Abstract
Background
Diabetes was identified as a tuberculosis (TB) risk factor mostly in retrospective studies with limited assessments of metabolic variables. The prospective Effects of Diabetes on Tuberculosis Severity study compared adults with pulmonary TB in Chennai, India, who were classified as having either diabetes or a normal glucose tolerance at enrollment.
Methods
Baseline TB severity, sputum conversion, and treatment outcomes (cure, failure, death, or loss to follow-up) were compared between groups with respect to glycemic status and body mass index (BMI).
Results
The cohort of 389 participants included 256 with diabetes and 133 with a normal glucose tolerance. Low BMIs (<18.5 kg/m2) were present in 99 (74.4%) of nondiabetic participants and 85 (33.2%) of those with diabetes. Among participants with normal or high BMIs, rates of cure, treatment failure, or death did not vary by glycemic status. Participants with low BMIs had the highest radiographic severity of disease, the longest time to sputum culture conversion, and the highest rates of treatment failure and death. Among participants with low BMIs, poorly controlled diabetes (glycohemoglobin [HbA1c] ≥8.0%) was unexpectedly associated with better TB treatment outcomes. A high visceral adiposity index was associated with adverse outcomes and, despite an overall correlation with HbA1c, was elevated in some low-BMI individuals with normal glucose tolerance.
Conclusions
In this South Indian cohort, a low BMI was significantly associated with an increased risk for adverse TB treatment outcomes, while comorbid, poorly controlled diabetes lessened that risk. A high visceral adiposity index, either with or without dysglycemia, might reflect a novel TB susceptibility mechanism linked to adipose tissue dysfunction.
Keywords: tuberculosis, diabetes, undernutrition, body mass index, treatment outcomes
In this prospective study of adults with pulmonary tuberculosis in Chennai, India, a body mass index (BMI) <18.5 kg/m2 was associated with higher odds of treatment failure or death. Among low-BMI individuals, comorbid diabetes was unexpectedly associated with better outcomes.
A body of mostly retrospective evidence indicates that a low body mass index (BMI) and poorly controlled diabetes mellitus (DM) are independently associated with increased risks for tuberculosis (TB) disease and adverse treatment outcomes [1–3]. The global population-attributable risk of malnutrition is 3 times that of human immunodeficiency virus (HIV), while the attributable risk of DM matches that of HIV [4]. Both conditions impede global TB elimination [5]. The mechanisms of TB susceptibility likely differ since a low BMI is associated with reduced levels of protective cytokines [6], while TB-DM comorbidity is associated with cytokine elevation [7]. The Effects of Diabetes on Tuberculosis Severity (EDOTS) study is prospectively investigating the TB-DM interaction in India, which has the greatest number of people living with both diseases [8].
The study enrolled adults newly diagnosed with culture-positive, pulmonary TB and classified them as having either DM or a normal glucose tolerance. Participants were followed through 6 months of TB treatment. Outcomes of cure, failure, death, or loss to follow-up were declared at or before the completion of TB treatment. Of 389 participants, 65.8% had DM and 47.3% had a BMI <18.5 kg/mg2. Within the low-BMI group, 46.2% had DM, reflecting the predisposition of South Asians to visceral adiposity and Type 2 DM at a younger age and with a lower BMI than Caucasians [9].
A low BMI and DM are independently associated with increased risks for TB disease and adverse TB outcomes through mechanisms that are incompletely understood. The EDOTS cohort provided an opportunity to compare the relative impacts of a low BMI or DM on TB outcomes, and to determine whether the relationship between DM and TB outcomes differed by the level of BMI. We anticipated finding an additive or synergistic, negative impact of a combined low BMI and DM but instead found that DM significantly weakened the association of a low BMI with treatment failure and mortality.
