Abstract
Background
Cytomegalovirus (CMV) infection remains an important cause of morbidity and mortality in allogeneic hematopoietic cell transplant (allo-HCT) recipients. CMV cell-mediated immunity (CMV-CMI) as determined by a peptide-based enzyme-linked immunospot (ELISPOT) CMV assay may identify patients at risk for clinically significant CMV infection (CS-CMVi).
Methods
The CS-CMVi was defined as CMV viremia and/or disease necessitating antiviral therapy. CMV-CMI was characterized as high when the intermediate-early 1 (IE-1) antigen spot counts (SPCs) were >100 (cutoff 1) or when the IE-1 and phosphoprotein 65 antigen SPCs were both >100 SPCs per 250 000 cells (cutoff 2), and a low CMV-CMI when SPCs were below these thresholds. In this prospective multicenter study, we evaluated CMV-CMI every 2 weeks from the pretransplant period until 6 months posttransplantation in 241 allo-HCT recipients with positive CMV serostatus. The primary endpoint was CS-CMVi occurring within 2 weeks of the last measurement of CMV-CMI.
Results
CS-CMVi occurred in 70 allo-HCT recipients (29%). CMV-CMI was low in patients who experienced CS-CMVi (94%), whereas those who had a high CMV-CMI were less likely to have CS-CMVi (P < .0001). Patients with CS-CMVi had higher all-cause mortality (P = .007), especially those with low CMV-CMI (P = .035). On multivariable analysis, CMV-CMI, sex, race, antithymocyte globulin, and steroid use were independent predictors of CS-CMVi, and the time from transplant to engraftment was the only predictor of mortality.
Conclusions
Measurement of CMV-CMI using a novel ELISPOT assay would be useful clinically to monitor allo-HCT recipients and distinguish between those at risk of developing CS-CMVi and requiring antiviral prophylaxis or therapy and those who are protected.
Keywords: cytomegalovirus, cell-mediated immunity, CMV ELISPOT assay, hematopoietic cell transplant, multicenter
Patients with low cytomegalovirus (CMV) cell-mediated immunity (CMI) were at risk for clinically significant CMV infection (CS-CMVi). CMV-CMI, sex, race, and steroid use were predictors of CS-CMVi, and patients with low CMV-CMI and CS-CMVi had the highest mortality.
(See the Major Article by Jarque et al on pages 2375–85 and the Editorial Commentary by Kim on pages 2386–8.)
Cytomegalovirus (CMV) can cause various end-organ diseases in immunocompromised hosts, in particular allogeneic hematopoietic cell transplant (allo-HCT) recipients. CMV has also been associated with graft-vs-host disease (GVHD) after HCT as well as secondary bacterial and fungal infections [1, 2]. After allo-HCT, the CMV reactivation rate may approach 70% when recipients are CMV seropositive [3]. Interestingly, recent data suggest that CMV viremia is associated with higher all-cause mortality and transplant-related mortality in allo-HCT recipients [4, 5]. Without prophylaxis, CMV reactivation typically occurs during the first 3 months after HCT, depending on several risk factors [6].
Monitoring CMV cell-mediated immunity (CMV-CMI) responses after HCT may be helpful in determining the population at risk for these viral infections and their prognosis. In fact, in the presence of the virus, CMV immediate-early 1 (IE-1) and phosphoprotein 65 (pp65) antigens have been identified as triggers that activate the immune response involving CD4+ and CD8+ T cells, producing and releasing interferon-gamma (IFN-γ), and other cytokines to protect the host against CMV infection [7]. The release of IFN-γ produced by CMV-responsive CD4+ and CD8+ T cells can be quantified using the modified enzyme-linked immunospot (ELISPOT) assay (T-SPOT.CMV; Oxford Immunotec USA) as a measurement of a patient’s CMV-specific immunity (spot counts [SPCs] per 2.5 × 105 peripheral blood mononuclear cells) [7]. Knowledge of a patient’s CMV immune status may assist clinicians in evaluating the likelihood of CMV reactivation and the patient’s ability to successfully clear or control an infection [8]. Recipients of allo-HCT as well as solid organ transplantation (SOT) who lack CMV-specific CD4+ and CD8+ T cells have a higher incidence of CMV infection, and restoring T-cell responses correlates with protection against CMV [9–12]. The utility of monitoring CMV-specific IFN-γ release using ELISPOT CMV assay has been studied in the SOT [13–16] as well as in the HCT population in single-center studies [7, 17]; however, no multicenter validation in HCT of any assay has been conducted to date.
