Abstract
We report a case of necrotizing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia complicated by a bronchopleural fistula and treated by decortication and salvage lobectomy. Owing to the unknown characteristics of the underlying SARS-CoV-2 infection, treatment of the abscess and bronchopleural fistula was delayed. This may have resulted in further deterioration of the patient, with ensuing multiple organ dysfunction. Complications of SARS-CoV-2 pneumonia, such as a bacterial abscess and a bronchopleural fistula, should be treated as if the patient were not infected with SARS-CoV-2.
A severe acute respiratory distress syndrome (ARDS) evolves in 3% to 5% of patients infected with severe acute respiratory syndrome coronavirus (SARS-CoV-2), requiring endotracheal intubation and mechanical ventilation. Respiratory viral infections are known to predispose to bacterial coinfections. These may result in empyema and other destructive lung infections. Administration of antiinflammatory drugs, such as steroids, may facilitate this.
A 36-year old White woman presented to the emergency department with cough, fever, and thoracic pain. She was 8 weeks pregnant, and the diagnosis of SARS-CoV-2 had been confirmed 4 days earlier by reverse transcription-polymerase chain reaction assay of a nasopharyngeal swab. Blood test revealed mild C-reactive protein increase (47 mg/L), hyperleukocytosis (20,200/mm3) with lymphopenia, and acute kidney injury (creatinine clearance of 52 mL/min/1.73m2). Blood gas analysis showed compensated lactic acidosis (pH 7.47; lactate, 3.7 mmol/L), a Po 2 of 69.80 mm Hg, and a Pco 2 of 28.40 mm Hg. There was a patchy infiltration of the right lung on chest roentgenogram, suggestive for infection.
The patient was admitted to the intensive care unit. Empiric treatment with ceftriaxone and hydroxychloroquine was initiated. Noninvasive respiratory support was provided by a high-flow nasal canula. Restrictive fluid management and blood pressure control by noradrenaline was started.
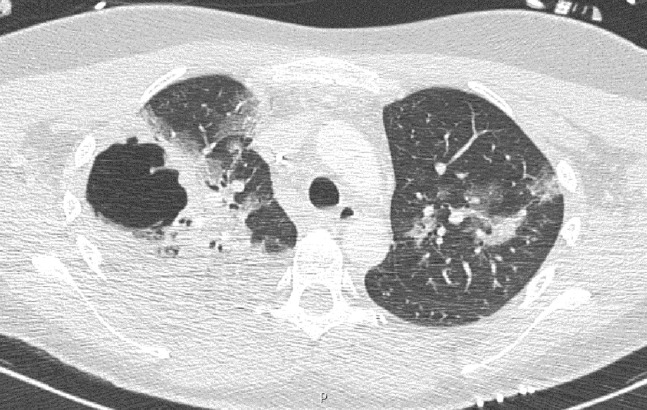
A computed tomographic (CT) scan revealed an extensive pneumonia with bilateral, multiple areas of consolidation, necrosis, and cavity formation in the right upper lobe (Figure 1 ). Fiberoptic bronchoscopy with bronchoalveolar lavage and biopsy was performed. Cultures were positive for Streptococcus mitis and Candida albicans. Empirically initiated ceftriaxone was switched to vancomycin and fluconazole.
Figure 1.
Chest computed tomographic scan shows the presence of bilateral, multiple areas of consolidation with extensive necrosis and abscess formation (5.8 × 5.6 cm) in the right upper lobe.
A spontaneous miscarriage occurred early during hospitalization. On day 9, the patient’s clinical condition deteriorated rapidly with necessity of high-flow nasal oxygen therapy at 100% fraction of inspired oxygen. An attempt at prone ventilation during noninvasive ventilation was unsuccessful, and the patient was sedated and intubated. Piperacillin/tazobactam was administered. Because of a hyperinflammatory syndrome, high-dose methylprednisolone was started.
Notwithstanding, refractory hypoxia worsened and became associated with hypercapnic failure. The next day she was placed on veno-venous extracorporeal membrane oxygenation (VV-ECMO). Cultures of endotracheal aspirate and bronchoalveolar lavage fluid were positive for extended-spectrum β-lactamase–producing Escherichia coli. Based on the antibiogram, antibiotics were changed to the combination of vancomycin and temocillin. Acyclovir was empirically added to cover potential opportunistic herpetic infections.
The deterioration of the ARDS also resulted in a pronounced coagulopathy with thrombocytopenia, which complicated the management of the anticoagulation for the VV-ECMO.
A CT scan repeated on day 20 showed a moderately large, right hydropneumothorax with leftward shift of the mediastinal structures. Two chest tubes were placed, with immediate drainage of 900 mL of blood and moderate air leak. Severe anemia developed 2 days later, with persistent need for transfusion. CT images showed a large hemothorax on the right side with compression atelectasis of the upper, middle, and lower lobe and consolidation of the entire right lung. The necrotic cavity in the right upper lobe remained unchanged.
Proning of the patient on VV-ECMO could not resolve the compression atelectasis of the right lung, and bronchoscopy could not detect any endobronchial hemorrhage. The administration of heparin was paused, which had its own risks because of the prothrombogenic nature of the SARS-CoV-2 infection and the VV-ECMO circuit.