METHODS
Study Population
Adults aged 25–60 years with pulmonary TB were screened for study participation [10]. The exclusion criteria were having a prior instance of TB, receiving >7 days of incident TB treatment, receiving >7 doses of a fluoroquinolone within 30 days of screening, having multidrug-resistant TB, being pregnant or nursing, living with HIV, taking immunosuppressive drugs, and having prediabetes. The glycemic status at enrollment was classified according to American Diabetes Association standards [11] by glycohemoglobin (HbA1c) for those with a prior DM history or by an oral glucose tolerance test for all others. Baseline chest X-rays were graded using a validated TB severity score [12]. The cohort of 389 individuals comprised all participants with evaluable data who were enrolled between January 2014 and June 2018 and for whom a treatment outcome (cure, failure, death, or loss to follow-up) was recorded. Participants were evaluated monthly through the 6-month course of TB treatment. Treatment failure was defined by a positive sputum culture at Month 5 or 6. Death comprised all-cause mortality. TB treatment was managed by government clinics according to Revised National Tuberculosis Control Program standards and DM was managed by local providers unrelated to the EDOTS study.
Visceral Adiposity Index
The visceral adiposity index (VAI) at baseline was calculated by the following formulae [13], where WC denotes waist circumference, TG denotes triglycerides, and HDL denotes high-density lipoprotein cholesterol:
Plasma Mediators
Plasma collected at enrollment was stored at −80oC prior to Luminex assay (Bio-Rad, Hercules, CA) for interferon-γ, tumor necrosis factor-α, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17A, IL-22, and granulocyte macrophage colony-stimulating factor. Transforming growth factor–β1 (R&D Systems) and IL-17F (BioLegend, San Diego, CA) were measured by enzyme-linked immunosorbent assay.
Data Analysis
Participant characteristics were summarized by glycemic status. Between-group differences were evaluated using Chi-squared tests for percentages or Wilcoxon rank-sum tests for continuous measures. In each of 4 groups—defined by low versus normal-high BMIs and normal glucose tolerance (NGT) versus DM—we calculated the percent of participants lost to follow-up and the percentages of those with known TB treatment outcomes. Between-group differences were evaluated using Chi-square tests or, for failure and death, Fisher’s exact tests.
The relative probability of an adverse outcome (treatment failure or death) by HbA1c and by BMI was portrayed using a locally weighted scatterplot smoothing function [14]. Cut points of HbA1c at 8.0% and BMI at 18.5 kg/m2 were chosen to define 4 subgroups for further analysis. Demographic and lifestyle characteristics, TB symptoms, and treatment outcomes were summarized for the 4 BMI/HbA1c groups. Between-group differences were evaluated using Pearson Chi-square tests for categorical variables, linear regression for continuous variables, or Cox proportional hazards models for time to sputum culture conversion. Associations of TB outcomes and symptoms within the BMI/HbA1c groups were estimated using regression models, adjusting for those covariates considered clinically plausible or previously documented in literature and statistically significant in bivariate association analyses. Key model assumptions and potential interactions among the covariates were tested. With respect to differences among the groups, the results were robust to covariate adjustment and under the wide range of models tested.
An unadjusted logistic regression was performed to evaluate the association of TB cures with possible contributing factors (serum albumin, 25-hydroxyvitamin D, and hemoglobin) [15–17] and with VAI data [13]. The association of metformin use with treatment outcomes was examined in unadjusted and adjusted logistic models among all participants with DM and among those with DM who were only diagnosed prior to incident TB. Analyses were conducted using Stata 14.2 (Stata Corp., College Station, TX). Any P values <.05 were regarded as statistically significant.
Ethics
This study was conducted according to the principles of the Declaration of Helsinki and approved by the Ethics Committee of the Prof. M. Viswanathan Diabetes Research Centre (ECR/51/INST/TN/2013/MVDRC/01). Written informed consent was obtained from all participants.
RESULTS
Study Population
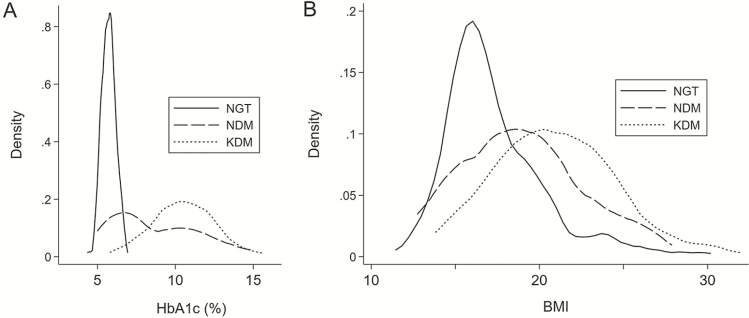
A total of 389 adult, urban Chennai residents with drug-sensitive pulmonary TB were followed through the 6-month course of anti-TB treatment or were recorded as deceased or lost from the study. At enrollment, 133 (34.2%) had NGT, 162 (41.6%) reported a DM diagnosis before incident TB (known DM [KDM]) and 94 (24.2%) had a new DM (NDM) diagnosis at study screening. Demographic, anthropometric, behavioral, and clinical laboratory variables are shown in Supplementary Table 1. Notably, 84 (33.2%) of the 256 participants with DM had BMIs <18.5 kg/m2, which is a cut-point for undernutrition [18]. Figure 1 shows the distribution of HbA1c and BMI data in NGT, KDM, and NDM participants.
