Abstract
Pancreatic cancer is an aggressive disease that responds poorly to conventional photon radiotherapy. Carbon‐ion (C‐ion) radiation has advantages compared with conventional radiotherapy, because it enables more accurate dose distribution and more efficient tumor cell killing. To elucidate the effects of local radiotherapy on the characteristics of metastatic tumors, it is necessary to understand the nature of motility in irradiated tumor cells; this will, in turn, facilitate the development of effective strategies to counter tumor cell motility, which can be used in combination with radiotherapy. The aim of the present study was to examine the invasiveness of pancreatic cancer cells exposed to C‐ion irradiation. We found that C‐ion irradiation suppressed the migration of MIAPaCa‐2, BxPC‐3 and AsPC‐1; diminished the invasiveness of MIAPaCa‐2; and tended to reduce the invasion of BxPC‐3 and AsPC‐1. However, C‐ion irradiation increased the invasiveness of PANC‐1 through the activation of plasmin and urokinase‐type plasiminogen activator. Administration of serine protease inhibitor (SerPI) alone failed to reduce C‐ion‐induced PANC‐1 invasiveness, whereas the combination of SerPI and Rho‐associated coiled‐coil forming protein kinase (ROCK) inhibitor suppressed it. Furthermore, PANC‐1 showed mesenchymal–amoeboid transition when we treated with SerPI alone. In conclusion, C‐ion irradiation is effective in suppressing the invasive potential of several pancreatic tumor cell lines, but not PANC‐1; this is the first study showing that C‐ion irradiation induces the invasive potential of a tumor cell line. Further in vivo studies are required to examine the therapeutic effectiveness of radiotherapy combined with inhibitors of both mesenchymal and amoeboid modes of tumor cell motility. (Cancer Sci 2012; 103: 677–683)
Pancreatic cancer is one of the most aggressive diseases, with an extremely low 5‐year survival rate.( 1 , 2 ) Most pancreatic cancer patients have advanced disease at the time of diagnosis, and the major clinical problem regarding its treatment is its inherent resistance to conventional chemotherapy and radiotherapy.( 1 , 2 , 3 ) Carbon‐ion (C‐ion) radiotherapy offers several advantages over conventional photon radiotherapy, including a more accurate dose distribution( 4 , 5 , 6 ) and higher biological efficiency in tumor cell killing.( 7 , 8 , 9 ) Thus, C‐ion irradiation is expected to improve the clinical outcome in pancreatic cancer patients.
It is unclear whether local radiotherapy affects the nature of subsequently appearing metastatic tumors. Both in vitro and in vivo studies have shown that photon irradiation enhances the metastatic potential of tumor cells, including pancreatic cancer cells.( 1 , 10 , 11 , 12 , 13 , 14 ) In contrast, C‐ion irradiation is known to diminish the invasive potential of several cell lines,( 14 , 15 , 16 ) but this effect has not been verified in pancreatic cancer cell lines. Under these circumstances, the characterization of pancreatic cancer cells exposed to anti‐tumor drugs and irradiation is essential to develop improved treatment strategies.
We have previously reported that conventional photon irradiation enhances the invasiveness of cells of the pancreatic cancer line, MIAPaCa‐2, through the induction of MMP2.( 13 ) Tumor cells with the mesenchymal mode of motility are known to use proteolytic enzymes, such as MMP, aspartic proteases and serine proteases (SerP), to create a path to move through the ECM.( 17 ) In contrast, cells with the amoeboid mode of motility, which are rounded, show a protease‐independent mechanism of invasion; this mechanism is based on actomyosin contractility and is dependent on ROCK/Rho signaling.( 17 , 18 , 19 , 20 , 21 , 22 ) Evidence shows that cells can shift between these two modes of motility, depending on the environmental conditions;( 22 ) this might limit the effectiveness of therapeutic agents, such as MMP inhibitors (MMPI), which are directed at inhibiting a single mode of tumor cell motility. MIAPaCa‐2 cells are morphologically heterogeneous, containing cells of both mesenchymal‐ and amoeboid‐type motility. Accordingly, our previous study showed that the invasiveness of MIAPaCa‐2 cells was suppressed by the combined use of MMPI and ROCK inhibitor (ROCKI), whereas the use of MMPI or ROCKI alone failed, or even enhanced, the invasiveness of these cells.( 13 ) The inappropriate use of inhibitors, that is, the use of MMPI or ROCKI alone, might lead to the induction of metastatic potential in some cell types. Therefore, an understanding of pancreatic cancer cell motility is critical for the effective use of inhibitors. To our knowledge, this is the first study on the invasiveness of C‐ion‐irradiated pancreatic cancer cells with mesenchymal and amoeboid motility.
