Abstract
Coronavirus disease 2019 (COVID-19) currently has few effective treatments. Given the uncertainty surrounding the effectiveness and uptake of a vaccine, it is important that the search for treatments continue. An exaggerated inflammatory state is likely responsible for much of the morbidity and mortality in COVID-19. Elevated levels of tumor necrosis factor (TNF), a key pro-inflammatory cytokine, have been shown to be associated with increased COVID-19 mortality. In patients with rheumatoid arthritis, TNF blockade reduces not only biologically active TNF but other pro-inflammatory cytokines important in COVID-19 hyperinflammation. Observational data from patients already on anti-TNF therapy show a reduced rate of COVID-19 poor outcomes and death compared with other immune-suppressing therapies. Anti-TNF has a long history of safe use, including in special at-risk populations, and is widely available. The case to adequately assess anti-TNF as a treatment for COVID-19 is compelling.
Keywords: coronavirus disease-2019, SARS-CoV-2, pandemic, tumor necrosis factor, glucocorticoids
Robinson et al. discuss the potential of TNF blockade in the management of COVID-19. The rationale for the therapeutic benefit of anti-TNF is outlined, including the pathogenic role of TNF in COVID-19, the mechanism of action of anti-TNF, animal data, and supportive observational data from those on anti-TNF who develop COVID-19.
Introduction
The novel coronavirus pandemic currently torments world society and will do so until we have effective vaccines and/or treatments. To date public health measures have been the most effective method of controlling the pandemic. However, these measures have proved difficult to institute and are proving even more difficult to sustain. Effective vaccines may take years to deliver to large populations, meaning that we will likely see ongoing coronavirus disease 2019 (COVID-19) infections for many years. Although the majority of patients recover from infection without evident consequence, many die or suffer long-term disability. Consequently, there is an urgent need to find effective treatments that reduce mortality and limit COVID-19-related damage.
Excess inflammation is emerging as a key driver of poor COVID-19 outcomes, including acute respiratory distress syndrome (ARDS), thrombosis, respiratory disease, neurological complications, and fatigue. Although the exact mechanism behind this excess inflammation is not fully understood, it appears to closely mirror other inflammatory disease processes about which far more is known. Therapeutic immunomodulation has proved to be very effective in the management of inflammatory diseases. Given the similarities between these diseases and COVID-19, therapeutic immunomodulation may also be effective in COVID-19.
There is an accelerated global effort to develop specific anti-SARS-CoV-2 antiviral drugs. However, drug development is typically a lengthy process because of the need to prove both clinical efficacy and safety. The same is true for vaccine development, particularly as any safety concerns will limit vaccine uptake. The lengthy timelines for drug and vaccine development make the repurposing of current therapeutics an extremely attractive option for the management of COVID-19. Key therapeutic agents that need to be evaluated are current immunomodulatory agents with proven efficacy in suppressing excess inflammation.1 The best therapeutic agents for mass implementation are those with well-understood safety profiles, including evaluation in diverse patient populations.2
Current immunomodulatory agents that have already been tested include therapies targeting the interleukin (IL)-6 pathway and dexamethasone. Agents targeting the IL-6 pathway were selected for clinical trials early given prima facie plausibility based on assumed biology, observed high circulating concentrations, and observational data. Disappointingly, multiple clinical trials of IL-6 blockade have largely shown limited benefit.3, 4, 5, 6, 7 Dexamethasone is effective only in critical cases in intensive care or in patients requiring oxygen. In addition, dexamethasone use in patients with less severe disease appears to worsen outcomes, meaning that patient selection is critical.8 There is thus an urgent need for therapeutics with greater effectiveness in COVID-19. The ideal agent is one that could be administered in the earlier stages of COVID-19 and could be given to high-risk subjects in an outpatient setting, such as a care home.
One immunomodulatory approach that holds great promise as a treatment for COVID-19 is tumor necrosis factor (TNF) inhibition.9 , 10 TNF is a pro-inflammatory cytokine that is intimately involved in excess inflammation. Levels of TNF are high in COVID-19, and early levels predict mortality.11 Observational data show that patients on anti-TNF for other indications do well following COVID-19 infection and in fact may fare better than those not on anti-TNF.12 , 13 Anti-TNF has been prescribed for more than 20 years to millions of individuals for many different indications and has a known safety profile and dose optimization in individual patients. As best-selling drugs, anti-TNF agents are widely available, with an effective supply chain. They are also largely off patent, with many more affordable biosimilar versions available at scale. From the perspective of plausibility and practicality, anti-TNF therapies are excellent candidates to repurpose for the treatment of COVID-19 hyperinflammation. In this review, we explore the role of TNF in COVID-19 pathophysiology as well as the effect of its inhibition in analogous models. We describe the potential of anti-TNF for modulating COVID-19-related damage and death and how that power might best be harnessed in practice.
