Abstract
PURPOSE
Treatment of children with CNS tumors (CNSTs) demands a complex, interdisciplinary approach that is rarely available in low- and middle-income countries. We established the Cross-Border Neuro-Oncology Program (CBNP) between Rady Children’s Hospital, San Diego (RCHSD), and Hospital General, Tijuana (HGT), Mexico, to provide access to neuro-oncology care, including neurosurgic services, for children with CNSTs diagnosed at HGT. Our purpose was to assess the feasibility of the CBNP across the United States-Mexico border and improve survival for children with CNSTs at HGT by implementing the CBNP.
PATIENTS AND METHODS
We prospectively assessed clinicopathologic profiles, the extent of resection, progression-free survival, and overall survival (OS) in children with CNSTs at HGT from 2010 to 2017.
RESULTS
Sixty patients with CNSTs participated in the CBNP during the study period. The most common diagnoses were low-grade glioma (24.5%) and medulloblastoma (22.4%). Of patients who were eligible for surgery, 49 underwent resection at RCHSD and returned to HGT for collaborative management. Gross total resection was achieved in 78% of cases at RCHSD compared with 0% at HGT (P < .001) and was a predictor of 5-year OS (hazard ratio, 0.250; 95% CI, 0.067 to 0.934; P = .024). Five-year OS improved from 0% before 2010 to 52% in 2017.
CONCLUSION
The CBNP facilitated access to complex neuro-oncology care for underserved children in Mexico through binational exchanges of resources and expertise. Survival for patients in the CBNP dramatically improved. Gross total resection at RCHSD was associated with higher OS, highlighting the critical role of experienced neurosurgeons in the treatment of CNSTs. The CBNP model offers an attractive alternative for children with CNSTs in low- and middle-income countries who require complex neuro-oncology care, particularly those in close proximity to institutions in high-income countries with extensive neuro-oncology expertise.
INTRODUCTION
Pediatric CNS tumors (CNSTs) remain one of the most challenging tumors to treat in low- and middle-income countries (LMICs)1 as a result of significant deficits in infrastructure, human resources, evidence-based treatments, and interdisciplinary teams trained in neuro-oncology.2-5 CNSTs comprise 15%-20% of all childhood neoplasms, are the second most common childhood malignancy after leukemia, and the first cause of mortality in children with cancer. However, after recent improvements in surgical interventions, imaging studies, and histopathologic classification systems, 5-year progression-free survival (PFS) for children with CNSTs in high-income countries (HICs) is as high as 70%-80%.1,3-7 Unfortunately, in LMICs, where 80% of the world’s children reside, 5-year overall survival (OS) for children with CNSTs is 0%-40%.1-5,8,9 Delivery of effective therapy for children with CNSTs demands a multimodal management of surgery, chemotherapy, and/or radiotherapy depending on the diagnosis,6 and poses a particular challenge in LMICs, such as Mexico, given the need for a comprehensive, interdisciplinary approach. This includes timely access to sophisticated neurosurgical and intensive care with experienced neuro-oncologists, pediatric neurosurgeons, neuro-radiologists, radiation oncologists, neuro-pathologists, and intensivists, which is frequently absent in LMICs.10,11 To deliver effective therapy for children with CNSTs in Mexico, we established the Cross-Border Neuro-Oncology Program (CBNP) in 2010 between the Hospital General, Tijuana (HGT), and Rady Children’s Hospital, San Diego (RCHSD), located 20 miles from HGT.
CONTEXT
Key Objective
The Cross-Border Neuro-Oncology Program was established across the United States-Mexico border to facilitate access to neuro-oncology care for children with CNS tumors (CNSTs) in Tijuana, Mexico, through binational exchanges of resources and expertise.
Knowledge Generated
Survival for patients in the Cross-Border Neuro-Oncology Program dramatically improved. Gross total resection through the partnership was associated with higher overall survival.
Relevance
The cross-border model provides complex neuro-oncology care to underserved children and serves as a feasible model for other border regions. Mentored neuro-oncology care of children with CNSTs vastly improved survival at Hospital General, Tijuana. Our model offers a suitable option for children with CNSTs in low- and middle-income countries who require surgical resection, particularly those in close proximity to high-income institutions capable of offering these complex services.
Our purpose was to assess the feasibility of the CBNP across the United States-Mexico border and improve survival for children with CNSTs at HGT. We leveraged the already established twinning program between RCHSD and HGT,12,13 and we provided comprehensive neuro-oncology care, including access to high-quality neurosurgical services at RCHSD, and targeted training and infrastructure enhancement at HGT. In this manuscript, we describe the implementation of the CBNP and clinical outcomes of patients who participated in the CBNP.
PATIENTS AND METHODS
CBNP Implementation
Mexico is one of the most populous countries in the world, with high poverty rates (41.9% in 2018)14 and profound socioeconomic disparities, including a Gini index of 45.4 (7th highest in the region, 2018).15 (The Gini index is a measure of statistical dispersion intended to represent the income or wealth distribution of a nation’s residents, and is the most commonly used measurement of inequality.)
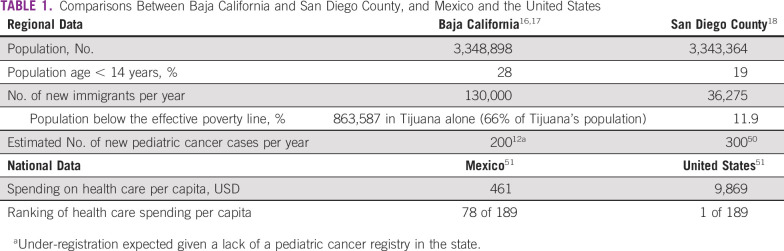
The border Mexican state of Baja California had 1 million children in 2015, 50% of whom were uninsured16,17 (Table 1). Tijuana is the 6th most populous city in Mexico16 and shares a 24-km border with San Diego (2nd largest Californian city and 8th largest in the United States).18 Sixty-eight million people cross this border annually, making it the world’s most transited border.19 There are vast cross-border health disparities14-18 (Table 1), which contribute to survival gaps in children with cancer.12,13,20
TABLE 1.
Comparisons Between Baja California and San Diego County, and Mexico and the United States
To address these disparities, we established a twinning program21 in 2008 between RCHSD, St Jude Children’s Research Hospital, and HGT to improve access to care and outcomes in children with cancer in Tijuana.12,13 Using this twinning program as a platform, we designed our CBNP model (Fig 1) by implementing key components of:
FIG 1.
