Abstract
Advances in ophthalmic diagnostics and results of interventional clinical trials are shifting diagnosis and management of idiopathic intracranial hypertension (IIH) to be more technology- and evidence-based. In this article, the evidence supporting current diagnostic criteria, evaluation, and medical and surgical management of IIH are reviewed.
Keywords: Idiopathic intracranial hypertension, pseudotumor cerebri, papilledema
Historical Note & Nomenclature
After the concept of unexplained high intracranial pressure (ICP) resulting in neurological and ophthalmic symptoms and signs was first described by Quincke as serous meningitis in the 1890s,1 our conceptualization of the disease has continued to evolve through the years. In 1904, ‘pseudotumor cerebri’ was coined by Nonne, and in 1937, Walter Dandy described 22 patients with ‘intracranial pressure without brain tumor.’2 In the 1950s, ‘benign intracranial hypertension’ was introduced, though this term fell out of favor due to the possibility of non-benign outcomes.3 ‘Idiopathic intracranial hypertension (IIH)’ was introduced by Corbett and Thompson in the late 1980s.4 The term IIH focuses on the idiopathic form, excluding all the non-tumor but known secondary causes of intracranial hypertension, such as venous sinus thrombosis and meningitis, that are included under the broader term pseudotumor cerebri syndrome.5 Pseudotumor cerebri remains a commonly used term, although there is some controversy over whether this nomenclature is of clinical utility over dividing intracranial hypertension into its primary (i.e., idiopathic) and secondary forms.5,6 From our perspective, pseudotumor cerebri (i.e. including secondary causes of intracranial hypertension) is advantageous from a diagnostic perspective because it focuses on identification of the high intracranial hypertension syndrome and maintains a broad differential diagnosis of secondary causes. On the other hand, IIH is advantageous from the perspective of targeted treatment, including clinical trials, since it explicitly excludes secondary causes.
Identifying Intracranial Hypertension
Identifying the syndrome of intracranial hypertension is important for three reasons. The first is to prompt diagnosis of secondary causes such as brain tumors, venous sinus thrombosis and infectious causes that require directed therapy. The second is to identify and address a pathophysiological state that puts patients at risk of blindness through compressive forces exerted on the optic nerve by high cerebrospinal fluid (CSF) pressure in the optic nerve sheath. The third is to identify a treatable cause of a patient’s symptoms such as headache, double vision and pulsatile tinnitus.
Clinical suspicion for intracranial hypertension is mainly based on symptoms described by the patient. However, some asymptomatic patients come to medical attention when papilledema is observed on routine ophthalmic examination or changes typical of high ICP are detected on neuroimaging studies done for other reasons. Symptoms, medications or past medical history associated with secondary causes of intracranial hypertension may also raise suspicion for this condition.7 Because most patients with IIH are females who are overweight or obese,8–10 and an increase of weight by 5–15% is often sufficient to increase the risk of developing IIH,11 some providers triage based on demographics and body habitus. However, this is imperfect since IIH does occur outside these demographics and secondary intracranial hypertension can occur in patients with typical IIH demographics.
Symptoms of intracranial hypertension
Our knowledge of the characteristics of symptoms in patients with IIH was informed by the IIH Treatment Trial (IIHTT).12 This trial included 165 subjects with IIH with mild vision loss,13 comprising the largest cohort of IIH patients rigorously studied to date. The baseline profile of IIHTT participants illustrates the prevalence of common symptoms (Table 2).14 Many of these symptoms are nonspecific, making clear that a diagnosis of secondary intracranial hypertension or IIH cannot be established based on symptoms alone.
Table 2:
Symptoms present at time of patient enrollment in the IIHTT14
| Symptom | Prevalence (n=165) |
|---|---|
| Headache | 84% |
| Vision changes | |
| Transient visual obscurations | 68% |
| Vision loss* | 32% |
| Diplopia | 18% |
| photophobia | 48% |
| Tinnitus | |
| Pulsatile | 52% |
| Non-pulsatile | 23% |
| Dizziness | 52% |
| Neck pain | 42% |
| Back pain | 53% |
| Cognitive disturbances | 20% |
All subjects in the trial had bilateral papilledema and mild vision loss on formal visual field testing as a trial entry criterion
Though presentations of IIH are typically subacute and some are chronic, a minority of patients (< 5%) have a fulminant presentation with rapidly progressive vision loss over the course of days to weeks. Identifying patients in this group and acting quickly to establish the diagnosis and institute appropriate therapy is of particular importance since visual outcomes are poor (50% remained legally blind, with visual acuity less than 20/200 in both eyes or severe visual field constriction despite aggressive treatment in one series).15
Headache
Headache is an important cause of morbidity in IIH, with IIHTT subjects reporting substantial to severe headache disability at baseline (mean score of 60 on the Headache Impact Test questionnaire, which surveys the frequency and impact of headache and ranges from 6–78).12 One-quarter of patients reported constant daily headache.16 While there are some headache features that suggest intracranial hypertension, including being worse in the morning and with lying flat, these characteristics are neither sensitive nor specific enough to be clinically helpful.17 In the IIHTT, headaches commonly fit the phenotype of primary headache disorders including migraine (52%) or tension-type headache (22%).16
Visual Symptoms
Visual loss is the main source of chronic morbidity in IIH.18 Central vision loss is rare except in advanced presentations, and most vision loss starts in the periphery. There can be a considerable range of visual deficits at presentation, most detectable on automated perimetry.14,19,20 However, some patients report nonspecific blurry or dim vision in the setting of normal visual function testing. This is likely due to impairment relative to personal baseline that remains within population normative data.14
Transient visual obscurations are short, lasting seconds to minutes, and characterized by monocular or binocular fogginess, black, white or grey out, usually triggered by head or eye movement.17 Some patients report episodic brief visual sparkles or flashes distinct from visual migraine aura.21 Position- and gaze-evoked symptoms are thought to originate from ischemia of the optic nerve induced by movement of the swollen optic nerve head.22 Similar symptoms occur in association with other causes of optic nerve head elevation, including pseudopapilledema due to optic nerve head drusen. Dimming or sparkles in vision induced by exposure to bright light is similar to what occurs in severe unilateral carotid stenosis and may represent ischemia due to increased metabolic demand that is not adequately met by the vascular supply to the retina or optic nerve.23
Constant or intermittent horizontal diplopia, resolving with occluding either eye, can occur due to unilateral or bilateral cranial nerve (CN) VI (abducens) palsy. This is a false localizing sign of high ICP states, occurring because of tension on the abducens nerve(s). Symptoms are more pronounced in lateral gaze and with distance focus. Unilateral or bilateral CN VI palsy can sometimes be subtle with esotropia or esophoria without clear abduction deficits. Other patterns of diplopia have been reported in IIH, but these are rare and should prompt additional investigation if they are present.24
Pulsatile Tinnitus
This consists of hearing either a unilateral or bilateral whooshing, whistling, humming, marching noise or heartbeat and is thought to represent auditory perception of turbulent pulsatile flow in intracranial vessels. Though 52% of subjects in the IIHTT reported this symptom, it is not specific for intracranial hypertension and also occurs due to underlying vascular abnormalities (including arteriovenous malformations, arterial stenoses, or arterial aneurysms), eustachian tube dysfunction, and other benign causes.25
Asymptomatic
A minority of patients with IIH may be asymptomatic.26–28 Some patients may have mild visual dysfunction they have not recognized, while others may be asymptomatic because there is no detectable vision loss.29 In the IIHTT, which required papilledema and mild visual impairment on automated perimetry to enroll, 68% patients reported no visual symptoms.
