Abstract
The scale at which humans can handle complex social situations is massively increased compared to other animals. However, the neural substrates of this scaling remain poorly understood. In this review, we discuss how the expansion and rearrangement of the temporoparietal junction and posterior superior temporal sulcus (TPJ-pSTS) may have played a key role in the growth of human social abilities. Comparing the function and anatomy of the TPJ-pSTS in humans and macaques, which are thought to be separated by 25 million years of evolution, we find that the expansion of this region in humans has shifted the architecture of the dorsal and ventral processing streams. The TPJ-pSTS contains areas related to face-emotion processing, attention, theory of mind operations, and memory; its expansion has allowed for the elaboration and rearrangement of the cortical areas contained within, and potentially the introduction of new cortical areas. Based on the arrangement and the function of these areas in the human, we propose that the TPJ-pSTS is the basis of a third frontoparietal processing stream that underlies the increased social abilities in humans. We then describe a model of how the TPJ-pSTS areas interact as a hub that coordinates the activities of multiple brain networks in the exploration of the complex dynamic social scenes typical of the human social experience.
Introduction
The ability to function in dynamic and complex social situations is one of the traits that make humans unique amongst other species (Buckner & Krienen, 2013). While other animals do function in social groups and hierarchies, none have been able to adapt to and thrive in the type of complex environments that characterize human social interactions, such as those posed by walking through Times Square in New York City or attending the 30,000+ Society for Neuroscience conference (Herrmann, Call, Hernàndez-Lloreda, Hare, & Tomasello, 2007). Social cognition consists of multiple domains, both verbal and non-verbal. Non-verbally, for instance, we must be able to quickly scan faces in a room to read ‘the mood’ of a social gathering or read the body positions of people as we enter a bar (Zaki & Ochsner, 2009). Verbally, we must be able to extract the hidden ‘emotional’ meaning of apparently neutral sentence during a job interview. While the brain areas involved in processing social stimuli are distributed across cortex, a region in the right temporoparietal junction-posterior superior temporal sulcus (TPJp-STS) appears especially important. The TPJ-pSTS brings into proximity posterior STS areas involved in processing biological motion (such as facial expressions), nodes of the ToM network, and nodes of the attention networks, along with other areas that may be relevant to processing social information. In this review, we will discuss how the expansion and rearrangement of this region in the course of evolution in humans vs. macaques supports these non-verbal social functions, and then propose a new framework for the dynamic interaction of these areas during social cognition.
Anatomy and evolution of TPJ-pSTS
In this review, we use the term TPJ-pSTS to encompass a set of cortical areas and structures that spans the inferior parietal lobule to the posterior STS in the dorsal-ventral axis, and the angular gyrus, and posterior aspect of the supramarginal gyrus in the caudal-rostral axis (see Figure 1A). We use this label to provide a unifying term for these regions and areas that are often discussed separately in other studies and reviews. In this section, we review the similarities and differences in the anatomy of the TPJ-pSTS in humans vs. macaques, the model system most often used to study cognition in primates.
Figure 1:
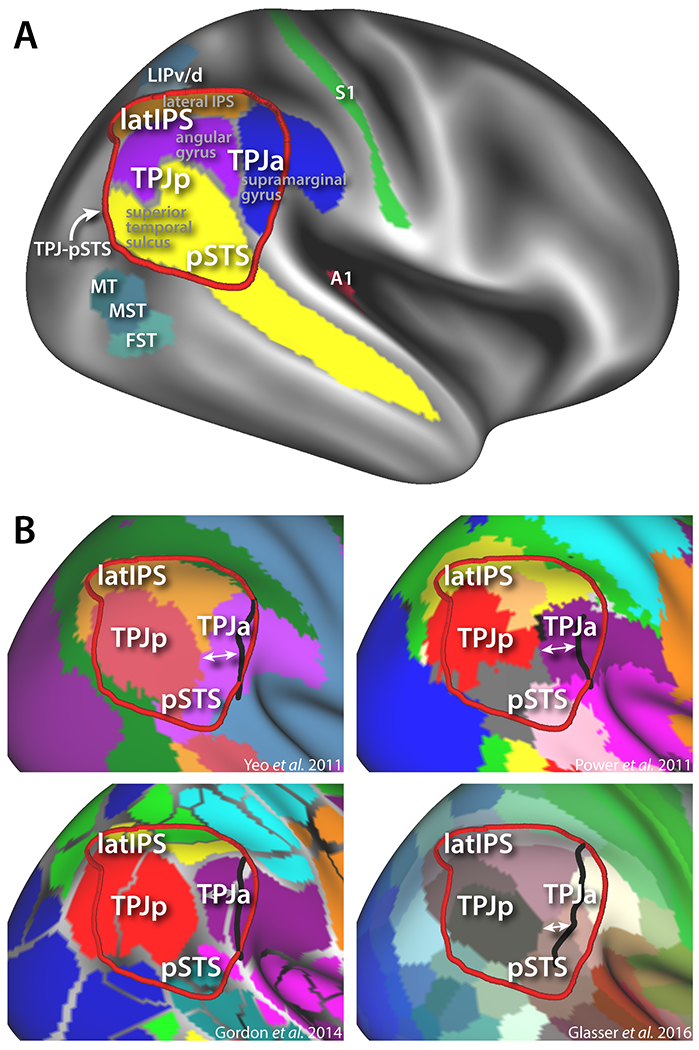
Structural and functional anatomy of the human TPJ-pSTS A) Approximate definition of this region compared to major sulcal/gyral features (derived from FreeSurfer labels) and functional areas (from HCP parcellation scheme (Glasser et al., 2016)). B) Different resting state parcellation schemes of TPJ-pSTS showing difference in posterior border of right anterior TPJ parcellation (white arrows) vs. left hemisphere homologues projected to right hemisphere (black border).
The human TPJp-STS is a large associative cortical region bordered by multiple sensory systems. Posteriorly and inferiorly to this region are extrastriate and ventral-occipital visual areas, such as the MT complex (MT/MST/FST in Figure 1A). Antero-ventrally to this region, in the Sylvian fissure, is auditory cortex (represented by A1), and antero-dorsal is the somatosensory system (represented by S1). Superior to this region are the parietal ocuolomotor/attention areas (LIPv/d). This region is one of the last to develop in humans, maturing in young adulthood at a similar time as prefrontal cortex (Hill et al., 2010; Sotiras et al., 2017). It is also one of the most morphologically variable between individuals (Croxson, Forkel, Cerliani, & Thiebaut de Schotten, 2017; Mueller et al., 2013; van Essen & Dierker, 2007a), perhaps a sign of its role in high-level integrative cognitive operations (Buckner & Krienen, 2013).
A number of parcellation schemes for dividing human cortex into individual areas have been recently proposed (Glasser et al., 2016; Gordon et al., 2014; Power et al., 2011; Yeo et al., 2011), with the aim of mapping the locations and boundaries of higher-level cortical areas that do not have clear macaque homologues. However, because of the individual morphological variability of this region, combined with its complex functional architecture, the parcellation of the TPJ-pSTS into cortical areas has been difficult. Numerous schemes have been proposed, often with little consensus (see Figure 1B). However, certain themes appears consistent. A major theme is a right-left asymmetry in the organization of the TPJ-pSTS. This is evident when comparing the effect of lesions to opposite hemispheres: whereas left TPJ strokes often result in language impairment (aphasia), right TPJ is one of the sites most commonly lesioned in strokes that cause visuospatial neglect (Bartolomeo, 2014; Corbetta & Shulman, 2011). Moreover, functional MRI studies in healthy subjects have found multiple functions to be asymmetrically located in the right TPJ-pSTS, including orienting of attention, theory of mind operations, and face-emotion recognition (reviewed in more detail below). Parcellation schemes based on resting state-functional connectivity and task fMRI studies have also found asymmetries in the organization of this region, with several studies finding large discrepancies in the border locations of purportedly homologous networks between hemispheres (Gordon et al., 2014; Power et al., 2011; Yeo et al., 2011).
This fits with the differences in the patterns of cortical expansion in the right versus left TPJ detailed in Van Essen et al. (van Essen, Glasser, Dierker, Harwell, & Coalson, 2011). In particular, the border of the anterior TPJ parcel (TPJa), usually located on the supramarginal gyrus, is often more posterior in the right versus left hemisphere (Figure 1B, the black line indicates the corresponding posterior border of anterior TPJ in the left hemisphere). Functionally, the TPJa is involved in the orienting of attention (Corbetta, Patel, & Shulman, 2008; Patel et al., 2015). Ventrally, in the pSTS, this parcel is either extended (Yeo et al., 2011) (Figure 1B top left panel), part of a series of areas running perpendicular to the STS (Glasser et al., 2016; Igelström, Webb, Kelly, & Graziano, 2016) (Figure 1B bottom right panel), or is ambiguous in areal assignment and difficult to parcellate (Power et al., 2011) (gray in Figure 1B top right panel). The pSTS overlaps with areas involved in face-emotion recognition and processing biological motion. Posterior to the TPJa on the angular gyrus is the TPJp, an area whose definition is generally agreed upon by task, functional connectivity, and DTI parcellation schemes, and is most often identified as a node of the default mode network (Raichle et al., 2001) or as part of the theory of mind network (Mars, Sallet, et al., 2012b). Superior to the TPJa/p on the lateral bank of the IPS (latIPS) is a region spanning the length of the inferior parietal lobule, implicated variously as a part of the frontoparietal task-control network (Dosenbach et al., 2007; Nelson et al., 2010) and additionally involved in retrieval of information from long-term memory (Sestieri, Shulman, & Corbetta, 2017). Architectonic parcellations may coarsely align with these subdivisions in the anterior-posterior axis, but do not appear to capture the dorsal-ventral organization (Caspers et al., 2008; Igelström & Graziano, 2017).
