Abstract
This purpose of this study was to retrospectively stratify the risks of malignancy according to the mammographic characteristics of Japanese women. We studied the mammographic findings of 1267 Japanese women. We characterized malignant phenotypes according to mass shape, margin and mass density, and by shape and distribution of calcified foci, and to obtain possible predictors for malignancies according to age groups. Lobular and irregular mass shape, no circumscribed margin and higher density turned out to be more powerful predictors for malignancy than other radiological factors (P < 0.001, respectively). The ratio of the cases detected as a mass in those between 21 and 49 years was lower than that of other age groups. In addition, the presence of calcifications and no mammographic abnormalities were the most powerful predictors for malignancies in the young age groups (P < 0.001, respectively). The peak age of breast cancer is between 40 and 49 years in Japan. In the present study, subtle differences were found in the mammographic results for young and old women, in contrast to those of women in the USA and Europe. The results of this study might enable more accurate prediction of biological behavior of the breast lesions in Japanese women. (Cancer Sci 2012; 103: 472–476)
The incidence of breast cancer continues to increase worldwide.( 1 ) Morbidity and death due to breast cancer are lower in Japan than in the USA and Europe, but their rates have been markedly increased in this decade.( 2 ) The effectiveness of screening mammography in reducing death from breast cancer has been established in both the USA, Europe and Japan.( 3 ) Worldwide, millions of mammographic examinations are performed each year, and mammography has become the gold standard for detecting breast disorders. It is important to continue to increase the mammographic screening ratio in order to reduce breast cancer deaths.
The American College of Radiology (ACR) has developed the Breast Imaging Reporting and Data System (BI‐RADS) to standardize mammographic reporting, to improve communication with clinicians, to reduce confusion regarding mammographic findings, to aid research and to facilitate outcomes monitoring.( 4 ) The use of BI‐RADS lexicon is increasing not only in the North American continent but also worldwide.( 5 ) However, some publications have raised the issue of observer variability of interpretation of the lexicon, and have even questioned the expressiveness of the BI‐RADS system.( 6 , 7 ) Some published studies propose classifications of features for the assignment of findings to the various BI‐RADS categories.( 6 , 7 )
Some published reports suggest possible differences in the biological characteristics of breast cancer between women in the USA and Europe and Japanese women.( 8 ) A striking difference is that the peak age for breast cancer is between 40 and 50 years in Japan, whereas the peak age in the USA and Europe is between 60 and 70 years.( 8 ) We have observed that the mammographic findings for Japanese women do not exactly correspond to those defined in BI‐RADS lexicon. The dense parenchyma in women before menopause can obscure tumor shadows, which results in the lower sensitivity of mammography screening in women 40–49 years of age.( 9 ) The purpose of the present study was to retrospectively stratify the risk of malignancy according to mammographic characteristics of Japanese women.
Materials and Methods
Patients. This is a retrospective study. We examined mammographical findings of 1267 Japanese women (707 malignant and 560 benign breast diseases) who underwent needle biopsies or surgical resection at the Tohoku University Hospital. We received informed consent from all patients and the protocol for this study was approved by the Ethics Committee at Tohoku University Graduate School of Medicine. The median age of the patients was 52 years (range, 21–89 years). The numbers of biopsy or surgical resection cases were: 151 for 21–39 years, 393 for 40–49 years, 355 for 50–59 years, 215 for 60–69 years and 153 for 70–89 years. The numbers of malignant cases were: 56 for 20–39 years, 205 for 40–49 years, 175 for 50–59 years, 147 for 60–69 years and 124 for 70–89 years. The criteria for performing a biopsy were BI‐RADS assessment categories of mammography and ultrasound category 4 or more.
Imaging and evaluation. All mammographic examinations were performed with dedicated machines. Analog mammographic examinations were performed with one unit (MAMMOMAT 3000 Nova; Siemens AG, Erlangen, Germany) and using a screen‐film technique (Min‐R 2000 Min‐R EV; Kodak Health Imaging, Rochester, NY, USA). Digital mammograms were acquired using a system with an amorphous selenium DirectRay digital detector (LOARD Selenia; Hologic, Waltham, MA, USA). The system was connected to a viewing monitor (MammoRead; TOYO Corporation, Tokyo, Japan).
