Abstract
Purpose:
To determine whether clinical features and visual outcomes of myelin oligodendrocyte glycoprotein antibody-associated optic neuritis (MOG-ON) differ between subjects who are White compared with those who are Asian.
Design:
Multicenter retrospective cohort.
Methods:
Setting: Multicenter.
Patient: 153 subjects who are White or Asian with history of adult-onset(age≥18years-old) optic neuritis and positive MOG-IgG serology by cell-based assay were enrolled from two unpublished cohorts(January 2017-November 2019) and nine published cohorts with case-level data available(2012-2018). Subjects with alternative etiologies of demyelinating disease and positive or lack of AQP-4-IgG serology result were excluded.
Main outcome measures: Clinical features and final visual outcomes.
Results:
One hundred and fifty-three subjects who are White(n=80) or Asian(n= 73) were included. 93(61%) were female, mean age of onset was 40.8±14.9 years-old, median follow up was 35.2(range1-432) months, which were all similar between White and Asian subjects. Subjects who are White were more likely to have recurrent optic neuritis(57(71%) vs. 20(27%);p=0.001) and extra-optic nerve manifestations(35(44%) vs. 8(11%);p=0.001). Optic disc swelling, neuroimaging findings, presenting visual acuity, treatment and final visual acuity did not differ according to subjects’ race. Despite the high prevalence of severe visual loss(<20/200) during nadir, the majority of subjects had good recovery of visual acuity(>20/40) at final examination(51(66%) of 77 subjects who are White vs. 52(74%) of 70 subjects who are Asian).
Conclusion:
Subjects with MOG-ON who are White were more likely to have recurrent disease and extra-optic nerve manifestations. Visual outcomes were similar between subjects who are White and those who are Asian.
INTRODUCTION
Optic neuritis (ON) is commonly associated with central nervous system (CNS) inflammatory demyelinating diseases. The discovery of novel serologic markers has allowed us to stratify some of the underlying disorders, which accounts for some of the observed heterogeneity in clinical characteristics and visual outcomes ranging from favorable in multiple sclerosis (MS) associated ON1 to poor in neuromyelitis optica spectrum disorders (NMOSD).2 Recently, myelin oligodendrocyte glycoprotein antibody (MOG-IgG), an autoantibody that targets the outer surface of oligodendrocytic myelin sheaths,3 has been identified as a marker for a subset of ON cases.4 Elevations in serum MOG-IgG are found in association with a range of clinical presentations including aquaporin-4-autoantibody (AQP4-IgG)-seronegative NMOSD phenotype, acute demyelinated encephalomyelitis (ADEM), particularly in children, and ON, which has been established as a separate disorder, MOG-IgG associated disorder (MOGAD).5-8 Since MOG-IgG testing has become more widely available, common characteristics including bilateral optic nerve involvement,5-7,9 optic disc swelling at onset,7,10,11 and a relapsing course of disease have been identified.4,5,7,9
Race is known to be an epidemiologic factor associated with differing clinical features and prognosis in demyelinating disease in general, and with visual prognosis in demyelinating ON.12-15 This study’s objective is to characterize differences in clinical features and visual outcomes of myelin oligodendrocyte glycoprotein antibody-associated optic neuritis (MOG-ON) between patients of White versus Asian race.
METHODS
Study design
This is a multicenter retrospective cohort of adult-onset MOG-ON. Case inclusion criteria were (1) adult subject (age ≥18-year-old) with history of one or more episodes of adult-onset(age ≥18-year-old) acute ON, (2) positive MOG-IgG serology by cell-based assay (CBA), interpreted according to reference standard of the reporting institution, (3) race/ethnicity data available. Exclusion criteria were (1) subjects with alternative etiologies of demyelinating disease, (2) positive or lack of AQP-4-IgG serology result. Following review of available case-level data, inclusion criteria were narrowed to White or Asian race due to limited numbers of case-level data for other races. Subjects were identified via three avenues: unpublished cases from the authors’ institutions, published cohorts with case-level data provided by the authors, and from published cohorts with publicly available case-level data. Details of how each of these was identified are provided below.
Unpublished cohorts
Stanford University (USA)
The Stanford Research Repository (STARR), Stanford Medicine’s resource for working with clinical data for research purposes, was used to identify adult patients (age ≥18-year-old), seen at Stanford Healthcare with a diagnosis of optic neuritis (ICD9 = 377.30, ICD10 = H46) and testing for MOG-IgG (Search terms of MOG-IgG1 and MOG FACS). These potential subjects were included in the study if chart review confirmed the clinical diagnosis of ON and positive serum MOG-IgG positive testing. Subjects with alternative etiologies of demyelinating disease, such as multiple sclerosis were excluded. Since MOG-IgG by CBA became commercially available to this center in 2017, all enrolled subjects were seen between January 1, 2017 and November 30, 2019. The study was approved with a waiver of informed consent by the Stanford University Institutional Review Board.
