Abstract
The present study examined long‐term efficacy outcomes in a subgroup of postmenopausal, estrogen receptor‐positive Japanese breast cancer patients from the Pre‐Operative “Arimidex” Compared with Tamoxifen trial, following pre‐operative (3 months) and post‐operative (5 years) adjuvant treatment with either anastrozole or tamoxifen. Patients with large, potentially operable, locally‐advanced breast cancer were randomized to receive anastrozole (1 mg/day) plus tamoxifen placebo or tamoxifen (20 mg/day) plus anastrozole placebo pre‐operatively. After surgery at 3 months, patients continued on the same study medication as adjuvant therapy for up to 5 years or until recurrence, intolerable toxicity or withdrawal of patient consent. Recurrence‐free survival and overall survival were measured from the date of randomization to the date of recurrence or death, whichever occurred first. Patients were monitored for adverse events throughout the study period and up to 30 days following administration of the last study medication. During post‐operative adjuvant therapy, 4/48 (8%) anastrozole and 25/49 (51%) tamoxifen patients experienced recurrence. There was a significant difference in recurrence‐free survival between the two groups (hazard ratio 0.14; 95% confidence interval 0.05–0.41; P = 0.0003). There was a significant increase in overall survival with anastrozole (0.21; 0.05–0.96; P = 0.0436) and there were 2/48 (4%) and 10/49 (20%) deaths with anastrozole and tamoxifen, respectively. Most patients responding to pre‐operative therapy remained recurrence‐free. Sequential pre‐operative/post‐operative treatment with anastrozole resulted in lower recurrence and death rates, compared with tamoxifen. (Cancer Sci 2012; 103: 491–496)
Although several pre‐operative trials comparing hormonal therapy with an aromatase inhibitor (AI) versus tamoxifen have reported the superior efficacy of AI in terms of primary tumor response, long‐term follow‐up outcomes from pre‐operative and subsequent post‐operative therapy have rarely been reported.( 1 , 2 , 3 , 4 , 5 ) For instance, in the P024 neoadjuvant endocrine therapy trial, which compared 4 months’ pre‐operative letrozole versus tamoxifen, patients were treated with post‐operative tamoxifen only, regardless of the randomized pre‐operative treatment received.( 6 ) Thus, it was not possible to evaluate the impact of a pre‐operative and subsequent post‐operative AI over tamoxifen on prognosis.
The Pre‐Operative Arimidex Compared with Tamoxifen (PROACT) trial was a randomized, double‐blind, double‐dummy, multicenter study, conducted in the USA (12 centers), in Europe and the rest of the world (44 centers) and in Japan (25 centers) that compared anastrozole versus tamoxifen as pre‐operative (12 weeks’ treatment prior to primary surgery), in terms of objective response (OR), and subsequent post‐operative (adjuvant) treatments in 451 postmenopausal women with large, operable, or potentially operable, locally‐advanced, hormone receptor‐positive (HR+) breast cancer. PROACT demonstrated that anastrozole was at least as effective as tamoxifen in the pre‐operative phase, in terms of primary tumor response rate (40% vs 35% for the anastrozole and tamoxifen treatment groups, respectively), and more patients in the anastrozole group showed an improvement between feasible surgery at the baseline and actual surgery compared with those in the tamoxifen group. The effect of ethnicity on the response to treatment was also investigated and Japanese centers were included to provide a cohort of non‐white patients. There were no specific treatment–ethnicity interactions, with respect to OR, between the Japanese patients and the rest of the population.( 7 ) In the post‐operative phase, PROACT was planned to continue for 5 years to investigate the long‐term efficacy of anastrozole and tamoxifen in terms of recurrence‐free survival (RFS: the time interval between randomization and disease recurrence or death, whichever occurred first) and overall survival (OS). However, when PROACT reached a median follow up of 3.8 years in the blinded phase, the study was unblinded and closed because the Arimidex, Tamoxifen, Alone and in Combination (ATAC) trial showed superior efficacy for anastrozole compared with tamoxifen in the post‐operative adjuvant setting.( 8 ) Furthermore, the combined analysis of data from two other prospective trials demonstrated the benefit of switching from adjuvant tamoxifen therapy to anastrozole after 2 years of treatment.( 9 )
In Japan, however, the PROACT study continued for a further 1.2 years, to meet regulatory commitments, stressing the importance of collecting data for anastrozole during post‐operative adjuvant use in Japanese women with HR+ early breast cancer. At unblinding, the Japanese PROACT patients had the option to re‐consent and receive either anastrozole or tamoxifen on an open‐label basis, thus allowing them to complete a total of 5 years’ follow‐up.