In the present study, we first analyzed the migration ability and invasiveness of cells of the pancreatic tumor lines MIAPaCa‐2, BxPC‐3, AsPC‐1 and PANC‐1 under C‐ion‐irradiated and non‐irradiated conditions. We found that C‐ion irradiation is effective in suppressing the metastatic potential of MIAPaCa‐2, BxPC‐3 and AsPC‐1 cells. However, the invasiveness of PANC‐1 cells was enhanced by irradiation through the activation of SerP, plasmin and urokinase‐type plasminogen activator (uPA). We found that irradiated PANC‐1 cells showed the ability to undergo mesenchymal–amoeboid transition. Therefore, to suppress the C‐ion‐induced invasiveness of PANC‐1 cells, the use of SerP inhibitor (SerPI) alone would be insufficient, and inhibition of both mesenchymal and amoeboid motility by using both SerPI and ROCKI is necessary.
Materials and Methods
Cell culture and reagents. Cells of the human pancreatic cancer cell lines MIAPaCa‐2, BxPC‐3, AsPC‐1 and PANC‐1 were purchased from ATCC (Manassas, VA, USA). GM6001, aprotinin, bestatin, E‐64, leupeptin and pepstatin A were purchased from Calbiochem‐Novabiochem International (La Jolla, CA, USA), and Y27632 was obtained from Wako (Osaka, Japan).
Irradiation. C‐ions were accelerated by the heavy‐ion medical accelerator in Chiba (HIMAC) at the National Institute of Radiological Sciences (NIRS), Japan.( 6 ) The initial energy of C‐ion beams was 290 MeV/u, and the LET value was 80 keV/μm; a mono‐energetic beam with narrow Bragg Peak was applied at a depth of 10 cm, and cells were irradiated with 0, 0.5, 1, 2 or 4 Gy. All irradiations were carried out at a dose rate of approximately 1 Gy/min. X‐ray irradiation was carried out as described previously.( 13 )
Immunoblotting. Immunoblotting was carried out as described previously.( 13 ) A primary Ab for human MMP2 (Daiichi Fine Chemical, Toyama, Japan), plasminogen (Santa Cruz Biotechnology, Santa Cruz, CA, USA), uPA (Santa Cruz Biotechnology), and GAPDH (Trevigen, Gaithersburg, MD, USA) with anti‐mouse IgG conjugated with horseradish peroxidase (Amersham Biosciences, Buckinghamshire, UK) were used for the analysis.
Quantitative real‐time polymerase chain reaction. Quantitative real‐time PCR (qRT–PCR) was carried out in a LightCycler 480 with the Probes Master Mix (Roche Diagnostics, Indianapolis, IN, USA), as described previously.( 23 ) The UPL probes and primer sequences used are listed in Table S1.
Migration ability and invasion assay. The migration ability and invasiveness of cells were examined by using transwell chambers containing a 6.5‐mm filter with a pore size of 8 μm (Corning, Horseheads, NY, USA),( 24 ) as described previously.( 13 ) MIAPaCa‐2 or PANC‐1 cells and BxPC‐3 or AsPC‐1 cells were suspended into the appropriate medium containing 0.35% BSA and seeded into each well at 1 × 105 and 2 × 105 cells/well, respectively, and the migration and invasion assays were carried out for 24 h (Fig. S1). On day 3, cells that reached the undersurface of the transwell membrane were fixed and stained with Diff Quick (Sysmex, Kobe, Japan). The number of migrated or invaded cells in four random fields was counted using a microscope. The percentages of both viable and dead cells were determined by a FACS with a cell‐viability double‐staining kit (Dojindo Molecular Technologies, Tokyo, Japan)( 25 ) and shown in Tables S2–S4. We calculated the number of viable cells among the seeded cells by multiplying the ratio of viable cells by the total number of seeded cells. We then determined the percentage of migrated or invaded cells.
Determination of SerP and uPA activity. To investigate the activity levels of SerP, a Serine Protease Detect Zymo Electrophoresis kit (Life Laboratory, Yamagata, Japan) was used. On day 2, the conditioned medium was replaced with serum‐free DMEM, and the cells were incubated for 24 h (Fig. S1). On day 3, the supernatant was collected, mixed with sample buffer without heating or reduction, and applied to casein‐polyacrylamide gels. Bands for plasmin, pancreas elastase chymotrypsin and trypsin were visualized at Mr values of 91, 26, 25 and 23 kDa, respectively. To assess uPA activity, the conditioned medium was analyzed by colorimetric assay in the presence of Spectorzyme UK (American Diagnostica, Stamford, CT, USA) as described previously.( 26 ) For inhibitor treatment, the uPA inhibitor, amiloride hydrochloride hydrate (Sigma‐Aldrich, St. Louis, MO, USA) was added to samples before analysis with Spectorzyme UK.
Statistical analysis. Statistical analyses were carried out using unpaired Student’s t‐test or Mann–Whitney U‐test. A P‐value of <0.05 was considered significant.
