INTRODUCTION
Brain metastases are common in patients with advanced ALK-rearranged lung cancers; ultimately CNS disease develops in 50%-60%of these patients.1,2 Second-generation tyrosine kinase inhibitors (TKIs) such as alectinib can effectively treat brain metastases,3,4 although intracranial and extracranial progression eventually occur.5 Lorlatinib is a third-generation ALK TKI that can re-establish disease control in this setting.6 Unfortunately, there are limited systemic options beyond standard chemotherapy and/or immunotherapy after disease progression while receiving treatment with lorlatinib.
Bevacizumab, a humanized, monoclonal antibody against the vascular endothelial growth factor (VEGF), can work effectively in the CNS. This is evidenced by its activity in gliomas7 and CNS radionecrosis.8 The antineoplastic effects of bevacizumab are produced through multiple mechanisms, including the inhibition of angiogenesis and regression of abnormal tumor vasculature, which may improve the delivery of cytotoxic agents to tumors and homogenize oxygen delivery.9,10 In advanced non–small-cell lung cancers (NSCLCs), adding bevacizumab to platinum-based chemotherapy improves systemic11,12 and intracranial progression-free survival (PFS).13
The precedent for combining antiangiogenic agents with TKIs in oncogene-driven lung cancers comes from data in EGFR-mutant NSCLCs. In this setting, the addition of bevacizumab or ramucirumab to erlotinib extends PFS.14-16 Similarly, data on oncogenic fusions suggest a potentially synergistic role. In mouse xenograft models with ALK-rearranged tumors, the combination of an anti-VEGFR antibody with alectinib enhanced tumor inhibition compared with alectinib alone.17 According to another report, adding bevacizumab-containing chemotherapy to alectinib in a case of ALK-rearranged NSCLC resulted in a clinical response after development of alectinib resistance.18 Interestingly, cell lines derived from the patient post-alectinib resistance retained ex vivo susceptibility to alectinib, indicating that bevacizumab may have improved drug delivery to the tumor.
We thus hypothesized that the addition of bevacizumab may re-establish disease control in patients with resistance to lorlatinib, and we treated two patients with the combination after disease progression on lorlatinib therapy. Patient consent was obtained for de-identified clinical information and images used in this case report.
CASE SERIES
Patient A is a 51-year-old woman without a smoking history who presented with a right-side lower lobe mass, multiple bilateral pulmonary nodules, and cerebellar metastasis. An ALK-rearranged adenocarcinoma was identified in a biopsy specimen of the lung mass, by pathologic examination and fluorescence in situ hybridization (FISH). The patient’s disease course over the next 2 years was characterized by intracranial disease that was relatively refractory to three ALK TKIs, radiation, and chemotherapy, with ongoing extracranial disease control. Despite stepwise treatment with crizotinib, alectinib, and brigatinib, in addition to whole-brain radiation therapy and stereotactic radiosurgery (SRS), imaging was remarkable for numerous new and growing brain metastases. Carboplatin and pemetrexed were added to brigatinib, with intracranial progression after four cycles.
Treatment with lorlatinib eventually resulted in a partial response with regression of the patient’s brain metastases, confirming ALK-dependent disease. Because of cognitive impairment, hypertriglyceridemia, and lipase elevation, however, she required a 25% dose reduction. Three months into lorlatinib therapy, worsening fecal incontinence was noted. Spinal magnetic resonance imaging (MRI) demonstrated enhancement in the conus medullaris, suggestive of new leptomeningeal disease, although the patient did not undergo a lumbar puncture. Lorlatinib dose was increased back to the full dose and spinal stereotactic body radiation therapy was administered. Although her fecal incontinence and leptomeningeal enhancement initially resolved with these measures, numerous brain metastases demonstrated slow growth 3 months later, along with substantial gait and balance issues.