Figure 1.
Distribution of (A) HbA1c and (B) BMI, stratified by glycemic classification as NGT, KDM prior to incident TB, and NDM, based on enrollment screening oral glucose tolerance test. Abbreviations: BMI, body mass index; DM, diabetes mellitus; HbA1c, glycohemoglobin; KDM, known DM; NDM, new DM; NGT, normal glucose tolerance; TB, tuberculosis.
Treatment Outcomes by Body Mass Index and Glycemic Status
We compared TB treatment outcomes grouped by BMIs <18.5 or ≥18.5 kg/m2 and by glycemic status as DM or NGT. Adverse outcomes of treatment failure or death were more frequent in low-BMI individuals, compared to those with normal-high BMIs (Table 1). No adverse effect of DM on TB outcomes was observed in either BMI group (Table 1). This was surprising, since BMI and DM were independently associated with adverse TB outcomes in prior studies [19, 20] and might have additive effects when combined.
Table 1.
Treatment Outcomes by Body Mass Index and Glycemic Status
| Outcome (%) | BMI <18.5 kg/m2 | BMI <18.5 kg/m2 | BMI ≥18.5 kg/m2 | BMI ≥18.5 kg/m2 | P Valuea |
|---|---|---|---|---|---|
| NGT, n = 99 | DM, n = 85 | NGT, n = 34 | DM, n = 171 | ||
| Lost | 17.2 | 21.2 | 17.6 | 11.7 | .23 |
| Not lost | n = 82 | n = 67 | n = 28 | n = 151 | |
| Cure | 78.1 | 82.1 | 96.4 | 92.7 | .003 |
| Failure | 14.6 | 11.9 | 3.6 | 6.0 | .09 |
| Death | 7.3 | 6.0 | 0 | 1.3 | .05 |
Abbreviations: BMI, body mass index; DM, diabetes mellitus; NGT, normal glucose tolerance.
aFor group differences using Fisher’s exact test for failure and death, chi-square test for lost and cure.
Low BMIs and low HbA1c levels were both associated with higher odds of bad outcomes (Supplementary Figure 1). To determine whether the relationships between DM and TB outcomes were different by the level of BMI, we compared demographic and behavioral characteristics, TB symptoms, radiographic severity, and time to sputum conversion in 4 groups: BMI <18.5 kg/m2 with HbA1c <8.0% (BMIlo/A1clo); BMI <18.5 kg/m2 with HbA1c ≥8.0% (BMIlo/A1chi); BMI ≥18.5 kg/m2 with HbA1c <8.0% (BMIhi/A1clo); and BMI ≥18.5 kg/m2 with HbA1c ≥8.0% (BMIhi/A1chi). Details of this group-wise comparison are shown in Supplementary Table 2.
In adjusted regression models, the BMIlo/A1clo group had the greatest radiographic severity and longest time to sputum conversion among the 4 groups (Table 2). The odds of being lost from the study (including through voluntary withdrawal) were similar for all groups. Among those not lost to follow-up, BMIlo/A1clo participants were more likely than those with normal-high BMIs to have died or failed treatment. The members of the BMIlo/A1chi group fared better in treatment outcomes than BMIlo/A1clo participants and were not significantly different from the 2 groups with normal-high BMIs. In adjusted models, the BMIhi/A1clo and BMIhi/A1chi groups did not differ from each other. Contrary to our prediction, poorly controlled DM was not associated with higher odds of adverse TB outcomes among participants with normal or high BMIs and unexpectedly reduced the odds for bad TB outcomes among those with low BMIs.
Table 2.
