Abstract
There is an urgent need to identify therapies that prevent SARS-CoV-2 infection and improve the outcome of COVID-19 patients. Although repurposed drugs with favorable safety profiles could have significant benefit, widely available prevention or treatment options for COVID-19 have yet to be identified. Efforts to identify approved drugs with in vitro activity against SARS-CoV-2 resulted in identification of antiviral sigma-1 receptor ligands, including antihistamines in the histamine-1 receptor binding class. We identified antihistamine candidates for repurposing by mining electronic health records of usage in population of more than 219,000 subjects tested for SARS-CoV-2. Usage of diphenhydramine, hydroxyzine and azelastine was associated with reduced incidence of SARS-CoV-2 positivity in subjects greater than age 61. We found diphenhydramine, hydroxyzine and azelastine to exhibit direct antiviral activity against SARS-CoV-2 in vitro. Although mechanisms by which specific antihistamines exert antiviral effects is not clear, hydroxyzine, and possibly azelastine, bind Angiotensin Converting Enzyme-2 (ACE2) and the sigma-1 receptor as off-targets. Clinical studies are needed to measure the effectiveness of diphenhydramine, hydroxyzine and azelastine for disease prevention, for early intervention, or as adjuvant therapy for severe COVID-19.
Keywords: Angiotensin converting Enzyme-2, Sigma-1 receptor, SARS-CoV-2, Repurposing, Docking
1. Introduction
SARS-CoV-2 has emerged as a major public health problem. There is an urgent need to identify safe drugs with activity against SARS-CoV-2 to prevent infection in at-risk populations [1]. Although known safety profiles of approved drugs permit rapid advancement to clinical trials, there has been limited success in identifying clinically effective small molecule drugs for COVID-19 therapy [[2], [3], [4], [5], [6], [7], [8]].
SARS-CoV-2 gains entry into cells by intermolecular interactions between the virus spike glycoprotein and Angiotensin converting enzyme 2 (ACE2) [9]. Therapeutic agents that interfere with the spike glycoprotein/ACE2 interaction neutralize SARS-CoV-2 infection. The interface between the receptor binding domain (RBD) of the SARS-CoV-2 spike glycoprotein and ACE2 is relatively flat [9].
ACE2 is a cell surface metalloprotease expressed in the lungs, arteries, heart, kidney, and intestines. ACE2 catalyzes the hydrolysis of angiotensin II (a vasoconstrictor peptide) into angiotensin (1–7) (a vasodilator). Structures of the apo and substrate bound forms of the ACE2 catalytic domain show large conformational changes that accompany catalysis within the active site, and distant from the active site [10]. The apo form of ACE2 exhibits an open conformation, whereas the substrate bound form of ACE2 shows a closed conformation. Since the SARS-CoV-2 spike protein was shown to bind the open form of ACE2, small molecule drugs that modify the ACE2 conformation to a closed form could inhibit RBD interactions.
We previously identified compounds that inhibit ACE2 catalytic activity [11], and drugs that enhance ACE2 catalytic activity [12]. We previously reported an ACE2 inhibitor that disrupted interaction of the SARS-CoV-1 spike glycoprotein with ACE2 [11], presumably through modifying the ACE2 conformation from an open to closed state. ACE2 inhibitors may be considered as candidates for repurposing drugs with antiviral effects. For example, hydroxyzine is a first generation histamine receptor (H1) antagonist that exhibits off-target ACE2 inhibitory activity [13].
In vitro evaluation of approved drugs for direct antiviral activity against SARS-CoV-2 using virus isolates has been used to identify candidates for further investigation and repurposing as therapeutic agents to prevent and treat COVID-19 [14,15]. The antihistamines clemastine, cloperastine [16], and astemizole [17] were shown to exhibit direct antiviral activity in vitro. Although antiviral mechanisms of action by antihistamines are not clear, clemastine and cloperastine were proposed to block the interaction between host sigma-1 receptor and SARS-CoV-2 nonstructural protein NSP6. The sigma-1 receptor is an integral chaperone protein localized to the endoplasmic reticulum (ER) and has broad roles in cellular stress signaling including regulating Ca2+ signaling, bioenergetics, and ER stress [6].
Usage of the antihistamine famotadine was associated with improved outcome in severe COVID-19 patients [18]. These data, and direct antiviral activity by clemastine, cloperastine, and astemizole in vitro [[16], [17]] [[,17], implicate specific antihistamines as repurposing candidates for prevention and treatment of SARS-CoV-2 infection.
Although mining of patient records was used to identify famotidine as a candidate for treatment of COVID-19, reports of associations between prescribed drug usage and SARS-CoV-2 infection are sparse. Prescribed drug usage associated with reduced incidence of SARS-CoV-2 positivity may be informative as indicators of low risk individuals, or because of direct drug effects on the virus and/or immune response. Although association studies are possible with all drugs captured in electronic medical records, associations for drugs used to treat rare diseases can be less informative compared to common drugs since the cohort size limits statistical significance.
