PURPOSE
While the immediate care and access disruptions associated with the COVID-19 pandemic have received growing attention in certain areas, the full range of gaps in cancer screenings and treatment is not yet well understood or well documented throughout the country comprehensively.
METHODS
This study used a large medical claims clearinghouse database representing 5%-7% of the Medicare fee-for-service population to characterize changes in the utilization of cancer care services and gain insight into the impact of COVID-19 on the US cancer population, including identification of new patients, gaps in access to care, and disruption of treatment journeys.
RESULTS
In March-July 2020, in comparison with the baseline period of March-July 2019, there is a substantial decrease in cancer screenings, visits, therapy, and surgeries, with variation by cancer type and site of service. At the peak of the pandemic in April, screenings for breast, colon, prostate, and lung cancers were lower by 85%, 75%, 74%, and 56%, respectively. Significant utilization reductions were observed in April for hospital outpatient evaluation and management (E&M) visits (−74%), new patient E&M visits (−70%), and established patient E&M visits (−60%). A decrease in billing frequency was observed for the top physician-administered oncology products, dropping in both April (−26%) and July (−31%). Mastectomies were reduced consistently in April through July, with colectomies similarly reduced in April and May and prostatectomies dipping in April and July.
CONCLUSION
The current impact of the COVID-19 pandemic on cancer care in the United States has resulted in decreases and delays in identifying new cancers and delivery of treatment. These problems, if unmitigated, will increase cancer morbidity and mortality for years to come.
INTRODUCTION
In late January 2020, the first cases of COVID-19 were diagnosed by the medical community in the United States, with the 7-day moving average number of new daily cases in the United States peaking in mid-April.1 In response to the increase in COVID-19 prevalence in the United States, the Centers for Disease Control and Prevention (CDC) implemented guidelines to diminish exposure, several states issued stay-at-home orders to reduce transmission risk, and across the country, individuals were encouraged to shelter in place, particularly those considered high risk for COVID-19, such as the elderly and immunocompromised.2 Many healthcare providers accommodated short-term adjustments to cancer care delivery, such as temporarily discontinuing nonemergent cancer screenings, shifting the delivery of care to telehealth, and delaying surgeries and other in-office cancer services to reduce transmission risk, build hospital capacity in anticipation of increasing rates of coronavirus infections, and comply with state regulations and guidelines from the CDC, Centers for Medicare and Medicaid Services (CMS), and professional societies.3-7 Professional societies have released guidance to amend care guidelines to compensate for these changes, such as beginning systemic therapy first to permit surgery to be delayed.8
CONTEXT
Key Objective
The COVID-19 pandemic is causing catastrophic changes to cancer care. This study including more than 6 million Medicare beneficiaries used a large claims database representing 5%-7% of the Medicare fee-for-service population to characterize changes in cancer care and gain insight into the impact of COVID-19 on the US cancer population.
Knowledge Generated
In March-July 2020 in comparison with 2019, there was a substantial decrease in cancer screenings, biopsies, surgeries, office visits, and therapy with variation by cancer type and site of service. At the peak of the pandemic in April, screenings for breast, colon, prostate, and lung cancers were lower by 85%, 75%, 74%, and 56%, respectively.
Relevance
Significant utilization reductions were observed in April for hospital outpatient evaluation and management (E&M) visits (−74%), new patient E&M visits (−70%), and established patient E&M visits (−60%). A decrease in the utilization of top physician-administered oncology products was observed, dropping in both April (−26%) and July (−31%).
By the end of September 2020, more than 7 million people in the United States had been infected with COVID-19.9 Given the focus on preserving health system capacity and also protecting high-risk patients from exposure to the virus, oncology patients have faced increased challenges in accessing care. The pandemic has resulted in substantial decreases in cancer screening, cancer management visits, and cancer surgeries, although variably by disease type across the country.10-14 An April analysis of 20 healthcare institutions part of the COVID and Cancer Research Network reported decreases in cancer encounters by 40%-50% for lung, colorectal, hematologic, breast, and prostate cancers as well as melanoma.15 Patients with cancer have reported delays in receiving cancer care, including follow-up clinic appointments and cancer therapies, such as radiation, infusion therapies, and surgical tumor removal.16,17
Although the stay-at-home orders were lifted across the country in May and June, the utilization of certain oncology services continues to lag behind 2019 levels, and the lasting impact on disease progression, cancer morbidity, and mortality remains unclear. The United Kingdom recently projected that delays in diagnosis and treatment may increase mortality from breast, colorectal, and lung cancers by as much as 9.6%, 16.6%, and 5.3%, respectively, after 5 years.18
How pervasive these changes are throughout the country is underappreciated. Our analysis sought to describe these trends of decreased utilization of cancer related services and explore their variability from month to month during the pandemic relative to utilization trends observed in 2019. In particular, by characterizing trends in cancer screenings and treatments, we hope to gain insight into potential solutions to better support seniors with cancer in the United States during the COVID-19 pandemic.
