Abstract
Background
Rates of early syphilis in US women are steadily increasing, but predictors of infection in this group are not clearly defined.
Methods
This retrospective analysis focused on women enrolled in the US CFAR Network of Integrated Clinical Systems cohort between January 2005 and December 2016 with syphilis testing performed. The primary outcome of incident syphilis infection was defined serologically as a newly positive test with positive confirmatory testing after a negative test or a 2-dilution increase in rapid plasma regain titer. Infection rates were calculated for each woman-year in care with testing. Predictors of syphilis were sought among sociodemographics, clinical information, and self-reported behaviors. Multivariable logistic regression models were created; a subgroup analysis assessed predictors in women of reproductive age.
Results
The annual rate of incident syphilis among 4416 women engaged in human immunodeficiency virus (HIV) care and tested during the 12-year study period was 760/100 000 person-years. Independent predictors of infection were injection drug use as a risk factor for HIV acquisition (aOR, 2.2; 95% CI, 1.3–3.9), hepatitis C infection (aOR, 1.9; 95% CI, 1.1–3.4), black race (aOR, 2.2; 95% CI, 1.3–3.7 compared with white race), and more recent entry to care (since 2005 compared with 1994–2004). Predictors were similar in women aged 18–49.
Conclusions
Syphilis infection is common among US women in HIV care. Syphilis screening and prevention efforts should focus on women reporting drug use and with hepatitis C coinfection. Future studies should identify specific behaviors that mediate syphilis acquisition risk in women who use drugs.
Keywords: CNICS, hepatitis C, HIV in women, injection drug use, syphilis
Incident syphilis infection was common among women who were tested in a multicenter US Human Immunodeficiency Virus (HIV) clinic cohort. Drug use, hepatitis C coinfection, black race, and recent entry to HIV care were independently associated with syphilis in women of all ages.
Primary and secondary syphilis rates in women and men in the United States rose in parallel in the early 1990s during the crack cocaine epidemic and then reached a nadir in 1999. After 2000, syphilis rates diverged by sex, with a steady increase in men followed by a more recent doubling of rates in women since 2013 [1, 2]. Syphilis infection in pregnancy increased at the same time, and congenital syphilis rates are at a 20-year high, with 1306 cases reported to the US Centers for Disease Control and Prevention (CDC) in 2018 [2–4]. A disproportionate number of early syphilis cases (48%) occur in men who have sex with men (MSM), but information about predictors of infection in women are limited [1, 2]. A 2019 CDC surveillance report was the first to show an association between rising syphilis rates in women and heterosexual men coincident with increasing rates of opioid and methamphetamine drug use [5]. Substance use disorder is a modifiable risk behavior that has also reached epidemic levels, with a prevalence as high as 58% in US women living with human immunodeficiency virus (HIV) [6–9]. A better understanding of modifiable factors contributing to syphilis acquisition risk in women can inform prevention strategies to improve outcomes in women and infants.
The CDC recommends annual syphilis screening for all sexually active adults with HIV [10]. Screening in women is critical since primary infection is often asymptomatic with a painless chancre at the site of inoculation [11]. In a study of incident syphilis predictors in the HIV Outpatient Study, 95% of cases occurred in men and predictors included younger age (18–30 years), black race, and being MSM [12]. These epidemiologic data are useful but limited in terms of discerning patterns and factors associated with syphilis infection in women and pregnancy.
The current study was designed to define rates and predictors of incident syphilis in a large, diverse multicenter cohort of women living with HIV in the United States. Based on historical trends, we hypothesized that predictors in women would include sexual behaviors (unprotected sex with multiple partners), black race, and problem drug or alcohol use.
METHODS
Study Design and Population
We examined adult women living with HIV and enrolled in the US Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS), a dynamic prospective clinical cohort of more than 32 000 adults receiving HIV care at 8 participating academic sites. Methods of data collection from the electronic medical record and patient-reported outcomes have been previously reported [13–15]. Briefly, comprehensive clinical data are collected through electronic medical records and other institutional data systems and undergo rigorous data-quality assessment with a central data repository that is updated quarterly. Visits occurred in HIV primary care clinics or women’s health clinics with syphilis screening and treatment provided as part of routine medical care. This retrospective study was limited to cis-gendered women with at least 1 HIV clinic visit between 1 January 2005 and 31 December 2016 and at least 1 syphilis test performed.
Study Outcomes
The primary study outcome was incident syphilis defined serologically as a positive test following a negative test (treponemal or nontreponemal specific) or a 2-dilution increase in rapid plasma regain (RPR) or Venereal Disease Research Laboratory (VDRL) titer (ie, from 1:16 to 1:64), both with positive confirmatory testing. The incident syphilis rate was calculated as the number of women with new cases among those who were tested during that calendar year. Women with positive testing who did not meet criteria for incident infection were categorized as having prevalent syphilis (positive treponemal and nontreponemal results on initial testing during the study period) or historical infection (isolated positive treponemal test with negative RPR). Secondary outcomes included annual testing rates (regardless of syphilis history) and reported risk behaviors.
