Supplemental Digital Content is available in the text.
Key Words: COVID-19, gastrointestinal symptoms, liver function, digestive system disease, meta-analysis
Abstract
Background:
The worldwide outbreak of COVID-19 infected millions of people. Some patients had gastrointestinal (GI) symptoms, abnormal liver function, digestive system disease and liver disease.
Aim:
To investigate the prevalence of GI symptoms, abnormal liver function, digestive system disease and liver disease in patients with COVID-19 by a systematic review and meta-analysis.
Methods:
We searched PubMed, Ovid Embase, Medline, and 2 Chinese databases. Primary outcomes were the prevalence of GI symptoms, abnormal liver function, digestive system disease, and liver disease. Different studies were included in different subset analysis. These outcomes were estimated with proportions, odds ratio, 95% confidence interval (CI) and P-value by Stata SE 15.1.
Results:
Thirty-one studies involving 4682 patients were included. The most significant GI symptoms were diarrhea (0.08, 95% CI: 0.06-0.11) and anorexia (0.17, 95% CI: 0.06-0.27). The most significant abnormal liver function was increased alanine aminotransferase (ALT) (0.25, 95% CI: 0.16-0.33). A total of 5% of the patients had digestive system disease (95% CI: 0.02-0.08). A total of 3% of the patients had liver disease (95% CI: 0.02-0.05). The prevalence of nausea and vomiting, diarrhea, abnormal liver function, digestive system disease, and liver disease was higher in Wuhan group. The prevalence of diarrhea was higher in non-China group. Patients in severe/intensive care unit group were more likely to have diarrhea, anorexia, abdominal pain increased aspartate aminotransferase, and increased ALT.
Conclusion:
The most significant GI symptoms were anorexia and diarrhea. The most significant abnormal liver function was increased ALT. Severe patients were more likely to have GI symptoms and abnormal liver function.
In December 2019, several cases of pneumonia with unknown reason emerged in Wuhan, Hubei province, China.1,2 Chinese Center for Disease Control and Prevention (China CDC) identified a novel coronavirus, which was named 2019 novel coronavirus (2019-nCoV).3 2019 novel coronavirus disease (COVID-19) spread through China rapidly in last 3 months, thus far, there were >80 thousand confirmed cases and >3 thousand dead cases in China. At present, the epidemic has been contained successfully in China with effective measures. However, COVID-19 is affecting many countries and territories outside China, as of April 3, 2020, >1 million cases of COVID-19 have been reported, among which >50 thousand patients have died.4 On March 11, 2020, World Health Organization (WHO) pronounced that COVID-19 could be characterized as pandemic.5
The severity of COVID-19 can vary from mild to severe, some patients have only influenza-like symptoms of fever, cough, myalgia, and fatigue, however, a few patients can progress rapidly to acute respiratory distress syndrome, acute respiratory failure, and other serious complications. Previous studies have shown that 5% of the patients were admitted to the intensive care unit (ICU), 2.3% of the patients need invasive mechanical ventilation and 1.4% of the patients died.6–8 Except for typical respiratory symptoms, gastrointestinal (GI) symptoms such as diarrhea, nausea, and vomiting are worthy of attention. A multicenter research in China shown that 3.8% of the patients had diarrhea, 5% of the patients had nausea or vomiting.8 In the study of Wang et al,9 10.1% of the patients had nausea and diarrhea at the onset of illness followed by fever and dyspnea. Besides, abnormal liver function was observed in a substantial portion of the patients. In the study of Chen et al,7 43% of the patients had differing degrees of liver insufficiency with increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT), 18% of the patients had increased total bilirubin (TBil). Another study found that liver damage was more likely to be observed in severe patients during the process of disease.10 Previous studies have shown that COVID-19 was more likely to affect populations with comorbidities. In the study of Chen and colleagues, 11% of the patients got COVID-19 with underlying digestive system disease. With the rapid spread of COVID-19, the better understanding of clinical features will facilitate efforts to control COVID-19. The aim of this meta-analysis is to identify the prevalence of GI symptoms, abnormal liver function, digestive system disease, and liver disease in patients with COVID-19, also to compare the difference based on region and severity of disease.
METHODS
Search Strategy
We systematically searched PubMed, Ovid Embase, Medline, 2 Chinese databases (CNKI and Wanfang Data) since inception up to April 6, 2020. Search terms were “COVID-19,” “SARS-CoV-2,” “2019-nCoV,” “2019 novel coronavirus,” and “novel coronavirus pneumonia.”
Inclusion Criteria and Exclusion Criteria
We included studies which met criteria as follows: (1) studies aimed at COVID-19; (2) studies provided information about GI symptoms, abnormal liver function, digestive system disease, and liver disease; (3) studies designed by randomized controlled trials (RCTs), prospective cohorts, retrospective cohorts, and open-label; (4) full text. The exclusion criteria were as follows: (1) studies published as reviews, letters, case reports, editorials, comments, conference abstracts, and family-based studies; (2) studies only describe these situations such as GI symptoms, abnormal liver function, digestive system disease, liver disease, and lacks specific data.