In this study, we identified candidate antihistamines for repurposing by mining electronic health records of over 219,000 patients tested for SARS-CoV-2 within the University of California Health System. To determine if specific antihistamines exhibit direct antiviral effects, drugs were tested in vitro using infectious SARS-CoV-2 cell-based drug susceptibility assays which measure inhibitory effects on the production of infectious virus over time. Molecular docking was used to identify potential binding sites for antiviral antihistamines on ACE2 and the sigma-1 receptor.
2. Materials and methods
2.1. Molecular docking
Molecular docking of ACE2 was performed as described [13]. Drugs were docked individually using AutoDock Vina [19] into the ACE2 crystal structure (PDB 1R4L). The SMILES string of each drug was obtained from PubChem and translated into 3 dimensional coordinates using the NCI/CADD translator (http://cactus.nci.nih.gov/translate/). AutoDock Tool assigned hydrogen atoms and calculated atom charges for AutoDock Vina. The crystal structure of human sigma 1 receptor PDB 5HK1 [20] was used to predict drug interactions. Atomic coordinates for ligand PD144418 and solvent molecules were extracted from the sigma 1 receptor structure and each drug was docked to the ligand binding site using AutoDock Vina. The top 9 scoring orientations were evaluated by visual inspection with the highest scoring poses reported.
2.2. Study population and association analysis based on electronic health records (EHRs)
Medical history and information related to SARS-CoV-2 infection tests from EHRs of over 219,000 individuals from the University of California COVID Research Data Set (UC CORDS) was obtained starting February 02, 2020. The UC CORDS is structured using the Observational Medical Outcome Partnership common data model (OMOP-CDM) [21] with standardized vocabularies representing diagnosis, medications, lab measurements and medical procedures associated with clinical encounter of individuals across UC Health. The SARS-CoV-2 positive patients were identified based on confirmed RT-qPCR test results among those tested across UC Health. We computed odds ratios representing the association between prior prescription of antihistamine and SARS-CoV-2 negative test results as primary outcome of interest using logistic regression adjusting for sex and age. The age of individuals was categorized into three groups 0–30 years, 31–60 years and 61 years and above. We used the glm function implemented in R statistical software [22] (27) to compute odds ratio along with confidence intervals and p-values. All the p-values were corrected for false discovery rate and were represented as q-values. The estimated odds ratio was considered significant if the confidence intervals did not span 1 and q-value ≤ 0.10.
2.3. IRB approval and medical record access
Access to the HIPAA Limited Data Set of medical records (deidentified except with dates) within the University of California Health system were obtained under permission by the UC Health IRB Directors and classified as non-human subject research (UCSF IRB 20–30889). The University of California Health COVID Research Data Set (UC CORDS) includes nearly all patients tested for SARS-CoV-2 and treated at any of the 12 UC Health hospitals across 5 academic medical centers (UCLA, UC San Francisco, UC San Diego, UC Irvine, and UC Davis).
2.4. Drug susceptibility assays
Drug susceptibility assays were performed for hydroxyzine against the USA-WA1/2020 strain of SARS-CoV-2 on human lung A549 cells that were transfected with hACE2 (ACE2-A549) cells. For these studies, ACE2-A549 cells were seeded into 6 well plates and allowed to attached overnight at 37 °C, 5% CO2. Cells were pre-treated with various concentrations of hydroxyzine for 4 h prior to infection. Confluent cell monolayers were then infected with USA-WA1/2020 at a multiplicity of infection (MOI) equivalent to 0.03 PFU/cell and permitted to adsorb for 1 h. Plates were shaken every 15 min to ensure even distribution of the virus. Unbound virus was removed by washing cell monolayers twice with warm PBS and medium containing various concentrations of hydroxyzine were added to each well of the 6-well plate. One well served as a no-treatment control. Cell supernatants were harvested on day 4 post-inoculation, the day in which peak viral burden is achieved for this MOI on ACE2-A549 cells, clarified by high-speed centrifugation, and frozen at −80 °C until the end of the study. Assays were conducted in triplicate in two independent experiments.
2.5. Viruses
The entire SARS-CoV-2 glycoprotein was synthesized by Genewiz (GeneBank: MT118835.1) and used for pseudotyping a lentivirus encoding CMV-GFP-PGK-puro (Cellomics Technology, LLC). Lentivirus was used at 2.5 X10 ^ 6 TU/ml. The SARS-CoV-2 strain UF-1 was previously isolated, sequenced, and deposited in GeneBank (MT295464.1). The USA-WA1/2020 strain of SARS-CoV-2 was deposited by the Centers for Disease Control and Prevention and obtained through BEI Resources, NIAID, NIH (NR-52281). All strains of SARS-CoV-2 was propagated in Vero E6 cells (ATCC).