In macaques, the candidate TPJ-pSTS region shares some anatomical similarities. In addition to a similar architectonic/connectivity based parcellation (Caspers et al., 2008; 2012; Gregoriou, Borra, Matelli, & Luppino, 2006; Rozzi et al., 2006), the macaque TPJ-pSTS, located in the macaque IPL, is also surrounded by visual, somatosensory, auditory, and attention/oculomotor areas (see Figure 2A and B). In addition, signatures of purportedly homologous functional areas have been observed within this region, including the lateral parietal node of the default mode network (Mantini et al., 2011; Vincent et al., 2007), a theory of mind area (Sliwa & Freiwald, 2017), and neurons demonstrating attention-reorienting activity (Constantinidis & Steinmetz, 2001). However, major anatomical differences are apparent when comparing this region between the two species. Specifically, the TPJ-pSTS is one of the most expanded cortical regions in humans versus macaques (see Figure 2C) (Hill et al., 2010). This is true regardless of whether the comparison is performed by landmark registration (Hill et al., 2010) or by cortical thickness (Sotiras et al., 2017).
Figure 2:
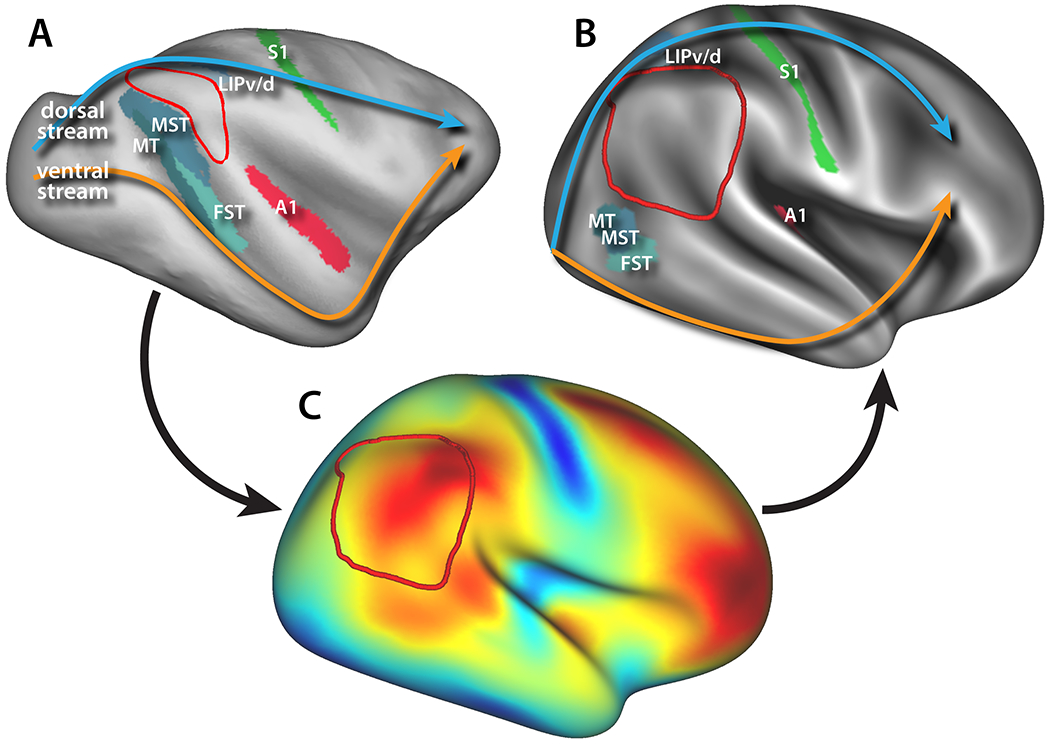
Location and extension of the TPJ-pSTS (red border) in A) macaques vs. B) humans compared to homologous sensory areas. Arrows schematically illustrate the dorsal (blue) and ventral (orange) visual pathways. C) Cortical deformation map for registering macaque cortex to human from Hill et al. (Hill et al., 2010).
The cortical expansion is most obvious when considering the relative positions of MT, LIP, and auditory cortex in macaques versus their purported homologues in humans (Figure 2A and B). While auditory cortex lies inside of the Sylvian fissure in both species, the other two areas have been pushed away from the TPJ-pSTS (Krubitzer, 2009). MT in macaques is in the posterior/inferior bank of the STS in macaques, but in humans the homologous areas have been pushed out of the STS ventrally to the junction of the inferior temporal and lateral occipital sulci (Orban, van Essen, & Vanduffel, 2004). Similarly, area LIP in macaques is on the lateral bank of the intraparietal sulcus and thus on the inferior parietal lobule, but the human homologues are pushed superiorly, out of the intraparietal sulcus and onto the dorsal aspect of the superior parietal lobule (Glasser et al., 2016; Patel et al., 2015). This massive expansion of the TPJ-pSTS had the effect of pushing apart the dorsal and ventral stream visual pathways (see arrows in Figure 2A and B), opening this region to elaboration and/or rearrangement of already-present areas or allowing for the emergence of new areas with new, high-level functions (Buckner & Krienen, 2013). The TPJ-pSTS is also one of the most morphologically variable between individuals, another potential indicator of its late evolution (Buckner & Krienen, 2013; Croxson, Forkel, Cerliani, & Thiebaut de Schotten, 2017; Mueller et al., 2013; van Essen & Dierker, 2007).
Below we discuss evidence that this expansion and rearrangement has allowed for the TPJ-pSTS to shift from sensory integration to the integration of the cognitive operations necessary for complex social functioning. It is important to note that it is not clear whether the need for increased social cognitive abilities drove the expansion and/or the rearrangement of the TPJ-pSTS, or that the TPJ-pSTS expanded first, which then allowed for the cortical areas to be expanded and rearranged to subserve social cognitive processes as social complexity increased. For excellent discussions of this point, see reviews by Buckner and Krienen (Buckner & Krienen, 2013), Krubitzer and Seelke (Krubitzer & Seelke, 2012), Dehaene and Cohen (Dehaene & Cohen, 2007), and Dunbar (Dunbar, 2016). Cortex appears to have expanded well before increased societal complexity driven by the agricultural revolution (Buckner & Krienen, 2013; Harari, 2015), suggesting cortical “recycling” (Dehaene & Cohen, 2007) may be a potential explanation for the evolution of the function of the TPJ-pSTS. On the other hand, the ability to form and maintain complex and dynamic dyadic relationships may have evolved first, reflected in the expansion of the TPJ-pSTS, which then allowed for the creation of the complex social structures necessary for the agricultural revolution (Dunbar, 2016). In any case, the discussion below does not depend on the ordering of these events in evolution.
Functional Reorganization of TPJ-pSTS
pSTS Face-Emotion Recognition Areas
One system in the TPJ-pSTS that has dramatically reorganized is the face processing network, and in particular the circuit responsible for face-emotion recognition. In macaques, the face processing network comprises of two parallel streams of areas (see Figure 3A), one running along the inferior temporal gyrus and the inferior bank of the STS (ML, AL, and AM, pink arrow), and one running superior to this in the fundus and superior bank of the STS (MF and AF, blue arrow) (Tsao, Moeller, & Freiwald, 2008). Of these two streams, the superior MF/AF streams appear more involved in motion processing, with MF being adjacent to MT/MST/FST complex and potentially overlapping with LST, another motion processing area (Nelissen, Vanduffel, & Orban, 2006; Polosecki et al., 2013). In addition, injection of muscimol into the fundus of STS in or near MF interferes with use of eye-gaze information to direct attention (Roy, Shepherd, & Platt, 2014), suggesting that this stream is more involved in the processing of moving facial expressions as opposed to facial identity. There also appears to be a posterior to anterior hierarchy in the face processing, with anterior areas (AF, AL, and AM) integrating information from posterior areas (ML and MF) (Freiwald & Tsao, 2010; Schwiedrzik, Zarco, Everling, & Freiwald, 2015). The posterior areas connect to the TPJ node of the macaque default-mode network, whereas the anterior areas demonstrate more frontal connectivity (Schwiedrzik et al., 2015).