METHODS
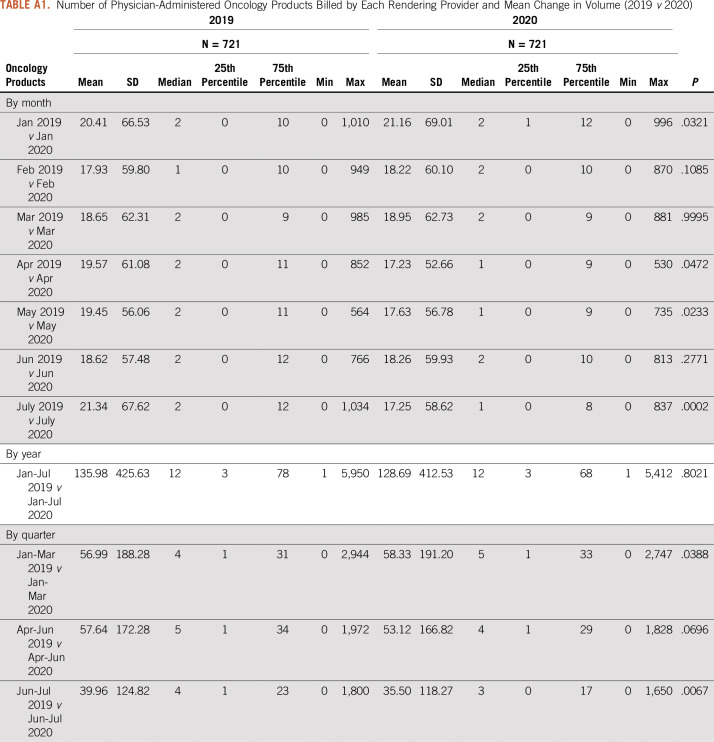
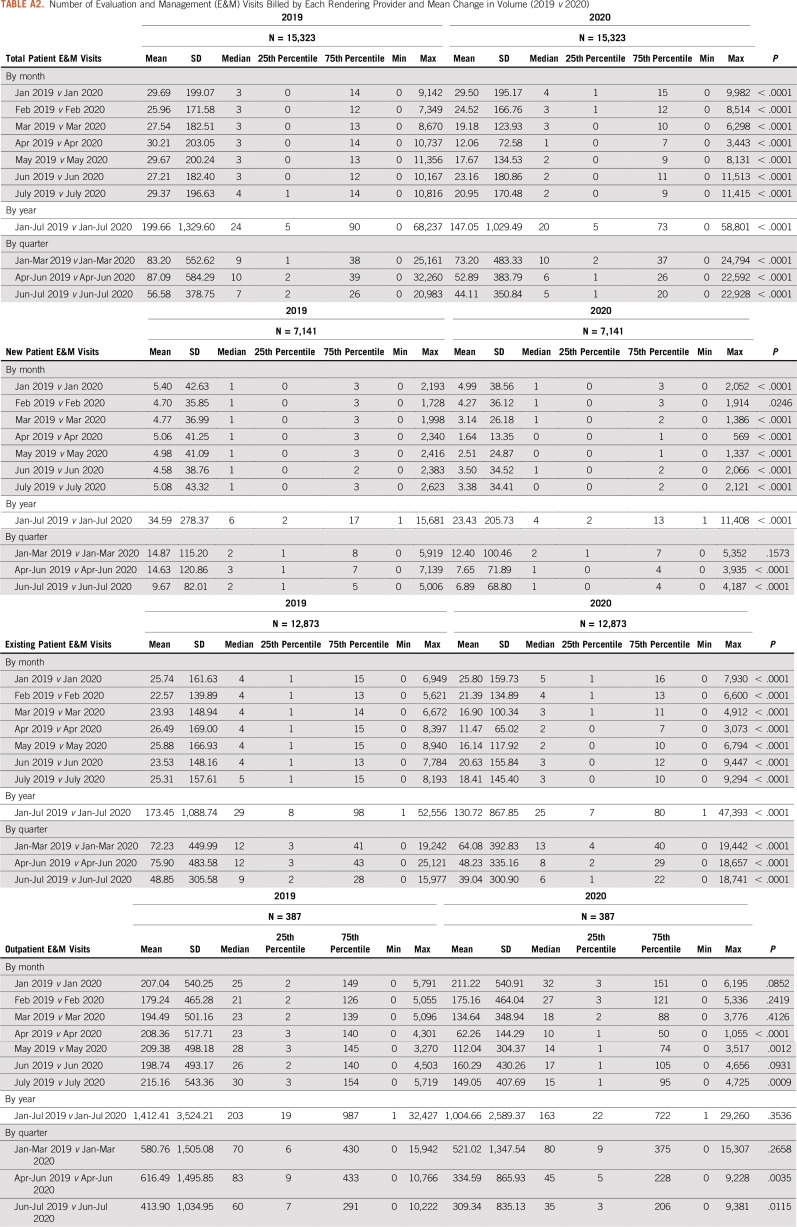
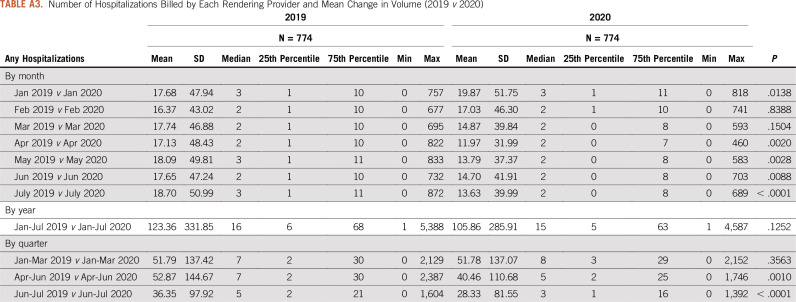
This retrospective analysis assessed whether variation in health service utilization was significantly impacted by the COVID-19 stay-at-home orders during the first half of 2020. To accomplish this task, data were sourced from a proprietary provider clearinghouse registry comprising approximately 5%-7% of all Medicare fee-for-service (FFS) claims that were submitted for adjudication between January 1, 2019, and July 31, 2020, inclusive. Included in the database are CMS-1450 claims from institutional providers, such as hospital-based cancer centers and hospital outpatient departments, and CMS-1500 claims from noninstitutional or professional providers, such as independent physician offices. Because of the additional regulatory and billing flexibilities finalized by the CMS during the public health emergency, setting of care was determined using the claim type rather than the site of service modifier, particularly for services delivered via telehealth. The full data set was then limited to claims that were (1) incurred during the first 7 months (January-July) of each year and (2) specific to targeted cancer-related services of interest, including diagnostic screening (eg, breast and colon), physician office visits, hospitalizations, surgeries, and infusion therapies administered in an outpatient setting. For each procedure or service category, the number of procedures billed by each rendering provider was tracked and trended, and the mean change in volume (2019 v 2020) was estimated and compared, statistically, using a Wilcoxon rank-sum test (Appendix Tables A1-A5). All data manipulation and statistical analyses were carried out using SAS 9.4 (SAS Institute, Cary, NC), assuming a P value (α) of .05.
RESULTS
