Abstract
Baseline CD4 testing rates declined from 73% to 21% between 2013 and 2018 with adoption of “Treat All” in Uganda. Advanced human immunodeficiency virus (HIV) disease (CD4 count < 200 cells/µL) remained common (24% of those tested in 2018, 83% of whom had World Health Organization stage I/II disease). Despite frequent presentation with advanced HIV disease, CD4 testing has declined dramatically.
Keywords: HIV, CD4, opportunistic infections, treat all, test-and-treat
(See the Editorial Commentary by Ford et al on pages 2500–1.)
In 2017, there were an estimated 19.6 million people living with human immunodeficiency virus (PLWH HIV) in sub-Saharan Africa (SSA), with less than two-thirds receiving antiretroviral therapy (ART) [1]. The introduction of ART has significantly reduced mortality associated with HIV globally including within SSA [2]. Previous guidelines on when to initiate ART among PLWH used CD4 T-cell count thresholds [3]. In 2015, the World Health Organization (WHO) recommended ART initiation for all PLWH regardless of CD4 count, or a “Treat All” strategy [4], with further recommendations for ART initiation within the same week [5]. These guidelines were intended to reduce barriers to ART initiation, reduce morbidity and mortality, and reduce the risk of ongoing HIV transmission. The Treat All strategy was launched in Uganda in December 2016 [6], with continued baseline CD4 count assessment recommended prior to ART initiation as in WHO guidelines [4].
While the Treat All approach reduces barriers to starting ART, recommendations to initiate ART regardless of CD4 cell count may lead to reductions in evaluation of baseline CD4 T-cell count. Baseline CD4 testing remains the best way to gauge degree of immunosuppression, to guide early screening and prophylaxis for clients at greatest risk of serious opportunistic infections (OIs) and death, and to monitor immune reconstitution in the large proportion of patients still presenting with advanced HIV/AIDS (CD4 < 200 cells/µL) [5]. Interventions that reduce mortality in individuals with advanced HIV/AIDS include cryptococcal antigen screening and preemptive fluconazole for the prevention of cryptococcal meningitis [7], intensified tuberculosis (TB) screening including point-of-care lipoarabinomannan and sputum assessment [8, 9], and co-trimoxazole prophylaxis to reduce incidence of Pneumocystis pneumonia and severe bacterial infections [10]. Routine clinical evaluation has poor accuracy in determining those with low CD4 counts who would benefit from differentiated management for advanced HIV including prevention and early diagnosis of common OIs [11, 12].
We evaluated changes in baseline CD4 testing over time including following rollout of Treat All in Uganda at the end of 2016 as well as factors associated with baseline CD4 assessment following Treat All adoption.
METHODS
Study Setting
The study was conducted at 6 urban public health facilities in Kampala, Uganda, in adults (age > 16 years) initiated on ART during 2013–2018. CD4 testing is available in 1 facility using BD Facscount (BD Biosciences, San Jose, California), and Alere Pima (Abbott, Lake Bluff, Illinois) testing is available at all facilities with same-day results.
Study Design and Analysis
We performed a cross-sectional study using routine clinical data from the Uganda electronic medical record system. Variables included sex, age, point of entry into care, and baseline body mass index (BMI), suspicion for TB disease, and WHO clinical stage.
We evaluated client characteristics using descriptive statistics. The proportion starting ART who had documented baseline CD4 testing by quarter was plotted and single-group interrupted time-series analysis performed using ordinary least squares regression with Newey-West standard errors to evaluate temporal changes [13]. No autocorrelation terms were added, informed by the Cumby-Huizinga test. In clients receiving testing, we evaluated the proportion with advanced (CD4 count < 200 cells/µL) and very advanced (CD4 count < 100 cells/µL) HIV by year. Logistic regression was used to assess whether WHO clinical stage 3 or 4, baseline TB suspicion, or being underweight (BMI < 18.5 kg/m2) were associated with baseline CD4 assessment adjusting for sex, age, and point of entry. Alpha was set at < .05. Analyses were performed using Stata version 13 software (StataCorp, College Station, Texas). Ethics approval was obtained from the School of Health Sciences Research Ethics Committee at Makerere University College of Health Sciences.
RESULTS
A total of 42 672 adults were initiated on ART during 2013–2018; 25% (10 784/42 672) were male and median age was 29 years (interquartile range [IQR], 24–35 years). Main points of clinic entry were the medical outpatient department (55% [22 054/40 193]) and prevention of mother-to-child transmission services (26% [10 450/40 193]). From 2013 through 2018, 58% (24 784/42 672) had baseline CD4 assessment with a median CD4 count of 342 cells/µL (IQR, 195–490 cells/μL); 26% (6331/24 784) had advanced and 13% (3280/24 784) very advanced HIV/AIDS. Seven percent (2751/37 355) with baseline WHO clinical staging were stage 3 or 4, including 17% (940/5606) with a CD4 count < 200 cells/µL and 22% (629/2913) with a CD4 count < 100 cells/µL.