COVID-19 Pathophysiology and the Role of TNF
SARS-CoV-2 binds to the cell surface receptor of ACE-2 via a trimeric virus spike glycoprotein that promotes entry into cells.14 The virus then replicates and disseminates throughout the body, infecting the many types of cells expressing ACE-2 and other viral receptors. As part of the usual response to infection, SARS-CoV-2 activates both the innate and adaptive immune responses. For most people infected with SARS-CoV-2, these immune responses clear the virus with only mild to moderate symptoms. Severe disease is associated with a hyperinflammatory response characterized by elevated circulating cytokine concentrations (a true cytokine “storm,” however, is uncommon). Age, obesity, diabetes, heart disease, and kidney disease are associated with increased risk for severe disease, although the precise mechanism behind this enhanced risk is not yet fully understood.15
Hyperinflammation and Cytokines in COVID-19
In post-mortem studies of patients with COVID-19, the predominant feature is diffuse alveolar damage with evidence of virus presence in the pneumocytes and an inflammatory infiltrate of macrophages in the alveolar lumen and lymphocytes in the interstitium.16 , 17 Abundant macrophages and monocytes play a key role in hyperinflammation in COVID-19 and contribute to the elevated pro-inflammatory cytokines reported.16 Although the exact mechanism of hyperinflammation is uncertain, there are many ongoing studies attempting to clarify the mechanisms by which the beneficial immune response is subverted in severe cases.18, 19, 20, 21 Multiple studies have shown that elevated cytokine levels are associated with poor outcome in patients with COVID-19. This was first extensively documented among 1,484 patients admitted to Mt. Sinai Hospital during the first wave of COVID-19, with high serum IL-6, IL-8, and TNF concentrations at the time of admission predicting poor outcomes.11 Levels of IL-1β did not predict outcomes and were relatively low.11 , 22 Patients who died of COVID-19 were found to have significantly higher levels of IL-2 receptor, IL-6, IL-8, IL-10, and TNF-α compared with patients who recovered.23 Similar findings were seen when comparing intensive care unit (ICU) and non-ICU patients, with levels of multiple pro-inflammatory cytokines being higher in those admitted to ICUs, including IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (GCSF), and TNF.24
High levels of cytokines contribute to clinical deterioration in COVID-19, as their actions of promoting cell recruitment, activation, and capillary leak influence clinical manifestations. Capillary leak is a major component of deteriorating lung function in COVID-19, resulting in ARDS.25 Capillary leak results from inflammation driven by key inflammatory cytokines, TNF, IL-1, IL-6, IL-8, and especially vascular endothelial growth factor (VEGF), which in the past was also called “vascular permeability factor.”26 Another key feature of severe COVID-19 is venous and arterial thrombosis in up to 31% of patients admitted to ICUs.27 D-dimer levels are associated with worse clinical outcomes.28 The pathogenesis of COVID-19 coagulopathy is not fully understood but may result from vascular damage exposing tissue factor and/or excess pro-inflammatory cytokines, as in rheumatoid arthritis (RA).29 In a study using gene set enrichment analysis, IL-1, IL-6, TNF, signal transducer and activator of transcription 3 (STAT3), nuclear factor κB (NF-κB), and lipopolysaccharide were found to be the top regulators of thrombosis-associated markers in patients with severe to critical COVID-19.30
The Importance of Targeting TNF
The biology of TNF has features that make it a promising target for therapy in patients with COVID-19. Its role in inflammatory disease has been extensively studied for more than 20 years, and the effectiveness and relative safety of anti-TNF in many diseases have led to major changes in medical practice.31 TNF has an important role in the initiation of the inflammatory cascade and is elevated early in the disease process; on this basis, early intervention may be more beneficial than late intervention.32 It coordinates cell recruitment via regulation of chemokines and adhesion molecules.32 , 33 Anti-TNF therapies neutralize TNF and prevent it from exerting its pro-inflammatory effects. Highly relevant is that anti-TNF treatment also leads to a reduction in the production of other pro-inflammatory cytokines, including IL-1 and IL-6, both in animal models of sepsis and in patients with RA.34 , 35 Thus, blocking TNF in patients with COVID-19 might reduce many of these potentially pathogenic cytokines. Anti-TNF also reduces granulocyte-macrophage colony-stimulating factor (GM-CSF) and VEGF both in patients with RA and in cells, meaning that anti-TNF therapies could potentially reduce COVID-19-induced capillary leak in the lungs.36 , 37 Acute-phase proteins are also reduced after administration of anti-TNF, with downregulation of C-reactive protein (CRP), serum amyloid A, haptoglobin, and fibrinogen seen in patients with RA.34 , 36 In addition there is an effect on clotting, with significant reductions seen in D-dimer and pro-thrombin fragments within 1 h of anti-TNF administration.38 Therefore, anti-TNF may also reduce COVID-19-induced thrombosis.
Further evidence supporting the importance of specifically targeting TNF in COVID-19 comes from a recent study evaluating the effect of pro-inflammatory cytokines on cell death. In a very elegant set of experiments, Karki et al.39 used subsets of the cytokines reported to be elevated in patients with COVID-19 to determine that the unique combination of TNF and interferon (IFN)-γ synergistically induces cell death characterized by pyroptosis, apoptosis, and necroptosis, which has been termed “PANoptosis.” Combinations of other cytokines, including IL-6, do not induce cell death, and neither does TNF or IFN-gamma when administered individually. The damage induced by TNF and IFN-gamma is mediated via the IFN regulatory factor 1/signal transducer and activator of transcription 1 pathway and involves the upregulation of inducible nitric oxide synthetase and nitric oxide. Very importantly, the authors found that in a mouse model of SARS-CoV-2, administration of a combination of neutralizing antibodies to TNF and IFN-γ increases survival from ∼14% to 50%. The authors concluded that blockade of these cytokines together or individually has the potential to prevent severe COVID-19.
Another argument for why TNF is a promising cytokine to target comes from studies in SARS-CoV-1. In vitro studies have shown that attachment of the SARS-CoV-1 spike protein to the ACE-2 receptor leads to increased TNF-converting enzyme (TACE) activity.40 The interaction between the ACE-2 receptor and TACE then facilitates viral entry into the cell. As discussed above, TACE is the enzyme that releases TNF into the circulation. This mechanism suggests that TNF could be released into the circulation very early in the course of infection at the point of viral entry into the cell and would predispose to excess TNF production. These studies have not been replicated in SARS-CoV-2, but if the same mechanism exists, it provides a strong rationale for the early use of anti-TNF in the disease course.