Optic nerve changes in intracranial hypertension
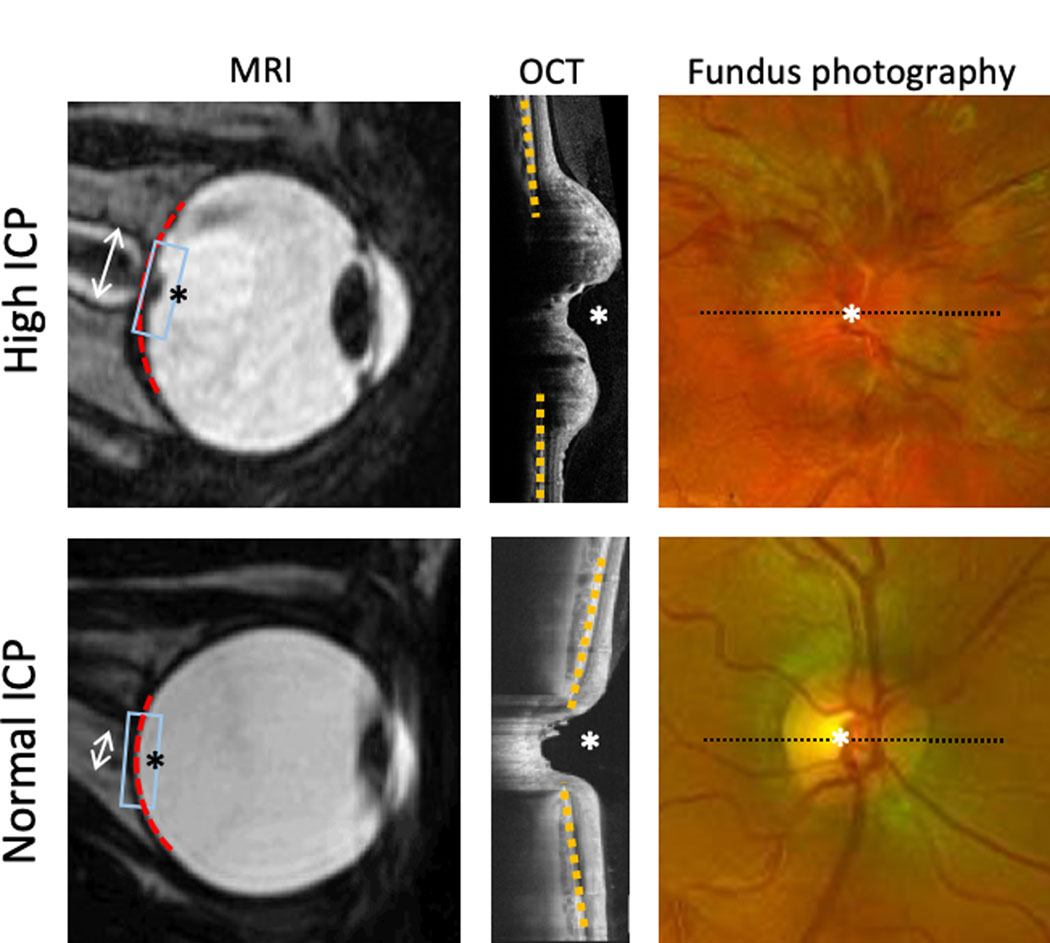
Funduscopic exam showing bilateral optic disc edema is very concerning for papilledema due to intracranial hypertension (figure). The observation of this finding, either incidentally or in a patient with otherwise unexplained headaches, should prompt urgent diagnostic evaluation. It is important, however, to note that optic disc edema is a nonspecific finding that can occur in other neurological and ophthalmic diseases. Other nonspecific findings of intracranial hypertension on fundus exam include venous stasis, hyperemia, hemorrhages, infarcts, cotton wool spots, choroidal and retinal folds and absence of spontaneous retinal venous pulsations.18,30–32 In chronic untreated cases, optic disc edema can be replaced by optic atrophy due to damage to the retinal ganglion cells.
Figure:
Findings associated with intracranial hypertension (top) compared with normal ICP (bottom) on ancillary testing including MRI (left), OCT (center) and fundus photography (right). Box on MRI indicates region of OCT. Dashed line on fundus photography indicated cross section of OCT. Optic nerve head (*), optic nerve sheath width (double arrow), globe flattening (red dashed line), eye shape change (yellow dashed line).
Neuroimaging changes in intracranial hypertension
A diagnosis of IIH is considered in some patients when findings associated with high ICP are found on magnetic resonance imaging or computed tomography of the brain done for other reasons. These include globe flattening, optic nerve protrusion into the vitreous, optic nerve widening with increased CSF spaces in the optic nerve sheath, empty sella and transverse venous sinus changes (figure).33 While suggestive, these findings are not diagnostic of active intracranial hypertension due to their occurrence in normal individuals34 as well as only partial normalization following treatment.35
Confirming Intracranial Hypertension
In cases of suspected intracranial hypertension, pathological elevation of the intracranial pressure is confirmed by measurement of opening pressure following lumbar puncture, so long as there is no mass lesion to preclude this procedure being done safely. For the purposes of diagnosing IIH, an opening pressure of ≥ 250 mm water is considered elevated and diagnostic in adults. This cutoff was established in a 1983 paper by Corbett and Mehta, which obtained opening pressure measurements from 4 groups of patients: healthy non-obese, healthy obese, acute pseudotumor cerebri, and chronic (previously diagnosed) pseudotumor cerebri.36 About 90% of the acute pseudotumor cerebri patients had an opening pressure of greater than or equal to 250 mm water. Some healthy obese (26%) and non-obese (7%) patients had an opening pressure of 200–250 mm water, and there was no correlation between the degree of obesity and opening pressure. These findings were in agreement with other studies of ICP in different populations.37,38 A more recent prospective study of lumbar puncture opening pressures obtained in the lateral decubitus position in over 200 outpatients without a high ICP-associated diagnosis defined a normal range from 100 mm water (2.5 percentile) to 250 mm water (97.5 percentile).39 A similar study in children found a higher 97.5 percentile of 280 mm water.40 However, it is important to note that these cut offs are not absolute, with 2.5% of normal adults having ICP above 250mm water in the population study39 and 10% of patients with acute pseudotumor cerebri having ICP less than 250mm water in the Corbett & Mehta study,36 leading to the possibility of both over and under diagnosis of intracranial hypertension based on lumbar puncture alone.