This analysis reports the long‐term outcomes for this subgroup of Japanese patients from PROACT who received anastrozole or tamoxifen as pre‐operative and subsequent post‐operative adjuvant treatment.
Patients and Methods
Study design and patients. The design of the main PROACT study (Clinicaltrials.gov identifier: NCT 00232661) has been previously described in detail.( 7 ) Briefly, PROACT was designed to compare pre‐operative therapy with anastrozole with tamoxifen in terms of OR in postmenopausal women with large, operable (T2 [≥3 cm], T3, N0–2, M0), or potentially operable, locally‐advanced (T4b, N0–2, M0), estrogen receptor‐positive (ER+) and/or progesterone‐receptor positive (PgR+) breast cancer (histologically and cytologically proven using needle‐biopsy specimens).( 10 ) Secondary objectives included the comparison of post‐operative therapy with anastrozole or tamoxifen in terms of RFS, OS, time to recurrence, and safety. Patients were randomized to receive anastrozole (1 mg/day) plus tamoxifen placebo or tamoxifen (20 mg/day) plus anastrozole placebo for 3 months before surgery (pre‐operative phase). Concomitant chemotherapy was permitted, if considered appropriate by the investigator. Concomitant ketoconazole (or related systemic compounds) and any drugs that affect sex hormone status (e.g. HRT) were not permitted from randomization through to cessation of study therapy.
Eligible patients were to receive surgery at 3 months, and then continue receiving the same study medication as adjuvant therapy for up to 5 years or until recurrence, intolerable toxicity or withdrawal of patient consent. However, due to data demonstrating the superiority of anastrozole compared with tamoxifen in the post‐operative setting,( 8 ) the main study was closed at unblinding in all countries except Japan. Japanese patients continued to be assessed according to the study protocol and in accordance with the Declaration of Helsinki and applicable regulatory requirements. The study protocol was approved by the institutional review board and written informed consent was obtained from all patients.
Assessments. In the main PROACT trial, objective response rate at 3 months (pre‐operative phase) was determined using ultrasound and caliper according to Response Evaluation Criteria in Solid Tumors (RECIST). The interaction between treatment and ethnicity (Japanese patients versus others) was also assessed for OR. Due to the continuation of PROACT in Japan, it was also possible to measure RFS and OS for this sub‐population. By definition, RFS could include any of the following: death by any cause, distant metastases, loco‐regional recurrence or disease progression (pre‐operative phase). DFS (the length of time after treatment during which no disease was found) was also measured. OS was measured from the date of randomization to the date of death. After surgery, follow‐up visits for RFS and OS occurred every 6 months for up to 5 years. Treatment compliance was determined by recording details of dispensed and unused medication for each individual patient. Patients were monitored for adverse events (AE) throughout the study period and up to 30 days following administration of the last study medication.
Statistical analysis. A logistic regression model with a treatment–ethnicity interaction term was used to calculate that 40 Japanese patients per treatment arm were required in order to detect, with 80% power, at a 5% significance level, a response rate of 15% improvement for patients receiving anastrozole versus tamoxifen in the overall study population, and a 15% decrease for patients receiving anastrozole versus tamoxifen in the Japanese patient subgroup.