Figure 3:
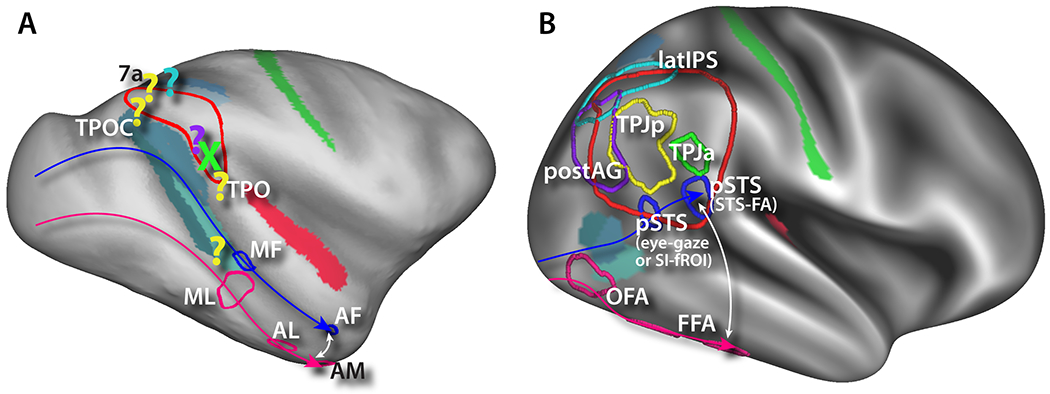
Functional architecture of TPJ-pSTS in macaques vs. humans. A) Macaque TPJ-pSTS homologues. Face patches derived from (Schwiedrzik et al., 2015). Other homologues locations projected from published cortical surface data (see text for citations). B) Human TPJ-pSTS functional areas. Face patches, TPJp, and TPJa derived from unpublished task fMRI localizer data. PostAG and latIPS borders projected from (Sestieri et al., 2010; 2011) and (Sestieri et al., 2014), respectively.
Like in macaques, the human pSTS appears to be involved in the processing of biological motion, with a major portion devoted to processing moving facial expressions (the STS-FA) (Grossman, Battelli, & Pascual-Leone, 2005; Pelphrey, Morris, Michelich, Allison, & McCarthy, 2005; Polosecki et al., 2013) (see Figure 3B). According to a recent theory, the pSTS is generally involved in making predictions on a short time-scale about biological mechanics—i.e. predicting the trajectory of an arm or facial feature once it has started to move (Koster-Hale & Saxe, 2013). In humans, however, this system has been substantially re-arranged. Not only have the inferior stream face-processing areas been moved ventrally to ventral occipitotemporal cortex (Figure 3B pink arrow), culminating in the fusiform face areas (FFA), but the superior stream has been angled to be perpendicular to the STS (blue arrow). Similar to macaques, this stream starts adjacent to the MT/MST/FST complex with an eye-gaze processing area (now on the lip of the inferior bank of the pSTS (Glasser et al., 2016; Marquardt, Ramezanpour, Dicke, & Thier, 2017), but instead of advancing along the STS, it culminates in the STS-FA on the opposite bank of the pSTS (Polosecki et al., 2013; Tsao et al., 2008). This puts the two ends of the streams centimeters apart in temporal cortex, potentially anchoring another massive expansion of cortex along the mid to anterior STS (compare white arrows in Figure 3A and 3B. This also puts the STS-FA much closer to the TPJ in humans than in macaques.
TPJa in Orienting of Attention
Immediately superior to the STS-FA in humans is the TPJa (Mars, Sallet, et al., 2012b), originally labeled the TPJ by Corbetta and Shulman (Corbetta et al., 2008; Corbetta & Shulman, 2002) (Figure 3B green border). This area was originally posited to be involved in the stimulus-driven reorienting of attention (Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000), but only to behaviorally relevant stimuli (Downar, Crawley, Mikulis, & Davis, 2000; Kincade, Abrams, Astafiev, Shulman, & Corbetta, 2005). This hypothesis was based on the stronger response of TPJ, and other regions involved in attention/eye movements, such as the parietal eye region (LIP) and FEF, to unexpected sensory stimuli that require a motor response. It was proposed that this region is involved in signaling the occurrence of a behaviorally relevant, yet unexpected stimuli (Downar et al., 2000; Serences et al., 2005; Shulman et al., 2009), and that this signal acts a ‘circuit-breaker’ interrupting ongoing activity in dorsal attention/eye movement regions re-directing the locus of processing to the new location of interest (Corbetta et al., 2008; Corbetta & Shulman, 2002). In addition, it was found that the magnitude of deactivation of this area predicts accuracy of detection on a foveal visual discrimination task (Shulman, Astafiev, McAvoy, d’Avossa, & Corbetta, 2007; Shulman et al., 2003; Todd & Marois, 2004). Our interpretation was that suppression of the mechanism for reorienting attention to irrelevant sensory stimuli (distracters) is active when subjects primarily focus their attention to important stimuli. Recent studies have further separated TPJa into two halves (Dugué, Merriam, Heeger, & Carrasco, 2017; Igelström et al., 2016), with the posterior half (labeled vTPJ in (Dugué et al., 2017)) specifically involved in attentional reorienting and corresponding to the original TPJ definition proposed by Corbetta and Shulman (Corbetta et al., 2008; Dugué et al., 2017; Igelström et al., 2016; Patel et al., 2015; Shulman et al., 2003; 2009), and the anterior portion selectively involved in target detection (Igelström et al., 2016).
In the macaque, however, the TPJa has no clear homologue. Single-unit electrophysiology studies initially found evidence of attention orienting signals in area 7a, the architectonic area that covers much of the macaque TPJ-pSTS on the dorsolateral surface of the IPL (Constantinidis & Steinmetz, 2001; Steinmetz, Connor, Constantinidis, & McLaughlin, 1994). A comparative fMRI study also found a macaque homologue that was roughly anatomically homologous and shared similarly movie-synchronized BOLD activity (Mantini, Corbetta, Romani, Orban, & Vanduffel, 2013) (Figure 3A green X). However, in a study specifically designed to elucidate the macaque TPJa homologue, Patel et al. did not find the target detection or search deactivation signals that robustly define the TPJa in humans (Patel et al., 2015).
Moreover, theories about the role of the TPJa in orienting of attention have continued to develop. For instance, the timing of task-related neural activity in the TPJa was found to be too delayed to be the source of reorienting signals. In fact, visually evoked responses by targets that induce reorienting of attention peak in TPJa relatively late at/near ~300 ms, a latency not compatible with latency of an eye movements and/or attention shift (~200 ms) (Corbetta et al., 2008; Daitch et al., 2013; Spadone et al., 2015). Accordingly, TPJa is now thought to be involved in higher-level cognitive operations than just stimulus-driven reorienting, including the post-perceptual updating of internal models of environmental context (Geng & Vossel, 2013). A version of this idea is that TPJa indicates the end of a task by sending a reset signal that interrupts ongoing activity in task-relevant regions (a sort of cognitive offset signal (Corbetta et al., 2008; Jack, Shulman, Snyder, McAvoy, & Corbetta, 2006; Shulman, Ollinger, Linenweber, Petersen, & Corbetta, 2001)). This theory still fits with the involvement of TPJa in attention- and detection-related processes, but additionally explains a) the late onset response of TPJ; b) the overall greater response to behaviorally relevant but unexpected stimuli (not necessarily indicating a mismatch in space); and c) the similarity between TPJ pattern of response and the P300 evoked response, which has been also interpreted as underlying late post-perceptual updating (Aston-Jones & Cohen, 2005; Corbetta et al., 2008; Geng & Vossel, 2013). A temporally delayed response and behaviorally relevant sensory-evoked modulation also fits prediction-error models of TPJp function (see below) (Koster-Hale & Saxe, 2013). Another not-incompatible theory is that the TPJa is involved in the conscious awareness of novel stimuli, with the neural processes underlying awareness lagging those of the actual visual processing of the stimulus (Beauchamp, Sun, Baum, Tolias, & Yoshor, 2012; Webb, Igelström, Schurger, & Graziano, 2016). These newer formulations elevate the complexity of the processes in TPJa from a low-level stimulus-driven reorienting mechanism to a mechanism related to network coordination or prediction (see below).
TPJp in Theory of Mind Operations
Posterior to the TPJa, across the STS and on the angular gyrus, is the TPJp (Decety & Lamm, 2007; Geng & Vossel, 2013; Mars, Sallet, et al., 2012b) (Figure 3B yellow border). This area is involved in the understanding of the mental states of other people, known commonly as theory of mind operations (Saxe & Kanwisher, 2003; Schurz, Radua, Aichhorn, Richlan, & Perner, 2014; Yang, Rosenblau, Keifer, & Pelphrey, 2015). The TPJp heavily overlaps with the anteroventral portion of the default-mode network angular gyrus node. In general, the default-mode network, a network originally defined in humans as an area that deactivates during externally oriented attention-demanding tasks, heavily overlaps with the theory of mind network (Christoff, Irving, Fox, Spreng, & Andrews-Hanna, 2016; Mars, Neubert, et al., 2012a; Raichle et al., 2001; Shulman et al., 1997; Yang et al., 2015).