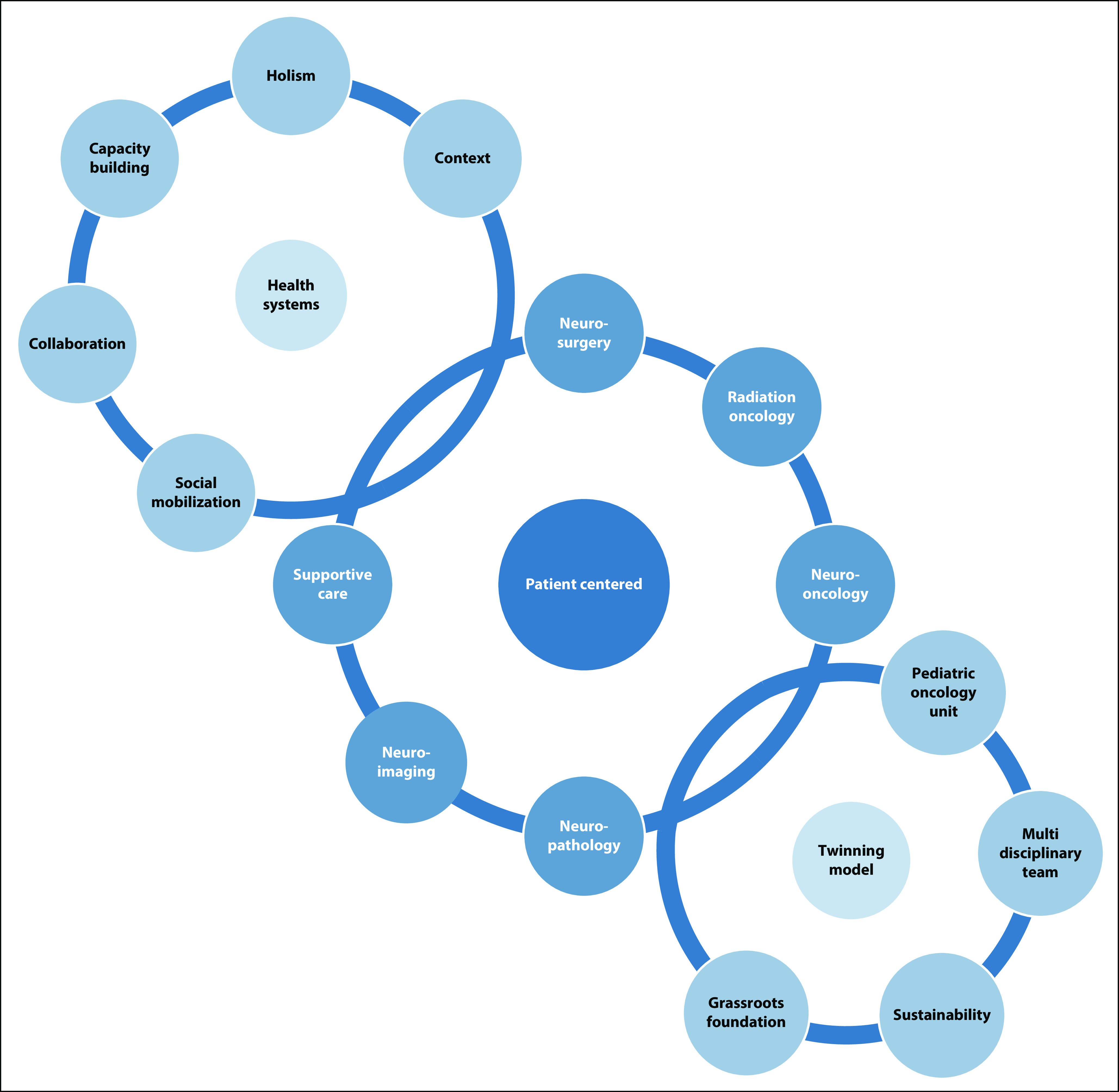
Cross-Border Neuro-Oncology Program and collaboration model between Rady Children’s Hospital, San Diego, and Hospital General, Tijuana, based on the Swanson’s health systems, twinning, and patient-centered neuro-oncology models.
Twinning in pediatric neuro-oncology, including telemedicine22-24
Patient-centered care
The global neurosurgery initiative from the 2015 Lancet Commission on Global Surgery.26-28
We adapted a needs assessment tool29 to identify key requirements for a neuro-oncology program.29 The needs assessment, completed in 2009, revealed that, although HGT had basic oncology, nursing, imaging, and radiotherapy services, it did not have neurosurgical equipment, experienced neurosurgeons, or a specialized interdisciplinary team to provide comprehensive neuro-oncology care.
Similar to barriers reported in other LMICs, we also found inadequate pediatric neuro-oncology training for health care providers, delays in diagnosis and radiotherapy, deficiencies in referral and diagnostic pathways, and limited access to accurate diagnosis and chemotherapy protocols.2,9,30,31 All patients with CNSTs (n = 16) diagnosed before CBNP implementation (2002-2009) died (data from HGT hospital-based cancer registry, unpublished).
By leveraging the unique characteristics of HGT and its close proximity to RCHSD, we developed an action plan that included (1) a patient evaluation and transfer workflow at HGT and RCHSD, and (2) targeted training and infrastructure enhancement at HGT, such that patients with CNSTs that required surgical resection could be transferred from RCHSD back to HGT for additional neuro-oncology management.
Study Population
We used the HGT registry database to prospectively identify eligible patients. Patients age 0-21 years were eligible to participate in the CBNP if they were diagnosed with a CNST amenable for neuro-surgical intervention between January 1, 2010 and December 31, 2017, and had not yet undergone surgical resection (Fig 2 and Appendix). Sixty-five patients were diagnosed with CNSTs during the study period. Five patients were excluded from the cohort for the following reasons: diagnosis of brainstem glioma after biopsy (n = 3), lost to follow up (n = 1), and diagnosis of a vascular malformation (n = 1). We defined being lost to follow up as a patient missing a scheduled appointment without medical justification during active therapy (treatment abandonment) or after finishing therapy and being noncontactable for 6 months. Our final cohort included 60 Mexican patients. The institutional review boards for the University of California San Diego/RCHSD and HGT approved this study.
FIG 2.