NETosis in COVID-19
There is massive neutrophilia in COVID-19, with elevated blood levels of major neutrophil constituent proteins including S-100A8/9.41 Neutrophil extracellular trap (NET) release, or NETosis, is a mechanism whereby neutrophils release sticky extracellular traps that help limit the spread of infection.42 Levels of NETs are higher in patients with COVID-19 compared with healthy controls, and SARs-CoV-2 virus has been shown to directly promote the release of NETs from neutrophils.43 NETs are prothrombotic and activate platelets and the clotting cascade, leading to the production of thrombi.44 In COVID-19 autopsies, NET-containing microthrombi have been found in the lung.45 , 46 NETs have also been shown to induce pulmonary epithelial cell death, leading to alveolar damage and fibrosis in COVID-19 patients.43 These findings have led authors to suggest that agents targeting NETosis may be effective in COVID-19.43 , 46 Of relevance, anti-TNF therapy reduces NET formation in vitro as well as in murine models and patients with immune mediated disease.47, 48, 49
The Beneficial Impact of Anti-TNF on Other Viral Infections in Animals
TNF is involved in the defense against viruses, so a key question is whether anti-TNF interferes with antiviral immunity.50 A number of viral infections have been treated with anti-TNF, including SARS-CoV-1, respiratory syncytial virus (RSV), dengue, and influenza. In all of these infections, anti-TNF was beneficial, with no evidence of a reduction in antiviral immunity. In an animal model of SARS-CoV-1, anti-TNF therapy resulted in a substantial reduction in mortality (approximately 75% survival with anti-TNF versus approximately 10% survival without; see Figure 1 ).51 Anti-TNF reduced weight loss and reduced the severity of illness in mice with RSV and influenza infection; in addition there was no inhibition of CD8 killer cells, which are key to viral immune defense.52 With respect to RSV infection, both clearance of RSV and production of RSV-specific antibodies were unaffected by anti-TNF treatment. In RSV-affected animals, the degree of perivascular and peribronchiolar infiltrate was also significantly reduced. Using anti-TNF in a universally lethal mouse model of dengue virus infection reduced mortality from 100% to 40%.53 These data support the observational human data that using anti-TNF could well be a net benefit in the treatment of COVID-19.
Figure 1.
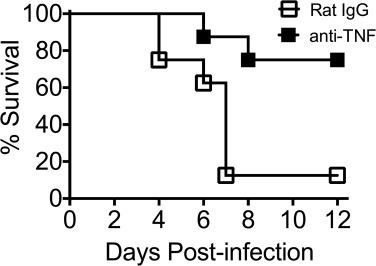
Survival Over 12 Days of BALB/c Mice Intranasally Infected with 3 × 104 Plaque-Forming Units of SARS-CoV and Then Given Either Anti-TNF or Rat IgG Isotype Control
From Channappanavar et al.51
Observational Data on the Impact of Anti-TNF on COVID-19
Observational human data, including small case series and larger databases, have shown possible benefit of anti-TNF therapy in patients with COVID-19. However, these data have clear limitations. Three large registries of individuals with immune-mediated inflammatory diseases have demonstrated an inverse association of anti-TNF use and COVID-19-related outcomes.12 , 54 , 55 The COVID-19 Global Rheumatology Alliance (GRA) registry collected information from clinicians on patients with rheumatic disease who were diagnosed with COVID-19.12 After controlling for important confounders, being on treatment with anti-TNF therapy for the underlying rheumatic disease was significantly associated with lower odds of hospitalization related to COVID-19, compared with being on no anti-TNF inhibitor therapy (adjusted odds ratio [OR] 0.40, 95% confidence interval [CI] 0.19–0.81). The PsoProtect registry included clinician-reported cases of individuals with psoriasis and COVID-19.54 Compared with biologic use, not using biologics was significantly associated with higher odds of hospitalization (OR 2.84, 95% CI 1.31–6.18), a finding that mirrored the GRA registry results. In the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, no significant association was seen with TNF inhibitor use (compared with no use) for the primary outcome of severe COVID-19, defined as the composite of ICU admission, ventilator use, and/or death (adjusted OR 0.9, 95% CI 0.4–2.2).55 However, anti-TNF use was inversely associated with the secondary outcome of hospitalization or death (adjusted OR 0.60, 95% CI 0.38–0.96), consistent with the other two registries. Other cohorts of patients with inflammatory bowel disease (IBD) on anti-TNF treatment doing favorably have also been reported.56 , 57 These reduced odds of poor outcome were not seen with other commonly prescribed treatments with different mechanisms of action. Additionally, a case series of 77 patients reported by North American infectious disease physicians described 16 patients using anti-TNF therapy prior to infection.58 In that series, no patients on anti-TNF required ventilator support or died, which was not the case with the remaining 61 patients on other cytokine-blocking biologics, JAK inhibitors, or other immunosuppressants.
The limitations of observational data need to be considered when translating these findings to clinical care. All three studies were based on registry data. There may be selection bias in terms of geographic variations in reporting and the possibility that more severe cases of COVID-19 were reported. Although these studies adjusted for important confounders, including known risk factors for COVID-19 outcomes such as age, sex, and comorbidities, unmeasured and residual confounding was potentially present. The underlying differences between individuals taking anti-TNF at baseline and those who were on alternative medications cannot be fully overcome by adjusting for measured confounders. There may be channeling bias (confounding by indication) whereby patients with certain characteristics that put them at higher risk for infection are not prescribed biologic medications.
Early feasibility data are also available for anti-TNF therapy. Stallmach et al.59 retrospectively studied seven patients with severe COVID-19 but no underlying immune-mediated inflammatory disease, who received a single dose of infliximab 5 mg/kg between 0 and 3 days after admission. They tracked a fall in pro-inflammatory cytokines over the first 10 days after receipt of infliximab. Only one patient of the seven died, noting that the median age was 60 years. Although it was an uncontrolled study, the authors used these data to cautiously promote further study of the therapeutic effects of anti-TNF therapy in patients with severe COVID-19.