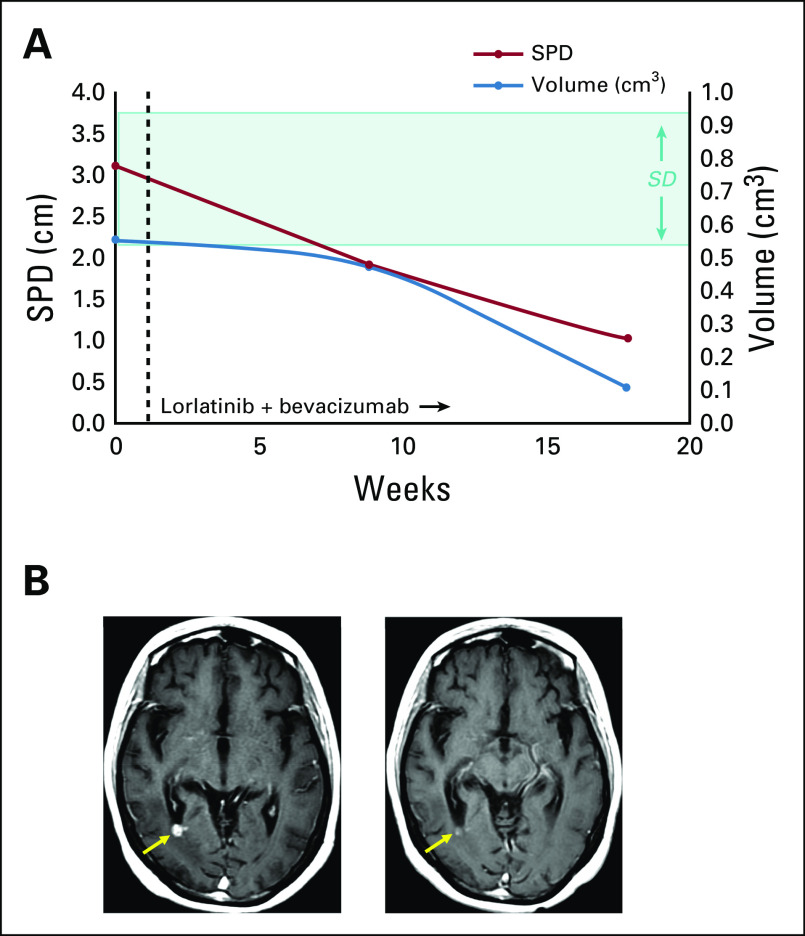
Bevacizumab 15 mg/kg every 3 weeks was then added to the lorlatinib treatment. Patient A achieved a partial response by Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria,19 with 68% shrinkage by the sum of product dimension (SPD) measurements and 82% shrinkage by volumetric analysis (Fig 1). Gait and imbalance improved and she had no clinically significant adverse effects from bevacizumab. The combination resulted in a total duration of disease control of 9.1 months. Thereafter, suspicion for progressive leptomeningeal disease prompted a switch to gemcitabine and vinorelbine.
FIG 1.
Response to lorlatinib and bevacizumab. (A) Volumetric and radiographic measurement show a decrease in intracranial disease for both metrics in patient A. A partial response by Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria (68% shrinkage by the sum of product dimension [SPD]; red line) and 82% shrinkage by volumetric analysis (blue line) were achieved. The highlighted area on the graph denotes SPD criteria for stable disease by RANO-BM criteria, which were exceeded in this case. Objective responses were assigned using SPD measurements calculated by a neuroradiologist using the sum of longest diameters of no more than five enhancing brain and/or dural metastases. Volumetric analysis was concurrently performed using commercially available software (iNtuition; TeraRecon, Foster City, CA), and results were used to generate three-dimensional depictions of intracranial disease. Volumetric analysis is not part of objective response criteria. (B) Representative brain magnetic resonance images. Axial contrast T1 weighted images of (left) baseline and (right) follow-up 26 weeks after combination treatment show nearly resolved metastasis in the right occipital lobe (arrow).
Patient B is a 56-year-old woman with a 20 pack-year former smoking history. She was treated with neoadjuvant cisplatin and pemetrexed, surgical resection, and postoperative radiation for stage IIIA disease. The surgical specimen was positive for an ALK rearrangement by FISH and ALK overexpression (D5F3 antibody immunohistochemistry). Unfortunately, 8 months later, symptomatic brain metastases developed without evidence of extracranial disease. These were treated with crizotinib, resection, and SRS. More than 2 years of disease control was achieved with crizotinib therapy, followed by nearly 2 years of treatment with alectinib before dural-based metastases developed.