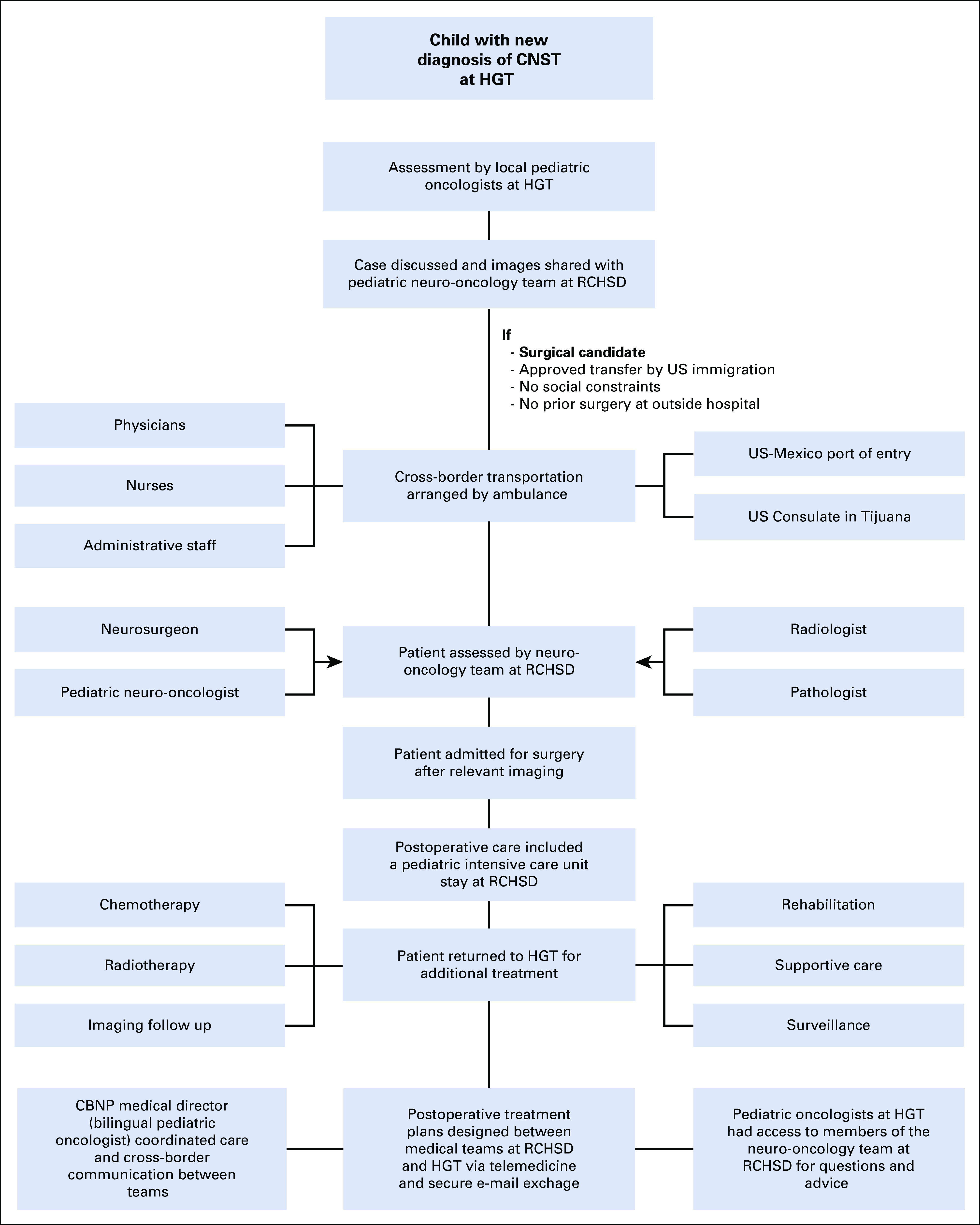
Workflow for patients participating in the Cross-Border Neuro-Oncology Program (CBNP) from diagnosis to collaborative treatment. CNST, CNS tumor; HGT, Hospital General, Tijuana; RCHSD, Rady Children’s Hospital, San Diego.
Data were collected from each patient’s medical record every 3 months. The following baseline variables were collected at presentation: age, sex, race/ethnicity, duration of symptoms, diagnosis date, tumor location, and neuro-imaging characteristics. After surgical resection, we collected the following variables: resection date, extent of surgical resection, histopathologic diagnosis per the 2007 WHO classification of brain tumors,32 and treatment received. The extent of surgical resection was determined by postoperative magnetic resonance imaging using response assessment criteria in pediatric neuro-oncology33 and was categorized into the following groups: gross total resection (GTR), near-total resection, subtotal resection, and partial resection.33
Clinical Outcomes
Clinical outcomes included death, tumor progression, and lost to follow-up. Progression was defined as radiographic (at least a 25% increase in two-dimensional measurements of the visible tumor(s) on imaging) and/or histopathologic evidence of disease recurrence, or progression after previously documented complete remission and/or stable disease. PFS was defined as the time that elapsed between treatment initiation and tumor progression. OS was calculated from the date of diagnosis to the date of last follow up, death by any cause, or lost to follow up if no vital status was known after the event. Data were analyzed in two eras—Era 1 (2010-2013) and Era 2 (2014-2017)—to compare outcomes before and after the CBNP was fully established (December 2013).
Statistical Analysis
We performed descriptive statistics, used Mann-Whitney U and Fisher exact tests to determine the statistical significance of differences in continuous and categorical variables between subgroups, and performed a Cox proportional hazards regression model for continuous predictors (age at diagnosis and duration of symptoms). Variables included death, progression, and PFS and OS at 3 and 5 years, whereas age, sex, race/ethnicity, tumor type, duration of symptoms, presence of metastasis, and extent of surgical resection were covariates.
Kaplan-Meier survival curves were generated for PFS and OS, and we calculated hazard ratios and 95% CIs. Log-rank tests were used to determine the statistical significance of differences in PFS and OS. Statistical analyses were conducted using R software (3.5.0).34 We considered a P value < .05 to indicate statistical significance.
RESULTS
Patient Evaluation and Transfer Workflow
Children with a new diagnosis of CNST at HGT were discussed and images were shared via secure Web-platform with the RCHSD neuro-oncology team. Upon arrival at RCHSD, patients were assessed by the neuro-oncology team and admitted for surgery after obtaining relevant neuro-imaging. Postoperative care included a pediatric intensive care unit stay at RCHSD. Upon stabilization, the patient returned to HGT for additional management, which included chemotherapy, supportive care, radiotherapy, rehabilitation, surveillance, and neuro-imaging follow up. After histopathology reports were completed at RCHSD, postoperative treatment plans were discussed and designed between the teams at RCHSD and HGT. Cross-border communication between teams, including review of postoperative and follow-up images, and additional treatment recommendations, was conducted via teleconference and secure e-mail exchange. Additional information is provided in Figure 2 and the Appendix.
Capacity Building: Targeted Training and Infrastructure Enhancement
To ensure high-level care before and after surgical resection, targeted neuro-oncology training (in person and virtual) was provided to the medical team at HGT, including pediatric oncologists (n = 4), pediatricians (n = 8), nurses (n = 32), and ancillary staff (n = 12). One initial challenge was the scarce number of pediatric oncologists who were trained to care for children with CNSTs. An advanced practice provider model was used as a solution for this shortage in which eight pediatricians received specialized training in neuro-oncology and pediatric intensive care. They now provide specialized care to critically ill patients in a dedicated pediatric intensive care oncology unit when patients with CNSTs are transferred back to HGT from RCHSD after their neurosurgical resection.
A structured plan to enhance infrastructure and provide access to medications, supplies, chemotherapy, radiology, and radiotherapy services was implemented, ensuring high-quality care for patients returning to HGT for postoperative care. Chemotherapy protocols were collaboratively developed by RCHSD and HGT teams on the basis of published recommendations for LMICs and were tailored to the local resources and availability of chemotherapy agents.11,23,35-37 Surgery services at RCHSD were provided as in kind. HGT leadership and the local foundation, Patronato HGT, committed to fund personnel, equipment, medications, supplies, and operational costs.
Patient Clinical Outcomes
A total of 60 Mexican patients at HGT were diagnosed with CNSTs during the study period, and 49 underwent surgery at RCHSD. Patient characteristics are listed in Table 2.
TABLE 2.