Ramathibodi Hospital (Thailand)
The Ramathibodi hospital neuro-ophthalmology clinic optic neuritis registry was used to identify adult patients seen in the clinic with a diagnosis of optic neuritis (ICD10 = H46) and MOG-IgG test result. Patients were included as subjects if chart review confirmed clinical diagnosis of ON, positive serum MOG-IgG testing, and the phenotype was consistent with MOGAD. Commercial MOG-IgG by CBA became available in early 2018, and all enrolled subjects were seen between January 1, 2018 and November 30, 2019. The optic neuritis registry was approved by Faculty of Medicine Ramathibodi Hospital Institutional Review Board and participants provided informed consent.
Published cohorts
To identify published cohorts, we used a systematic review approach to identify articles published between January 1, 2011 and September 1, 2019 (Figure1). Queries of MEDLINE and PUBMED using the terms (MOG or myelin oligodendrocyte glycoprotein) and (antibody or IgG) and optic neuritis identified 330 publications. Based on abstract review, this was narrowed to 59 English language publications that included clinical characteristics of ON associated with MOG-IgG seropositivity in adult human subjects. The other 271 abstracts were excluded due to non-original study, no clinical data, pediatric age group, or publication language other than English. Through full-text review of the 59 publications corresponding to the identified abstracts, 18 publications met our publication selection criteria which included report of 1) clinical characteristics and visual acuity outcomes of adult-onset MOG-ON, 2) MOG-IgG serology was tested by CBA, 3) homogeneous Asian or White study population or mixed-race study population with clinical data reported by race. Of these 18 selected publications, 7 publications had publicly available case-level data and these subjects were included in our study.
Figure1:
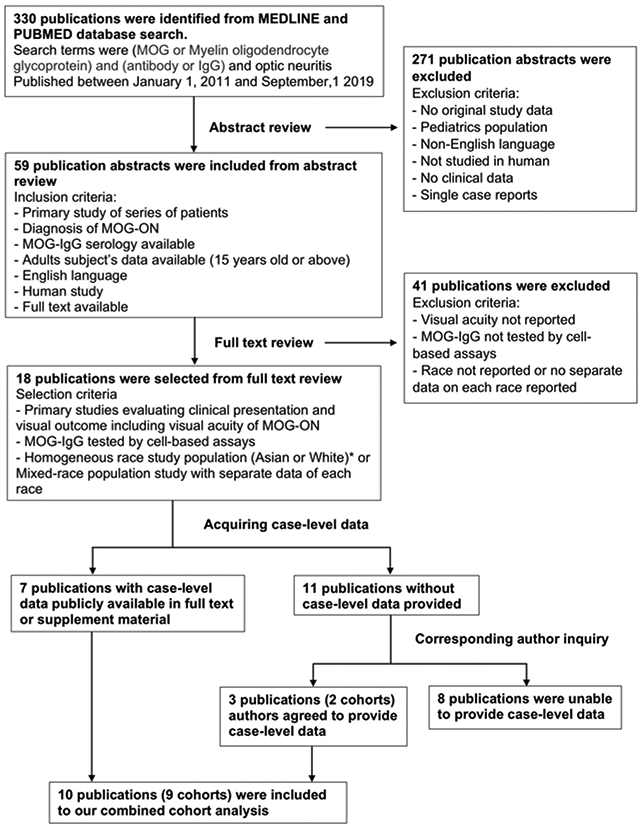
Flow chart showing process to select previously published cohorts. Nine eligible published cohorts with case-level data were included in our combined retrospective cohort study.
Abbreviation: MOG-ON=Myelin oligodendrocyte glycoprotein antibody associated optic neuritis, MOG-IgG=Myelin oligodendrocyte glycoprotein immunoglobulin G
* Review of available case-level data was narrowed to subjects who are White or Asian due to limited numbers of case-level data for other races in the literature.
For the remaining 11 publications, the corresponding authors were contacted by e-mail. Two authors of 3 publications shared de-identified case-level data for the purposes of this study. We were unable to acquire case-level data from the remaining 8 publications due to restriction of international data sharing,16 or no response to inquiry e-mail. Thus 9 cohorts from 10 publications (2 publications shared the same subjects) were included in our study.6-8,10,17-22 Study population, inclusion/exclusion criteria and geographic distribution of each cohorts are summarized in the supplementary table. (Supplementary material available at AJO.com)
Variable definition
Clinical characteristics and visual outcomes for each subject were abstracted from the medical records of the unpublished cohorts and from de-identified research records for the published cohorts. The primary outcomes of interest were final visual acuity and clinical phenotype.