Adjusted Multivariate Models of Tuberculosis Treatment Outcomes
| BMI <18.5 kg/m2 | Total n in Model | ||||
|---|---|---|---|---|---|
| HbA1c <8.0% | HbA1c ≥8.0% | HbA1c <8.0% | HbA1c ≥8.0% | ||
| CXR score, coefficienta | 14.99 (7.27–22.71) | 9.24 (−.58 to 19.05) | 2.32 (−7.18 to 11.82) | 0 | 331 |
| TTC, HRb | .64 (.44–.93) | 1 | .70 (.45–1.09) | .72 (.49–1.05) | 352 |
| Lost, OR | 1 | 1.07 (.40–2.86) | 1.24 (.49–3.16) | 1.01 (.47–2.21) | 371 |
| Cure, OR | 1 | 3.04 (.92–10.05) | 4.40 (1.17–16.55) | 4.49 (1.77–11.35) | 312 |
| Failure/death, OR | 4.99 (1.77–11.36) | 1.48 (.42–5.19) | 1.02 (.25–4.09) | 1 | 312 |
Data are presented as n (95% confidence interval). Data were adjusted for age, sex, height, household income, smoking, and alcohol consumption.
Abbreviations: BMI, body mass index; CXR, chest x-ray; HbA1c, glycohemogloblin; HR, hazard ratio; OR, odds ratio; TTC, time to sputum conversion.
aCoefficient (higher score indicates greater radiographic severity of disease).
bHigher HR indicates faster sputum conversion.
Nutritional Biomarkers
The TB risk in people with low BMIs is commonly attributed to undernutrition, but the mechanisms of susceptibility and resistance associated with extremes of BMIs are poorly understood. In our cohort, there was an association of low serum albumin with a low BMI that might reflect undernutrition (Supplementary Figure 2). Albumin was negatively associated with radiographic severity at enrollment and was positively associated with being cured (P = .001). In contrast, there were no associations of adverse TB outcomes with serum 25-hydroxyvitamin D or hemoglobin.
Potential Countervailing Mechanisms Associated with Diabetes Mellitus
The moderating effect of DM on TB outcomes in low-BMI individuals might be explained by behaviors, but this was not evident in the data. Durations of coughs before enrollment were similar between groups and there was no consistent trend for greater symptoms in any group (Supplementary Table 2). This suggests that there was no difference between groups in the time from TB onset to diagnosis. More BMIlo/A1clo participants were lost compared to other groups, but adherence was no different between groups for those reaching a treatment endpoint. Metformin was reported to reduce TB mortality in Type 2 DM patients [21] and was used by 116 (71.6%) of KDM participants in our study. However, there were no associations of metformin with TB outcomes in analyses based on all participants with HbA1c ≥8.0%, all DM (KDM plus NDM) participants, or KDM participants only. Among 162 KDM participants, cures were recorded for 75.9% on metformin and 89.1% of those not on metformin (P = .57, adjusted for age, sex, BMI, stature, income, alcohol, and smoking).
In prior studies, TB-DM comorbidity was associated with elevated plasma cytokine levels, compared to TB without DM [7], while a low BMI was associated with low cytokine levels [6]. To assess whether cytokine elevation with DM could offset the immunosuppressive effect of a low BMI, we measured levels of 15 cytokines in plasma from 256 participants with available samples. There was no consistent pattern of cytokine differences between the low-BMI participants with or without poorly controlled DM that could explain the relative protection enjoyed by the former (Supplementary Figure 3).
