Abstract
Background
Bedaquiline and delamanid are newly available drugs for treating multidrug-resistant tuberculosis (MDR-TB); however, there are limited data guiding their use and no comparison studies.
Methods
We conducted a prospective, observational study among patients with MDR-TB in Georgia who were receiving a bedaquiline- or delamanid-based treatment regimen. Monthly sputum cultures, minimal inhibitory concentration testing, and adverse event monitoring were performed. Primary outcomes were culture conversion rates and clinical outcomes. Targeted maximum likelihood estimation and super learning were utilized to produce a covariate-adjusted proportion of outcomes for each regimen.
Results
Among 156 patients with MDR-TB, 100 were enrolled and 95 were receiving a bedaquiline-based (n = 64) or delamanid-based (n = 31) regimen. Most were male (82%) and the median age was 38 years. Rates of previous treatment (56%) and cavitary disease (61%) were high. The most common companion drugs included linezolid, clofazimine, cycloserine, and a fluoroquinolone. The median numbers of effective drugs received among patients on bedaquiline-based (4; interquartile range [IQR], 4–4) and delamanid-based (4; IQR, 3.5–5) regimens were similar. Rates of acquired drug resistance were significantly higher among patients receiving delamanid versus bedaquiline (36% vs 10%, respectively; P < .01). Adjusted rates of sputum culture conversion at 2 months (67% vs 47%, respectively; P = .10) and 6 months (95% vs 74%, respectively; P < .01), as well as more favorable clinical outcomes (96% vs 72%, respectively; P < .01), were higher among patients receiving bedaquiline versus delamanid.
Conclusions
Among patients with MDR-TB, bedaquiline-based regimens were associated with higher rates of sputum culture conversion, more favorable outcomes, and a lower rate of acquired drug resistance versus delamanid-based regimens.
Keywords: bedaquiline, delamanid, multidrug-resistant, tuberculosis, culture conversion
In a prospective, observational, cohort study of patients receiving bedaquiline- versus delamanid-based regimens for multidrug-resistant tuberculosis, those receiving bedaquiline had higher rates of sputum culture conversion and favorable outcomes and were less likely to develop acquired drug resistance.
While the scourge of multidrug-resistant tuberculosis (MDR-TB) constitutes a global public health crisis, the recent implementation of new and repurposed drugs has provided hope for patients and health-care providers [1]. Bedaquiline is the first anti-tuberculosis agent to be approved by the Food and Drug Administration in >50 years and delamanid has received “conditional approval” by the European Medicines Agency. Both drugs have appealing properties, including unique mechanisms of action, the ability to kill replicating and nonreplicating Mycobacterium tuberculosis organisms, and narrow spectrums of action limited predominantly to Mycobacteria species [2]. The repurposing of linezolid and the reemergence of clofazimine have also offered key additional agents for MDR-TB. While guidance and recommendations for the use of the above-mentioned drugs has been included in recent World Health Organization (WHO) guidelines [3] there remains limited data on the clinical outcomes of patients treated with these agents under programmatic conditions, including no comparison of patients treated with bedaquiline- versus delamanid-based regimens.
Evidence for bedaquiline use stems from early in vitro studies displaying bactericidal activity, to Phase II and IIb clinical trials and a recent individual patient data meta-analysis demonstrating improved clinical outcomes [1, 4, 5]. In contrast, positive results from a Phase II study of delamanid were not replicated in a Phase III trial, which found no difference in clinical outcomes with or without delamanid [6, 7]. Of note, the programmatic roll out and use of bedaquiline has been more widespread and, consequently, there are more reports on the clinical outcomes of patients using bedaquiline and scarce reports of delamanid (precluding evaluation in the above-mentioned meta-analysis) [5, 8]. Further experience with delamanid will better define its role and, moreover, comparisons of bedaquiline and delamanid will help determine the relative efficacy and safety of each agent.
Our study was conducted in Georgia; similar to other former Soviet Union republics, Georgia has one of the highest rates of MDR-TB in the world [1]. With support from government and various agencies, the Georgian National Tuberculosis Program (NTP) implemented bedaquiline, delamanid, linezolid, and clofazimine into programmatic use for the treatment of MDR-TB and extensively drug-resistant (XDR) TB in 2015. We concurrently developed a prospective, observational study to evaluate clinical outcomes among patients receiving these drugs, with a main aim of comparing outcomes among patients receiving bedaquiline versus delamanid. Our overall goal was to provide valuable data on the programmatic use of new drugs to help define their appropriate and responsible use.
METHODS
Setting
The study was carried out at the National Center for Tuberculosis and Lung Diseases (NCTLD) in Tbilisi, Georgia. Patients ≥16 years old with a sputum culture positive for M. tuberculosis and confirmed multidrug resistance who started bedaquiline, linezolid, clofazimine, and/or delamanid from December 2015 through May 2017 were eligible for inclusion. Written informed consent was required and ethics approvals were obtained from the institutional review boards of Emory University and the NCTLD.