Results
Migration ability and invasiveness of pancreatic cancer cells after C‐ion irradiation. AsPC‐1, BxPC‐3, MIAPaCa‐2 and PANC‐1 cells were assayed for the effect of C‐ion irradiation on their migration ability and invasiveness. C‐ion irradiation (2 Gy) suppressed the migration of AsPC‐1, BxPC‐3 and MIAPaCa‐2 cells, and diminished MIAPaCa‐2 cell invasion (Fig. 1A). In contrast, 2 Gy irradiation induced invasiveness in the PANC‐1 cells, without altering their migration ability (Fig. 1A). Analysis by radiation doses showed that among the tested doses, just 4 Gy affected the migration of PANC‐1 cells, causing a significant reduction in their migration ability (Fig. 1B). In addition, C‐ion irradiation at 1, 2 and 4 Gy enhanced PANC‐1 cell invasion. We also sought to test the effects of 6 and 8 Gy C‐ion irradiation; however, most of the cells were dead and could not be used for the assay (data not shown). To investigate the factors involved in the induction of invasiveness in PANC‐1 cells by C‐ion irradiation, we further investigated these cells.
Figure 1.
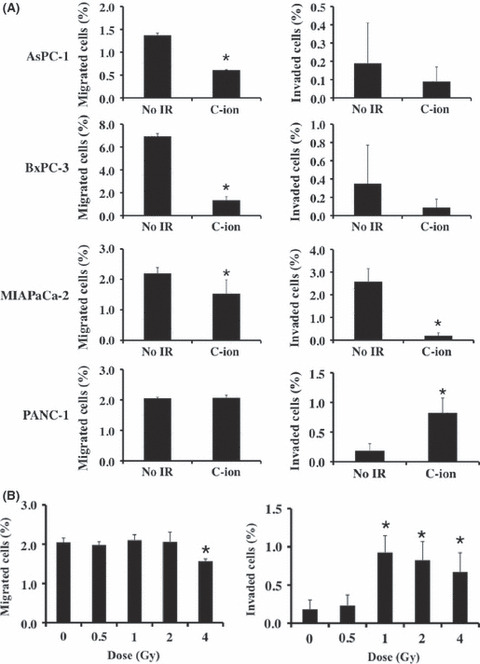
(A) Effects of carbon‐ion (C‐ion) irradiation (2 Gy) on the migration and invasiveness of AsPC‐1, BxPC‐3, MIAPaC‐2 and PANC‐1 cells. (B) Responses of the migration ability and invasiveness of PANC‐1 by radiation dose. The percentages of migrated cells (left panel) and invaded cells (right panel) are shown. Data are presented as mean ± SD of samples (n = 5–6). *P < 0.05 versus control. No_IR, no irradiation.
Effects of MMP2 on C‐ion‐induced invasiveness of PANC‐1 cells. A recent report showed that conventional photon irradiation with γ‐rays increases the invasiveness of PANC‐1 cells through the activation of MMP2.( 12 ) We have previously reported that photon irradiation with X‐rays caused a slight increase (1.75‐fold) in the invasiveness of PANC‐1 cells.( 13 ) In this study,( 13 ) we examined the role of MMP2 in the X‐ray‐induced increase in the invasiveness of PANC‐1 cells. We observed that the expression of the MMP2 protein was indeed enhanced in X‐ray‐irradiated PANC‐1 cells and that the resultant increase in invasiveness was suppressed by the MMPI (Fig. S2).
To clarify the involvement of MMP2 in the C‐ion‐induced PANC‐1 cell invasion, we first investigated the expressions of MMP2 protein in irradiated PANC‐1 cells. Unlike the case with X‐ray irradiation, protein expression of pro‐MMP2 in the conditioned medium of PANC‐1 cells exposed to C‐ion irradiation remained the same as that in the medium of non‐irradiated cells, and no signals corresponding to the molecular weight of active MMP2 were detected (Fig. 2A). In addition to MMP2, other MMP were also significant in cell invasion,( 10 ) thus we then investigated the effect of MMP on the invasiveness of C‐ion‐irradiated PANC‐1 cells by using MMPI; GM6001, which inhibits the activity of MMP1 (Ki = 400 pM), MMP2 (Ki = 500 pM), MMP3 (Ki = 27 nM), MMP8 (Ki = 100 pM) and MMP9 (Ki = 200 pM). MMPI did not attenuate the C‐ion‐induced invasiveness of PANC‐1 cells, as shown in Figure 2(B).
Figure 2.
