Abstract
Around 71 million people are chronically infected with HCV worldwide. HCV antiviral drug development has been remarkable. The availability of pangenotypic direct-acting antivirals with excellent efficacy and good tolerability profiles offer a unique opportunity to achieve HCV elimination worldwide. IFN-free DAA combinations can now cure HCV in more than 95% of patients with HCV infection after 8-12 weeks of treatment. Programmes to eliminate HCV must include increased screening (risk-based and universal), linkage to care, as well as increased access to treatment worldwide. In this paper, we will review the available data on recently approved direct-acting antiviral agents, with sustained virological response that reaches almost 100%.
Keywords: chronic hepatitis C, compliance, genotype, HCV elimination, people who inject drugs, screening
1 |. INTRODUCTION
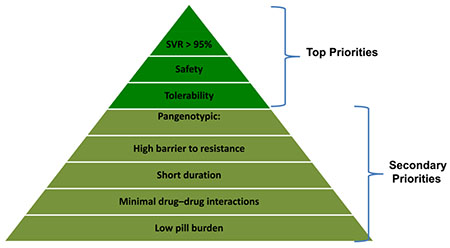
A remarkable revolution has recently occurred with the availability of direct-acting antivirals (DAAs) with different modes of action, leading to high chance of cure and a good tolerance profile1,2 (Figure 1A,B). The primary goal of treatment is to achieve a sustained virological response (SVR) defined as undetectable serum HCV RNA 12 weeks after the end of treatment.3 An SVR has been shown to be durable during long-term follow-up and associated with the eradication of HCV infection confirmed by undetectable HCV RNA in serum and the liver.4 An SVR indicates that viral infection has been cured. In addition, viral eradication is associated with the regression of fibrosis, the reversal of cirrhosis and a significant improvement in clinical outcome and survival with a decreased incidence of complications, especially hepatocellular carcinoma (HCC; Box 1). Persons with HCV chronic infection and compensated or decompensated cirrhosis who achieve an SVR can have clinically significant reductions in hepatic venous pressure gradient during long-term follow-up.5 The benefit to survival of DAAs in subjects with decompensated cirrhosis was recently confirmed.6 Treating HCV infection with newly developed DAAs frequently improves perceived fatigue and patient-reported outcome.7 Top priorities for DAAs are high efficacy (>95%) and favourable safety and tolerance profiles (Box 2).