In the main PROACT study, no formal statistical analysis could be performed on the data from the adjuvant period due to the early closure of the trial. However, in the Japanese substudy, RFS and OS were summarized by randomized study treatment in the intent‐to‐treat population by estimating the hazard ratio (HR) and two‐sided 95% confidence intervals (CI) for anastrozole versus tamoxifen, derived from a Cox regression model. Overall RFS and OS were summarized using Kaplan–Meier methods. For time to recurrence, any patient who had not recurred was censored at the date of their last visit. For OS, any patient who had not died was censored at the date of last contact. Safety data were analyzed on the basis of treatment first received.
Results
Patients. The first patient entered the PROACT study in October 2000. The last patient entered in 2002, finishing in December 2007. In total, 97 Japanese patients were randomized to receive neoadjuvant treatment with anastrozole (n = 48) or tamoxifen (n = 49). Patient demographics and characteristics at the baseline were well balanced in both groups, consistent with the overall population (Table 1). The disposition of patients throughout the study is summarized in Figure 1. During the pre‐operative phase, 82 (85%) patients received endocrine therapy alone and 15 (15%) patients received chemotherapy in addition to endocrine treatment.( 11 )
Table 1.
Patient baseline characteristics in the Japanese intent‐to‐treat population and concomitant therapies during the adjuvant study
| Japanese patient cohort | Overall population | |||
|---|---|---|---|---|
| Anastrozole (n = 48) | Tamoxifen (n = 49)† | Anastrozole (n = 228) | Tamoxifen (n = 223) | |
| Median age, years (range) | 61.5 (51.4–84.7) | 61.6 (51.4–81.3) | 67.3 (48.7–91.5) | 66.7 (44.1–95.9) |
| Median height, cm (range) | 153.1 (140.0–167.5) | 152.3 (138.0–163.1) | 157.2 (140.0–178.0) | 156.4 (137.0–173.0) |
| Median weight, kg (range) | 55.5 (35.0–76.0) | 55.0 (42.0–79.0) | 67.3 (35.0–144.0) | 67.3 (38.0–118.0) |
| Median body mass index, kg/m2 (range) | 23.7 (15.7–32.0) | 23.6 (18.9–35.2) | 27.3 (15.2–60.7) | 27.5 (16.3–48.6) |
| Tumor dimension by ultrasound | ||||
| Mean, cm (range) | 3.8 (1.7–9.0) | 3.8 (1.8–6.3) | 3.6 (1.1–9.5) | 3.6 (0.4–8.9) |
| <4 cm, n (%) | 29 (60.4) | 32 (65.3) | 147 (64.5) | 148 (66.4) |
| ≥4 cm, n (%) | 19 (39.6) | 17 (34.7) | 81 (35.5) | 75 (33.6) |
| ER and PgR status, n (%) | ||||
| ER+ and PgR+ | 32 (66.7) | 27 (55.1) | 159 (69.7) | 152 (68.2) |
| ER+ and PgR− | 15 (31.3) | 21 (42.9) | 56 (24.6) | 59 (26.5) |
| ER− and PgR+ | 1 (2.1) | 1 (2.0) | 3 (1.3) | 2 (0.9) |
| Feasible surgery, n (%) | ||||
| Breast‐conserving | 2 (4.2) | 2 (4.1) | 26 (11.4) | 38 (17.0) |
| Mastectomy | 46 (95.8) | 47 (95.9) | 185 (81.1) | 168 (75.3) |
| Inoperable | 0 (0) | 0 (0) | 17 (7.5) | 16 (7.2) |
| Concomitant therapy received during the adjuvant study | Anastrozole (n = 48) | Tamoxifen (n = 43)† | ||
| Any chemotherapy‡ | 10 (23.3) | 20 (46.5) | – | – |
| Radiotherapy | 18 (41.9) | 17 (39.5) | – | – |
| Any chemotherapy in combination with radiotherapy | 4 (9.3) | 8 (18.6) | – | – |
†Includes 12 patients who switched from randomized tamoxifen to receive open‐label anastrozole. ‡Some patients received more than one type of chemotherapy. ER, estrogen receptor; PgR, progesterone receptor.