Patient Demographics Overall (N = 60)
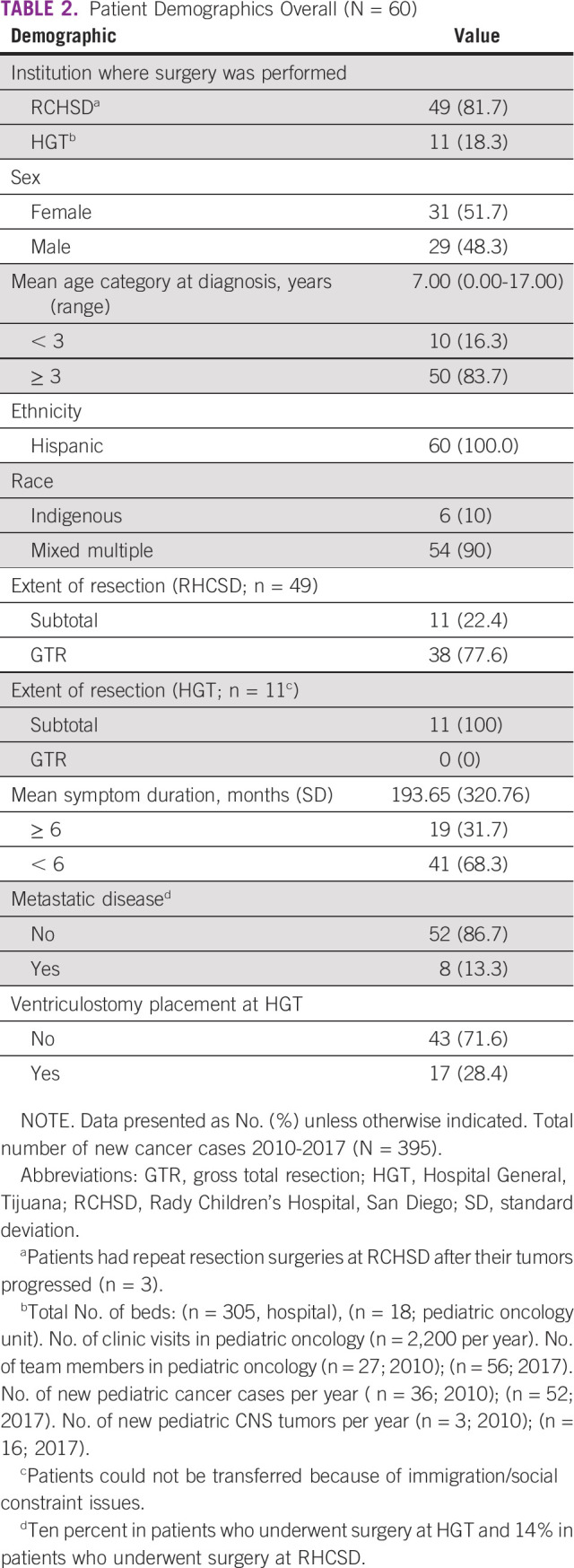
The most common diagnoses were low-grade glioma (n = 15; 24.5%), medulloblastoma (n = 13; 22.4%), ependymoma (n = 8; 13.3%), craniopharyngioma (n = 6; 10%), and other (n = 18; 28.6%). The most common clinical features at presentation were emesis (53%), headache (51%), balance disturbances (25%), visual impairment (22%), papilledema (8%), seizures (8%), and vertigo (7%). The majority of patients had symptoms for more than 6 months (n = 34; 68.3%), did not have metastasis at presentation (n = 52; 86.7%), and did not have presurgical complications, such as respiratory or metabolic aberrations (n = 51; 85.7%). Seventeen patients (28.4%) had a ventriculostomy placement at HGT before transfer to RCHSD. Of note, none of the patients’ parents requested to continue care at RCHSD after surgery, and expressed that they were very satisfied with the follow-up care at HGT when surveyed upon their return to HGT.
Extent of Resection and Survival Outcomes
The majority (n = 38; 77.6%) of patients who achieved GTR had surgery performed at RCHSD. Having surgery done at RCHSD, compared with HGT, was a significant determinant of GTR (P < .001). None of the patients who underwent surgery at HGT (n = 11) achieved GTR (Table 3).
TABLE 3.
Factors Associated With Extent of Resection
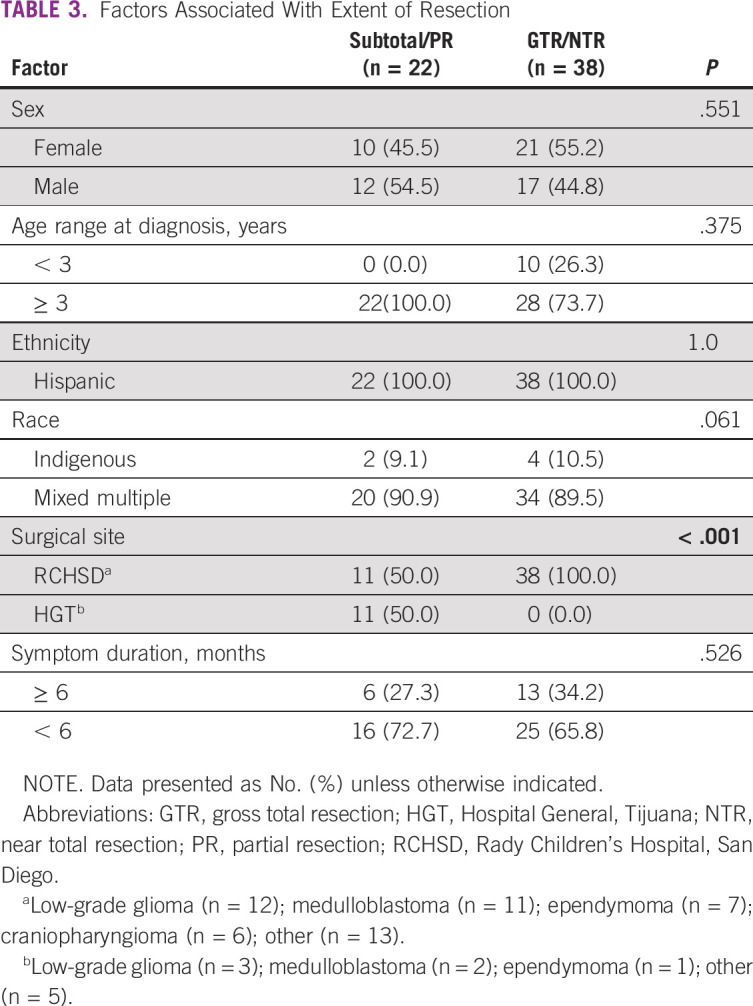
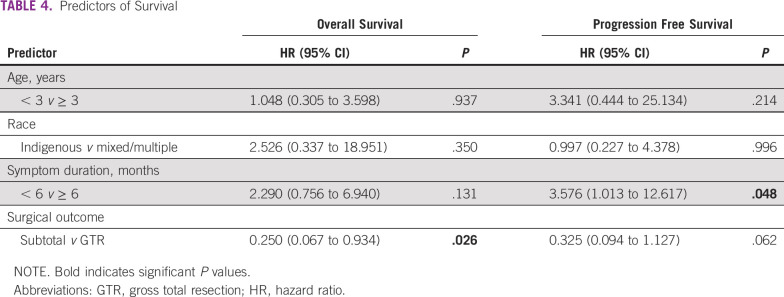
GTR was a significant predictor of OS (hazard ratio, 0.250; 95% CI, 0.067 to 0.934; P = .026), and symptom duration of ≥ 6 months at initial presentation was a significant predictor of decreased PFS (hazard ratio, 3.576; 95% CI, 1.013 to 12.617). Age and race were not predictors of PFS or OS. Five-year OS improved from 0% before 2010 to 52% in 2017 (Fig 3A). Survival by diagnosis is shown in Figure 3. There was an incremental improvement in 3-year OS, from 37% at the end of Era 1 (2010-2013) to 53% in Era 2 (2014-2017; P = .023; Fig 3B and Table 4).