Laboratory
All sputum specimens underwent acid-fast bacilli (AFB) smear microscopy, and AFB sputum cultures were performed at the National Reference Laboratory using Löwenstein-Jensen–based solid medium and the BACTEC mycobacterial growth indicator tube 960 broth culture system. Positive cultures were confirmed to be M. tuberculosis complex using the M. tuberculosis related antigen test. Phenotypic first- and second-line drug susceptibility testing (DST) were carried out as previously described, and the MTBDRplus assay was performed on positive culture isolates [9]. Pyrazinamide DST was conducted per manufacturer’s instructions using a critical concentration of 100 µg/ml. M. tuberculosis isolates were frozen at −80°C for later minimal inhibitory concentration (MIC) testing, which was performed using customized Thermo Scientific Sensititre Mycobacterium tuberculosis MYCOTB MIC plates [10]. Additional drugs added to the standard M. tuberculosis MIC panel included capreomycin, clofazimine, levofloxacin, linezolid, and bedaquiline. The reported MIC was the lowest antibiotic concentration to inhibit visible growth. Full methodology and plate configurations are available in the Supplementary Materials. Sputum samples were collected monthly until culture conversion and at least through the first 12 months. Patients were asked to return approximately 6 months posttreatment for follow-up and to provide a sputum sample.
Treatment
Patients were hospitalized at the initiation of MDR-TB treatment and were advised to remain hospitalized until sputum smear microscopy conversion and clinical improvement. Treatment regimens were individualized based on DST results and following NTP and WHO guidlines [11–13]. All treatment regimens were reviewed and decided upon by the NTP Drug Resistance Committee, which meets twice weekly. Bedaquiline use was preferred for patients with pre-XDR-TB and XDR-TB; delamanid used was preferred for patients with an albumin <3 grams/dL and in patients with human during delaminid use was preferred in patients with human immunodeficiency virus, hepatitis C virus infection, and diabetes, due to less potential for hepatotoxicity and drug-drug interactions. The standard of care for treatment duration during the study period was 20–24 months. All treatment was administered through directly observed therapy.
Data Management
Baseline information on patient demographics, medical history, clinical presentation, radiology, and laboratory data was collected and prospectively gathered for sputum culture and laboratory results and for adverse event and final treatment outcomes. Adverse event monitoring, including laboratory and electrocardiogram monitoring, was carried out through the NTP pharmacovigilance monitoring system [14]. An effective drug was defined as a drug received and with susceptibility confirmed by MIC testing [10, 15], with 2 exceptions for drugs without susceptibility testing: delamanid was always considered an effective drug, while unknown pyrazinamide results were considered resistant [16].
The time to sputum culture conversion was defined as the number of days from the initiation of bedaquiline or delamanid to the first of 2 consecutive, negative sputum culture results ≥28 days apart [17]. Acquired drug resistance was defined either as a drug going from susceptible on baseline MIC testing to resistant on follow-up MIC testing or as a 2-fold increase in MIC value. Clinical treatment outcomes were defined using WHO criteria [11], with 1 exception: patients with a loss to follow-up (LFU) were reclassified as having a poor outcome if they had a documented positive culture after LFU or as having a favorable outcome if they had initial culture conversion and a subsequent posttreatment negative sputum culture. The NCTLD database was checked on 1 March 2019 to evaluate for relapses. Patients with a relapse were defined as having a poor outcome regardless of the initial (end of treatment) treatment outcome.
Statistical Analysis
Our primary outcome analyses examined differences between bedaquiline- and delamanid-based treatment regimens in regards to sputum culture conversion by 2 and 6 months and to clinical treatment outcomes. A targeted maximum likelihood estimation (TMLE) was used to estimate the covariate-adjusted proportion of outcomes under each regimen [18]. TMLE is a robust analytic method that, for our analysis, combined 2 models to estimate an adjusted proportion of sputum culture conversion over time under bedaquiline- versus delaminid-based regimens. These quantities were estimated using super learning, a flexible regression technique that selects the combination of pre-specified regression algorithms that provides the best estimated fit to the data [19]. Pre-specified regressions are listed in the Supplementary Materials. We used a level 0.05 Wald test of the null hypothesis of no difference in proportion of the outcome. In secondary analyses, we estimated the weekly cumulative probability of sputum culture conversion over the duration of the study and pointwise 95% confidence intervals. We conducted sensitivity analyses by computing E-values [20]. The E-value is an estimate of the minimum strength of association between an unmeasured cofounder and treatment and between the same confounder and the outcome that would negate the observed treatment-outcome association. Large E-values indicate a strong unmeasured confounder would be needed to negate the observed association. Analyses were carried out using R [21] software, using drtmle [22] and SuperLearner [19] packages.