FIGURE 1.
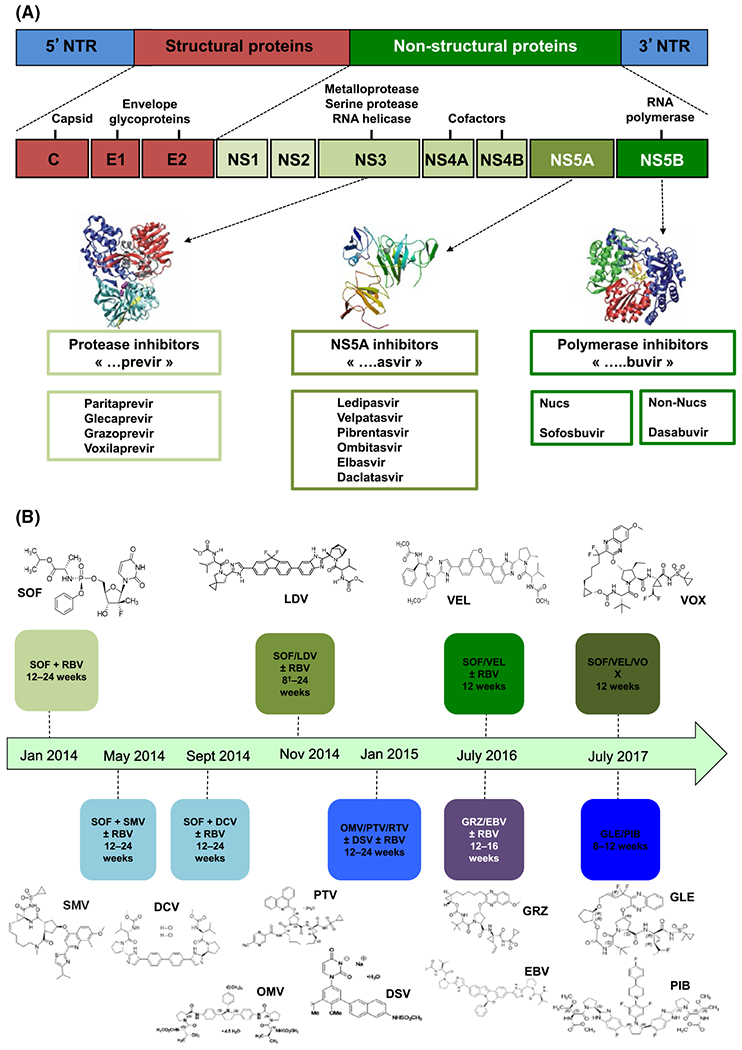
(A) Direct-acting antiviral agents (DAAs) with different mode of actions. HCV is curable with many DAAs available that specifically target the HCV virus replication cycle to inhibit replication (NS3 protease inhibitors, nucleoside/nucleotide analogues and non-nucleoside inhibitors of the RNA-dependent RNA polymerase and NS5A inhibitors). (B) Development milestones of approved direct-acting antiviral agents. DCV, daclatasvir; DSV, dasabuvir; EBV, elbasvir; GLE, glecaprevir; GRZ, grazoprevir; LDV, ledipasvir; OMV, ombitasvir; PIB, pibrentasvir; PTV, paritaprevir; RTV, ritonavir; SMV, simeprevir; SOF, sofosbuvir; VEL, velpatasvir
Box 1. Clinical benefits obtained by achieving sustained virological response.
Eradicate the virus (HCV cure)
Reduce necroinflammation
Stop fibrosis progression
Prevent cirrhosis and complications
Prevent hepatocellular carcinoma
Reduce extra-hepatic manifestations
Increase survival
Improve quality of life
Box 2. Requirements of direct-acting antiviral agents.
2 |. SOFOSBUVIR/VELPATASVIR (EPCLUSA)
The HCV non-structural (NS) 5B uridine nucleotide polymerase inhibitor, sofosbuvir (SOF), has nanomolar in vitro activity against all HCV genotypes. It has a favourable safety profile, and a high genetic barrier to resistance.8 Velpatasvir (VEL) is a second-generation HCV NS5A inhibitor with antiviral activity against HCV replicons in genotypes 1 through 6.9
The combination of SOF/VEL for treating chronic HCV has been evaluated in several studies.10–12 ASTRAL-1,-2 and-3 each evaluated a once-daily, fixed-dose combination regimen of SOF/VEL for 12 weeks, and collectively the studies covered a broad range of HCV populations, including HCV genotypes 1-6. The overall response rate in more than 1000 patients was high, with 98% achieving SVR12 (Figure 2A).
FIGURE 2.
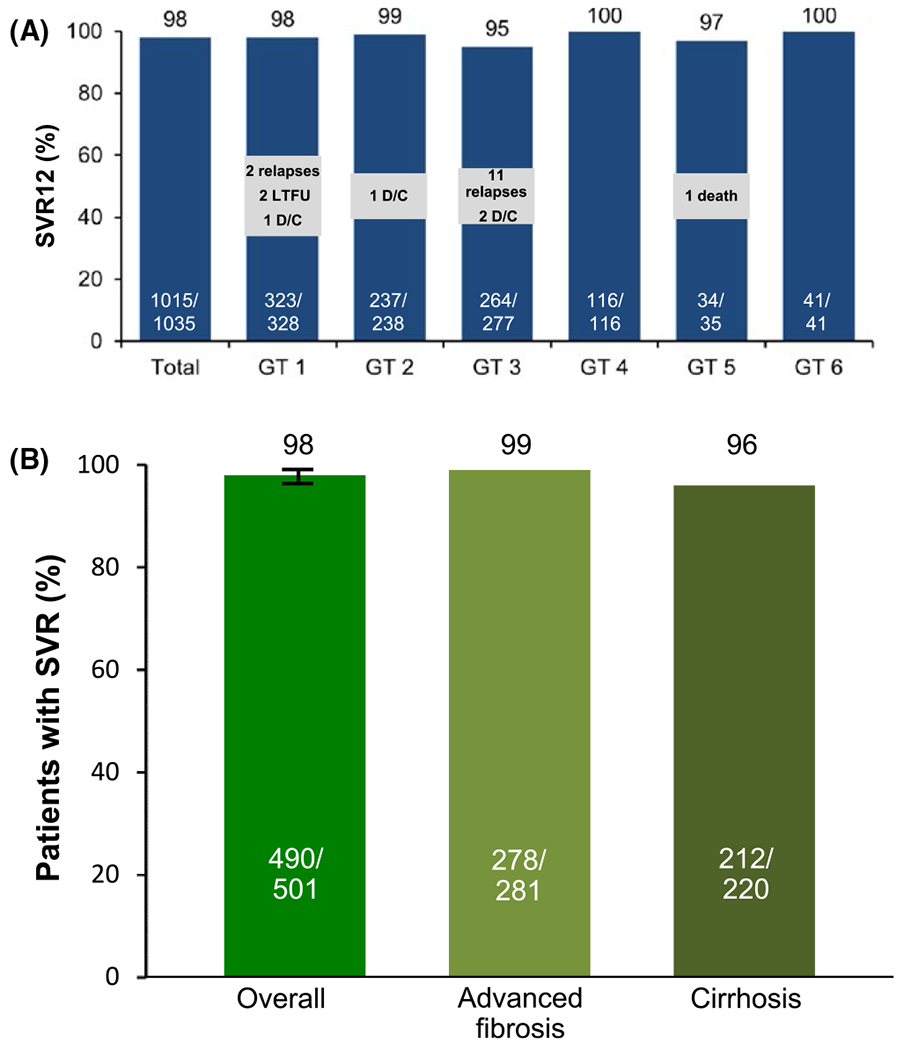