Demographic variables included age at the time of first ON event, gender, and race. Race information was based on self-reported race as abstracted from the medical record in unpublished cohorts and published cohorts with data provided by co-authors. Remaining published cohorts did not indicate means of used to identify the race of subjects in the publication. (Supplementary table available at AJO.com) Historical variables included presence of prior non-optic neuritis neurological attacks. Features concurrent with ON events, including presence of pain, presence of optic disc swelling, visual acuity (VA) at nadir of the worst ON attack, magnetic resonance imaging (MRI) findings, and other associated neurological symptoms were recorded. In order to create a cohesive data set, VA was converted to logarithm of minimum angle of resolution (logMAR) values. The following logMAR values were used for nonnumeric visual acuities: no light perception = 3.0, light perception = 2.3, hand motion = 2.0, and count fingers = 1.7.23 MRI was categorized according to involvement of each of 4 optic nerve segments (intraorbital, intracanalicular, prechiasmatic, and optic chiasm), presence of perioptic nerve sheath enhancement and involvement of more than half of the optic nerve length. For the unpublished cohorts this was based on review of radiology reports for the first ON episode. For the published cohorts this was based on available data as provided by the investigators or publication.
Follow up variables included follow up duration from onset of ON event, VA at final examination (not included if the final examination was within 3 months of the last ON attacks), presence and number of subsequent episodes of ON, bilaterality of optic nerve involvement, and type(s) of acute and/or maintenance therapies. Bilateral involvement during course of disease was defined when both optic nerves were affected either simultaneously or sequentially during the course of a subject’s follow up. ON relapse was defined as an episode of acute ON occurring more than one month after a previous attack. For subjects with at least one year follow up duration, ON annualized relapsed rate (ARR) was calculated as the number of relapsed episodes (excluding initial presenting episode) divided by follow up in years.
The final clinical phenotype was categorized as single episode vs. recurrent disease and isolated ON vs. ON with other neurological symptoms based on a review of available data for each subject. Isolated ON referred to disease limited to optic nerve without other neurological features. This includes both single episode and recurrent isolated ON. Chronic relapsing idiopathic optic neuropathy (CRION), steroid-dependent and steroid-responsive recurrent ON with other etiology excluded,24 were included in recurrent isolated ON category. Non-isolated ON was further stratified as AQP-4-IgG negative NMOSD, defined according to 2015 international consensus diagnostic criteria25 and ADEM , characterized by a widespread central nervous system inflammatory demyelination with compatible neuroimaging characteristic.26 Subjects with other neurological symptoms not fulfilling these diagnostic criteria were defined as ON with other neurological symptoms.
Statistical analysis
Subjects were grouped according to race (Asian, White). Features were compared between subjects who were White and subjects who were Asian using generalized estimating equation models (GEE) to account for correlation within cohorts, age at the time of first ON event and gender. P values < .05 for adjusted parameter coefficients were considered statistically significant. Statistical analyses were completed using SPSS Statistics version 26 software (IBM, Armonk, NY, USA).
RESULTS
One hundred and fifty-three subjects who are White or Asian with adult-onset MOG-ON meeting the inclusion criteria were included in the multicenter retrospective cohort. Twenty-four subjects were enrolled from unpublished cohort (18 patients from Stanford University and 6 subjects from Ramathibodi hospital). Seventy-six subjects were enrolled from published cohorts that provided case-level data through collaboration. Additionally, 53 patients’ data were acquired from 7 publications which publicly provided case-level data. There were 7 subjects from contributing cohorts who are not White or Asian and were therefore excluded from our study. Geographic distribution of the multicenter cohort includes subjects from USA (California, Minnesota, Florida, and Arizona), UK, Germany, Denmark, Thailand, Japan, China, and South Korea. (Supplementary table available at AJO.com)
Of the included subjects, eighty (52%) were White and seventy-three (48%) were Asian. Overall, 93 (61%) patients were female with age at first ON event of 40.8 ±14.9 (mean ± SD) years. Follow up duration ranged from 1 to 432 (median = 35.2) months.
Comparisons of clinical features and visual outcomes of MOG-ON between subjects of White vs Asian race are summarized in Table1. Demographic parameters including age of onset, gender, and follow up duration were similar between subjects who are White and those who are Asian. With regards to ON features, subjects who are White were more likely to experience ocular pain during the acute ON episodes, while subjects of both races had a similar prevalence of optic disc swelling and simultaneous bilateral ON. Subjects who are Asian were more likely to have ON as a presenting symptom of MOGAD, either isolated or accompanied by other concurrent neurological symptoms.
