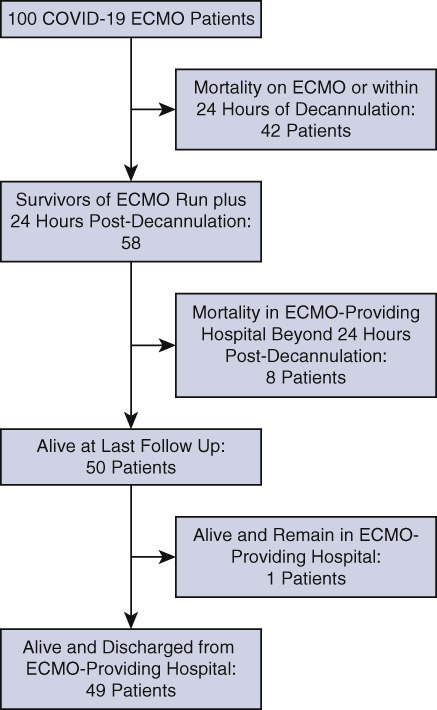
The distribution by category of outcome of 100 patients with COVID-19 supported with ECMO at 20 hospitals. All 100 patients have since been separated from ECMO; 50 patients survived, and 50 patients died. Of 50 survivors, 49 have been discharged from the hospital and 1 remains hospitalized at the ECMO-providing hospital.1
Central Message.
ECMO is an important therapeutic option for a subset of critically ill patients with COVID-19. Shih and colleagues provide important data about the role of ECMO in the care of these challenging patients.
See Article page 1071.
Shih and colleagues2 are to be congratulated for their thoughtful analysis of “37 patients admitted with severe acute respiratory distress syndrome associated with COVID-19 [coronavirus disease 2019]” who “were initiated on VV ECMO [venovenous extracorporeal membrane oxygenation] support at 1 of 4 ECMO referral hospitals within a large health care system. Initiation of ECMO occurred on median day 11.5 following admission, and, of the successfully decannulated patients, median time on ECMO was 17 days. Survival to discharge from ECMO center has occurred in 21 of 37 patients (56.8%).”2 These findings are important, and the authors have made an extremely important contribution in these challenging times.
All of us have had our very existence affected by the COVID-19 pandemic; indeed, life as we know it has changed dramatically, stunningly, rapidly, and globally. The evolution of the treatment of patients with COVID-19 is unlike any event ever seen in medicine. As of November 19, 2020, 56,754,669 patients around the world have been diagnosed with COVID-19, with 1,357,641 associated deaths to date (2.39% mortality worldwide).3 In the United States, as of November 19, 2020, 11,689,545 patients have been diagnosed with COVID-19, with 252,290 associated deaths to date (2.16% mortality in the United States).3 Most deaths in patients with COVID-19 are due to severe respiratory failure, with a smaller group dying of combined pulmonary and cardiac failure. Several recent publications have documented that ECMO facilitates salvage and survival of select critically ill patients with COVID-19.2 , 4, 5, 6 Early data from Wuhan, China, reported an alarmingly high rate of mortality of 83% (5/6) in patients with COVID-19 supported with ECMO7 , 8; however, more recent data reveal improved survival of patients with COVID-19 supported with ECMO.1 , 2 , 4, 5, 6 , 9 , 10 Both individual institutional reports5 and recent reports from multi-institutional registries.1 , 4 , 6 , 9 , 10 have demonstrated promising results and important salvage of critically ill patients.
One of the earliest publications of multi-institutional data concerning patients with COVID-19 supported with ECMO4 was a 24-day analysis of all patients with COVID-19 supported with ECMO entered into the SpecialtyCare Operative Procedural REgistry (https://specialtycareus.com/). This early report describes a series of 32 consecutive patients with COVID-19 who were placed on ECMO at 9 different hospitals, with an analytic window starting March 17, 2020, when the first patient with COVID-19 in this series was placed on ECMO, and ending April 9, 2020. An update of this multi-institutional cohort was recently presented at the 2020 annual meeting of The Southern Thoracic Surgical Association and included 100 patients with COVID-19 supported with ECMO at 20 hospitals.1 All 100 patients have since been separated from ECMO; 50 patients survived, and 50 patients died. Of 50 survivors, 49 had been discharged from the hospital and 1 remained hospitalized at the ECMO-providing hospital. Survival with venovenous ECMO was 49 of 96 patients (51%), and survival with venoarterial ECMO was 1 of 4 patients (25%). The median time from COVID diagnosis to intubation was 3.5 days, and the medium time from intubation to ECMO cannulation was 4 days. The median time on ECMO was 12 days (interquartile range, 8-22 days). The medium time on ECMO was 10.5 days in survivors and 14 days in nonsurvivors. Survivors were generally younger, with a lower median age (47 vs 56.5 years, P = .014). Another update of this cohort will be presented at the 2021 annual meeting of The Society of Thoracic Surgeons and will include more than 200 patients with COVID-19 supported with ECMO at 20 hospitals.9
Ample evidence exists that ECMO facilitates salvage and survival of select critically ill patients with COVID-19. As of November 19, 2020, the Extracorporeal Life Support Organization COVID-19 Registry Dashboard includes 3344 patients worldwide with suspected or confirmed COVID-19 supported with ECMO and 3330 patients with confirmed COVID-19 supported with ECMO.10 Of these patients, 2505 with confirmed COVID-19 had ECMO initiated at least 90 days before November 19, 2020, and these patients have an in-hospital mortality of 44%. Clearly, it is a fact that much remains to be learned about the treatment of patients with COVID-19 and the role of ECMO in this treatment.