Data Extraction
Information was extracted from each study, including baseline characteristics such as age, sex, region, severity of disease, clinical outcome, date of cases collection, GI symptoms such as nausea, vomiting, diarrhea, abdominal pain, anorexia, belching and constipation, abnormal liver function such as increased AST, ALT, and TBil, digestive system disease, and liver disease. We defined studies performed in Wuhan as Wuhan group, studies performed outside Wuhan in China as non-Wuhan group, studies performed in China as China group and studies performed outside China as non-China group. We defined the patients who had severe symptoms or received ICU care as severe/ICU group, the patients who had general symptoms or did not receive ICU care as general/non-ICU group. Primary outcome was the prevalence of GI symptoms. Secondary outcomes were the prevalence of abnormal liver function, digestive system disease, and liver disease.
Statistical Analysis
The data was analyzed by Stata SE 15.1, the proportion and 95% confidence interval (CI) was used to calculate the prevalence. Forest plots were used to present data visually. Heterogeneity was evaluated using the Cochran’s Q test and I2 statistics, P-value<0.10 or I2>50% means the heterogeneity was significant. The random-effects model was used if heterogeneity was significant, otherwise, the fixed-effects model was adopted. Publication bias was assessed with funnel plots and Egger’s test. Subgroup analysis were performed based on region. Odds ratio (OR), 95% CI, and P-value were computed to compare the difference based on severity of disease. P-value <0.05 means the difference between groups was statistically significant. Different studies were included in different subset analysis.
RESULTS
Study Selection and Baseline Characteristics
We initially retrieved 3154 unique citations from PubMed, Ovid Embase, Medline, and 2 Chinese databases (CNKI and Wanfang Data). A total of 569 studies were excluded in the first screening because of duplication. After reading the titles, abstracts, and full-text of citations, 2554 literatures were excluded and 31 studies with a total of 4682 patients were included (Fig. 1). In this meta-analysis, the time span of cases collection was from December 11, 2019 to February 28, 2020. The median age of patients ranged from 36 to 62 years, 55% of the patients were male (Table 1).
FIGURE 1.
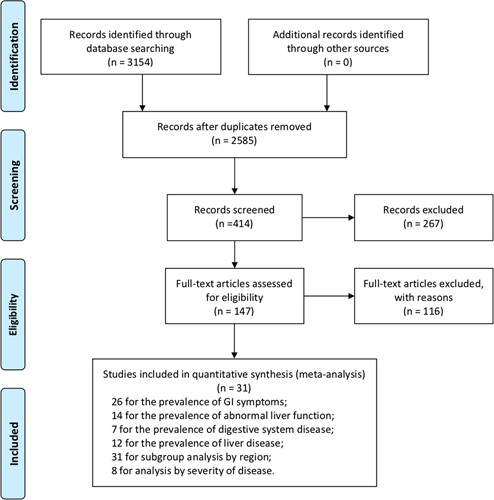
A flow diagram of articles retrieved and inclusion progress through the stage of meta-analysis.
TABLE 1.
Baseline Characteristics and Prevalence of GI Symptoms, Abnormal Liver Function, Digestive System Disease, and Liver Disease
| GI Symptoms | Abnormal Liver Function | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| References | Date (mm dd, yy) | Sample Size | Age, Mean | Sex, Male | Nausea and Vomiting | Diarrhea | Abdominal Pain | Anorexia | Increased AST | Increased ALT | Increased Tbil | Digestive System Disease | Liver Disease |
| Bai et al11 | Jan 6, 2020-Feb 20, 2020 | 219 | 44.8 | 119 | 6 | ||||||||
| Chen et al 7 | Jan 1, 2020-Jan 20, 2020 | 99 | 55.5 | 67 | 1 | 2 | 35 | 28 | 18 | 11 | |||
| Chen et al12 | Jan 13, 2020-Feb 28, 2020 | 274 | 62 | 171 | 40 | 77 | 19 | 66 | 84 | 3 | |||
| Chu et al13 | Jan 7, 2020-Feb 11, 2020 | 54 | 39 | 36 | 1 | 3 | 3 | ||||||
| Chung et al14 | Jan 18, 2020-Jan 27, 2020 | 21 | 51 | 13 | 1 | ||||||||
| Dong et al15 | Jan 20, 2020-Feb 14, 2020 | 11 | 36.6 | 5 | 1 | 2 | 2 | ||||||
| Easom et al16 | Jan 29, 2020-Feb 24, 2020 | 68 | 42.5 | 32 | 2 | 9 | 2 | 3 | |||||
| Guan et al9 | Dec 11, 2019-Jan 29, 2020 | 1099 | 47 | 637/1096 | 55 | 42 | 168/757 | 158/741 | 76/722 | ||||