2.6. Cell culture
HEK293T cells overexpressing human ACE2 (HEK293T-hACE2) were purchased from GeneCopoeia. Cell media was 10% FBS in DMEM (high glucose, pyruvate) with 1X penicillin/streptomycin. Cells were grown on rat-tail type 1 collagen coated 100 mm plastic dishes or on 18 mm collagen-coated coverslips (Neuvitro) in 6-well plates in a 37 °C and 5% CO2 humidified incubator. Transgene expression was maintained by hygromycin B [100 ng/ml]; hygromycin B was removed 24 h prior to addition of drugs/lentivirus and remained absent during the entire experiment. Cells were 70–80% confluent at time of treatment and drug exposure was for 5–10 min prior to lentivirus infection. Cells were harvested and fixed in 2% paraformaldehyde 72 h later. Studies were performed 6 independent times. Vero E6 cells purchased from ATCC and grown in DMEM+2%FBS+ 1X penicillin/streptomycin at 37 °C and 5% CO2 in a humidified incubator.
Studies performed with the USA-WA1/2020 strain of SARS-CoV-2 utilized Vero E6 cells purchased from ATCC that were propagated in Eagle’s Minimum Essential Medium (EMEM; Hyclone) supplemented with 10% FBS (Hyclone) and 1X penicillin/streptomycin solution (Hyclone). hACE2-A549 cells [23] (a kind gift from Dr. Shinji Makino) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS and 1X penicillin/streptomycin solution. Vero E6 and hACE2-A549 cells were incubated at 37 °C, 5% CO2 and split twice-weekly.
2.7. Microscopy
Coverslips containing cells HEK293T-hACE2 were imaged on a Zeiss Axio Zoom V16 microscope [24]. The same magnification was used across experiments. Two to three image fields were examined per coverslip and number of GFP-positive cells counted and averaged. Nuclei were counterstained using Hoechst [24].
2.8. Viral plaque assay
Vero E6 cells were plated in 6 well plates with replicates on different plates. Monolayers were infected with virus master mix at 100 PFU/ml aliquoted in separate tubes with drugs at designated concentrations for 1 h (Herzog et al., 2008). Monolayers were overlaid with MEM in 1.5% low-melt agarose with drugs at concentrations indicated above. Plaques were enumerated at 72 h post infection. For dose response curves, virus was diluted and allowed to infect Vero E6 across the indicated drug concentrations in DMEM+ 10%FBS in triplicate for 1 h with gentle rocking every 10 min. Solution was removed, then cells overlain with MEM+5%FBS in 1.5% low melt agarose containing the indicated drug concentrations and incubated at 37 °C with 5% CO2 for 3 dpi. 200 μl PBS containing 0.03% neural red was added to wells and plaques counted 3–6 h later to determine apparent PFU/ml.
2.9. Drugs
Drugs were dissolved in 100% DMSO and diluted with 0.9% saline for a 1000X stock; DMSO concentration in stocks was 5%. All stocks were diluted into HEK media. Recombinant spike protein (Sino Biological) was diluted in media for a final concentration of 13.5 ng/ml. DMSO was a control for all drugs. Screening of drugs occurred in a blinded fashion. Hydroxyzine dihydrochloride (Sigma-Aldrich), cetirizine dihydrochloride (Sigma-Aldrich), diphenhydramine hydrochloride (Spectrum), loratadine (Spectrum), and azelastine hydrochloride (TCI America) were studied.
2.10. Statistical analysis
One-way ANOVA was performed, followed by a Dunnett multiple comparison post hoc test, to determine differences in assays relative to DMSO vehicle control group. For association analysis based on EHRs, odds ratio, confidence interval, and p-value were calculated using logistic regression, and p-values were corrected for false discovery rate and represented as q-values. Statistical significance was determined by q-value ≤ 0.10.
3. Results
3.1. Usage of specific antihistamines was associated with reduced incidence of SARS-CoV-2 positivity
We utilized medical history and information related to SARS-CoV-2 infection test from EHRs of over 219,000 individuals from the University of California COVID Research Data Set (UC CORDS) starting February 02, 2020, and computed odds ratios representing the association between prior prescription of antihistamine and SARS-CoV-2 negative test results as primary outcome of interest using logistic regression adjusting for sex and age. The age of individuals was categorized into three groups 0–30 years, 31–60 years and 61 years and above. Prior usage of loratadine, diphenhydramine, cetirizine, hydroxyzine, and azelastine was associated with a reduced incidence of positive SARS-CoV-2 test results in individuals 61 years and above in a statistically significant manner (Supplemental Table 1). Although numerous factors confound the observed association of antihistamine usage with SARS-CoV-2 positivity (e.g., socio-economic status, co-morbidities), we asked if associated drugs may exert direct antiviral activity against SARS-CoV-2 as a possible contributing factor.