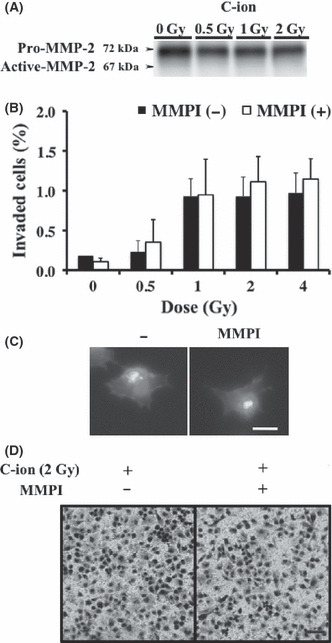
Effects of MMP inhibitors (MMPI) on the invasiveness of carbon‐ion (C‐ion) irradiated PANC‐1 cells. (A) The expression of MMP2 protein in irradiated PANC‐1. (B) Invasion assay was carried out with addition of MMPI (GM6001, 25 μM). Data represent the mean ± SD of samples (n = 5). (C) Morphology of PANC‐1 after incubation for 5 h with GM6001, 25 μM. Bar, 10 μm. (D) Morphology of invaded PANC‐1 cells with or without pretreatment of MMPI (GM6001, 25 μM) are shown. Bar, 200 μm.
We have previously shown that the use of MMPI or ROCKI alone failed to reduce, or even enhance, the invasiveness of MIAPaCa‐2 cells, because MIAPaCa‐2 cells might have mesenchymal or amoeboid motility and can also undergo mesenchymal–amoeboid transition depending the environmental conditions; therefore, they can escape attack from therapeutic agents directed at controlling a single mode of tumor cell motility.( 13 ) PANC‐1 cells are not rounded, which suggests that these cells have mesenchymal‐type motility and that their invasiveness is dependent on proteolysis. Therefore, we examined whether MMPI treatment leads to changes in PANC‐1 morphology, conferring them with the ability for amoeboid motility and thereby causing them to avoid the effects of MMPI. Treatment of MMPI resulted in no morphological alterations being observed in PANC‐1 cells cultured on poly‐l‐lysine‐coated cover glass stained with phalloidine and Hoechst 33258 (Fig. 2C) or the invaded irradiated‐PANC‐1 cells reaching the undersurface of the transwell membrane (Fig. 2D). Thus, MMPI treatment did not result in any transition.
Effect of other proteases on C‐ion‐induced PANC‐1 cell invasion. Five human protease classes have been characterized by catalytic mechanism: aspartic, cysteine, serine, threonine and MMP. All these enzymes contribute to cellular invasion by facilitating the degradation and remodeling of the ECM.( 17 , 27 ) In order to investigate the involvement of proteases other than MMP in the C‐ion‐induced PANC‐1 cell invasion, we evaluated the effect of the protease inhibitors, aprotinin, bestatin, E‐64 and leupeptin, and pepstatin A for serine, aminopeptidase, cysteine and aspartic proteases, respectively, on the invasiveness of irradiated PANC‐1 cells. Suppression of the C‐ion‐induced invasiveness of PANC‐1 cells was not noted with the treatment of any of the inhibitors, except pepstatin A, which reduced the invasiveness slightly (Fig. 3A). Surprisingly, the treatment of cells with aprotinin or pepstatin A resulted in the formation of rounded cells among the invaded cells (Fig. 3B).
Figure 3.
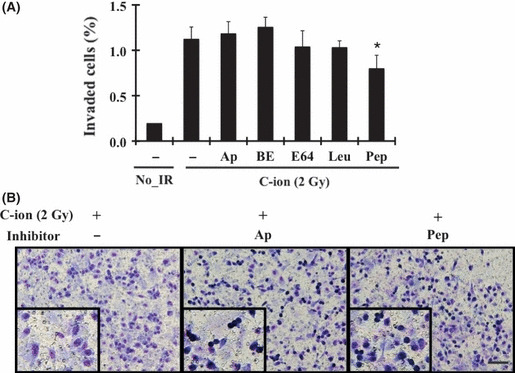
Effects of other proteases on carbon‐ion (C‐ion) irradiated PANC‐1 invasion at the dose of 2 Gy. (A) Invasion assay was carried out with the addition of protease inhibitors, aprotinin (Ap; 0.4 μM), bestatin (Be; 25 μM), E‐64 (E64; 7.5 μM), leupeptin (Leu; 10 μM) and pepstatin A (Pep; 5 μM). Data are represented as mean ± SD of samples (n = 6). *P < 0.05 versus control. (B) Invaded cells of irradiated PANC‐1 with or without pretreatment of aprotinin or pepstatin A are shown. Bar, 200 μm. No_IR, no irradiation.
SerP and aspartic proteases in C‐ion‐irradiated PANC‐1 cells. Treatment with aprotinin or pepstatin A alone resulted in an increase in the number of rounded cells within the invaded cells, which showed that these inhibitors induced the mesenchymal–amoeboid transition. Thus, serine or aspartic proteases might be involved in the induction of PANC‐1 cell invasion by C‐ion irradiation. In order to identify the key protease responsible for PANC‐1 cell invasion, we first examined the enzyme activities of SerP by using the Serine Protease Detect Zymo Electrophoresis kit, which could detect the protease activities of plasmin, pancreas elastase, chymotrypsin and trypsin. The band at Mr 91 kDa was only detected in C‐ion‐irradiated PANC‐1 cells (2 Gy), showing that the activation of plasmin was enhanced by C‐ion irradiation (Fig. 4A). Consistent with this finding, increased protein expression and its activity of uPA, which generates plasmin from plasminogen, was increased in irradiated cells, of which activation were reduced by the treatment of the uPA inhibitor, amiloride hydrochloride hydrate (Fig. 4B,C). The levels of plasminogen, however, were not altered by C‐ion irradiation (Fig. 4B). In addition to activating plasminogen, uPA has several other functions; it binds to the urokinase plasminogen activator receptor (uPAR) and initiates integrin‐mediated signals to alter cell adhesion, migration and invasion.( 28 , 29 ) Similarly, in the present study, the transcript expression of uPA and uPAR were also elevated in C‐ion‐irradiated PANC‐1 cells (Fig. S3).