Further uncertainty is introduced by the concern that normal ICP ranges established with lateral decubitus positioning may not apply for different lumbar puncture techniques. While a previous study suggested that prone CSF pressure measurement may overestimate ICP by about 20 mm water compared to lumbar decubitus positioning,41 a 2014 study suggested that there was no statistically or clinically significant difference between opening pressures measured in the prone and lateral decubitus position, and that body mass index (BMI) did not affect measurements in either position.42 What is likely most important is that the manometer zero is positioned level with the foramen magnum regardless of patient positioning. This is underscored by a study of invasive ICP monitoring that demonstrated relatively stable ICP (within 5mm Hg) in supine versus seated and standing positions in normal subjects.43 Interestingly IIH and hydrocephalus subjects had larger changes in ICP, with supine and lateral decubitus ICP being higher than when sitting or vertical.
CSF pressure measurement via lumbar puncture is a point measurement of a fluctuating system. This, and challenges with CSF pressure measurement procedures related to both procedural (e.g. needle positioning) and patient factors (e.g. valsalva), raise the possibility of false positive and false negative ICP measurements. As with any medical test result, the measured pressure must be interpreted in the context of the patient’s symptoms, examination, and other diagnostic test results.
Diagnosis of IIH
Dandy’s original suggestion of separating IIH from other causes of raised intracranial pressure including brain tumors was formalized as the ‘modified Dandy criteria’ by J.L. Smith in 1985.44 Neuroimaging to exclude tumor and venous sinus thrombosis, CSF analysis, and review of medications and past medical history is typically undertaken to exclude secondary causes (Table 3).7 Cross sectional imaging of the brain (MRI or CT) is necessary to assess for secondary causes such as tumors and hydrocephalus. Venous sinus thrombosis cannot be distinguished from IIH on the basis of history,45,46 and though it may be evident on brain MRI, MR or CT venography (MRV or CTV) are more sensitive.47 Venous imaging may have an additional role in identifying impaired outflow, and arterial imaging may help to identify dural arteriovenous fistulae contributing to intracranial hypertension.48 Spinal imaging can identify spinal lesions, a rare secondary cause of intracranial hypertension.49 A challenge is that while there are some conditions such as hydrocephalus, brain tumors, and venous sinus thrombosis, as well as some medications such as steroid withdrawal or tetracycline antibiotics for which a causal relationship with intracranial hypertension is likely, the causal relationship for other conditions and medications reported in association with intracranial hypertension is murkier.
Table 3:
Secondary causes of intracranial hypertension*
| Past medical history | Anemia |
| Addison’s disease | |
| Adrenal insufficiency | |
| Acute & chronic inflammatory demyelinating polyneuropathy | |
| Down syndrome | |
| Head trauma | |
| Lupus | |
| Obstructive sleep apnea | |
| Past subarachnoid hemorrhage or meningitis | |
| Renal failure | |
| Superior vena cava syndrome | |
| Systemic venous hypertension | |
| Turner syndrome | |
| Medications | Tetracycline, minocycline, doxycycline |
| Vitamin A and retinoids | |
| Corticosteroid withdrawal | |
| Growth hormone | |
| Lithium | |
| Many others implicated but unproven | |
| Laboratory tests (serum) | Anemia |
| Laboratory tests (CSF) | Meningitis (infections, inflammatory or neoplastic) |
| Elevated protein | |
| Neuro-imaging | Intracranial mass lesions |
| Hydrocephalus | |
| Venous sinus thrombosis or obstruction | |
| Arterio-venous fistula | |
| Bilateral jugular venous blockage | |
| Sub-arachnoid hemorrhage | |
| Craniosynostoses | |
| Spinal cord tumor | |
Presence of a secondary cause of intracranial hypertension is not diagnostic of intracranial hypertension
Friedman and Jacobson formalized the cutoff of 250 mm H2O for high ICP in their diagnostic criteria published in 2002.50 2013 criteria for pseudotumor cerebri syndrome included a cut off of 280 mm water in children.5 To account for the possibility of patients with the clinical syndrome of intracranial hypertension having ICP below this cutoff, current criteria allow for borderline ICP of 200–250 mm H2O when symptoms (pulsatile tinnitus), ophthalmic examination findings (Frisen grade 2 papilledema or sixth nerve palsy) or high ICP-associated neuroimaging findings are present (Table 1).13 Though false normal ICP measures can occur for a variety of reasons, including recently attempted LP with CSF leak, poor needle positioning, and physiological fluctuation, caution is urged when making an IIH diagnosis without confirmation of intracranial hypertension, including cases where an LP has not been attempted. Response of symptoms and examination findings to directed treatment can be helpful in indeterminate cases.
Table 1:
IIHTT Modified Dandy Criteria for IIH13
| CSF pressure | > 25 cm H2O | 20–25 cm H2O Fulfill all requirements for CSF pressure > 25cm H2O plus at least one * item |
|---|---|---|
| Symptoms | headaches, nausea, vomiting, transient obscurations of vision, papilledema Awake and alert |
*Pulse synchronous tinnitus |
| Neurological exam | Absence of localizing findings on neurologic examination (except unilateral or bilateral cranial nerve VI palsies) | *CN VI palsy *Frisèn Grade II papilledema and no pseudopapilledema |
| CSF analysis | normal constituents | |
| Neuro-imaging | Absence of deformity, displacement, or obstruction of the ventricular system No abnormal neuroimaging except for empty sella turcica, optic nerve sheath with filled out CSF spaces, and smooth-walled non flow-related venous sinus stenosis or collapse |
*MRV (Magnetic Resonance Venography) with lateral sinus collapse or stenosis *Partially empty sella with unfolded perioptic nerve CSF spaces |
| Disclaimer | No other cause of increased intracranial pressure present |
Special care is advised when diagnosing intracranial hypertension in the absence of disc swelling due to the possibility of falsely elevated ICP measurement and the common occurrence of primary headache disorders.7,51 Friedman et al included these cases of IIH without papilledema in a ‘probable’ diagnostic category,5 and similar guidelines were included in recently published consensus criteria.7 While IIH without papilledema is physiologically possible, given that unilateral papilledema can occur, this diagnosis should be made with caution since it is more likely that an ICP measurement is falsely elevated in a patient with a chronic primary headache disorder than for a patient with intracranial hypertension to not have papilledema. There is also an important prognostic distinction, since IIH without papilledema does not threaten vision.