Visceral Adiposity Index is Associated with Adverse Tuberculosis Outcomes
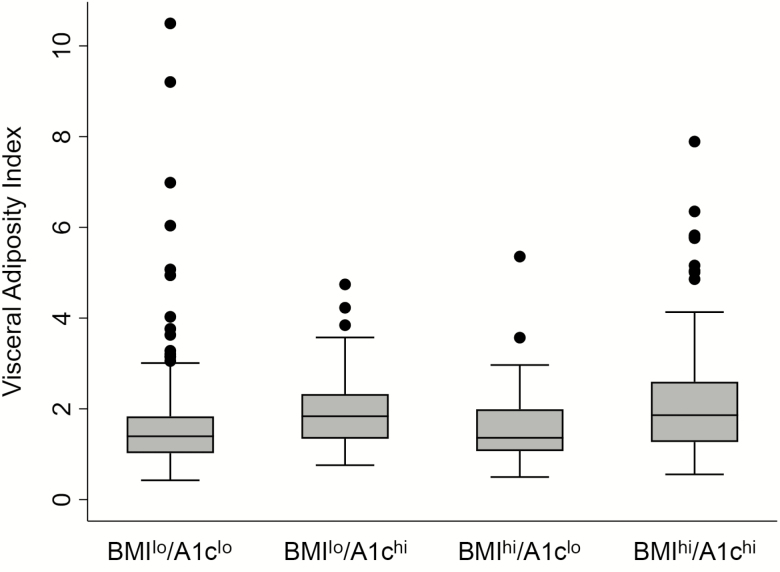
Visceral adiposity is a hallmark of DM and, in South Asians, may be present despite a normal or low BMI. White adipose tissue might provide an energy reserve to fuel immunity and is a niche for pathogen-specific memory T cells [22]. Despite those considerations, we found no associations of waist circumference or waist-height ratio with TB outcomes. The VAI reflects both visceral fat mass and metabolic dysfunction [13]. There was an expected, overall-positive correlation of VAI results with HbA1c in our cohort, although VAIs declined at the highest HbA1c levels (Supplementary Figure 4). We tested the association of VAIs with adverse TB outcomes in the 4 BMI/A1c groups. As shown in Table 3, a significant effect was evident in BMIlo/A1clo participants, but not in the other 3 groups. Among all participants, a VAI result >5.0 was strongly associated with the combined outcomes of treatment failure or death, with an odds ratio (OR) of 5.1 and a 95% confidence interval (CI) of 1.4–19.0 (P = .015). This association remained significant when restricted to participants with BMIs <18.5 kg/m2 (OR = 13.5; 95% CI 1.4–135.0; P = .027). A groupwise scatter plot of VAIs surprisingly revealed that 12 (8.7%) participants in the BMIlo/A1clo group were outliers, having high VAIs despite normal glucose tolerance (Figure 2). We concluded that a high VAI, which is associated with cardiovascular disease risk [23], reflects a TB susceptibility mechanism among individuals with low BMIs.
Table 3.
Association Between Visceral Adiposity Index and Adverse Tuberculosis Outcomes
| BMI <18.5 kg/m2 | ||||
|---|---|---|---|---|
| HbA1c <8.0% | HbA1c ≥8.0% | HbA1c <8.0% | HbA1c ≥8.0% | |
| VAI per unit (OR) | 1.53 (.02) | 1.08 (.90) | .84 (.88) | 1.12 (.67) |
| n per group | 112 | 37 | 44 | 135 |
Data are for combined outcomes of treatment failure or death among participants not lost to follow-up. Data are shown by BMI-HbA1c group.
Abbreviations: BMI, body mass index; HbA1c, glycohemogloblin; OR, odds ratio; VAI, visceral adiposity index.
Figure 2.
Visceral Adiposity Index (VAI), stratified by BMIs and HbA1c levels. The VAI was calculated for the following 4 groups: BMI <18.5 kg/m2 and HbA1c <8.0% (BMIlo/A1clo; n = 138); BMI <18.5 kg/m2 with HbA1c ≥8.0% (BMIlo/A1chi; n = 44); BMI ≥18.5 kg/m2 and HbA1c <8.0% (BMIhi/A1clo; n = 50); and BMI ≥18.5 kg/m2 and HbA1c ≥8.0% (BMIhi/A1chi; n = 151). The horizontal lines indicate the medians, the boxes show the interquartile ranges, the whiskers show the highest and lowest values, and the dots represent outliers. Abbreviations: BMI, body mass index; HbA1c, glycohemoglobin.