3.2. Hydroxyzine, diphenhydramine and azelastine exhibit direct antiviral activity against SARS-CoV-2 in vitro
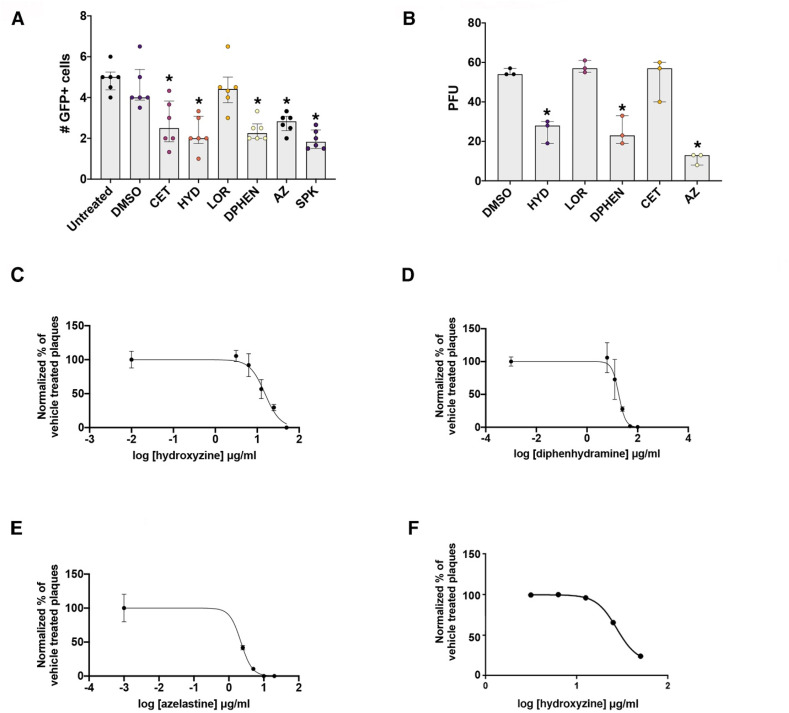
Each associated drug was tested for the ability to disrupt SARS-CoV-2/ACE2 interactions using a lentivirus pseudotyped to express the SARS-CoV-2 spike protein (Fig. 1 A) using recombinant spike protein and the DMSO vehicle as positive and negative controls, respectively. Associated drugs were also tested for direct antiviral activity by measuring effects on infection of Vero E6 cells with SARS-CoV-2 isolate USA-UF-1/2020 (Fig. 1B). Hydroxyzine, diphenhydramine and azelastine exhibited direct antiviral effects against SARS-CoV-2 in vitro. Drug concentrations effective for 50% inhibition of SARS-CoV-2 isolate USA-UF-1/2020 infection (EC50) of Vero E6 cells were 15.3 μg/ml for hydroxyzine (Fig. 1C), 17.4 μg/ml for diphenhydramine (Fig. 1D), and 2.24 μg/ml for azelastine (Fig. 1E). The drug concentration effective for 50% inhibition (EC50) for hydroxyzine against another SARS-CoV-2 isolate USA-WA1/2020 in the human lung cell line A549 overexpressing ACE2 was estimated to be 27.34 μg/ml for (Fig. 1F). These data show that specific common antihistamines exhibit antiviral effects on distinct isolates of SARS-CoV-2 in multiple cell types in vitro.
Fig. 1.
Antiviral activity of specific antihistamines against SARS-CoV-2 isolates in vitro. Panel A. An engineered GFP-expressing lentivirus pseudotyped with the SARS-CoV-2 surface glycoprotein was used to infect ACE2 expressing HEK293 cells in the presence and absence of histamine-1 receptor binding antihistamines. The number of GFP positive cells per image field 72 h after a single application of drugs in HEK293T cells overexpressing human ACE2 is shown. n = 6 independent experiments. ∗p = 0.0044 DMSO vs CET ∗; p = 0.0002 DMSO vs HYD; ∗p = 0.0006 DMSO vs DIPH; ∗p = 0.0052 DMSO vs AZ; p < 0.0001 DMSO vs SPK. Panel B. Number of SARS-CoV-2 isolate USA-UF-1/2020 viral plaques present in Vero E6 cells 3 dpi following exposure to antihistamines or DMSO vehicle control. n = 3 independent experiments. ∗p = 0.0003 DMSO vs HYD ∗; p = 0.0003 DMSO vs DIPH; ∗p < 0.0001 DMSO vs AZ. Dose response curves against SARS-CoV-2 isolate USA-UF-1/2020 in Vero E6 cells for hydroxyzine (C), diphenhydramine (D) and azelastine (E). n = 3 independent experiments. Dose response curve against SARS-CoV-2 isolate USA-WA1/2020 in human lung A549 cells for hydroxyzine (F). n = 2 independent experiments. Abbreviations: cetirizine (CET), hydroxyzine (HYD), diphenhydramine (DIPH), loratadine (LOR), azelastine (AZ), plaque forming units (PFU), spike protein (SPK). Drug concentrations in Panels A and B in μg/ml: hydroxyzine [10]; cetirizine [10]; loratadine [1.5]; diphenhydramine hydrochloride [25]; azelastine hydrochloride [7.0]. Spike protein used at 13.5 ng/ml. Data were analyzed by a one-way ANOVA followed by a Dunnett multiple comparison test. In panel A B, the median + interquartile range are shown.