Covariates of Interest
We examined baseline demographic and clinical characteristics including the following: age (continuous and categorical), race (black, white, other/unknown), ethnicity (Hispanic/non-Hispanic), CNICS site (Case Western University, Fenway at Harvard University, Johns Hopkins University, University of Alabama at Birmingham, University of California San Diego, University of California San Francisco, University of North Carolina at Chapel Hill, University of Washington), risk factors for HIV acquisition (heterosexual, injection drug use [IDU], other, unknown), hepatitis B (HBsAg), and hepatitis C (hepatitis C virus [HCV] antibody, viral load) testing. Hepatitis B and C were included since infections have been associated with drug use and sexually transmitted infection (STI) risk. Year of entry to HIV care at the site was used as a proxy measure for the timing of HIV diagnosis (which was not collected). We included CD4 count (cells/mm3) and HIV viral load (copies/mL) ± 180 days from the date of the syphilis infection or test date selected for analysis. We defined “in care” as having at least 1 CNICS visit in a given year. “Engagement” in HIV care was defined as having 2 HIV visits separated by 90 days in a calendar year.
Patients used touch-screen devices to complete an assessment of patient-reported measures and outcomes (PROs) every ~4–6 months during routine visits [16]. Seven of the 8 CNICS sites incorporated PROs during the study period and the year of initiation varied (median start in 2008). PRO data (±365 days) closest to the syphilis testing or diagnosis date were included [17]. Sexual behaviors were collected in the modified HIV Risk Assessment for Positives (HRAP) survey and referred to the past 6 months. The HRAP survey asks about sexual partner number, condom use with vaginal/anal sex, partner HIV status (infected, not infected, or unknown), and sex after drugs or alcohol use. We defined consistent condom use as 100%. A proxy variable was created for a high number of sex partners (>6 partners in the past 6 months) as a possible indicator of transactional sex since a dedicated query about sex in exchange for drugs or money was not added to the PRO assessment until 2017. Current drug use in the past 3 months was assessed using the validated modified Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) questionnaire, with responses categorized into current, prior, and never use [18, 19]. We assessed 4 classes of illicit drug use: opioid use including heroin, methamphetamines, cocaine use including crack, and marijuana use. Opioid use referred specifically to medications that were not prescribed for the participant. A composite drug-use measure included opioid, cocaine, and/or methamphetamines. We also evaluated any IDU during follow-up and the term people who inject drugs (PWID) refers to women who reported IDU. The validated Alcohol Use Disorders Identification Test (AUDIT-C) instrument categorized alcohol use in the past 12 months into 3 groups: high risk (score ≥ 4), low risk, and not at risk [20]. The term “problem alcohol use” refers to high-risk use.
Syphilis Testing
Most clinical sites used the traditional algorithm for syphilis testing (nontreponemal screening test with RPR/VDRL) and reflex confirmatory treponemal testing (with syphilis immunoglobulin G, enzyme immunoassay, Treponema pallidum particle agglutination, T. pallidum hemagglutination assay, micro-hemagglutination assay, or fluorescent treponemal antibody test after absorption). A few laboratories switched to the reverse algorithm in the latter study period, with the treponemal screening test followed by reflex RPR. Some laboratories performed qualitative RPR testing. Most indeterminate, equivocal, and borderline test results were repeated per laboratory protocol and results were considered negative if follow-up testing was not definitive.
Statistical Analysis
The annual incidence rate was calculated as the number of newly diagnosed syphilis cases per year with a denominator of women-years with syphilis testing performed in that calendar year. To minimize selection bias caused by loss to follow-up, each year in care was analyzed separately for outcomes of interest. This allowed for variability in predictors (ie, sexual behaviors) over time. The main study outcome was incident syphilis; women were allowed to have more than 1 incident infection during follow-up. Models were based on univariate (UV) and multivariable (MV) logistic regression with generalized estimating equations and auto-regressive correlation structure to account for women who contributed data in more than 1 calendar year. For time-dependent variables, an index date was selected per year in care. This index date was the date of the first positive syphilis test, first negative syphilis test, or the first visit. Variables were chosen for the MV model based on prior studies, data completeness, and collinearity considerations. We anticipated potential collinearity between IDU and HCV coinfection. In the absence of collinearity, we planned to incorporate CNICS site in the model to address differences in patient population and screening practices. Test of trend for predictor variables over time used the Cochrane-Armitage test. Because PRO assessments began during the study period, models with PRO data are limited to the years when surveys were administered. Sensitivity analyses of varying PRO windows were conducted to improve issues with missing data. A preplanned analysis restricted the analysis to women of reproductive age (18–49 years) to assess for unique predictors among women at risk of congenital syphilis and adverse birth outcomes. Statistical significance was set at a 2-sided P < .05, and analysis was performed using SAS version 9.4 (SAS Institute).
Ethics
All women enrolled in the CNICS study completed written informed consent. Institutional review boards (IRBs) at each site approved the cohort protocol, and this study was approved by the University of Alabama at Birmingham IRB.