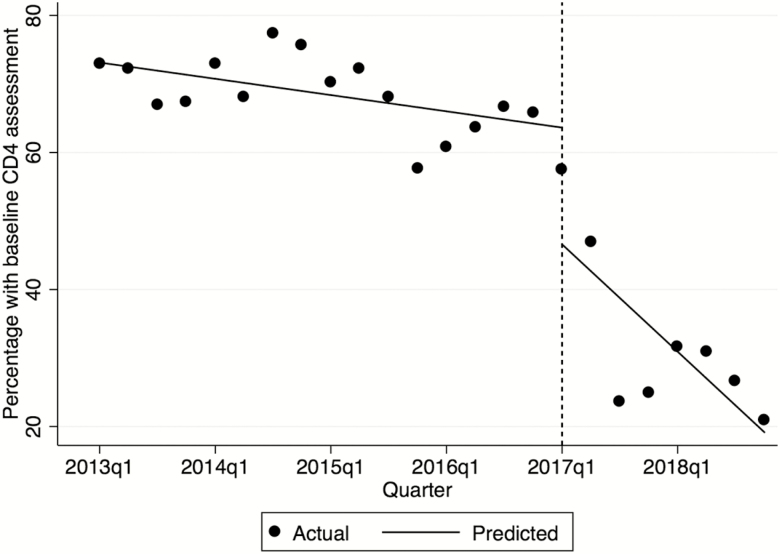
The proportion of new clients with baseline CD4 assessment declined from 73% (1469/2005) in quarter 1 of 2013 to 21% (192/909) in quarter 4 of 2018 (Figure 1). Before Treat All, baseline CD4 T-cell count assessment declined marginally from quarter 1 of 2013 to quarter 4 of 2016 (−0.5% per quarter; 95% confidence interval [95% CI], −.9 to −.2; P = .009), with a decline in testing in quarter 1 of 2017, immediately after adoption of Treat All (−17.8%; 95% CI, −33.4 to −2.2; P = .027), and subsequent decline through quarter 4 of 2018 (−3.5% per quarter; 95% CI, −6.2 to −.8; P = .013). Among clients with baseline CD4 assessment, in the years 2013 and 2018 the proportions with advanced HIV were 29% (1278/4444) and 24% (420/1737), and with very advanced HIV 14% (635/4444) and 12% (216/1737), respectively.
Figure 1.
Trends in baseline CD4 T-cell count assessment in clients initiating antiretroviral therapy during 2013–2018, by quarter. The dotted vertical line represents quarter 1 of 2017 after Treat All was implemented. Dates are presented on the x-axis by the year followed by quarter (q).
After adoption of Treat All, clients who received baseline CD4 assessment were similar to those without in age and baseline BMI. Similar proportions with and without baseline CD4 assessment had suspected TB (6% [318/5274] vs 5% [433/9301] and underweight (9% [131/1449] vs 10% [400/4131]), but clients with baseline CD4 assessment were less likely to have WHO clinical stage 3 or 4 disease compared to those without (6% [306/5005] vs 9% [704/8068]). Suspected TB (adjusted odds ratio [aOR], 1.29; 95% CI, 1.11–1.50; P = .001) was associated with greater receipt of baseline CD4 assessment and WHO stage 3 or 4 disease with lower likelihood (aOR, 0.66; 95% CI, .57–.76; P < .001), while being underweight was not associated with testing (aOR, 0.99; 95% CI, .80–1.22; P = .92).
DISCUSSION
From a programmatic assessment of > 40 000 clients initiating ART in 6 urban clinics in Uganda, we provide important insights into the impact of the HIV Treat All strategy on CD4 testing. A dramatic decline in baseline CD4 assessment was observed following the adoption of Treat All, dropping from almost three-quarters of clients starting ART in 2013 to less than one-quarter by the end of 2018. Furthermore, of clients who received baseline CD4 assessment, the proportion with advanced HIV/AIDS (CD4 < 200 cells/µL) declined only marginally: One-quarter of those with a baseline CD4 in 2018 had a CD4 count < 200 cells/µL, in line with other findings in the region [14, 15]. It is possible that selective baseline CD4 assessment could, in part, explain this ongoing large proportion of clients with advanced HIV disease. However, the overall lack of CD4 testing and similarities between clients who received and did not receive baseline CD4 assessment suggest instead that clinics are prioritizing rapid ART initiation with frequent missed opportunities to provide differentiated care to clients with advanced HIV/AIDS in greatest need for OI screening and prophylaxis.
Our findings highlight the poor sensitivity of WHO clinical staging alone in identifying clients in need of OI screening and prophylaxis. Even under standardized clinical trial conditions of patients with advanced HIV [7, 11], a significant proportion of PLWH initiating ART have stage 1 or 2 disease. Under routine care settings in Uganda, we found that 83% of individuals with a baseline CD4 T-cell count < 200 cells/µL and 78% with a CD4 < 100 cells/µL were initially assessed as having stage 1 or 2 HIV disease, with few if any clinical manifestations of advanced immunosuppression. Surprisingly, following adoption of Treat All, WHO clinical stage 3 or 4 was associated with a lower likelihood of receiving baseline CD4 testing after adjusting for other factors (aOR, 0.66; P < .001). Initial suspicion for TB, however, was associated with greater completion of CD4 testing (aOR, 1.29; P = .001), in line with clinical care guidelines recommending TB screening for all and delayed initiation of ART up to 8 weeks after starting anti-TB therapy in individuals with prevalent TB disease [4]. As baseline CD4 testing is essential for guiding differentiated care, our findings suggest a need for routine rapid CD4 assessment, ideally using point-of-care testing, or better algorithms for determining the likelihood of advanced immunodeficiency where rapid CD4 testing is not readily available.