We first examined the correlation between mammographic findings and the ratio of malignant cases. Two of the authors independently evaluated mammographic findings. These two investigators were blinded to the clinical outcome of the patients. The presence of mass, calcification and the other findings, including architectural distortion, focal asymmetric density and asymmetric breast tissue (ABT) were each recorded. As for mass, shape was tentatively classified as round, oval, lobular or irregular. Margin was classified as circumscribed, microlobulated, indistinct or spiculated. Density was classified into higher, equivalent or lower. Shape of calcification was tentatively classified as punctuate, amorphous, pleomorphic or linear. Distribution was classified as diffuse, grouped or segmental. We classified FAD and ABT into those with or without architectural distortion. We first examined the correlation between mammographic findings and the ratio of malignant cases. We then examined the ratio of malignant cases according to mammographic findings. We devised classification system for predicting. In addition, we attempted to obtain possible predictors for malignancies according to age groups: 21–39, 40–49, 50–59, 60–69 and 70–89 years. If there were two or more findings in a mammography, we determined the priority for mammographic findings as follows: mass, calcification, other findings including architectural distortion, FAD and ABT, and no mammographic abnormality, and recorded a prior finding as a possible predictor for malignancy.
Statistical analysis. Statistical analyses were performed using StatMate IV for Windows (ATMS, Tokyo, Japan). Results were considered significant at P < 0.05.
Results
Sensitivity, specificity and PPV by age groups. Of the 1040 cases with mammographic findings, 656 were malignant and 384 were benign, whereas 51 of the 227 cases without any mammographic findings were malignant. Sensitivity, specificity and positive predictive value were 92.8, 31.4 and 63.1%, respectively. In addition, sensitivity, specificity and PPV by age groups were: 87.5, 40.0 and 46.2% for 21–39 years; 86.8, 35.2 and 51.5% for 40–49 years; 96.1, 29.3 and 66.6% for 50–59 years, 96.6%, 17.6 and 71.7% for 60–69 years; and 93.5, 31.0 and 85.3% for 70–89 years, respectively.
Correlation between mammographic findings and ratio of malignant cases. The ratio of malignant cases according to the mass shape were 46.7% (28/60) of round, 45.1% (23/51) of oval, 83.3% (62/74) of lobular and 94.9% (131/138) of irregular lesions, respectively (Fig. 1A). There were statistically significant differences between round or oval and lobular or irregular shape (P < 0.001, respectively). The ratio of malignant cases according to the margin were 19.3% (11/57) of circumscribed, 75.5% (74/98) of microlobulated, 87.3% (62/71) of indistinct and 100% (97/97) of spiculated margin, respectively (Fig. 1B). There were statistically significant differences between circumscribed and the other characteristics, and microlobulated and spiculated margin (P < 0.001, respectively). The ratios of malignant cases according to mass density were 81.7% (210/257) of higher, 55.9% (33/59) of equivalent and 14.3% (1/7) of lower mass density, respectively (Fig. 1C). There were statistically significant differences between higher and equivalent or lower mass density (P < 0.001). The ratios of malignant cases according to calcification type were 28.1% (25/89) of punctuate, 48.3% (129/267) of amorphous, 88.4% (137/155) of pleomorphic and 100% (20/20) of linear type, respectively (Fig. 2A). There were statistically significant differences between punctuate or amorphous and any calcification shapes (P = 0.001 for between punctuate and amorphous, P < 0.001 for the other combinations). The ratios of malignant cases according to distribution of calcification were 16.7% (1/6) of diffuse, 53.1% (146/275) of grouped and 65.6% (164/250) of segmental (Fig. 2B). There were statistically significant differences between segmental and diffuse or grouped cases (P = 0.041 and P = 0.005, respectively).
Figure 1.