| Huang et al6 | Dec 16, 2019-Jan 2, 2020 | 41 | 49 | 30 | 1/38 | 15 | 1 | ||||||
| Lian et al17 | Jan 17, 2020-Feb 12, 2020 | 788 | 407 | 31 | |||||||||
| Lin et al18 | Jan 17, 2020-Feb 15, 2020 | 95 | 45.3 | 45 | 21 | 23 | 17 | 4 | 5 | 22 | |||
| Liu et al10 | Jan 23, 2020-Feb 8, 2020 | 32 | 38.5 | 20 | 2 | 9 | 1 | ||||||
| Liu et al19 | Dec 30, 2019-Jan 24, 2020 | 137 | 55 | 61 | 11 | ||||||||
| Liu et al20 | Dec 26, 2019-Jan 21, 2020 | 12 | 53.7 | 8 | 2 | 2 | 3 | 2 | |||||
| Mo et al21 | Jan 1, 2020-Feb 5, 2020 | 155 | 54 | 86 | 6 | 7 | 3 | 7 | |||||
| Peng et al22 | Jan 20, 2020-Feb 15, 2020 | 112 | 62 | 53 | 15 | ||||||||
| Shi et al23 | Dec 20, 2019-Jan 23, 2020 | 81 | 49.5 | 42 | 4 | 3 | 1 | 43 | 7 | ||||
| Wang et al8 | Jan 1, 2020- Jan 28, 2020 | 138 | 56 | 75 | 19 | 14 | 3 | 55 | 4 | ||||
| Wang et al24 | Jan 21, 2020-Jan 24, 2020 | 4 | 44.3 | 3 | 1 | ||||||||
| Wu et al25 | Jan 22, 2020- Feb 14, 2020 | 80 | 46.1 | 39 | 1 | 1 | 3 | 1 | |||||
| Xu et al26 | Jan 23, 2020-Feb 4, 2020 | 90 | 50 | 39 | 7 | 5 | |||||||
| Xu et al27 | Jan 10, 2020-Jan 26, 2020 | 62 | 41 | 36 | 3 | 10 | 7 | ||||||
| Yang et al28 | Jan 17, 2020-Feb 10, 2020 | 149 | 45.1 | 81 | 2 | 11 | 27 | 18 | 4 | 8 | |||
| Yang et al29 | Dec 24, 2019-Jan 26, 2020 | 52 | 59.7 | 35 | 2 | ||||||||
| Zhang et al30 | Jan 16, 2020-Feb 25, 2020 | 95 | 49 | 53 | 45 | 52 | |||||||
| Zhang et al31 | Jan 16, 2020-Feb 3, 2020 | 140 | 57 | 71 | 31/139 | 18/139 | 8/139 | 17/139 | 13 | 8 | |||
| Zhang et al32 | Jan 18, 2020-Feb 3, 2020 | 9 | 36 | 5 | 1 | ||||||||
| Zhao et al33 | Jan 23, 2020-Feb 5, 2020 | 19 | 48 | 11 | 1 | 5/18 | 5/18 | ||||||
| Zhao et al34 | 101 | 44.4 | 56 | 2 | 3 | 6 | |||||||
| Zhou et al35 | Dec 29, 2019-Jan 31, 2020 | 191 | 56 | 119 | 7 | 9 | 59/189 | ||||||
| Zhou et al36 | Dec 20, 2019-Feb 9, 2020 | 254 | 50 | 115 | 36 | 46 | 3 | 3 | |||||
| Total | 4682 | 2570 | 241 | 309 | 36 | 161 | 443 | 336 | 120 | 47 | 77 | ||
| Propotion (%) | 55 | 7 | 8 | 3 | 17 | 24 | 25 | 13 | 5 | 3 | |||
| 95% CI | (0.04-0.09) | (0.06-0.11) | (0.01-0.05) | (0.06-0.27) | (0.16-0.32) | (0.16-0.33) | (0.05-0.20) | (0.02-0.08) | (0.02-0.05) | ||||
| I2 | 85.80% | 86.40% | 73.40% | 95.60% | 94.70% | 92.90% | 92.50% | 76.50% | 50.60% | ||||
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; GI, gastrointestinal; Tbil, total bilirubin.
Prevalence of GI Symptoms
In total, 26 studies were included to analyze the prevalence of nausea, vomiting, diarrhea, abdominal pain, and anorexia which we focused on in this meta-analysis. In addition, the study of Zhang et al31 reported 7 patients with belching. The study of Wang et al24 reported a patient with constipation. The prevalence of nausea and vomiting was 7% (95% CI: 0.04-0.09), heterogeneity was significant (P<0.0001, I2=85.8%), the funnel plot and Egger’s test revealed evidence of publication bias (t=2.57, 95% CI: 0.46-4.55, P=0.019). The prevalence of diarrhea was 8% (95% CI: 0.06-0.11), heterogeneity was significant (P<0.0001, I2=86.4%) (Fig. 2), the funnel plot and Egger’s test revealed the evidence of publication bias (t=3.02, 95% CI: 0.77-4.13, P=0.006). The prevalence of abdominal pain was 3% (95% CI: 0.01-0.05), heterogeneity was significant (P=0.005, I2=73.4%), the funnel plot and Egger’s test revealed no evidence of publication bias (t=2.86, 95% CI: −0.46 to 8.77, P=0.064). The prevalence of anorexia was 17% (95% CI: 0.06-0.27), heterogeneity was significant (P<0.0001, I2=95.6%) (Fig. S1 in Supplementary Appendix, Supplemental Digital Content 1, http://links.lww.com/JCG/A622), the funnel plot and Egger’s test revealed no evidence of publication bias (t=2.10, 95% CI: −1.31 to 13.34, P=0.089). We utilized random-effect model to analyze the prevalence of GI symptoms.
FIGURE 2.