Table 1:
Comparison of demographic and clinical features of myelin oligodendrocyte glycoprotein antibody-associated optic neuritis between subjects of White and Asian race
| Clinical features | White (n=80) | Asian (n=73) | Adjusted p-valuea |
|---|---|---|---|
| Age of ON onset, year, mean (SD) | 40.84 (13.94) | 40.85 (16.05) | .95 |
| Gender, female, n (%) | 51 (64) | 42 (58) | .47 |
| Duration of follow up after onset, months, median (range) | 35.12 (1-432) | 36.00 (3-276) | .17 |
| Follow up duration after onset | None Missing | None Missing | |
| ≥ 1 year follow up duration, n (%) | 60 (75) | 57 (78) | .80 |
| < 1 year follow up duration, n (%) | 20 (25) | 16 (22) | |
| Optic neuritis features | |||
| ON as an initial presentation of MOG | (n = 79), missing 1 | none missing | |
| Present, n (%) | 65 (82) | 72 (99) | .005 |
| Absent, n (%) | 14 (18) | 1 (1) | |
| Ocular pain during ON episode | (n=70), missing 10 | (n=34), missing 39 | |
| Present, n (%) | 63 (90) | 25 (74) | <.001 |
| Absent, n (%) | 7 (10) | 9 (26) | |
| Optic disc swelling during ON episode | (n=42), missing 38 | (n=22), missing 51 | |
| Present, n (%) | 35 (83) | 16 (73) | .25 |
| Absent, n (%) | 7 (17) | 6 (26) | |
| Simultaneous bilateral ON (≥ 1 episode) | (n=77), missing 3 | (n=69), missing 4 | |
| Present, n (%) | 32 (42) | 29 (43) | .95 |
| Absent, n (%) | 45 (58) | 39 (57) | |
| Visual acuity (VA) at nadir | (n=49), missing 31 | (n=57), missing 16 | |
| Mild visual loss (>20/40) | 6 (12) | 3 (5) | ref |
| Moderate visual loss (20/40-20/200) | 11 (23) | 21 (37) | .01 |
| Severe visual loss (<20/200) | 32 (65) | 33 (58) | .07 |
| Disease course features | |||
| Visual acuity (VA) at last follow up | (n=77), missing 3 | (n=70), missing 3 | |
| Mild visual loss (>20/40), n (%) | 51 (66) | 52 (74) | ref |
| Moderate visual loss (20/40-20/200), n (%) | 19 (25) | 13 (19) | .39 |
| Severe visual loss (<20/200), n (%) | 7 (9) | 5 (7) | .36 |
| ON involvement during course of disease | none missing | none missing | |
| Bilateral, n (%) | 61 (76) | 33 (45) | <.001 |
| Unilateral, n (%) | 19 (24) | 40 (55) | |
| ON Recurrence | none missing | none missing | |
| ≥ 2 episodes of ON, n (%) | 57 (71) | 20 (27) | .001 |
| 1 episode of ON, n (%) | 23 (29) | 53 (73) | |
| Spatial involvement | none missing | none missing | |
| Isolated ON, n (%) | 45 (56) | 65 (89) | .001 |
| ON with extra-optic nerve manifestation, n (%) | 35 (44) | 8 (11) | |
| Final clinical phenotype | none missing | none missing | |
| Isolated single episode of ON, n (%) | 12 (15) | 48 (66) | <.001 |
| Isolated recurrent ON (including CRION), n(%) | 33 (41) | 17 (23) | .04 |
| ADEM, n (%) | 6 (8) | 2 (3) | .11 |
| NMOSD, n (%) | 25 (31) | 4 (5) | .001 |
| ON + other neurological symptoms, n(%) | 4 (5) | 2 (3) | .54 |
Abbreviation: ARR = annualized relapsed rate, ON = optic neuritis, CRION = chronic relapsing idiopathic optic neuropathy, ADEM= acute demyelinating encephalomyelitis, NMOSD = neuromyelitis optica spectrum disorder
P-value for variable coefficient using generalized estimating equation (GEE) adjusting for age of onset, gender and correlation within cohort
Subjects who are White had similar visual outcomes as those who are Asian. There were no significant differences in VA at nadir phase (White, logMAR VA 1.70(0.60 – 2.30) vs. Asian, logMAR VA 1.50 (0.70 – 2.00) (median(IQR)); p=.22) or last follow up (White, logMAR VA 0.00 (0.00 – 0.48) vs. Asian, logMAR VA 0.00 (0.00 – 0.30) (median(IQR)); p =.16). Subjects had comparable rates of visual recovery at last follow up examination regardless of race. The relationship between final VA and race was not changed when accounting for follow up duration (p=0.36). Prevalence of poor visual outcomes (final VA <20/200) were not different between subjects with less than 1 year and at least 1 year follow up duration (<1 year, 2(6%) of 33 subjects vs. ≥1 year, 10(9%) of 114 subjects; p=1.000, Fisher’s exact). There was no difference in involvement of optic nerve segments on neuroimaging between subjects of different races. The most frequently affected segment of optic nerve was the intraorbital part. (Table2)