RESULTS
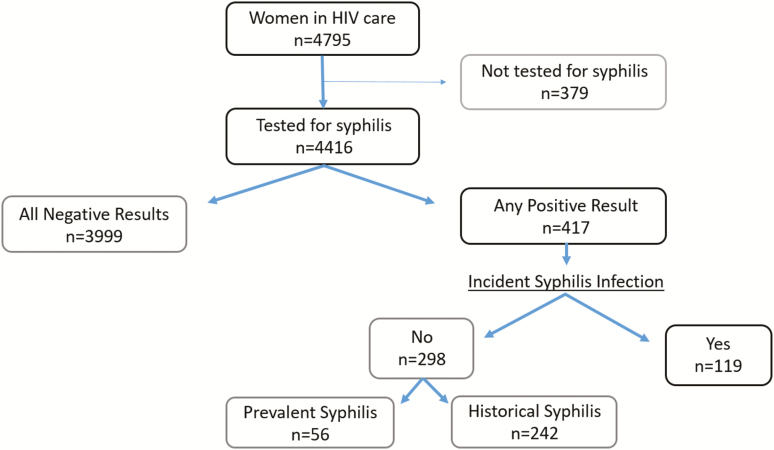
Of 4795 women in the CNICS HIV cohort seen between 2005 and 2016, 4416 (92.1%) were tested for syphilis and comprise the study population. These 4416 women contributed 26 316 person-years in care and 16 447 years (62.5%) with syphilis testing. Annual syphilis testing rates ranged from 55% to 69% by site, with no discernable testing trend over time. A total of 417 women (9.4%) had at least 1 positive syphilis test. Among them, 119 women had 125 new syphilis cases diagnosed during follow-up, the primary outcome of interest. The annual rate of incident syphilis was 760 cases per 100 000 person-years with testing. Among women aged 18–49 years (n = 3416), this rate was 716 cases per 100 000 person-years (Figure 1).
Figure 1.
Flow diagram of women with HIV and incident syphilis in a US CFAR CNICS cohort: 2005–2016. Abbreviations: CFAR, Centers for AIDS Research; CNICS, Centers for AIDS Research Network of Integrated Clinical Systems; HIV, human immunodeficiency virus.
Participant Characteristics
Characteristics of study participants during their most recent year in care and stratified by syphilis test results are shown in Table 1. The median age at last year in care was 47 years (range, 18–97 years; interquartile range [IQR], 38–54 years) and 64% were black. Injection drug use was the HIV-acquisition risk factor in 18% of participants. These cohort characteristics are representative of the US HIV epidemic in women [21]. Engagement in care was 65% overall; on average, women had 3 clinic visits during the most recent year in care and an average of 5.7 years in care during the 12-year study period. Median CD4 count was 532 cells/mm3, and 71% had an HIV viral load (VL) of less than 500 copies/mL. The HCV coinfection rate was 23%: 75% (671/899) of women with HCV RNA VL testing available were viremic (median VL, 1.9 million copies/mL). Women with incident syphilis were older than women with negative serologies (49 vs 47 years; P = .03), and a higher proportion were black (77% vs 62%) and more likely to have IDU as an HIV risk factor (39% vs 17%). Injection drug use as the HIV-transmission risk factor varied significantly by geographic location of the CNICS site, with proportions ranging from 5% in Cleveland to more than 35% in Boston and San Francisco. Patient-reported outcomes are also listed in Table 1.
Table 1.
Characteristics of Women With Human Immunodeficiency Virus According to Syphilis Seroreactivity
| Incident Syphilis Infection (n = 119) | Prevalent or Historical Syphilis Infection (n = 298) | Negative Syphilis Testing (n = 3999) | P a | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Median age (IQR), years | 49 (42–56) | 50 (42–55) | 47 (38–54) | .03 |
| Age | .18 | |||
| 18–29 years | 8 (6.7) | 7 (2.4) | 286 (7.2) | |
| 30–39 years | 18 (15.1) | 41 (13.8) | 871 (21.8) | |
| 40–49 years | 34 (28.6) | 96 (32.2) | 1218 (30.5) | |
| ≥50 years | 59 (49.6) | 153 (51.3) | 1620 (40.5) | |
| Race | <.01 | |||
| Black | 92 (77.3) | 243 (81.5) | 2491 (62.3) | |
| White | 21 (17.7) | 45 (15.1) | 1181 (29.5) | |
| Other/unknown | 6 (5.0) | 10 (3.4) | 327 (8.2) | |
| Hispanic ethnicity | 11 (9.7) | 12 (4.7) | 333 (9.0) | .80 |
| CNICS site | <.01 | |||
| A | 56 (47.1) | 84 (28.2) | 834 (20.9) | |
| B | 22 (18.5) | 104 (34.9) | 952 (23.8) | |
| C | 15 (12.6) | 22 (7.4) | 495 (12.4) | |
| D | 8 (6.7) | 2 (0.7) | 366 (9.2) | |
| E | 9 (7.6) | 30 (10.1) | 595 (14.9) | |
| F | 3 (2.5) | 16 (5.4) | 425 (10.6) | |
| G | 6 (5.0) | 40 (13.4) | 288 (7.2) | |
| H | 0 (0) | 0 (0) | 44 (1.1) | |
| HIV and comorbidities | ||||
| HIV risk factor | <.01 | |||
| Heterosexual sex | 71 (59.7) | 202 (67.8) | 3054 (76.4) | |
| IDU | 46 (38.7) | 77 (25.8) | 680 (17.0) | |
| Other/unknown | 2 (1.7) | 19 (6.4) | 265 (6.6) | |
| Year of initial CNICS HIV visit | .07 | |||
| 1995–2004 | 39 (32.8) | 119 (39.9) | 1430 (35.8) | |
| 2005–2010 | 55 (46.2) | 73 (24.5) | 1458 (36.5) | |
| 2011–2016 | 25 (21.0) | 106 (35.6) | 1111 (27.8) | |
| Most recent year in care at CNICS site | <.01 | |||
| 2005–2011 | 15 (12.6) | 56 (18.8) | 986 (24.7) | |
| 2012–2015 | 47 (39.5) | 66 (22.2) | 1124 (28.1) | |
| 2016 | 57 (47.9) | 176 (59.1) | 1889 (47.2) | |