Figure 4.
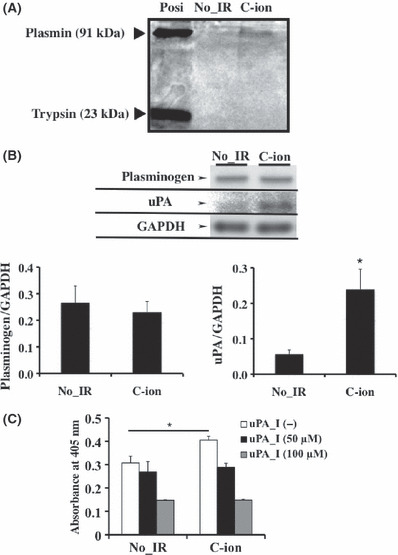
The key protease responsible for the carbon‐ion (C‐ion)‐irradiated PANC‐1 invasion at the dose of 2 Gy. (A) Enzyme activities of serine proteases (SerP) were determined. (B) Protein expression of plasminogen and urokinase‐type plasminogen activator (uPA) were determined by western blotting. Quantitative densitometric results of plasminogen and uPA were shown. These data represent the mean ± SD of samples (n = 5). *P < 0.05 versus control. (C) Enzyme activity of uPA was investigated. Amiloride hydrochloride hydrate was used for uPA inhibitor (uPA_I). Data represent the mean ± SD of samples (n = 3). *P < 0.05 versus control. No_IR, no irradiation.
In addition to SerP, aspartic proteases, such as cathepsin B and cathepsin D, are also known to be involved in cell invasion through the degradation of the ECM; these proteases are upregulated in several cancer types, including pancreatic cancer.( 30 ) It is also reported that cathepsins can activate pro‐uPA,( 31 ) and might thus be involved in the maintenance of cell invasiveness, possibly through uPA activation. The expression of cathepsin B and cathepsin D was detected in PANC‐1 cells, but the levels of these enzymes were not altered by C‐ion exposure (Fig. S4); this finding showed that pepstatin A treatment resulted in a reduction in the invasiveness induced by C‐ion‐irradiation, possibly through the inhibition of uPA activation.
Significance of SerP and aspartic proteases on the invasiveness of C‐ion‐irradiated PANC‐1 cells. To evaluate the significance of the activities of serine and aspartic proteases on C‐ion‐induced PANC‐1 cell invasion, we treated the cells with aprotinin plus ROCKI or pepstatin A plus ROCKI and examined whether the invasiveness could be suppressed by blocking the key factor involved in mesenchymal and amoeboid motility. Treatment with aprotinin plus ROCKI or pepstatin A plus ROCKI reduced the population of rounded cells within the invaded cells; that is, the mesenchymal–amoeboid transition was blocked by aprotinin plus ROCKI or pepstatin A plus ROCKI treatment (Fig. 5A). However, pepstatin A plus ROCKI treatment failed to suppress the invasiveness of irradiated PANC‐1, showing that aspartic proteases are not the key proteases for C‐ion‐induced PANC‐1 invasion. In contrast, aprotinin plus ROCKI effectively suppressed PANC‐1 cell invasion (Fig. 5B).
Figure 5.
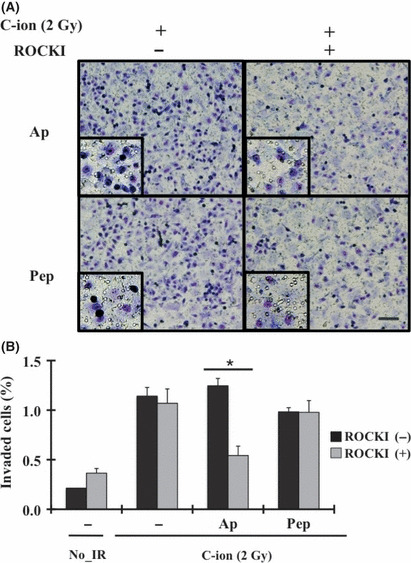
Effects of aprotinin or pepstatin A, and Rho‐associated coiled‐coil forming protein kinase inhibitor (ROCKI) on the invasiveness of carbon‐ion (C‐ion)‐irradiated PANC‐1 at the dose of 2 Gy. (A) Morphology of invaded PANC‐1 cells with inhibitors, aprotinin (Ap; 0.4 μM) or pepstatin A (Pep; 5 μM) with or without the addition of ROCKI (Y27632; 10 μM). Bar, 200 μm. (B) Invasion assay was carried out with the addition of ROCKI, or ROCKI plus aprotinin or pepstatin A. Data represent the mean ± SD of samples (n = 6). *P < 0.05 versus control. No_IR, no irradiation.