RESULTS
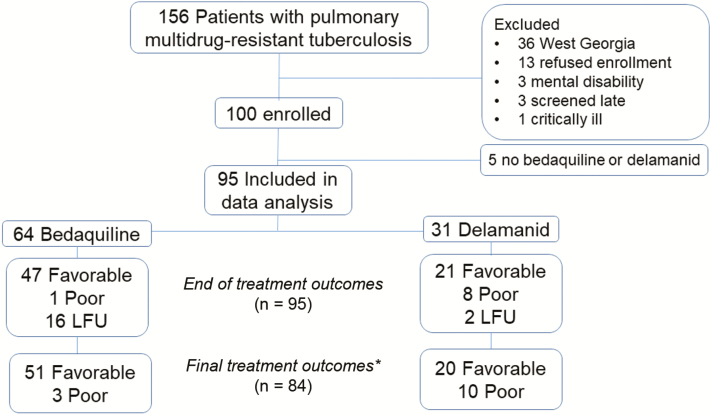
Among 156 patients with MDR-TB who were approached for enrollment, 100 agreed to participate and 95 initiated treatment with bedaquiline or delamanid and were included in analyses (Figure 1). There were 64 patients (67%) initiating treatment with a bedaquiline-based regimen and 31 (33%) initiating treatment with a delamanid-based regimen. Patients initiating treatment with either a bedaquiline- or delamanid-based regimen were similar in regards to age, body mass index, rates of drug use, and comorbidities; the 2 patients coinfected with human immunodeficiency virus received bedaquiline, given they were taking albumin at <3 g/dl (Table 1). Rates of smear positivity and bilateral or cavitary disease were not significantly different. Table 1 contains a comparison of available baseline characteristics. Baseline MIC testing revealed no significant difference in rates of XDR-TB (Supplementary Table S1).
Figure 1.
Study flow diagram including patient clinical treatment outcomes. *Patients with LFU during treatment and no post treatment follow-up were excluded while those with post treatment follow-up were included and final outcomes were determined by follow up culture results. Abbreviation: LFU, loss to follow-up.
Table 1.
Characteristics of Patients with Multidrug-resistant Tuberculosis Receiving a Bedaquiline- or Delamanid-Based Treatment Regimen
| Characteristic | Delamanid, n = 31, n (%) | Bedaquiline, n = 64, n (%) | P a |
|---|---|---|---|
| Median age, years (IQR) | 38.3 (28.9–47.4) | 37.3 (27.7–50.7) | .98 |
| Median BMI, kg/m2 (IQR) | 20.4 (18.3–23.5) | 19.8 (18.4–22.1) | .66 |
| Male | 21 (68) | 57 (89) | .01 |
| From Tbilisi | 23 (74) | 39 (61) | .20 |
| History of imprisonment | 7 (31) | 16 (25) | .80 |
| Any tobacco use | 14 (45) | 35 (55) | .38 |
| ≥1 pack | 9 (29) | 16 (25) | |
| Any alcohol use | 11 (36) | 19 (30) | .38 |
| Heavyb | 4 (13) | 8 (13) | |
| Diabetes mellitus | 4 (13) | 8 (13) | .96 |
| Hepatitis C antibody positive | 7 (23) | 13 (20) | .80 |
| HIV infection | 0 | 2 (3)c | .32 |
| Known mental health disorder | 0 | 0 | |
| Tuberculosis presentation | |||
| Case definition | .25 | ||
| New | 10 (32) | 32 (50) | |
| Prior treatment with first-line drugs | 6 (19) | 8 (13) | |
| Prior treatment with second-line drugs | 15 (48) | 24 (38) | |
| Disease location | .98 | ||
| Pulmonary only | 30 (97) | 62 (97) | |
| Pulmonary and extrapulmonaryd | 1 (3) | 2 (3) | |
| Chest radiology | |||
| Multilobar | 20 (65) | 52 (81) | .07 |
| Bilateral | 18 (58) | 37 (58) | .98 |
| Cavity | 21 (68) | 37 (58) | .35 |
| Bilateral cavities | 5 (16) | 13 (20) | .63 |
| AFB sputum smear positivee | 24 (77) | 48 (75) | .80 |
| Drug resistance | |||
| Extensive drug resistance | 9 (29) | 12 (19) | .26 |
| Median baseline laboratory values (IQR) | |||
| White blood cell count | 9.4 (7.1–12.8) | 8.8 (7.4–11.0) | .33 |
| Hemoglobin | 12.0 (10.9–14.1) | 12.8 (11.4–14.0) | .30 |
| Platelets | 371 (283–466) | 371 (293–430) | .97 |
| Creatinine | .77 (.70–.87) | .69 (.60–.84) | .03 |
| Alanine transaminase | 17 (11–24) | 17 (12–25) | .88 |
| Albumin, n = 37 | 3.6 (3.4–4.0) | 3.6 (3.0–4.0) | .72 |
Abbreviations: AFB, acid-fast bacilli; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range.
aDifferences in categorical variables were tested using the Chi-square test and differences in continuous variables were tested using a 2-sample t test.
b>15 drinks per week for men, >8 drinks per week for women.
cCD4 counts of 4 and 7, not on antiretroviral therapy.
dBone, joint, and ear involvement.
eAt time of new drug initiation.