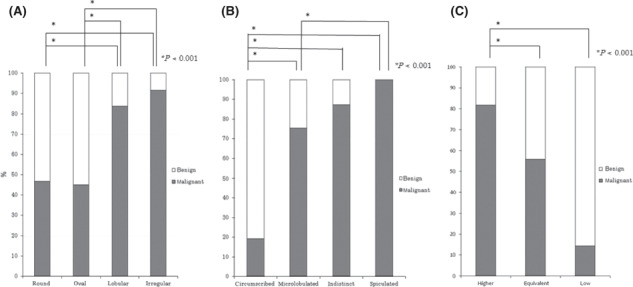
Correlation between mammographic findings of masses and malignant ratio: (A) mass shape, (B) margin and (C) mass density.
Figure 2.
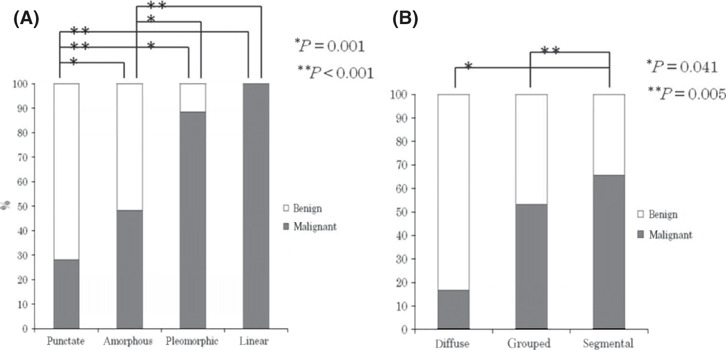
Correlation between mammographic findings of calcifications and malignant ratio: (A) characteristics of calcification and (B) distribution.
Classifications for predicting malignancies. We attempted to devise a classification system predicting malignancies (Table 1). The masses with spiculated or microlobulated margins, and the masses with lobular or irregular mass shapes and indistinct margins turned out to be more powerful predictors of malignancies than the other radiological factors (P < 0.001). In contrast, the masses with circumscribed margins and equivalent or lower mass densities were less powerful predictors of malignancies (P = 0.154).
Table 1.
Classifications for predicting malignancies
| Malignant ratio (%) | Mammographic findings | Total number | No. malignancies | Ratio of malignant cases (%) | P‐value | Odds ratio | ||
|---|---|---|---|---|---|---|---|---|
| Class I | 0–20 | Mass | Any mass shape‐circumscribed‐equivalent or lower | 25 | 2 | 8.0 | 0.154 | 0.30 |
| Calcification | Punctate‐diffuse or grouped | 42 | 7 | 16.7 | 0.525 | 0.69 | ||
| Class II | 20–40 | Mass | Round‐circumscribed‐higher‐with calcification | 19 | 5 | 26.3 | 0.921 | 1.23 |
| Oval‐circumscribed‐higher | 12 | 3 | 25.0 | 0.881 | 1.15 | |||
| Oval‐microlobulated‐equivalent | 8 | 3 | 37.5 | 0.572 | 2.07 | |||
| Calcification | Punctate‐segmental | 28 | 6 | 21.4 | 0.907 | 0.94 | ||
| Amorphous‐diffuse or grouped | 106 | 30 | 28.3 | 0.308 | 1.36 | |||
| Class III | 40–60 | Mass | Round‐indistinct‐higher | 4 | 2 | 50.0 | 0.485 | 3.45 |
| Lobular‐microlobulated or indistinct‐equivalent | 6 | 3 | 50.0 | 0.277 | 3.45 | |||
| Calcification | Punctate‐grouped‐with FAD | 9 | 5 | 55.6 | 0.589 | 4.31 | ||
| Amorphous‐segmental | 86 | 37 | 43.0 | <0.001 | 2.61 | |||
| FAD | Without distortion | 123 | 50 | 40.7 | <0.001 | 2.36 | ||
| Class IV | 60–80 | Mass | Irregular, round or lobular‐microlobulated‐higher | 62 | 46 | 74.2 | <0.001 | 9.92 |
| Calcification | Punctate‐segmental‐with FAD | 6 | 4 | 66.7 | 0.420 | 6.90 | ||
| Amorphous‐grouped or segmental‐with FAD | 53 | 41 | 77.4 | <0.001 | 11.79 | |||
| Pleomorphic‐grouped | 43 | 33 | 76.7 | <0.001 | 11.39 | |||
| ABT | Without distortion | 5 | 4 | 80.0 | 0.014 | 13.80 | ||
| Class V | 80–100 | Mass | Any mass shape‐spiculated‐any density | 100 | 100 | 100.0 | <0.001 | – |