Discussion
An understanding of the effects of irradiation on the metastatic potential of different cell lines is important for improving the clinical outcome of cancer radiotherapy. In the present in vitro study, we found that C‐ion irradiation suppresses the invasive potential of cells of three pancreatic cancer lines, namely, MIAPaCa‐2, BxPC‐3 and AsPC‐1, but fails to reduce the invasiveness of PANC‐1 cells. In fact, C‐ion irradiation increased the invasiveness of PANC‐1 cells through the activation of SerP, plasmin and uPA. We observed that the use of SerPI alone resulted in the induction of a mesenchymal–amoeboid transition in PANC‐1 cells, whereby the cells can continue to invade adjacent tissues through protease‐independent mechanisms. Inhibition of both mesenchymal and amoeboid motility of tumor cells by the combined administration of SerPI and ROCKI was required to suppress the C‐ion‐induced PANC‐1 cell invasion.
Among SerP, plasmin is thought to facilitate cellular invasion by degrading several ECM components, such as fibronectin, laminin, vitronectin, type IV collagen, proteoglycans and fibrin, directly and/or through the activation of latent MMP.( 28 ) Thus, the activation of plasmin observed in the irradiated PANC‐1 cells might cause the degradation of the ECM. In addition to plasmin, uPA is also involved in cellular invasion.( 28 , 29 ) Significant overexpression of uPA has been reported in pancreatic adenocarcinoma and is thought to be involved in tumor progression.( 32 , 33 ) In addition to activating plasminogen, secreted uPA binds to uPAR in the extracellular space and induces integrin‐dependent activation of the MAPK Erk1 and Erk2, which in turn stimulates tumor cell migration and invasion through myosin light‐chain kinase (MLCK) phosphorylation. (34) In the present study, we found that the expressions of both uPA and uPAR transcripts were elevated in irradiated PANC‐1 cells. The increased uPA activity found in irradiated PANC‐1 cells might be involved not only in the generation of plasmin, but also integrin‐associated stimulation of tumor cell invasiveness. In fact, preliminary studies showed that the inhibition of MLCK activity by using the MLCK inhibitor, ML‐7, resulted in a 57% reduction in the invasiveness of C‐ion‐irradiated PANC‐1 cells (data not shown).
The transcription factors, Ap1 and nuclear factor of kappa light polypeptide gene enhancer B cell (NF‐κB), are involved in the transcription of the uPA and uPAR genes.( 35 ) Recent evidence has shown that ionizing radiation causes the activation of the JNK signaling pathway, which phosphorylates the nuclear protein c‐Jun – a major component of the transcription factor, AP1, in lymphoblastoid cells and in normal fibroblasts.( 36 , 37 ) In addition, particle irradiation induces NF‐κB activation in the radioresistant cells through the degradation of inhibitor of NF‐κB (IκB) by its phosphorylation. (38) In our preliminary studies based on DNA microarray analysis, we found that the expression of neither AP1 nor NF‐κB was altered in irradiated PANC‐1 cells (data not shown). The mechanism underlying the induction of uPA and uPAR expression in C‐ion‐irradiated PANC‐1 cells remains unclear and requires further analysis.
MMP2 is a key factor involved in X‐ray‐induced MIAPaCa‐2 cell invasion.( 13 ) In the present study, we confirmed that MMP2 is also an important mediator of PANC‐1 cell invasion induced by X‐ray irradiation. In contrast, SerP, such as uPA and uPAR, rather than MMP2, appear to be the key factors involved in C‐ion‐induced PANC‐1 cell invasion. Notably, the expressions of uPA and uPAR were reduced in C‐ion‐irradiated MIAPaCa‐2 cells (data not shown); however, we have not yet determined whether these reductions played any role in the C‐ion‐induced suppression of MIAPaCa‐2 cell invasion. It is still unclear how C‐ion irradiation generates such cell‐specific effects, and further investigations, such as research on the genetic background of target tumors,( 39 , 40 ) will be required to improve the clinical outcome with C‐ion irradiation.
In conclusion, this is the first study showing that C‐ion irradiation enhances invasive potential in a pancreatic cancer cell line, PANC‐1 in vitro, through the induction of plasmin and uPA activity; this increase in invasiveness of PANC‐1 cells was reduced by combined treatment with inhibitors of SerP and ROCK. We have not yet examined the effects of C‐ion irradiation‐induced invasiveness of PANC‐1 cells on the metastatic potential in xenografts, and further in vivo studies are required to clarify this.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. An outline of the experimental procedures.