3.3. Antihistamines with antiviral properties bind ACE2 and the sigma-1 receptor
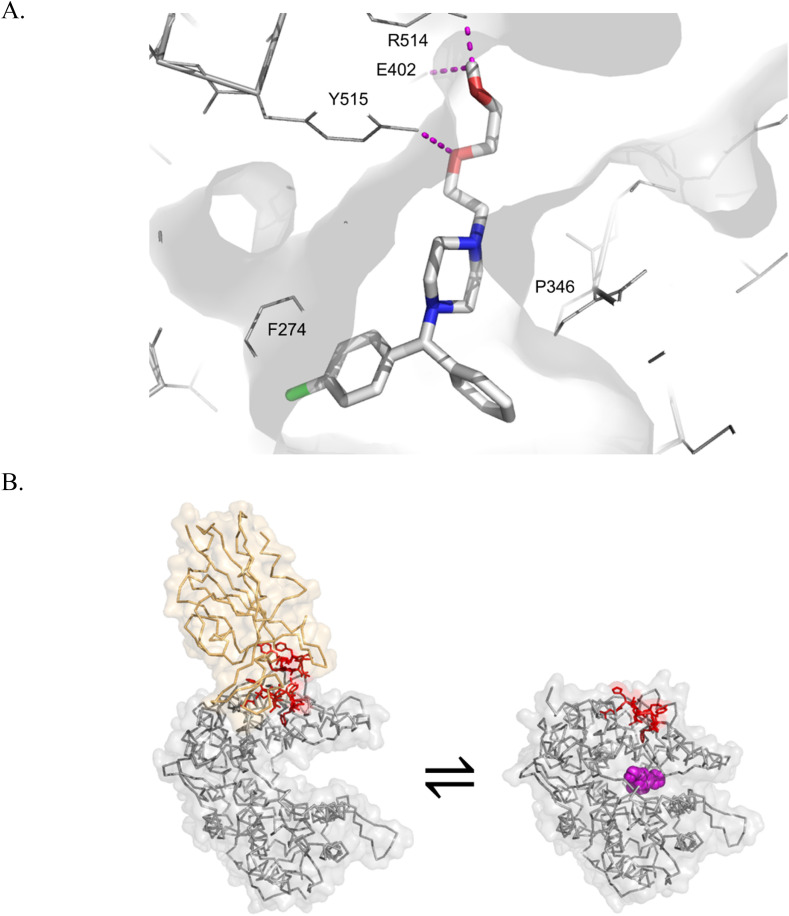
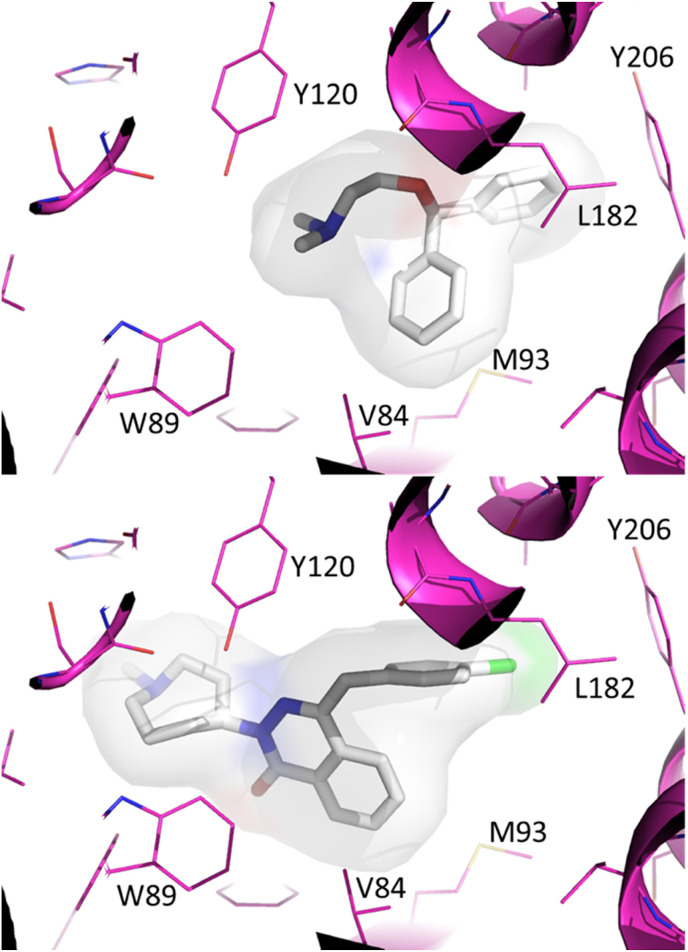
We used molecular docking to define potential interactions between hydroxyzine, diphenhydramine, azelastine and off-targets ACE2 and the sigma-1 receptor. Hydroxyzine was previously shown to inhibit ACE2 catalytic activity and exhibited a molecular docking ΔG score of −8.6 kcal/mol (Fig. 2 ). Azelastine was predicted to bind the active site of ACE2 (ΔG -9.9 kcal/mol), whereas diphenhydramine was not predicted to bind ACE2 with high affinity (ΔG 6.9 kcal/mol). The affinities of hydroxyzine and diphenhydramine for sigma-1 receptor were previously measured [25], showing an estimated Kd of 192 nM for hydroxyzine, 1.7 μM for diphenhydramine. Molecular docking showed that azelastine was predicted to bind sigma-1 receptor with a higher affinity (ΔG -11.3 kcal/mol) than diphenhydramine (ΔG -8.2 kcal/mol)(Fig. 3 ). These data suggest that ACE2 and sigma-1 receptor binding mechanisms may regulate antiviral effects mediated by hydroxyzine and azelastine.