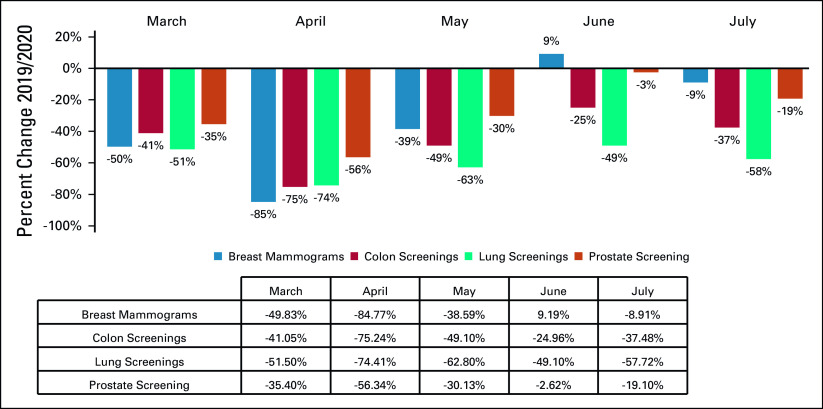
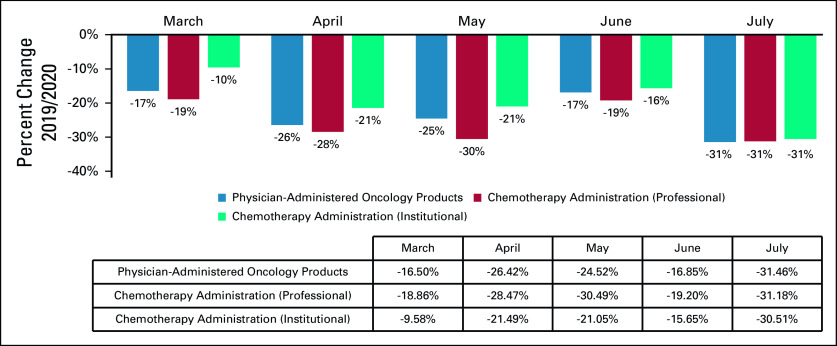
A total of 6,227,474 Medicare FFS claims were captured by the analysis. Significant decreases in screening for breast, colon, prostate, and lung cancers were observed in 2020 compared with 2019, with the most significant reduction occurring in April for mammograms (−85%) and lung (−75%), colon (−74%), and prostate (−56%) screenings (Table and Fig 1). A decrease in billing frequency was observed for the top physician-administered oncology products, dropping in both April (−26%) and July (−31%) (Table and Fig 2). Similarly, billing for chemotherapy administration services in both the professional and institutional settings dropped in April and May as well as in July (−28% and −21% in April, −30% and −21% in May, −31% and −31% in July, respectively) (Table and Fig 2). Reductions in cancer biopsies were also observed in both April and July for breast (−71% and −31%), colon (−79% and −33%), and lung (−58% and −47%) biopsies (Table and Fig 3). Mastectomies were reduced consistently in April through July, with colectomies similarly reduced in April and May and prostatectomies dipping in April and July (Table and Fig 3).
FIG 1.
Relative change in billing frequencies for select cancer screening procedures (March-July 2019/2020). Billing frequencies were determined by the following procedure codes: breast mammograms (77061, 77062, 77063, 77065, 77066, 77067); colon screening (45330, 81528, 82270, 82272, 82274, G0104, G0105, G0121, G0328); lung screening (31624, G0296, G0297); prostate screening (G0103).
FIG 2.