Discussion
The EDOTS study was designed to compare presenting features and treatment responses of pulmonary TB in participants rigorously characterized as having either a NGT or Type 2 DM. India has the highest number of DM cases linked to TB [24], and the study site in Chennai is at the epicenter of this syndemic, reflected by the 54.1% prevalence of DM in EDOTS participants with TB that was reported in an interim analysis when recruitment was sequential [10]. We now report the longitudinal follow-up of the first 389 participants with a TB treatment outcome determined. We anticipated finding greater TB severity and worse treatment outcomes in the DM group, particularly in view of the poor glycemic control in this population, with a median HbA1c level of 10.5%. Instead, a BMI <18.5 kg/m2 was the strongest negative factor for the combined outcomes of treatment failure or all-cause mortality (adjusted OR, 4.49; 95% CI 1.77–1.35; Table 2). Contrary to expectations, poorly controlled DM, classified as an HbA1c level ≥8.0%, was paradoxically associated with better outcomes in low-BMI participants, resulting in sputum conversion and cure rates closer to those of diabetic and nondiabetic participants with normal-high BMIs.
Our findings were consistent with evidence linking low BMIs to greater risks for TB treatment failure and mortality [25]. Over half of the TB incidence in India is attributable to undernutrition [26]. The association of TB risk and BMI is commonly ascribed to undernutrition, but additional factors may be involved, since the log linear correlation of BMI with susceptibility and protection extends through normal BMIs to obesity [27]. Epidemiological evidence suggests that the protective effect of a high BMI may be tempered in diabetic obesity [28], although that was not evident in our cohort. There was a strong correlation between BMI and serum albumin (Supplementary Figure 2), and low albumin levels were associated with adverse TB outcomes, suggesting that undernutrition was a factor in the poor treatment response of the BMIlo/A1clo group. However, albumin is an imperfect biomarker of nutritional status, since levels fall with systemic inflammation [29]. We found no association of treatment outcomes with levels of vitamin D or hemoglobin, which are alternative indicators of nutritional status [30]. Short stature is a marker of childhood and inter-generational undernutrition [31], but we found no relations between stature and TB treatment outcomes in this cohort. Having a low BMI was the major risk factor for poor TB outcomes in our cohort and may have influenced the TB-DM interaction in ways that differ from other populations, where this comorbidity more commonly occurs in overweight individuals [20, 32].
Poorly controlled DM had a protective effect in individuals with low BMIs and TB in our study (Table 2). We considered that participants with DM might have had a shorter interval between TB onset and diagnosis, due to contact with medical providers for DM care and greater health awareness. That possibility could not be excluded with the available data, but there was no difference in the duration of cough prior to a TB diagnosis across all BMI/A1c groups. We considered that demographic or behavioral variables might account for the protective effect of poorly controlled DM in low-BMI individuals, but this association was maintained after adjustments for potentially confounding variables that influence TB susceptibility, including age [33], sex [34], smoking [35], and alcohol consumption [36]. This association was also maintained after an adjustment for family income, which is an indicator of food insecurity [37]. Treatment adherence is the most important factor influencing TB outcomes, but did not differ between groups in this cohort (Supplementary Table 2).
Abundant clinical and basic research evidence supports the hypothesis that chronic hyperglycemia impairs TB defense [38]. The apparent lack of negative consequences for high HbA1c levels in EDOTS participants with normal to high BMIs, and protection in those with low BMIs, might reflect population-specific factors that obscured the well-described adverse effects of diabetic immunopathy in TB [38]. We questioned whether relative protection in the underweight participants with DM was attributable to a DM-associated factor other than chronic hyperglycemia. It has been proposed that metabolic syndrome reflects an evolutionary response to build the fat energy reserves needed for defense against chronic infectious diseases, including TB [39]. Visceral adiposity, a hallmark of metabolic syndrome, might provide such a protective energy reserve. Moreover, white adipose tissue is a niche for protective, pathogen-specific, memory T cells [22]. Despite these considerations, we found no associations of TB outcomes with waist circumference alone or as a ratio with height. Waist circumference does not distinguish between subcutaneous and visceral adipose tissue, nor does it indicate adipose tissue dysfunction. We therefore calculated VAIs that included serum lipid parameters linked to metabolic syndrome. This metric, which reflects visceral adipose tissue mass and function, is a biomarker for cardiovascular disease risk [13]. While VAIs were generally correlated with HbA1c levels in our cohort, they declined at the highest HbA1c values and were surprisingly elevated in 8.7% of BMIlo/A1clo individuals who had normal oral glucose tolerance tests. We anticipated that higher VAIs would be associated with protection, but the opposite was observed; participants with VAIs >5.0 had significantly greater odds of adverse TB outcomes. When analyzed as a continuous variable versus in the 4 BMI/A1c groups, the association of VAI with adverse TB outcomes was restricted to BMIlo/A1clo individuals. To our knowledge, VAI was not previously investigated in the context of TB.
