor (PR), Ki-67, and human epidermal growth factor receptor 2 (HER2). Expression of ER, PR, HER2 and Ki-67 was first examined by immunohistochemistry and scored by pathologists. Sections with ≥ 1% of cells expressing ER or PR were scored as positive. The tumors were considered HER2-positive if immunohistochemical analysis score was greater than 3 or amplification of HER2 gene was confirmed by fluorescence in situ hybridization (FISH), as the American Society of Clinical Oncology guidelines. Ki-67 staining was scored as the percentage of cells with positive nuclear signals (0–100%). Sections with > 20% of positive cells were defined as having a high level of Ki-67 expression. Molecular subtypes were defined following St. Gallen Breast Classification 2017.
In determination of pathological efficacy, pathological complete response (pCR) was defined as complete remission of the invasive components of cancer in the breast and lymph nodes25.
MLPA method
BRCAness was detected by examination of formalin-fixed, paraffin-embedded (FFPE) core needle biopsy (CNB) specimens and/or surgical specimens. DNA was isolated from the tumor using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) after macro dissection. BRCAness was classified by multiplex ligation-dependent probe amplification (MLPA) with the Probemix P376-BRCA1ness (MRC-Holland, Amsterdam, The Netherlands)26. MLPA was performed at Falco Biosystems (Kyoto, Japan) according to the manufacturer’s instructions. BRCAness assays for the first 73 patients were performed at no cost under a collaborative study contract. The relative copy number ratios for the 38 target-specific probes, compared with the reference samples of human genomic DNA (Promega, Madison, WI), were calculated by Coffalyser.Net software and were used for the prediction analysis for microarrays, with the training set generated by MRC-Holland. Laboratory scientists who analyzed BRCAness status were unaware of the patients’ clinical information. The cutoff ratio for BRCAness positivity in this study was 0.5. For patients who had both BRCAness results of CNB specimen and surgical specimen, if one of the results was positive, the patient was defined as BRCAness positive.
Germline BRCA analysis
Sixty-three patients who were suspected of having germline BRCA mutations by physicians or genetic counsellors had received genetic detection according to National Comprehensive Cancer Network guidelines. Detection was performed at Falco Biosystems (Kyoto, Japan) using direct sequencing method on blood samples. Positivity of germline BRCA mutation was defined as presence of pathogenic mutations and/or possible pathogenic mutations.
Statistical analysis
Data was processed using a Statistical Package for the Social Sciences (version 20.0). Survival curves were plotted using GraphPad Prism (version 6.0). Categorical variables were compared using a Chi-square test. Age difference between groups was analyzed by a non-parametric test. 5-year DFS and OS were generated using the Kaplan–Meier method and were compared using the log-rank test. Hazard ratios were calculated using Cox proportional hazards regression. P-value lower than 0.05 was considered significant.
Acknowledgments
Special thanks to Falco Biosystems (Kyoto, Japan) for BRCAness assays for the first 73 patients at no cost under a collaborative study contract.
Abbreviations
- MLPA
Multiplex ligation-dependent probe amplification
- DFS
Disease free survival
- OS
Overall survival
- DSB
Double-strand DNA breaks
- HR
Homologous recombination
- HRD
Homologous recombination deficient
- PARP
Particularly poly (ADP-ribose) polymerase
- TNBC
Triple negative breast cancer
- NAC
Neoadjuvant chemotherapy
- MRI
Magnetic resonance imaging
- cRR
Clinical response rate
- cCR
Clinical complete response
- cPR
Clinical partial response
- ER
Estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor 2
- FISH
Fluorescence in situ hybridization
- pCR
Pathological complete response
- FFPE
Formalin-fixed, paraffin-embedded
- CNB
Core needle biopsy
Author contributions
L.L. wrote the main manuscript text, analyzed the data and prepared all the figures. S.N. made substantial contributions to the conception and design of the work. Y.M. and S.A.-T. acquired and interpreted all the data of BRCAness. J.T. and H.M. helped to draft the work and substantively revised the manuscript. M.I. acquired and interpreted the data of BRCA germline mutations. Y.I., R.H., C.W., K.T., T.S., H.O., A.A., T.K., S.N. and Y.T. helped to achieve clinical data, reviewed the outline and put forward their suggestions for revision. All authors had reviewed the manuscript, approved the submitted version.