| Median number of years with HIV visits (IQR) | 7 (4–10) | 5 (3–8) | 5 (3–9) | <.01 |
| Engaged in HIV care (HRSA HAB) | 85 (71.4) | 206 (69.1) | 2568 (64.2) | .11 |
| Median number of HIV visits in year (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | .31 |
| Median CD4 count (IQR), cells/mm3 | 535 (343–827) | 509 (258–782) | 532 (298–794) | .70 |
| CD4 count (cells/mm3) | .57 | |||
| <200 | 18 (16.7) | 48 (17.1) | 604 (16.5) | |
| 200–350 | 11 (10.2) | 46 (16.4) | 500 (13.7) | |
| >350 | 79 (73.2) | 186 (66.4) | 2551 (69.8) | |
| HIV viral load <500 copies/mL | 84 (76.4) | 194 (67.8) | 2615 (71.6) | .27 |
| Death recorded | 14 (11.8) | 40 (13.4) | 522 (13.1) | .68 |
| Hepatitis B coinfection (HBsAg+) | 6 (5.0) | 15 (5.0) | 148 (3.7) | .45 |
| Hepatitis C coinfection (HCV Ab+) | 53 (44.5) | 87 (29.2) | 859 (21.5) | <.01 |
| Patient-reported outcomes—sexb | ||||
| Number of sex partners (n = 1759) | .04 | |||
| 0 | 19 (31.2) | 46 (38.0) | 643 (40.8) | |
| 1 | 33 (54.1) | 66 (54.6) | 825 (52.3) | |
| ≥2 | 9 (14.8) | 9 (7.4) | 109 (6.9) | |
| More than 6 sex partners in past 6 months on any PRO | 6 (7.8) | 5 (3.2) | 83 (3.9) | .09 |
| Sex partner characteristics (n = 1566) | ||||
| HIV-positive | 11 (19.0) | 14 (13.5) | 215 (15.4) | .47 |
| Unknown HIV status | 3 (5.1) | 9 (8.3) | 105 (7.4) | .51 |
| Consistent condom use (n = 1601) | 20 (57.1) | 38 (64.4) | 386 (50.9) | .47 |
| Sex after drugs or alcohol (n = 1617) | 11 (19.0) | 11 (10.3) | 200 (13.8) | .26 |
| Anal sex (ever) (n = 1611) | 19 (32.2) | 34 (31.2) | 474 (32.9) | .92 |
| Patient-reported outcomes—drugs and alcoholb | ||||
| Substance abuse composite (n = 1759) (cocaine, opioid, methamphetamine) | <.01 | |||
| Current | 10 (17.0) | 23 (19.2) | 160 (10.1) | |
| Prior | 24 (40.7) | 46 (38.3) | 439 (27.8) | |
| Never | 25 (42.4) | 51 (42.5) | 981 (62.1) | |
| Cocaine use (n = 1723) | <.01 | |||
| Current | 9 (15.5) | 20 (17.2) | 107 (6.9) | |
| Prior | 23 (39.7) | 42 (36.2) | 408 (26.3) | |
| Never | 26 (44.8) | 54 (46.6) | 1034 (66.8) | |
| Opioid use (nonprescribed) (n = 1330) | .71 | |||
| Current | 0 (0) | 3 (3.7) | 38 (3.1) | |
| Prior | 3 (14.3) | 17 (21.0) | 160 (13.0) | |
| Never | 18 (85.7) | 61 (75.3) | 1030 (83.9) | |
| Methamphetamine use (n = 1700) | .93 | |||
| Current | 1 (1.8) | 1 (0.9) | 41 (2.7) | |
| Prior | 6 (10.9) | 11 (10.1) | 169 (11.0) | |
| Never | 48 (87.3) | 97 (89.0) | 1326 (86.3) | |
| Marijuana use (n = 1712) | .81 | |||
| Current | 13 (22.0) | 18 (16.4) | 289 (18.7) | |
| Prior | 16 (27.1) | 31 (28.2) | 442 (28.7) | |
| Never | 30 (50.9) | 61 (55.5) | 812 (52.6) | |
| IDU on any PRO (n = 2295) | 20 (27.4) | 36 (24.0) | 370 (17.9) | .04 |
| Problem alcohol use (n = 1801) | 7 (11.5) | 16 (13.0) | 214 (13.2) | .69 |
Data are presented as n (%) unless otherwise noted and refer to the most recent year in care for time-dependent variables (age, most recent year in care, engagement in care, CD4, viral load, PRO). N = 4416. Missing n = 5 for age, 362 for ethnicity, 373 for CD4, 366 for HIV viral load, and 2058 for >6 sex partners in 3–6 months.
Abbreviations: Ab+, antibody positive; CNICS, Centers for AIDS Research Network of Integrated Clinical Systems; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HRSA HAB, Health Resources and Services Administration HIV/AIDS Bureau; IDU, injection drug use; IQR, interquartile range; PRO, patient-reported measure and outcome.
a P value comparing the incident syphilis group with the negative syphilis testing group. χ2 tests were used for categorical variables. Wilcoxon rank sum tests were used for continuous variables.
bBehaviors refer to the past 12 months for alcohol, past 6 months for sex, and past 3 months for drug use. Condom use is among sexually active women.
Syphilis Testing Rates
Annual syphilis testing rates by site ranged from 55% to 69%, and incident syphilis rates ranged from 290 to 1560 cases per 100 000 woman-years. To assess potential screening bias, we measured syphilis rates at the 3 centers with the highest testing rates (>75%). We found that infection rates at these sites were similar to infection rates overall. Older women were tested less frequently for syphilis (71% in ages 18–29 years vs 58% in ages ≥50 years), and testing rates were similar by race (62% black vs 64% white).