Table2:
Comparison of magnetic resonance imaging (MRI) findings of myelin oligodendrocyte glycoprotein antibody-associated optic neuritis between subjects of White and Asian race
| MRI findings | White | Asian |
Adjusted
p-valuea |
|---|---|---|---|
| Optic nerve segment involved | (n = 39), missing 41 | (n = 41), missing 32 | |
| Intraorbital optic nerve, n (%) | 34 (87) | 37 (90) | .20 |
| Intracanalicular optic nerve, n (%) | 25 (64) | 34 (83) | .26 |
| Prechiasmatic optic nerve, n (%) | 25 (64) | 30 (73) | .42 |
| Optic chiasm, n (%) | 7 (18) | 10 (24) | .62 |
| Perioptic nerve sheath enhancement | (n = 38), missing 42 | (n = 34), missing 39 | |
| Present, n (%) | 22 (58) | 21 (62) | .65 |
| Long segment involvement | (n = 40), missing 40 | (n = 41), missing 32 | |
| Present, n (%) | 28 (70) | 32 (78) | .29 |
P-value for variable coefficient (present vs. absent) by generalized estimating equation (GEE) adjusting for age of onset, gender and correlation within cohort
The vast majority of subjects received acute treatment for MOG-ON. Overall, Intravenous methylprednisolone (IVMP) was the most common acute treatment followed by plasma exchange (PLEX) plus IVMP. Other acute treatment regimens, such as intravenous immunoglobulin (IVIG) and oral prednisone, were used in a minority of subjects. Adjusting for age of onset, gender, and correlation within cohort, there was no difference in acute treatment pattern between subjects of both races. Maintenance immunosuppressants were prescribed in approximately half of the subjects regardless of race. (Table3)
Table3:
Comparison of myelin oligodendrocyte glycoprotein antibody-associated optic neuritis treatment between subjects of White and Asian race
| Treatment | White (n = 80) |
Asian (n = 73) |
Adjusted p-valuea |
|---|---|---|---|
| No acute treatment, n (%) | 3 (4) | 3 (4) | .91 |
| Acute treatment present | (n = 77) | (n = 70) | |
| Monotherapy, n (%) | 60 (78) | 67 (96) | .058b |
| IVMP, n (%) | 57 (74) | 64 (92) | |
| Oral prednisone, n (%) | 2 (3) | 3 (4) | |
| IVIG, n (%) | 1 (1) | 0 (0) | |
| Combined therapy, n(%) | 17 (22) | 3 (4) | |
| IVMP + PLEX, n (%) | 16 (21) | 3 (4) | |
| IVMP + IVIG, n (%) | 1 (1) | 0 (0) | |
| Maintenance treatment | |||
| Present, n (%) | 46 (58) | 30 (41) | .11 |
| Absent, n (%) | 34 (42) | 43 (59) |
Abbreviation: IVMP = intravenous methylprednisolone, PLEX = plasma exchange, IVIG = intravenous immunoglobulin
P-value for variable coefficient by generalized estimating equation (GEE) adjusting for age of onset, gender and correlation within cohort
P-value for comparison of monotherapy vs. combined therapy between subjects who were White and those who were Asian
During the course of disease, MOG-ON in subjects who are Asian tended to have disease isolated to the optic nerve, whereas MOG-ON in subjects who are White were more likely to have extra-optic nerve CNS manifestations (35(44%) of 80 subjects vs. 8(11%) of 73 subjects; p=.001). Subjects who are White had a higher proportion of recurrent ON (57(71%) OF 80 subjects vs. 20(27%) 73 subjects; p=.001), despite similar follow up duration, with correspondingly higher ON annualized relapsed rate (ARR) in White subjects (0.62 (0.07 – 0.96) vs. 0.00 (0.00 – 0.17) (median(IQR)) relapsed episodes per year; p<.001). Although bilateral optic nerve involvement during the disease course was more common in subjects who are White (61(76%) of 80 subjects vs 33(45%) of 73 subjects; p<.001), the proportion of subjects who had at least 1 episode of simultaneous bilateral ON was similar in both races.