Monitoring IIH
Though this chapter focuses on IIH, many of the monitoring techniques are also applicable in patients with papilledema due to secondary causes of intracranial hypertension who are at risk of visual impairment.
Monitoring vision
Visual acuity with each eye tested separately with best correction for testing distance is essential but does not replace peripheral vision assessment since central vision is impacted late in the disease. Confrontation visual field testing is of limited sensitivity for mild to moderate visual field loss.52 Quantitative visual field testing is preferred for guiding management and can be accomplished with automated perimetry, most commonly a Humphrey Visual Field. This method maps a patient’s ability to see varying intensities of stimuli throughout the visual field. This test is critical for establishing visual function and monitoring response to treatment. As such, it has been both an enrollment criterion and primary outcome measure in IIH treatment trials.53
Peripheral visual defects are the most common pattern of visual loss, and develop from optic nerve dysfunction related to compression by high CSF pressure in the optic nerve sheath associated with papilledema.54,55 Earliest changes are usually seen in the inferior nasal periphery.56 Blind spot enlargement is due to peripapillary hyperopia (a refractive deficit), and less commonly, from chorio-retinal folds due to optic nerve sheath distension or fluid tracking beneath the retina from the optic disc.57 Central vision is not affected until there is advanced optic nerve dysfunction and peripheral vision loss encroaches on fixation, or there is tracking of subretinal fluid from the swollen optic nerve to the fovea. A pattern of central more than peripheral vision loss should prompt consideration of other causes of optic neuropathy.50 There is also the potential for functional (i.e. non-physiologic) visual field loss or poor testing performance impacting testing results. The former should be suspected when there is incongruence between the physical exam and pattern of vision loss on automated testing. The latter should be suspected when test indices show high false positive responses, false negative responses, or fixation errors.
Papilledema monitoring
Fundus examination
Papilledema appearance is a useful metric and is generally graded with the Frisen classification despite its poor inter-rater reliability.58,59 Use of ophthalmic imaging (see below) has practical applications with regard to documentation and comparison between visits. Optic nerve pallor suggests permanent injury to the optic nerve has occurred.
Fundus photography
This can be performed quickly and without pupil dilation to generate images of the optic nerve head (figure). It is widely available in ophthalmic practice and actively being studied in neurology and emergency room settings.60 It has a higher sensitivity than direct ophthalmoscopy61 and medical students are more accurate in ophthalmic pathology identification using fundus photography compared to direct ophthalmoscopy.62 Fundus photography is an effective method of assessing the fundus in an emergency setting in which providers may have difficulty identifying key findings using the direct ophthalmoscope.63,64 Even for examiners skilled in the ophthalmoscopic physical exam, fundus photos capture many ophthalmic features of IIH and can serve as the basis for comparison between exams.65,66
Optical Coherence Tomography (OCT)
OCT generates non-invasive high-resolution cross-sectional images of the retina using a near infrared light source through undilated pupils and is widely used in ophthalmic practice (figure). Many neuro-ophthalmologists incorporate OCT in monitoring of IIH patients, though there remains disagreement whether this is associated with improved outcomes.67
The optic nerve contour, which can be difficult to appreciate with non-stereoscopic direct ophthalmoscopy, is readily apparent on OCT. It can identify, classify and provide longitudinal assessment of optic nerve swelling, though it does not capture some of the qualitative vascular features seen on ophthalmoscopy and fundus photography.68 There may even be subtle changes in patients with IIH without frank papilledema.69 Another finding on OCT relevant to IIH management is quantification of the thickness of the retinal ganglion cell layers in the macula, a decrease in which can reflect irreversible injury to the optic nerve.70 Detection of such atrophy should alert the provider that the patient is at risk of permanent vision loss.
The shape of the back of the eye and of the scleral opening around the optic nerve, visible on OCT, assume a concave shape in disc swelling due to intracranial hypertension compared with other causes.71 Research studies have demonstrated that quantitative OCT parameters of the optic nerve head such as optic nerve head volume and eye shape relate to other optic nerve head measures and CSF pressure.72,73 Furthermore, OCT measures of optic nerve swelling normalized in association with treatment in the IIHTT.74 However, these are not yet established diagnostic tests.
Management of IIH
Management of IIH is based on symptomatic and ophthalmic outcomes as described above. Diagnosis of secondary intracranial hypertension does not preclude the use of IIH management strategies, since these can be important to improve symptoms and visual outcomes in addition to or instead of treatment of the secondary cause. Examples include directed ICP treatment in patients with venous sinus thrombosis or in patients for whom cessation of a (possibly) causal medication may pose undue risk.
Management should be guided by disease outcomes, with vision loss, optic nerve injury or disability prompting escalation of therapy and shorter follow up intervals. A recent set of guidelines includes discussion of controversies and available evidence.7
Weight Loss
In the IIHTT, all enrolled subjects received a comprehensive weight loss program with dietary, exercise, and behavioral interventions. Though there was a greater improvement in the visual field outcome in the group that also received acetazolamide, the placebo group also showed improvement in outcomes of vision, headache and quality of life.12 In a previous study, a weight loss diet was associated with sustained improvement in ICP, symptoms, and papilledema.75 Bariatric surgery has been associated with CSF pressure reduction and papilledema resolution in non-randomized studies.76 Weight loss is therefore an important consideration as part of any IIH treatment regimen. In patients without optic nerve injury, weight loss intervention can be considered as monotherapy, but close ophthalmic monitoring is imperative as 6 of 7 treatment failures in the IIHTT occurred in the group that received weight intervention only.12 However, given that weight loss cannot be realistically accomplished in a short time frame, additional treatment in those with vision loss or severe headache disability is often necessary.