(A) SOF/VEL SVR rates for 12 weeks across all genotypes (ASTRAL-1-2-3).10,11 These studies encompassed a broad range of HCV populations, including HCV genotypes 1-6. The overall SVR rate in more than 1000 patients was high, with 98% achieving SVR12. AE, adverse event; D/C, discontinuation; LTFU, lost to followup; SAE, serious adverse event. (B) Sustained virological response rates overall and by cirrhosis or advanced fibrosis status.13 (ASTRAL-1-2-3). SVR12 was achieved by 98% of patients (490/501; 95% CI, 96-99). Patients with cirrhosis had an SVR12 rate of 96% (212/220), and those with advanced fibrosis had an SVR12 rate of 99% (278/281)
Treatment had a favourable safety and tolerability; 2% of patients experienced one or more serious adverse events (SAE) and no SAEs were considered study drug related. Two patients discontinued treatment due to AEs.
In a retrospective analysis of more than 500 patients, SOF/VEL was highly effective as a pangenotypic treatment for HCV patients with advanced fibrosis or compensated cirrhosis, a population historically considered difficult to cure and with higher risks of safety issues.13 Treatment with sofosbuvir plus velpatasvir for 12 weeks resulted in and SVR12 in 98% of patients infected with HCV genotypes 1-6 (Figure 2B).
3 |. SOFOSBUVIR/VELPATASVIR/VOXILAPREVIR (VOSEVI)
Voxilaprevir (VOX) is a macrocyclic, pangenotypic inhibitor of the NS3/4A protease with picomolar antiviral activity against HCV GT 1-6 and an improved resistance profile compared to earlier protease inhibitors.
The POLARIS-2 trial enrolled patients infected with all HCV genotypes. Compensated cirrhosis was allowed except for patients with HCV genotype 3 infection.14 Patients received either a fixed-dose combination of SOF/VEL/VOX once daily for 8 weeks or a fixed-dose combination of 400 mg of SOF/VEL once daily for 12 weeks. The overall SVR rate was 95% for the 8 week 3-DAAs regimen and 98% for the 12 week 2-DAAs regimen. Hence, the primary efficacy endpoint of non-inferiority (5% margin) was not met.
4 |. GLECAPREVIR-PIBRENTASVIR (MAVIRET)
Glecaprevir (GLE) is a potent NS3/4A protease inhibitor with nanomolar antiviral activity against HCV GT 1-6 and most known NS3 RASs.15 Pibrentasvir (PIB) is an NS5A inhibitor with picomolar antiviral activity against HCV GT 1-6 and most NS5A RASs. This fixed-dose combination demonstrated synergistic antiviral activity and a high barrier to resistance. Efficacy results of GLE/PIB are represented in Figure 3A,B.16,17
FIGURE 3.