Figure 1.
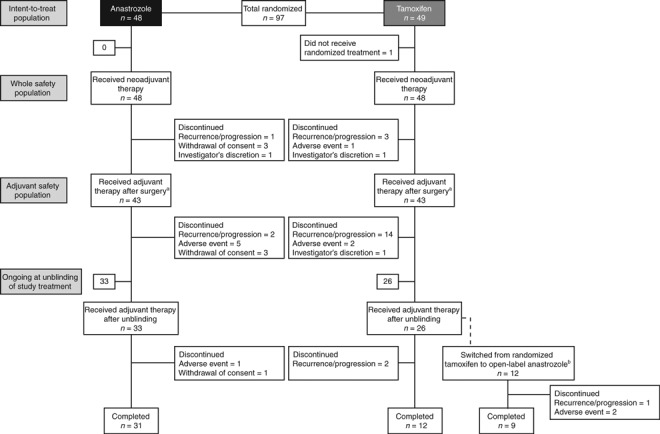
Patient disposition (completion or discontinuation). aThe date of surgery was used to define the end of the neoadjuvant period, as detailed in the statistical analysis plan. bTwelve patients who received randomized tamoxifen before unblinding opted to switch to receive open‐label anastrozole. No patients who received randomized anastrozole before unblinding opted to switch to receive open‐label tamoxifen.
Following pre‐operative therapy and surgery, 86 (89%) patients (43 in each group) entered the post‐operative phase of the study. After unblinding, 12 patients switched from tamoxifen to anastrozole and all other patients continued on their prior therapy. A total of 10 (23%) anastrozole and 20 (47%) tamoxifen patients received concomitant chemotherapy during the post‐operative phase, although a similar number of patients received radiotherapy: 18 (42%) patients in the anastrozole group and 17 (40%) patients in the tamoxifen group. Treatment compliance throughout the whole study was high in both groups (94% in the anastrozole group and 95% in the tamoxifen group).
Recurrence‐free survival. At a median follow‐up of 62 months for both groups, 29 events had occurred, with recurrence rates of 8% (4/48) in the anastrozole group and 51% (25/49) in the tamoxifen group. All four events in the anastrozole group and 20/25 (80%) events in the tamoxifen group were confirmed before unblinding. There was a significant difference in RFS between patients in the anastrozole versus tamoxifen groups (HR 0.14, 95% CI 0.05–0.41; P = 0.0003) (Fig. 2a).
Figure 2.
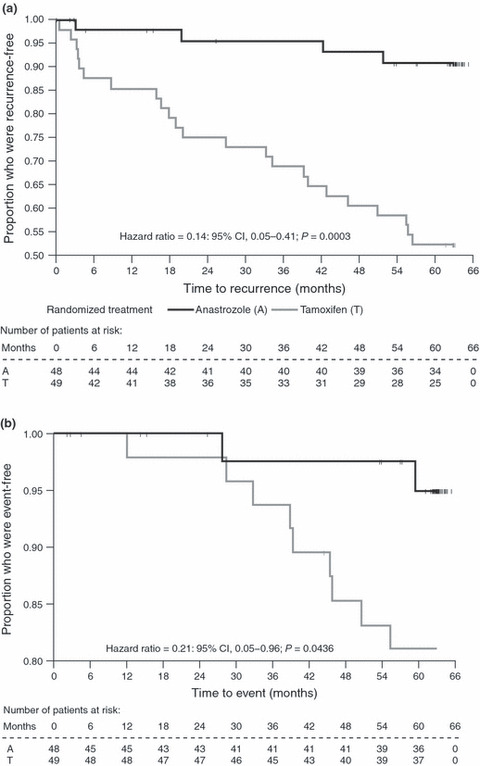
Kaplan–Meier plot for: (a) recurrence‐free survival (all randomized Japanese patients) and (b) overall survival (all randomized Japanese patients). Recurrence‐free survival is defined as time to first event, where event is either death or recurrence. Overall survival is defined as time to death. Patients who did not recur or die have been censored; tick marks indicated censored patients. CI, confidence interval.