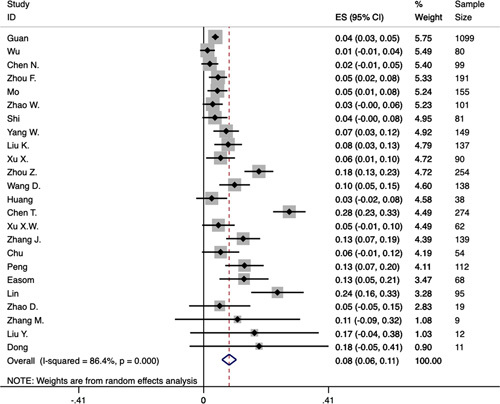
Forest plot of prevalence of diarrhea in patients with COVID-19. CI indicates confidence interval; ES, effect size.
Prevalence of Abnormal Liver Function
Fourteen studies were included to analyze the prevalence of increased AST, ALT, and TBil. The upper limit of normal value of AST, ALT, and TBil was 40 U/L, 40 to 64 U/L, and 17.1 to 26 μmol/L, respectively. In total, 24% of the patients had increased AST (95% CI: 0.16-0.32), heterogeneity was significant (P<0.0001, I2=94.7%) (Fig. S2 in Supplementary Appendix, Supplemental Digital Content 2, http://links.lww.com/JCG/A623), the funnel plot and Egger’s test revealed no evidence of publication bias (t=1.49, 95% CI: −1.61 to 8.30, P=0.165). A total of 25% of the patients had increased ALT (95% CI: 0.16-0.33), heterogeneity was significant (P<0.0001, I2=92.9%) (Fig. 3), the funnel plot and Egger’s test revealed no evidence of publication bias (t=0.99, 95% CI: −3.21 to 7.80, P=0.356). A toal of 13% of the patients had increased TBil (95% CI: 0.05-0.20), heterogeneity was significant (P<0.0001, I2=92.5%), the funnel plot and Egger’s test revealed evidence of publication bias (t=1.12, 95% CI: −11.69 to 19.89, P=0.38). All the results were analyzed by random-effect model.
FIGURE 3.
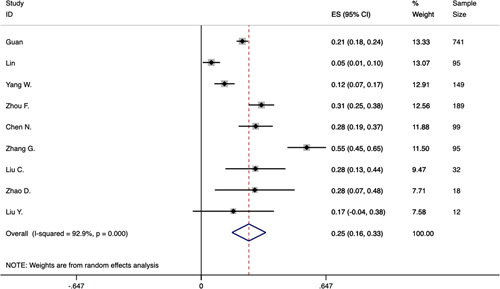
Forest plot of prevalence of increased alanine aminotransferase in patients with COVID-19. CI indicates confidence interval; ES, effect size.
Prevalence of Digestive System Disease and Liver Disease
Seventeen studies were included to analyze the prevalence of digestive system disease and liver disease. The disease categories were not mentioned in most studies. The study of Easom et al16 reported 3 patients with gastroenteritis. The study of Wang et al24 reported 1 patient with fatty liver. The study of Zhang et al31 reported 8 patients with fatty liver and abnormal liver function, 7 patients with chronic gastritis and gastric ulcer, and 6 patients with cholelithiasis. In this meta-analysis, 5% of the patients had digestive system disease (95% CI: 0.02-0.08), heterogeneity was significant (P<0.0001, I2=76.5%) (Fig. 4), the funnel plot and Egger’s test revealed evidence of publication bias (t=5.61, 95% CI: 1.68-4.53, P=0.002). In total, 3% of the patients had liver disease (95% CI: 0.02-0.05), heterogeneity was significant (P=0.023, I2=50.6%) (Fig. 5), the funnel plot and Egger’s test revealed no evidence of publication bias (t=2.18, 95% CI: −0.03 to 2.83, P=0.054). Both the results were analyzed by fixed-effects model.
FIGURE 4.
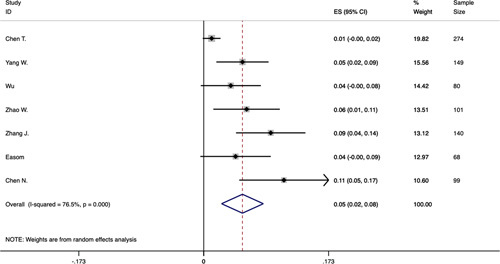
Forest plot of prevalence of digestive system disease in patients with COVID-19. CI indicates confidence interval; ES, effect size.
FIGURE 5.
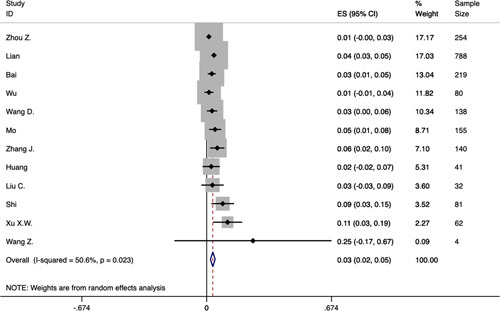
Forest plot of prevalence of liver disease in patients with COVID-19. CI indicates confidence interval; ES, effect size.