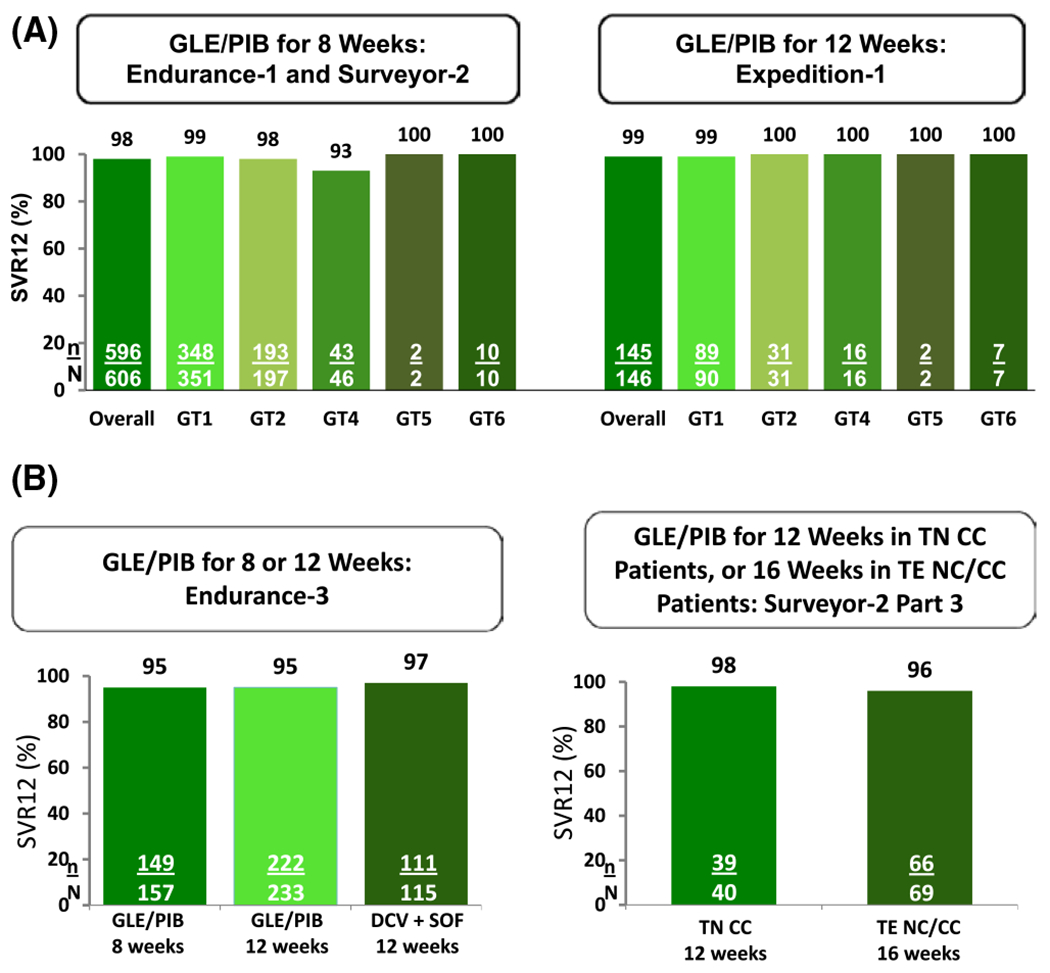
(A) Studies evaluating the efficacy of GLE/PIB in patients with HCV GT1, 2, 4-6 Infection with or without compensated cirrhosis. GLE/PIB for 8 weeks (Endurance-1 and Surveyor-2) in patients without cirrhosis resulted overall in an SVR of 98% (596/606); and for GT1 an SVR of 99% (348/351).(B) Studies evaluating the efficacy of GLE/PIB in patients with HCV GT3 infection with or without compensated cirrhosis. Genotype 3-infected patients treated for 12 weeks achieved an SVR12 rate of 95.3% with GLE/PIB, compared to a 96.5% SVR12 rate with SOF/DCV; 8-week GLE/PIB yielded an SVR12 rate of 94.9%
The efficacy and safety of 8- and 12-week treatment with GLE/PIB in patients without cirrhosis with HCV genotype 1 or 3 infection was evaluated.17 Endurance-1 and Endurance-3 were phase 3, randomized, open-label, multicentre studies. Patients with genotype 1 infection were randomized 1:1 to receive once-daily GLE/PIB for either 8 or 12 weeks. Patients with genotype 3 infection were randomized 2:1 to receive 12 weeks of GLE/PIB or SOF plus daclatasvir (DCV). An additional group of patients with genotype 3 infection was then enrolled in a non-randomized arm and treated for 8 weeks with GLE/PIB.