Fig. 2.
In silico modeling of the ACE2 catalytic inhibitor hydroxyzine suggests binding within the active site. A. Hydroxyzine was posed by molecular docking with AutoDock Vina. Hydroxyzine is shown as sticks, white for carbon, blue for nitrogen, red for oxygen, green for chlorine. Polar interactions (e.g., H bonds) shown as magenta dashes. B. The SARS-CoV-2 spike glycoprotein binds the open conformation of ACE2. The SARS-CoV-2 spike protein is shown in orange (from PDB 6M17). ACE2 is shown in grey in the open conformation (left, from PDB 6M17) and the closed conformation (right, PDB 1R4L). Inhibitor binding prevents intermolecular contacts between ACE2 and SARS-CoV-2 spike protein (shown as sticks in red). Hydroxyzine is shown as spheres in magenta, as posed by AutoDock Vina.
Fig. 3.
Molecular docking predicts binding of diphenhydramine and azelastine to the ligand binding pocket of the Sigma-1 receptor. The crystal structure of the human sigma-1 receptor bound to a ligand was used as the basis for molecular docking with AutoDock Vina. The ligand PD144418 was extracted from the coordinates of PDB code 5HK1, and diphenhydramine was docked to this site (upper panel), estimated ΔG -8.2 kcal/mol. Azelastine was docked to the ligand binding site of the sigma 1 receptor (lower panel), estimated ΔG -11.3 kcal/mol.
4. Discussion
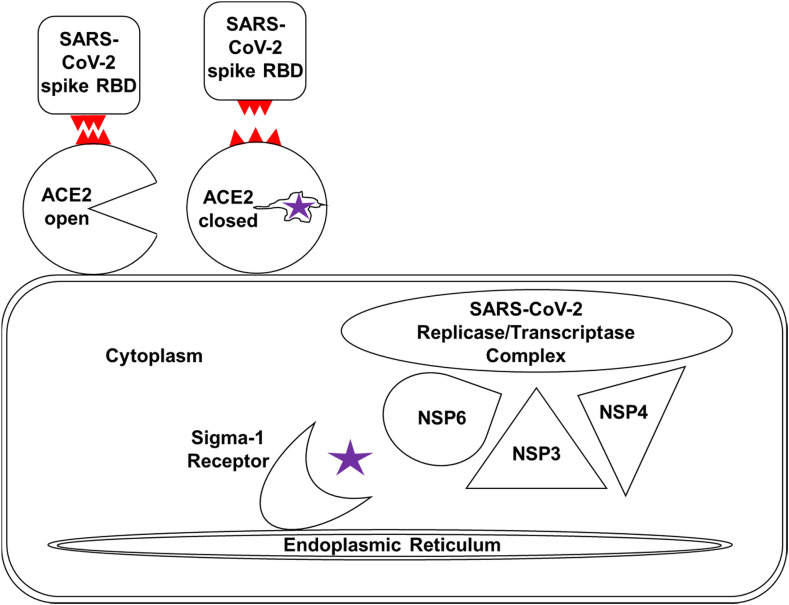
Hydroxyzine, diphenhydramine and azelastine exhibited direct antiviral activity against SARS-CoV-2 isolates in vitro (Fig. 1). These data are consistent with direct antiviral activity demonstrated by antihistamines clemastine [16], cloperastine [16], and astemizole [17]. Although mechanisms underlying antiviral activity of specific antihistamines are not well characterized, antihistamines that inhibit SARS-CoV-2 infection have been shown to bind off-targets including ACE2 (for hydroxyzine [13])(Fig. 2), and the sigma receptor-1 (Fig. 3)(for hydroxyzine and diphenhydramine [25]). The sigma receptor-1 is an endoplasmic reticulum resident chaperone protein shown to bind the SARS-CoV-2 protein nonstructural protein 6 (NSP6) [16]. The sigma-1 receptor links the SARS-CoV-2 replicase/transcriptase complex to the ER membrane by binding directly to NSP6, which forms a complex with NSP3 and NSP4. NSP6 is also thought to inhibit degradation of SARS-CoV-2 proteins by preventing autolysosome expansion [26]. Antihistamines that bind sigma-1 receptors, such as hydroxyzine, may exert antiviral activity by preventing NSP6 binding and pathological autolysosome effects. Alternatively, antihistamines that bind ACE2, such as hydroxyzine, have the potential to exert antiviral activity by interfering with SARS-CoV-2 spike protein interactions [13,27](Fig. 4 ). Antiviral mechanisms of specific antihistamines remain to be clearly elucidated.