Given the association between weight gain and IIH recurrence,77 emphasis should be on a long-term, sustainable weight management plan. In the IIHTT, this was accomplished using a telephone-based program in a multidisciplinary collaboration.78 Bariatric surgery is an option in obese patients and this should be pursued in a bariatric surgery center.79 A randomized study of surgical and non-surgical approaches to weight loss for IIH treatment is being planned.80
Medical Treatment
Acetazolamide is a carbonic anhydrase inhibitor that is thought to reduce CSF production by the choroid plexus. In oral and IV forms it reduces ICP81 and has been first line therapy for IIH many years. The IIHTT provided level II evidence by demonstrating that a combination of weight loss intervention and oral acetazolamide was associated with improved vision, papilledema, and quality of life over 6 months.12 Mediation analysis confirmed a drug effect after accounting for more weight loss in the acetazolamide group. Interestingly there was no drug effect on headaches, which improved similarly in both groups. In a 6 month extension of the IIHTT, subjects who had not improved on placebo were transitioned to open label acetazolamide and subsequently had significant improvement in papilledema and headache.82 Doses of up to 4 g daily were tolerated, with common symptoms reported by patients including paresthesias, dysgeusia, nausea, vomiting, diarrhea, and fatigue. Hypokalemia and metabolic acidosis occurred in 4 and 5% of acetazolamide treated subjects, respectively.83 Acetazolamide is a diuretic and can put patients with venous sinus thrombosis at risk of clot propagation due to dehydration if hydration is not maintained. While it may have a role for ICP management in cases of venous sinus thrombosis, careful monitoring is necessary. It commonly causes a mild metabolic acidosis which can exacerbate respiratory and metabolic acidosis from other causes (e.g. myasthenia gravis or diabeters). It can also cause hypokalemia, which can be easily managed with potassium supplementation.
Topiramate is a weak carbonic anhydrase inhibitor that was non-inferior in a randomized open label trial against 1–1.5g of acetazolamide.84 It also has efficacy in migraine and weight-reduction effects. Though the carbonic anhydrase side effects are milder than with acetazolamide, it may cause cognitive slowing in a subset of patients. A recent study demonstrated topiramate to be more effective at lowering ICP than acetazolamide in healthy rodents.85 However, it is not known if this translates to humans.
Furosemide was associated with lower CSF pressure when combined with acetazolamide in a small pediatric case series.86 It is sometimes used as add-on therapy when acetazolamide response is inadequate. It is included in the medical therapy regimen for the surgical IIH treatment trial (NCT03501966).
Symptomatic headache management may be an important adjunct to improve disability concurrent with ICP lowering therapy.7 In patients who have no subjective or objective visual impairment and no evidence of visual pathway injury including those without papilledema, symptomatic headache therapy without ICP lowering therapy may be considered with close ophthalmic monitoring.
Procedural Treatment
This is generally reserved for medically refractory or fulminant IIH, particularly in patients with vision-threatening disease. Enrollment is ongoing for the Surgical IIH Treatment Trial (NCT03501966) which will compare medical management alone, optic nerve sheath fenestration and ventriculoperitoneal (VP) shunt. A cerebral venous sinus stenting trial is also planned.87
Optic nerve sheath fenestration involves cutting slits or a window in the nerve sheath, thereby reducing CSF pressure in the subarachnoid space around the optic nerve in an effort to improve or stabilize vision. A rare though significant complication is vision loss.88 In a meta-analysis of published cases, vision improved in a majority and worsened in 11%.89 In some cases there is reported improvement in headache and in fellow eye papilledema following a unilateral procedure.
CSF diversion aims to acutely and directly lower ICP using VP shunts or lumboperitoneal shunts and is associated with improvement in vision.90 Long-term outcomes suggest better headache remission in IIH patients with papilledema and with shorter term (<2 year) headache duration.91 There are long-term risks related to indwelling hardware, in addition to immediate procedural complications. Lumbar drains may have application for short-term emergent treatment. Serial LPs are not in routine use but may have application as a temporizing measure in certain cases.7
Cerebral venous sinus stenting aims to reduce cerebral venous hypertension and by extension intracranial hypertension by opening a stenosis in one of the transverse sinuses. Stenoses, either primary or secondary to high ICP, can be detected on MRV or CTV. Catheter venography is used to confirm a pressure gradient across the stenosis and deploy the stent. Experience is increasing with this relatively new procedure, which appears to be safe and effective in the short term in carefully selected patients.92 Long term follow up is not yet available. This is discussed in detail elsewhere in this issue.
Delivery of Care
IIH diagnosis and monitoring relies heavily on the ophthalmoscopic exam. Trainees and non-ophthalmologists (including neurologists) often perform this with poor proficiency.93,94 In addition, most emergency, inpatient and non-eye care outpatient facilities lack the necessary equipment for visual field testing, an important IIH outcome. While some of these barriers can be overcome using ophthalmic imaging such as fundus photography, another strategy is partnership with an ophthalmologist or optometrist who is skilled in collection of this data.
In most cases, IIH is managed in the outpatient setting. Thus, effective transitions from emergency and inpatient settings where the initial diagnostic work up often occurs95 are critical to optimize outcomes. Challenges relate to social deprivation that exists among some patients,96 which in turn is associated with poorer access to health care. Limited availability of subspecialists with expertise in its diagnosis and management is another important concern.97
Conclusion
Symptoms of intracranial hypertension are broad and it is important to maintain a high clinical suspicion in order to diagnose patients with secondary causes that need treatment and patients who are at risk of irreversible optic nerve injury. Confirmation of the diagnosis through measurement of ICP as well as careful assessment of optic nerve function and structure are important to guide management with the goal of preserving vision and controlling symptoms. Weight loss and medical therapy are well tolerated and effective in IIH. Procedural interventions are generally reserved for medically refractory or fulminant presentations.
Acknowledgements
National Institute of Health K23 EY 024345, P30 EY026877 and an unrestricted grant from Research to Prevent Blindness to the Stanford Department of Ophthalmology.
Abbreviations
- BMI
body mass index
- CN
cranial nerve
- CSF
cerebrospinal fluid
- CT
computed tomography
- CTV
computed tomography venogram
- ICP
intracranial pressure
- IIH
Idiopathic intracranial hypertension
- IIHTT
Idiopathic intracranial hypertension treatment trial
- LP
Lumbar puncture
- MRI
magnetic resonance imaging
- MRV
magnetic resonance venogram
Contributor Information
Sarah R. Ahmad, Resident in Neurology, Department of Neurology & Neurological Sciences, Stanford University, 300 Pasteur Dr,; Palo Alto, CA 94304.