A total of 1208 patients were included. The SVR12 rate in genotype 1-infected patients was 99.1% in the 8-week group and 99.7% in the 12-week group. Genotype 3-infected patients treated for 12 weeks achieved an SVR12 rate of 95.3% with GLE/PIB, compared to a 96.5% SVR12 rate with SOF/DCV; 8-week GLE/PIB resulted in an SVR12 rate of 94.9%. Adverse events led to discontinuation in ≤1% of patients.
In summary, once-daily treatment with GLE/PIB for 8 or 12 weeks achieved high SVR rates in patients with HCV genotype 1 or 3 infection without cirrhosis.
We evaluated the safety and efficacy of 8 and 12 weeks of treatment with GLE/PIB in patients with HCV genotype 2,4,5 or 6 infection without cirrhosis in 3 separate phase 3 trials.18 We performed 2 open-label, single-arm studies (Surveyor-II, Part 4 and Endurance-4) and a randomized, double-blind, placebo-controlled study (Endurance-2).
In the Endurance-2 study, adult patients with untreated or previously treated HCV genotype 2 infection without cirrhosis were randomly assigned (2:1) to groups given once-daily oral GLE/PIB (n = 202; 300 mg/120 mg) or placebo (n = 101) for 12 weeks. In the Surveyor-II, Part 4 and Endurance-4 studies, adult patients with untreated or previously treated patients with HCV genotypes 2, 4, 5 or 6 infection, without cirrhosis, were given once-daily oral GLE/PIB (n = 121 in Endurance-4 and n = 145 in SURVEYOR-II) for 12 or 8 weeks respectively.
The SVR12 rates in patients receiving GLE/PIB for 8 weeks were 98% in patients infected with HCV genotype 2% and 93% in those infected with HCV genotypes 4, 5 or 6. The SVR12 rates in patients receiving GLE/PIB for 12 weeks, were 99.5% in those infected with HCV genotype 2% and 99% in those infected with HCV genotype 4, 5 or 6. No virological failures occurred in patients with HCV genotypes 4, 5 or 6 infections. The frequency and severity of adverse events in patients receiving GLE/PIB were similar to those of patients who received placebo.
Finally, in 3 Phase 3 studies, 8 weeks of treatment with GLE/PIB induced an SVR12 in at least 93% of patients with chronic HCV genotypes 2, 4, 5 or 6 infection without cirrhosis, with a virological failure in less than 1%. The drug combination had a safety profile comparable to 12 weeks of treatment with GLE/PIB.
To increase the data on GLE/PIB for genotypes 5 and 6, an open-label, multicentre study is evaluating the safety and optimum duration of G/P in 80 patients with HCV genotypes 5 and 6 infection (NCT02966795).
Another study evaluated patients with both HCV infection and advanced chronic kidney disease who have limited treatment options. A multicentre, open-label, phase 3 trial was performed to evaluate the efficacy and safety of treatment with GLE/PIB for 12 weeks in adults who had HCV genotypes 1, 2, 3, 4, 5 or 6 infection and also had compensated liver disease (with or without cirrhosis) with severe renal impairment, dependence on dialysis or both.19 Patients had stage 4 or 5 chronic kidney disease and either had received no previous treatment for HCV infection or had received previous treatment.
The SVR rate was 98% (102/104 patients). None of the patients had virological failures during treatment, and none had a virological relapse after the end of treatment. Adverse events were reported in at least 10% of the patients including pruritus, fatigue and nausea. Serious adverse events were reported in 24% of the patients. Four patients discontinued the trial treatment prematurely because of adverse events; three of these patients had an SVR.
Finally, treatment with GLE/PIB for 12 weeks resulted in a high rate of SVR in patients with stage 4 or 5 chronic kidney diseases and HCV infection.
5 |. ELBASVIR/GRAZOPREVIR (ZEPATIER)
Elbasvir (EBV), an NS5A inhibitor and Grazoprevir (GZR), an NS3/4A protease inhibitor, have demonstrated high in vitro potency against HCV genotype 1 and 4 replicons, as well as resistance-associated substitutions (RASs) that confer resistance to first-generation protease inhibitors and RASs related to treatment failures on DCV and LDV.20
The C-Edge study evaluated the efficacy and safety profile of EBR/GZR in a Phase 3 study in treatment-naïve patients, with and without cirrhosis, with genotypes 1,4 or 6 infection.21 SVR12 was achieved in 95% of patients. High efficacy was demonstrated in genotypes 1 and 4 HCV infection. High efficacy was reported in patients with compensated cirrhosis (SVR12 = 97.1%).