Subgroup Analysis by Region
Fourteen studies were performed in Wuhan, 14 studies were performed outside Wuhan in China. Only 1 study was performed in United Kingdom. Two studies including patients in and outside Wuhan in China, however, the difference of clinical characteristics between patients in and outside Wuhan in China were not mentioned. Ultimately, 28 studies were included to analyze the difference between Wuhan group and non-Wuhan group. And 31 studies were included to analyze the difference between China group and non-China group.
In Wuhan group, 58% of the patients were male. The prevalence of nausea and vomiting was 8% (95% CI: 0.04-0.12), the prevalence of diarrhea was 10% (95% CI: 0.05-0.14), and the prevalence of anorexia was 17% (95% CI: 0.05-0.28). The prevalence of increased AST, ALT, and TBil were 40% (95% CI: 0.31-0.49), 38% (95% CI: 0.23-0.52), and 18% (95% CI: 0.11-0.26), respectively. The prevalence of digestive system disease and liver disease were 7% (95% CI: 0.00-0.14) and 4% (95% CI: 0.02-0.06), respectively. In non-Wuhan group, 53% of the patients were male. The prevalence of nausea and vomiting was 5% (95% CI: 0.02-0.09), the prevalence of diarrhea was 7% (95% CI: 0.03-0.10), and the prevalence of anorexia was 18% (95% CI: 0.10-0.26). The prevalence of increased AST, ALT, and TBil were 13% (95% CI: 0.06-0.21), 14% (95% CI: 0.06-0.22), and 13% (95% CI: −0.08 to 0.33), respectively. The prevalence of digestive system disease and liver disease were 5% (95% CI: 0.03-0.07) and 3% (95% CI: 0.02-0.05), respectively (Fig. S3-S8 in Supplementary Appendix, Supplemental Digital Content 3, http://links.lww.com/JCG/A624, Supplemental Digital Content 4, http://links.lww.com/JCG/A625, Supplemental Digital Content 5, http://links.lww.com/JCG/A626, Supplemental Digital Content 6, http://links.lww.com/JCG/A627, Supplemental Digital Content 7, http://links.lww.com/JCG/A628, Supplemental Digital Content 8, http://links.lww.com/JCG/A629).
In China group, 55% of the patients were male. The prevalence of nausea and vomiting was 7% (95% CI: 0.05-0.09), the prevalence of diarrhea was 8% (95% CI: 0.06-0.11). The prevalence of increased AST and digestive system disease were 26% (95% CI: 0.18-0.34) and 6% (95% CI: 0.02-0.09), respectively. In non-China group, 47% of the patients were male. The prevalence of nausea and vomiting was 3% (95% CI: −0.01 to 0.07), the prevalence of diarrhea was 13% (95% CI: 0.05-0.21) (Fig. S9, Supplemental Digital Content 9, http://links.lww.com/JCG/A630). The prevalence of increased AST and digestive system disease were 3% (95% CI: −0.01 to 0.07) and 4% (95% CI: 0.00-0.09).
Difference Between General/Non-ICU Group and Severe/ICU Group
Only 8 studies were included, the prevalence of nausea and vomiting, diarrhea, abdominal pain, anorexia, increased AST, increased ALT, and liver disease were analyzed. There was no significant difference between general/non-ICU group and severe/ICU group for the prevalence of nausea and vomiting (OR=0.97, 95% CI: 0.45-2.08, P=0.927) and liver disease (OR=1.20, 95% CI: 0.48-2.97, P=0.695). Patients in severe/ICU group were more likely to have diarrhea (OR=1.65, 95% CI: 1.04-2.62, P=0.033), anorexia (OR=2.19, 95% CI: 1.21-3.93, P=0.009), abdominal pain (OR=6.38, 95% CI: 1.77-22.91, P=0.005), increased AST (OR=2.98, 95% CI: 2.11-4.21, P<0.0001) and increased ALT (OR=2.66, 95% CI: 1.11-6.37, P=0.029) compared with the patients in general/non-ICU group (Fig. S10-S14 in Supplementary Appendix, Supplemental Digital Content 10, http://links.lww.com/JCG/A631, Supplemental Digital Content 11, http://links.lww.com/JCG/A632, Supplemental Digital Content 12, http://links.lww.com/JCG/A633, Supplemental Digital Content 13, http://links.lww.com/JCG/A634, Supplemental Digital Content 14, http://links.lww.com/JCG/A635). Heterogeneity was not significant in the above analysis apart from the prevalence of nausea and vomiting and increased ALT.
DISCUSSION
In the last 2 decades, the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) had a great effect on global health, the emergence of 2019-nCoV marked the third introduction of a highly morbific coronavirus which could cause epidemics with variable severity of respiratory and extra-respiratory symptoms into the human population.37 2019-nCoV is the seventh member of the human-infecting coronaviruses family which had 88% identity with 2 bat-derived SARS-like coronaviruses, bat-SL-CoVZC45, and bat-SL-CoVZXC21, and was 79% related to SARS-associated coronavirus (SARS-CoV), 50% related to MERS-associated coronavirus (MERS-CoV), respectively.38,39 The main mode of COVID-19 transmission is via droplets and contact, positive reverse-transcription polymerase chain reaction (RT-PCR) test for viral RNA from oropharyngeal or nasopharyngeal swabs can help doctors make a quick diagnosis. Notably, laboratories in China declared that they isolated 2019-CoV from stool specimens of patients with COVID-19.40 A study of 18 patients in Singapore shown that 2019-nCoV was detectable in the stools in 50% of the patients.41 The first confirmed case in the United State had a history of nausea and vomiting for 2 days followed by diarrhea and abdominal discomfort.42 Patients could have negative PCR test in oropharyngeal swabs together with positive results in stools. The existence duration of viral RNA in patients’ stool specimens is longer than in oropharyngeal swabs, which was essential during convalescence.43 Besides, angiotensin converting enzyme II (ACE2) which was the cell entry receptor of SARS-CoV has also been confirmed as the cell entry receptor of 2019-nCoV, which could influence the expression of neutral amino acid transporters in gut, change the composition of the gut microbiota, and cause diarrhea and intestinal inflammation.44,45 All the information revealed that COVID-19 could influence GI manifestations, and fecal-oral might contribute to the transmission of COVID-19.
