Abstract
Background
Treatment initiation with integrase strand transfer inhibitors (INSTIs) has been associated with excess weight gain. Whether similar gains are seen after switch to INSTIs among virologically suppressed persons is less clear. We assessed pre/post-INSTI weight changes from AIDS Clinical Trials Group participants (A5001 and A5322).
Methods
Participants who were in follow-up from 1997–2017 and switched to INSTI-based antiretroviral regimens were included. Piecewise linear mixed-effects models adjusting for age, sex, race/ethnicity, baseline BMI, nadir and current CD4+ T-cell count, smoking, diabetes and follow-up time with suppressed HIV-1 RNA examined weight and waist circumference change before and after first switch to INSTIs. Linear spline models with a single knot at time of switch accounted for nonlinear trends.
Results
The 972 participants who switched to INSTIs were 81% male and 50% nonwhite with a median age at switch of 50 years, CD4+ T-cell count 512 cells/μL, and BMI 26.4 kg/m2. Restricting to persons with suppressed HIV-1 RNA at switch (n = 691), women, blacks, and persons ≥60 years experienced greater weight gain in the 2 years after versus before switch. In adjusted models, white or black race, age ≥60, and BMI ≥30 kg/m2 at switch were associated with greater weight gain following switch among women; age ≥60 was the greatest risk factor among men. Trends for waist circumference were similar.
Conclusions
Yearly weight gain increased following switch to INSTIs, particularly for women, blacks, and persons aged ≥60. Concomitant increases in waist circumference suggest that this weight gain is associated with an increase in fat mass.
Keywords: integrase inhibitor, weight gain, women, HIV dolutegravir
We report the first data on within-person weight trajectories following switch to integrase inhibitor-based antiretroviral therapy while virologically suppressed, suggesting a risk of greater weight gain for some people with human immunodeficiency virus, particularly for women and with dolutegravir use.
Weight gain following antiretroviral therapy (ART) initiation is common with most modern ART regimens; however, recent reports from both real-world and randomized clinical trial settings suggest that integrase strand transfer inhibitor (INSTI)–based ART use may be associated with excess weight gain, particularly when compared with the use of nonnucleoside reverse transcriptase inhibitor (NNRTI)–based ART. For example, in a pooled analysis of 8 randomized controlled trials involving ART initiation, INSTI use was associated with greater weight gain than NNRTI- and protease inhibitor (PI)–based ART use, with risk factors for weight gain including lower pre-ART CD4+ T-cell count, higher pre-ART human immunodeficiency virus (HIV)-1 RNA, no injection drug use, female sex, and black race [1]. Sex-based differences in risk of excess weight gain following any ART and INSTI initiation has been observed in other studies [2, 3], but the mechanism(s) remains unknown.
Most data examining weight change with INSTIs have focused on the period immediately following ART initiation, but analysis of weight gain in this period is associated with confounding due to the return to health phenomenon. As INSTI-based ART has increasingly become the standard of care for most persons with HIV (PWH), switch to INSTI-based ART from NNRTI- and PI-based regimens has become a frequent occurrence in clinical care. Whether switch to INSTI-based ART in virologically suppressed individuals is also associated with excess weight gain, and which patients are at the greatest risk of such weight gain following switch to INSTI-based ART, remains unclear [4, 5]. To address this issue, we assessed weight changes following switch to INSTI-based ART among AIDS Clinical Trials Group (ACTG) participants in protocols A5001 and A5322, which provide long-term, observational follow-up of individuals previously enrolled in ACTG randomized interventional trials. We hypothesized that, in virologically suppressed individuals, the switch to INSTIs would be associated with increased within-person, annualized weight gain compared with the pre-switch time period.
METHODS
Study Population
Two sequential, longitudinal, observational ACTG-sponsored cohorts, A5001 and A5322, provided more than 20 years of participant follow-up. In 2000, A5001 (ACTG Longitudinal Linked Randomized Trials [ALLRT]) [6] began enrolling participants previously enrolled into ACTG randomized interventional trials. Parent trials included both ART initiation in treatment-naive individuals and change to salvage therapy. A5001 followed participants every 4 months through 2013. In 2013, A5001 follow-up ended and participants 40 years of age or older from treatment-naive parent trials were offered enrollment into A5322 (the HIV Infection, Aging, Immune Function Long-Term Observational Study [HAILO]), which follows participants every 6 months. For this analysis, INSTI-naive A5001 and A5322 participants who switched to INSTI-based ART by the end of 2017 and had available weight data were included.