The purpose of this study was to validate, in a multicenter setting, the ability of the ELISPOT CMV assay in CMV-seropositive allo-HCT recipients to evaluate the relationship between the strength of the T-cell response as measured by the assay and the subsequent occurrence of CMV reactivation and/or disease.
METHODS
Study Design
In this prospective, noninterventional multicenter observational study, 241 CMV-seropositive adult allo-HCT recipients were enrolled. The study was conducted at 13 HCT centers in the United States, Canada, the United Kingdom, and Sweden. All enrolled patients underwent an allo-HCT from matched or haploidentical related donors, matched or mismatched unrelated donors, and patients who underwent a cord blood transplant. Patients who had active CMV infection within 1 month prior to enrollment or during the study’s prescreening period, patients who received any antiviral therapy active against CMV other than acyclovir and valacyclovir prior to enrollment, and those known to be positive for hepatitis B, hepatitis C, or human immunodeficiency virus were excluded from the study. All pediatric patients (≤18 years of age) were excluded. After enrollment, patients were followed up within 14 days before transplantation, at day 14 and day 28 after transplantation, and then every 2 weeks after transplantation through day 182 (about 14 visits over 6 months). Institutional review board approval was granted at each site, and each participant provided written informed consent.
Definitions and Endpoints
CMV disease and CMV infection were defined according to Ljungman et al [18]. In brief and specifically for this study, CMV DNAemia is defined as the detection and quantification of CMV DNA by nucleic acid amplification techniques in plasma. The definition of CMV disease required clinical symptoms and/or signs of end organ disease combined with documented CMV in tissue by different methods including virus isolation, rapid culture, histopathology, or DNA hybridization techniques. The primary endpoint was clinically significant CMV infection (CS-CMVi), defined as the first CMV reactivation or CMV disease after HCT necessitating the start of anti-CMV therapy by the treating physician according to each center’s institutional treatment protocols [19]. In brief, each center employed the preemptive approach and monitored for CMV by polymerase chain reaction (PCR) at least once a week, starting from the time of transplantation up to day 100 after transplantation. All-cause mortality was an exploratory endpoint.
Laboratory Analysis
Blood samples were collected up to 14 days before transplantation and after transplantation at day 14, day 28, and every 2 weeks for up to 6 months. At each time point, peptide-based ELISPOT CMV assays were performed. Samples were shipped overnight to Oxford Diagnostic Laboratories (Memphis, Tennessee). The CMV-specific ELISPOT assay was performed within 32 hours of blood specimen collection, in accordance with validated test procedures [20]. T-cell immune activity was assessed by detecting the number of IFN-γ–producing CD4+ and CD8+ T cells (reported as SPCs per 250 000 cells) following ex vivo stimulation with CMV antigens IE-1 and pp65 as reported elsewhere [7]. The results of the assays were not made available to the treating physicians; thus, CMV management was not based on the results of the assays. Additionally, CMV viral load was monitored in the plasma, and bronchoalveolar lavage, if sampled, in each center using different CMV assays calibrated to the World Health Organization International Standard for Human CMV, and recorded in international units per milliliter.
Data Collection
Patients’ demographics, medical history, baseline transplant data (eg, type of donor, histocompatibility data), time to engraftment (engraftment defined as the first of 3 days with stable neutrophil count >0.5 × 109/L), study procedure–related adverse events, and relevant transplant-related laboratory data, including results of quantitative PCR for CMV viremia performed at the site’s local laboratory, were obtained. In addition, data on pretransplant conditioning regimens, GVHD prophylaxis and management, CS-CMVi and management, and all-cause mortality were collected. De-identified data of participants from all sites were captured on a password-protected online database designed for the sponsor of the trial (Oxford Immunotec USA).
Statistical Analyses
The ELISPOT CMV assay, as described above, targets the 2 antigens pp65 and IE-1. In the statistical analyses, the repeated CMV-CMI measurements were treated as time-dependent variables and classified into 2 categories, high and low, based on either of the 2 cutoffs. We classified patients as having a high CMV-CMI when the IE-1 SPCs were >100 per 250 000 cells (cutoff 1) or when the IE-1 and pp65 SPCs were both >100 per 250 000 cells (cutoff 2), and as having a low CMV-CMI when the SPCs of IE-1 (cutoff 1) or either antigen (cutoff 2) were below the aforementioned thresholds. When evaluating the diagnostic performance of the ELISPOT CMV assay for patients who did not experience CS-CMVi, the maximum pp65 and IE-1 SPCs over 10 weeks from transplant (the period of time when most of the CMV events occurred in this cohort) was used to distinguish low and high CMV-CMI. For patients who experienced CS-CMVi, the CMV-specific pp65 and IE-1 SPCs occurring 14 days to >2 days before the infection were used to determine if the patient had a high or a low CMV-CMI.