| Any mass shape‐microlobulated‐any density‐with calcification | 18 | 17 | 94.4 | <0.001 | 58.67 | |||
| Lobular or irregular‐obscured or indistinct‐any findings | 64 | 58 | 90.6 | <0.001 | 33.36 | |||
| Calcification | Pleomorphic‐grouped or segmental with FAD | 32 | 29 | 90.6 | <0.001 | 33.36 | ||
| Pleomorphic‐segmental | 58 | 53 | 91.4 | <0.001 | 36.58 | |||
| Linear‐any distribution | 16 | 16 | 100.0 | <0.001 | – | |||
| FAD | With distortion | 90 | 77 | 85.6 | <0.001 | 20.44 | ||
| ABT | With distortion | 20 | 20 | 100.0 | <0.001 | – |
Mammographic findings of all the cases by age groups. Mammographic findings of all cases by age group are summarized in Figure 3A. The ratios of the cases detected as a mass were 18.5% for 21–39 years, 16.8% for 40–49 years, 25.4% for 50–59 years, 31.6% for 60–69 years and 46.4% for 70–89 years, respectively. There were statistically significant differences between 21 and 39 and 60–69 (P = 0.007) or 70–89 years (P < 0.001), 40–49 and 50–59 years (P = 0.005), 60–69 (P < 0.001) or 70–89 years (P < 0.001), 50–59 and 70–89 years (P < 0.001), and 60–69 and 70–89 years (P = 0.006). The ratios of calcification were 29.8% for 21–39 years, 41.7% for 40–49 years, 41.4% for 50–59 years, 39.5% for 60–69 years and 24.8% for 70–89 years, respectively. There were statistically significant differences between 20 and 39 and 40–49 (P = 0.014) or 50–59 years (P = 0.021), 40–49 and 70–89 years (P < 0.001), 50–59 and 70–89 years (P < 0.001), and 60–69 and 70–89 years (P = 0.005). The ratios of the other findings, including architectural distortion, FAD and ABT were 21.9% for 21–39 years, 16.3% for 40–49 years, 19.7% for 50–59 years, 20.9% for 60–69 years and 17.6% for 70–89 years, respectively. There were no statistically significant differences among age groups. The ratios of the cases without mammographic abnormalities were 29.8% for 21–39 years, 25.4% for 40–49 years, 13.8% for 50–59 years, 7.9% for 60–69 years and 11.1% for 70–89 years, respectively. There were statistically significant differences between 21 and 39 and 50–59 (P < 0.001), 60–69 (P < 0.001) or 70–89 years years (P < 0.001), 40–49 and 50–59 (P < 0.001), 60–69 (P < 0.001) or 70–89 years (P < 0.001), and 50–59 and 60–69 years (P = 0.046).
Figure 3.
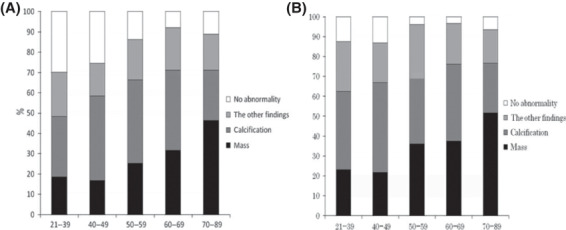
The ratio of mammographic findings by age groups: (A) mammographic findings of all cases by age groups and (B) mammographic findings of malignant cases by age groups.