Assessments
Standardized weights was captured at every 4-month visit on A5001 and yearly in A5322, and waist circumference was measured initially every 4 months then yearly in A5001 and in A5322. Medical history and medications were updated every 4 months in A5001 and every 6 months in A5322. Fasting lipid and glucose and CD4+ T-cell counts were measured annually during A5322, 2–3 times every year during A5001, and HIV-1 RNA was measured every 6 months at local, certified laboratories.
Statistical Analysis
Weight and waist circumference trajectories were plotted overall and by individual INSTI agent using locally estimated scatterplot smoothing (LOESS) curves. Annualized, within-person weight and waist circumference changes were estimated pre- and post-switch to INSTIs, allowing participants to serve as their own controls for estimation of background/age-related weight gain. Participants who subsequently stopped the INSTIs were censored at the time of cessation (8.5% of all switchers). Those switching to a different INSTI during follow-up remained in the study (8.7% of switchers). Piecewise linear mixed-effects models adjusting for age, sex, race/ethnicity (black, non-Hispanic; white, non-Hispanic; Hispanic; other), parent study, baseline body mass index (BMI), and their interactions with time; nadir CD4+ T-cell count; smoking; diagnosis of diabetes; and percentage of follow-up time (>75% vs ≤75%) with suppressed (<200 copies/mL) HIV-1 RNA were fit to examine factors associated with weight and waist circumference change before and after first switch to INSTIs. Linear spline models with a single knot at the time of switch accounted for nonlinear trends.
Two additional analyses examining weight were conducted. First, we modeled slope over all available follow-up time pre- and post-INSTI switch. Second, we compared the post-INSTI switch slope to that of propensity score–matched participants with suppressed HIV-1 RNA who did not switch to INSTIs.
Linear mixed-effects models were also fit to assess the effect of post-switch weight gain on metabolic parameters, including lipids and glucose concentrations. Models were adjusted for time since switch, most recent lipid or glucose levels prior to switch, sex, age, race, history of diabetes mellitus, and current CD4+ T-cell count.
RESULTS
Study Population
Adults (n = 972) who switched to INSTIs (68% from PI, 31% NNRTI, 2% other non-INSTI) at a median of 7.8 years after parent trial entry were primarily male (81%) and 50% were nonwhite (Table 1). The median age at switch was 50 years, CD4+ T-cell count was 512 cells/μL, and BMI was 26.4 kg/m2; 539 switched to raltegravir (RAL), 222 to elvitegravir (EVG), and 211 to dolutegravir (DTG). Timing of follow-up did not allow for inclusion of persons switching to bictegravir (BIC)-based ART. Demographic and clinical characteristics of the 691 persons with suppressed HIV-1 RNA (<200 copies/mL) at the time of switch were similar to those of the larger cohort (Table 1). To avoid the confounding effects of viremia-induced catabolism, further data presented here are restricted to persons who had suppressed HIV-1 RNA at the time of switch to an INSTI (n = 691).
Table 1.
Baseline Demographics and Clinical Characteristics of Participants
| All Switchers (n = 972) | Suppressed at Switch (n = 691) | |
|---|---|---|
| Male sex, % | 81 | 82 |
| Age, years | 50 (45, 57) | 51 (46, 57) |
| Black race, % | 29 | 26 |
| Hispanic ethnicity, % | 19 | 19 |
| Body mass index, kg/m2 | 26.4 (23.5, 29.9) | 26.9 (23.7, 30.3) |
| Weight, kg | 79.1 (69.1, 91.7) | 79.9 (69.9, 93.3) |
| Body mass index category, % | ||
| Underweight | 2 | 1 |
| Normal | 37 | 34 |
| Overweight | 37 | 37 |
| Obese | 25 | 27 |
| Waist circumference, cm | 93.5 (86.0, 102.1) | 94.2 (86.9, 103.5) |
| Waist circumference elevated for sex,a % | 52 | 56 |
| Total cholesterol, mg/dL | 180 (156, 211) | 183 (159, 213) |
| HDL cholesterol, mg/dL | 43 (35, 53) | 45 (37, 55) |
| Triglycerides, mg/dL | 139 (97, 214) | 137 (96, 209) |
| LDL cholesterol, mg/dL | 103 (80, 126) | 105 (83, 129) |
| Fasting glucose, mg/dL | 90 (83, 100) | 91 (84, 102) |
| Hemoglobin A1c, % | 5.5 (5.2, 5.9) | 5.5 (5.2, 5.9) |
| History of diabetes prior to switch, % | 11 | 12 |
| History of smoking prior to switch, % | 58 | 57 |
| Current CD4+ T-cell count, cells/μL | 512 (321, 749) | 610 (439, 816) |
| Nadir CD4+ T-cell count ≤200 cells/μL, % | 59 | 54 |
| HIV-1 RNA prior to switch, copies/mL | 40 (39, 1,077) | … |
| ART regimen prior to switch, % | ||
| PI-based | 68 | 63 |
| NNRTI-based | 31 | 35 |
| Other | 5 | 2 |
| Main NRTI component prior to switch, % | ||
| ABC | 20 | 25 |
| TDF | 53 | 49 |
| TAF | 11 | 14 |
Data are presented as medians (interquartile range) or percentages. Values are at time of/prior to switch unless indicated.