The C-Surfer study evaluated EBR/GZR in HCV infected patients with creatinine clearance <30 mL/min, including patients on haemodialysis, chronic kidney disease (CKD) stage 4/5 (±haemodialysis dependence); CKD stage 4: eGFR 15–29 mL/min/1.73 m2; CKD stage 5: eGFR < 15 mL/min/1.73 m2 or on dialysis,22 targeting 20% non-haemodialysis patients. In an ITT analysis, 94% (115/122) achieved an SVR12. In the modified full analysis set (non-virological failures excluded) 99% (115/116) achieved an SVR12. Once daily GZR/EBR for 12 weeks was highly effective for the treatment of HCV genotype 1 infection in patients with CKD stage 4/5. Efficacy is consistent across different subpopulations: genotypes 1a and 1b, with diabetes, and patients on haemodialysis. Once daily GZR/EBR for 12 weeks was generally well-tolerated in this study population of patients with advanced kidney disease.
Furthermore, treatment with DAAs results in high rates of cure in people who inject drugs. A major study has demonstrated that patients with HCV infection who were receiving opiate agonist therapy and treated with EBR/GZR had high rates of SVR12, regardless of ongoing drug use23 (Figure 4). In these studies 0%-3% of patients discontinued treatment because of adverse events. Compliance was excellent, even in patients using illicit drugs while on treatment. Risk of reinfection was rare.
FIGURE 4.
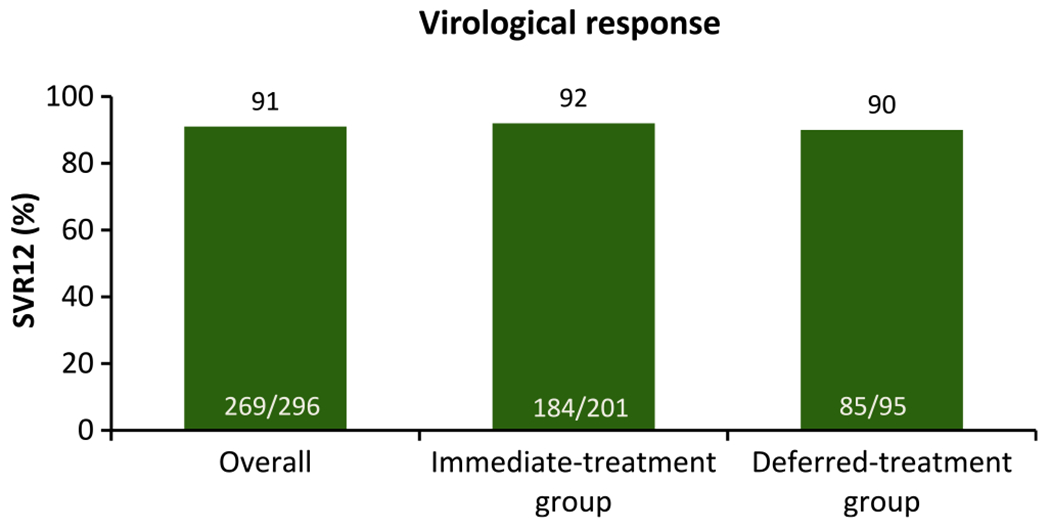
C-EDGE COSTAR: efficacy of 12 weeks GRZ/EBV in GT 1, 4 or 6 patients receiving OST.23 DAAs results in high rates of SVR in people who inject drugs; 92% (184/201) in the immediate treatment group. In this study, 0%-3% of patients discontinued treatment because of adverse events. Among 18 patients with post-treatment viral recurrence through 24-week follow-up, 6 had probable reinfection
We conducted an integrated analysis to assess the efficacy of the once-daily combination of EBR/GZR, with and without RBV in HCV genotype 4-infected patients enrolled in the Phase 2/3 clinical programme with EBR/GZR.24 Overall, the SVR12 efficacy rates among patients treated with 12 or 16 weeks of EBR/GZR ± RBV were 96.4% (107/111) in treatment-naïve patients and 88.6% (39/44) in treatment-experienced patients. The SVR12 rates were 96.0% (97/101) in treatment-naïve patients treated with 12 weeks of EBR/GZR, and 100% (8/8) in treatment-experienced patients treated with 16 weeks of EBR/GZR plus RBV.24 Efficacy was not influenced by subtype. Finally, the regimens of 12 weeks of EBR/GZR without RBV, and 16 weeks of EBR/GZR plus RBV were effective in HCV genotype 4-infected treatment-naïve and treatment-experienced patients respectively. Baseline NS5A resistance-associated substitutions did not influence the efficacy of EBR/GZR in genotype 4-infected patients.