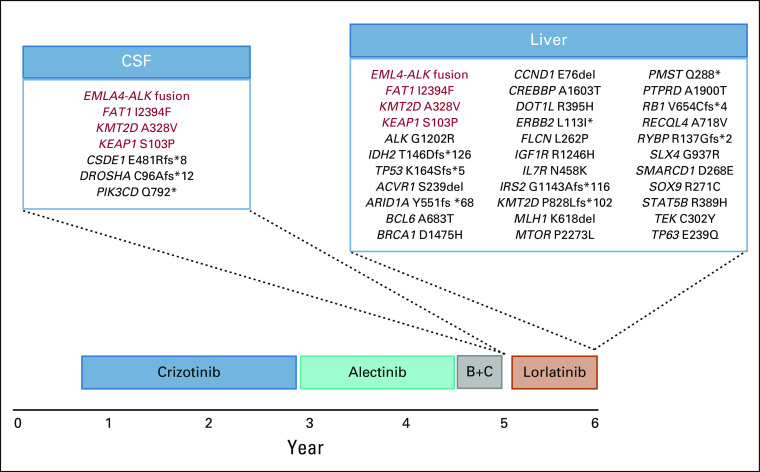
Brigatinib therapy was initiated; however, enlarging brain metastases were noted after 2 months. Carboplatin, pemetrexed, and bevacizumab were then added to the brigatinib regimen, but brigatinib and carboplatin were stopped after three cycles for intractable nausea. After two additional cycles of bevacizumab and pemetrexed without brigatinib, a brain MRI demonstrated new leptomeningeal enhancement at the left sylvian fissure in the setting of ongoing intolerable headaches. Lumbar puncture yielded negative cytology but capture-based next-generation sequencing of cell-free DNA from cerebrospinal fluid using the Memorial Sloan Kettering Integrated Molecular Profiling of Actionable Cancer Targets19 revealed EML4-ALK fusion. Six somatic mutations were also detected (Appendix Fig A1), none of which have been associated with ALK TKI resistance.20,21
Lorlatinib initiation resulted in almost immediate improvement in patient B’s headaches and nausea. A partial response by RANO-BM criteria was achieved with 32% shrinkage. Her only adverse effect from lorlatinib was mild hand swelling. After 3 months, however, asymptomatic brain metastases increased by 25%, suggesting rapid lorlatinib resistance.
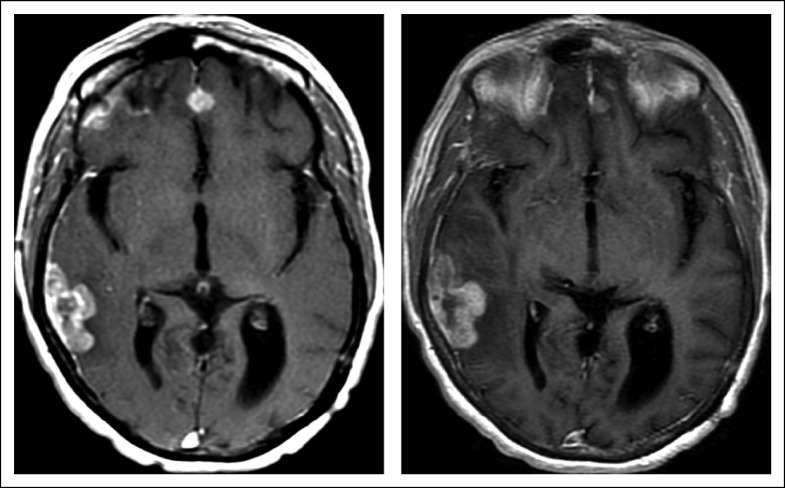
Bevacizumab 10 mg/kg every 3 weeks was then added to her lorlatinib regimen; this dose was chosen because of concurrent full-dose anticoagulation. Despite her cancer being previously refractory to bevacizumab, the combination of lorlatinib and bevacizumab re-established disease control. Her best response was stable disease, with 8% shrinkage by volumetric analysis (Fig 2). There was no recurrence of previously debilitating headaches or nausea. Disease control with the combination lasted 5.4 months, almost twice as long as she had been receiving single-agent lorlatinib treatment.