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; HOMA-IR, homeostasis model assessment–insulin resistance; LDL, low-density lipoprotein; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside/-tide reverse transcriptase inhibitor; PI, protease inhibitor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
aGreater than 94 cm for men and 80 cm for women.
Unadjusted Weight Trajectories
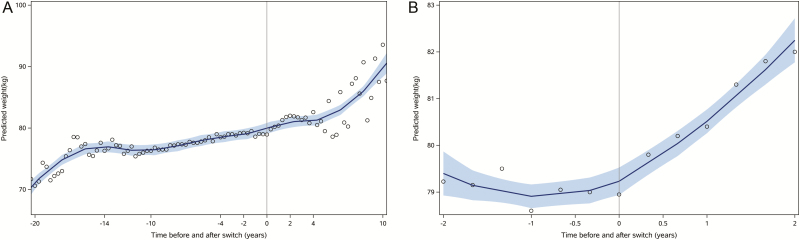
Predicted weights over the complete follow-up period for participants switched to an INSTI are shown in Figure 1A, with weight trajectories for the 2 years prior to and following switch to INSTIs shown in Figure 1B. Overall annualized weight gain for the cohort was 0.4 kg/year prior to INSTI initiation and 0.6 kg/year following INSTI initiation. However, women, persons of black race, and persons aged 60 years or older experienced significantly greater weight gain in the 2 years following switch to INSTIs versus the 2 years prior to switch, with 1.3, 0.9, and 1.2 kg annualized weight gain after switch for these groups, respectively. Women and persons of black race also experienced significant increases in waist circumference following switch to INSTIs (data not shown). Men and persons aged less than 40 years experienced similar or less weight gain following switch to INSTIs compared with pre-INSTI weight changes (Table 2).
Figure 1.
Change in weight before and after switch to an integrase strand transfer inhibitor, over the total duration of follow-up (A) including antiretroviral therapy initiation and limited to the 2 years before and after switch (B). The predicted weight is represented by the line and the 95% confidence interval by the shaded area. Abbreviations: ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor.
Table 2.
Annual Rate of Weight Change Pre-/Post-switch to Integrase Strand Transfer Inhibitors
| All | Women | Men | White Racea | Black Race | Age <40 Yearsb | Age ≥60 Years | BMI <18.5 kg/m2b | BMI >30 kg/m2 | |
|---|---|---|---|---|---|---|---|---|---|
| Pre-INSTI | 0.4 (<.001) | 0.3 (.05) | 0.5 (<.001) | 0.4 (<.001) | 0.3 (.04) | 1.1 (<.001) | −0.03 (.8) | 0.8 (.7) | 0.02 (.9) |
| Post-INSTI | 0.6 (<.001) | 1.6 (<.0001) | 0.4 (.0009) | 0.4 (.002) | 1.2 (<.001) | −0.3 (.42) | 1.2 (<.001) | 1.4 (.03) | 0.5 (.05) |
| Pre-post difference | 0.2 (.22) | 1.3 (<.001) | −0.1 (.6) | 0.01 (.97) | 0.9 (.002) | −1.4 (.01) | 1.2 (.001) | 0.5 (.6) | 0.5 (.2) |
Weight change shown in kilograms/year (P value) for 2 years before and after switch to INSTIs in virologically suppressed adults (n = 691).
Abbreviations: BMI, body mass index; INSTI, integrase strand transfer inhibitor.
aResults for Hispanic ethnicity were similar to those for white race.
bNo significant change in slope of weight gain among persons 40–60 years of age or for BMI 18.5–30 kg/m2.