Models for Incident Syphilis
Table 2 shows results for the univariate association between PRO variables and incident syphilis among those who completed a PRO assessment within ±365 days. Predictors were similar in all women and women of reproductive age. They included active drug use (odds ratio [OR], 3.3; 95% confidence interval [CI], 1.3–8.6), history of drug use (OR, 2.2; 95% CI, 1.0–4.8), IDU during clinic follow-up (OR, 1.7; 95% CI, 1.0–2.8), and multiple sex partners in the past 6 months (OR, 1.9; 95% CI, .9–4.3). Adjusted PRO models could not be created due to missing data, and sensitivity analyses with wider PRO windows showed similar results.
Table 2.
Predictors of Incident Syphilis in Women with Human Immunodeficiency Virus: Patient-Reported Outcomes, 2008–2016
| Women Aged 18–49 Years | All Women | |||
|---|---|---|---|---|
| Variablea | Crude OR (95% CI) | P value | Crude OR (95% CI) | P value |
| Drug-use composite | ||||
| Current | 3.2 (.9–11.0) | .06 | 3.3 (1.3–8.6) | .01 |
| Prior | 1.9 (.6–6.0) | .27 | 2.2 (1.0–4.8) | .06 |
| Never | Ref | Ref | ||
| Cocaine/crack use | ||||
| Current | 4.2 (1.3–13.9) | .02 | 2.7 (1.0–7.5) | .06 |
| Prior | 1.4 (.4–4.8) | .55 | 1.7 (.8–3.6) | .20 |
| Never | Ref | Ref | ||
| Opioid abuse | ||||
| Current | 0 (0–0) | <.01 | 3.4 (.8–14.5) | .10 |
| Prior | 0.8 (.1–5.9) | .79 | 1.8 (.6–5.0) | .27 |
| Never | Ref | Ref | ||
| Methamphetamine use | ||||
| Current | 0 (0–0) | <.01 | 1.5 (.2–11.3) | .70 |
| Prior | 1.9 (.5–6.8) | .31 | 2.5 (1.1–5.8) | .03 |
| Never | Ref | Ref | ||
| Marijuana use | ||||
| Current | 0.5 (.1–2.4) | .27 | 1.0 (.4–2.9) | .94 |
| Prior | 0.8 (.2–2.5) | .68 | 1.0 (.5–2.4) | .94 |
| Never | Ref | Ref | ||
| IDU during HIV clinic follow-up | 1.7 (.9 – 3.3) | .12 | 1.7 (1.0–2.8) | .049 |
| Problem alcohol use in the past 12 months | 0.3 (.04–2.1) | .21 | 0.5 (.2–1.5) | .21 |
| Number of sex partners in past 6 months | ||||
| 0 | Ref | .95 | Ref | .42 |
| 1 | 1.0 (.4–2.9) | .16 | 1.3 (.7–2.7) | .06 |
| ≥2 | 2.5 (.7–8.5) | 2.6 (1.0–6.9) | ||
| More than 6 sex partners in 6-month period | 2.0 (.8–5.0) | .13 | 1.9 (.9–4.3) | .12 |
PRO surveys started after 2008.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IDU, injection drug use; OR, odds ratio; PRO, patient-reported measure and outcome; Ref, reference.
aThe number of syphilis cases in the model varied according to data availability for each PRO variable: composite drug use = 29 cases in 8327 woman-years; cocaine/crack = 29 cases in 8168 person-years; opioid = 23 cases in 7161 woman-years; methamphetamine = 28 cases in 8130 woman-years; marijuana = 27 cases in 7973 woman-years; IDU = 28 cases in 8158 woman-years; alcohol = 46 cases in 9165 woman-years; sex partner number = 39 cases in 8746 women-years; maximum partner number = 81 cases in 16 963 woman-years.
Crude and adjusted models for incident syphilis infection between 2005 and 2016 are shown in Table 3. Models are based on 122 cases of incident syphilis in all women (n = 4407 and 25 468 woman-years) and 77 cases of incident syphilis (n = 3406 and 16 419 woman years) in the subgroup of women aged 18–49 years. Predictors were very similar overall in both groups. Multivariable models in all women demonstrated 4 independent predictors: IDU HIV risk factor (adjusted OR [aOR], 2.2; 95% CI, 1.3–3.9), HCV coinfection (aOR, 1.9; 95% CI, 1.1–3.4), black race (compared with white race; aOR, 2.2; 95% CI, 1.3–3.7), and more recent establishment of care (for 2011–2016 compared with 1994–2004; aOR, 2.3; 95% CI, 1.4–4.0). Detectable HIV viremia was associated with syphilis in women aged 18–49 years (aOR, 1.5; 95% CI, 1.0–2.4) and women aged 18–29 years were somewhat more likely to have incident syphilis compared with women over age 50 years (aOR, 1.9; 95% CI, .9–3.7) (P = .08). Since models with and without CNICS clinic site had similar results and no collinearity, site was retained in the final models.
Table 3.