In regards to treatment characteristics, over half of the patients in each group received bedaquiline (53%) or delamanid (61%) within 2 weeks of any TB treatment initiation; the median numbers of days from treatment initiation were 4 days for delamanid and 15 days for bedaquiline (P = .14) (Table 2). There was a total of 64 unique, initial treatment regimens (Supplementary Table S2), with the most commonly used companion drugs being linezolid, cycloserine, clofazimine, and an injectable agent. More patients on delamanid received clofazimine, but the difference was not statistically significant (81% vs 67% on bedaquiline; P = .09). Cycloserine was more likely to be used among those on bedaquiline, compared to delamanid (91% vs 65%, respectively; P < .01). The median overall number of effective drugs received per patient was 4 in each treatment group (P = .98), while the median number of effective Class A or B drugs received was 2 in each group (P = .43). There were 6 patients who underwent adjunctive surgical resection, including 1 (3%) receiving delamanid and 5 (8%) receiving bedaquiline (P = .39). The median overall treatment durations were 549 days for patients receiving a bedaquiline-based regimen and 533 days for patients receiving a delamanid-based regimen.
Table 2.
Treatment Characteristics and Clinical Outcomes of Patients With Multidrug-resistant Tuberculosis Receiving a Bedaquiline- or Delamanid-Based Treatment Regimen
| Characteristic, median (IQR) | Delamanid, n = 31 | Bedaquiline, n = 64 | P |
|---|---|---|---|
| Days from SLD initiation to starting bedaquiline or delamanid | 4 (0–31) | 15 (0–71) | .14 |
| Initiated bedaquiline or delamanid <15 days from treatment start | 19 (61) | 34 (53) | .45 |
| Bedaquiline or delamanid duration, days | 182 (173–206) | 171 (166–190) | |
| Initial hospitalization duration, days | 111 (72–209) | 103 (64–174) | .27 |
| Subsequent hospitalizations | 1 (1–2) | 1 (1, 2) | .49 |
| Follow-up sputum cultures | 11 (9–13) | 12 (9–14) | .83 |
| Adjunctive surgical resection, n (%) | 1 (3) | 5 (8) | .39 |
| Treatment duration, days | 533 (283–608) | 549 (394–609) | |
| Initial companion drugs, n (%) | |||
| Linezolid | 25 (81) | 50 (78) | .78 |
| Clofazamine | 26 (81) | 43 (67) | .09 |
| Imipenem | 11 (36) | 9 (14) | .01 |
| Pyrazinamide | 4 (13) | 7 (11) | .78 |
| Ethambutol | 1 (3) | 5 (8) | .39 |
| Levofloxacin or moxifloxacin | 11 (36) | 25 (39) | .74 |
| Capreomycin or kanamycin | 19 (61) | 43 (67) | .57 |
| Para-aminosalicylic acid | 5 (16) | 15 (23) | .41 |
| Cycloserine | 20 (65) | 58 (91) | .002 |
| Prothionamide | 6 (19) | 26 (41) | .04 |
| Median drugs received, n (IQR) | 5 (4–6) | 5 (5, 6) | .17 |
| Median effective drugs received, n (IQR) | 4.0 (3.5–5) | 4.0 (4) | .98 |
| Effective Class A or B drugs received | 2 (2–3) | 2 (2, 3) | .43 |
| Clinical outcomes, n (%) | |||
| Median follow-up time since treatment initation, days (IQR) | 905 (812–992) | 855 (716–1016) | .72 |
| Median follow-up time post–treatment outcome, days (IQR) | 396 (258–579) | 367 (230–545) | .57 |
| Sputum culture conversion, n = 91 | 23 (74) | 59 (98) | |
| Culture conversion within 60 days, n = 91 | 15 (48) | 38 (63) | a |
| Culture conversion within 180 days, n = 91 | 23 (74) | 57 (95) | a |
| Acquired drug resistance | 11 (36) | 6 (10) | <.01 |
| Initial treatment outcome | |||
| Cured | 17 (54) | 42 (66) | |
| Completed | 4 (13) | 5 (8) | |
| Loss to follow-up | 2 (7) | 16 (25) | |
| Failure | 6 (19) | 1 (2) | |
| Death | 2 (7) | 0 | |
| Loss to follow-up with recategorized outcomes, n = 18 | |||
| Poor | 1 (3) | 1 (2) | |
| Favorable | 0 | 5 (8) | |
| Relapse | 1 (3) | 1 (2) | |
| Overall treatment outcomes, n = 84 | … | … | a |
| Poor | 10 (33) | 3 (6) | |
| Favorable | 20 (67) | 51 (94) |
There were 68 patients (72%) with MDR-TB who had an initial favorable outcome; the remaining 27 patients (28%) had poor outcomes, including death (2%), failure (7%), and LFU (19%).