Although observational data on anti-TNF use are suggestive of a therapeutic benefit in patients with COVID-19, randomized controlled trials (RCTs) are needed to confirm this benefit. Observational data must be interpreted with caution. Observational data for IL-6 inhibition and COVID-19 treatment were largely positive, with two meta-analyses suggesting mortality benefit.60 , 61 However, published RCTs for tocilizumab have largely not shown benefit.3, 4, 5, 6, 7 Although the observational data for anti-TNF may be used to help guide the development of further research and encourage the performance of RCTs, the results from RCTs are still needed.
Current Clinical Trials of Anti-TNF in COVID-19
There are a number of trials of anti-TNF in COVID-19 (see Table 1 ). These trials are using two off-patent anti-TNF agents, infliximab and adalimumab, and are mostly small.
Table 1.
Clinical Trials of Anti-TNF in COVID-19
| Trial | No. | Design | Intervention | Comparator | Patient Cohort | Cases/Controls | Status |
|---|---|---|---|---|---|---|---|
| CATALYST | ISRCTN40580903 | randomized controlled platform study | SOC + infliximab | SOC | admitted patients | 60 patients per intervention arm, 1:1 intervention/control | enrolling |
| ACTIV-1 | NCT04593940 | randomized controlled platform study | remdesivir + infliximab 5 mg/kg | SOC | admitted patients | 2,190 total across 3 intervention arms and 1 control arm | enrolling |
| Tufts | NCT04425538 | uncontrolled single arm | infliximab 5 mg/kg | Nil | admitted patients | 17:0 | unknown |
| Xu | ChiCTR2000030089 | randomized controlled | SOC + adalimumab | SOC | severe or critical illness | 30:30 | suspended |
| AVID-CC | ISRCTN33260034 | randomized controlled | SOC + adalimumab 80 or 160 mg | SOC | outpatients | 375:375 | pre-enrollment |
SOC, standard of care.
Infliximab
There is currently an actively recruiting trial of infliximab in hospitalized patients with COVID-19 in the United Kingdom as an arm of the CATALYST phase 2 proof-of-principle platform (ISRCTN40580903) (the other active treatment arm is namilumab, an anti-GM-CSF agent). Hospitalized adult patients (≥16 years of age) with a clinical picture strongly suggestive of SARS-CoV-2 pneumonia (confirmed by chest radiography or computed tomographic [CT] scan, with or without a positive reverse transcription polymerase chain reaction [RT-PCR] assay) and elevated CRP (>40 mg/L) are randomized to usual care plus a single dose of infliximab 5 mg/kg. Preliminary data are anticipated in the next few months. There is also a small (n = 17) uncontrolled study of infliximab 5 mg/kg in the recruitment phase at Tufts Medical Center in Boston for hospitalized patients with an oxygen requirement (NCT04425538).
As part of the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiative, part of the U.S. government’s Operation Warp Speed, a trial (ACTIV-1) has been announced of remdesivir in combination with either infliximab, abatacept (a T cell co-stimulatory blocker) or cenicriviroc (a CCR2-CCR5 dual antagonist). The trial is currently enrolling and is expected to enroll 2,190 patients (NCT04593940).
Adalimumab
A planned Chinese trial of adalimumab (30 adalimumab-treated patients and 30 controls) was scheduled to start recruiting on February 28, 2020 (ChiCTR2000030089), but was unable to recruit any patients because of the pandemic being controlled in Wuhan (Huji Xu, personal communication). A trial of adalimumab in the community setting (AVID-CC) to prevent progression from community infection to respiratory failure or death is in the pre-enrollment phase (ISRCTN33260034).62 In order to achieve therapeutic concentrations rapidly, the protocol uses a loading dose and is examining two dose levels (80 and 160 mg).
Future Trial Considerations
It is important to collect biological samples during clinical trials of anti-TNF in COVID-19 to enable detailed immune profiling of both the disease and the impact of therapy. It is very important to establish not only the pharmacology (e.g., pharmacokinetics) in these ill patients but also whether the effects of anti-TNF seen in prior studies in RA of reductions in IL-6 and VEGF do occur in patients with COVID-19. And importantly, it should be determined whether these effects differ between patients who respond and those who do not.
It is also important that careful consideration go into the timing of the intervention. Although corticosteroids have been shown to potentially be of harm when administered early in the COVID-19 disease course, the same may not be true for anti-TNF. In fact, anti-TNF therapy may be more effective when administered early, as by targeting the early production of TNF, the hyperinflammation seen in moderate to severe disease may be prevented. Certainly the evidence from studies of the immunology of the phases of illness points to the biggest change happening in the mild-to-moderate transition as opposed to the moderate-to-severe transition.21 The premise of community trials of anti-TNF therapy such as AVID-CC is that preventing the development of hyperinflammation may be more beneficial than treating it once it has evolved to the full phenotype that is often present on admission to the hospital.
Pharmacology of Anti-TNF Therapy
Given the considerations around intervention early in the disease course and the rapid progression of COVID-19, treatment should aim to achieve therapeutic concentrations quickly. For intravenous drugs such as infliximab, this is readily achieved. The usual clinical dose of 5 mg/kg rapidly reaches concentrations in considerable excess of circulating concentrations of TNF, although the associated tissue-concentration ratios are unknown, but excess is predicted. There is a risk for hypersensitivity reactions with infliximab; this and the requirement for intravenous administration make it a less suitable option for patients outside the hospital (Table 2 ). Subcutaneous administration is more straightforward outside the hospital but has a slower rise in plasma drug concentration. Use of a loading-dose regimen may address this issue and achieve therapeutic concentrations sufficiently rapidly.
Table 2.