The final clinical phenotype varied between subjects of different races. Single episode isolated ON was more common in subjects who are Asian than those who are White. In contrast, recurrent isolated ON, including CRION, was more common in subjects who are White. AQP-4-IgG negative NMOSD was more prevalent in subjects who were White than those who were Asian. Few MOG-ON subjects in both races had other neurological symptoms including transverse myelitis (not compatible with NMOSD criteria), and other brainstem syndromes. ADEM was rare in our adult cohort in both races.
DISCUSSION
During the past decade, multiple large case series have characterized the clinical features and visual outcomes of MOG-ON. However, most of the cohorts have been of homogeneous race and accordingly, the relationship between race, clinical features and outcomes has received little attention, despite well described variations in other forms of ON between subjects who are of different races. Accordingly, this study aimed to directly compared clinical features and visual outcomes of MOG-ON between subjects of White versus Asian race.
Several features of MOG-ON were similar in subjects of both races including demographic characteristics, optic nerve appearance, neuroimaging findings and visual acuity outcomes. The gender distribution in our cohort reinforces previous findings of MOG-ON having less female predominance than AQP-4-IgG associated ON.16,27-30 The finding of a high proportion of subjects with optic disc swelling reinforces optic disc swelling as a common feature in MOG-ON regardless of race.29,31,32 MRI findings were comparable between subjects of different race with common involvement of a long segment of the intraorbital optic nerve and presence of perioptic nerve sheath enhancement in the majority of cases, in contrast to what has been reported for AQP-4-IgG or MS associated ON.8 These imaging features are consistent with what has been described as typical for MOG-ON.32 Visual outcomes were excellent with the majority of subjects of either race recovering final visual acuity >20/40 despite having severe visual loss (<20/200) during the nadir phase. Only 7(9%) of 77 subjects who are White and 5(7%) of 70 subjects who are Asian had final visual acuity in either eye <20/200. Consistent with prior reports, these visual outcomes are better than reported in AQP-4-IgG associated ON, in which approximately one-third of patients had severe visual loss after recovery (<20/200).2,33
A notable difference in MOG-ON attack characteristics between subjects of White and Asian race was a higher frequency of ocular pain during ON episode in subjects who are White compared with those who are Asian. A similarly lower prevalence of pain has also been reported in other Chinese MOG-ON cohorts, which met criteria for inclusion in our cohort, but for which case-level data could not be obtained.16,34 However, it is difficult to conclude if differences in pain, a subjective perception, are a result of disease differences or other differences including a cultural difference in pain perception and evaluation. Interestingly, pain perception studies report lower pain threshold and tolerance levels in subjects who are Asian than for those who are White, which would not account for our observation.35
We found four additional interesting differences in disease course between subjects of White versus Asian race. First, though ON was a common presenting manifestation of MOG-ON in subjects of both races, subjects who are White were more likely to have a clinical syndrome other than ON as their initial presentation. However, it should be noted that our case inclusion criteria requiring ON likely biased towards ON as the initial presenting syndrome in comparison to other studies that considered non-optic neuritis presentations of MOGAD,5,36,37 though this bias is likely similar for subjects of either race. Second, subjects who are White were more likely to have recurrent ON, both based on proportion of subjects with recurrence and annualized relapse rate (ARR). Nonetheless, the comparison may be overstated as the rate of recurrent ON in our subjects who are Asian was lower than reported in other Asian-predominant cohorts not included in our study in which more than half of subjects had recurrence.29,31,38 Third, subjects who are White were more likely to have bilateral optic nerve involvement during the course of their disease. However, this may be an artifact of recurrent disease since both races had similar prevalence of simultaneous bilateral ON. Fourth, the majority of subjects who are Asian (89%) had an isolated ON clinical phenotype compared to approximately two-thirds of subjects who are White. This difference persisted when excluding cases from two cohorts that excluded patients with seronegative NMOSD phenotype.10,17
The results should be interpreted in the context of how race information in this study was derived, namely by self-report without subclassification. It was demonstrated in prior studies that self-reported race correlated well with predominant genetic ancestry,39,40 but there is heterogeneity as a result. Furthermore, subjects of both races in this study may not adequately represent the diverse subgroups in each race. For instance, our Asian study population derived from East Asia (Japan, China, South Korea) and Southeast Asia (Thailand), with minimal representation from Central Asia or South Asia. This limitation also applies to White study subjects, with the term White for identification of race applying to a heterogeneous population in terms of ancestry, geographic origin, and birthplace.41 Differences in clinical features of MOG-ON between races in this study may be confounded by geographic distribution as mediated by differences in healthcare system and environment. Further study with consideration of racial subgroups and geographic location would help to clarify the role of these factors.