Abbreviations: IQR, interquartile range; LFU, loss to follow-up; MDR-TB, multidrug-resistant tuberculosis; SLD, second-line drugs.
aSee Table 4 for adjusted analyses.
There were 4 patients who had a negative culture when initiating bedaquiline or delamanid; thus, 91 patients were evaluated for sputum culture conversion. The median numbers of serial sputum cultures collected were 11 for patients on delamanid and 12 for those on bedaquiline. The sputum culture conversion rates for the whole cohort at 2 and 6 months and overall were 58%, 88%, and 90%, respectively (Table 2). In an adjusted analysis, the estimated proportions of patients achieving sputum culture conversion were higher in patients receiving a bedaquiline-based regimen, as compared to a delamanid-based regimen, at 2 months (67% vs 47%, respectively; P = .10) and at 6 months (95% vs 74%, respectively; P < .01; Table 3; Figure 2). The E-value for the 6-month culture conversion was 1.56 (an unmeasured confounder would need an odds ratio of ≥1.56 with both treatment drug [predictor] and culture conversion (outcome] to negate the association). Acquired drug resistance occurred in 17 patients (Supplementary Table S3) and was more common among patients receiving a delamanid- versus bedaquiline-based regimen (36% vs 10%, respectively; P < .01).
Table 3.
Adverse Events Among Patients With Multidrug-resistant Tuberculosis Receiving a Bedaquiline- or Delamanid-Based Treatment Regimen
| Characteristic | Delamanid, n = 31, n (%) | Bedaquiline, n = 64, n (%) |
|---|---|---|
| Premature permanent discontinuation secondary to adverse event | 1 (3)a | 2 (3)b |
| EKG results | ||
| Mean baseline QTc | 397 (27) | 400 (22) |
| Mean follow-up EKGs (SD) | 9 (3) | 8 (4) |
| Highest mean follow-up QTc (SD) | 448 (26) | 432 (31) |
| Patients with follow-up QTc ≥ 500 ms | 2 (7)c | 1 (2)d |
| Mean largest QTc increase from baseline (SD) | 50 (31) | 31 (34) |
| Patients with QTc increase ≥ 60 ms | 9 (29) | 12 (19) |
| Laboratory follow-up results | ||
| Mean follow-up laboratory tests (SD) | 9 (3) | 8 (4) |
| White blood cell count < 2000/mm3 | 0 | 0 |
| Hemoglobin ≤ 7.9 g/dl | 2 (7) | 1 (2) |
| Platelets < 100 000 per microliter | 3 (10) | 6 (9) |
| Increase in serum creatinine > 2 times baseline | 5 (16) | 15 (23) |
| Potassium < 3.4 mEq/L | 0 | 0 |
| ALT > 3x time upper limit of normal | 4 (13) | 5 (8) |
| ALT > 5x time upper limit of normal | 1 (3) | 3 (5) |
Abbreviations: ALT, alanine transaminase; EKG, electrocardiogram; QT, Q wave to the end of the T wave; QTc, corrected QT interval; SD, standard deviation.
aReceived total of 18 weeks of delamanid, which was stopped along with all other treatment due to hepatotoxicity.
bBedaquiline stopped along with other treatment due to hepatoxicity and exfoliative dermatitis after 23 and 13 weeks, respectively.
cOccurred at 3 months (515 ms) and 5 months (501 ms); both cases had decrease of QTc 1 month later and did not have interruption of delamanid.
dOccurred at 3 months (508 ms) and had decreased QTc 1 month later; no interruption of bedaquiline.
Figure 2.
Sputum culture conversion among patients with multidrug-resistant tuberculosis receiving a bedaquiline- or delamanid-based treatment regimen. A, Adjusted proportion of culture conversion. B, Adjusted difference in proportion of culture conversion. Bars represent 95% confidence intervals. Abbreviations: BDQ, bedaquiline; DLM, delamanid.