Relevant Pharmacokinetic Properties of Selected Anti-TNF Agents, in Healthy Individuals (as per Registration Data)
| Anti-TNF Therapeutic Agent |
|||
|---|---|---|---|
| Etanercept | Adalimumab | Infliximab | |
| Route of administration | subcutaneous | subcutaneous | intravenous |
| Time to maximum concentration (days) | 2.9 ± 1.4 | 5.5 ± 2.3 | 0 |
| Optimal clinical scenario for application | new COVID-19 infection with risk for delayed hyperinflammation in 3–15 days | established hyperinflammation secondary to COVID-19 infection | |
| Half-life (days) | 4.3 ± 1.3 | 10–20 | 8.0–9.5 |
| Structure | fusion protein | human monoclonal antibody | chimeric monoclonal antibody, murine Fv, rest human |
| Risk for tuberculosis with long-term use | lesser increase | greater increase | |
COVID-19 causes end-organ damage, including kidney and liver damage, which may limit the extent to which some therapeutics can be used. Anti-TNF agents have well-established favorable pharmacological properties in these settings. Anti-TNF agents are cleared via the reticuloendothelial system and do not require dose modification in renal or hepatic failure. Although early immunogenicity, particularly the formation of anti-drug antibodies, can be problematic for some therapeutic monoclonal antibodies, anti-TNF agents do not demonstrate clinically relevant immunogenicity during time frames relevant to COVID-19 treatment. Certain high-risk patient populations in COVID-19 may have altered pharmacokinetic properties relevant to anti-TNF use. For example, obesity may shorten drug half-life through increased clearance and may necessitate more rapid repeat dosing. Anti-TNF therapy can be reassuringly and readily optimized outside of a clinical trial population without the need for further investigation because of its well-established pharmacological properties in varied populations.
Safety of Anti-TNF Therapy
General Infection and Anti-TNF Therapy in Patients with COVID-19
A concern with the use of anti-TNF in patients with COVID-19 is the potential for increased secondary infections given that TNF plays an important role in host defense. Reassuringly, the risk for infection with anti-TNF therapy has been low in clinical practice over the past 20 years.63, 64, 65 In addition, the risk for serious infection is cumulatively related to exposure, and given the short-term nature of any therapy in patients with COVID-19, the risk for infection is likely to be lower than that seen in patients on long-term anti-TNF therapy.66 Autoimmune disease registry data demonstrate that the early increased risk is equally spread over 6 months, further supporting the safety of very short term use.67 Furthermore, in these registry data, early risk is explained by a number of other factors not relevant to COVID-19 therapy: early dropout of high-risk patients leading to a subsequent healthy-user effect, time-dependent changes in autoimmune disease activity-related pathways relevant to infection risk, and higher use of concomitant chronic glucocorticoids (with attendant infection risk) early in autoimmune disease course.
In early trials using infliximab in the treatment of bacterial septic shock, mortality did not significantly improve; however, infective parameters also did not deteriorate.68 , 69 Subsequent observational data of patients receiving anti-TNF therapy for multiple autoimmune disease indications, particularly in COVID-19-infected populations, provide further reassurance.12 , 54 , 55 , 58
Finally, although some early clinical data suggested increased infection from high doses of infliximab, this has not been demonstrated in short courses of therapy. In particular, high-dose infliximab induction in IBD has not been demonstrated to confer increased rates of infection versus lower doses or placebo.70 This experience from autoimmune disease would suggest that high doses in short courses of therapy, as proposed in the AVID-CC trial (discussed above), are unlikely to incur any additional risk.
Tuberculosis and Anti-TNF Therapy in Patients with COVID-19
Latent tuberculosis (TB) is common in many regions where COVID-19 is problematic, such as the Indian subcontinent and South America. Similar to high-dose glucocorticoids, anti-TNF therapy can lead to destabilization of granulomas and subsequent reactivation of latent TB.71 , 72 Despite this, TB reactivation following anti-TNF therapy does not increase mortality, and there is widespread use of anti-TNF therapy in these regions.73 Importantly, the risk for latent TB reactivation increases over time, with only ∼10% of the reactivation risk of long-term infliximab therapy incurred in the first month. Of note, etanercept confers a markedly lower overall risk for TB reactivation compared with other anti-TNF agents and an even smaller proportion of its risk in the first month of use, probably because of its reduced capacity for granuloma destabilization.71 Hence, the risk for latent TB reactivation might be mitigated by preferential use of etanercept in countries with high TB prevalence, along with review of incidental pulmonary imaging and concomitant isoniazid therapy.
Anti-TNF Therapy for COVID-19 in At-Risk Populations
Anti-TNF therapy has been shown to be safe in many population groups with known increased risk for poor outcomes from COVID-19.74, 75, 76 Anti-TNF therapy is currently widely used in older populations for autoimmune disease, and there are data showing that it reduces cardiovascular risk.77 , 78 It is also demonstrably safe in pregnancy, with no association seen between maternal anti-TNF use and adverse pregnancy outcomes despite known active transport across the placenta for most anti-TNF agents.79 No developmental delay has been demonstrated in children born to mothers treated with anti-TNF agents, and breastfeeding is also considered safe.80 , 81 When used to treat complications of cancer immunotherapy, short-term anti-TNF therapy has not been associated with an increased rate of cancer progression.82 Anti-TNF agents can therefore be used with confidence in those patient groups most likely to benefit from their use.
Conclusion
COVID-19 represents the most significant medical problem of our time. Already there has been mass loss of life, health systems are overwhelmed, and economies have entered recession. Ongoing infections are inevitable even if a relatively effective vaccine is developed within the next year. Consequently, there is an urgent need for a highly effective treatment, ideally one with a demonstrated safety profile with an existing capacity for affordable mass production.