An ongoing multisite, open-label, partially randomized trial on the efficacy and safety of EBR/GZR in French subjects with chronic HCV genotype 4 infection is evaluating 8 weeks of treatment (NCT03111108).
6 |. RESCUE THERAPY FOR PATIENTS WHO FAILED DAAS
In case of failure, compliance must first be confirmed. In case of true virological failures resistance testing might be performed. Patients who failed previous treatment and developed resistance-associated variants to DAAs should be rescued with the newly approved combinations.25,26
7 |. CONCLUSION
There has been a major revolution in the treatment of HCV infection, with availability of several oral regimens combining DAAs from different mode of actions. These treatments result in an increase in SVR which reaches almost 100% and reduces the duration of treatment to 8 or 12 weeks. These treatments have a favourable safety profile and a good tolerability.
Treatment recommendations should include a healthy lifestyle (Mediterranean diet and physical activity). Patients with cirrhosis will require surveillance (screening for HCC with ultrasound performed every 6 months). Programmes to eliminate HCV must include increased screening, referral to care, as well as improving access to treatment worldwide (Box 3). The accuracy, affordability and acceptability of testing must be improved worldwide. Point-of-care testing in countries with a high prevalence of HCV could also improve referral to care. Reducing the cost of DAAs will also be important. Real-life data on treatment efficacy, tolerability and adherence should be obtained. Finally, reducing the duration of treatment from 8-12 weeks to 3-6 weeks with potent triple or quadruple DAAs could greatly improve compliance and cost to help reach global HCV eradication.27
Box 3. How to achieve HCV elimination worldwide.
Key points.
Combining DAAs results in a high SVR (> 95%) and a favourable safety profile.
HCV elimination will require improvement in screening, prevention, referral to care and access to treatment.
DAAs results in high rates of cure and good compliance in people who inject drugs.
Treatment recommendations should include a healthy lifestyle (in case of excess weight, diet and physical activity).
Patients with cirrhosis will require surveillance (screening for HCC with ultrasound performed every 6 months).
Abbreviations:
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CHC
chronic hepatitis C
- DAAs
direct-acting antivirals
- DCV
daclatasvir
- DSV
dasabuvir
- EBV
elbasvir
- GLE
glecaprevir
- GRZ
grazoprevir
- GT
genotype
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IFN
interferon
- LDV
ledipasvir
- OMV
ombitasvir
- PEG-IFN
pegylated-interferon
- PIB
pibrentasvir
- PI
protease inhibitors
- PTV
paritaprevir
- PWID
People who inject drugs
- QD
once daily
- RAV
resistance-associated variants
- RBV
ribavirin
- RdRp
RNA-dependent RNA polymerase
- RTV
ritonavir
- SMV
simeprevir
- SOF
sofosbuvir
- STR
single tabletregimen
- SVR
sustained virological response
- VEL
velpatasvir
- VOX
voxilaprevir
Footnotes
CONFLICTS OF INTEREST
Tarik Asselah is a speaker and investigator for AbbVie, Bristol-Myers Squibb, Janssen, Gilead, Roche and Merck. Patrick Marcellin is a speaker for AbbVie, Bristol-Myers Squibb, Janssen, Gilead, Roche and Merck. Raymond Schinazi is the founder and major shareholder of Cocrystal Pharma, Inc.