There were 68 patients (72%) with MDR-TB who had an initial favorable outcome, and the remaining 27 patients (28%) had poor outcomes, including death (2%), failure (7%), and LFU (19%). Both deaths and 6 of 7 treatment failures were among patients receiving a delamanid-based regimen. The 1 patient with treatment failure who was receiving bedaquiline had culture conversion at 3 months with clinical improvement, but a positive end-of-treatment 24-month sputum culture. The median follow-up time after the initial treatment outcome was 367 days for patients receiving bedaquiline and 396 days for those receiving delamanid. There was 1 case of relapse detected in each group during the study surveillance period. Of the 18 LFU patients, 7 presented for a follow-up evaluation and were able to be re-classified as either a poor outcome (n = 2) based on a positive sputum culture or a favorable outcome (n = 5) based on the lack of symptoms and a negative sputum culture (Table 2; Supplementary Table S4). The remaining 11 LFU patients were not included in the final treatment outcome analysis. In an adjusted analysis, the proportion of patients with a favorable outcome was higher in patients receiving a bedaquiline- versus delamanid-based regimen (96% vs 72%, respectively; P < .01). The E-value for our treatment outcome was 1.98 (Table 3).
There were 2 patients who required premature discontinuation of bedaquiline (hepatoxicity and severe rash) and 1 patient who required premature discontinuation of delamanid (hepatoxicity; Table 4). Only 3 patients had a corrected QT interval (QTc) >500 ms (501, 508, and 515 ms) and neither drug was discontinued due to prolonged QTc. There were 4 patients who developed severe hepatitis (alanine transaminase > 5x time normal), including 1 on delamanid and 3 on bedaquiline (1 patient required temporary bedaquiline interruption).
Table 4.
Adjusted Sputum Culture Conversion Rates and Treatment Outcomes Among Patients With Multidrug-resistant Tuberculosis Receiving a Bedaquiline- or Delamanid-Based Treatment Regimen
| Outcome | Delamanid (95% CI) | Bedaquiline (95% CI) | P Value | E Value |
|---|---|---|---|---|
| SCC at 2 months, n = 91 | .47 (.27–.68) | .67 (.56–.78) | .10 | 1.23 |
| SCC at 6 months, n = 91 | .74 (.60–.88) | .95 (.89–1.00) | <.01 | 1.56 |
| Favorable outcomes, n = 84 | .72 (.61–.82) | .96 (.91–1.00) | <.01 | 1.98 |
Variables adjusted for in all analyses included age, body mass index, gender, history of imprisonment, tobacco and alcohol use, diabetes mellitus, hepatitis C, human immunodeficiency virus infection, new versus prior treatment case, sputum smear status, cavitary disease, and number of effective drugs.
Abbreviations: CI, confidence interval; SCC, sputum culture conversion.
DISCUSSION
Our prospective, observational cohort study found high rates of favorable outcomes among patients with pulmonary MDR-TB who were treated with new and repurposed drugs. In an adjusted analysis, bedaquiline-containing regimens were associated with improved clinical outcomes, as compared to delamanid-based regimens (96% vs 72%, respectively; P < .01). To our knowledge, this is the first study comparing clinical outcomes among patients receiving bedaquiline- versus delamanid-based regimens, and our results support recent WHO guidelines including bedaquiline as a Class A priority drug for all patients with MDR-TB and delamanid as a Class C agent recommended for use when there are not enough Class A and B drugs available [3]. The strengths of our study include a well-characterized prospective cohort with intensive follow-up, including MIC testing, and our robust analytic approach. Our observational cohort study adds novel data to the evidence base of clinical outcomes with newly approved anti-TB drugs.
The development of new treatments for MDR-TB has been urgently needed given poor outcomes and high rates of adverse events with previously used regimens [23]. Observational clinical study data is an important adjunct to clinical trial data to help inform the optimal use of new drugs and various different drug regimens. This is highlighted by the recent WHO drug-resistant treatment guidelines, which were informed by an individual patient meta-analysis of 50 mainly observational cohort studies [5]. Our prospective, observational cohort study adds important data to the field and demonstrates that among a group of patients with MDR-TB with high rates of previous treatment, cavitary disease, and drug resistance, high rates of successful outcomes can be achieved. It also demonstrates that bedaquiline-based regimens may be superior to delamanid-based regimens. In looking at various outcomes, bedaquiline use was associated with higher rates of sputum culture conversion at 2 and 6 months, better clinical treatment outcomes, and less development of acquired drug resistance. In the absence of existing data from randomized controlled trials of bedaquiline versus delamanid, the results from our study help inform clinicians and national tuberculosis programs on the relative efficacy of bedaquiline versus delamanid. Our analytic approach utilized a robust TMLE method to control for all measured confounders and evaluate numerous models, and our sensitivity calculation of E-values found that a moderate to strong unmeasured confounder would have been needed to negate the association of bedaquiline with favorable clinical outcomes. Ongoing clinical trials that will help further define bedaquiline and delamanid use include an AIDS clinical trials group study looking at the safety and tolerability of combining both drugs (NCT02583048) and the endTB study (NCT02754765), which is utilizing an adaptive study design to compare clinical treatment outcomes in bedaquiline- versus delamanid-based regimens.