Relative change in billing frequencies for select physician-administered oncology products (at any site of services) and relative change in chemotherapy administration (by the site of service) (March-July 2019/2020). Billing frequency of the top 23 physician-administered oncology products (and respective biosimilars) as determined by 2018 Medicare Part B spend: Prolia/Xgeva, Neupogen (Zarxio, Nivestym), Somatuline Depot, Aloxi, Neulasta (Fulphila, Udenyca, Ziextenzo), Tecentriq, Bendeka, Avastin (Mvasi, Zirabev), Velcade, Adcetris, Kyprolis, Erbitux, Cyclophosphamide, Darzalex, Yervoy, Abraxane, Keytruda, Opdivo, Alimta, Perjeta, Rituxan (Truxima, Ruxience), Kadcyla, Herceptin (Ontruzant, Hersuma, Ogivri, Trazimera, Kanjinti).
FIG 3.
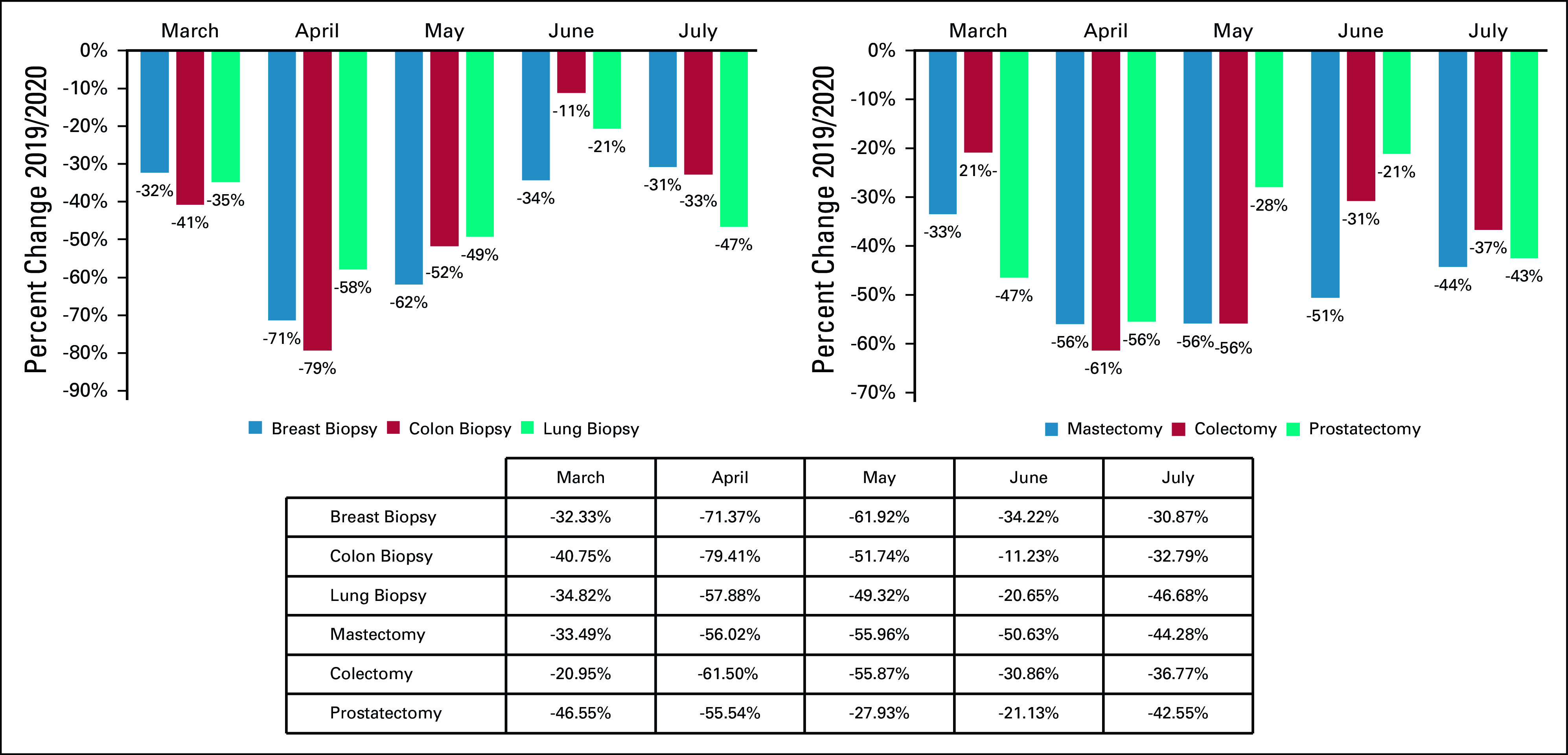
Relative change in billing frequencies for cancer-related biopsies and surgeries (March-July 2019/2020). Billing frequencies were classified by type of claim and identified by the following procedure codes: chemotherapy injection (96401, 96402, 96405, 96409); chemotherapy infusion (96413, 96415-96417); and chemotherapy administration (96420, 96425).
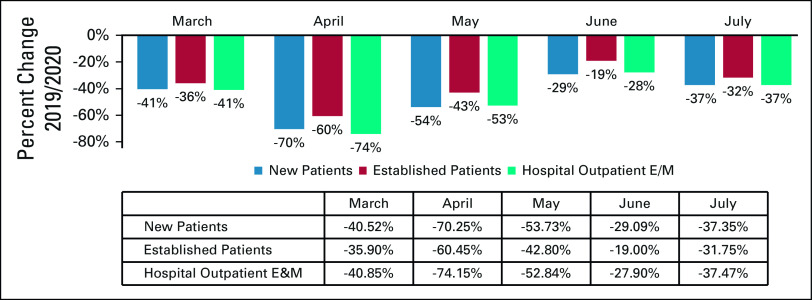
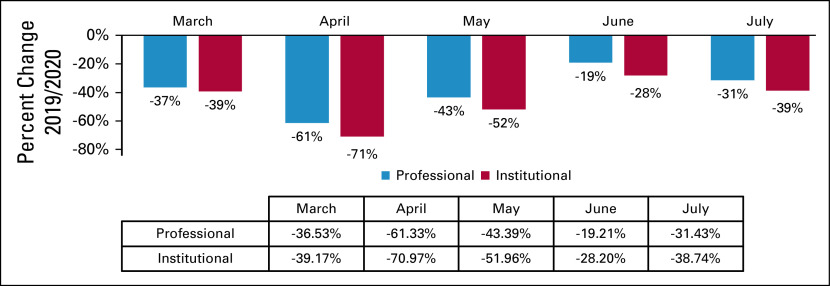
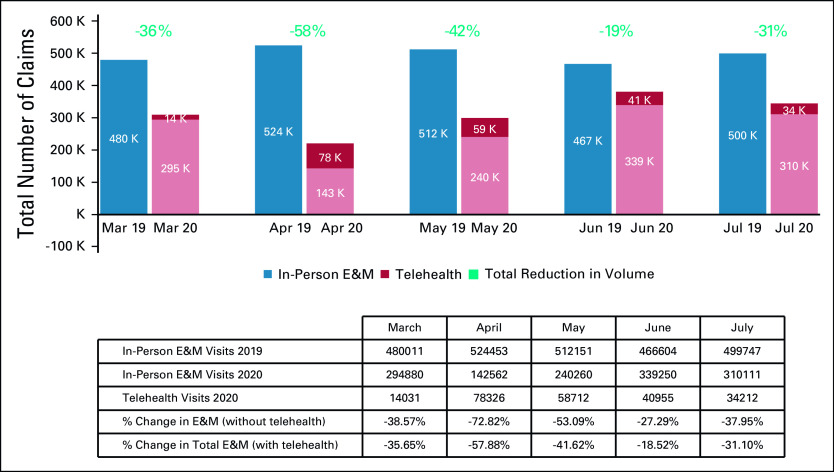
Significant utilization reductions were observed in patient evaluation and management (E&M) visits, with the greatest reduction in April hospital outpatient E&M visits (−74%) (Table and Fig 4). Drops in utilization were higher for new patient E&M visits (−70% in April) than established patient E&M visits (−60% in April) (Table and Fig 4). Cancer-related hospitalizations also declined in March (−30%), April (−41%), May (−36%), June (−31%), and July (−38%). Institutional providers experienced greater reductions in delivery of cancer care, with the greatest reduction in E&M visits in April among institutional providers and professional providers (−71% v −61%, respectively) (Table and Fig 5). Moreover, even with the expanded use of telemedicine, the delivery of E&M services via telehealth was only able to mitigate the drop in E&M utilization in April from −73% to −58% (Table and Fig 6). Utilization of telehealth has been almost entirely driven by professional providers, who delivered approximately 95% of telehealth E&M services in April through July.