FIG 3.
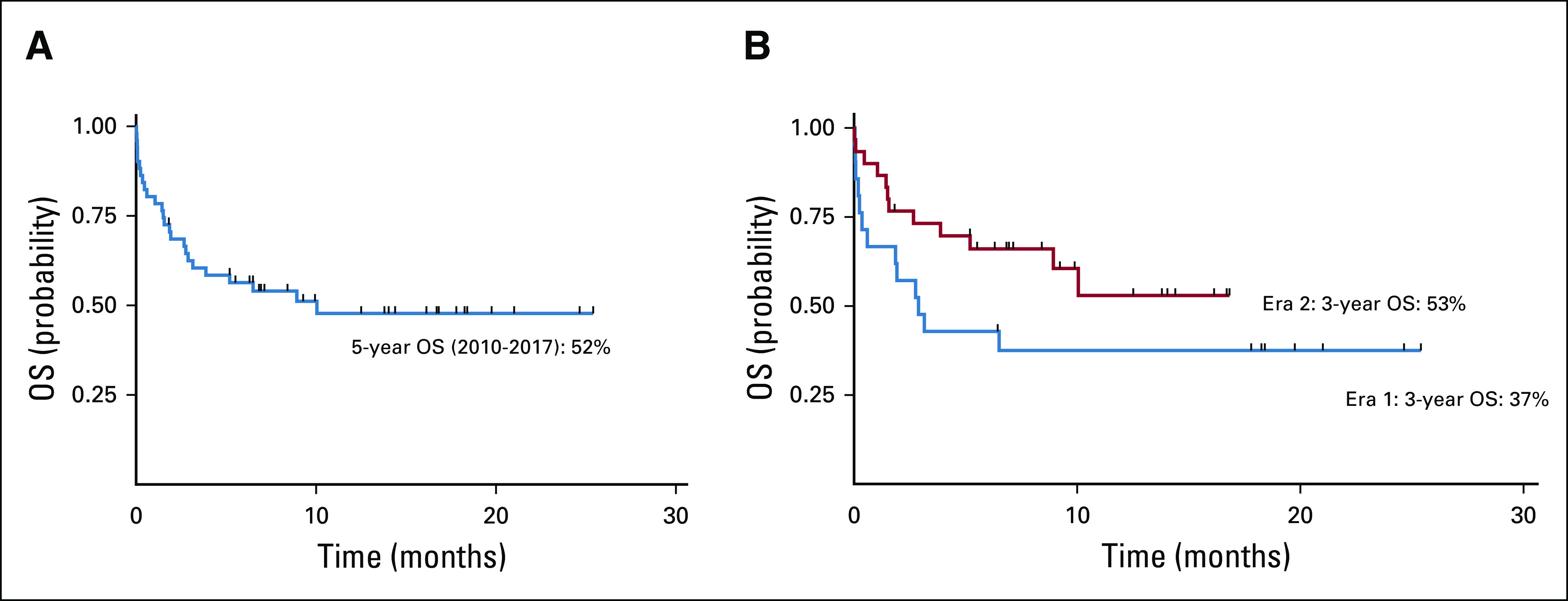
Kaplan-Meier survival curves for 5-year overall survival (OS) in the Cross-Border Neuro-Oncology Program. (A) Five-year OS (2010-2017): All CNS tumors (CNSTs), 52%; medulloblastoma, 40%; ependymoma, 44%; low-grade glioma, 91%; craniopharyngioma, 75%; other, 25%. (B) Three-year overall survival by Era (Era 1: 2010-2013; Era 2: 2014-2017). Era 1 3-year OS: 37%; Era 2 3-year OS: 53% (P = .23). Three-year OS (2010-2017): All CNSTs, 60%; medulloblastoma, 55%; ependymoma, 67%; low-grade glioma, 91%; craniopharyngioma, 75%; other, 25%.
TABLE 4.
Predictors of Survival
DISCUSSION
The CBNP led to dramatic improvements in the quality of pediatric neuro-oncology care for children with CNSTs diagnosed at HGT, including access to sophisticated neurosurgical management and increased survival.
Most global neuro-oncology efforts have focused on surgical camps to provide charity neurosurgical services.26 Our model leveraged the already-established pediatric oncology twinning program at HGT,12,13 responded to the 2015 Lancet Commission in Global Surgery,28 and focused on local health system strengthening, targeted training, capacity building, and advocacy.12,13,25 Faced with a neurosurgeon and neuro-oncologist ratio gap in Tijuana that was impossible to close expeditiously, as well as the imminent need to treat patients with CNSTs, the CBNP was a logical, temporary solution that capitalized on the close proximity between RCHSD and HGT across the United States-Mexico border.
Capacity building resulted in the implementation of disease-specific treatment guidelines and in a highly trained team able to provide high-quality intensive care expeditiously. Moreover, delays to timely neuro-imaging, diagnosis, neurosurgery, and radiotherapy have overall decreased. Communication and integration among health care teams has also improved. As reported by Qaddoumi et al,22 in addition to case discussions via online meetings, regular e-mails contributed to enhanced trust by the local team and helped reinforce the concepts discussed during telemedicine sessions.
Whereas published data on childhood cancer epidemiology in Mexico are relatively nascent, a recent report suggests that the estimated incidence of childhood cancer is 156.9 cases per 1 million per year and seems to be continuing to increase annually.38 CNSTs (9.1%) are the third most common pediatric cancer after leukemias (49.8%) and lymphomas (9.9%), and the most common cause of death in children age 5 to 14 years.38 These statistics are consistent with those reported at HGT in our study, where 12% of pediatric oncology patients between 2010-2017 were diagnosed with CNSTs. The diagnosis make-up in our cohort is consistent with the findings of other publications in HICs and LMICs.1,4,6,30
Five-year OS for all CNSTs at HGT improved from 0% (data from HGT hospital-based cancer registry, unpublished) before 2010 to 52% in 2017 after the implementation of the CBNP. There was a significant incremental improvement of 16% in 3-year OS from Era 1 (2010-2013) to Era 2 (2014-2017), highlighting the escalating positive impact of the CBNP. This increase is consistent with reports from other LMICs when long-standing twinning programs are implemented.23,24
GTR has an important role in improving the prognosis of patients with certain CNSTs, particularly medulloblastoma and low-grade glioma.7,11,37,39-42 Only children who underwent resection at RCHSD achieved GTR compared with patients who underwent surgery exclusively at HGT. This highlights the critical role of surgical expertise and adequate infrastructure and equipment to effectively cure CNSTs.2,11,26,27,43 Lower rates of GTR may reflect gaps in neurosurgery expertise and infrastructure in Tijuana and in similar settings in Mexico. Moreover, GTR was associated with better OS, which is consistent with the literature across many CNST subtypes,39-45 and supports the importance of GTR whenever possible.1,4,8,23,46,47 LMICs have not benefited from global advancements in neurosurgery, with most having minimal or no neurosurgical capacity.26 Moreover, there is a dramatic disparity in access to trained pediatric neurosurgeons in LMICs. There are an estimated 2,297 pediatric neurosurgeons in practice globally, with 85% operating in HICs, leaving only 350 pediatric neurosurgeons to care for a total population of 1.2 billion in LMICs with a ratio of one pediatric neurosurgeon per 3.6 million children.48
As reported in prior studies, in most CNSTs—other than certain medulloblastoma subgroups and certain low-grade gliomas1,6—patients with longer symptom duration at initial presentation had overall poorer outcomes. This suggests that timely diagnosis and swift cross-border transfer of pediatric patients with CNSTs to a site capable of providing vital, high-quality surgical resection afforded these patients the best chances of survival.