Continuous variables were compared using the Wilcoxon rank-sum test. Categorical variables were compared using the χ 2 or Fisher exact test, as appropriate. The Cox proportional hazards regression model was used to evaluate the independent predicting effects of low/high CMV-CMI on CS-CMVi and all-cause mortality. Cumulative incidence curves were estimated for CS-CMVi and mortality using the Simon and Makuch method [21] treating CMV-CMI as a time-dependent variable, and the curves were compared between patients with low and high CMV-CMI using univariate Cox regression analysis. Cumulative survival curves were estimated for and compared between patients with low and high CMV-CMI, as well as among the following 4 groups: patients with low CMV-CMI with and without CS-CMVi and patients with high CMV-CMI with and without CS-CMVi, where both CMV-CMI (low/high) and CS-CMVi were treated as time-dependent variables. Week 2 post-HCT was chosen as time 0 in all Cox regression analyses and cumulative incidence curve analyses in order to identify predictors of CS-CMVi and mortality posttransplantation. We also constructed the IE-1–predicted probability curves of protection again CS-CMVi based on a multivariable logistic regression model with repeated measures using the method of generalized estimating equations. Box plot was used to compare ELISPOT CMV data between CS-CMVi and no CS-CMVi occurrences. The correlation between IE-1 and pp65 was measured using the Spearman correlation coefficient. All tests were 2-sided with a significance level of .05. Statistical analyses were performed using SAS version 9.3 software (SAS Institute, Cary, North Carolina).
RESULTS
Clinical Characteristics
Two hundred fifty patients were enrolled in this multicenter study. Of these patients, 9 were excluded because of withdrawal and/or unavailability of CMV-CMI results. Overall, 241 patients were followed for 6 months (Figure 1), and 70 (29%) patients experienced a CS-CMVi. Median time from CMV-CMI testing to CS-CMVi was 9 days (interquartile range, 6–13 