Common GI symptoms include nausea, vomiting, diarrhea, abdominal pain, and anorexia. The study of Guan et al8 showed that 5% of the patients had nausea and vomiting and 3.8% of the patients had diarrhea. The study of Zhang et al31 showed that 12.2% of the patients had anorexia, 5.8% of the patients had abdominal pain, and 5% of the patients had belching. However, the study of Wang et al9 showed that 10.1% of the patients had nausea and diarrhea, 39.9% of the patients had anorexia. In this meta-analysis, 7% of the patients had nausea and vomiting, 8% of the patients had diarrhea, 3% of the patients had abdominal pain, and 17% of the patients had anorexia. After reviewing previous studies, we found that the prevalence of GI symptoms of COVID-19 was lower than that of SARS and MERS. Previous studies showed that 40% to 70% of the patients had self-limited watery diarrhea, and 20% to 35% of the patients had nausea and vomiting in SARS infection.46,47 Besides, at least one-third of the patients got GI symptoms in MERS infection.48 Just as the description in above paragraph, ACE2 was identified as cell receptor of 2019-nCoV. Previous study showed that abundant presentation of ACE2 was discovered in esophagus keratinocytes, stomach epithelial cells, intestinal epithelial cells, and colon colonocytes. ACE2 might provide possible routes of entry for 2019-nCoV and played an important role in the GI infection of COVID-19.49,50 In order to treat the disease, multiple types of antiviral agents and antibiotic agents were used. Pharmacy expert consensus in China indicated that lopinavir and ritonavir could cause nausea, diarrhea, and vomiting.51 A previous study showed that the most common adverse events (AEs) of combination therapy of oseltamivir, amantadine, and ribavirin for the treatment of influenza were nausea, diarrhea, and vomiting.52 The use of antibiotic agents could change the composition and metabolic function of the intestinal microflora, which was essential to the immunity and metabolism. Antibiotic agents might cause dysbacteriosis and contributed to antibiotic-associated diarrhea (AAD).53,54 The clinical manifestations of AAD varied from mild to severe, severe AAD could have nausea, fever, abdominal pain, and severe watery diarrhea, some patients could have megacolon, even death. Common opportunistic pathogen of severe AAD was Clostridium difficile, a meta-analysis revealed that the most commonly used antibiotic agents were clindamycin, fluoroquinolones, and cephalosporins in clinical practice, and the frequency of clostridium difficile among AAD was 20.2%.55 Therefore, it is necessary to avoid blindly using medicine, especially the abuse of combination therapy of antiviral agents or antibiotics. A meta-analysis shown that probiotics could reduce risk of AAD in adults, however, the effect in elderly population was not significant.56 It reminded us that probiotics could be used to relieve GI symptoms.
A considerable number of patients could have abnormal liver function, primarily presented as increased AST, ALT, and TBil. Besides, some patients with normal liver function at baseline could have liver insufficiency during the disease process. In the study of Guan et al,8 the prevalence of increased AST, ALT, TBil were 22%, 21.3%, and 10.5%, respectively. In the study of Chen et al,7 1 patient had severe liver function damage with extremely increased ALT (7590 U/L) and AST (1445 U/L). Besides, the study of Zhao et al33 reported 44.44% of the patients had increased γ-glutamyl, and compared with other types of pneumonia, COVID-19 could cause liver dysfunction more frequently. The study of Liu et al20 indicated that 17% of the patients, who did not have liver damage at baseline, got liver dysfunction during the process of disease. In this meta-analysis, 24% of the patients had increased AST, 25% of the patients had increased ALT, and 13% of the patients had increased TBil. Reviewing previous studies of SARS and MERS, we found that up to 60% patients could have abnormal liver function in SARS, large numbers of virus particles was found in liver, the liver function impairment was associated with the direct attack of coronavirus or the immune response of viral infection in SARS.57,58 The study of Assiri et al59 shown that 15% of the patients had increased AST and 11% of the patients had increased ALT in MERS. MERS-CoV could bind with host cell dipeptidyl peptidase 4 (DPP4) receptor to enter host cells, and DPP4 was widely expressed on the tissues of liver, which might explain the liver impairment by MERS-CoV.60,61 In COVID-19, the liver damage might be associated with several factors. The previous studies shown that ACE2 could express in liver cells and biliary epithelial cells, the level of expression in biliary epithelial cells was similar to that in alveolar type 2 cells in lung, and higher than that in liver cells, which might explain the liver damage in COVID-19.50,62 Besides, pharmacy expert consensus in China indicated that lopinavir and ribavirin should been used with caution with the AEs of liver damage.51 There was a case report of liver dysfunction and alimentary tract hemorrhage caused by oseltamivir in a child, although the AEs of the drug were mostly mild.63 Therefore, it is essential to monitor the liver function and avoid intensive use of drugs which could cause liver damage, especially in severe patients. Protecting liver therapy could be applied if necessary.