Fig. 4.
Model for antiviral mechanisms mediated by specific antihistamines. Hydroxyzine and related antihistamines have the potential to inhibit SARS-CoV-2 entry (by binding ACE2), and virus replication (by binding the sigma-1 receptor). Hydroxyzine (purple) exhibits off-target inhibitory ACE2 activity by forming intermolecular interactions with the active site, inducing a conformational change from the open conformation to the closed conformation. The conformational change shifts the position of ACE2 at positions that contact the SARS-CoV-2 spike glycoprotein receptor binding domain (RBD), shown in red, which results in loss of intermolecular interactions at the ACE2/RBD interface. The sigma-1 receptor is a membrane bound chaperone highjacked by SARS-CoV-2 to link the replicase/transcriptase complex to the endoplasmic reticulum by binding directly to nonstructural protein NSP6, which forms a complex with NSP3 and NSP4. Hydroxyzine binds the sigma-1 receptor with high affinity, potentially interfering with the virus life cycle by blocking protein-protein interactions with NSP6.
The EC50 values for in vitro antiviral activity are above concentrations expected in plasma following recommended dosing for hydroxyzine [28] and diphenhydramine [29]. The azelastine concentrations used in the study effectively inhibit SARS-CoV-2 below prescribed nasal spray doses [30]. Combining an antiviral antihistamine with antiviral drugs that bind distinct targets may provide additive or synergistic antiviral effects and decrease the likelihood of SARS-CoV-2 resistance to a single drug. However, antihistamine use should occur as directed and determining whether antihistamines should be combined requires feedback from a licensed medical provider.
Specific antihistamine drugs may influence SARS-CoV-2 infection or cause benefit in COVID-19 patients by multiple interrelated mechanisms [17]. Although our data suggest antihistamines exhibit direct antiviral effects, on-target effects of antihistamines (binding histamine-1 receptors for hydroxyzine, diphenhydramine and azelastine) may inhibit inflammatory responses that increase mortality [31]. Underlying genetic or cellular factors including allergic responses might also contribute to risk of SARS-CoV-2 infection. A recent report indicating a lower than expected representation of patients with asthma and COVID-19 is consistent with this idea [32].
5. Conclusion
We found that usage of select antihistamines, including diphenhydramine, hydroxyzine, and azelastine, was associated with reduced incidence of SARS-CoV-2 positivity in a large population. These three drugs exhibited direct antiviral activity in vitro. Randomized placebo controlled clinical trials will be required to determine if specific antihistamines have beneficial effects for prevention or treatment of COVID-19.
Acknowledgements
The authors also thank Dr. Paul McCray, Dr. Chris Vulpe, Dr. Don Bolser, and Dr. Patrick Concannon for helpful discussions and advice. We would like to thank Dr. John Lednicky for providing the UF-1 SARS-CoV-2 isolate. Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427, and the UCSF Florida Clinical and Translational Science Institute supported in part by the National Center for Advancing Translational Sciences under award number UL1TR001872. Other support provided by the National Institutes of Health include: R00HL119560 (PI, LRR), OT2OD026582 (PI, LRR), and OT2OD023854 (Co–I, LRR) and the Center for Data Driven Insight and Innovation in the University of California Health.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bbrc.2020.11.095.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Rogosnitzky M., Berkowitz E., Jadad A.R. Delivering benefits at speed through real-world repurposing of off-patent drugs: the COVID-19 pandemic as a case in point. JMIR Public Health Surveill. 2020;6(2) doi: 10.2196/19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gautret P., Lagier J.C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. S0924-8579(20)30099-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Liu J., Cao R., Xu M., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6 doi: 10.1038/s41421-020-0156-0. 16-020-0156-0. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent M.J., Bergeron E., Benjannet S., et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2 doi: 10.1186/1743-422X-2-69. 69-422X-2-69. doi: 1743-422X-2-69 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon D.E., Jang G.M., Bouhaddou M., et al. 2020. A SARS-CoV-2-Human Protein-Protein Interaction Map reveals Drug Targets and Potential Drug-repurposing. bioRxiv. 2020.03.22.002386 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyall J., Gross R., Kindrachuk J., et al. Middle east respiratory syndrome and severe acute respiratory syndrome: current therapeutic options and potential targets for novel therapies. Drugs. 