Heather E. Moss, Department of Ophthalmology, Department of Neurology & Neurological Sciences, Stanford University, 2370 Watson Court, suite 200, MC 5353; Palo Alto, CA 94303.
References
- 1.Quincke HI. Über meningitis serosa. Samml Klin Vortr Innere Med. 1893;23:655–694. [Google Scholar]
- 2.Dandy WE. INTRACRANIAL PRESSURE WITHOUT BRAIN TUMOR: DIAGNOSIS AND TREATMENT. Annals of surgery. 1937;106(4):492–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley J. Benign forms of intracranial hypertension; toxic and otitic hydrocephalus. Brain : a journal of neurology. 1955;78(1):1–41. [DOI] [PubMed] [Google Scholar]
- 4.Corbett JJ, Thompson HS. The rational management of idiopathic intracranial hypertension. Archives of neurology. 1989;46(10):1049–1051. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159–1165. [DOI] [PubMed] [Google Scholar]
- 6.Wall M, Corbett JJ. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2014;83(2):198–199. [DOI] [PubMed] [Google Scholar]
- 7.Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. Journal of neurology, neurosurgery, and psychiatry. 2018;89(10):1088–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe FJ, Sarkies NJ. The relationship between obesity and idiopathic intracranial hypertension. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23(1):54–59. [DOI] [PubMed] [Google Scholar]
- 9.Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. The Lancet Neurology. 2016;15(1):78–91. [DOI] [PubMed] [Google Scholar]
- 10.Szewka AJ, Bruce BB, Newman NJ, Biousse V. Idiopathic intracranial hypertension: relation between obesity and visual outcomes. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2013;33(1):4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels AB, Liu GT, Volpe NJ, et al. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). American journal of ophthalmology. 2007;143(4):635–641. [DOI] [PubMed] [Google Scholar]
- 12.Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. Jama. 2014;311(16):1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman DI, McDermott MP, Kieburtz K, et al. The idiopathic intracranial hypertension treatment trial: design considerations and methods. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2014;34(2):107–117. [DOI] [PubMed] [Google Scholar]
- 14.Wall M, Kupersmith MJ, Kieburtz KD, et al. The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA neurology. 2014;71(6):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68(3):229–232. [DOI] [PubMed] [Google Scholar]
- 16.Friedman DI, Quiros PA, Subramanian PS, et al. Headache in Idiopathic Intracranial Hypertension: Findings From the Idiopathic Intracranial Hypertension Treatment Trial. Headache. 2017;57(8):1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman DI. Headaches Due to Low and High Intracranial Pressure. Continuum (Minneapolis, Minn). 2018;24(4, Headache):1066–1091. [DOI] [PubMed] [Google Scholar]
- 18.Corbett JJ, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39(8):461–474. [DOI] [PubMed] [Google Scholar]
- 19.Corbett JJ, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Archives of neurology. 1982;39(8):461–474. [DOI] [PubMed] [Google Scholar]
- 20.Orcutt J, Page N, Sanders M. Factors affecting visual loss in benign intracranial hypertension. Ophthalmology. 1984;91(11):1303. [DOI] [PubMed] [Google Scholar]
- 21.Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case-control study. Neurology. 1991;41(2 ( Pt 1)):239–244. [DOI] [PubMed] [Google Scholar]
- 22.Sadun AA, Currie JN, Lessell S. Transient visual obscurations with elevated optic discs. Annals of neurology. 1984;16(4):489–494. [DOI] [PubMed] [Google Scholar]
- 23.Kaiboriboon K, Piriyawat P, Selhorst JB. Light-induced amaurosis fugax. Am J Ophthalmol. 2001;131(5):674–676. [DOI] [PubMed] [Google Scholar]
- 24.Bruce BB, Newman NJ, Biousse V. Ophthalmoparesis in idiopathic intracranial hypertension. Am J Ophthalmol. 2006;142(5):878–880. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann E, Behr R, Neumann-Haefelin T, Schwager K. Pulsatile tinnitus: imaging and differential diagnosis. Deutsches Arzteblatt international. 2013;110(26):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassan H, Berkner L, Stolovitch C, Kesler A. Asymptomatic idiopathic intracranial hypertension in children. Acta neurologica Scandinavica. 2008;118(4):251–255. [DOI] [PubMed] [Google Scholar]
- 27.Bandyopadhyay S, Jacobson DM. Clinical features of late-onset pseudotumor cerebri fulfilling the modified dandy criteria. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2002;22(1):9–11. [DOI] [PubMed] [Google Scholar]
- 28.Galvin JA, Van Stavern GP. Clinical characterization of idiopathic intracranial hypertension at the Detroit Medical Center. J Neurol Sci. 2004;223(2):157–160. [DOI] [PubMed] [Google Scholar]
- 29.Rowe FJ, Sarkies NJ. Assessment of visual function in idiopathic intracranial hypertension: a prospective study. Eye. 1998;12(1):111–118. [DOI] [PubMed] [Google Scholar]
- 30.Kupersmith MJ, Sibony PA, Feldon SE, Wang JK, Garvin M, Kardon R. The Effect of Treatment of Idiopathic Intracranial Hypertension on Prevalence of Retinal and Choroidal Folds. American journal of ophthalmology. 2017;176:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sibony PA, Kupersmith MJ, Feldon SE, Wang JK, Garvin M. Retinal and Choroidal Folds in Papilledema. Investigative ophthalmology & visual science. 2015;56(10):5670–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacks AS, Miller NR. Spontaneous retinal venous pulsation: aetiology and significance. Journal of neurology, neurosurgery, and psychiatry. 2003;74(1):7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR American journal of neuroradiology. 2011;32(11):1986–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly LP, Saindane AM, Bruce BB, et al. Does bilateral transverse cerebral venous sinus stenosis exist in patients without increased intracranial pressure? Clin Neurol Neurosurg. 2013;115(8):1215–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranganathan S, Lee SH, Checkver A, et al. Magnetic resonance imaging finding of empty sella in obesity related idiopathic intracranial hypertension is associated with enlarged sella turcica. Neuroradiology. 2013;55(8):955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbett JJ, Mehta MP. Cerebrospinal fluid pressure in normal obese subjects and patients with pseudotumor cerebri. Neurology. 1983;33(10):1386–1388. [DOI] [PubMed] [Google Scholar]
- 37.Gilland O, Tourtellotte WW, O’Tauma L, Henderson WG. Normal cerebrospinal fluid pressure. Journal of neurosurgery. 1974;40(5):587–593. [DOI] [PubMed] [Google Scholar]