This collaboration was not unilateral as RCHSD benefited significantly from this partnership. Culturally appropriate strategies to ensure treatment compliance at RCHSD were adopted from HGT, specifically in Hispanic patients. In addition, pediatric oncologists at RCHSD are now more cognizant of resource conservation, as medications and personnel are often perceived as unlimited in the United States and these resources can be scarce in LMICs.
Our study must be considered in light of certain limitations. Our smaller-than-expected number of patients (based on the population size of Tijuana), related underdiagnosis of cases regionally, possible referral bias, and the evolving nature of the CBNP preclude an in-depth and consistent statistical analysis of patient outcomes over the study period. Moreover, assessing the outcome of patients who were transported to RCHSD is difficult without comparison with a control; however, denying children access to life-saving surgery for control purposes would be unethical. Instead, we may only compare outcomes with the small pool of patients who presented before the program was established. Furthermore, as a result of the lack of a well-established hospital-based registry (before the initiation of our program), specific data on imaging, diagnosis, neurosurgery, and radiotherapy delays were not systematically collected. Lastly, although the CBNP is an effective solution for a problem that was leading to the deaths of many children, we recognize that it is only a short-term step toward an established neuro-oncology infrastructure in Mexico, that it may impede the development of local neurosurgery services, and that the training of local pediatric neurosurgeons would be a long-term solution.
Many factors may hamper the structured growth of the CBNP in Baja California, including political and socioeconomic instability and a rapidly growing population. Ideally, a single regional center in an LMIC that is fully equipped and staffed could act as a referral center for pediatric neuro-oncology to improve the level of care.47,49
Future goals involve (1) growing philanthropic support for the CBNP (2) expanding access for the increasing number of patients and transitioning all facets of neuro-oncology care to HGT, (3) expanding cross-border collaboration among anesthesiologists and rehabilitation physicians, and (4) training local pathologists in neuropathology and a local neurosurgeon by instituting a neurosurgery residency program in Tijuana. Long-term sustainability requires cross-border commitment from many stakeholders and consideration of the local sociocultural perspectives. Global neuro-oncology programs should balance local challenges and opportunities and engage in capacity building through the development of training programs and formalized neuro-oncology and neurosurgery skills transfer to health care professionals in LMICs. This approach will advance their ability to effectively care for underserved children with CNSTs. Long-lasting improvements in disparate outcomes in LMICs will require cohesive health system planning with multiple stakeholders and establishing partnerships between institutions at HICs and LIMCs aimed at developing large-scale collaborative projects and research that have the potential to change national and international policy.
In conclusion, through bidirectional collaboration between cross-border communities and stakeholders, the creation of programs like the CBNP is feasible and may lead to significantly improved survival in underserved children with CNSTs. There are few twinning initiatives in pediatric neuro-oncology, and the CBNP has engendered an open and bilateral exchange of resources, expertise, and cross-border access. A health systems–strengthening approach facilitated interdisciplinary collaboration, enhanced infrastructure, and allowed for local capacity building. The CBNP offers opportunities for replication in HIC institutions in close proximity to LMICs across the United States-Mexico border and throughout the world.
ACKNOWLEDGMENT
The authors thank the entire team at Hospital General, Tijuana, especially Jose Bustamante, MD; Jose Robles, MD; Clemente Zuniga, MD; Alfredo Ornelas, MD; Alberto Reyes, MD; Angelica Martinez, MD; Adriana Loera, MD; Laura Nuno, MD; Martha Magdaleno, MD; Gabriela Tamayo, MD; Magdalena Perez, MD; Gabriela Aguiar, MD; Dara Torres, MD; Rebeca Banales, MD; Alicia Sanchez, RN; Marco Aguilera, RN; Mitzi Romano, RN; Leslie Armenta, MA; Gabriela Murillo, BA; Dulce Sanchez, BA; Raquel Ruiz, MA; Marcela Baltazar, MA; Dora Bastidas; and Martha Garcia, LSW, for their support and hard work. The authors thank Patronato HGT and Casa de la Amistad foundations, especially Alfonso Valenzuela, MD; Gloria Monforte, MD; Salma Amaya, MA; Pedro Perez, PhD; Miriam Foglio; Consuelo Barcenas; Alberto Torres, MS; Silvia Ornelas, MA; and Leonardo Arana, MS, for their continued support. The authors thank James Proudfoot, MS, at the University of California, San Diego, for his biostatistical support. At Rady Children’s Hospital, San Diego, the authors thank the neuro-oncology team (Jennifer Elster, MD; Megan Paul, MD; Katherine Pehlivan, MD; JoAnne Auger, RN; Kimberly Bower, MD; Lanipua Yeh-Nayre, NP; Nathalie Le Floch, NP; Karen Miller, RN; and Cara Lee Stephenson, NP); the pathology team (Robert Newbury, MD; Jun Mo, MD, PhD; Suzanne Tucker, MD; and Katie Shayan, MD); the international team (Joaquin Zavala and Carlos Valenzuela); the leadership team (Patrick A. Frias, MD; Nicholas Holmes, MD, MBA; and Gail Knight, MD, MMM); and pediatric oncology case managers and nurse practitioners. The authors thank the team at St Jude Children’s Research Hospital-Global Pediatric Medicine, especially Carlos Rodriguez-Galindo, MD, and Raul C. Ribeiro, MD, for sharing their knowledge. The support and advice of Margareta Norton, Chief Administrative Officer at Rady Children’s Hospital, San Diego, has been extremely valuable and is greatly appreciated.
Appendix
Patient Evaluation and Transfer Workflow
Children with a new diagnosis of CNS tumors at Hospital General, Tijuana (HGT), were initially assessed by the local pediatric oncologist. Each case was discussed and images were shared via secure Web platform with the pediatric neuro-oncology team at Rady Children’s Hospital, San Diego (RCHSD).
If the patient had neuroradiographic evidence of a tumor that was amenable to resection, after discussions between the neuro-oncology and neuro-surgery teams, and there was hospital administration approval, cross-border transportation was arranged by ambulance. Patients were considered eligible if they were age 0-21 years, were deemed appropriate for neuro-surgical resection, did not have prior surgery outside of RCHSD, were approved for transfer by US immigration, and did not have any social constraints, such as a caregiver unable to cross to San Diego because of family demands in Tijuana, a legal guardian unable to accompany patient because they did not reside in Tijuana, or a caregiver with health/mental conditions, among others.
This complex coordination involved negotiations with various stakeholders at both hospitals, including physicians, nurses, ambulance operators, administrative staff, hospital leadership, and at the United States-Mexico port of entry and US Consulate in Tijuana.