Our study had several limitations. The sample size of NGT participants with BMIs ≥18.5 kg/m2 was small, which may have limited our ability to identify the expected adverse effect of poorly controlled DM on TB outcomes in those with normal-high BMIs. The magnitude of the impact that low BMIs had in this cohort was not anticipated, so a detailed nutrition survey was not included in the research protocol and vitamin A levels [40] were not measured. The association of VAI with TB outcomes was also not anticipated, so potentially relevant studies—such as homeostatic model assessments for insulin resistance or measurements of free fatty acids or plasma glycerol—were not performed. Despite these limitations, our study identified low BMI as a more important risk factor for adverse TB outcomes than DM, and it revealed a surprising, protective effect of comorbid, poorly controlled DM in underweight individuals. Future studies evaluating other DM-associated variables, such as adipose tissue inflammation or gut microbiomes, might clarify the mechanisms of susceptibility and protection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. H. K., S. B., and V. V. obtained funding for the project and designed the study. S. B. S. managed the study team at the field sites. K. W. managed the database and data quality. E. P.-G., K. K., and W. L. developed the statistical analysis plan and performed the analyses. H. K., E. P.-G., and V. V. drafted the manuscript. All authors contributed to the data collection and quality assurance, critiqued the manuscript for important intellectual content, contributed to the manuscript revisions, and approved the final version.
Acknowledgments. The authors thank the Greater Chennai Corporation, Dr Senthil Kumar (City Health Officer), and Dr Lavanya J (Revised National Tuberculosis Control Program Program Officer) for permission to conduct the study in the Tuberculosis Units of Chennai and Drs Matthew Magee (Emory University) and Gregory Bisson (University of Pennsylvania) for providing invaluable advice on the analysis and presentation of this report.
Financial support. This work was supported by the Indian Department of Biotechnology (grant number USB1-31149-XX-13 to V. V. and H. K.), the Indian Council of Medical Research, and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and was administered by Civilian Research and Development Foundation Global.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: a systematic review. PLOS One 2017; 12:e0175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ugarte-Gil C, Alisjahbana B, Ronacher K, et al. Diabetes mellitus among pulmonary tuberculosis patients from four TB-endemic countries: the TANDEM study. Clin Infect Dis 2019;. pii:ciz284. doi:10.1093/cid/ciz284 [DOI] [PubMed] [Google Scholar]
- 3. Huangfu P, Ugarte-Gil C, Golub J, Pearson F, Critchley J. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis 2019; 23:783–96. [DOI] [PubMed] [Google Scholar]
- 4. Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med 2013; 2013:28939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Odone A, Houben RM, White RG, Lonnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol 2014; 2:754–64. [DOI] [PubMed] [Google Scholar]
- 6. Shaviya N, Budambula V, Webale MK, Were T. Circulating interferon-gamma levels are associated with low body weight in newly diagnosed Kenyan non-substance using tuberculosis individuals. Interdiscip Perspect Infect Dis 2016; 2016:9415364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prada-Medina CA, Fukutani KF, Pavan Kumar N, et al. Systems immunology of diabetes-tuberculosis comorbidity reveals signatures of disease complications. Sci Rep 2017; 7:1999–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harries AD, Kumar AM, Satyanarayana S, et al. Addressing diabetes mellitus as part of the strategy for ending TB. Trans R Soc Trop Med Hyg 2016; 110:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unnikrishnan R, Anjana RM, Mohan V. Diabetes in South Asians: is the phenotype different? Diabetes 2014; 63:53–5. [DOI] [PubMed] [Google Scholar]
- 10. Kornfeld H, West K, Kane K, et al. High prevalence and heterogeneity of diabetes in patients with TB in South India: a report from the effects of diabetes on tuberculosis severity (EDOTS) study. Chest 2016; 149:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 2018; 41(Suppl 1):S13–27. [DOI] [PubMed] [Google Scholar]
- 12. Ralph AP, Ardian M, Wiguna A, et al. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax 2010; 65:863–9. [DOI] [PubMed] [Google Scholar]
- 13. Amato MC, Giordano C, Galia M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010; 33:920–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1974; 74:829–36. [Google Scholar]