In regard to delamanid, our results provide needed data on use in a programmatic setting. Beyond the Phase III randomized clinical trial, which found no difference in outcomes whether delamanid was or was not added an optimized background regimen (76.5% vs 77.2%, respectively) [7], there is little reported experience. A retrospective cohort of 19 MDR-TB patients from Latvia who were receiving delamanid found 16 patients with a cure and 3 patients LFU [24]. Another retrospective cohort from South Africa reporting on 12-month interim outcomes among 46 MDR-TB patients found 2- and 6-month culture conversion rates of 52% and 81%, respectively, and favorable outcomes in 61% [25]. Our study clinical outcomes were most consistent with the larger study from South Africa and similarly showed that delamanid was well tolerated and safe. Our results provide novel data on the rate of acquired drug resistance, which was high and may have been underestimated given our lack of delamanid MIC testing. Further clinical outcomes data are needed from other settings to see whether similar rates of treatment failure and acquired drug resistance are seen with delamanid use.
The rates of culture conversion and favorable outcomes with the use of bedaquiline were extremely high, with almost all patients achieving culture conversion (96%) and, after excluding patients LFU, achieving a favorable outcome (95%). These results compare favorably with a trend towards better outcomes than the bedaquiline Phase IIB clinical trial (24-week culture conversion 79%; cure rate 58%) [26] and with another large multicounty cohort of 537 patients [27]. The use of linezolid, clofazimine, and beta-lactams may have contributed to improved outcomes in our cohort, as compared to the Phase IIb study, as these drugs were not part of routine trial regimens. Treatment regimens in the cohort study were more similar to ours, as were results including a 6-month culture conversion rate of 78% and, when excluding patients LFU, a treatment success rate of 80%. Utilizing an active drug safety and monitoring program, we also found reassuring data on the safety and tolerability of bedaquiline. The high rates of LFU in our study (19%) and in the above-mentioned cohort study (15%) are worth mentioning and highlight the challenges of completing treatment. Further observational study will be important to evaluate whether the implementation of injectable-free and shorter-duration regimens decrease LFU.
There are additional important implications from our study. Among 95 patients, there were 64 unique initial treatment regimens utilized. This finding is remarkable in demonstrating the breadth of possible regimens and showing that when using a common backbone, there are many effective regimens, thus allowing clinicians the ability to adjust treatment based on clinical assessments and to provide patient-centered care. Through MIC testing and posttreatment surveillance, we detected 1 bedaquiline- and clofazimine-treated case with disease relapse, with a 3-fold increase in bedaquiline (0.03 → 0.25) and clofazimine (0.06 → 0.50) MICs. This case illustrates that bedaquiline and clofazimine resistance commonly occur together, which is thought to be due to mutations in efflux pumps and brings attention to the concern that the long half life (~5 months) of bedaquiline may increase the risk of increasing MIC values in patients who relapse [28].
Our observational study design introduces the possibility of bias by unmeasured or uncontrolled confounding. This potential for bias was mitigated by the fact that patients receiving each regimen had similar, commonly measured characteristics and by using a robust TMLE approach. TMLE utilizes flexible regression techniques to estimate both outcome regression and propensity scores, and it can yield consistent estimates even in small samples. Additionally, in performing a sensitivity analysis, we found E-values that indicate a moderate to strong unknown confounder would be needed to negate our findings. The lack of delamanid MIC testing also prevented us from determining baseline and acquired drug resistance to the drug. Based on other reports [29], and no prior delamanid use in Georgia, it is unlikely that baseline delamanid resistance existed. However, if baseline resistance existed, this may in part explain treatment failures on delaminid. Forthcoming whole-genome sequencing data will also allow us to infer phenotypic resistance.
In summary, we provide novel and encouraging clinical data on the treatment outcomes among patients receiving bedaquiline- versus delamanid-based regimens and, notably in a programmatic setting, excellent clinical outcomes with the use of bedaquiline.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the physicians, nurses, and staff at the National Center for Tuberculosis and Lung Diseases in Tbilisi, Georgia, who provided care for the patients with multidrug-resistant tuberculosis in this study.