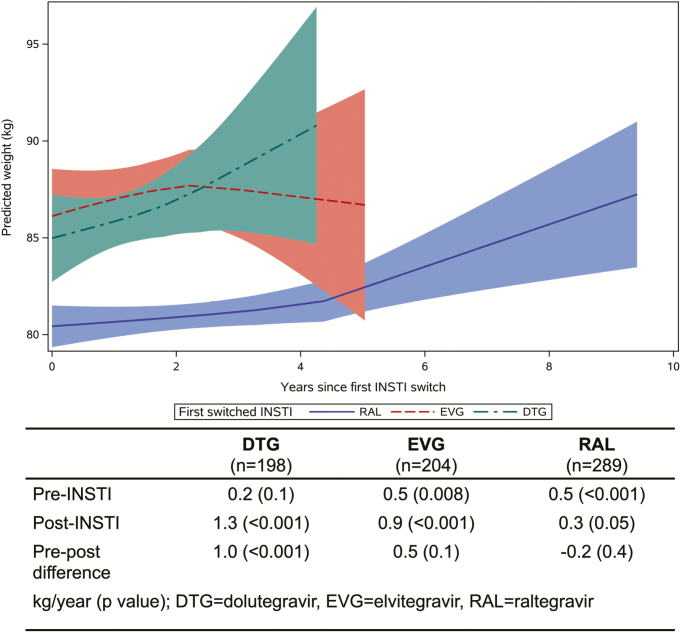
Although subgroup sample sizes limited the ability to fully explore individual drug effects, persons switched to DTG gained 1.0 kg more per year following the switch, whereas persons switched to RAL or EVG had more neutral to slightly reduced annualized weight gain over the follow-up period (Figure 2). Increases in annualized change in waist circumference were also greatest for DTG versus RAL and EVG, although the pre/post differences were not statistically significant (data not shown).
Figure 2.
Weight after switch to an INSTI, by DTG (green), EVG (red), or RAL (blue). Corresponding estimates of weight change and P values are also shown. The predicted weight is represented by the line and the 95% confidence interval by the shaded area. Abbreviations: DTG, dolutegravir; EVG, elvitegravir; INSTI, integrase strand transfer inhibitor; RAL, raltegravir.
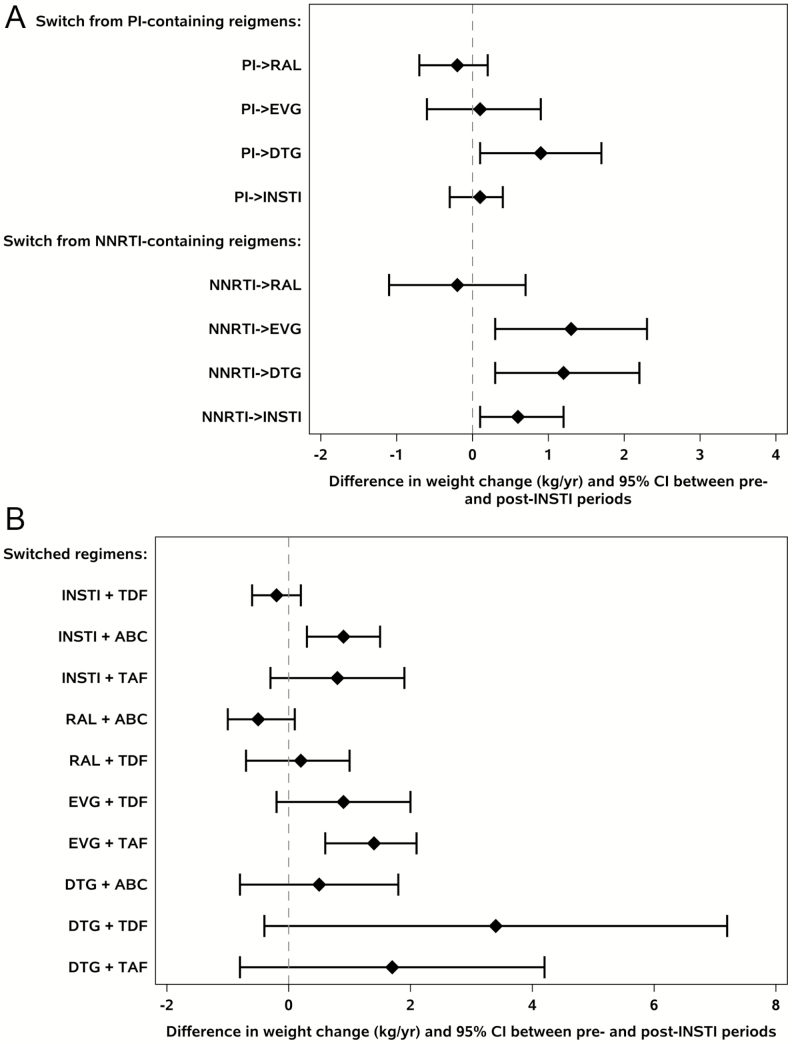
Weight gain following switch to INSTIs also varied by pre-switch ART class and agent and by nucleoside reverse transcriptase inhibitor (NRTI) at time of switch. Switch from NNRTI to both DTG and EVG was associated with significant increases in annualized weight gain (DTG: +1.2 [95% confidence interval [CI] .3, 2.2] kg/year, P = .01; EVG: +1.3 [.3, 2.3] kg/year, P = .01), whereas switch from NNRTI to RAL was not (−.2 [−1.1, .7] kg/year, P = .6). When switching from a PI, only switch to DTG was associated with significant increases in annualized weight gain (DTG: +0.9 [95% CI, .06, 1.7] kg/year, P = .04; EVG: +0.1 [−.7, .9] kg/year, P = .8; RAL: −.2 [−.7, .2] kg/year, P = .3) (Figure 3A). Similar trends in waist circumference were seen when switching from NNRTI to INSTI, whereas a switch from PI to INSTI was not associated with significant annualized changes in waist circumference (data not shown).