Upon arrival at RCHSD, patients were assessed by the neuro-oncology team and admitted for surgery after obtaining relevant neuro-imaging. Postoperative care included a pediatric intensive care unit stay at RCHSD. Upon stabilization, the patient returned to HGT via ambulance for additional treatment, including chemotherapy, supportive care, radiotherapy, rehabilitation, surveillance, and neuro-imaging follow up. After histopathology reports were completed at RCHSD, postoperative treatment plans were discussed and designed between the medical teams at RCHSD and HGT. All pathology services were completed at RCHSD, and medulloblastoma cases were subgrouped according to immunohistochemistry. Future planning will include using molecular studies to further enhance CNS tumor diagnostics. Pediatric oncologists at HGT had access to members of the neuro-oncology team at RCHSD for questions and advice. A bilingual (English/Spanish), bicultural (Anglo/Hispanic) pediatric oncologist (P.A.) at RCHSD was appointed the Cross-Border Neuro-Oncology Program medical director and coordinated care. Cross-border communication between teams, including review of postoperative and follow-up images and additional treatment recommendations, was provided to the HGT medical team via teleconference and secure e-mail exchange.
SUPPORT
Supported in part by National Cancer Institute Grant No. K08-CA230306 (P.A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Cancer Institute.
AUTHOR CONTRIBUTIONS
Conception and design: Paula Aristizabal, Luke P. Burns, Mario A. Ornelas, William Roberts, Michael L. Levy, John R. Crawford
Administrative support: Bianca P. Perdomo
Collection and assembly of data: Paula Aristizabal, Rebeca Rivera-Gomez, Mario A. Ornelas
Data analysis and interpretation: Paula Aristizabal, Nikhil V. Kumar, Bianca P. Perdomo, Mario A. Ornelas, David Gonda, Denise Malicki, Courtney D. Thornburg, William Roberts, Michael L. Levy, John R. Crawford
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David Gonda
Consulting or Advisory Role: Medtronic, Monteris Neuroblate, PTC Therapeutics
Courtney D. Thornburg
Honoraria: Biomarin, Genentech
Consulting or Advisory Role: Bluebird Bio, Iron Pharmaceuticals, Biomarin, Spark Therapeutics, Novo Nordisk, Genzyme
Research Funding: Genzyme (Inst), Novo Nordisk (Inst), Bayer (Inst)
Travel, Accommodations, Expenses: Genentech, Biomarin
John R. Crawford
Honoraria: Illumina
Speakers' Bureau: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gajjar A, Reaman G, Racadio J, et al., editors. Brain Tumors in Children. Memphis, TN: Springer; 2018. Global challenges in pediatric neuro-oncology pp. 403–426. (eds): , in: [Google Scholar]
- 2.Helal AE, Abouzahra H, Fayed AA, et al. Socioeconomic restraints and brain tumor surgery in low-income countries. Neurosurg Focus. 2018;45:E11. doi: 10.3171/2018.7.FOCUS18258. [DOI] [PubMed] [Google Scholar]
- 3.Ezzat S, Kamal M, El-Khateeb N, et al. Pediatric brain tumors in a low/middle income country: Does it differ from that in developed world? J Neurooncol. 2016;126:371–376. doi: 10.1007/s11060-015-1979-7. [DOI] [PubMed] [Google Scholar]
- 4.Gupta T, Achari R, Chatterjee A, et al. Comparison of epidemiology and outcomes in neuro-oncology between the East and the West: Challenges and opportunities. Clin Oncol (R Coll Radiol) 2019;31:539–548. doi: 10.1016/j.clon.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Galindo C, Friedrich P, Morrissey L, et al. Global challenges in pediatric oncology. Curr Opin Pediatr. 2013;25:3–15. doi: 10.1097/MOP.0b013e32835c1cbe. [DOI] [PubMed] [Google Scholar]
- 6.Crawford J. Childhood brain tumors. Pediatr Rev. 2013;34:63–78. doi: 10.1542/pir.34-2-63. [DOI] [PubMed] [Google Scholar]
- 7.Packer RJ, Zhou T, Holmes E, et al. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: Results of Children’s Oncology Group trial A9961. Neuro-oncol. 2013;15:97–103. doi: 10.1093/neuonc/nos267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan MH, Boop F, Qaddoumi I. Challenges and opportunities to advance pediatric neuro-oncology care in the developing world. Childs Nerv Syst. 2015;31:1227–1237. doi: 10.1007/s00381-015-2771-x. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich P, Ortiz R, Fuentes S, et al. Barriers to effective treatment of pediatric solid tumors in middle-income countries: Can we make sense of the spectrum of nonbiologic factors that influence outcomes? Cancer. 2014;120:112–125. doi: 10.1002/cncr.28339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: A call to action. Lancet. 2010;376:1186–1193. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 11.Aristizabal P, Burns L, Rivera-Gomez R, et al. Medulloblastoma with extensive nodularity: Tailored therapy in a low-resource setting. J Pediatr Hematol Oncol. 2017;39:299–301. doi: 10.1097/MPH.0000000000000798. [DOI] [PubMed] [Google Scholar]
- 12.Aristizabal P, Fuller S, Rivera R, et al. Improving pediatric cancer care disparities across the United States-Mexico border: Lessons learned from a transcultural partnership between San Diego and Tijuana. Front Public Health. 2015;3:159. doi: 10.3389/fpubh.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aristizabal P, Fuller S, Rivera-Gomez R, et al. Addressing regional disparities in pediatric oncology: Results of a collaborative initiative across the Mexican-North American border. Pediatr Blood Cancer. 2017;64:e26387. doi: 10.1002/pbc.26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Bank Mexico: Poverty headcount ratio at national poverty lines (% of population) https://data.worldbank.org/indicator/SI.POV.NAHC?locations=MX
- 15.World Bank Mexico: GINI index (World Bank estimate) https://data.worldbank.org/indicator/SI.POV.GINI?locations=MX
- 16.Instituto Nacional de Estadísticas y Geografía Baja California: Distribución de la población. https://www.inegi.org.mx/app/areasgeograficas/?ag=02
- 17.Consejo Nacional de Evaluación de la Política de Desarrollo Social Baja California: Pobreza municipal. https://www.coneval.org.mx/Medicion/Paginas/Pobreza-municipal.aspx
- 18.US Census Bureau Quick Facts: San Diego County, California. https://www.census.gov/quickfacts/fact/table/sandiegocountycalifornia,CA/PST045219
- 19.Department of Transportation Border crossing/entry data https://explore.dot.gov/views/BorderCrossingData/CrossingRank?:isGuestRedirectFromVizportal=y&:embed=y
- 20.Nuño-Vázquez L, Rivera-Gomez R, Ornelas-Sánchez M, et al. Capacity-building initiative to improve clinical outcomes in pediatric acute lymphoblastic leukemia in the US-Mexico border region. Blood Adv. 2017;1(suppl):70–73. [Google Scholar]
- 21.Veerman AJ, Sutaryo, Sumadiono Twinning: A rewarding scenario for development of oncology services in transitional countries. Pediatr Blood Cancer. 2005;45:103–106. doi: 10.1002/pbc.20390. [DOI] [PubMed] [Google Scholar]