There is a compelling argument for adequately investigating anti-TNF therapy in the management of COVID-19. Observational data from multiple sources suggest that anti-TNF therapies confer morbidity and mortality benefit in those infected with COVID-19. In addition, anti-TNF therapies have a well-demonstrated ability to reduce inflammation and specifically reduce levels of pro-inflammatory cytokines associated with poor COVID-19 outcomes. More than 20 years of clinical experience provides reassurance of drug safety, particularly in patients with infection and those at high risk for clinical deterioration. A range of different anti-TNF therapies are currently available, many with differing pharmacokinetics that would enable clinicians to individualize treatment, particularly with reference to the timing of administration.
Adequately powered randomized clinical trials of anti-TNF administered at different time points in the disease course of COVID-19 are required. A small number of trials are in progress, but more are essential if we are to progress from interesting hypotheses to important therapeutic benefit.
Acknowledgments
No funding was received for this work.
Author Contributions
P.C.R., J.W.L., D.F.L.L., H.L.T., and S.S. wrote the first draft. All authors revised the manuscript for critical content and approved the final version for submission.
Declaration of Interests
P.C.R. has received personal fees from AbbVie, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Roche, and UCB; research grant funding from UCB, Janssen, Pfizer, and Novartis; and non-financial support from Bristol Myers Squibb (all unrelated to this work). D.R. has received personal fees for consultancy on drug safety from GlaxoSmithKline unrelated to the topic of this work. M.F. has held patents, now expired, on the use of infliximab and methotrexate in inflammatory arthritis and has received royalties (now ceased) from Johnson & Johnson, AbbVie, Amgen, and UCB, unrelated to this work.
References
- 1.Amigues I., Pearlman A.H., Patel A., Reid P., Robinson P.C., Sinha R., Kim A.H., Youngstein T., Jayatilleke A., Konig M.F. Coronavirus disease 2019: investigational therapies in the prevention and treatment of hyperinflammation. Expert Rev. Clin. Immunol. 2020 doi: 10.1080/1744666X.2021.1847084. Published online November 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monaco C., Nanchahal J., Taylor P., Feldmann M. Anti-TNF therapy: past, present and future. Int. Immunol. 2015;27:55–62. doi: 10.1093/intimm/dxu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M., BACC Bay Tocilizumab Trial Investigators Efficacy of tocilizumab in patients hospitalized with COVID-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2028836. Published online October 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermine O., Mariette X., Tharaux P.-L., Resche-Rigon M., Porcher R., Ravaud P., CORIMUNO-19 Collaborative Group Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.6820. Published online October 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., Bruzzi P., Boni F., Braglia L., Turrà C., RCT-TCZ-COVID-19 Study Group Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.6615. Published online October 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosas I., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S. Tocilizumab in hospitalized patients with COVID-19 pneumonia. medRxiv. 2020 doi: 10.1101/2020.08.27.20183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roche . 2020. Roche’s phase III EMPACTA study showed Actemra/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID-19 associated pneumonia Roche Pharmaceuticals.https://www.roche.com/media/releases/med-cor-2020-09-18.htm [Google Scholar]
- 8.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19—preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. Published online July 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson P.C., Richards D., Tanner H.L., Feldmann M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020;2:E653–E655. doi: 10.1016/S2665-9913(20)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., Richards D., Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianfrancesco M., Hyrich K.L., Al-Adely S., Carmona L., Danila M.I., Gossec L., Izadi Z., Jacobsohn L., Katz P., Lawson-Tovey S., COVID-19 Global Rheumatology Alliance Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianfrancesco M., Yazdany J., Robinson P.C. Epidemiology and outcomes of novel coronavirus 2019 in patients with immune-mediated inflammatory diseases. Curr. Opin. Rheumatol. 2020;32:434–440. doi: 10.1097/BOR.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 14.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U S A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H., Najafian B., Deutsch G., Lacy J.M., Williams T. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect. Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B., Yale IMPACT Team Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overmyer K.A., Shishkova E., Miller I.J., Balnis J., Bernstein M.N., Peters-Clarke T.M., Meyer J.G., Quan Q., Muehlbauer L.K., Trujillo E.A. Large-scale multi-omic analysis of COVID-19 severity. Cell Syst. 2020 doi: 10.1016/j.cels.2020.10.003. Published online October 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su Y., Chen D., Yuan D., Lausted C., Choi J., Dai C.L., Voillet V., Duvvuri V.R., Scherler K., Troisch P. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.10.037. Published online October 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann E.R., Menon M., Knight S.B., Konkel J.E., Jagger C., Shaw T.N., Krishnan S., Rattray M., Ustianowski A., Bakerly N.D., NIHR Respiratory TRC. CIRCO Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci. Immunol. 2020;5:eabd6197. doi: 10.1126/sciimmunol.abd6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dvorak H.F., Brown L.F., Detmar M., Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 27.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M. The unique characteristics of COVID-19 coagulopathy. Crit. Care. 2020;24:360. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aid M., Busman-Sahay K., Vidal S.J., Maliga Z., Bondoc S., Starke C., Terry M., Jacobson C.A., Wrijil L., Ducat S. Vascular disease and thrombosis in SARS-CoV-2-infected rhesus macaques. Cell. 2020;183:1354–1366.e13. doi: 10.1016/j.cell.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldmann M., Maini R.N. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat. Med. 2003;9:1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 32.Feldmann M., Brennan F.M., Maini R.N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 33.Feldmann M. Translating molecular insights in autoimmunity into effective therapy. Annu. Rev. Immunol. 2009;27:1–27. doi: 10.1146/annurev-immunol-082708-100732. [DOI] [PubMed] [Google Scholar]