Figure 3.
Annual weight gain (kg/year) by pre-switch PI- or NNRTI-based regimens to specific INSTI agents (A). Annual weight gain (kg/year) by the post-switch combination of INSTI and NRTI (B). Abbreviations: ABC, abacavir; CI, confidence interval; DTG, dolutegravir; EVG, elvitegravir; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RAL, raltegravir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Since these were observational data, participants were not required to maintain their NRTI backbone when switching to an INSTI, although 62% of participants on abacavir (ABC), 61% on tenofovir disoproxil fumarate (TDF), and 57% on tenofovir alafenamide (TAF) prior to switch maintained this NRTI backbone at switch to INSTI. Given this, switch to any INSTI with ABC was associated with a significant increase in annualized weight gain (+.9 [95% CI, .3–1.5] kg/year, P = .004) with a similar effect size with TAF (0.8 [−.3 to 1.9] kg/year, P = .1), while switch to INSTI with TDF showed no significant weight gain (−.2 [−.6 to .2] kg/year, P = .3). As certain NRTI/INSTI combinations were more likely to occur as part of switch, particularly when switching to a single-tablet regimen, individual combinations were further explored, and switch to any INSTI with ABC and switch to EVG with TAF were associated with significant increases in annualized weight gain compared with pre-switch (Figure 3B).
Adjusted Weight Trajectories
In adjusted models, white or black race, age 60 or older, and BMI of 30 kg/m2 or greater were associated with significantly greater annualized weight gain (0.9 to 2.0 kg) following switch among women (Table 3). Among men, age 60 or older was the greatest risk factor, with men in that age group experiencing a 0.8 kg greater annualized weight gain compared with pre-switch. Trends for waist circumference were similar, particularly for women who were black or obese prior to switch to an INSTI (data not shown).
Table 3.
Adjusted Annual Rate of Weight Change by Sex, Race, Age, and Body Mass Index at Switch to Integrase Strand Transfer Inhibitors
| Women | Men | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Black | White | Black | White | Age ≤40 | Age ≥60 | Age ≤40 | Age ≥60 | Women: BMI ≥30 kg/m2 | |
| Pre-INSTI | 0.4 (.08) | 0.6 (.03) | 0.4 (.02) | 0.4 (<.001) | 1.5 (.01) | −0.2 (.61) | 0.8 (.009) | 0.1 (.5) | 0.2 (.5) |
| Post-INSTI | 1.3 (<.001) | 2.0 (<.001) | 1.0 (.002) | 0.2 (.09) | −1.0 (.2) | 1.8 (<.001) | −0.1 (.9) | 0.9 (<.001) | 1.9 (<.001) |
| Pre-post difference | 0.9 (.04) | 1.4 (.02) | 0.6 (.11) | −0.2 (.4) | −2.5 (.02) | 2.0 (.008) | −0.9 (.20) | 0.8 (.04) | 1.7 (.002) |
Weight change shown in kilograms/year (P value) for 2 years before and after switch to INSTIs (n = 691). Adjusted for age, sex, race/ethnicity, parent study, baseline BMI, and their interactions; nadir CD4+ T-cell count; smoking; diabetes; and percentage of follow-up time with suppressed (<200 copies/mL) HIV-1 RNA. There was no significant change for Hispanic men or women. Women aged 40–49 years, not significant; 50–59 years similar to ≥60 years. Men aged 40–49 years similar to ≤40 years; 50–59 years, not significant. Nonobese women and men of any BMI category, not significant.