This study has some limitations. Combining retrospective case-level data from multiple sources captures the selection and ascertainment bias from each source due to differences in inclusion criteria and data measurement. We addressed this statistically using models accounting for correlation within cohorts. The majority of patients included in this study were from tertiary centers, which could bias the cohort toward more severe or relapsing disease. However, this should bias should be equal between White and Asian subjects and therefore should not influence the comparison between the two races. Missing data in certain parameters such as ocular pain, optic disc swelling, and MRI features in some cohorts also limits analysis of these features. Performance of this study illustrated systematic barriers to work of this kind related to issues with data sharing and race reporting. For example, in some countries collection of race and ethnicity data for research purposes is prohibited by regulation. Additionally, depending on the country and institution, international data sharing may be prohibited by government regulations.
CONCLUSION
In this multicenter retrospective cohort study, we found differences in clinical features of MOG-ON between subjects who are White and Asian race. Subjects who are White were more likely to report pain, have recurrent attacks, and have bilateral optic nerve involvement during the disease course. Subjects who are Asian were more likely to have disease phenotype limited to the optic nerve. Visual outcomes and other clinical features were similar between subjects of different races and similar to what is typically associated with MOG-ON. It is a matter of speculation whether differences between subjects of different races reflect genetic or environmental influences related to residential location. A multicenter prospective population-based study is warranted in order to gain further insight into associations between MOG-ON and different races.
Supplementary Material
Highlights.
White MOG-ON subjects reported a higher incidence of pain and recurrent optic neuritis.
Asian MOG-ON subjects were more likely to have an isolated optic neuritis phenotype.
The majority of both races had optic disc swelling on the fundus exam.
Both races had similar MOG-ON visual outcomes.
ACKNOWLEDGEMENT/DISCLOSURE
Funding: This work was supported by the National Institutes of Health [NIH P30 026877], Research to Prevent Blindness unrestricted grant both to the Stanford Department of Ophthalmology.
Financial disclosure:
Dr.Padungkiatsagul, Dr.Moss, Dr.Chen, Dr.Jindahra, Dr.Akaishi, Dr.Takeshita have no conflicts of interest.
Dr.Takahashi has received speaker honoraria from Cosmic Corporation.
Dr.Nakashima has received funding for travel and received speaker honoraria from Mitsubishi Tanabe Pharma, Biogen Japan, Novartis Pharma, Alexion Pharma, Takeda Pharmaceutical Company, and has received research funding from LSI Medience and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI 17K09772).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Visual function 15 years after optic neuritis: a final follow-up report from the Optic Neuritis Treatment Trial. Ophthalmology. 2008;115(6):1079–1082.e1075. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes DB, Ramos Rde I, Falcochio C, Apostolos-Pereira S, Callegaro D, Monteiro ML. Comparison of visual acuity and automated perimetry findings in patients with neuromyelitis optica or multiple sclerosis after single or multiple attacks of optic neuritis. J Neuroophthalmol. 2012;32(2):102–106. [DOI] [PubMed] [Google Scholar]
- 3.Reindl M, Di Pauli F, Rostasy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol. 2013;9(8):455–461. [DOI] [PubMed] [Google Scholar]
- 4.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140(12):3128–3138. [DOI] [PubMed] [Google Scholar]
- 6.Pache F, Zimmermann H, Mikolajczak J, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation. 2016;13(1 ):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JJ, Flanagan EP, Jitprapaikulsan J, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Positive Optic Neuritis: Clinical Characteristics, Radiologic Clues, and Outcome. Am J Ophthalmol. 2018;195:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S-M, Woodhall MR, Kim J-S, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurology - Neuroimmunology Neuroinflammation. 2015;2(6):e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramanathan S, Reddel SW, Henderson A, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm. 2014;1(4):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akaishi T, Sato DK, Nakashima I, et al. MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study. J Neurol Neurosurg Psychiatry. 2016;87(4):446–448. [DOI] [PubMed] [Google Scholar]