REFERENCES
- 1.Asselah T, Boyer N, Saadoun D, et al. Direct-acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver Int. 2016;36(Suppl 1):47–57. [DOI] [PubMed] [Google Scholar]
- 2.Schinazi RF. Asselah T. From HCV to HBV cure. Liver Int. 2017;37(Suppl1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinot-Peignoux M, Stern C, Maylin S, et al. Twelve weeks post treatment follow-up is as relevant as 24 weeks to determine the sustained virologic response in patients with hepatitis C virus receiving pegylated interferon and ribavirin. Hepatology. 2010;51:1122–1126. [DOI] [PubMed] [Google Scholar]
- 4.Maylin S, Martinot-Peignoux M, Moucari R, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology. 2008;135:821–829. [DOI] [PubMed] [Google Scholar]
- 5.Afdhal N, Everson GT, Calleja JL, et al. Effect of viral suppression on hepaticvenous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J Viral Hepat. 2017;24:823–831. [DOI] [PubMed] [Google Scholar]
- 6.Kim RW, Mannalithara A, Lee H, Osinusi A, Schall REA, Brainard DM. Survival benefit of direct acting antiviral therapy in patients with decompensated cirrhosis. Hepatology. 2017;66:LB27. [Google Scholar]
- 7.Younossi ZM, Stepanova M, Jacobson IM, et al. Changes in patient-reported outcomes in direct acting antiviral-naive chronic hepatitis C patients with or without cirrhosis: the impact of sofosbuvir and velpatasvir with or without voxilaprevir. Aliment Pharmacol Ther. 2018;47:259–267. [DOI] [PubMed] [Google Scholar]
- 8.Asselah T Sofosbuvir for the treatment of hepatitis C virus. Expert Opin Pharmacother. 2014;15:121–130. [DOI] [PubMed] [Google Scholar]
- 9.Cheng G, Yu M, Peng B, et al. GS-5816, a second-generation HCV NS5A inhibitor with potent antiviral activity, broad genotypic coverage and a high resistance barrier. J Hepatol. 2013;58(Suppl 1):S484. [Google Scholar]
- 10.Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599–2607. [DOI] [PubMed] [Google Scholar]
- 11.Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617. [DOI] [PubMed] [Google Scholar]
- 12.Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618–2628. [DOI] [PubMed] [Google Scholar]
- 13.Asselah T, Bourgeois S, Pianko S, et al. Sofosbuvir/velpatasvir in patients with hepatitis C virus genotypes 1-6 and compensated cirrhosis or advanced fibrosis. Liver Int. 2018; in press, 10.1111/liv.13534. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson IM, Lawitz E, Gane EJ, et al. Efficacy of 8 weeks of Sofosbuvir, Velpatasvir, and Voxilaprevir in patients with chronic HCV infection: 2 Phase 3 Randomized Trials. Gastroenterology. 2017;153: 113–122. [DOI] [PubMed] [Google Scholar]
- 15.Tl Ng, Tripathi R, Reisch T, et al. In vitro antiviral activity and resistance profile of the next-generation hepatitis C virus NS3/4A protease inhibitor glecaprevir. Antimicrob Agents Chemother. 2017;62:pii: AAC.01620-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maviret Summary of Product Characteristics; Accessed August 2017.
- 17.Zeuzem S, Foster GR, Wang S, et al. 8- or 12-week Glecaprevir/Pibrentasvir in Non-cirrhotic HCV Genotype 1 or 3. NEJM. 2018; in press. [DOI] [PubMed] [Google Scholar]
- 18.Asselah T, Kowdley KV, Zadeikis N, et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with HCV genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. 2018. In press. [DOI] [PubMed] [Google Scholar]
- 19.Gane E, Lawitz E, Pugatch D, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. 2017;377:1448–1455. [DOI] [PubMed] [Google Scholar]
- 20.Asante-Appiah E, Curry S, McMonagle P, et al. Antiviral activity and resistance analysis of NS3/4A protease inhibitor grazoprevir and NS5A inhibitor Elbasvir in hepatitis C virus GT4 Replicons. Antimicrob Agents Cbemotber. 2017;61:pii: e00363–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163:1–13. [DOI] [PubMed] [Google Scholar]
- 22.Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–1545. [DOI] [PubMed] [Google Scholar]
- 23.Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165:625–634. [DOI] [PubMed] [Google Scholar]
- 24.Asselah T, Reesink H, Gerstoft J, et al. High efficacy of Elbasvir and grazoprevir with or without Ribavirin in 103 treatment-naive and experienced patients with HCV genotype 4 infection: A pooled analysis. In: 66th Annual Meeting of the American Association for the Study of Liver Diseases, November 1317, San Francisco, USA; abstract 251. [Google Scholar]
- 25.Bourlière M, Gordon SC, Flamm SL, et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376:2134–2146. [DOI] [PubMed] [Google Scholar]
- 26.Poordad F, Felizarta F, Asatryan A, et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment. Hepatology. 2017;66:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau G Shortening HCV therapy: science meets public health. Lancet Gastroenterol Hepatol. 2017;2:771–772. [DOI] [PubMed] [Google Scholar]