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor.
When we included all available pre- and post-switch weight data (median time pre-switch = 7.2 years and post-switch = 3.8 years), the difference between pre- and post-switch slope was not significantly different among women (−.03 [95% CI, −.2, .2] kg/year), persons of black race (−.1 [−.3, .05] kg/year), or black women (−.1 [−.5, .2] kg/year); age 60 or older remained associated with greater post-switch weight gain (.4 [.2, .6] kg/year).
To further validate our findings, we compared the post-switch weight slope for INSTI switchers with that of virally suppressed, propensity score–matched participants from A5001 and A5322 over the same time interval who did not switch to an INSTI regimen (n = 694). Compared with the INSTI-switch slope (0.5 [95% CI, .2, .8] kg), the slope for those who did not switch was significantly less steep (.1 [−.03, .3] kg).
Cardiometabolic Impact of Weight Gain
Cardiometabolic effects of weight gain following switch to an INSTI were explored (Table 4). In models adjusting for time since switch to an INSTI, most recent fasting lipid or glucose prior to switch, sex, age, race, history of diabetes mellitus, and nadir CD4+ T-cell count, each 1-kg increase in weight was associated with a 0.14 mg/dL decline in high-density lipoprotein cholesterol, a 0.26-mg/dL increase in low-density lipoprotein cholesterol, a 0.36-mg/dL increase in total cholesterol, a 1.10-mg/dL increase in triglycerides (P < .001 for all lipids), and a 0.06-mg/dL increase in fasting glucose (P = .007). Post-switch to an INSTI, changes in fasting lipid and glucose values were similar (data not shown).
Table 4.
Effect of Integrase Strand Transfer Inhibitor Weight Change on Fasting Lipid and Glucose Parameters
| HDL Cholesterol | LDL Cholesterol | Total Cholesterol | Triglycerides | Glucose | |
|---|---|---|---|---|---|
| Change in lipid/glucose parameter (mg/dL) per 1-kg change in weight after INSTI switch | −0.14 [−.16, −.12] (<.001) | 0.26 [.20, .32] (<.001) | 0.36 [.29, .44] (<.001) | 1.10 [.83, 1.37] (<.001) | 0.06 [.02, .10] (.007) |
Data are presented as B-estimates [95% CI] (P value). Reference values: female sex, black race, <40 years of age, no diabetes. Models were adjusted for time after INSTI switch, baseline lipid/glucose value, sex, race/ethnicity, age, nadir CD4+ T-cell count, proportion of time of viral suppressed, and history of diabetes.
Abbreviations: BMI, body mass index; CI, confidence interval; INSTI, integrase strand transfer inhibitor.
DISCUSSION
We observed small but statistically significant increases in annualized, within-person weight gain among virologically suppressed persons switched to INSTI-based ART. Specifically, women of white or black race, aged 60 years or older or with a BMI of 30 kg/m2 or greater were at greatest risk of increased weight gain following switch to an INSTI, while age 60 or older was the predominant risk factor for male participants. When compared with pre-switch weight changes on stable suppressive ART, and weight changes among persons not switching to INSTI, and given concomitant increases in waist circumference, these data suggest that increases in weight and fat mass are greater than expected for increasing age for some PWH.
Our data are unique in that they reflect the first switch data published from persons originally enrolled in randomized controlled ART trials and followed (83% since first ART initiation) every 6 months since the conclusion of their trial, the detailed participant characterization allowed for complete ART history, and comorbidity/concomitant medication/social factor characterization of participants. While reasons for switching to INSTI-based therapy while virologically suppressed were not captured, for most participants this likely reflected the evolving standard of care and/or efforts to achieve regimen simplification/improved side-effect profiles.
An important strength of our study was the ability to measure the pre-switch trajectory of weight for persons on virally suppressive ART that occurred after the initial period of weight change following ART initiation, thus approximating individual trajectories of “age-related” weight gain while receiving stable ART. Evaluation of within-person weight trajectories pre- and post-switch is also more relevant than comparisons between persons who did or did not switch to INSTI-based therapy, which may be confounded by multiple, difficult-to-measure variables (physician preference, ART resistance, patient preference) in addition to between-person differences in genetics and metabolism.