Predictors of Incident Syphilis in US Women Tested in Human Immunodeficiency Virus Clinics, 2005–2016
| Women Aged 18–49 Yearsa | All Womenb | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Crude OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Crude OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
| Age (years) | ||||||||
| 18–29 | 1.4 (.8–2.5) | .29 | 1.6 (.8–3.2) | .17 | 1.4 (.8–2.5) | .30 | 1.9 (.9–3.7) | .08 |
| 30–39 | .7 (.4–1.2) | .23 | .8 (.5–1.4) | .41 | .7 (.4–1.2) | .21 | .9 (.5–1.6) | .73 |
| 40–49 | Ref | Ref | 1.0 (0.7–1.5) | .95 | 1.1 (.7–1.7) | .71 | ||
| ≥50 | N/A | … | Ref | Ref | ||||
| Race | ||||||||
| White | Ref | .17 | Ref | .01 | Ref | .02 | Ref | <.01 |
| Black | 1.5 (.9–2.5) | .60 | 2.4 (1.2–4.7) | .36 | 1.8 (1.1–2.9) | .97 | 2.2 (1.3–3.7) | .39 |
| Other/unknown | .8 (.3–2.2) | 1.2 (.4–3.6) | 1.0 (.4–2.5) | 1.5 (.6–3.7) | ||||
| Hispanic ethnicity | 1.0 (.5–2.0) | .92 | Not included | N/A | 1.1 (.6–2.0) | .83 | Not included | N/A |
| Initial visit at site | ||||||||
| 1994–2004 | Ref | <.01 | Ref | <.01 | Ref | Ref | <.01 | |
| 2005–2010 | 2.8 (1.6–5.0) | <.01 | 2.9 (1.6–5.4) | <.01 | 1.9 (1.2–2.8) | 1.9 (1.2–2.9) | <.01 | |
| 2011–2016 | 2.7 (1.4–5.3) | 3.4 (1.7–7.1) | 1.8 (1.1–3.0) | 2.3 (1.4–4.0) | ||||
| IDU HIV acquisition risk factor | 3.4 (2.2–5.5) | <.01 | 2.8 (1.3–6.1) | .01 | 3.2 (2.2–4.7) | <.01 | 2.2 (1.3–3.9) | <.01 |
| CD4 count (cells/mm3) | ||||||||
| <200 | 1.2 (.7–2.2) | .53 | Not included | N/A | 1.1 (.7–1.9) | .61 | Not included | N/A |
| 200–350 | 1.4 (.8–2.5) | .27 | … | 1.2 (.8–2.0) | .41 | … | ||
| >350 | Ref | … | Ref | … | ||||
| HIV viral load >500 copies/mL | 1.8 (1.2–2.8) | <.01 | 1.5 (1.0–2.4) | .06 | 1.6 (1.1–2.2) | .02 | 1.3 (.9–1.9) | .14 |
| Hepatitis B infection | 1.3 (.5–3.5) | .63 | Not included | N/A | 1.2 (.5–2.8) | .63 | Not included | N/A |
| Hepatitis C infection | 3.3 (2.1–5.2) | <.01 | 2.3 (1.1–5.1) | .04 | 3.0 (2.1–4.3) | <.01 | 1.9 (1.1–3.4) | .02 |
Model adjusted for variables as noted and CNICS site.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IDU, injection drug use; N/A, not applicable; OR, odds ratio; Ref, reference.
aAdjusted model includes 3406 women with 16 419 woman years and 77 cases of incident syphilis.
bAdjusted model includes 4407 women with 25 468 woman years and 122 cases of incident syphilis.
DISCUSSION
The annual rate of incident syphilis diagnoses among 4416 women tested at 8 urban US HIV clinics between 2005 and 2016 was 760 cases per 100 000 person-years with no trend over time. Nearly 1 in 10 women followed in the CNICS cohort had evidence of active or prior syphilis infection. Historical IDU, hepatitis C coinfection, black race, and recent entry to HIV care were strong and independent predictors of incident syphilis in all women and in younger women who are at risk of adverse pregnancy outcomes.
Annual syphilis testing rates of 55–69% in this study are comparable to other studies of women in HIV care (48–59% in the CDC Medical Monitoring Project) [12, 22]. Screening is recommended in all sexually active adults with HIV, yet 40% of women reported no sex in the past 6 months [10]. As a result, observed rates may have underestimated the proportion of sexually active women screened per CDC guidelines [23]. Incident syphilis rates were similar across age groups. This finding is important and unique compared with chlamydia and gonorrhea infections, which are more prevalent in women with HIV aged less than 30 years [24, 25]. It also highlights the importance of annual syphilis screening in all sexually active women with HIV. The incident syphilis rate reported here is consistent with the HIV Outpatient Study (900 cases per 100 000 woman-years) [12]. In our study, incident syphilis was also more common in women who enrolled more recently in CNICS. These studies indicate a pattern of increasing STI among women with excellent HIV control and access to care—a trend that is not attributable to increased screening alone [1, 25–27].
Predictors of syphilis infection in women with HIV identified in this study align with national trends showing a link between rising syphilis infection rates among heterosexuals and the drug-use epidemic [28–31]. Risk factors for syphilis acquisition in women and pregnancy in the general population include the following: black race, residence in high-incidence areas, and drug use [3, 5, 23]. Illicit drug use continues to be prevalent among women with HIV; this likely contributes to high rates of incident syphilis. More than 1 in 3 women (37%) in the Women’s Interagency HIV Cohort Study between 1996 and 2004 reported IDU [32, 33]. Drug use is also common among pregnant women with HIV (23% in 2012) [34]. Since drug use and IDU were associated with incident syphilis in this study these behaviors are relevant targets for syphilis and congenital syphilis-prevention efforts. We hypothesize that transactional sex is 1 mediator of the association between drug use and incident syphilis in women. Hepatitis C virus coinfection as an independent predictor of syphilis in women warrants further study. Most US women acquire HCV from IDU use but partner characteristics (PWID, HCV status) are also important [35, 36].