FIG 2.
Disease stabilization with lorlatinib and bevacizumab. In patient B, axial contrast T1 weighted images of (left) baseline and (right) follow-up after 17 weeks of combination treatment are shown. The combination of lorlatinib and bevacizumab resulted in stabilization of anterior parafalcine dural metastasis with only a slight increase in a right temporal occipital dural metastasis, recognizing that the patient’s intracranial disease had substantially progressed while receiving prior treatment with single-agent lorlatinib.
Intracranial (40% volumetric increase) and extracranial progression (new hepatic metastasis) was noted thereafter. She stopped treatment with bevacizumab and began gemcitabine therapy, in addition to lorlatinib.
DISCUSSION
We describe, to our knowledge, the first reports of bevacizumab added to lorlatinib in patients with ALK-rearranged lung cancer after lorlatinib progression. Both patients had symptomatic CNS disease and were heavily pretreated with and relatively refractory to all three generations of ALK TKIs, chemotherapy, and radiation, leaving few additional treatment options. The combination of bevacizumab and lorlatinib resulted in disease regression and/or control that lasted 5-9 months and notably exceeded the duration of prior single-agent lorlatinib therapy. Combination therapy was well tolerated in both cases. Because patients with ALK-rearranged lung cancers have no additional approved targeted therapies after progression on lorlatinib, exploring the activity of this combination prospectively in the setting of lorlatinib failure would be welcome.
In patients who have experienced prior disease progression with lorlatinib therapy, the combination of lorlatinib and bevacizumab may help circumvent both on-target and off-target resistance. On-target resistance takes the form of ALK kinase domain mutations.22 In particular, serial ALK TKI therapy can result in the acquisition of double or triple compound ALK mutations that may not be amenable to therapy with any currently available ALK TKI alone. Furthermore, serial ALK TKI use may result in a higher frequency of ALK-independent or fusion-extrinsic resistance. This includes EGFR/MAPK pathway activation and upregulation of epithelial-mesenchymal transition,20,23,24 rather than secondary ALK mutations that develop after treatment with first- and second-generation TKIs.
Beyond acquired resistance to lorlatinib, the combination could also be considered for patients who are lorlatinib naïve, either in the setting of prior first- and/or second-generation ALK TKI progression or in ALK TKI-naïve patients. Furthermore, lorlatinib is an active drug in TKI-naïve and TKI-pretreated patients with ROS1-rearranged lung cancers. CROWN, a first-line trial comparing crizotinib versus lorlatinib in untreated patients with ALK and ROS1 fusions is underway, with preliminary results reported in 2020 (ClinicalTrials.gov identifier: NCT03052608). Given the potential role of anti-VEGF agents in modulating tumor microenvironment, adding anti-VEGF agents to third-generation TKIs may be well suited to enhance response and extend survival. A similar strategy is already under investigation with the combination of bevacizumab and osimertinib in EGFR TKI-naïve patients with EGFR-mutant lung cancers25; the combination has been demonstrated to be safe and active.
In conclusion, the combination of lorlatinib and bevacizumab demonstrated activity and was safe in patients with heavily pretreated, ALK-rearranged lung cancers that had progressed on single-agent lorlatinib immediately before the combination. These findings add to a growing body of literature on the potential utility of antiangiogenic therapy with TKI therapy in oncogene-driven cancers.
APPENDIX
FIG A1.
Mutational analysis. Patient B underwent next-generation sequencing of circulating free DNA in cerebrospinal fluid (CSF) after progression of dural-based metastases while receiving a combination of brigatinib and chemotherapy (B+C), using Memorial Sloan Kettering Integrated Molecular Profiling of Actionable Cancer Targets. This demonstrated six somatic mutations, in addition to EML4-ALK fusion. She later underwent liver biopsy after disease progression on lorlatinib. This demonstrated a total of 32 mutations, in addition to EML4-ALK fusion, including ALK G1202R.
SUPPORT
Supported by the Craig Thompson Cancer Center grant from National Institutes of Health (P30 CA008748).