Overall survival. At a median follow up of 63 months for both groups, there had been two (4%) deaths in the anastrozole group (one before unblinding) and ten (20%) deaths in the tamoxifen group (seven before unblinding), resulting in a significant increase in OS in the anastrozole group compared with the tamoxifen group (HR 0.21, 95% CI 0.05–0.96; P = 0.0436) (Fig. 2b).
Correlation between pre‐operative objective response and recurrence‐free survival. Of the 31 patients who responded to pre‐operative anastrozole or tamoxifen therapy, 26 (84%) remained recurrence‐free (15/17 [88%] and 11/14 [79%] in the anastrozole and tamoxifen groups, respectively). The number of patients with stable disease was the same in both groups (n = 26). All 26 patients with stable disease remained recurrence‐free in the anastrozole group, whereas 13/26 (50%) of patients had a recurrence event in the tamoxifen group (Table 2).
Table 2.
Correlation between pre‐operative objective response (Response Evaluation Criteria in Solid Tumors by ultrasound) and recurrence‐free survival
| Number of patients (%) | ||||
|---|---|---|---|---|
| Anastrozole (n = 43) Recurrence‐free survival | Tamoxifen (n = 43) Recurrence‐free survival | |||
| Yes | No | Yes | No | |
| Objective response | ||||
| Complete response | 0 (0.0) | 0 (0.0) | 1 (2.3) | 1 (2.3) |
| Partial response | 15 (34.8) | 2 (4.7) | 10 (23.3) | 2 (4.7) |
| Stable disease | 26 (60.5) | 0 (0.0) | 13 (30.2) | 13 (30.2) |
| Progressive disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (7.0) |
Responders are defined as those patients being assessed as having a complete or partial response. Non‐responders are defined as those patients being assessed as progressing or having stable disease or not evaluable.
Tolerability. As one patient (randomized to tamoxifen) did not receive treatment, 96 patients comprised the safety population. Fewer patients reported AE and serious AE (SAE) in the anastrozole group than in the tamoxifen group. Hot flashes were the most commonly reported treatment‐related AE, with a similar incidence in each group (Table 3). Of the SAE, cerebral infarction and endometrial hyperplasia were observed in two patients from the tamoxifen group, while no SAE were reported in the anastrozole group. There were no myocardial infarctions, fractures or thrombolytic events in either treatment group. AE leading to death were seen in one patient with pancreatic carcinoma and in another patient with cerebral infarction in the tamoxifen group.
Table 3.
Most commonly reported treatment‐related adverse events (AE) (≥2%),† whole safety population
| Number of patients (%)‡ | ||
|---|---|---|
| Anastrozole (n = 48) | Tamoxifen (n = 48)§ | |
| Hot flash | 8 (17) | 10 (21) |
| Aspartate aminotransferase increased | 2 (4) | 7 (15) |
| Alanine aminotransferase increased | 2 (4) | 6 (13) |
| Hepatic steatosis | 3 (6) | 4 (8) |
| Hyperhidrosis | 3 (6) | 4 (8) |
| Arthralgia | 3 (6) | 3 (6) |
| Genital discharge | 0 (0) | 5 (10) |
| Headache | 5 (10) | 0 (0) |
| Nausea | 2 (4) | 3 (6) |
| Alopecia | 0 (0) | 4 (8) |
| Back pain | 1 (2) | 3 (6) |
| Dizziness | 3 (6) | 1 (2) |
| Hypertension | 3 (6) | 1 (2) |
| Osteoporosis | 3 (6) | 1 (2) |
| Endometrial hypertrophy | 0 (0) | 3 (6) |
| Musculoskeletal stiffness | 3 (6) | 0 (0) |
†Considered by the investigator to be related to randomized drug treatment. AE categorized according to the National Cancer Institute Common Terminology Criteria Version 2.0 wherever possible. ‡Patients with multiple events in the same category are counted only once in that category. Patients with events in more than one category are counted once in each of those categories. §Includes 12 patients who switched from randomized tamoxifen to receive open‐label anastrozole.