Some patients had digestive system disease and liver disease at the baseline. The study of Chen et al7 showed that 11% of the patients had digestive system disease. The study of Zhang et al31 showed that 5.7% of the patients had fatty liver and abnormal liver function, 5% of the patients had chronic gastritis and gastric ulcer, 4.3% patients had cholelithiasis, and 6.4% of the patients received cholecystectomy at the baseline. Compared with other types of viral pneumonia, patients infected by COVID-19 had similar prevalence for liver disease in the study of Bai et al.11 In this meta-analysis, 5% of the patients had digestive system disease and 3% of the patients had liver disease. A meta-analysis revealed that the pooled mean prevalence of fatty liver disease was 16.73% in China.64 The prevalence of gastro-oesophageal reflux symptoms was 2.5% in China.65 The prevalence of gallstone diseases ranged from 4% to 73% around the world.66 Although we did not acquire all the epidemiological data of digestive system disease and liver disease, it seemed that we could not say people with digestive system disease and liver disease were more likely to have COVID-19.
Compared with non-Wuhan group, the prevalence of nausea and vomiting, diarrhea, abnormal liver function, and digestive system disease was higher in Wuhan group. In Wuhan region, severe patients were admitted to designated hospitals in concentrated way, such as Jinyintan Hospital and Zhongnan Hospital, and the studies included in Wuhan group were mostly from these designated hospitals. The proportion of severe patients in these hospitals might be higher than hospitals in other provinces whose patients were more sporadic. The study of Huang et al6 which included patients infected early in the COVID-19 outbreak showed that the mortality rate of COVID-19 was 15%. However, in the study of Guan et al8 which included 1099 cases from 552 hospitals in 30 provinces, autonomous regions in China, the mortality rate is 1.4%. The variation might because of the emergence of more mild-moderate patients.
Compared with general/non-ICU group, the patients in severe/ICU group were more likely to have diarrhea, anorexia, abdominal pain, increased AST, and increased ALT in this meta-analysis. However, there were no significant differences between severe/ICU group and general/non-ICU group for the prevalence of nausea and vomiting and liver disease. The study of Wang and colleagues reported 5% of the patients had vomiting, 14% of the patients had diarrhea or nausea, and there was no significant difference between ICU patients and non-ICU patients. However, patients in ICU were more likely to have abdominal pain, anorexia, and abnormal liver function.9 In the study of Zhang et al,31 there was no difference for the prevalence of underlying digestive system disease between severe patients and nonsevere patients. Study of Yang et al29 showed 29% of the ICU patients had liver dysfunction. The patients with hepatic insufficiency usually had multiple complications such as acute respiratory distress syndrome, respiratory failure, and renal insufficiency, therefore, the liver damage might be the reflection of disease severity and be induced by cytokine release syndrome (CRS). CRS was a systemic inflammatory response caused by a variety of factors such as infection and drugs which could lead to multiorgan system failure, hepatomegaly, hypofibrinogeniemia, liver failure, and abnormal liver enzymes were common in CRS.67 Besides, the severe patients usually received combination therapy of antivirals agents and antibiotics which could increase the burden of liver.
There were limitations in this meta-analysis. First, classification of digestive system disease and liver disease was unclear in most studies. Second, few studies have performed colonoscopy to observe colon directly, or biopsy of liver and colon to identified the pathologic features. Third, quite a part of included studies had small samples. Most studies were published in China, studies from other countries outside China were needed for further analysis. Besides, the heterogeneity between several studies were high because of varies sample size and different severity among the cases.
In conclusion, we have analyzed the prevalence of GI symptoms, abnormal liver function, digestive system disease, and liver disease using different set of studies and patients. Diarrhea and anorexia were the most common GI symptoms in patients with COVID-19. COVID-19 could cause liver damage in different extent, the most significant abnormal liver function was increased ALT. The prevalence of digestive system disease and liver disease was 5% and 3%, respectively. The prevalence of nausea and vomiting, diarrhea, abnormal liver function, digestive system disease, and liver disease was higher in Wuhan group. Severe patients were more likely to have GI symptoms and abnormal liver function. More researches were needed to explore the relevant mechanism of the outcomes.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.
Footnotes
Z.-y.D.: carried out the literature search, selection, validity assessment, data abstraction and data analysis. B.-j.X. and M.-j.S.: provided relevant support. Z.-y.D. and C.D.: wrote the paper and incorporated the comments from other authors and peer reviewers. C.D. and M.J. had the original idea for the paper, formulated the protocol, and contributed to data abstraction and analysis.
Supported by Liaoning Science and Technology Foundation (No 20170541052), but the work was independent of it.
The authors declare that they have nothing to disclose.
REFERENCES
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;94:401–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui DS, I Azhar E, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it [World Health Organization]. Available at: www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. Accessed April 6 2020.