Financial support. This work was supported by the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (grant numbers K23AI103044 and R21AI122001 to R. R. K.); the NIH Fogarty International Center (grant number D43TW007124); the Georgia Clinical and Translational Science Alliance (grant number UL1TR002378); the International Science and Technology Center; and the Emory Global Health Institute.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009; 360:2397–405. [DOI] [PubMed] [Google Scholar]
- 2. Esposito S, Bianchini S, Blasi F. Bedaquiline and delamanid in tuberculosis. Expert Opin Pharmacother 2015; 16:2319–30. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva, Switzerland: World Health Organization, 2019. [PubMed] [Google Scholar]
- 4. Diacon AH, Donald PR. The early bactericidal activity of antituberculosis drugs. Expert Rev Anti Infect Ther 2014; 12:223–37. [DOI] [PubMed] [Google Scholar]
- 5. Ahmad N, Ahuja SD, et al. The Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment-2017 Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 2018; 392:821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 2012; 366:2151–60. [DOI] [PubMed] [Google Scholar]
- 7. von Groote-Bidlingmaier F, Patientia R, Sanchez E, et al. Efficacy and safety of delamanid in combination with an optimised background regimen for treatment of multidrug-resistant tuberculosis: a multicentre, randomised, double-blind, placebo-controlled, parallel group Phase 3 trial. Lancet Respir Med 2019; 7:249–59. [DOI] [PubMed] [Google Scholar]
- 8. Cox V, Brigden G, Crespo RH, et al. Global programmatic use of bedaquiline and delamanid for the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2018; 22:407–12. [DOI] [PubMed] [Google Scholar]
- 9. Tukvadze N, Kempker RR, Kalandadze I, et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLOS One 2012; 7:e31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee J, Armstrong DT, Ssengooba W, et al. Sensititre MYCOTB MIC plate for testing Mycobacterium tuberculosis susceptibility to first- and second-line drugs. Antimicrob Agents Chemother 2014; 58:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: World Health Organization, 2014. [PubMed] [Google Scholar]
- 12. World Health Organization. The use of delamanid in the treatment of multidrug-resistant tuberculosis: interim policy guidance. Geneva, Switzerland: World Health Organization, 2014. [PubMed] [Google Scholar]
- 13. World Health Organization. Introduction of bedaquiline for the treatment of multidrug-resistant tuberculosis at country level: implementation plan. Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 14. Guglielmetti L, Hewison C, Avaliani Z, et al. Examples of bedaquiline introduction for the management of multidrug-resistant tuberculosis in five countries. Int J Tuberc Lung Dis 2017; 21:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Technical report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis [WHO/CDS/TB/2018.5]. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 16. Allana S, Shashkina E, Mathema B, et al. pncA gene mutations associated with pyrazinamide resistance in drug-resistant tuberculosis, South Africa and Georgia. Emerg Infect Dis 2017; 23:491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US Department of Health and Human Services, Food and Drug Administration. Pulmonary tuberculosis: developing drugs for treatment. Rockville, MD: Center for Drug Evaluation and Research, 2013. Available at: https://www.fda.gov/media/87194/download [Google Scholar]
- 18. Laan MJvd, Rose S. Targeted learning: causal inference for observational and experimental data. Spring: Springer Series in Statistics, 2011. Available at: https://www.amazon.com/Targeted-Learning-Observational-Experimental-Statistics/dp/1441997814
- 19. van der Laan MJ, Polley EC, Hubbard AE. Super learner. Stat Appl Genet Mol Biol 2007; 6:Article25. doi:10.2202/1544-6115.1309 [DOI] [PubMed] [Google Scholar]
- 20. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167:268–74. [DOI] [PubMed] [Google Scholar]
- 21. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. Available at: http://www.R-project.org/ [Google Scholar]
- 22. Benkeser D, Carone M, Laan MJV, Gilbert PB. Doubly robust nonparametric inference on the average treatment effect. Biometrika 2017; 104:863–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lange C, Aarnoutse RE, Alffenaar JWC, et al. Management of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2019; 23:645–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuksa L, Barkane L, Hittel N, Gupta R. Final treatment outcomes of multidrug- and extensively drug-resistant tuberculosis patients in Latvia receiving delamanid-containing regimens. Eur Respir J 2017; 50:1701105. doi:10.1183/13993003.01105-2017 [DOI] [PubMed] [Google Scholar]
- 25. Mohr E, Hughes J, Reuter A, et al. Delamanid for rifampicin-resistant tuberculosis: a retrospective study from South Africa. Eur Respir J 2018; 51:1800017. doi:10.1183/13993003.00017-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diacon AH, Pym A, Grobusch MP, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 2014; 371:723–32. [DOI] [PubMed] [Google Scholar]
- 27. Mbuagbaw L, Guglielmetti L, Hewison C, et al. Outcomes of bedaquiline treatment in patients with multidrug-resistant tuberculosis. Emerg Infect Dis 2019; 25:936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen TVA, Anthony RM, Bañuls AL, Nguyen TVA, Vu DH, Alffenaar JC. Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis 2018; 66:1625–30. [DOI] [PubMed] [Google Scholar]
- 29. Stinson K, Kurepina N, Venter A, et al. MIC of delamanid (OPC-67683) against Mycobacterium tuberculosis clinical isolates and a proposed critical concentration. Antimicrob Agents Chemother 2016; 60:3316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.