It has been proposed that the TPJp is involved in making model-based predictions about other people’s beliefs and desires as well as predictions about bio-mechanical movements on a time-scale longer than immediate perception, and in further updating the model based on new incoming sensory information (Geng & Vossel, 2013; Koster-Hale & Saxe, 2013). These prediction-error theories are based on the observation that predicted stimuli evoke lower-amplitude responses compared to unpredicted stimuli; the theory posits that the extra neural activity is generated by the updating the internal model (Koster-Hale & Saxe, 2013). While the terminology may differ, prediction-error theories of TPJp and circuit-breaker theories of TPJa empirically make equivalent hypotheses: unpredicted or unexpected stimuli will evoke a higher amplitude of activity than those that are predicted or expected. The TPJp then communicates with other areas within the theory of mind or default-mode networks, including a medial prefrontal area thought to be involved in making even longer time-scale model-based predictions (Koster-Hale & Saxe, 2013). The default-mode network has been recently linked to the generation of sensory-independent but internally constrained cognition (Andrews-Hanna, Reidler, Huang, & Buckner, 2010; Christoff et al., 2016), consistent with the internal generation and maintenance of internal models of the intentions of other people (Buckner & Carroll, 2007; Mars, Neubert, et al., 2012a).
Multiple studies have located a putative macaque homologue of the default mode network angular gyrus area with an area at the junction of the IPL and superior terminus of the STS, labeled as TPOC (Mantini et al., 2011; Teichert, Grinband, Hirsch, & Ferrera, 2010; Vincent et al., 2007) (Figure 3A yellow question marks). A recent study also found an area just superior of this in area 7a to be activated by social interactions (Sliwa & Freiwald, 2017). However, the overlap between the default mode network and areas activated by social interactions was an area far anterior on the STG, area TPO, which is superior to MF (Schwiedrzik et al., 2015; Sliwa & Freiwald, 2017). This may be similar to the “social interaction” area (SI-fROI) observed in humans in (Isik, Koldewyn, Beeler, & Kanwisher, 2017) in the STS near the STS-FA. Mars et al. found a more extreme discrepancy when searching for macaque TPJ homologues using functional connectivity, with the identified area located in mid-STS cortex near MT/FST, MF and ML (Mars, Sallet, Neubert, & Rushworth, 2013). These observations underscore the expansion and rearrangement of the TPJ-pSTS in humans versus macaques: 1) human TPJp demonstrates robust activation by watching movies of conspecifics interacting or acting independently, whereas the purported macaque homologue in TPOC does not, and 2) macaque TPO is considered to be a part of the default mode network, whereas human SI-fROI is not.
AG and latIPS in Memory Retrieval
Overlapping or posterior to human TPJp (Mars, Sallet, et al., 2012b; Yang et al., 2015) lays the remaining posterodorsal aspect of the DMN angular gyrus node, corresponding to cytoarchitectonic areas PGa and PGp (Caspers et al., 2013; Christoff et al., 2016), here labeled as the postAG (Figure 3B purple border). This area contains heteromodal cortex associated with multiple high level functions (Humphreys & Lambon Ralph, 2015; Seghier, 2013). Several lines of evidence converge on the role of this region in the representation of information retrieved from episodic (Rugg & King, 2017; Sestieri et al., 2017) and semantic (Binder & Desai, 2011) memory, perhaps allowing the construction of multi-purposes event/situation models (Cohn-Sheehy & Ranganath, 2017). This function relies on the presence of functional (Kahn, Andrews-Hanna, Vincent, Snyder, & Buckner, 2008; Vincent et al., 2006) and structural (Kravitz, Saleem, Baker, & Mishkin, 2011; Mufson & Pandya, 1984) connections with medial temporal structures traditionally involved in memory functions (Kravitz et al., 2011; Squire, 1992).
Memory retrieval in humans is associated with activity of another parietal region (Vilberg & Rugg, 2008; Wagner, Shannon, Kahn, & Buckner, 2005), labeled here as latIPS (Sestieri et al., 2017; Vilberg & Rugg, 2008) (Figure 3B cyan border) and located dorsally to areas TPJa and TPJp (roughly corresponding to cytoarchitectonic area hIP1 (Caspers et al., 2013)). The latIPS overlaps with the so-called frontoparietal control network (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Duncan, 2010), which is anatomically interposed between the dorsal attention network and the default-mode network (Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013). The role of the latIPS in memory retrieval differs from that of the postAG in several ways. First, latIPS tracks the feeling of oldness (familiarity) rather than recollection (Hutchinson et al., 2012; Sestieri et al., 2014). Second, it shows sustained activity consistent with a role in active maintenance and manipulation of memories according to task demands (Sestieri et al., 2017; Sestieri, Corbetta, Romani, & Shulman, 2011). Third, it overlaps with the parietal node of the frontoparietal control network, which is thought to regulate the balance between the processing of external (perceptual) information in the dorsal attention network and of internal (introspective) information in the default-mode network (Dixon et al., 2018; Sestieri, Corbetta, Spadone, Romani, & Shulman, 2014a). Finally, it is functionally and anatomically connected with prefrontal, rather than medial temporal, regions (Uddin et al., 2010).
While activity during memory retrieval is typically observed in the left parietal cortex (Duarte, Henson, & Graham, 2011; Guerin & Miller, 2009), retrieval-related activity is also observed in homologue regions of the right hemisphere (e.g. (Klostermann, Loui, & Shimamura, 2009; Sestieri, Shulman, & Corbetta, 2010)), and the degree of hemispheric asymmetry has not been assessed with quantitative indices (Seghier, 2008). On the basis of the importance of memory information for social interactions (Buckner & Carroll, 2007; Spreng, 2013), it is possible to hypothesize that retrieval-related activity in the right parietal areas serve adaptive social processing implemented in adjacent regions, such as the TPJ-pSTS. In particular, while memory representations in the AG might contribute to social knowledge retrieval (Y. Wang et al., 2017) and memory-guided inference of other’s mental states (Spreng & Mar, 2012), the latIPS might help to regulate the balance between exploration of the external world (dorsal attention) and accessing internally stored models of social agents and situations (TPJ-pSTS) in cognition (Dixon et al., 2018).
Compared to humans, evidence for the involvement of monkey parietal lobes in memory functions is scarce. Based on resting-state functional connectivity data, one study hypothesized that that in macaques the area would be on the posterior STG (Vincent, Kahn, van Essen, & Buckner, 2010). Evidence supporting this hypothesis was described in a recent fMRI study employing a serial probe-recognition task in macaques (Miyamoto et al., 2013). The functional and structural properties of two parietal regions, area PG/PGOp (Figure 3A purple question mark) of the inferior parietal lobule and PEa/DIP located in the medial bank of the intraparietal sulcus (cyan question mark) resemble that of the postAG and latIPS in humans, respectively (Miyamoto et al., 2014). If these homologies prove to be true, this would indicate that in humans the parietal site supporting episodic recallection (the postAG) has shifted posteriorly compared to macaque PG/PGOp, while the site supporting the sense of familiarity (latIPS) has shifted ventrally and anteriorly compared to macaque PEa/DIP. However, the homologous relationships are not straightforward: the macaque episodic recall area (Miyamoto et al., 2013) is clearly anterior to the macaque angular gyrus node homologue, TPOC (Mantini et al., 2011); and PEa/DIP is located in macaque areas typically implicated in visual processing, attention, or motor planning (Patel, Kaplan, & Snyder, 2014) whose homologues are in the human SPL (Patel et al., 2015). A study comparing functional connectivity networks in humans and macaques has also found that the fronto-parietal networks that comprise areas involved in memory retrieval may be unique to humans (Mantini et al., 2013).
The TPJ-pSTS: A Newly Evolved Stream of Processing
Based on early macaque histology and neurophysiology studies, visual processing has traditionally been divided into two streams: a dorsal spatially-oriented processing stream and a ventral feature-based processing stream (Kravitz et al., 2011; Mishkin, Ungerleider, & Macko, 1983). Both pathways originate in striate cortex (V1), share a common substrate in extrastriate areas (V2-V4), and then diverge from lateral occipital cortex (V4) towards either parietal (spatial/action) or temporal (feature) cortex (Baizer, Ungerleider, & Desimone, 1991; Ungerleider, Galkin, Desimone, & Gattass, 2008). The dorsal pathway connects to the dorsal attention/oculomotor network areas in parietal and prefrontal cortex (Goldman-Rakic, 1988), and the ventral to the prefrontal cortex via a separate pathway (Kravitz, Saleem, Baker, Ungerleider, & Mishkin, 2013; Webster, Bachevalier, & Ungerleider, 1994). These two pathways are densely interconnected and interact with each other in visual processing (Cloutman, 2013). This has the effect of having mid-level sensory processing areas from all modalities in close proximity to each other around the macaque TPJ-pSTS, and the TPJ-pSTS in close proximity (on opposite sides of a gyrus) to attention/ocuolomotor (LIP) and other motor planning areas (VIP, AIP).