- 4.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 3 April 2020 [World Health Organization]. 2020. Available at: www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19--3-april-2020. Accessed April 6 2020.
- 5.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [World Health Organization]. 2020. Available at: www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed April 6 2020.
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W, Ni Z, Hu Y, et al. China medical treatment expert group for C. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Jiang ZC, Shao CX, et al. Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study [Chinese]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:148–152. [DOI] [PubMed] [Google Scholar]
- 11.Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020;296:E46–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu J, Yang N, Wei Y, et al. Clinical characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan, China. J Med Virol. 2020;92:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020;295:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong X, Cao YY, Lu XX, et al. Eleven faces of coronavirus disease 2019. Allergy. 2020;75:1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Easom N, Moss P, Barlow G, et al. 68 consecutive patients assessed for COVID-19 infection; experience from a UK regional infectious disease unit. Influenza And Other Respiratory Viruses. 2020;14:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lian J, Jin X, Hao S, et al. Analysis of epidemiological and clinical features in older patients with Corona Virus Disease 2019 (COVID-19) out of Wuhan. Clin Infect Dis. 2020;71:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. [DOI] [PubMed] [Google Scholar]
- 19.Liu K, Fang Y-Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133:1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV [Chinese]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:450–455. [DOI] [PubMed] [Google Scholar]
- 23.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Chen X, Lu Y, et al. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–68. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020;71:706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Wu X, Jiang X, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang G, Zhang J, Wang B, et al. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. [DOI] [PubMed] [Google Scholar]
- 32.Zhang MQ, Wang XH, Chen YL, et al. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:215–218. [DOI] [PubMed] [Google Scholar]
- 33.Zhao D, Yao F, Wang L, et al. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis. 2020;71:756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W, Zhong Z, Xie X, et al. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) Pneumonia: A Multicenter Study. AJR. 2020;214:1072–1077. [DOI] [PubMed] [Google Scholar]
- 35.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z, Zhao N, Shu Y, et al. Effect of gastrointestinal symptoms on patients infected with COVID-19. Gastroenterology. 2020;158:2294–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Wit E, van Doremalen N, Darryl F, et al. SARS and MERS: recent insights into emerging coronaviruses. Nat ev Microbiol. 2016;14:523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158:1518–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young BE, Ong SWX, Kalimuddin S, et al. Singapore novel coronavirus outbreak research T. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J. 2020;28:1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perlot T, Penninger JM. ACE2 - from the renin-angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15:866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung WK, To KF, Chan PK, et al. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical Features. Infect Dis Clin North Am. 2019;33:869–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Group WM-CR. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi F, Qian S, Zhang S, et al. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chinese Pharmaceutical Association. Novel coronavirus infection: guidance and prevention in hospital pharmacy expert consensus on control strategy (First Edition). [Chinese Pharmaceutical Association]. 2020. Available at: http://www.cpa.org.cn/?do=info&cid=75148 Accessed April 6 2020.
- 52.Beigel J, Bao Y, Beeler J, et al. Oseltamivir, amantadine, and ribavirin combination antiviral therapy versus oseltamivir monotherapy for the treatment of influenza: a multicentre, double-blind, randomised phase 2 trial. Lancet Infect Dis. 2017;17:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silverman MA, Konnikova L, Gerber JS. Impact of antibiotics on necrotizing enterocolitis and antibiotic-associated diarrhea. Gastroenterol Clin North Am. 2017;46:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neuman H, Forsythe P, Uzan A, et al. Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol Rev. 2018;42:489–499. [DOI] [PubMed] [Google Scholar]
- 55.Nasiri M, Goudarzi M, Hajikhani B, et al. Clostridioides (Clostridium) difficile infection in hospitalized patients with antibiotic-associated diarrhea: a systematic review and meta-analysis. Anaerobe. 2018;50:32–37. [DOI] [PubMed] [Google Scholar]
- 56.Jafarnejad S, Shab-Bidar S, Speakman J, et al. Probiotics reduce the risk of antibiotic-associated diarrhea in adults (18-64 years) but not the elderly (>65 years): a meta-analysis. Nutr Clin Pract. 2016;31:502–513. [DOI] [PubMed] [Google Scholar]
- 57.Chau T, Lee K, Yao H, et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. Journal Pathol. 2004;203:622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boonacker E, Van Noorden CJ. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol. 2003;82:53–73. [DOI] [PubMed] [Google Scholar]
- 61.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020. [Epub ahead of print]. [Google Scholar]
- 63.Fang S, Qi L, Zhou N, et al. Case report on alimentary tract hemorrhage and liver injury after therapy with oseltamivir: a case report. Medicine. 2018;97:e12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J, Zhou Q, Wang Y, et al. Prevalence of fatty liver disease and the economy in China: a systematic review. World J Gastroenterol. 2015;21:5695–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67:430–440. [DOI] [PubMed] [Google Scholar]
- 66.Cen L, Pan J, Zhou B, et al. Helicobacter pylori infection of the gallbladder and the risk of chronic cholecystitis and cholelithiasis: a systematic review and meta-analysis. Helicobacter. 2018;23:12457. [DOI] [PubMed] [Google Scholar]
- 67.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.