Discussion
While the superiority of the AI (including anastrozole and letrozole) over tamoxifen is well established in the post‐operative adjuvant setting, the efficacy of the AI over tamoxifen given as pre‐operative and subsequent post‐operative treatments has yet to be reported. We believe that this is the first randomized study to make such a comparison. At 5 years’ follow‐up in Japanese patients, we found that in the anastrozole group, 92% of patients were free from recurrence and 96% of patients were still alive. In the tamoxifen group, 49% of patients were free from recurrence and 80% of patients were still alive after 5 years. Thus, patients treated with anastrozole had significantly lower risk of disease recurrence and death than those treated with tamoxifen.
Unexpectedly, the difference in RFS between anastrozole and tamoxifen was substantial, and a significant difference in OS was also observed. The reduction in RFS (HR 0.14, 95% CI 0.05–0.41; P = 0.0003) with anastrozole versus tamoxifen observed in this study was much greater than that in the ATAC trial at 5 years’ follow‐up (HR 0.87, 95% CI 0.78–0.97; P = 0.01).( 8 ) Furthermore, no significant difference in OS was reported in the ATAC trial between anastrozole and tamoxifen.( 8 ) The latest analysis of ATAC data (at a 10‐year median follow up) has confirmed a significant difference for anastrozole versus tamoxifen in RFS (HR 0.91, 95% CI 0.83–0.99; P = 0.04) in the overall population, but no significant differences in OS (HR 0.97, 95% CI 0.88–1.08; P = 0.6).( 12 )
The reason why the efficacy of anastrozole was so great in this long‐term follow up for a cohort of Japanese patients from the PROACT trial is unclear. One potential suggestion is that a number of patients were not included in the adjuvant treatment phase who did not respond to neoadjuvant treatment (5/48 patients in the anastrozole group and 6/49 patients in the tamoxifen group). It is possible that this extra level of selection or censorship might, therefore, have led to a slightly higher OS and RFS rate compared with the ATAC trial. Another possible explanation lies in the difference in the proportion of patients with CYP2D6 genotypes of decreased or no activity between Asians (approximately 30%) and white people (approximately 10%),( 13 ) because recently these genotypes have been shown to be associated with a poor response to tamoxifen,( 14 , 15 , 16 ) although such an association remains to be established.( 17 )
In this study, twice as many tamoxifen patients received concomitant chemotherapy and more tamoxifen patients were ER+/PgR− or ER−/PgR+, compared with anastrozole patients. In general, concomitant chemotherapy would be expected to improve clinical response. In the main PROACT study, in both treatment arms, OR rates were numerically higher for patients who received both endocrine and chemotherapy compared with patients who received endocrine therapy alone, indicating that concomitant chemotherapy improves response.( 7 ) Therefore, the greater use of concomitant chemotherapy in the tamoxifen arm of the Japanese cohort would be expected to improve response compared with the anastrozole arm, which, if anything, would underestimate the effect of anastrozole. These HR imbalances between the two groups could have had a bearing on the RFS and OS results. However, an adjusted Cox regression analysis was not performed due to the small patient population. Because the PROACT study design did not include a comparison of Japanese versus matched non‐Japanese data, we must emphasize that the RFS and OS benefits with anastrozole observed in this post‐hoc analysis might only apply to the subgroup of Japanese patients examined in this study.