Mammographic findings of malignant cases by age groups. Mammographic findings of malignant cases by age group are summarized in Figure 3B. The ratios of the cases detected as a mass were 23.2% for 21–39 years, 21.7% for 40–49 years, 36.1% for 50–59 years, 37.4% for 60–69 years and 51.6% for 70–89 years, respectively. There were statistically significant differences between 21 and 39 and 70–89 years (P < 0.001), 40–49 and 50–59 (P = 0.003), 60–69 (P = 0.003) or 70–89 years (P < 0.001), 50–59 and 70–89 years (P = 0.008), and 60–69 and 70–89 years (P = 0.026). The ratios of calcification were 39.3% for 21–39 years, 45.1% for 40–49 years, 35.1% for 50–59 years, 38.8% for 60–69 years and 25.0% for 70–89 years, respectively. The only statistically significant differences ware between 40 and 49 and 70–89 years (P < 0.001). The ratios of the other findings including architectural distortion, FAD and ABT were 25.0% for 21–39 years, 19.4% for 40–49 years, 24.9% for 50–59 years, 20.4% for 60–69 years and 22.1% for 70–89 years. There were statistically significant differences between 50 and 59 and 70–89 years (P = 0.046). The ratios of the cases without mammographic abnormalities were 12.5% for 21–39 years, 13.1% for 40–49 years, 3.9% for 50–59 years, 3.4% for 60–69 years and 6.5% for 70–89 years, respectively. There were statistically significant differences between 21 and 39 and 50–59 (P = 0.034) or 60–69 years (P = 0.034), and 40–49 and 50–59 (P = 0.002) or 60–69 years (P = 0.004).
Mammographic predictors for malignancy by age groups. The distinctive mammographic predictors for malignancy by age group are summarized in Table 2. The masses not only spiculated but also with microlobulated and indistinct margins turned out to be powerful predictors for malignancies in the groups of 50–59 years and above (Table 2).
Table 2.
Mammographic predictors for malignancy by age groups
| Years | Mammographic findings | Total number | No. malignancies | Ratio of malignant cases (%) | P‐value | Odds ratio | |
|---|---|---|---|---|---|---|---|
| 21–39 | Mass | Irregular‐spiculated: with calcification | 4 | 4 | 100.0 | 0.001 | – |
| Calcification | Amorphous‐segmental‐with FAD | 4 | 4 | 100.0 | 0.001 | – | |
| Pleomorphic‐any distribution | 11 | 9 | 81.8 | <0.001 | 15.53 | ||
| FAD | With distortion | 11 | 8 | 72.7 | <0.001 | 14.1 | |
| 40–49 | Mass | Lobular‐indistinct or spiculated‐high | 5 | 5 | 100.0 | 0.001 | – |
| Irregular‐indistinct‐high | 10 | 8 | 80.0 | <0.001 | 13.8 | ||
| Irregular‐spiculated‐any density | 13 | 13 | 100.0 | <0.001 | – | ||
| Calcification | Amorphous‐segmental | 52 | 26 | 50.0 | 0.001 | 3.35 | |
| Pleomorphic‐any distribution | 37 | 35 | 94.6 | <0.001 | 58.59 | ||
| Linear‐segmental | 4 | 4 | 100.0 | 0.004 | – | ||
| FAD | With distortion | 23 | 19 | 82.6 | <0.001 | 15.9 | |
| ABT | With distortion | 4 | 4 | 100.0 | 0.004 | – | |
| 50–59 | Mass | Round‐indistinct or microlobulated‐equivalent or high | 6 | 6 | 100.0 | <0.001 | – |
| Oval‐microlobulated‐high | 8 | 6 | 75.0 | 0.002 | 15.35 | ||
| Lobular‐indistinct or microlobulated‐high | 14 | 13 | 92.9 | <0.001 | 44.86 | ||
| Irregular‐indistinct, obscured or spiculated | 43 | 43 | 100.0 | <0.001 | – | ||