- 34.Charles P., Elliott M.J., Davis D., Potter A., Kalden J.R., Antoni C., Breedveld F.C., Smolen J.S., Eberl G., deWoody K. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J. Immunol. 1999;163:1521–1528. [PubMed] [Google Scholar]
- 35.Fong Y., Tracey K.J., Moldawer L.L., Hesse D.G., Manogue K.B., Kenney J.S., Lee A.T., Kuo G.C., Allison A.C., Lowry S.F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J. Exp. Med. 1989;170:1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haworth C., Brennan F.M., Chantry D., Turner M., Maini R.N., Feldmann M. Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid arthritis: regulation by tumor necrosis factor-α. Eur. J. Immunol. 1991;21:2575–2579. doi: 10.1002/eji.1830211039. [DOI] [PubMed] [Google Scholar]
- 37.Paleolog E.M., Young S., Stark A.C., McCloskey R.V., Feldmann M., Maini R.N. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor α and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998;41:1258–1265. doi: 10.1002/1529-0131(199807)41:7<1258::AID-ART17>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Ingegnoli F., Fantini F., Favalli E.G., Soldi A., Griffini S., Galbiati V., Meroni P.L., Cugno M. Inflammatory and prothrombotic biomarkers in patients with rheumatoid arthritis: effects of tumor necrosis factor-alpha blockade. J. Autoimmun. 2008;31:175–179. doi: 10.1016/j.jaut.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.K.S. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2020 doi: 10.1016/j.cell.2020.11.025. Published online November 19, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc. Natl. Acad. Sci. U S A. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L., Long X., Xu Q., Tan J., Wang G., Cao Y., Wei J., Luo H., Zhu H., Huang L. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:992–994. doi: 10.1038/s41423-020-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 43.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetité D., Tavares L.A., Paiva I.M. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thålin C., Hisada Y., Lundström S., Mackman N., Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019;39:1724–1738. doi: 10.1161/ATVBAHA.119.312463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bösmüller H., Traxler S., Bitzer M., Häberle H., Raiser W., Nann D., Frauenfeld L., Vogelsberg A., Klingel K., Fend F. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Arch. 2020;477:349–357. doi: 10.1007/s00428-020-02881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Middleton E.A., He X.-Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz-Limon P., Ladehesa-Pineda M.L., Castro-Villegas M.D.C., Abalos-Aguilera M.D.C., Lopez-Medina C., Lopez-Pedrera C., Barbarroja N., Espejo-Peralbo D., Gonzalez-Reyes J.A., Villalba J.M. Enhanced NETosis generation in radiographic axial spondyloarthritis: utility as biomarker for disease activity and anti-TNF-α therapy effectiveness. J. Biomed. Sci. 2020;27:54. doi: 10.1186/s12929-020-00634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pérez-Sánchez C., Ruiz-Limón P., Aguirre M.A., Jiménez-Gómez Y., Arias-de la Rosa I., Ábalos-Aguilera M.C., Rodriguez-Ariza A., Castro-Villegas M.C., Ortega-Castro R., Segui P. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in Rheumatoid Arthritis patients. J. Autoimmun. 2017;82:31–40. doi: 10.1016/j.jaut.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Sudo M., Iida K., Tsutsui H., Mitani K., Jimbo M., Hatano E., Fujimoto J. Blockade of tumor necrosis factor by etanercept prevents postoperative adhesion formation in mice. Cell. Physiol. Biochem. 2020;54:1041–1053. doi: 10.33594/000000286. [DOI] [PubMed] [Google Scholar]
- 50.Seo S.H., Webster R.G. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J. Virol. 2002;76:1071–1076. doi: 10.1128/JVI.76.3.1071-1076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hussell T., Pennycook A., Openshaw P.J. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 53.Atrasheuskaya A., Petzelbauer P., Fredeking T.M., Ignatyev G. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol. Med. Microbiol. 2003;35:33–42. doi: 10.1111/j.1574-695X.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 54.Mahil S.K., Dand N., Mason K.J., Yiu Z.Z.N., Tsakok T., Meynell F., Coker B., McAteer H., Moorhead L., Mackenzie T., PsoProtect study group Factors associated with adverse COVID-19 outcomes in patients with psoriasis-insights from a global registry-based study. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.10.007. Published online October 16, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brenner E.J., Ungaro R.C., Gearry R.B., Kaplan G.G., Kissous-Hunt M., Lewis J.D., Ng S.C., Rahier J.-F., Reinisch W., Ruemmele F.M. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodríguez-Lago I., Ramírez de la Piscina P., Elorza A., Merino O., Ortiz de Zárate J., Cabriada J.L. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque country (Spain) Gastroenterology. 2020;159:781–783. doi: 10.1053/j.gastro.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waggershauser C.H., Tillack-Schreiber C., Berchtold-Benchieb C., Szokodi D., Howaldt S., Ochsenkühn T. Letter: immunotherapy in IBD patients in a SARS-CoV-2 endemic area. Aliment. Pharmacol. Ther. 2020;52:898–899. doi: 10.1111/apt.15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winthrop K.L., Brunton A.E., Beekmann S., Polgreen P., Baddley J., Saag K.G., Calabrese C., Calabrese L., Robinson P.C., Wallace Z.S., Curtis J.R., COVID-19 Study Team SARS CoV-2 infection among patients using immunomodulatory therapies. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218580. Published online August 5, 2020. [DOI] [PubMed] [Google Scholar]