Clinical guidelines for the management of patients with COVID-19 have been released by the World Health Organization11 and the Centers for Disease Control and Prevention of the United States.12 The Extracorporeal Life Support Organization13 and American Society for Artificial Internal Organs14 have also published guidelines regarding the role of ECMO in treating patients with COVID-19. Nevertheless, the role of ECMO in the management of these challenging patients remains promising but unclear. Areas that require ongoing investigation include (1) the identification of subsets of patients with COVID-19 most likely to benefit from ECMO; (2) the role of adjuvant therapies in the care of these patients before, during, and after ECMO, including anti–interleukin-6 receptor monoclonal antibodies (tocilizumab or sarilumab), antiviral medications (remdesivir), convalescent plasma, hydroxychloroquine, prostaglandin (Flolan; GlaxoSmithKline, Research Triangle Park, NC), and intravenous steroids; and (3) the ideal type of ECMO support in these patients (eg, anticoagulation strategy, cannulation strategy, type of circuit, type of oxygenator, and type of pump).
Much remains to be learned about the treatment of COVID-19 and the role of ECMO in these challenging patients. It is clear that ECMO offers an important therapeutic option for a subset of critically ill patients with COVID-19. Given the increasing use of ECMO in patients with COVID-19 acute respiratory distress syndrome, it is conceivable to believe that our community will face a growing number of patients who have recovered from the viral illness but are left with advanced irreversible lung damage. Recently, successful transition from the initial intent of bridge to recovery to subsequent bridge to lung transplantation has been described in a small number of patients with COVID-19.15 , 16 Further understanding the use of ECMO for these patients and fostering a multidisciplinary decision-making environment will be of utmost importance not only to allow for the possibility of native lung recovery whenever appropriate but also to consider life-saving lung transplantation for patients who would otherwise be offered withdrawal of life sustaining therapies.17 In summary, Shih and colleagues1 have provided important data showing how cardiothoracic surgeons can help meet the challenges of the COVID-19 pandemic. As the treatment options for COVID-19 mature and evolve, cardiothoracic surgeons will continue to serve a key role.
Footnotes
Disclosures: J.P.J. is a consultant for SpecialtyCare. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Jacobs JP, Stammers AH, St. Louis J, Awori Hayanga JW, Firstenberg MS, Mongero LB, et al. Experience with 100 patients with COVID-19 and Severe Pulmonary Compromise treated with Extracorporeal Membrane Oxygenation. Accepted for presentation at the 67th Annual Meeting of the Southern Thoracic Surgical Association (STSA). STSA 67th Annual Meeting, Loews Royal Pacific Resort at Universal Orlando ResortTM, Orlando, FL. November 4-7, 2020. For presentation Thursday, November 5, 2020. Live Program cancelled secondary to COVID-19 Pandemic. Presented at STSA Webinar on November 5, 2020.
- 2.Shih E., DiMaio J.M., Squiers J.J., Banwait J.K., Meyer D.M., George T.J., et al. the Baylor Scott & White ECMO for COVID Group Venovenous extracorporeal membrane oxygenation for patients with refractory coronavirus disease 2019 (COVID-19): multicenter experience of referral hospitals in a large healthcare system. J Thorac Cardiovasc Surg. 2022;163:1071–1079.e3. doi: 10.1016/j.jtcvs.2020.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) https://coronavirus.jhu.edu/map.html Available at:
- 4.Jacobs J.P., Stammers A.H., St Louis J., Hayanga J.W.A., Firstenberg M.S., Mongero L.B., et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: experience with 32 patients. ASAIO J. 2020;66:722–730. doi: 10.1097/MAT.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kon Z.N., Smith D.E., Chang S.H., Goldenberg R.M., Angel L.F., Carillo J.A., et al. Extracorporeal membrane oxygenation support in severe COVID-19. Ann Thorac Surg. July 17, 2020 doi: 10.1016/j.athoracsur.2020.07.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E., et al. Extracorporeal Life Support Organization Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. Erratum in: Lancet Respir Med. 2020;8:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry B.M. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8:e24. doi: 10.1016/S2213-2600(20)30119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs JP, Stammers AH, St. Louis J, Awori Hayanga JW, Firstenberg MS, Mongero LB, et al. Abstract Number: 925242. Analysis of 153 consecutive patients with COVID-19 and Severe Pulmonary Compromise treated with Extracorporeal Membrane Oxygenation (ECMO): Outcomes and Trends Over Time. Accepted for presentation as an oral livestream presentation at The Society of Thoracic Surgeons 2021 Annual Scientific Meeting, which will be held virtually, January 29-31, 2021.
- 10.Full COVID-19 Registry Dashboard. https://www.elso.org/Registry/FullCOVID19RegistryDashboard.aspx Available at:
- 11.World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance. 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf Available at:
- 12.Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html Available at:
- 13.Bartlett R.H., Ogino M.T., Brodie D., McMullan D.M., Lorusso R., MacLaren G., et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopal K., Keller S., Akhanti B., Bime C., Loyalka P., Cheema F.H., et al. Advanced pulmonary and cardiac support of COVID-19 patients: emerging recommendations from ASAIO—a “Living Working Document”. ASAIO J. 2020;66:588–598. doi: 10.1097/MAT.0000000000001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang C., Jaksch P., Hoda M.A., Lang G., Staudinger T., Tschernko E., et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir. 2020;8:1057–1060. doi: 10.1016/S2213-2600(20)30361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han W., Zhu M., Chen J., Zhang J., Zhu S., Li T., et al. Lung transplantation for elderly patients with end-stage COVID-19 pneumonia. Ann Surg. 2020;272:e33–e34. doi: 10.1097/SLA.0000000000003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machuca TN, Cypel M, Bharat A. Comment on let's build bridges to recovery in COVID-19 ARDS, not burn them! Ann Surg. November 17 [Epub ahead of print]. [DOI] [PubMed]