| Calcification | Amorphous‐grouped‐with FAD | 8 | 8 | 100.0 | <0.001 | – | |
| Amorphous‐segmental | 27 | 14 | 51.9 | 0.002 | 3.72 | ||
| Pleomorphic‐any distribution | 43 | 33 | 76.7 | <0.001 | 11.39 | ||
| FAD | With or without distortion | 63 | 44 | 69.8 | <0.001 | 7.99 | |
| ABT | With distortion | 7 | 7 | 100.0 | <0.001 | – | |
| 60–69 | Mass | Round‐circumscribed or microlobulated‐high | 6 | 6 | 100.0 | 0.012 | – |
| Lobular‐microlobulated or indistinct‐any density | 11 | 10 | 90.9 | <0.001 | 34.51 | ||
| Irregular‐any periphery‐any density | 31 | 31 | 100.0 | <0.001 | – | ||
| Calcification | Amorphous‐segmental‐with FAD | 5 | 5 | 100.0 | 0.025 | – | |
| Pleomorphic‐any distribution | 28 | 26 | 92.9 | <0.001 | 31.2 | ||
| Linear‐any distribution | 5 | 5 | 100.0 | 0.045 | – | ||
| FAD | With distortion | 19 | 17 | 89.5 | <0.001 | 20.4 | |
| ABT | With or without distortion | 4 | 4 | 100.0 | 0.045 | – | |
| 70–89 | Mass | Lobular or irregular, any periphery, any density | 52 | 49 | 94.2 | <0.001 | 56.37 |
| Calcification | Pleomorphic, any distribution | 14 | 13 | 92.9 | 0.013 | 16.25 |
Discussion
The BI‐RADS lexicon was created and has evolved to help capture predictive mammographic descriptors in a standardized manner in the USA and Europe.( 4 , 10 ) Various biological features of breast cancer have been found to be different between women in the USA and Europe and Japanese women.( 8 , 11 ) Mammographic findings of Japanese women do not exactly correspond to those defined in BI‐RADS lexicon for women in the USA and Europe.
The results of this study demonstrate that lobular and irregular mass shape, no circumscribed margin and higher density are more powerful predictors of malignancy than the other findings with statistical significance. As for calcification, a statistically significant difference was detected between necrotic and secretary calcification. FAD combined with architectural distortion represented a high malignant ratio of up to 80%. These results were similar to those in the BI‐RADS lexicon.
However, the striking difference between Japanese and the USA and Europe breast cancer patients is that the peak age for breast cancer was between 40 and 50 years in the Japan, whereas the peak age in the USA and Europe was between 60 and 70 years.( 8 ) The results of our present study reveals that the peak age of breast cancer was 40 years. There were subtle differences in mammographic findings between young and old women, which are different from those of women in the USA and Europe. Previous studies have demonstrated that mass is the more powerful predictor in women in the USA and Europe.( 12 , 13 ) However, the results in the present study demonstrate that the ratio of the cases detected as a mass between 21 and 49 years was lower than that of the other age groups. In addition, the masses that were spiculated and had microlobulated and indistinct margins turned out to be powerful predictors for malignancies in those of 50 years and above, whereas only limited masses were predictors for those under 49 years old. Therefore, the presence of a mammographic mass is not necessarily the most powerful predictor for malignancy in Japanese women.