Of the INSTIs, DTG appeared to be associated with the greatest increase in annualized weight gain, which occurred in persons switched from both PIs and NNRTIs. We were limited in our ability to assess the concomitant effects of switching NRTI backbone drugs on weight gain given that some persons switched only the third drug, while others switched both the third agent and NRTI component simultaneously; these subgroup analyses were limited by sample size. However, given the evolution of INSTIs and single-tablet regimen availability at the time of data collection, many switches to DTG were accompanied by stable or new ABC use, whereas EVG use was accompanied by TDF or TAF use.
The available (and minimally) published data on weight gain following switch to INSTIs do not diminish the unique contribution of our data. For example, in a retrospective single-center analysis, Burns et al [4] did not observe significant weight gain in persons switched to RAL- or DTG-based ART. However, compared with our diverse cohort, their cohort was primarily male and white, and the median number of weight measurements over the pre- and post-switch follow-up periods combined was only 4 per participant. Similar to our findings, they observed an increase in weight of 1.6 kg/year in men aged 65 and older following ART switch, although this was not statistically significant. Similar to other published studies, our data also demonstrate the relevance of the prior regimen, with the most pronounced weight gains when switching from efavirenz to an INSTI [1, 7]. An observational comparison of women switching to INSTIs versus not switching in the Women’s Interagency HIV study did demonstrate increased weight gain in switchers compared with nonswitchers [5] but did not assess within-person trajectories pre-/post-switch to an INSTI, which is a more relevant comparison given potential differences in populations undergoing switches through routine care.
In ART initiation studies, DTG does seem to be associated with the greatest weight gain among INSTIs [2, 8], and EVG with the least amount of weight gain [8], demonstrating considerable heterogeneity within the INSTI class rather than a homogenous class effect. In addition, observed weight gain does seem to be associated with gains in fat mass, particularly for women [2, 9]. This sex-based difference may contribute to the apparent postmarketing awareness of weight gain with INSTI-based ART, as many registrational trials enrolled relatively low numbers of women or did not present sex-stratified weight analyses, but the mechanism behind this difference by sex is unknown.
One possible contributor to DTG-related weight gain is its documented in vitro inhibition of the melanocortin-4 receptor (MC4R) activity [10]. MC4R plays a role in human energy homeostasis, and MC4R-knockout mice are severely obese when both alleles are nonfunctional and moderately obese when 1 allele is compromised [11]. In the general population, women have higher circulating leptin levels [12], subcutaneous adipose tissue leptin mRNA expression [13], subcutaneous adipose tissue metabolic rates [14], and more hypothalamic-to-subcutaneous adipose tissue neuronal connections than men [15], and leptin stimulates production of pro-opiomelanocortin peptides (POMCs), which reduce food intake and body weight through MC4R agonism [16, 17]. Additionally, leptin receptor expression on POMC neurons may be required for modulation of fat distribution in women but not in men [18]. Early animal and human studies of MC4R/POMC deficiency suggest that pharmacologic MC4R agonism induces weight loss and that persons with POMC deficiency benefit more than MCR4-deficient persons [19]. Among PWH, data are conflicting [20, 21] in their reporting of sex differences in leptin insufficiency/resistance or do not report sex-stratified results, but it is reasonable to postulate that women could have a differential or more exacerbated response to DTG-induced MC4R functional insufficiency.
Limitations to our study include the lack of available data regarding ART switches to BIC and the confounding issue of the effects of NRTI backbone. In the ADVANCE trial, ART-naive PWH in South Africa were randomized to receive DTG + TAF/emtricitabine (FTC), DTG + TDF/FTC, or the standard-of-care EFV + TDF/FTC. Both DTG groups gained more weight than the EFV group, but DTG + TAF/FTC-treated participants gained more weight than DTG + TDF/FTC-treated persons, and without a plateau in weight after 48 weeks. This trend was particularly evident among female participants, although weight data more than 2 years following switch in our cohort were limited. Increases in both lean and fat mass occurred following ART initiation, and similar to previous studies, lower CD4+ T-cell count, higher baseline HIV-1 RNA, and older age were associated with treatment-emergent obesity [2]. We were unable to fully control for simultaneous switches to INSTIs with a change in NRTI backbone. However, the majority of persons on ABC and TDF in our cohort did not switch NRTI at the time of switch to an INSTI, and most switches to ABC occurred in the context of switching to DTG/ABC/lamivudine (3TC). Among those switching from EVG/cobicistat/TDF/FTC to EVG/cobicistat/TAF/FTC, this included both a change to INSTI and to TAF, as prior receipt of an INSTI was an exclusion criterion for this parent trial. Last, although we compared within-person changes pre– and post–INSTI switch, it is possible that our analyses reflect age-associated weight change that may also steepen with increasing age.