FIG 4.
Relative change in billing frequencies for cancer-related evaluation and management (E&M) services (March-July 2019/2020). Billing frequencies were determined using a subset of highest-volume procedure codes for each of the following services: breast biopsy (19081-19085); colon biopsy (44389, 45380); lung biopsy (32405, 39402).
FIG 5.
Relative change in billing frequencies for cancer-related evaluation and management (E&M) services by setting of care (March-July 2019/2020). Billing frequencies were determined using a subset of highest-volume procedure codes for each of the following services: mastectomy (19301-19303, 19307); colectomy (44139, 44140, 44143, 44160, 44204, 44205, 44207, 44213); prostatectomy (52601, 52648, 55821, 55840, 55866).
FIG 6.
Total number of claims for cancer-related in-office evaluation and management (E&M) v telehealth E&M services and relative change in billing frequencies (March-July 2019/2020). Billing frequencies were determined by the following procedure codes: new patient E&M (99201-99205); established patient E&M (99211-99215); hospital outpatient (G0463).
DISCUSSION
The COVID-19 pandemic and associated stay-at-home orders established across the US limited patient access to in-person care and, in turn, impacted patients' ability to receive cancer care. Reduced access caused significant short-term disruptions in care delivery and, as an unintended consequence, may have long-term morbidity and survival implications for patients who missed cancer screenings and surgeries during this period. Delays in diagnosis can allow cancer to grow and progress to a more advanced stage, resulting in higher mortality rates.