The results of the present study also demonstrated that 7.2% (51/707) of malignant cases had mammographic abnormalities. In addition, the ratios of malignant cases in those of 20, 30 and 40 years without mammographic abnormalities were statistically higher than the ratios of the other age groups. There was dense parenchyma in women before menopause. A previous study demonstrated that the percentage of extremely dense and dense breast were 76.3% of 40 years, 51.5% of 50 years and 17.6% of 60 years in Japanese breast cancer cases, respectively.( 9 ) Breast masses are indicated by their density in the mammography. In addition, the mass findings are often hidden in a dense breast. Previous study has demonstrated that screening mammography is effective for women aged 50 years and over to detect malignancy, whereas the effectiveness for women under 50 years has not been proven.( 3 ) Ohuchi et al. ( 14 ) suggested that screening mammography alone for Japanese women aged 40 years and over is insufficient. For the purpose of complementing this weakness of mammography, the effectiveness of ultrasound screening for women aged 40 years and over has been evaluated in relation to detecting and reducing death as a result of breast cancer in Japan.( 14 )
In the present study, we attempted to establish mammographic criteria for Japanese women for predicting breast malignancy. We examined the mammographic characteristics according to age groups. Lobular and irregular mass shape, no circumscribed margin and higher density turned out to be more powerful predictors for malignancy than other radiological factors. In addition, the ratio of the cases detected as a mass between 21 and 49 years was lower than that for other age groups. The presence of calcifications and no mammographic abnormalities was one of the most powerful predictors for malignancies in the young age groups. However, this study was retrospective and took place in a single institute. Therefore, it is probable that further investigation not only with Japanese women but also with other Asian women will confirm the new mammographic criteria. The results of this study might enable more accurate prediction of the biological behavior of the breast lesions in Japanese women.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported in part by a Grant‐in‐Aid from the Kurokawa Cancer Research Foundation.
References
- 1. Sutela A, Vanninen R, Sudah M et al. Surgical specimen can be replaced by core samples in assessment of ER, PR and HER‐2 for invasive breast cancer. Acta Oncol 2008; 47: 38–46. [DOI] [PubMed] [Google Scholar]
- 2. Endo T. International exchange activities with East Japanese countries through mammography. Breast Cancer 2008; 16: 10–17. [DOI] [PubMed] [Google Scholar]
- 3. Kawai M, Kuriyama S, Suzuki A et al. Effect of screening mammography on breast cancer survival in comparison to other detection methods: a retrospective cohort study. Cancer Sci 2009; 100: 1479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American College of Radiology . Breast imaging reporting and data system (BI‐RADS), 4th edn Reston, VA: American College of Radiology, 2003. [Google Scholar]
- 5. Stines J. BI‐RADS: use in the French radiologic community. How to overcome with some difficulties. Eur J Radiol 2007; 61: 224–34. [DOI] [PubMed] [Google Scholar]
- 6. Burnside ES, Ochsner JE, Fowler KJ et al. Use of microcalcification descriptors in BI‐RADS 4th edition to stratify risk of malignancy. Radiology 2007; 242: 388–95. [DOI] [PubMed] [Google Scholar]
- 7. Berube M, Curpen B, Ugolini P et al. Level of suspicion of a mammographic lesion: use of features defined by BI‐RADS lexicon and correlation with large‐core breast biopsy. Can Assoc Radiol J 1998; 49: 223–8. [PubMed] [Google Scholar]
- 8. Leong SP, Shen ZZ, Liu TJ et al. Is breast cancer the same disease in Japanese and Western countries? World J Surg 2010; 34: 2308–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki A, Kuriyama S, Kawai M et al. Age‐specific interval breast cancers in Japan: estimation of the proper sensitivity of screening using a population‐based cancer registry. Cancer Sci 2008; 99: 2264–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American College of Radiology . Breast Imaging Reporting and Data System (BI‐RADS). 4th edn Reston, VA: American College of Radiology, 1998. [Google Scholar]
- 11. Hortobagyi GN, De la Garza Salazar J, Pritchard K et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer 2005; 6: 391–401. [DOI] [PubMed] [Google Scholar]
- 12. Piasso ED, Fajardo LL, Tsimikas J et al. Rate of insufficient samples for fine‐needle aspiration for nonpalpable breast lesions in a multicentral clinical trial. Cancer 1998; 82: 679–88. [DOI] [PubMed] [Google Scholar]
- 13. Thurfjell MG, Lindgren A, Thurfjell E. Nonpalpable breast cancer: mammographic appearance as predictor of histologic type. Radiology 2002; 222: 165–70. [DOI] [PubMed] [Google Scholar]
- 14. Ohuchi N, Ishida T, Kawai M et al. Randomized controlled trial on effectiveness of ultrasonography screening for breast cancer in women aged 40–49 (J‐START): research design. Jpn J Clin Oncol 2011; 41: 275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