- 22.Qaddoumi I, Bouffet E. Supplementation of a successful pediatric neuro-oncology telemedicine-based twinning program by e-mails. Telemed J E Health. 2009;15:975–982. doi: 10.1089/tmj.2009.0043. [DOI] [PubMed] [Google Scholar]
- 23.Qaddoumi I, Musharbash A, Elayyan M, et al. Closing the survival gap: Implementation of medulloblastoma protocols in a low-income country through a twinning program. Int J Cancer. 2008;122:1203–1206. doi: 10.1002/ijc.23160. [DOI] [PubMed] [Google Scholar]
- 24.Amayiri N, Swaidan M, Abuirmeileh N, et al. Video-teleconferencing in pediatric neuro-oncology: Ten years of experience. J Glob Oncol. 2018;4:1–7. doi: 10.1200/JGO.2016.008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson RC, Atun R, Best A, et al. Strengthening health systems in low-income countries by enhancing organizational capacities and improving institutions. Global Health. 2015;11:5. doi: 10.1186/s12992-015-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haglund MM, Fuller AT. Global neurosurgery: Innovators, strategies, and the way forward. J Neurosurg. 2019;131:993–999. doi: 10.3171/2019.4.JNS181747. [DOI] [PubMed] [Google Scholar]
- 27.Servadei F, Tropeano MP, Spaggiari R, et al. Footprint of reports from low- and low- to middle-income countries in the neurosurgical data: A study from 2015 to 2017. World Neurosurg. 2019;130:e822–e830. doi: 10.1016/j.wneu.2019.06.230. [DOI] [PubMed] [Google Scholar]
- 28.Meara JG, Greenberg SL, The Lancet Commission on Global Surgery Global surgery 2030: Evidence and solutions for achieving health, welfare and economic development. Surgery. 2015;157:834–835. doi: 10.1016/j.surg.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Baskin JL, Lezcano E, Kim BS, et al. Management of children with brain tumors in Paraguay. Neuro-oncol. 2013;15:235–241. doi: 10.1093/neuonc/nos291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehrvar A, Tashvighi M, Hedayati Asl AA, et al. Management and outcomes of treating pediatric medulloblastoma: An eight years’ experience in an Iranian pediatric center. Childs Nerv Syst. 2018;34:639–647. doi: 10.1007/s00381-017-3672-y. [DOI] [PubMed] [Google Scholar]
- 31.Seah T, Zhang C, Halbert J, et al. The magnitude and predictors of therapy abandonment in pediatric central nervous system tumors in low- and middle-income countries: Systematic review and meta-analysis. Pediatr Blood Cancer. 2019;66:e27692. doi: 10.1002/pbc.27692. [DOI] [PubMed] [Google Scholar]
- 32.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaspan T, Morgan PS, Warmuth-Metz M, et al. Response assessment in pediatric neuro-oncology: Implementation and expansion of the RANO criteria in a randomized phase II trial of pediatric patients with newly diagnosed high-grade gliomas. AJNR Am J Neuroradiol. 2016;37:1581–1587. doi: 10.3174/ajnr.A4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The R Foundation R version 3.5.0. https://www.r-project.org/
- 35.Hessissen L, Parkes J, Amayiri N, et al. SIOP PODC adapted treatment guidelines for low grade gliomas in low and middle income settings. Pediatr Blood Cancer. 2017;64(suppl 5):e26737. doi: 10.1002/pbc.26737. [DOI] [PubMed] [Google Scholar]
- 36.Gajjar A, Finlay JL. The management of children and adolescents with medulloblastoma in low and middle income countries. Pediatr Blood Cancer. 2015;62:549–550. doi: 10.1002/pbc.25371. [DOI] [PubMed] [Google Scholar]
- 37.Parkes J, Hendricks M, Ssenyonga P, et al. SIOP PODC adapted treatment recommendations for standard-risk medulloblastoma in low and middle income settings. Pediatr Blood Cancer. 2015;62:553–564. doi: 10.1002/pbc.25313. [DOI] [PubMed] [Google Scholar]
- 38.Rivera-Luna R, Shalkow-Klincovstein J, Velasco-Hidalgo L, et al. Descriptive epidemiology in Mexican children with cancer under an open national public health insurance program. BMC Cancer. 2014;14:790. doi: 10.1186/1471-2407-14-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson EM, Hielscher T, Bouffet E, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: A retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17:484–495. doi: 10.1016/S1470-2045(15)00581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akyurek S, Chang EL, Yu TK, et al. Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncol. 2006;80:177–183. doi: 10.1007/s11060-006-9169-2. [DOI] [PubMed] [Google Scholar]
- 41.Leeper H, Felicella MM, Walbert T. Recent advances in the classification and treatment of ependymomas. Curr Treat Options Oncol. 2017;18:55. doi: 10.1007/s11864-017-0496-7. [DOI] [PubMed] [Google Scholar]
- 42.Pica A, Miller R, Villà S, et al. The results of surgery, with or without radiotherapy, for primary spinal myxopapillary ependymoma: A retrospective study from the rare cancer network. Int J Radiat Oncol Biol Phys. 2009;74:1114–1120. doi: 10.1016/j.ijrobp.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 43.Amayiri N, Swaidan M, Yousef Y, et al. Review of management and morbidity of pediatric craniopharyngioma patients in a low-middle-income country: A 12-year experience. Childs Nerv Syst. 2017;33:941–950. doi: 10.1007/s00381-017-3411-4. [DOI] [PubMed] [Google Scholar]
- 44.Kong Z, Wang Y, Dai C, et al. Central nervous system germ cell tumors: A review of the literature. J Child Neurol. 2018;33:610–620. doi: 10.1177/0883073818772470. [DOI] [PubMed] [Google Scholar]
- 45.Delgado-López PD, Corrales-García EM. Survival in glioblastoma: A review on the impact of treatment modalities. Clin Transl Oncol. 2016;18:1062–1071. doi: 10.1007/s12094-016-1497-x. [DOI] [PubMed] [Google Scholar]
- 46.Rajagopal R, Abd-Ghafar S, Ganesan D, et al. Challenges of treating childhood medulloblastoma in a country with limited resources: 20 years of experience at a single tertiary center in Malaysia. J Glob Oncol. 2016;3:143–156. doi: 10.1200/JGO.2015.002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaghloul MS. Single pediatric neuro-oncology center may make difference in low/middle-income countries. Childs Nerv Syst. 2016;32:241–242. doi: 10.1007/s00381-015-2987-9. [DOI] [PubMed] [Google Scholar]
- 48.Dewan MC, Baticulon RE, Rattani A, et al. Pediatric neurosurgical workforce, access to care, equipment and training needs worldwide. Neurosurg Focus. 2018;45:E13. doi: 10.3171/2018.7.FOCUS18272. [DOI] [PubMed] [Google Scholar]
- 49.Yousef YA, Al-Nawaiseh I, Mehyar M, et al. How telemedicine and centralized care changed the natural history of retinoblastoma in a developing country: Analysis of 478 patients. Ophthalmology. doi: 10.1016/j.ophtha.2020.07.026. epub ahead of print on July 16, 2020. [DOI] [PubMed] [Google Scholar]
- 50.Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 51.World Bank Current health expenditure per capita (current US$) https://data.worldbank.org/indicator/SH.XPD.CHEX.PC.CD