- 59.Stallmach A., Kortgen A., Gonnert F., Coldewey S.M., Reuken P., Bauer M. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure-a cautionary case series. Crit. Care. 2020;24:444. doi: 10.1186/s13054-020-03158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aziz M., Fatima R., Assaly R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25948. Published online April 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malgie J., Schoones J.W., Pijls B.G. Decreased mortality in COVID-19 patients treated with Tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1445. Published online September 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahase E. Covid-19: Anti-TNF drug adalimumab to be trialled for patients in the community. BMJ. 2020;371:m3847. doi: 10.1136/bmj.m3847. [DOI] [PubMed] [Google Scholar]
- 63.Grijalva C.G., Chen L., Delzell E., Baddley J.W., Beukelman T., Winthrop K.L., Griffin M.R., Herrinton L.J., Liu L., Ouellet-Hellstrom R. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306:2331–2339. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh J.A., Wells G.A., Christensen R., Tanjong Ghogomu E., Maxwell L., Macdonald J.K., Filippini G., Skoetz N., Francis D., Lopes L.C. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst. Rev. 2011;(2):CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rutherford A.I., Subesinghe S., Hyrich K.L., Galloway J.B. Serious infection across biologic-treated patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 2018;77:905–910. doi: 10.1136/annrheumdis-2017-212825. [DOI] [PubMed] [Google Scholar]
- 66.Li X., Andersen K.M., Chang H.-Y., Curtis J.R., Alexander G.C. Comparative risk of serious infections among real-world users of biologics for psoriasis or psoriatic arthritis. Ann. Rheum. Dis. 2020;79:285–291. doi: 10.1136/annrheumdis-2019-216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galloway J.B., Hyrich K.L., Mercer L.K., Dixon W.G., Fu B., Ustianowski A.P., Watson K.D., Lunt M., Symmons D.P.M., BSRBR Control Centre Consortium. British Society for Rheumatology Biologics Register Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011;50:124–131. doi: 10.1093/rheumatology/keq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abraham E., Wunderink R., Silverman H., Perl T.M., Nasraway S., Levy H., Bone R., Wenzel R.P., Balk R., Allred R. Efficacy and safety of monoclonal antibody to human tumor necrosis factor α in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-α MAb Sepsis Study Group. JAMA. 1995;273:934–941. [PubMed] [Google Scholar]
- 69.Cohen J., Carlet J., International Sepsis Trial Study Group INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. Crit. Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Rutgeerts P., Sandborn W.J., Feagan B.G., Reinisch W., Olson A., Johanns J., Travers S., Rachmilewitz D., Hanauer S.B., Lichtenstein G.R. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 71.Mezouar S., Diarra I., Roudier J., Desnues B., Mege J.-L. Tumor necrosis factor-alpha antagonist interferes with the formation of granulomatous multinucleated giant cells: new insights into Mycobacterium tuberculosis infection. Front. Immunol. 2019;10:1947. doi: 10.3389/fimmu.2019.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallis R.S., Broder M.S., Wong J.Y., Hanson M.E., Beenhouwer D.O. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin. Infect. Dis. 2004;38:1261–1265. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 73.Sartori N.S., Picon P., Papke A., Neyeloff J.L., da Silva Chakr R.M. A population-based study of tuberculosis incidence among rheumatic disease patients under anti-TNF treatment. PLoS ONE. 2019;14:e0224963. doi: 10.1371/journal.pone.0224963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawashima H., Kagami S.-I., Kashiwakuma D., Takahashi K., Yokota M., Furuta S., Iwamoto I. Long-term use of biologic agents does not increase the risk of serious infections in elderly patients with rheumatoid arthritis. Rheumatol. Int. 2017;37:369–376. doi: 10.1007/s00296-016-3631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sumida K., Molnar M.Z., Potukuchi P.K., Hassan F., Thomas F., Yamagata K., Kalantar-Zadeh K., Kovesdy C.P. Treatment of rheumatoid arthritis with biologic agents lowers the risk of incident chronic kidney disease. Kidney Int. 2018;93:1207–1216. doi: 10.1016/j.kint.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim H.W., Lee C.-K., Cha H.-S., Choe J.-Y., Park E.-J., Kim J. Effect of anti-tumor necrosis factor alpha treatment of rheumatoid arthritis and chronic kidney disease. Rheumatol. Int. 2015;35:727–734. doi: 10.1007/s00296-014-3146-4. [DOI] [PubMed] [Google Scholar]
- 77.Nurmohamed M., Bao Y., Signorovitch J., Trahey A., Mulani P., Furst D.E. Longer durations of antitumour necrosis factor treatment are associated with reduced risk of cardiovascular events in patients with rheumatoid arthritis. RMD Open. 2015;1:e000080. doi: 10.1136/rmdopen-2015-000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greenberg J.D., Kremer J.M., Curtis J.R., Hochberg M.C., Reed G., Tsao P., Farkouh M.E., Nasir A., Setoguchi S., Solomon D.H., CORRONA Investigators Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann. Rheum. Dis. 2011;70:576–582. doi: 10.1136/ard.2010.129916. [DOI] [PubMed] [Google Scholar]
- 79.Komaki F., Komaki Y., Micic D., Ido A., Sakuraba A. Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-α use in females with immune mediated diseases; a systematic review and meta-analysis. J. Autoimmun. 2017;76:38–52. doi: 10.1016/j.jaut.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 80.Clowse M.E.B., Förger F., Hwang C., Thorp J., Dolhain R.J., van Tubergen A., Shaughnessy L., Simpson J., Teil M., Toublanc N. Minimal to no transfer of certolizumab pegol into breast milk: results from CRADLE, a prospective, postmarketing, multicentre, pharmacokinetic study. Ann. Rheum. Dis. 2017;76:1890–1896. doi: 10.1136/annrheumdis-2017-211384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mariette X., Förger F., Abraham B., Flynn A.D., Moltó A., Flipo R.-M., van Tubergen A., Shaughnessy L., Simpson J., Teil M. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann. Rheum. Dis. 2018;77:228–233. doi: 10.1136/annrheumdis-2017-212196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Esfahani K., Elkrief A., Calabrese C., Lapointe R., Hudson M., Routy B., Miller W.H., Jr., Calabrese L. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 2020;17:504–515. doi: 10.1038/s41571-020-0352-8. [DOI] [PubMed] [Google Scholar]