- 38.Tourtellotte WW. A selected review of reactions of the cerebrospinal fluid to disease In: Fields WS, ed. Neurological Diagnostic Techniques. Springfield, IL: Charles C Thomas; 1966:25–26. [Google Scholar]
- 39.Whiteley W, Al-Shahi R, Warlow CP, Zeidler M, Lueck CJ. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67(9):1690–1691. [DOI] [PubMed] [Google Scholar]
- 40.Avery RA, Shah SS, Licht DJ, et al. Reference range for cerebrospinal fluid opening pressure in children. The New England journal of medicine. 2010;363(9):891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz KM, Luetmer PH, Hunt CH, et al. Position-related variability of CSF opening pressure measurements. AJNR American journal of neuroradiology. 2013;34(4):904–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abel AS, Brace JR, McKinney AM, et al. Effect of patient positioning on cerebrospinal fluid opening pressure. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2014;34(3):218–222. [DOI] [PubMed] [Google Scholar]
- 43.Andresen M, Hadi A, Petersen LG, Juhler M. Effect of postural changes on ICP in healthy and ill subjects. Acta neurochirurgica. 2015;157(1):109–113. [DOI] [PubMed] [Google Scholar]
- 44.Smith JL. Whence pseudotumor cerebri? Journal of clinical neuro-ophthalmology. 1985;5(1):55–56. [PubMed] [Google Scholar]
- 45.Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664–670. [DOI] [PubMed] [Google Scholar]
- 46.Ferro JM, Canhao P, Stam J, et al. Delay in the diagnosis of cerebral vein and dural sinus thrombosis: influence on outcome. Stroke. 2009;40(9):3133–3138. [DOI] [PubMed] [Google Scholar]
- 47.Dinkin M, Moss HE. Should Magnetic Resonance Venography be Performed Routinely in all Patients Undergoing Evaluation for Idiopathic Intracranial Hypertension? Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2015;35(4):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon BJ, Han MH, Kang HS, Chang KH. MR imaging findings of intracranial dural arteriovenous fistulas: relations with venous drainage patterns. AJNR American journal of neuroradiology. 2005;26(10):2500–2507. [PMC free article] [PubMed] [Google Scholar]
- 49.Rekate HL. Why would a spinal tumor cause increased intracranial pressure? Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2002;22(3):197–198. [DOI] [PubMed] [Google Scholar]
- 50.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59(10):1492–1495. [DOI] [PubMed] [Google Scholar]
- 51.Digre KB, Nakamoto BK, Warner JEA, Langeberg WJ, Baggaley SK, Katz BJ. A comparison of idiopathic intracranial hypertension with and without papilledema. Headache. 2009;49(2):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kerr NM, Chew SS, Eady EK, Gamble GD, Danesh-Meyer HV. Diagnostic accuracy of confrontation visual field tests. Neurology. 2010;74(15):1184–1190. [DOI] [PubMed] [Google Scholar]
- 53.Wall M. The importance of visual field testing in idiopathic intracranial hypertension. Continuum (Minneapolis, Minn). 2014;20(4 Neuro-ophthalmology):1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayreh SS. Optic disc edema in raised intracranial pressure. V. Pathogenesis. Archives of ophthalmology (Chicago, Ill : 1960). 1977;95(9):1553–1565. [DOI] [PubMed] [Google Scholar]
- 55.Tso MO, Hayreh SS. Optic disc edema in raised intracranial pressure. III. A pathologic study of experimental papilledema. Archives of ophthalmology (Chicago, Ill : 1960). 1977;95(8):1448–1457. [DOI] [PubMed] [Google Scholar]
- 56.Keltner JL, Johnson CA, Cello KE, Wall M. Baseline visual field findings in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT). Invest Ophthalmol Vis Sci. 2014;55(5):3200–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corbett JJ, Jacobson DM, Mauer RC, Thompson HS. Enlargement of the blind spot caused by papilledema. American journal of ophthalmology. 1988;105(3):261–265. [DOI] [PubMed] [Google Scholar]
- 58.Frisen L. Swelling of the optic nerve head: a staging scheme. Journal of neurology, neurosurgery, and psychiatry. 1982;45(1):13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinclair AJ, Burdon MA, Nightingale PG, et al. Rating papilloedema: an evaluation of the Frisen classification in idiopathic intracranial hypertension. J Neurol. 2012;259(7):1406–1412. [DOI] [PubMed] [Google Scholar]
- 60.Bruce BB, Lamirel C, Biousse V, et al. Feasibility of nonmydriatic ocular fundus photography in the emergency department: Phase I of the FOTO-ED study. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2011;18(9):928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruce BB, Biousse V, Newman NJ. Nonmydriatic ocular fundus photography in neurologic emergencies. JAMA neurology. 2015;72(4):455–459. [DOI] [PubMed] [Google Scholar]
- 62.Kelly LP, Garza PS, Bruce BB, Graubart EB, Newman NJ, Biousse V. Teaching ophthalmoscopy to medical students (the TOTeMS study). American journal of ophthalmology. 2013;156(5):1056–1061.e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thulasi P, Fraser CL, Biousse V, Wright DW, Newman NJ, Bruce BB. Nonmydriatic ocular fundus photography among headache patients in an emergency department. Neurology. 2013;80(5):432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bruce BB, Thulasi P, Fraser CL, et al. Diagnostic accuracy and use of nonmydriatic ocular fundus photography by emergency physicians: phase II of the FOTO-ED study. Annals of emergency medicine. 2013;62(1):28–33.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischer WS, Wall M, McDermott MP, Kupersmith MJ, Feldon SE. Photographic Reading Center of the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT): Methods and Baseline Results. Invest Ophthalmol Vis Sci. 2015;56(5):3292–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biousse V, Bruce BB, Newman NJ. Ophthalmoscopy in the 21st century: The 2017 H. Houston Merritt Lecture. Neurology. 2018;90(4):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen JJ, Trobe JD. Optical Coherence Tomography Should Be Used Routinely to Monitor Patients With Idiopathic Intracranial Hypertension. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2016;36(4):453–459. [DOI] [PubMed] [Google Scholar]
- 68.Scott CJ, Kardon RH, Lee AG, Frisen L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010;128(6):705. [DOI] [PubMed] [Google Scholar]
- 69.Huang-Link Y, Eleftheriou A, Yang G, et al. Optical coherence tomography represents a sensitive and reliable tool for routine monitoring of idiopathic intracranial hypertension with and without papilledema. European journal of neurology. 2018. [DOI] [PubMed] [Google Scholar]