Of the patients who responded to pre‐operative treatment with anastrozole or tamoxifen, the majority remained recurrence‐free; that is DFS appeared to be similar between the anastrozole (88%) and tamoxifen groups (79%). However, among patients who had stable disease in the pre‐operative phase, DFS in the tamoxifen group (50%) was much worse than for the anastrozole group (100%). In the results of the National Surgical Adjuvant Breast and Bowel Project protocols B‐18 and B‐27, pathological response, rather than clinical objective response to pre‐operative chemotherapy, was significantly correlated with treatment outcome in terms of RFS and OS.( 18 , 19 ) We have previously reported the results of a comparison of objective clinical responses versus histopathological tumor responses in the same cohort of Japanese patients from PROACT.( 11 ) Although the objective clinical response rate (according to RECIST assessed by ultrasound) in the pre‐operative phase was similar for anastrozole versus tamoxifen (40% and 33%, respectively; Table 2), the difference in the histopathological response rate (i.e. a degenerative change in one‐third or more of constituent carcinoma cells, according to Pathological Response Criteria for Breast Cancer as defined by the Japanese Breast Cancer Society)( 10 ) was numerically greater (35% vs 12%, respectively), although there was no pathological complete response in either treatment arm.( 11 ) This difference in histopathological response between the two groups might reflect the effectiveness of the post‐operative treatment. The superiority of the AI over tamoxifen in the pre‐operative and post‐operative settings has also been corroborated in previous studies comparing letrozole versus tamoxifen, in which letrozole has led to statistically significant improvements in overall response and breast‐conserving surgery in the pre‐operative setting( 1 , 20 ) and fewer early relapses in the post‐operative setting, although OS was not significantly different to tamoxifen.( 21 ) In this study, the safety profiles of anastrozole and tamoxifen for a Japanese patient cohort were similar and consistent with those observed in previous studies.( 5 , 8 )
In conclusion, this long‐term follow up of the PROACT study has shown that pre‐operative and subsequent post‐operative adjuvant therapy with anastrozole might lead to a lower recurrence and death rate compared to tamoxifen in a Japanese patient subgroup. Because this study started in 2000, it was not possible to further classify the participating patients into luminal type A or B categories, or to determine their human epidermal growth factor receptor 2 or Ki67 status. Nonetheless, the results from this trial suggest that sequential pre‐operative and post‐operative anastrozole treatment is a suitable treatment strategy for HR+ postmenopausal breast cancer patients, in particular those with relatively large tumors and lower‐risk oncotype profiles. Further research involving a greater number of patients is needed to confirm our present observations, particularly in relation to any confounding factors such as concomitant chemotherapy.
Disclosure Statement
Professor Noguchi has received honoraria from AstraZeneca, and is currently conducting research sponsored by AstraZeneca. Dr Fujiwara is also conducting research sponsored by AstraZeneca and Dr Iwata has received honoraria from AstraZeneca. All other authors state that they have no conflicts of interest.
Acknowledgments
This study was sponsored by AstraZeneca. We would like to acknowledge Professors Aman Buzdar and Luigi Cataliotti for their role as International Coordinating Investigators in the main PROACT trial. We are grateful to all the patients and investigators who participated in the study. We also thank Varinia Muñoz, PhD, from Complete Medical Communications, for providing editorial support funded by AstraZeneca.