In humans, these two streams begin to diverge more posteriorly, making room for the expanded TPJ-pSTS: the dorsal stream has moved even more dorsally onto the SPL, and the ventral stream more ventrally to the ventral occipitotemporal cortex. As mentioned before, the pSTS stream in humans has angled perpendicularly to the STS and bisects this expansion. These pSTS areas connect to the TPJa/p (Ethofer et al., 2013), introducing a novel pathway for sensory information to reach the TPJ-pSTS (and filling in the visual cortex to TPJ connection in the Corbetta/Shulman model (Corbetta et al., 2008)). The right TPJ-pSTS is also connected to prefrontal areas through the more ventro-lateral branches of the superior longitudinal fasciculus (Croxson et al., 2005; Thiebaut de Schotten, Dell’Acqua, Valabregue, & Catani, 2012) and/or the extreme capsule (also known as the inferior fronto-occipital fascicle) (Mars et al., 2016; Sarubbo, De Benedictis, Maldonado, Basso, & Duffau, 2013), paralleling the motif of the anatomical pathways connecting language areas in the left hemisphere (Margulies & Petrides, 2013; Thiebaut de Schotten et al., 2012). This anatomical pathway provides a direct communication channel between visual, TPJ-pSTS, and prefrontal areas that is independent of the dorsal and ventral pathways. While this TPJ-pSTS pathway is different from the third visual pathway proposed by Haak and Beckmann (Haak & Beckmann, 2018); they may be related: their third visual pathway extends across lateral occipital cortex towards the pSTS and may serve as an entry point of visual information into the TPJ-pSTS pathway. The TPJ-pSTS pathway is also distinct from the parieto-medial temporal pathway described in Kravitz et al., though both originate in the TPJ-pSTS (Kravitz et al., 2011).
We propose that the TPJ-pSTS has emerged as a hub for social cognitive processes, bringing together information gleaned from exploration of the external sensory world with internally generated models of social factors (see Figure 4). Specifically, the TPJ-pSTS receives information related to facial emotions from different cortical regions and other biological motion signals (body, eye) via the posterior STS, theory of mind operations via TPJp, attention via TPJa, and recollection and familiarity signals related to previous experiences via the AG and latIPS (Kravitz et al., 2011). We believe that with this collection of functions, the TPJ-pSTS serves as a hub in a hybrid top-down/bottom-up processing pathway that underlies the quick detection and use of social cues to guide exploration of and interaction with the surrounding social environment (Corbetta et al., 2008; Serences et al., 2005). This pathway operates independently of the separable top-down and bottom up processes instantiated in the dorsal/ventral visual pathways in the TPJ-pSTS. The “bottom-up” or stimulus-driven functions of this pathway are instantiated by the bias towards the processing of salient biological motion and features through the pSTS. The “top-down” or goal-directed functions of this pathway originate in the TPJp, supported by access to relevant explicit memory through the postAG/latIPS complex. This information is then used to guide visual scanning exploration of the environment (TPJa and dorsal attention network).
Figure 4:
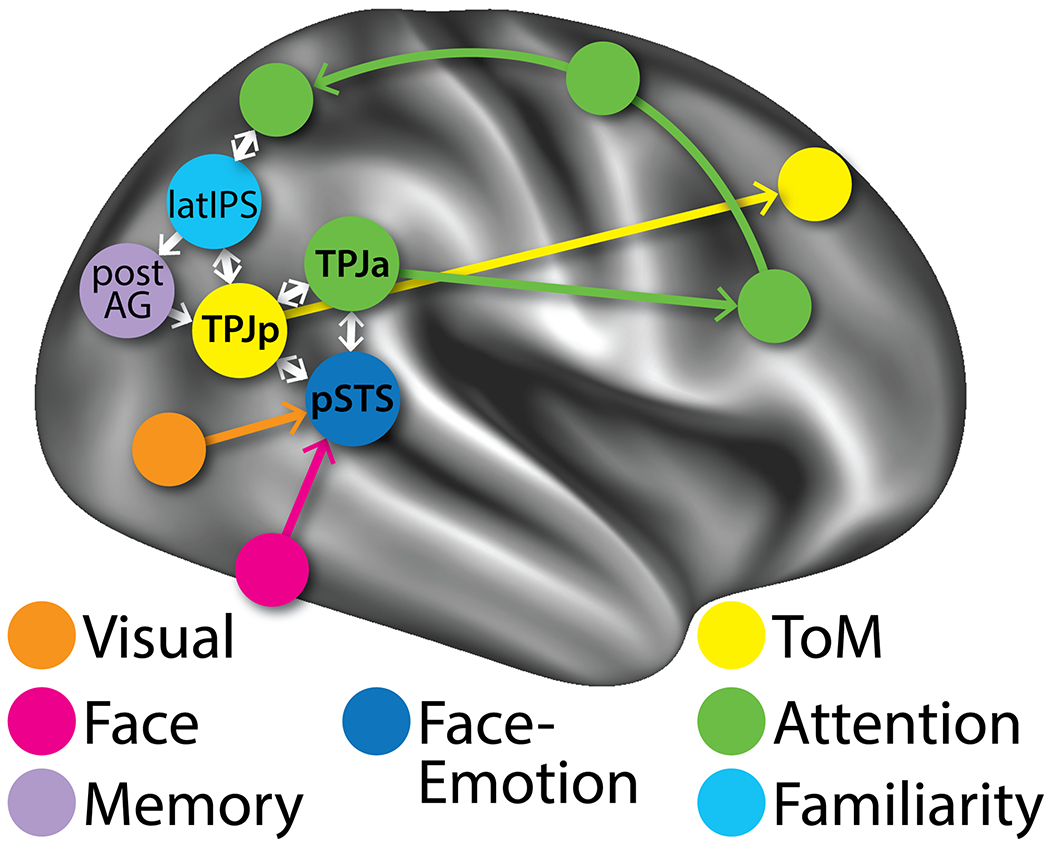
Map of human TPJ-pSTS hybrid pathway showing how visual and face processing areas connect through the TPJ-pSTS to prefrontal cortex.
An alternative hypothesis put forth by Genovesio et al. is that prefrontal-parietal networks have elaborated from the dorsal processing stream in the course of evolution to support the socially related relational metrics (order, number, duration, distance, etc.) necessary for social “foraging” or exploration (Genovesio, Wise, & Passingham, 2014). We note, however, the presence of an anatomical discrepancy between areas belonging to the traditional dorsal processing stream (e.g., area LIP and its human homologue on the superior parietal lobule) and areas involved in computing relational metrics (located on different portions of the inferior parietal lobule in both species). Moreover, inputs into this area appear to be a combination of dorsal (MT) and ventral (FFA) visual processing stream areas, while outputs appear to be directly to ventrolateral prefrontal cortex and not the FEF, further differentiating the TPJ-pSTS pathway from the dorsal pathway.
The sequence of processing through the TPJ-pSTS might follow one of two pathways (Figure 5A and B). Both would start with incoming biological motion sensory information, such as a moving facial expression in the visual periphery. Sensory processing would involve areas such as MT and FFA, culminating in the activation of the pSTS STS-FA (5A:1, 5B:1). At this point the possibilities diverge. One pathway may involve communication of the facial expression information (still in the periphery) with the TPJp as a possible updating of the internal model of the mental states of the people in the social scene (5A:2). If the social situation is familiar, this then activates the latIPS (5A:3), which serves two functions: a) trigger the recall of associated stored memories via the AG (5A:4a) to be used in the theory of mind operations in TPJp and elsewhere (5A:5), and 2) bridge the internally and externally oriented operations in the TPJp and dorsal attention network respectively (5A:4b). If the changes warrant further exploration, the TPJp communicates with the TPJa (5A:6) to trigger the orienting of attention to the face currently in the periphery (5A:7). An alternative hypothesis is that the external sensory information from the pSTS (5B:2) and the internal model information from the TPJp (5B:7) combine in the TPJa to trigger a model updating if the facial expressions violate the expectation, which then triggers further visual exploration through the dorsal attention network.
Figure 5:
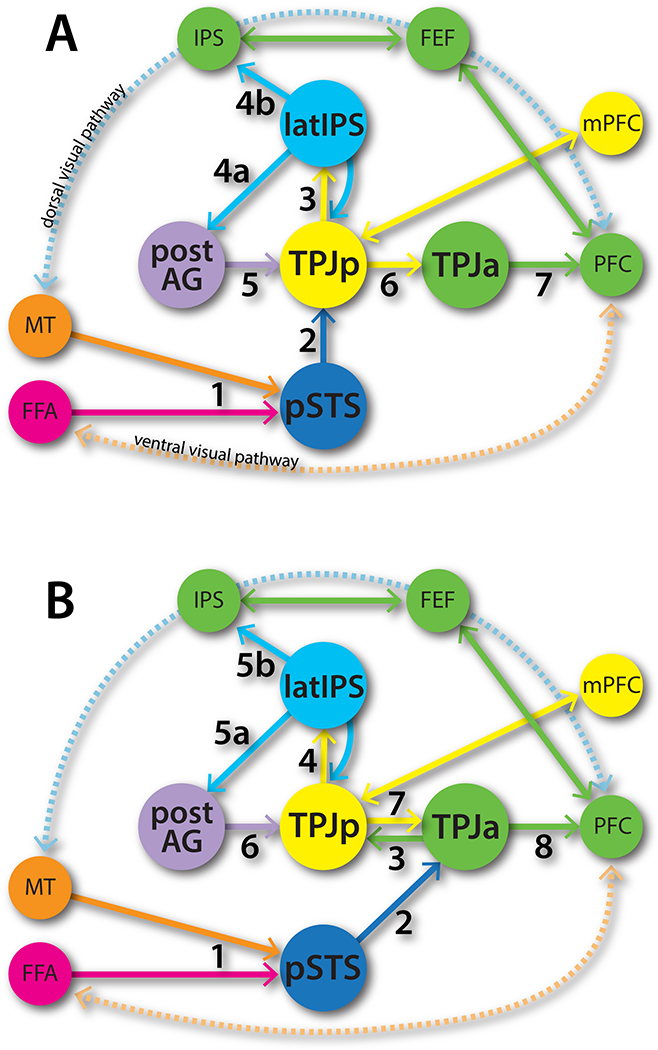
Potential sequence of interactions and transmission of information to prefrontal cortex if A) TPJa activation is late or B) early.