- 70.Chen JJ, Thurtell MJ, Longmuir RA, et al. Causes and Prognosis of Visual Acuity Loss at the Time of Initial Presentation in Idiopathic Intracranial Hypertension. Invest Ophthalmol Vis Sci. 2015;56(6):3850–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sibony P, Kupersmith MJ, Rohlf FJ. Shape analysis of the peripapillary RPE layer in papilledema and ischemic optic neuropathy. Investigative Ophthalmology & Visual Science. 2011;52(11):7987–7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheils CR, Fischer WS, Hollar RA, Blanchard LM, Feldon SE. The Relationship Between Optic Disc Volume, Area, and Frisen Score in Patients With Idiopathic Intracranial Hypertension. Am J Ophthalmol. 2018;195:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gampa A, Vangipuram G, Shirazi Z, Moss HE. Quantitative Association Between Peripapillary Bruch’s Membrane Shape and Intracranial Pressure. Invest Ophthalmol Vis Sci. 2017;58(5):2739–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang JK, Kardon RH, Ledolter J, Sibony PA, Kupersmith MJ, Garvin MK. Peripapillary Retinal Pigment Epithelium Layer Shape Changes From Acetazolamide Treatment in the Idiopathic Intracranial Hypertension Treatment Trial. Invest Ophthalmol Vis Sci. 2017;58(5):2554–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ (Clinical research ed). 2010;341:c2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manfield JH, Yu KK, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. Bariatric Surgery or Non-surgical Weight Loss for Idiopathic Intracranial Hypertension? A Systematic Review and Comparison of Meta-analyses. Obesity surgery. 2017;27(2):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ko MW, Chang SC, Ridha MA, et al. Weight gain and recurrence in idiopathic intracranial hypertension: a case-control study. Neurology. 2011;76(18):1564–1567. [DOI] [PubMed] [Google Scholar]
- 78.Weil R, Kovacs B, Miller N, et al. A 6-month telephone-based weight loss intervention in overweight and obese subjects with idiopathic intracranial hypertension. Obesity science & practice. 2016;2(2):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moss HE. Bariatric Surgery and the Neuro-Ophthalmologist. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2016;36(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ottridge R, Mollan SP, Botfield H, et al. Randomised controlled trial of bariatric surgery versus a community weight loss programme for the sustained treatment of idiopathic intracranial hypertension: the Idiopathic Intracranial Hypertension Weight Trial (IIH:WT) protocol. BMJ open. 2017;7(9):e017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gucer G, Viernstein L. Long-term intracranial pressure recording in the management of pseudotumor cerebri. Journal of Neurosurgery. 1978;49(2):256–263. [DOI] [PubMed] [Google Scholar]
- 82.Wall M, Kupersmith MJ, Thurtell MJ, Moss HE, Moss EA, Auinger P. The Longitudinal Idiopathic Intracranial Hypertension Trial: Outcomes From Months 6–12. Am J Ophthalmol. 2017;176:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.ten Hove MW, Friedman DI, Patel AD, Irrcher I, Wall M, McDermott MP. Safety and Tolerability of Acetazolamide in the Idiopathic Intracranial Hypertension Treatment Trial. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2016;36(1):13–19. [DOI] [PubMed] [Google Scholar]
- 84.Celebisoy N, Gokcay F, Sirin H, Akyurekli O. Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open-label study. Acta neurologica Scandinavica. 2007;116(5):322–327. [DOI] [PubMed] [Google Scholar]
- 85.Scotton WJ, Botfield HF, Westgate CS, et al. Topiramate is more effective than acetazolamide at lowering intracranial pressure. Cephalalgia : an international journal of headache. 2019;39(2):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schoeman JF. Childhood pseudotumor cerebri: clinical and intracranial pressure response to acetazolamide and furosemide treatment in a case series. Journal of child neurology. 1994;9(2):130–134. [DOI] [PubMed] [Google Scholar]
- 87.Chandran A, Pulhorn H, McMahon C. Idiopathic intracranial hypertension VISION (venous intervention versus shunting in IIH for optic nerve disc swelling) trial: patient perspective questionnaire. Br J Neurosurg. 2017:1–5. [DOI] [PubMed] [Google Scholar]
- 88.Gilbert AL, Chwalisz B, Mallery R. Complications of Optic Nerve Sheath Fenestration as a Treatment for Idiopathic Intracranial Hypertension. Seminars in ophthalmology. 2018;33(1):36–41. [DOI] [PubMed] [Google Scholar]
- 89.Feldon SE. Visual outcomes comparing surgical techniques for management of severe idiopathic intracranial hypertension. Neurosurgical focus. 2007;23(5):E6. [DOI] [PubMed] [Google Scholar]
- 90.Hickman SJ, Raoof N, Panesar H, McMullan JM, Pepper IM, Sharrack B. Visual Outcomes from Shunting for Idiopathic Intracranial Hypertension. Neuro-ophthalmology (Aeolus Press). 2014;38(6):310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGirt MJ, Woodworth G, Thomas G, Miller N, Williams M, Rigamonti D. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: predictors of treatment response and an analysis of long-term outcomes. J Neurosurg. 2004;101(4):627–632. [DOI] [PubMed] [Google Scholar]
- 92.Dinkin MJ, Patsalides A. Venous Sinus Stenting in Idiopathic Intracranial Hypertension: Results of a Prospective Trial. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2017;37(2):113–121. [DOI] [PubMed] [Google Scholar]
- 93.Lippa LM, Boker J, Duke A, Amin A. A novel 3-year longitudinal pilot study of medical students’ acquisition and retention of screening eye examination skills. Ophthalmology. 2006;113(1):133–139. [DOI] [PubMed] [Google Scholar]
- 94.Bruce BB, Lamirel C, Wright DW, et al. Nonmydriatic ocular fundus photography in the emergency department. The New England journal of medicine. 2011;364(4):387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koerner JC, Friedman DI. Inpatient and emergency service utilization in patients with idiopathic intracranial hypertension. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2014;34(3):229–232. [DOI] [PubMed] [Google Scholar]
- 96.Mollan SP, Aguiar M, Evison F, Frew E, Sinclair AJ. The expanding burden of idiopathic intracranial hypertension. Eye (London, England). 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frohman LP. Neuro-Ophthalmology: Transitioning From Old to New Models of Health Care Delivery. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2017;37(2):206–209. [DOI] [PubMed] [Google Scholar]