- 11.Ramanathan S, Mohammad S, Tantsis E, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89(2):127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarius S, Wildemann B, Paul F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin Exp Immunol. 2014;176(2):149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss HE, Gao W, Balcer LJ, Joslin CE. Association of race/ethnicity with visual outcomes following acute optic neuritis: an analysis of the Optic Neuritis Treatment Trial. JAMA Ophthalmol. 2014;132(4):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, Mealy MA, Levy M, et al. Racial differences in neuromyelitis optica spectrum disorder. Neurology. 2018;91(22):e2089–e2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitley J, Leite MI, Nakashima I, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain. 2012;135(Pt 6):1834–1849. [DOI] [PubMed] [Google Scholar]
- 16.Zhao G, Chen Q, Huang Y, et al. Clinical characteristics of myelin oligodendrocyte glycoprotein seropositive optic neuritis: a cohort study in Shanghai, China. J Neurol. 2018;265(1):33–40. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima H, Motomura M, Tanaka K, et al. Antibodies to myelin oligodendrocyte glycoprotein in idiopathic optic neuritis. BMJ Open. 2015;5(4):e007766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siritho S, Sato DK, Kaneko K, Fujihara K, Prayoonwiwat N. The clinical spectrum associated with myelin oligodendrocyte glycoprotein antibodies (anti-MOG-Ab) in Thai patients. Mult Scler. 2016;22(7):964–968. [DOI] [PubMed] [Google Scholar]
- 19.Jitprapaikulsan J, Chen JJ, Flanagan EP, et al. Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein Autoantibody Status Predict Outcome of Recurrent Optic Neuritis. Ophthalmology. 2018;125(10):1628–1637. [DOI] [PubMed] [Google Scholar]
- 20.Chen JJ, Tobin WO, Majed M, et al. Prevalence of Myelin Oligodendrocyte Glycoprotein and Aquaporin-4-IgG in Patients in the Optic Neuritis Treatment Trial. JAMA Ophthalmol. 2018;136(4):419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79(12):1273–1277. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Huang Y, Li H, et al. MOG-antibody associated demyelinating disease of the CNS: A clinical and pathological study in Chinese Han patients. J Neuroimmunol. 2017;305:19–28. [DOI] [PubMed] [Google Scholar]
- 23.Danesh-Meyer H, Savino PJ, Gamble GG. Poor prognosis of visual outcome after visual loss from giant cell arteritis. Ophthalmology. 2005; 112(6):1098–1103. [DOI] [PubMed] [Google Scholar]
- 24.Petzold A, Plant GT. Chronic relapsing inflammatory optic neuropathy: a systematic review of 122 cases reported. J Neurol. 2014;261(1):17–26. [DOI] [PubMed] [Google Scholar]
- 25.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261–1267. [DOI] [PubMed] [Google Scholar]
- 27.Mader S, Gredler V, Schanda K, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation. 2011. ;8:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82(6):474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Tan S, Chan TCY, et al. Clinical features of demyelinating optic neuritis with seropositive myelin oligodendrocyte glycoprotein antibody in Chinese patients. Br J Ophthalmol. 2018;102(10):1372–1377. [DOI] [PubMed] [Google Scholar]
- 30.Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. 2014;71(3):276–283. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Zhou H, Wang J, et al. The prevalence and prognostic value of myelin oligodendrocyte glycoprotein antibody in adult optic neuritis. J Neurol Sci. 2019;396:225–231. [DOI] [PubMed] [Google Scholar]
- 32.Ramanathan S, Prelog K, Barnes EH, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22(4):470–482. [DOI] [PubMed] [Google Scholar]
- 33.Jarius S, Frederikson J, Waters P, et al. Frequency and prognostic impact of antibodies to aquaporin-4 in patients with optic neuritis. J Neurol Sci. 2010;298(1-2):158–162. [DOI] [PubMed] [Google Scholar]
- 34.Kang H, Liu Z, Li H, et al. Simultaneous bilateral optic neuritis in China: clinical, serological and prognostic characteristics. Acta Ophthalmol. 2019;97(3):e426–e434. [DOI] [PubMed] [Google Scholar]
- 35.Rowell LN, Mechlin B, Ji E, Addamo M, Girdler SS. Asians differ from non-Hispanic Whites in experimental pain sensitivity. Eur J Pain. 2011;15(7):764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyun JW, Woodhall MR, Kim SH, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry. 2017;88(10):811–817. [DOI] [PubMed] [Google Scholar]
- 37.Mariotto S, Ferrari S, Monaco S, et al. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study. J Neurol. 2017;264(12):2420–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Jia X, Yang H, et al. Myelin oligodendrocyte glycoprotein antibody-associated demyelination: comparison between onset phenotypes. Eur J Neurol. 2019;26(1):175–183. [DOI] [PubMed] [Google Scholar]
- 39.Sinha M, Larkin EK, Elston RC, Redline S. Self-reported race and genetic admixture. N Engl J Med. 2006;354(4):421–422. [DOI] [PubMed] [Google Scholar]
- 40.Tang H, Quertermous T, Rodriguez B, et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet. 2005;76(2):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhopal R, Donaldson L. White, European, Western, Caucasian, or what? Inappropriate labeling in research on race, ethnicity, and health. American Journal of Public Health. 1998;88(9):1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