As a result of the progressive deterioration with suspicion of active bleeding and the lack of other therapeutic options, emergency right thoracotomy was eventually performed on day 27. During thoracotomy, a massive hemothorax and a large abscess in the upper lobe was found. On opening of the abscess, there was a central bronchopleural fistula. Owing to the size of the fistula and poor quality of the surrounding tissues, closure was not possible. An extensive decortication and right-sided upper-lobe resection were performed.
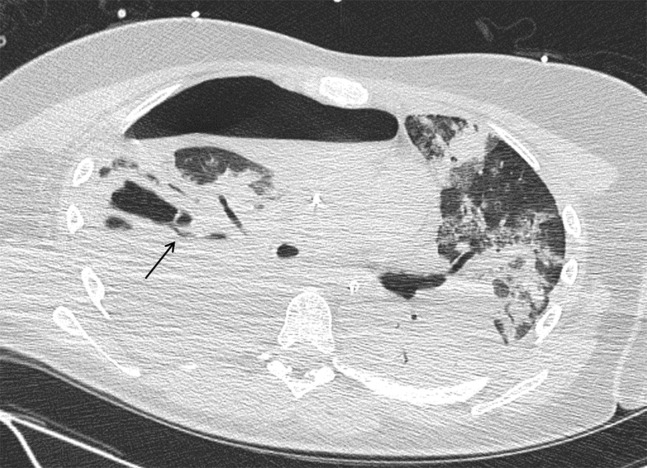
Hemodynamic instability and ongoing blood loss through the chest tubes warranted a revision that same night. There was only diffuse oozing because of coagulation derangements (mainly low fibrinogen levels), and the thoracic cavity was packed. After this intervention, the patient’s hemodynamics significantly improved. The patient underwent repeat thoracotomy and removal of the packs 3 days later. When we retrospectively reviewed the CT images, we were able to identify the bronchopleural fistula arising from the posterior wall of the abscess cavity (Figure 2 ).
Figure 2.
Retrospective review of the computed tomographic scan shows a bronchopleural fistula arising from the posterior wall of the abscess cavity.
Histopathologic examination revealed diffuse alveolar damage in the acute and organizing phase and a large necrotic abscess. Intraoperative cultures remained negative.
On day 31, weaning from ECMO was initiated. A tracheostomy was placed 2 days later to support weaning from mechanical ventilation. This was complicated by bleeding, initially treated with pharyngeal packing but a complete obstruction of the trachea by blood clots ultimately required surgical revision. On day 40, after 20 days on ECMO, she was successfully decannulated and separated from ECMO, and weaning from mechanical ventilation was initiated. The chest drains were removed on day 47 and day 51. Removal of the tracheostomy was only possible after intensive physiotherapy for severe muscle weakness acquired in the intensive care unit after 48 days of mechanical ventilation. The patient was discharged to a non coronavirus disease 2019 ward 59 days after being admitted to the hospital.
Comment
A hydropneumothorax developed in our patient due to a bronchopleural fistula that occurred as a complication of an extensive necrotizing SARS-CoV-2 pneumonia. Necrotizing pneumonia with secondary bronchopleural fistula and hydropneumothorax is a rare complication.
Goursaud and colleagues1 described a severe necrotizing pneumonia caused by SARS-CoV-2 and treated with ECMO. They reported the development of hemorrhagic shock related to a spontaneous left hemothorax. Treatment consisted of thoracic drainage and massive blood component transfusion. Despite these therapeutics and other supportive measures, the patient died of vasoplegic refractory shock with multiple organ failure related to a major systemic inflammatory response syndrome.
In critically ill patients with SARS-CoV-2, significant abnormalities in coagulation parameters have been reported.2 Emphasis has been placed on prevention of thromboembolic complications by prophylactic treatment with low-molecular-weight heparin.3 Although the incidence of hemorrhagic complications in patients with SARS-CoV-2 appears to be low, we noticed several bleeding complications. We therefore support the suggestion of Goursaud and colleagues1 that ECMO and escalated doses of anticoagulation for thromboembolic event prophylaxis in severe SARS-CoV-2 patients must be considered with caution.
We report SARS-CoV-2 pneumonia treated with ECMO and ultimately decortication and salvage lobectomy. Aiolfi and colleagues4 reported the management of persistent iatrogenic pneumothorax with thoracoscopy and bleb resection in 2 intubated SARS-CoV-2 patients. However, after a thorough and comprehensive literature search, we found no other reports of surgical treatment of complicated SARS-CoV-2 pneumonia. Although we do not recommend this treatment as a first-line option, decortication and salvage lobectomy were successful as salvage therapy.
In conclusion, complications of a severe viral pneumonia, such as bacterial superinfection, abscess formation, and bronchopleural fistula, need to be promptly treated. It appears that SARS-CoV-2 infection should not play a determining factor in delaying necessary treatments because it only allows multiple organ failure to further develop.
References
- 1.Goursaud S., Mombrun M., du Cheyron D. COVID-19 necrotising pneumonia and extracorporeal membrane oxygenation: a challenge for anticoagulation. ERJ Open Res. 2020;6:00182–2020. doi: 10.1183/23120541.00182-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patient with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi M., Thachil J., Iba T., Levy J. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiolfi A., Biraghi T., Montisci A., et al. Management of persistent pneumothorax with thoracoscopy and blebs resection in COVID-19 patients. Ann Thorac Surg. 2020;110:e413–e415. doi: 10.1016/j.athoracsur.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]