AUTHOR CONTRIBUTIONS
Conception and design: Noura J. Choudhury, Alexandra Miller, Alexander Drilon
Provision of study material or patients: Alexandra Miller, Alexander Drilon
Collection and assembly of data: Noura J. Choudhury, Robert J. Young, Matthew Sellitti, Alexandra Miller, Alexander Drilon
Data analysis and interpretation: Noura J. Choudhury, Robert J. Young, Alexandra Miller, Alexander Drilon
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Robert J. Young
Stock and Other Ownership Interests: Alexion Pharmaceuticals, Agios, Biogen, Celgene, Gilead Sciences, Karyopharm Therapeutics, Spark Therapeutics, Regeneron, Stemline Therapeutics, Vertex, Merck, Amgen
Consulting or Advisory Role: Agios, Puma Biotechnology, NordicNeuroLab, ICON Clinical Research
Research Funding: Agios (Inst)
Alexander Drilon
Honoraria: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, Peerview
Consulting or Advisory Role: Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Roche, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Verastem, Takeda/Ariad/Millenium, BerGenBio, MORE Health, Eli Lilly, Verastem, AbbVie, 14ner/Elevation Oncology, Remedica, Archer Biosciences, Monopteros
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Zhang I, Zaorsky NG, Palmer JD, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 2015;16:e510–e521. doi: 10.1016/S1470-2045(15)00013-3. [DOI] [PubMed] [Google Scholar]
- 2.Johung KL, Yeh N, Desai NB, et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol. 2016;34:123–129. doi: 10.1200/JCO.2015.62.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer TM, Shaw AT, Johnson ML, et al. Brain penetration of lorlatinib: Cumulative incidences of CNS and non-cns progression with lorlatinib in patients with previously treated ALK-positive non-small-cell lung cancer. Target Oncol. 2020;15:55–65. doi: 10.1007/s11523-020-00702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto FM, Fine HA. Bevacizumab for malignant gliomas. Arch Neurol. 2010;67:285–288. doi: 10.1001/archneurol.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [Erratum: Int J Radiat Oncol Biol Phys 84:6, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis LM. Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol. 2006;33(suppl 10):S1–S7. doi: 10.1053/j.seminoncol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 13.Tian Y, Zhai X, Tian H, et al. Bevacizumab in combination with pemetrexed and platinum significantly improved the clinical outcome of patients with advanced adenocarcinoma NSCLC and brain metastases. Cancer Manag Res. 2019;11:10083–10092. doi: 10.2147/CMAR.S222910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): An open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 16.Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe H, Ichihara E, Kayatani H, et al. Significant combination benefit of anti-VEGFR antibody and oncogene-targeted agents in EGFR or ALK mutant NSCLC cells. Cancer Res. 2019;79:2131. (suppl 13) [Google Scholar]
- 18.Nakasuka T, Ichihara E, Makimoto G, et al. Primary resistance to alectinib was lost after bevacizumab combined chemotherapy in ALK-rearranged lung adenocarcinoma. J Thorac Oncol. 2019;14:e168–e169. doi: 10.1016/j.jtho.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015;16:e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 20.Recondo G, Mezquita L, Facchinetti F, et al. Diverse resistance mechanisms to the third-generation ALK inhibitor lorlatinib in ALK-rearranged lung cancer. Clin Cancer Res. 2020;26:242–255. doi: 10.1158/1078-0432.CCR-19-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw AT, Solomon BJ, Besse B, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dagogo-Jack I, Rooney MM, Lin JJ, et al. Treatment with next-generation ALK inhibitors fuels plasma ALK mutation diversity. Clin Cancer Res. 2019;25:6662–6670. doi: 10.1158/1078-0432.CCR-19-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redaelli S, Ceccon M, Zappa M, et al. Lorlatinib Treatment elicits multiple on- and off-target mechanisms of resistance in ALK-driven cancer. Cancer Res. 2018;78:6866–6880. doi: 10.1158/0008-5472.CAN-18-1867. [DOI] [PubMed] [Google Scholar]
- 24.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu HA, Kim R, Makhnin A, et al. A phase 1/2 study of osimertinib and bevacizumab as initial treatment for patients with metastatic EGFR-mutant lung cancers. J Clin Oncol. 2019;37(15_suppl):9086. [Google Scholar]