Racial disparities in syphilis rates were noted in this study and persist in the United States [1]. We were unable to adjust for socioeconomic status and partner characteristics, which likely account for part of the association. Sexual networks, regional syphilis rates, access to health care, and social marginalization form part of the complex causal web to explain why women with HIV remain vulnerable to syphilis [37].
Limitations and Strengths
Our syphilis case definition was limited to laboratory data since clinical symptoms and physical examination findings were not available systematically for syphilis staging. Also, missing PRO data, particularly in earlier years, and a lack of information about pregnancy and birth outcomes are important limitations. This study focused on women seen in HIV clinics who were tested for syphilis. This selection bias excluded some high-risk women, but findings should be generalizable to many US women in HIV care. In addition, women may have been misclassified as syphilis “negative” due to missed screening opportunities or testing at an outside clinic. This information bias would underestimate actual incidence rates. Social desirability bias is inherent with self-report—a bias that may be reduced by the computer-assisted PRO data collection in CNICS [38]. The current study is limited to cis-gender women since transgender women are being analyzed separately. Strengths of the cohort include its size and representation of a diverse group of US women living with HIV.
Study Implications and Next Steps
This study shows a link between the syphilis epidemic and drug-use epidemic in US women in HIV care. A coordinated response to the drug-use epidemic in women with HIV should include syphilis prevention. Current syphilis screening recommendations in women with HIV may be inadequate, particularly for sexually active women who are at risk of adverse birth outcomes if they become pregnant. Medical professionals should ask women with HIV about drug use at each visit and screen for syphilis accordingly. In the midst of an STI epidemic, HIV cohort studies should advance the scientific agenda by improving data capture for STI and pregnancy outcomes in women. Finally, novel interventions to prevent syphilis in women with HIV are needed: these will be most effective if they focus on modifiable and contributing factors among women at greatest risk.
Conclusions
Incident syphilis infection was common among US women tested as part of routine HIV care. Predictors of infection included drug use, HCV coinfection, black race, and more recent entry to care. Study findings support an intersection between current syphilis and drug-use epidemics in women. Efforts to prevent syphilis in women and infants should prioritize women with HIV and drug use or HCV coinfection.
Notes
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number K23 1HD090993; to JD) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant numbers CFAR P30 A1027767 [University of Alabama at Birmingham], P30 AI094189 [Johns Hopkins University], U01 DA036935 [to R. D. M.], and CFAR Network of Integrated Clinical Systems [CNICS] R24 A1067039).
Potential conflicts of interest. M. K. has received research funding to the institution from Gilead Sciences and ViiV Healthcare and has participated in an advisory board for Gilead Sciences, outside the submitted work. K. C. reports grants from Gilead Sciences, outside the submitted work. H. M. C. reports grants from the National Institutes of Health, Patient-Centered Outcomes Research Institute, and ViiV Healthcare, outside the submitted work. J. D.-O. reports grants from the University of Washington, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: An oral abstract at the 2019 Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washington.
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. Atlanta: US Department of Health and Human Services, 2018. [Google Scholar]
- 2. Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2018. Atlanta: US Department of Health and Human Services, 2019. doi:10.15620/cdc.79370 [Google Scholar]
- 3. Trivedi S, Williams C, Torrone E, Kidd S. National trends and reported risk factors among pregnant women with syphilis in the United States, 2012-2016. Obstet Gynecol 2019; 133:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aslam MV, Owusu-Edusei K, Kidd SE, Torrone EA, Dietz PM. Increasing syphilis diagnoses among females giving birth in US hospitals, 2010–2014. Sex Transm Dis 2018; 46:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kidd SE, Grey JA, Torrone EA, Weinstock HS. Increased methamphetamine, injection drug, and heroin use among women and heterosexual men with primary and secondary syphilis—United States, 2013-2017. MMWR Morb Mortal Wkly Rep 2019; 68:144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cook JA, Burke-Miller JK, Steigman PJ, et al. Prevalence, comorbidity, and correlates of psychiatric and substance use disorders and associations with HIV risk behaviors in a multisite cohort of women living with HIV. AIDS Behav 2018; 22:3141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chirikov VV, Marx SE, Manthena SR, Strezewski JP, Saab S. Development of a comprehensive dataset of hepatitis C patients and examination of disease epidemiology in the United States, 2013-2016. Adv Ther 2018; 35:1087–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whittle HJ, Sheira LA, Frongillo EA, et al. Longitudinal associations between food insecurity and substance use in a cohort of women with or at risk for HIV in the United States. Addiction 2019; 114:127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. VanHouten JP, Rudd RA, Ballesteros MF, Mack KA. Drug overdose deaths among women aged 30-64 years—United States, 1999-2017. MMWR Morb Mortal Wkly Rep 2019; 68:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Workowski KA. Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis 2015; 61(Suppl 8):S759–62. [DOI] [PubMed] [Google Scholar]
- 11. Workowski K, Bolan G. The MMWR series of publications is published by the Center for Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, GA 30329-4027. MMWR Recomm Rep 2015; 64. [Google Scholar]