One major issue that remains to be resolved in this model of TPJ-pSTS areal interactions in social cognition is the role of the TPJa. While there is evidence that TPJa activity follows attention reorienting, this may just be an artifact of the sparse and static stimuli used in these tasks. TPJa activity may be superfluous to these artificial tasks, with attention orienting in these tasks controlled by the classic top-down/bottom-up mechanisms instantiated in the dorsal and ventral visual pathways. After all, macaques are able to perform these tasks without a TPJa. However, in more naturalistic tasks, where the complex integration described above is necessary, activity in TPJa may actually precede attention orienting and the resulting exploratory saccades, based on the decision to update the internal models maintained in TPJp and elsewhere. Saccades occur about twice per second in the exploration of a naturalistic scene (H. X. Wang, Freeman, Merriam, Hasson, & Heeger, 2012), which is plenty of time to accommodate the TPJa signals (~300ms) and still drive a saccade through the dorsal attention network.
The lack of a similarly organized TPJ-pSTS in macaques makes it difficult to resolve these issues. However, the study of the macaque TPJ-pSTS may yet reveal some hints about the integrative function of this hub. In macaques, the areas surrounding the TPJ-pSTS tend to be more secondary sensory areas involved in basic motion detection (MT/MST), somatosensory and auditory functioning, and motor planning in the IPS. Perhaps in the course of 25 million years of parallel evolution, then, the TPJ-pSTS in macaques has been purposed to support quick navigation of their environment, populated less by other macaques and more by trees and other non-macaque stimuli (van Schaik, Isler, & Burkart, 2012). This may be supported by the presence of neural signals in macaque area 7a related to optic flow patterns (Raffi & Siegel, 2007) and maze navigation (Crowe, Chafee, Averbeck, & Georgopoulos, 2004).
However, given the disparities between the macaque and human TPJ-pSTS, future investigations of the interactions posed in this anatomical framework will require direct study of this region in humans using techniques with high temporal and spatial resolution, such as multiband fMRI, magnetoencephalography (MEG) or electrocorticography (ECoG), combined with use of stimuli that simultaneously activate these areas in naturalistic ways. These may include naturalistic videos of social situations, virtual reality environments, or even simultaneous fMRI scanning of two people interacting (Hasson, Ghazanfar, Galantucci, Garrod, & Keysers, 2012). With these techniques we will hopefully be able to understand how this cortical region gives rise to the behaviors that make us unique as humans.
Acknowledgements:
We would like to acknowledge Caspar Schwiedrzik for his assistance with Figure 3. We would also like to acknowledge our funding sources—GHP: NIMH (K23MH108711), Brain & Behavior Research Foundation, American Psychiatric Foundation, and the Sydney J. Baer Foundation; MC: Strategic Grant from the University of Padova and NIH (R01NS095741).
Financial Disclosures: GHP receives income and equity from Pfizer, Inc through family. CS and MC declare no competing financial interests.
References
- Andrews-Hanna JR, Reidler JS, Huang C, & Buckner RL (2010). Evidence for the default network’s role in spontaneous cognition. Journal of Neurophysiology, 104(1), 322–335. 10.1152/jn.00830.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, & Cohen JD (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403–450. 10.1146/annurev.neuro.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- Baizer JS, Ungerleider LG, & Desimone R (1991). Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 11(1), 168–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo P (2014). Attention Disorders After Right Brain Damage. New York: Springer; 10.1007/978-1-4471-5649-9_1 [DOI] [Google Scholar]
- Beauchamp MS, Sun P, Baum SH, Tolias AS, & Yoshor D (2012). Electrocorticography links human temporoparietal junction to visual perception. Nature Neuroscience, 15(7), 957–959. 10.1038/nn.3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, & Desai RH (2011). The neurobiology of semantic memory. Trends in Cognitive Sciences, 15(11), 527–536. 10.1016/j.tics.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, & Carroll DC (2007). Self-projection and the brain. Trends in Cognitive Sciences, 11(2), 49–57. 10.1016/j.tics.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Buckner RL, & Krienen FM (2013). The evolution of distributed association networks in the human brain. Trends in Cognitive Sciences, 17(12), 648–665. 10.1016/j.tics.2013.09.017 [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, & Amunts K (2008). The human inferior parietal lobule in stereotaxic space. Brain Structure and Function, 212(6), 481–495. 10.1007/s00429-008-0195-z [DOI] [PubMed] [Google Scholar]
- Caspers S, Schleicher A, Bacha-Trams M, Palomero-Gallagher N, Amunts K, & Zilles K (2012). Organization of the Human Inferior Parietal Lobule Based on Receptor Architectonics. 10.1093/cercor/bhs048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Schleicher A, Bacha-Trams M, Palomero-Gallagher N, Amunts K, & Zilles K (2013). Organization of the human inferior parietal lobule based on receptor architectonics. Cerebral Cortex (New York, N.Y. : 1991), 23(3), 615–628. 10.1093/cercor/bhs048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Irving ZC, Fox KCR, Spreng RN, & Andrews-Hanna JR (2016). Mind-wandering as spontaneous thought: a dynamic framework. Nature Reviews Neuroscience, 17(11), 718–731. 10.1038/nrn.2016.113 [DOI] [PubMed] [Google Scholar]
- Cloutman LL (2013). Interaction between dorsal and ventral processing streams: where, when and how? Brain and Language, 127(2), 251–263. 10.1016/j.bandl.2012.08.003 [DOI] [PubMed] [Google Scholar]
- Cohn-Sheehy BI, & Ranganath C (2017). Time regained: how the human brain constructs memory for time. Current Opinion in Behavioral Sciences, 17, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, & Steinmetz MA (2001). Neuronal responses in area 7a to multiple-stimulus displays: I. neurons encode the location of the salient stimulus., 11(7), 581–591. [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2011). Spatial neglect and attention networks. Annual Review of Neuroscience, 34, 569–599. 10.1146/annurev-neuro-061010-113731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, & Shulman GL (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience, 3(3), 292–297. 10.1038/73009 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel GH, & Shulman GL (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron, 58(3), 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe DA, Chafee MV, Averbeck BB, & Georgopoulos AP (2004). Neural activity in primate parietal area 7a related to spatial analysis of visual mazes, 14(1), 23–34. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Forkel SJ, Cerliani L, & Thiebaut de Schotten M (2017). Structural Variability Across the Primate Brain: A Cross-Species Comparison., 1–13. 10.1093/cercor/bhx244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TEJ, Robson MD, Pinsk MA, Gross CG, et al. (2005). Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 25(39), 8854–8866. 10.1523/JNEUROSCI.1311-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitch AL, Sharma M, Roland JL, Astafiev SV, Bundy DT, Gaona CM, et al. (2013). Frequency-specific mechanism links human brain networks for spatial attention. Proceedings of the National Academy of Sciences of the United States of America, 110(48), 19585–19590. 10.1073/pnas.1307947110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, & Lamm C (2007). The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. The Neuroscientist : a Review Journal Bringing Neurobiology, Neurology and Psychiatry, 13(6), 580–593. 10.1177/1073858407304654 [DOI] [PubMed] [Google Scholar]
- Dehaene S, & Cohen L (2007). Cultural recycling of cortical maps. Neuron, 56(2), 384–398. 10.1016/j.neuron.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Dixon ML, La Vega, De A, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, & Christoff K (2018). Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proceedings of the National Academy of Sciences of the United States of America, 115(7), E1598–E1607. 10.1073/pnas.1715766115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, & Davis KD (2000). A multimodal cortical network for the detection of changes in the sensory environment. Nature Neuroscience, 3(3), 277–283. 10.1038/72991 [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, & Graham KS (2011). Stimulus content and the neural correlates of source memory. Brain Research, 1373, 110–123. 10.1016/j.brainres.2010.11.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué L, Merriam EP, Heeger DJ, & Carrasco M (2017). Specific Visual Subregions of TPJ Mediate Reorienting of Spatial Attention, 1–16. 10.1093/cercor/bhx140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM (2016). The Social Brain Hypothesis and Human Evolution In Oxford Research Encyclopedia of Psychology (Vol. 1). Oxford University Press; 10.1093/acrefore/9780190236557.013.44 [DOI] [Google Scholar]
- Duncan J (2010). The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences, 14(4), 172–179. 10.1016/j.tics.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Ethofer T, Bretscher J, Wiethoff S, Bisch J, Schlipf S, Wildgruber D, & Kreifelts B (2013). Functional responses and structural connections of cortical areas for processing faces and voices in the superior temporal sulcus. NeuroImage, 76, 45–56. 10.1016/j.neuroimage.2013.02.064 [DOI] [PubMed] [Google Scholar]