2017;77(18):1935–1966. doi: 10.1007/s40265-017-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alessandro S., Scaccabarozzi D., Signorini L., et al. The use of antimalarial drugs against viral infection. Microorganisms. 2020;8(1) doi: 10.3390/microorganisms8010085. E85 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Towler P., Staker B., Prasad S.G., et al. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279(17):17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huentelman M.J., Zubcevic J., Hernandez Prada J.A., et al. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44(6):903–906. doi: 10.1161/01.HYP.0000146120.29648.36. 01.HYP.0000146120.29648.36 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Hernandez Prada J.A., Ferreira A.J., Katovich M.J., et al. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51(5):1312–1317. doi: 10.1161/HYPERTENSIONAHA.107.108944. [DOI] [PubMed] [Google Scholar]
- 13.Kulemina L.V., Ostrov D.A. Prediction of off-target effects on angiotensin-converting enzyme 2. J. Biomol. Screen. 2011;16(8):878–885. doi: 10.1177/1087057111413919. [DOI] [PubMed] [Google Scholar]
- 14.Joshi S., Joshi M., Degani M.S. Tackling SARS-CoV-2: proposed targets and repurposed drugs. Future Med. Chem. 2020;12(17):1579–1601. doi: 10.4155/fmc-2020-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poduri R., Joshi G., Jagadeesh G. Drugs targeting various stages of the SARS-CoV-2 life cycle: exploring promising drugs for the treatment of covid-19. Cell. Signal. 2020;74:109721. doi: 10.1016/j.cellsig.2020.109721. S0898-6568(20)30198-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon D.E., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riva L., Yuan S., Yin X., et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020 doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedberg D.E., Conigliaro J., Wang T.C., et al. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.053. S0016-5085(20)34706-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris G.M., Huey R., Lindstrom W., et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt H.R., Zheng S., Gurpinar E., Koehl A., Manglik A., Kruse A.C. Crystal structure of the human sigma1 receptor. Nature. 2016;532(7600):527–530. doi: 10.1038/nature17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klionsky D.J., Abdelmohsen K., Abe A., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing.http://www.R-project.org/ URL. [Google Scholar]
- 23.Mossel E.C., Huang C., Narayanan K., Makino S., Tesh R.B., Peters C.J. Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. J. Virol. 2005;79(6):3846–3850. doi: 10.1128/JVI.79.6.3846-3850.2005. 79/6/3846 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao Y.S.J., Kuan S.P., Guevara M.V., et al. Acid exposure disrupts mucus secretion and impairs mucociliary transport in neonatal piglet airways. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;318(5):L873–L887. doi: 10.1152/ajplung.00025.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basile A.S., Paul I.A., Mirchevich A., Kuijpers G., De Costa B. Modulation of (+)-[3H]pentazocine binding to Guinea pig cerebellum by divalent cations. Mol. Pharmacol. 1992;42(5):882–889. [PubMed] [Google Scholar]
- 26.Cottam E.M., Whelband M.C., Wileman T. Coronavirus NSP6 restricts autophagosome expansion. Autophagy. 2014;10(8):1426–1441. doi: 10.4161/auto.29309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huentelman M.J., Zubcevic J., Hernandez Prada J.A., et al. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44(6):903–906. doi: 10.1161/01.HYP.0000146120.29648.36. 01.HYP.0000146120.29648.36 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Fouda H.G., Hobbs D.C., Stambaugh J.E. Sensitive assay for determination of hydroxyzine in plasma and its human pharmacokinetics. J. Pharmaceut. Sci. 1979;68(11):1456–1458. doi: 10.1002/jps.2600681134. S0022-3549(15)42933-8 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Blyden G.T., Greenblatt D.J., Scavone J.M., Shader R.I. Pharmacokinetics of diphenhydramine and a demethylated metabolite following intravenous and oral administration. J. Clin. Pharmacol. 1986;26(7):529–533. doi: 10.1002/j.1552-4604.1986.tb02946.x. [DOI] [PubMed] [Google Scholar]
- 30.Derendorf H., Munzel U., Petzold U., et al. Bioavailability and disposition of azelastine and fluticasone propionate when delivered by MP29-02, a novel aqueous nasal spray. Br. J. Clin. Pharmacol. 2012;74(1):125–133. doi: 10.1111/j.1365-2125.2012.04222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berlin D.A., Gulick R.M., Martinez F.J. Severe covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman-Cribbin W., Rapp J., Alpert N., Tuminello S., Taioli E. The impact of asthma on mortality in patients with COVID-19. Chest. 2020 doi: 10.1016/j.chest.2020.05.575. S0012-3692(20)31645-31647 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.