References
- 1. Eiermann W, Paepke S, Appfelstaedt J et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double‐blind multicenter study. Ann Oncol 2001; 12: 1527–32. [DOI] [PubMed] [Google Scholar]
- 2. Ellis MJ, Coop A, Singh B et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB‐1‐and/or ErbB‐2‐positive, estrogen receptor‐positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol 2001; 19: 3808–16. [DOI] [PubMed] [Google Scholar]
- 3. Semiglazov V, Semiglazov VV, Ivanov VG et al. Anastrozole (A) vs tamoxifen (T) vs combine (A + T) as neoadjuvant endocrine therapy of postmenopausal breast cancer patients. Breast 2003; 12: 39–87, abs 3538. [Google Scholar]
- 4. Huober J, Krainick‐Strobel U, Kurek R, Wallwiener D. Neoadjuvant endocrine therapy in primary breast cancer. Clin Breast Cancer 2004; 5: 341–7. [DOI] [PubMed] [Google Scholar]
- 5. Dixon JM, Renshaw L, Bellamy C, Stuart M, Hoctin‐Boes G, Miller WR. The effects of neoadjuvant anastrozole (Arimidex) on tumor volume in postmenopausal women with breast cancer: a randomized, double‐blind, single‐center study. Clin Cancer Res 2000; 6: 2229–35. [PubMed] [Google Scholar]
- 6. Ellis MJ, Tao Y, Luo J et al. Outcome prediction for estrogen receptor‐positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008; 100: 1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cataliotti L, Buzdar AU, Noguchi S et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor‐positive breast cancer: the pre‐operative “Arimidex” compared to tamoxifen (PROACT) trial. Cancer 2006; 106: 2095–103. [DOI] [PubMed] [Google Scholar]
- 8. The ATAC Trialists’ Group . Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005; 365: 60–2. [DOI] [PubMed] [Google Scholar]
- 9. Jakesz R, Jonat W, Gnant M et al. Switching of postmenopausal women with endocrine‐responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 2005; 366: 455–62. [DOI] [PubMed] [Google Scholar]
- 10. Kurosumi M, Akiyama F, Iwase T, Motomura K, Okazaki M, Tsuda H. Histopathological criteria for assessment of therapeutic response in breast cancer. Breast Cancer 2001; 8: 1–2. [DOI] [PubMed] [Google Scholar]
- 11. Kurosumi M, Takatsuka Y, Watanabe T et al. Histopathological assessment of anastrozole and tamoxifen as preoperative (neoadjuvant) treatment in postmenopausal Japanese women with hormone receptor‐positive breast cancer in the PROACT trial. J Cancer Res Clin Oncol 2008; 134: 715–22. [DOI] [PubMed] [Google Scholar]
- 12. Cuzick J, Sestak I, Baum M et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early‐stage breast cancer: 10‐year analysis of the ATAC trial. Lancet Oncol 2010; 11: 1135–41. [DOI] [PubMed] [Google Scholar]
- 13. Goetz MP, Rae JM, Suman VJ et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 2005; 23: 9312–8. [DOI] [PubMed] [Google Scholar]
- 14. Lim HS, Ju Lee H, Seok Lee K, Sook Lee E, Jang IJ, Ro J. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol 2007; 25: 3837–45. [DOI] [PubMed] [Google Scholar]
- 15. Kiyotani K, Mushiroda T, Imamura CK et al. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol 2010; 28: 1287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimizu T, Ochiai H, Asell F et al. Bioinformatics research on inter‐racial difference in drug metabolism I. Analysis on frequencies of mutant alleles and poor metabolizers on CYP2D6 and CYP2C19. Drug Metab Pharmacokinet 2003; 18: 48–70. [DOI] [PubMed] [Google Scholar]
- 17. de Souza JA, Olopade OI. CYP2D6 genotyping and tamoxifen: an unfinished story in the quest for personalized medicine. Semin Oncol 2011; 38: 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fisher B, Brown A, Mamounas E et al. Effect of preoperative chemotherapy on local‐regional disease in women with operative breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B‐18. J Clin Oncol 1997; 15: 2483–93. [DOI] [PubMed] [Google Scholar]
- 19. Bear HD, Anderson S, Brown A et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B‐27. J Clin Oncol 2003; 21: 4165–74. [DOI] [PubMed] [Google Scholar]
- 20. Dellapasqua S, Colleoni M. Letrozole. Expert Opin Drug Metab Toxicol 2010; 6: 251–9. [DOI] [PubMed] [Google Scholar]
- 21. The BIG 1‐98 Collaborative Group . Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med 2009; 361: 766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]