There were limitations to this study. We used routinely collected clinic data that may have errors. To minimize these, data were reviewed by a data collection team for completeness prior to analysis. Second, detailed reasons for lack of CD4 testing after Treat All rollout were not captured, including healthcare providers’ knowledge of the continued need for baseline CD4 assessment or issues with CD4 assay stockout or machine malfunction. Third, as an exploratory analysis of routinely collected data, we did not capture more detailed information on differences between clients who received and did not receive baseline CD4 assessment, instead focusing on clinically relevant baseline factors. Finally, our study was limited to urban clinic settings in Kampala, Uganda, and may not be broadly generalizable throughout SSA.
In conclusion, a steep decline in baseline CD4 testing was observed following national Treat All adoption. As CD4 count determination is essential in determining clients with advanced HIV and low pre-ART CD4 count remains common, opportunities are missed to reduce early mortality after starting ART through targeted screening of opportunistic infections and preventive therapy. Health systems should prioritize rapid baseline CD4 assessment to reduce early mortality in clients accessing ART services.
Notes
Acknowledgments. The authors acknowledge the health workers at the facilities where these data was collected; the data collectors who collected and cleaned the data; and the United States President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention and Makerere University’s Infectious Diseases Institute for the support of the public health facilities.
Disclaimer. The views expressed are not necessarily those of the US National Institutes of Health (NIH), the National Health Service, the National Institute for Health Research (NIHR), or the Department of Health and Social Care.
Financial support. M. W. T. reports salary support from the NIH (grant numbers T32AI007044 and F32AI140511), outside the submitted work. Salary support for Y. C. M. is provided through the NIH, Fogarty International Center (grant numbers D43TW009771 and D43TW010132). J. N. J. has received support from the UK NIHR using Official Development Assistance funding through a Global Health Professorship (award number RP-2017-08-ST2-012), the Wellcome Trust, the European and Developing Countries Clinical Trials Partnership, and the Medical Research Council.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Joint United Nations Programme on HIV/AIDS. Fact sheet—World AIDS day 2018 Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed 10 May 2019.
- 2. Lessells RJ, Mutevedzi PC, Iwuji CC, Newell ML. Reduction in early mortality on antiretroviral therapy for adults in rural South Africa since change in CD4+ cell count eligibility criteria. J Acquir Immune Defic Syndr 2014; 65:e17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection Available at: https://www.who.int/hiv/pub/guidelines/arv2013/en/. Accessed 10 May 2019.
- 4. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV Available at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. Accessed 10 May 2019. [PubMed]
- 5. World Health Organization. Guidelines for managing advanced HIV disease and early initiation of antiretroviral therapy Available at: http://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/. Accessed 10 May 2019. [PubMed]
- 6. Republic of Uganda Ministry of Health. Consolidated guidelines for prevention and treatment of HIV in Uganda Available at: https://aidsfree.usaid.gov/sites/default/files/uganda_hiv_gl_2016.pdf. Accessed 10 May 2019.
- 7. Mfinanga S, Chanda D, Kivuyo SL, et al. . REMSTART Trial Team Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 2015; 385:2173–82. [DOI] [PubMed] [Google Scholar]
- 8. Gupta-Wright A, Corbett EL, van Oosterhout JJ, et al. . Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018; 392:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peter JG, Zijenah LS, Chanda D, et al. . Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016; 387:1187–97. [DOI] [PubMed] [Google Scholar]
- 10. Walker AS, Ford D, Gilks CF, et al. . Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet 2010; 375:1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hakim J, Musiime V, Szubert AJ, et al. . REALITY Trial Team Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. N Engl J Med 2017; 377:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munthali C, Taegtmeyer M, Garner PG, et al. . Diagnostic accuracy of the WHO clinical staging system for defining eligibility for ART in sub-Saharan Africa: a systematic review and meta-analysis. J Int AIDS Soc 2014; 17:18932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J 2015; 15:480–500. [Google Scholar]
- 14. Carmona S, Bor J, Nattey C, et al. . Persistent high burden of advanced HIV disease among patients seeking care in South Africa’s National HIV program: data from a nationwide laboratory cohort. Clin Infect Dis 2018; 66:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kujawski SA, Lamb MR, Lahuerta M, et al. . Advanced human immunodeficiency virus disease at diagnosis in Mozambique and Swaziland. Open Forum Infect Dis 2017; 4:ofx156. [DOI] [PMC free article] [PubMed] [Google Scholar]