- Freiwald WA, & Tsao DY (2010). Functional compartmentalization and viewpoint generalization within the macaque face-processing system. Science (New York, NY), 330(6005), 845–851. 10.1126/science.1194908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ, & Vossel S (2013). Re-evaluating the role of TPJ in attentional control: contextual updating? Neuroscience and Biobehavioral Reviews, 37(10 Pt 2), 2608–2620. 10.1016/j.neubiorev.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovesio A, Wise SP, & Passingham RE (2014). Prefrontal-parietal function: from foraging to foresight. Trends in Cognitive Sciences, 18(2), 72–81. 10.1016/j.tics.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. (2016). A multi-modal parcellation of human cerebral cortex. Nature. 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1988). Topography of Cognition: Parallel Distributed Networks in Primate Association Cortex. Annual Review of Neuroscience, 11(1), 137–156. 10.1146/annurev.ne.11.030188.001033 [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, & Petersen SE (2014). Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. 10.1093/cercor/bhu239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Borra E, Matelli M, & Luppino G (2006). Architectonic organization of the inferior parietal convexity of the macaque monkey. The Journal of Comparative Neurology, 496(3), 422–451. 10.1002/cne.20933 [DOI] [PubMed] [Google Scholar]
- Grossman ED, Battelli L, & Pascual-Leone A (2005). Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Research, 45(22), 2847–2853. 10.1016/j.visres.2005.05.027 [DOI] [PubMed] [Google Scholar]
- Guerin SA, & Miller MB (2009). Lateralization of the parietal old/new effect: an event-related fMRI study comparing recognition memory for words and faces. NeuroImage, 44(1), 232–242. 10.1016/j.neuroimage.2008.08.035 [DOI] [PubMed] [Google Scholar]
- Haak KV, & Beckmann CF (2018). Objective analysis of the topological organization of the human cortical visual connectome suggests three visual pathways. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 98, 73–83. 10.1016/j.cortex.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari YN (2015). Sapiens. Harper Collins. [Google Scholar]
- Hasson U, Ghazanfar AA, Galantucci B, Garrod S, & Keysers C (2012). Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends in Cognitive Sciences, 16(2), 114–121. 10.1016/j.tics.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E, Call J, Hernàndez-Lloreda MV, Hare B, & Tomasello M (2007). Humans Have Evolved Specialized Skills of Social Cognition: The Cultural Intelligence Hypothesis. Science (New York, NY), 317(5843), 1360–1366. 10.1126/science.1146282 [DOI] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, & van Essen DC (2010). Similar patterns of cortical expansion during human development and evolution. Proceedings of the National Academy of Sciences of the United States of America, 107(29), 13135–13140. 10.1073/pnas.1001229107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, & Lambon Ralph MA (2015). Fusion and Fission of Cognitive Functions in the Human Parietal Cortex, 25(10), 3547–3560. 10.1093/cercor/bhu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Weiner KS, Bressler DW, Silver MA, Preston AR, & Wagner AD (2012). Functional Heterogeneity in Posterior Parietal Cortex Across Attention and Episodic Memory Retrieval, 24(1), 49–66. 10.1093/cercor/bhs278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelström KM, & Graziano MSA (2017). The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia. 10.1016/j.neuropsychologia.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Igelström KM, Webb TW, Kelly YT, & Graziano MSA (2016). Topographical Organization of Attentional, Social, and Memory Processes in the Human Temporoparietal Cortex. eNeuro, 3(2). 10.1523/ENEURO.0060-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik L, Koldewyn K, Beeler D, & Kanwisher NG (2017). Perceiving social interactions in the posterior superior temporal sulcus. Proceedings of the National Academy of Sciences of the United States of America, 70, 201714471 10.3758/BF03206542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack AI, Shulman GL, Snyder AZ, McAvoy M, & Corbetta M (2006). Separate modulations of human V1 associated with spatial attention and task structure. Neuron, 51(1), 135–147. 10.1016/j.neuron.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, & Buckner RL (2008). Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100(1), 129–139. 10.1152/jn.00077.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, & Corbetta M (2005). An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 25(18), 4593–4604. 10.1523/JNEUROSCI.0236-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann EC, Loui P, & Shimamura AP (2009). Activation of right parietal cortex during memory retrieval of nonlinguistic auditory stimuli. Cogn Affect Behav Neurosci, 9(3), 242–248. 10.3758/CABN.9.3.242 [DOI] [PubMed] [Google Scholar]
- Koster-Hale J, & Saxe R (2013). Theory of Mind: A Neural Prediction Problem. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, & Mishkin M (2011). A new neural framework for visuospatial processing. Nature Reviews Neuroscience, 12(4), 217–230. 10.1038/nrn3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, & Mishkin M (2013). The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends in Cognitive Sciences, 17(1), 26–49. 10.1016/j.tics.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA (2009). In search of a unifying theory of complex brain evolution. Annals of the New York Academy of Sciences, 1156, 44–67. 10.1111/j.1749-6632.2009.04421.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, & Seelke AMH (2012). Cortical evolution in mammals: the bane and beauty of phenotypic variability. Proceedings of the National Academy of Sciences of the United States of America, 109 Suppl 1, 10647–10654. 10.1073/pnas.1201891109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Romani GL, Orban GA, & Vanduffel W (2013). Evolutionarily Novel Functional Networks in the Human Brain? The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 33(8), 3259–3275. 10.1523/JNEUROSCI.4392-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Gerits A, Nelissen K, Durand J-B, Joly O, Simone L, et al. (2011). Default mode of brain function in monkeys. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 31(36), 12954–12962. 10.1523/JNEUROSCI.2318-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, & Petrides M (2013). Distinct parietal and temporal connectivity profiles of ventrolateral frontal areas involved in language production. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 33(42), 16846–16852. 10.1523/JNEUROSCI.2259-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Ramezanpour H, Dicke PW, & Thier P (2017). Following Eye Gaze Activates a Patch in the Posterior Temporal Cortex That Is not Part of the Human “Face Patch” System. eNeuro, 4(2). 10.1523/ENEURO.0317-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Foxley S, Verhagen L, Jbabdi S, Sallet J, Noonan MP, et al. (2016). The extreme capsule fiber complex in humans and macaque monkeys: a comparative diffusion MRI tractography study. Brain Structure and Function, 221(8), 4059–4071. 10.1007/s00429-015-1146-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, & Rushworth MFS (2012a). On the relationship between the “default mode network” and the “social brain”. Frontiers in Human Neuroscience, 6, 189 10.3389/fnhum.2012.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Neubert F-X, & Rushworth MFS (2013). Connectivity profiles reveal the relationship between brain areas for social cognition in human and monkey temporoparietal cortex. Proceedings of the National Academy of Sciences of the United States of America, 110(26), 10806–10811. 10.1073/pnas.1302956110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schüffelgen U, Jbabdi S, Toni I, & Rushworth MFS (2012b). Connectivity-based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks., 22(8), 1894–1903. 10.1093/cercor/bhr268 [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, & Macko KA (1983). Object vision and spatial vision: two cortical pathways. Trends in Neurosciences, 6, 414–417. 10.1016/0166-2236(83)90190-X [DOI] [Google Scholar]
- Miyamoto K, Adachi Y, Osada T, Watanabe T, Kimura HM, Setsuie R, & Miyashita Y (2014). Dissociable Memory Traces within the Macaque Medial Temporal Lobe Predict Subsequent Recognition Performance. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 34(5), 1988–1997. 10.1523/JNEUROSCI.4048-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Osada T, Adachi Y, Matsui T, Kimura HM, & Miyashita Y (2013). Functional Differentiation of Memory Retrieval Network in Macaque Posterior Parietal Cortex. Neuron, 77(4), 787–799. 10.1016/j.neuron.2012.12.019 [DOI] [PubMed] [Google Scholar]
- Mueller S, Wang D, Fox MD, Yeo BTT, Sepulcre J, Sabuncu MR, et al. (2013). Individual variability in functional connectivity architecture of the human brain. Neuron, 77(3), 586–595. 10.1016/j.neuron.2012.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, & Pandya DN (1984). Some observations on the course and composition of the cingulum bundle in the rhesus monkey. The Journal of Comparative Neurology, 225(1), 31–43. 10.1002/cne.902250105 [DOI] [PubMed] [Google Scholar]
- Nelissen K, Vanduffel W, & Orban GA (2006). Charting the lower superior temporal region, a new motion-sensitive region in monkey superior temporal sulcus. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 26(22), 5929–5947. 10.1523/JNEUROSCI.0824-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, et al. (2010). A parcellation scheme for human left lateral parietal cortex. Neuron, 67(1), 156–170. 10.1016/j.neuron.2010.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, van Essen DC, & Vanduffel W (2004). Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences, 8(7), 315–324. 10.1016/j.tics.2004.05.009 [DOI] [PubMed] [Google Scholar]
- Patel GH, Kaplan DM, & Snyder LH (2014). Topographic organization in the brain: searching for general principles. Trends in Cognitive Sciences, 18(7), 351–363. 10.1016/j.tics.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel GH, Yang D, Jamerson EC, Snyder LH, Corbetta M, & Ferrera VP (2015). Functional evolution of new and expanded attention networks in humans. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1420395112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, & McCarthy G (2005). Functional anatomy of biological motion perception in posterior temporal cortex: an FMRI study of eye, mouth and hand movements., 15(12), 1866–1876. 10.1093/cercor/bhi064 [DOI] [PubMed] [Google Scholar]