In summary, we report the first data describing within-person weight trajectories following switch to INSTIs while on virologically suppressive ART. These data confirm ART initiation data suggesting a risk of greater weight gain on INSTIs for some PWH, particularly for women and with DTG use. Although the weight gain over the 2-year period was small, with decades of ART the potential long-term consequences could be considerable if the trajectory is sustained. Over just the first 2 years following switch, weight gain on INSTIs was accompanied by worsening of lipid and glucose profiles, as anticipated with obesity in the general population. Future work will focus on understanding the mechanism of weight gain with INSTI use in susceptible persons.
Notes
Acknowledgments. The authors thank the participants and study staff for their commitment and participation.
Financial support. This work was funded in part by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant numbers K23AI110532 [to J. E. L.], UM1 AI068634, and UM1 AI068636, UM1 AI106701) and the National Institute on Aging (grant number R01 AG054366; to K. M. E.).
Potential conflicts of interest. J. E. L. has served as a consultant to Merck and Gilead Sciences and receives research support from Gilead Sciences. F. J. P. is a consultant and/or on the speakers bureau for Gilead Sciences, Janssen, ViiV, and Merck. S. H. B. receives research support from Gilead Sciences. G. M. serves as consultant for Merck, ViiV, and Gilead and has received research funding from Gilead, Merck, ViiV, Roche, Tetraphase, and Astellas. K. M. E. is a consultant for Gilead Sciences and ViiV and receives research support from Gilead Sciences. J. R. K. reports grants and personal fees from Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2019. pii:ciz999. Epub ahead of print. doi: 10.1093/cid/ciz999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 3. Bares SH, Smeaton LM, Xu A, Godfrey C, McComsey GA. HIV-infected women gain more weight than HIV-Infected men following the initiation of antiretroviral therapy. J Womens Health (Larchmt) 2018; 27:1162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burns JE, Stirrup OT, Dunn D, et al. No overall change in the rate of weight gain after switching to an integrase-inhibitor in virologically suppressed adults with HIV. AIDS 2020; 34:109–14. [DOI] [PubMed] [Google Scholar]
- 5. Kerchberger AM, Sheth AN, Angert CD, et al. Weight gain associated with integrase stand transfer inhibitor use in women. Clin Infect Dis 2019. pii:ciz853. Epub ahead of print. doi: 10.1093/cid/ciz853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smurzynski M, Collier AC, Koletar SL, et al. AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials 2008; 9:269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Norwood J, Turner M, Bofill C, et al. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2019. pii:ciz407. Epub ahead of print. doi: 10.1093/cid/ciz407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Debroy P, Sim M, Erlandson KM, et al. Progressive increases in fat mass occur in adults living with HIV on antiretroviral therapy, but patterns differ by sex and anatomic depot. J Antimicrob Chemother 2019; 74:1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Medicines Agency. European Medicines Agency Assessment Report of Dolutegravir (Tivicay) Available at: www.ema.europa.eu/documents/assessment-report/tivicay-epar-public-assessment-report_en.pdf. Accessed 9 December 2019.
- 11. Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997; 88:131–41. [DOI] [PubMed] [Google Scholar]
- 12. Saad MF, Damani S, Gingerich RL, et al. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab 1997; 82:579–84. [DOI] [PubMed] [Google Scholar]
- 13. Montague CT, Prins JB, Sanders L, Digby JE, O’Rahilly S. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes 1997; 46:342–7. [DOI] [PubMed] [Google Scholar]
- 14. Nookaew I, Svensson PA, Jacobson P, et al. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J Clin Endocrinol Metab 2013; 98:E370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adler ES, Hollis JH, Clarke IJ, Grattan DR, Oldfield BJ. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J Neurosci 2012; 32:15913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 2005; 8:571–8. [DOI] [PubMed] [Google Scholar]
- 17. Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell 2015; 161:133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab 2008; 294:E630–9. [DOI] [PubMed] [Google Scholar]
- 19. Collet TH, Dubern B, Mokrosinski J, et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol Metab 2017; 6:1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tiliscan C, Aramă V, Mihăilescu R, et al. Leptin expression in HIV-infected patients during antiretroviral therapy. Germs 2015; 5:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Onyemelukwe GC, Ogoina D, Bakari AG. Serum leptin levels in antiretroviral therapy naive HIV-1 infected patients in Zaria, Nigeria. Int J Endocrinol Metabolism 2009; 7:162–9. [Google Scholar]