Fig. S2. The role of MMP2 activity in X‐ray‐induced PANC‐1 cells invasiveness.
Fig. S3. The expression of urokinase‐type plasminogen activator and urokinase plasminogen activator receptor transcripts in carbon‐ion‐irradiated PANC‐1 cells.
Fig. S4. The expression of cathepsin transcripts in carbon‐ion‐irradiated PANC‐1 cells.
Table S1. Primer sequences and probe numbers.
Table S2. Percentages of viable cells used for migration and invasion assays for Figure 2.
Table S3. Percentages of viable cells used for migration and invasion assays for Figure 3.
Table S4. Percentages of viable cells used for migration and invasion assays for Figure 5.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
This work was supported in part by a Grant‐in‐Aid for Scientific Research (C) (grant no. 22591394 to TI) and for Young Scientists (B) (grant no. 23791467 for MF) from the Japan Society for the Promotion of Science. We thank Drs YM, KI, TS and FN for helpful discussions and advice.
References
- 1. Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol 2009; 6: 412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lau WY, Lai EC. Development and controversies of adjuvant therapy for pancreatic cancer. Hepatobiliary Pancreat Dis Int 2008; 7: 121–5. [PubMed] [Google Scholar]
- 3. Krishnan S, Rena V, Janjan NA et al. Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer 2006; 107: 2589–96. [DOI] [PubMed] [Google Scholar]
- 4. Okada T, Kamada T, Tsuji H et al. Carbon ion radiotherapy: clinical experiences at National Institute of Radiological Science (NIRS). J Radiat Res 2010; 51: 355–64. [DOI] [PubMed] [Google Scholar]
- 5. Fokas E, Kraft G, An H, Engenhart‐Cabillic R. Ion beam radiobiology and cancer: time to update ourselves. Biochim Biophys Acta 2009; 1796: 216–29. [DOI] [PubMed] [Google Scholar]
- 6. Schulz‐Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol 2007; 25: 953–64. [DOI] [PubMed] [Google Scholar]
- 7. Tobias CA, Blakely EA, Alpen EL et al. Molecular and cellular radiobiology of heavy ions. Int J Radiat Oncol Biol Phys 1982; 8: 2109–20. [DOI] [PubMed] [Google Scholar]
- 8. Matsui Y, Asano T, Kenmochi T, Iwakawa M, Imai T, Ochiai T. Effects of carbon‐ion beams on human pancreatic cancer cell lines that differ in genetic status. Am J Clin Oncol 2004; 27: 24–8. [DOI] [PubMed] [Google Scholar]
- 9. Hamada N, Imaoka T, Masunaga S et al. Recent advances in the biology of heavy‐ion cancer therapy. J Radiat Res 2010; 51: 365–83. [DOI] [PubMed] [Google Scholar]
- 10. Kleg S, Buchler P, Ludwig R, Buchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer 2003; 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wild‐Bode C, Weller M, Rimber A, Dichigans J, Wick W. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 2001; 61: 2744–50. [PubMed] [Google Scholar]
- 12. Qian LW, Mizumoto K, Urashima T et al. Radiation‐induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin Cancer Res 2002; 8: 1223–7. [PubMed] [Google Scholar]
- 13. Fujita M, Otsuka Y, Yamada S, Iwakawa M, Imai T. X‐ray irradiation and Rho‐kinase inhibitor additively induce invasiveness of the cells of the pancreatic cancer line, MIAPaCa‐2, which exhibits mesenchymal and amoeboid motility. Cancer Sci 2011; 102: 792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogata T, Teshima T, Kagawa K et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res 2005; 65: 113–20. [PubMed] [Google Scholar]
- 15. Akino Y, Teshima T, Kihara A et al. Carbon‐ion beam irradiation effectively suppresses migration and invasion of human non‐small‐cell lung cancer cells. Int J Radiat Oncol Biol Phys 2009; 75: 475–81. [DOI] [PubMed] [Google Scholar]
- 16. Goetze K, Scholz M, Taucher‐Scholz G, Mueller‐Klieser W. The impact of conventional and heavy ion irradiation on tumor cell migration in vitro. Int J Radiat Biol 2007; 83: 889–96. [DOI] [PubMed] [Google Scholar]
- 17. Wolf K, Mazo I, Leung H et al. Compensation mechanism in tumor cell migration: mesenchymal‐amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 2003; 160: 267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanz‐Morero V, Gadea G, Ahn J et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell 2008; 135: 510–23. [DOI] [PubMed] [Google Scholar]
- 19. Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol 2008; 10: 127–37. [DOI] [PubMed] [Google Scholar]
- 20. Yamazaki D, Kurisu S, Takenawa T. Involvement of Rac and Rho signaling in cancer cell motility in 3D substrates. Oncogene 2009; 28: 1570–83. [DOI] [PubMed] [Google Scholar]
- 21. Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev 2009; 28: 65–76. [DOI] [PubMed] [Google Scholar]