- Polosecki P, Moeller S, Schweers N, Romanski LM, Tsao DY, & Freiwald WA (2013). Faces in Motion: Selectivity of Macaque and Human Face Processing Areas for Dynamic Stimuli. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 33(29), 11768–11773. 10.1523/JNEUROSCI.5402-11.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffi M, & Siegel RM (2007). A functional architecture of optic flow in the inferior parietal lobule of the behaving monkey. PLoS ONE, 2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Shepherd SV, & Platt ML (2014). Reversible inactivation of pSTS suppresses social gaze following in the macaque (Macaca mulatta). Social Cognitive and Affective Neuroscience, 9(2), 209–217. 10.1093/scan/nss123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, & Luppino G (2006). Cortical connections of the inferior parietal cortical convexity of the macaque monkey., 16(10), 1389–1417. 10.1093/cercor/bhj076 [DOI] [PubMed] [Google Scholar]
- Rugg MD, & King DR (2017). ScienceDirect, 1–13. 10.1016/j.cortex.2017.07.012 [DOI] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado IL, Basso G, & Duffau H (2013). Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Structure and Function, 218(1), 21–37. 10.1007/s00429-011-0372-3 [DOI] [PubMed] [Google Scholar]
- Saxe R, & Kanwisher NG (2003). People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind.” NeuroImage, 19(4), 1835–1842. [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, & Perner J (2014). Neuroscience and Biobehavioral Reviews. Neuroscience and Biobehavioral Reviews, 42, 9–34. 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Schwiedrzik CM, Zarco W, Everling S, & Freiwald WA (2015). Face Patch Resting State Networks Link Face Processing to Social Cognition. PLoS Biology, 13(9), e1002245 10.1371/journal.pbio.1002245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML (2008). Laterality index in functional MRI: methodological issues. Magn Reson Imaging, 26(5), 594–601. 10.1016/j.mri.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML (2013). The angular gyrus: multiple functions and multiple subdivisions. The Neuroscientist : a Review Journal Bringing Neurobiology, Neurology and Psychiatry, 19(1), 43–61. 10.1177/1073858412440596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, & Yantis S (2005). Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychological Science : a Journal of the American Psychological Society / APS, 16(2), 114–122. 10.1111/j.0956-7976.2005.00791.x [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, & Shulman GL (2011). Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 31(12), 4407–4420. 10.1523/JNEUROSCI.3335-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, & Corbetta M (2010). Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 30(25), 8445–8456. 10.1523/JNEUROSCI.4719-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, & Corbetta M (2017). The contribution of the human posterior parietal cortex to episodic memory. Nature Reviews Neuroscience, 18(3), 183–192. 10.1038/nrn.2017.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Tosoni A, Mignogna V, McAvoy MP, Shulman GL, Corbetta M, & Romani GL (2014). Memory accumulation mechanisms in human cortex are independent of motor intentions. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 34(20), 6993–7006. 10.1523/JNEUROSCI.3911-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DLW, Snyder AZ, McAvoy MP, & Corbetta M (2009). Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 29(14), 4392–4407. 10.1523/JNEUROSCI.5609-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, McAvoy MP, d’Avossa G, & Corbetta M (2007). Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis., 17(11), 2625–2633. 10.1093/cercor/bhl170 [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, & Petersen SE (1997). Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. Journal of Cognitive Neuroscience, 9(5), 648–663. 10.1162/jocn.1997.9.5.648 [DOI] [PubMed] [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d’Avossa G, & Corbetta M (2003). Quantitative analysis of attention and detection signals during visual search. Journal of Neurophysiology, 90(5), 3384–3397. 10.1152/jn.00343.2003 [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Linenweber M, Petersen SE, & Corbetta M (2001). Multiple neural correlates of detection in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 98(1), 313–318. 10.1073/pnas.021381198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa J, & Freiwald WA (2017). A dedicated network for social interaction processing in the primate brain. Science (New York, NY), 356(6339), 745–749. 10.1126/science.aam6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiras A, Toledo JB, Gur RE, Gur RC, Satterthwaite TD, & Davatzikos C (2017). Patterns of coordinated cortical remodeling during adolescence and their associations with functional specialization and evolutionary expansion. Proceedings of the National Academy of Sciences of the United States of America, 114(13), 3527–3532. 10.1073/pnas.1620928114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadone S, Penna Della, S, Sestieri C, Betti V, Tosoni A, Perrucci MG, et al. (2015). Dynamic reorganization of human resting-state networks during visuospatial attention. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1415439112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN (2013). Examining the role of memory in social cognition. Frontiers in Psychology, 4, 437 10.3389/fpsyg.2013.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, & Mar RA (2012). I remember you: a role for memory in social cognition and the functional neuroanatomy of their interaction. Brain Research, 1428, 43–50. 10.1016/j.brainres.2010.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, & Schacter DL (2013). Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci, 25(1), 74–86. 10.1162/jocn_a_00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR (1992). Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev, 99(2), 195–231. [DOI] [PubMed] [Google Scholar]
- Steinmetz MA, Connor CE, Constantinidis C, & McLaughlin JR (1994). Covert attention suppresses neuronal responses in area 7a of the posterior parietal cortex. Journal of Neurophysiology, 72(2), 1020–1023. [DOI] [PubMed] [Google Scholar]
- Teichert T, Grinband J, Hirsch J, & Ferrera VP (2010). Effects of heartbeat and respiration on macaque fMRI: implications for functional connectivity. Neuropsychologia, 48(7), 1886–1894. 10.1016/j.neuropsychologia.2009.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, & Catani M (2012). Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 48(1), 82–96. 10.1016/j.cortex.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Todd JJ, & Marois R (2004). Capacity limit of visual short-term memory in human posterior parietal cortex. Nature, 428(6984), 751–754. 10.1038/nature02466 [DOI] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, & Freiwald WA (2008). Comparing face patch systems in macaques and humans. Proceedings of the National Academy of Sciences of the United States of America, 105(49), 19514–19519. 10.1073/pnas.0809662105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, & Menon V (2010). Dissociable Connectivity within Human Angular Gyrus and Intraparietal Sulcus: Evidence from Functional and Structural Connectivity. Cerebral Cortex (New York, N.Y. : 1991), 20(11), 2636–2646. 10.1093/cercor/bhq011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Galkin TW, Desimone R, & Gattass R (2008). Cortical connections of area V4 in the macaque., 18(3), 477–499. 10.1093/cercor/bhm061 [DOI] [PubMed] [Google Scholar]
- van Essen DC, & Dierker DL (2007). Surface-based and probabilistic atlases of primate cerebral cortex. Neuron, 56(2), 209–225. 10.1016/j.neuron.2007.10.015 [DOI] [PubMed] [Google Scholar]
- van Essen DC, Glasser MF, Dierker DL, Harwell J, & Coalson T (2011). Parcellations and Hemispheric Asymmetries of Human Cerebral Cortex Analyzed on Surface-Based Atlases. 10.1093/cercor/bhr291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik CP, Isler K, & Burkart JM (2012). Explaining brain size variation: from social to cultural brain. Trends in Cognitive Sciences, 16(5), 277–284. 10.1016/j.tics.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Vilberg KL, & Rugg MD (2008). Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia, 46(7), 1787–1799. 10.1016/j.neuropsychologia.2008.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, van Essen DC, & Buckner RL (2010). Functional connectivity of the macaque posterior parahippocampal cortex. Journal of Neurophysiology, 103(2), 793–800. 10.1152/jn.00546.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, van Essen DC, et al. (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature, 447(7140), 83–86. 10.1038/nature05758 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, & Buckner RL (2006). Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology, 96(6), 3517–3531. 10.1152/jn.00048.2006 [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, & Buckner RL (2005). Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences, 9(9), 445–453. 10.1016/j.tics.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Wang HX, Freeman J, Merriam EP, Hasson U, & Heeger DJ (2012). Temporal eye movement strategies during naturalistic viewing. Journal of Vision, 12(1), 16–16. 10.1167/12.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Collins JA, Koski J, Nugiel T, Metoki A, & Olson IR (2017). Dynamic neural architecture for social knowledge retrieval. Proceedings of the National Academy of Sciences of the United States of America, 114(16), E3305–E3314. 10.1073/pnas.1621234114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TW, Igelström KM, Schurger A, & Graziano MSA (2016). Cortical networks involved in visual awareness independent of visual attention. Proceedings of the National Academy of Sciences of the United States of America, 113(48), 13923–13928. 10.1073/pnas.1611505113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, & Ungerleider LG (1994). Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys, 4(5), 470–483. [DOI] [PubMed] [Google Scholar]
- Yang DYJ, Rosenblau G, Keifer C, & Pelphrey KA (2015). Neuroscience and Biobehavioral Reviews. Neuroscience and Biobehavioral Reviews, 51, 263–275. 10.1016/j.neubiorev.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, & Ochsner KN (2009). The need for a cognitive neuroscience of naturalistic social cognition. Annals of the New York Academy of Sciences, 1167, 16–30. 10.1111/j.1749-6632.2009.04601.x [DOI] [PMC free article] [PubMed] [Google Scholar]