days). Table 1 compares the demographic and clinical characteristics of patients who experienced CS-CMVi and patients who did not. Patients who received corticosteroids (P < .0001), those who received antithymocyte globulin (ATG) (P = .02), and those who had GVHD (P = .017) at any time during the study were more likely to have CS-CMVi than patients who did not.
Figure 1.
Flowchart showing the number of patients enrolled in the study. Abbreviations: CMV, cytomegalovirus; CS-CMVi, clinically significant CMV infection; ELISPOT, enzyme-linked immunospot assay; HCT, hematopoietic cell transplant.
Table 1.
Clinical and Demographic Characteristics of Study Participants
| Characteristic | CS-CMVi (n = 70) | No CS-CMVi (n = 171) | P Value |
|---|---|---|---|
| Age, y, median (range) | 57 (18–80) | 56 (18–78) | .89 |
| Sex | .007 | ||
| Male | 30 (43) | 106 (62) | |
| Female | 40 (57) | 65 (38) | |
| Race | .11 | ||
| White | 48/67 (72) | 129/153 (84) | |
| African American | 6/67 (9) | 8/153 (5) | |
| Asian | 6/67 (9) | 10/153 (7) | |
| Other | 7/67 (10) | 6/153 (4) | |
| Unknown | 3 | 18 | |
| Type of transplant | .06 | ||
| Matched related donor | 17/69 (25) | 70/169 (41) | |
| Matched/mismatched unrelated | 41/69 (59) | 72/169 (43) | |
| Cord blood | 1/69 (1) | 2/169 (1) | |
| Haploidentical | 10/69 (14) | 25/169 (15) | |
| Unknown | 1 | 2 | |
| HCT donor status | .50 | ||
| CMV seropositive | 37/69 (54) | 94/161 (58) | |
| CMV seronegative | 32/69 (46) | 67/161 (42) | |
| Unknown | 1 | 10 | |
| Conditioning regimen | .46 | ||
| Myeloablative | 36/69 (52) | 76/162 (47) | |
| Nonmyeloablative | 33/69 (48) | 86/162 (53) | |
| Unknown | 1 | 9 | |
| Time from HCT to engraftment, d, median (range) | 13 (3–42) | 14 (0–42) | .92 |
| Steroid use (at any time during the study period) | 67 (96) | 119 (70) | <.0001 |
| Acute GVHD (at any time during the study period) | 43 (61) | 76 (44) | .017 |
| Antithymocyte globulin | 17 (24) | 21 (12) | .02 |
| Cyclophosphamide | 20 (29) | 45 (26) | .72 |
| Mycophenolate | 11 (16) | 32 (19) | .58 |