- 22. Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol 2010; 188: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imadome K, Iwakawa M, Nojiri K et al. Upregulation of stress‐response genes with cell cycle arrest induced by carbon ion irradiation in multiple murine tumors models. Cancer Biol Ther 2008; 7: 208–17. [DOI] [PubMed] [Google Scholar]
- 24. Albini AY, Iwamoto H, Kleinman KG et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987; 47: 3239–45. [PubMed] [Google Scholar]
- 25. Kaneshiro ES, Wyder MA, Wu YP, Cushion MT. Reliability of calcein acetoxy methyl ester and ethidium homodimer or propidium iodide for viability assessment of microbes. J Microbiol Methods 1993; 17: 1–16. [Google Scholar]
- 26. Deshet N, Lupu‐Meiri M, Espinoza I et al. Plasminogen‐induced aggregation of PANC‐1 cells requires conversion to plasmin and is inhibited by endogenous plasminogen activator inhibitor‐1. J Cell Physiol 2008; 216: 632–9. [DOI] [PubMed] [Google Scholar]
- 27. Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol 2011; 21: 228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 2002; 3: 932–43. [DOI] [PubMed] [Google Scholar]
- 29. Ossowski L, Aguirre‐Ghiso JA. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Curr Opin Cell Biol 2000; 12: 613–20. [DOI] [PubMed] [Google Scholar]
- 30. Skrzydlewska E, Sulkowsa M, Koda M, Sulkowski S. Proteolytic‐antiproteolytic balance and its regulation in carcinogenesis. World J Gastroenterol 2005; 11: 1251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whiteman HJ, Weeks ME, Dowen SE et al. The role of S100P in the invasion of pancreatic cancer cells is mediated through cytoskeletal changes and regulation of cathepsin D. Cancer Res 2011; 67: 8633–42. [DOI] [PubMed] [Google Scholar]
- 32. Cantero D, Friess H, Deflorin J et al. Enhanced expression of urokinase plasminogen activator and its receptor in pancreatic carcinoma. Br J Cancer 1997; 75: 388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nielsen A, Scarlett CJ, Samra JS et al. Significant overexpression of urokinase‐type plasminogen activator in pancreatic adenocarcinoma using real‐time quantitative reverse transcription polymerase chain reaction. J Gastroenterol Hepatol 2005; 20: 256–63. [DOI] [PubMed] [Google Scholar]
- 34. Nguyen DH, Catling AD, Webb DJ et al. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of the urokinase‐type plasminogen activator‐stimulated cells in an integrin‐selective manner. J Cell Biol 1999; 146: 149–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ulisse S, Baldini E, Sorrenti S, D’Armiento M. The urokinase plasminogen activator system: a target for anti‐cancer therapy. Curr Cancer Drug Targets 2009; 9: 32–71. [DOI] [PubMed] [Google Scholar]
- 36. Shafman TD, Saleem A, Kyriakis J, Weichselbaum R, Kharbanda S, Kufe DW. Defective induction of stress‐activated protein kinase activity in ataxia‐telangiectasia cells exposed to ionizing radiation. Cancer Res 1995; 55: 3242–5. [PubMed] [Google Scholar]
- 37. Lee SA, Dritschilo A, Jung M. Impaired ionizing radiation‐induced activation of a nuclear signal essential for phosphorylation of c‐Jun by dually phosphorylated c‐Jun amino‐terminal kinases in ataxia telangiectasia fibroblasts. J Biol Chem 1998; 273: 32889–94. [DOI] [PubMed] [Google Scholar]
- 38. Ahmed KM, Li JJ. NF‐kappa B‐mediated adaptive resistance to ionizing radiation. Free Radic Biol Med 2008; 44: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pakneshan P, Szyf M, Farias‐Eisner R, Rabbani SA. Reversal of the hypomethylation status of urokinase (uPA) promoter blocks breast cancer growth and metastasis. J Biol Chem 2004; 279: 31735–44. [DOI] [PubMed] [Google Scholar]
- 40. Pulukuri SM, Estes N, Patel J, Rao JS. Demethylation‐linked activation of urokinase plasminogen activator is involved in progression of prostate cancer. Cancer Res 2007; 67: 930–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. An outline of the experimental procedures.
Fig. S2. The role of MMP2 activity in X‐ray‐induced PANC‐1 cells invasiveness.
Fig. S3. The expression of urokinase‐type plasminogen activator and urokinase plasminogen activator receptor transcripts in carbon‐ion‐irradiated PANC‐1 cells.
Fig. S4. The expression of cathepsin transcripts in carbon‐ion‐irradiated PANC‐1 cells.
Table S1. Primer sequences and probe numbers.
Table S2. Percentages of viable cells used for migration and invasion assays for Figure 2.
Table S3. Percentages of viable cells used for migration and invasion assays for Figure 3.
Table S4. Percentages of viable cells used for migration and invasion assays for Figure 5.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