Institutional providers have had greater reductions in delivery of cancer care, likely because of more limited supply of hospital resources caused by an influx of acutely ill patients with COVID-19 and patient reluctance to visit hospital outpatient clinics amid concerns regarding the transmission of COVID-19. Similarly, physician offices and other noninstitutional provider settings have struggled to adapt to state-specific reopening requirements and overcome patient fears about COVID-19 exposure. Overall, institutional settings of care experienced greater decreases in care delivery and were unable to adopt telehealth services to the same extent as providers in professional settings of care. This difference could be due to, among other factors, the overwhelming strain of COVID-19 on limited hospital resources and the ability of smaller community oncology providers to adapt quickly to changing care dynamics while maintaining connections with existing patients.
The COVID-19 pandemic has therefore impacted cancer care delivery. Fewer patients are undergoing screenings, with many providers and patients choosing to reschedule or completely forego screenings during the months of the pandemic, leading to fewer cancer diagnoses. This is particularly true for cancers that rely on routine preventive screenings to detect a large portion of asymptomatic tumors. The natural consequence of disruption in cancer screenings and delays in diagnosis and treatment is that cancer will present at a later stage and often require more complex care, lowering the likelihood that patients will respond to therapy and be cured of the disease. In the years after the pandemic, we anticipate that the effects of COVID-19 on access to cancer care will result in a stage migration to higher stages of disease and an overall increase in cancer mortality.