- 15. Murakami R, Matsuo N, Ueda K, Nakazawa M. Epidemiological and spatial factors for tuberculosis: a matched case-control study in Nagata, Japan. Int J Tuberc Lung Dis 2019; 23:181–6. [DOI] [PubMed] [Google Scholar]
- 16. Hella J, Cercamondi CI, Mhimbira F, et al. Anemia in tuberculosis cases and household controls from Tanzania: contribution of disease, coinfections, and the role of hepcidin. PLOS One 2018; 13:e0195985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aibana O, Huang CC, Aboud S, et al. Vitamin D status and risk of incident tuberculosis disease: a nested case-control study, systematic review, and individual-participant data meta-analysis. PLOS Med 2019; 16:e1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferro-Luzzi A, Sette S, Franklin M, James WP. A simplified approach of assessing adult chronic energy deficiency. Eur J Clin Nutr 1992; 46:173–86. [PubMed] [Google Scholar]
- 19. Yen YF, Chuang PH, Yen MY, et al. Association of body mass index with tuberculosis mortality: a population-based follow-up study. Medicine (Baltimore) 2016; 95:e2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiménez-Corona ME, Cruz-Hervert LP, García-García L, et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax 2013; 68:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Degner NR, Wang JY, Golub JE, Karakousis PC. Metformin use reverses the increased mortality associated with diabetes mellitus during tuberculosis treatment. Clin Infect Dis 2018; 66:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han SJ, Glatman Zaretsky A, Andrade-Oliveira V, et al. White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity 2017; 47:1154–68.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kouli GM, Panagiotakos DB, Kyrou I, et al. Visceral adiposity index and 10-year cardiovascular disease incidence: the ATTICA study. Nutr Metab Cardiovasc Dis 2017; 27:881–9. [DOI] [PubMed] [Google Scholar]
- 24. Lönnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol 2014; 2:730–9. [DOI] [PubMed] [Google Scholar]
- 25. Bhargava A, Chatterjee M, Jain Y, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLOS One 2013; 8:e77979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhargava A, Benedetti A, Oxlade O, Pai M, Menzies D. Undernutrition and the incidence of tuberculosis in India: national and subnational estimates of the population-attributable fraction related to undernutrition. Natl Med J India 2014; 27:128–33. [PubMed] [Google Scholar]
- 27. Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2010; 39:149–55. [DOI] [PubMed] [Google Scholar]
- 28. Lin HH, Wu CY, Wang CH, et al. Association of obesity, diabetes, and risk of tuberculosis: two population-based cohorts. Clin Infect Dis 2018; 66:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004; 17:432–7. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients 2017; 9:E829–E848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martorell R, Zongrone A. Intergenerational influences on child growth and undernutrition. Paediatr Perinat Epidemiol 2012; 26(Suppl 1):302–14. [DOI] [PubMed] [Google Scholar]
- 32. Magee MJ, Kempker RR, Kipiani M, et al. Diabetes mellitus is associated with cavities, smear grade, and multidrug-resistant tuberculosis in Georgia. Int J Tuberc Lung Dis 2015; 19:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schaaf HS, Collins A, Bekker A, Davies PD. Tuberculosis at extremes of age. Respirology 2010; 15:747–63. [DOI] [PubMed] [Google Scholar]
- 34. Horton KC, MacPherson P, Houben RM, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLOS Med 2016; 13:e1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slama K, Chiang CY, Enarson DA, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis 2007; 11:1049–61. [PubMed] [Google Scholar]
- 36. Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). a systematic review. BMC Public Health 2009; 9:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chinnakali P, Upadhyay RP, Shokeen D, et al. Prevalence of household-level food insecurity and its determinants in an urban resettlement colony in north India. J Health Popul Nutr 2014; 32:227–36. [PMC free article] [PubMed] [Google Scholar]
- 38. Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol 2014; 44:617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roth J, Szulc AL, Danoff A. Energy, evolution, and human diseases: an overview. Am J Clin Nutr 2011; 93:875s–83. [DOI] [PubMed] [Google Scholar]
- 40. Aibana O, Franke MF, Huang CC, et al. Impact of vitamin A and carotenoids on the risk of tuberculosis progression. Clin Infect Dis 2017; 65:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.