- 12. Novak RM, Ghanem A, Hart R, Ward D, Armon C, Buchacz K; HIV Outpatient Study Investigators Risk factors and incidence of syphilis in human immunodeficiency virus (HIV)-infected persons: the HIV outpatient study, 1999-2015. Clin Infect Dis 2018; 67:1750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mimiaga MJ, Reisner SL, Grasso C, et al. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of integrated clinical systems cohort. Am J Public Health 2013; 103:1457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hutton HE, Lesko CR, Li X, et al. Alcohol use patterns and subsequent sexual behaviors among women, men who have sex with men and men who have sex with women engaged in routine HIV care in the United States. AIDS Behav 2019; 23:1634–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of integrated clinical systems. Int J Epidemiol 2008; 37:948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fredericksen R, Crane PK, Tufano J, et al. Integrating a web-based, patient-administered assessment into primary care for HIV-infected adults. J AIDS HIV Res 2012; 4:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crane HM, Lober W, Webster E, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Curr HIV Res 2007; 5:109–18. [DOI] [PubMed] [Google Scholar]
- 18. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). Addiction 2008; 103:1039–47. [DOI] [PubMed] [Google Scholar]
- 19. Wolff N, Shi J. Screening for substance use disorder among incarcerated men with the Alcohol, Smoking, Substance Involvement Screening Test (ASSIST): a comparative analysis of computer-administered and interviewer-administered modalities. J Subst Abuse Treat 2015; 53:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujii H, Nishimoto N, Yamaguchi S, et al. The Alcohol Use Disorders Identification Test for Consumption (AUDIT-C) is more useful than pre-existing laboratory tests for predicting hazardous drinking: a cross-sectional study. BMC Public Health 2016; 16:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. HIV Surveillance Supplemental Report. Estimated HIV inc idence and prevalence in the United States, 2010–2016. Atlanta, Georgia: Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, 2019; 64:629–34. [Google Scholar]
- 22. Mattson CL, Bradley H, Beer L, Johnson C, Pearson WS, Shouse RL. Increased STD testing among sexually active persons receiving medical care for HIV infection in the United States, 2009-2013. Clin Infect Dis 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cantor AG, Pappas M, Daeges M, Nelson HD. Screening for syphilis: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315:2328–37. [DOI] [PubMed] [Google Scholar]
- 24. Dionne-Odom J, Westfall AO, Van Der Pol B, Fry K, Marrazzo J. Sexually transmitted infection prevalence in women with HIV: is there a role for targeted screening? Sex Transm Dis 2018; 45:762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raifman JR, Gebo KA, Mathews WC, et al. ; HIV Research Network Gonorrhea and chlamydia case detection increased when testing increased in a multisite US HIV cohort, 2004-2014. J Acquir Immune Defic Syndr 2017; 76:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dean BB, Scott M, Hart R, et al. ; HIV Outpatient Study (HOPS) Investigators Sexually transmitted disease testing of human immunodeficiency virus-infected men who have sex with men: room for improvement. Sex Transm Dis 2017; 44:678–84. [DOI] [PubMed] [Google Scholar]
- 27. Newman DR, Rahman MM, Brantley A, Peterman TA. Rates of new HIV diagnoses after reported STI, women in Louisiana 2000–2015: implications for HIV prevention. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hand DJ, Short VL, Abatemarco DJ. Substance use, treatment, and demographic characteristics of pregnant women entering treatment for opioid use disorder differ by United States census region. J Subst Abuse Treat 2017; 76:58–63. [DOI] [PubMed] [Google Scholar]
- 29. Friedman SR, Mateu-Gelabert P, Ruggles KV, et al. Sexual risk and transmission behaviors, partnerships and settings among young adult nonmedical opioid users in New York City. AIDS Behav 2017; 21:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Acheampong AB, Striley CW, Cottler LB. Prescription opioid use, illicit drug use, and sexually transmitted infections among participants from a community engagement program in North Central Florida. J Subst Use 2017; 22:90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brookmeyer KA, Haderxhanaj LT, Hogben M, Leichliter J. Sexual risk behaviors and STDs among persons who inject drugs: a national study. Prev Med 2019; 126:105779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morris JD, Golub ET, Mehta SH, Jacobson LP, Gange SJ. Injection drug use and patterns of highly active antiretroviral therapy use: an analysis of ALIVE, WIHS, and MACS cohorts. AIDS Res Ther 2007; 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DʼSouza G, Matson PA, Grady CD, et al. Medicinal and recreational marijuana use among HIV-infected women in the Women’s Interagency HIV Study (WIHS) cohort, 1994-2010. J Acquir Immune Defic Syndr 2012; 61:618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rough K, Tassiopoulos K, Kacanek D, et al. ; Pediatric HIVAIDS Cohort Study Dramatic decline in substance use by HIV-infected pregnant women in the United States from 1990 to 2012. AIDS 2015; 29:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frederick T, Burian P, Terrault N, et al. Factors associated with prevalent hepatitis C infection among HIV-infected women with no reported history of injection drug use: the Women’s Interagency HIV Study (WIHS). AIDS Patient Care STDS 2009; 23:915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tieu HV, Laeyendecker O, Nandi V, et al. Prevalence and mapping of hepatitis C infections among men who have sex with men in New York City. PLoS One 2018; 13:e0200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shokoohi M, Bauer GR, Kaida A, et al. ; CHIWOS Research Team Patterns of social determinants of health associated with drug use among women living with HIV in Canada: a latent class analysis. Addiction 2019; 114:1214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Islam MM, Topp L, Conigrave KM, et al. The reliability of sensitive information provided by injecting drug users in a clinical setting: clinician-administered versus audio computer-assisted self-interviewing (ACASI). AIDS Care 2012; 24:1496–503. [DOI] [PubMed] [Google Scholar]