Notably, the considerable drops in screenings in April have subsequent implications for the number of new patient E&M visits, biopsies, and treatment dynamics in later months, as these patients could now have delayed diagnoses until their next scheduled cancer screening or until their disease becomes symptomatic. Because of the extended timeline of a patient's cancer treatment journey, there is an expected lag of up to 3 months between when a patient is screened for cancer and when a patient is subsequently scheduled for a biopsy and then eventually receives treatment. Coupled with delays in surgeries in April and May, a second wave of the decreased utilization of cancer services in the following months should be anticipated, as observed in the July figures reported in this study and likely to be continued in subsequent months. This second wave is characterized in the trends in delivery of chemotherapy administration, which first dropped in April and May in response to stay-at-home orders, patient hesitancy to seek care, and providers struggling to adapt to CDC guidelines for remaining open, followed by a secondary drop in July, possibly attributable to the after-effect of delayed or postponed screenings and biopsies, resulting in delayed therapy initiation or failure to identify cancer patients with asymptomatic disease. Fewer patients are also undergoing surgeries, receiving physician-administered treatment, and seeking chronic follow-up for existing cancers.
While cancer screening has improved in much of the United States, routine testing was disrupted for at least 6 months of 2020, and most rates remain diminished today. To effectively diagnose and manage cancer, stakeholders should consider how to heighten the awareness of the dangers of medical distancing and recover seniors' confidence in their ability to seek safe and appropriate care. This care includes routine cancer screenings and appropriate treatment required to avoid significant negative impacts on cancer mortality in the United States. Decline in cancer screening rates, physician E&M visits, and administration of cancer therapies will likely translate into both a stage migration to more advanced cancer at diagnosis and higher cancer mortality among senior citizens in the United States. Policies to promote access to cancer care and support the cancer ecosystem have the potential to reduce the expected morbidity and mortality in this patient population. Further studies should be conducted to understand the impact on specific patient populations.
Appendix
TABLE A1.
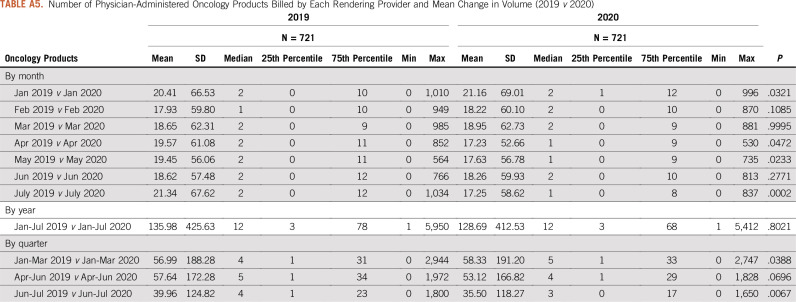
Number of Physician-Administered Oncology Products Billed by Each Rendering Provider and Mean Change in Volume (2019 v 2020)
TABLE A2.
Number of Evaluation and Management (E&M) Visits Billed by Each Rendering Provider and Mean Change in Volume (2019 v 2020)
TABLE A3.
Number of Hospitalizations Billed by Each Rendering Provider and Mean Change in Volume (2019 v 2020)
TABLE A4.
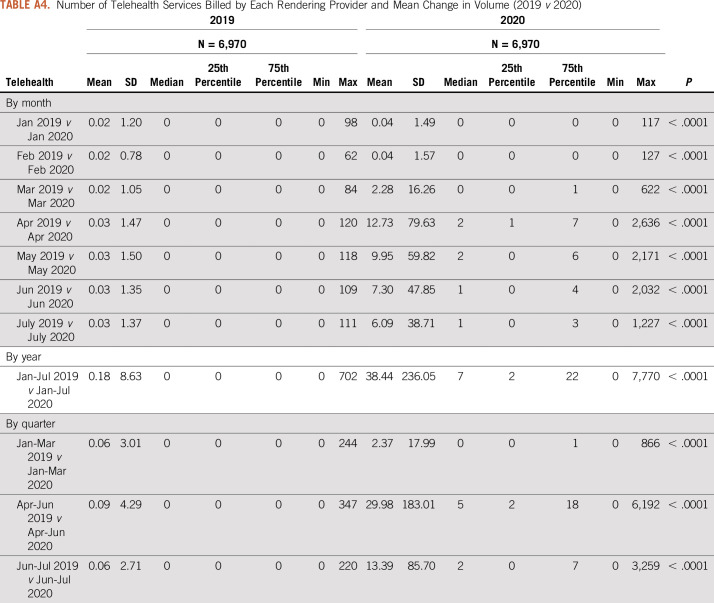
Number of Telehealth Services Billed by Each Rendering Provider and Mean Change in Volume (2019 v 2020)
TABLE A5.