Data availability
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miki Y, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 3.King MC. "The race" to clone BRCA1. Science. 2014;343(6178):1462–1465. doi: 10.1126/science.1251900. [DOI] [PubMed] [Google Scholar]
- 4.Lord CJ, Ashworth A. BRCAness revisited. Nat. Rev. Cancer. 2016;16(2):110–120. doi: 10.1038/nrc.2015.21. [DOI] [PubMed] [Google Scholar]
- 5.Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nat. Rev. Cancer. 2004;4(10):814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi S, et al. Clinicopathologic analysis of BRCA1- or BRCA2-associated hereditary breast carcinoma in Japanese women. Cancer. 1999;85(10):2200–2205. doi: 10.1002/(SICI)1097-0142(19990515)85:10<2200::AID-CNCR14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Akashi-Tanaka S, et al. BRCAness predicts resistance to taxane-containing regimens in triple negative breast cancer during neoadjuvant chemotherapy. Clin. Breast. Cancer. 2015;15(1):80–85. doi: 10.1016/j.clbc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Foulkes WD, et al. Primary node negative breast cancer in BRCA1 mutation carriers has a poor outcome. Ann. Oncol. 2000;11(3):307–313. doi: 10.1023/A:1008340723974. [DOI] [PubMed] [Google Scholar]
- 9.Huzarski T, et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J. Clin. Oncol. 2013;31(26):3191–3196. doi: 10.1200/JCO.2012.45.3571. [DOI] [PubMed] [Google Scholar]
- 10.Zhong Q, Peng HL, Zhao X, Zhang L, Hwang WT. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin. Cancer Res. 2015;21(1):211–220. doi: 10.1158/1078-0432.CCR-14-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortesi L, et al. Favourable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC Cancer. 2010;10:90. doi: 10.1186/1471-2407-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin PJ, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J. Clin. Oncol. 2012;30(1):19–26. doi: 10.1200/JCO.2010.33.0068. [DOI] [PubMed] [Google Scholar]
- 13.Mori H, et al. BRCAness as a biomarker for predicting prognosis and response to anthracycline-based adjuvant chemotherapy for patients with triple-negative breast cancer. PLoS ONE. 2016;11(12):e0167016. doi: 10.1371/journal.pone.0167016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanino H, et al. BRCAness and prognosis in triple-negative breast cancer patients treated with neoadjuvant chemotherapy. PLoS ONE. 2016;11(12):e0165721. doi: 10.1371/journal.pone.0165721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakhani SR, et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002;20(9):2310–2318. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Loman N, et al. Steroid receptors in hereditary breast carcinomas associated with BRCA1 or BRCA2 mutations or unknown susceptibility genes. Cancer. 1998;83(2):310–319. doi: 10.1002/(SICI)1097-0142(19980715)83:2<310::AID-CNCR15>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Phillips KA, Andrulis IL, Goodwin PJ. Breast carcinomas arising in carriers of mutations in BRCA1 or BRCA2: are they prognostically different? J. Clin. Oncol. 1999;17(11):3653–3663. doi: 10.1200/JCO.1999.17.11.3653. [DOI] [PubMed] [Google Scholar]
- 18.Eisinger F, Nogues C, Birnbaum D, Jacquemier J, Sobol H. BRCA1 and medullary breast cancer. JAMA. 1998;280(14):1227–1228. doi: 10.1001/jama.280.14.1227. [DOI] [PubMed] [Google Scholar]
- 19.Marcus JN, et al. Hereditary breast cancer: pathobiology, prognosis, and BRCA1 and BRCA2 gene linkage. Cancer. 1996;77(4):697–709. doi: 10.1002/(SICI)1097-0142(19960215)77:4<697::AID-CNCR16>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 20.Armes JE, et al. The histologic phenotypes of breast carcinoma occurring before age 40 years in women with and without BRCA1 or BRCA2 germline mutations: a population-based study. Cancer. 1998;83(11):2335–2345. doi: 10.1002/(SICI)1097-0142(19981201)83:11<2335::AID-CNCR13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Kriege M, et al. The efficacy of taxane chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer. 2012;118(4):899–907. doi: 10.1002/cncr.26351. [DOI] [PubMed] [Google Scholar]
- 22.Rottenberg S, et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc. Natl. Acad. Sci. USA. 2007;104(29):12117–12122. doi: 10.1073/pnas.0702955104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tutt A, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat. Med. 2018;24(5):628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Shigenaga R, Akashi-Tanaka S. Comparison among Japanese general rules for clinical and pathological recording of breast cancer 16th eds and UICC TNM classification 7th eds. Nihon Rinsho. 2012;70 Suppl 7:191–194. [PubMed] [Google Scholar]
- 26.Oonk AM, et al. Clinical correlates of 'BRCAness' in triple-negative breast cancer of patients receiving adjuvant chemotherapy. Ann. Oncol. 2012;23(9):2301–2305. doi: 10.1093/annonc/mdr621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.