Number of Physician-Administered Oncology Products Billed by Each Rendering Provider and Mean Change in Volume (2019 v 2020)
AUTHOR CONTRIBUTIONS
Conception and design: Debra Patt, Lucio Gordan, Michael Diaz, Lance Grady, Merrill Harmison, Ted Okon, Nathan Markward
Financial support: Debra Patt, Lucio Gordan, Michael Diaz, Lance Grady, Merrill Harmison, Ted Okon, Nathan Markward
Administrative support: Lance Grady, Merrill Harmison
Collection and assembly of data: Debra Patt, Lucio Gordan, Michael Diaz, Ted Okon, Lance Grady, Merrill Harmison, Nathan Markward
Data analysis and interpretation: Lucio Gordan, Michael Diaz, Ted Okon, Lance Grady, Merrill Harmison, Nathan Markward, Milena Sullivan, Jing Peng, Anan Zhou
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Debra Patt
Employment: Texas Oncology, McKesson, MedNax
Leadership: McKesson, Mednax, Texas Oncology
Stock and Other Ownership Interests: Mednax
Consulting or Advisory Role: Pfizer, Roche, AstraZeneca
Research Funding: Merck, Eisai, Seattle Genetics, Lilly
Travel, Accommodations, Expenses: McKesson
Lucio Gordan
Employment: Florida Cancer Specialists
Leadership: Florida Cancer Specialists
Honoraria: Ameris Pharma
Consulting or Advisory Role: Janssen Oncology
Speakers' Bureau: Myriad Genetics
Lance Grady
Employment: Avalere
Stock and Other Ownership Interests: INOVALON
Merrill Harmison
Employment: Avalere Health
Anan Zhou
Employment: Avalere Health, Analysis Group
No other potential conflicts of interest were reported.
REFERENCES
- 1.Stokes EK Zambrano LD Anderson KN, et al. : Coronavirus disease 2019 case surveillance—United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 69:759, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus Disease 2019 (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/index.html [Google Scholar]
- 3.Broom A Kenny K Page A, et al. : The paradoxical effects of COVID-19 on cancer care: Current context and potential lasting impacts. Clin Cancer Res, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Forbes N Smith ZL Spitzer RL, et al. : Changes in gastroenterology and endoscopy practices in response to the coronavirus disease 2019 pandemic: Results from a North American survey. Gastroenterology 159:772-774.e13, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagar H, Formenti SC: Cancer and COVID-19—Potentially deleterious effects of delaying radiotherapy. Nat Rev Clin Oncol 17:332-334, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera A Ohri N Thomas E, et al. : The impact of COVID-19 on radiation oncology clinics and cancer patients in the US. Adv Radiat Oncol 5:538-543, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Quteimat OM, Amer AM: The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol 43:452-455, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al‐Shamsi HO Alhazzani W Alhuraiji A, et al. : A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID‐19) pandemic: An International Collaborative Group. Oncologist 25:e936, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coronavirus Disease 2019 (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html [Google Scholar]
- 10.Anand S Walling A Wenger N, et al. : Impact of the COVID-19 pandemic on medical oncology utilization at a busy urban academic medical center. Clin Cancer Res 26, 2020(18 suppl; abstr PO-023) [Google Scholar]
- 11.Epstein MM Sundaresan D Fair M, et al. : Impact of COVID-19 on breast and prostate cancer screening and early detection in a large health care provider group. Clin Cancer Res 26, 2020(18 suppl; abstr S11-03) [Google Scholar]
- 12.Carethers JM Sengupta R Blakey R, et al. : Disparities in cancer prevention in the COVID-19 era. Cancer Prev Res (Phila) 13:893-896, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman HW Chen Z Niles J, et al. : Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open 3:e2017267, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fedewa SA Yabroff KR Zheng Z, et al. : Unemployment and cancer screening: Baseline estimates to inform health care provision in the context of COVID-19 economic distress. Clin Cancer Res 26, 2020(18 suppl; abstr S09-04) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.London JW Fazio-Eynullayeva E Palchuk MB, et al. : Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform 4:657-665, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warner ET Restrepo E Benjamin C, et al. : Patient-reported impact of the COVID-19 pandemic on breast cancer screening, diagnosis, and treatment: A national survey. Clin Cancer Res 26, 2020(18 suppl; abstr S11-02) [Google Scholar]
- 17.Papautsky EL, Hamlish T: Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat 184:249-254, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maringe C Spicer J Morris M, et al. : The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol 21:1023-1034, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]