| CMV disease | 10 (14) | … | |
| Peak CMV PCR, IU/mL, median (range)a | 1586 (323–26 364) | … | |
| All-cause mortality | 16 (23) | 23 (13) | .07 |
| CMV-CMI | |||
| Cutoff 1b | |||
| High | 4 (6) | 55 (32) | <.0001 |
| Low | 66 (94) | 116 (68) | |
| Cutoff 2 | |||
| High | 3 (4) | 55 (32) | <.0001 |
| Low | 67 (96) | 116 (68) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: CMI, cell-mediated immunity; CMV, cytomegalovirus; CS-CMVi, clinically significant CMV infection; GVHD, graft-vs-host disease; HCT, hematopoietic cell transplant; PCR, polymerase chain reaction.
aCMV PCR was provided for all patients who experienced CMV viremia (68 patients), excluding the 2 patients who experienced CMV disease as primary endpoint.
bHigh CMV-CMI is defined as immediate-early 1 antigen (IE-1) >100 (cutoff 1) or both IE-1 and phosphoprotein 65 (pp65) antigens >100 (cutoff 2). Low CMV-CMI is defined as an IE-1 ≤100 (cutoff 1) or either IE-1 or pp65 ≤100 (cutoff 2).
Clinically Significant CMV Infections
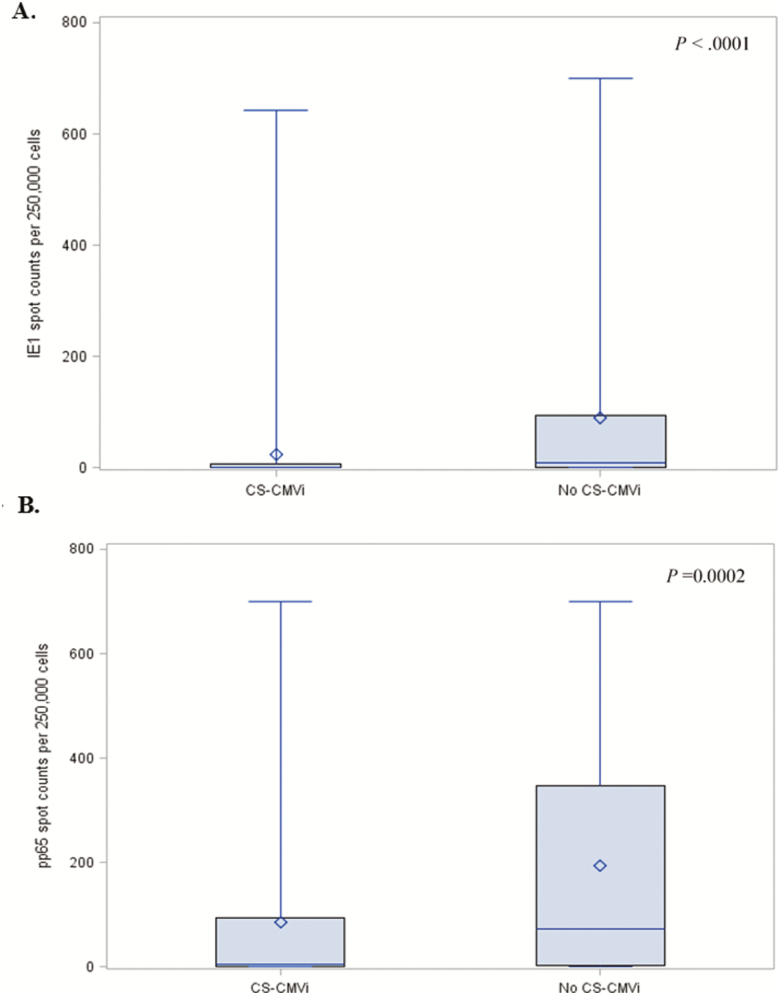
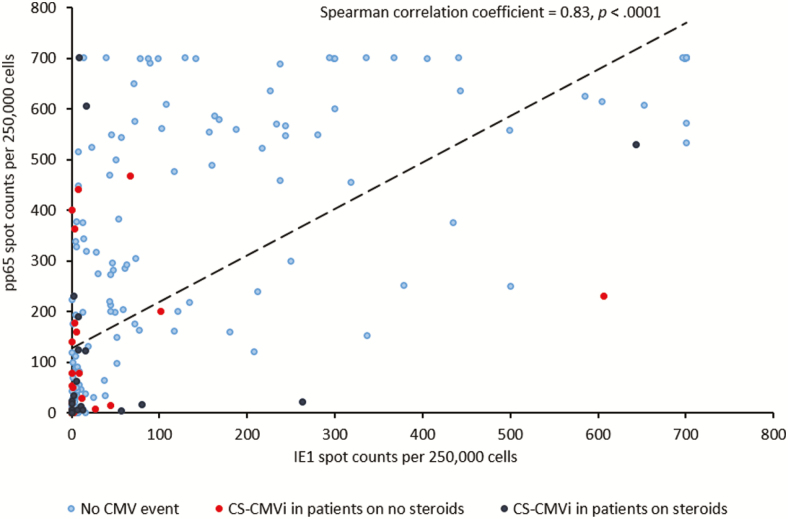
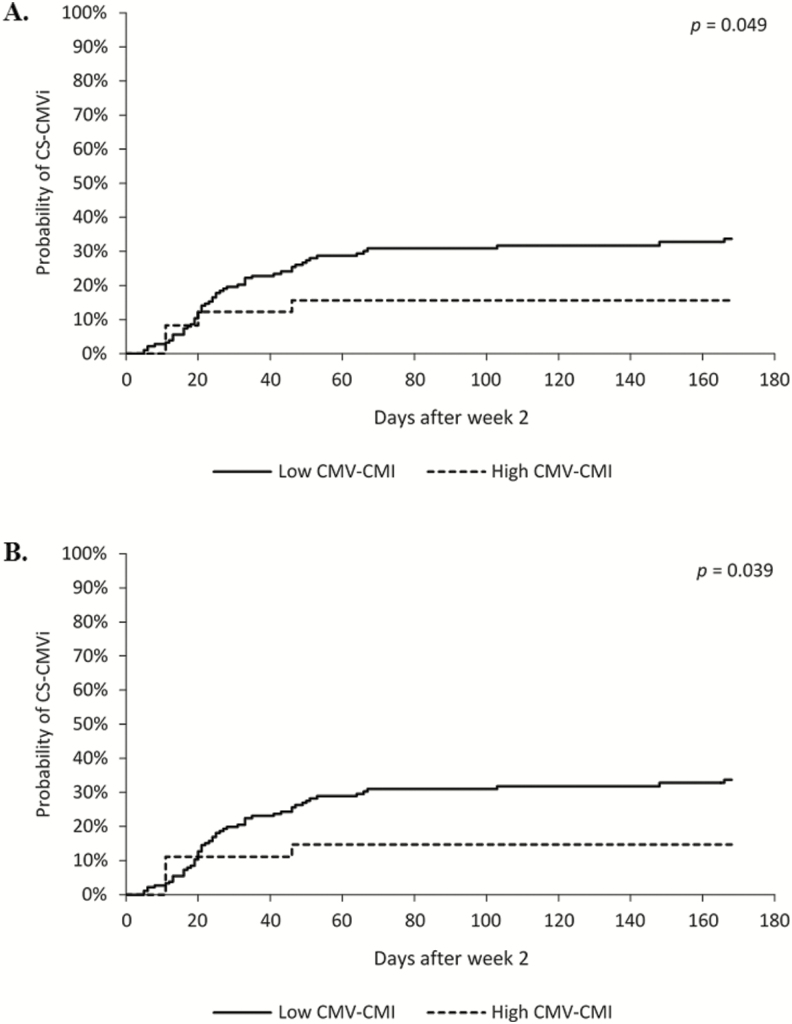
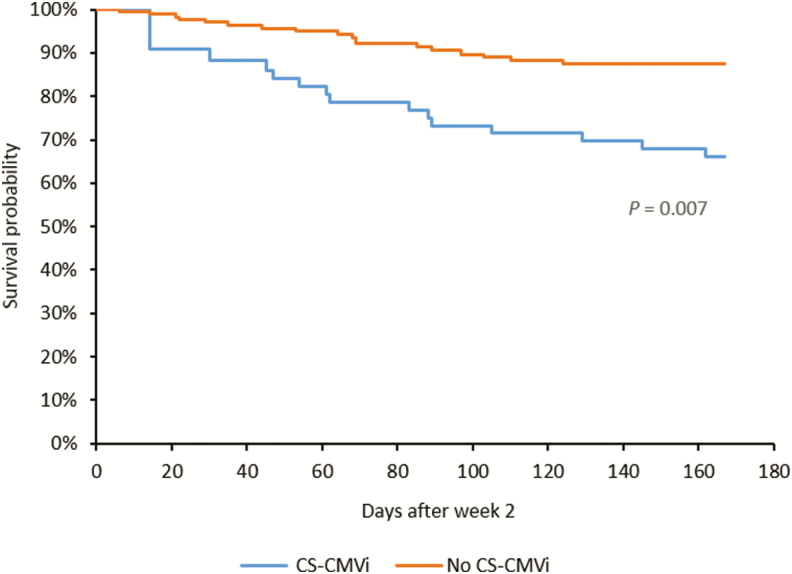
The CMV-CMI results with high background nil control (nil control >10) were not reported and occurred in 37 of the total 2728 samples tested, resulting in an indeterminate rate of 1.35%. Of the 70 (29%) patients who experienced CS-CMVi within the 6-month study period, 66 (94%) had low CMV-CMI according to cutoff 1 (IE-1 SPCs ≤100; Table 1). The 4 remaining patients who had a high CMV-CMI but had CS-CMVi were receiving corticosteroids (n = 2), had IE-1 SPCs very close to the cutoff (ie, 102 SPCs per 250 000 cells) (n = 1), or had a CS-CMVi before their transplant date (n = 1). Additionally, patients who had a low CMV-CMI were more likely to experience CS-CMVi than were patients with a high CMV-CMI (relative risk, 5.3 [95% confidence interval {CI}, 2.0–14.0]; P < .0001). The IE-1 SPCs were significantly higher in patients who did not have CS-CMVi (mean, 91 SPCs per 250 000 cells [range, 0–700]) than in those who had CS-CMVi (mean, 24 SPCs per 250 000 cells [range, 0–643]) (P < .0001; Figure 2A); similar results were observed with pp65 SPCs (P = .0002; Figure 2B). The Spearman statistical correlation analysis shown in Figure 3 revealed a positive correlation between IE-1 and pp65 SPCs (P < .0001). However, only IE-1 SPCs correlated with CS-CMVi, as most occurred in patients with IE-1 SPCs <100 (Figure 3). Cumulative incidence curves of CS-CMVi showed an association between a low CMV-CMI with either cutoff (Figure 4A and 4B) and having CS-CMVi (P < .0001). Survival curves showed no association or correlation between low CMV-CMI and death (P = .97) with either of the 2 cutoffs (Supplementary Figure 1). Of the 241 enrolled patients, 10 had CMV disease, including 2 patients who had CMV pneumonia as the primary endpoint of the study at day 58 and day 163 after transplantation. The remaining 8 patients experienced CS-CMVi requiring preemptive therapy followed by CMV disease at a median of 40 days after transplant (range, 22–77 days). The CMV-CMI of the 10 patients with CMV disease was low, with a median IE-1 of 5 SPCs (range, 0–44 SPCs) and a median pp65 of 156 SPCs (range, 0–606 SPCs). Patients who had CS-CMVi were 2.7 times more likely to die relative to patients without CS-CMVi (hazard ratio, 2.7 [95% CI, 1.31–5.75]; P = .007; Figure 5). Last, median SPCs per 250 000 cells for IE-1 and pp65 for each time point in patients with or without CS-CMVi are depicted in Supplementary Figure 2.
Figure 2.
Box plot of the number of spots produced in the enzyme-linked immunospot (ELISPOT) cytomegalovirus (CMV) assay for patients with and without clinically significant CMV infection (CS-CMVi) for the intermediate-early 1 (IE-1; A) and phosphoprotein 65 (pp65; B) antigens. The length of box represents the interquartile range (the distance between the 25th and 75th percentiles). The diamond in the box represents the group mean. The horizontal line in the box represents the group median. The vertical lines originating from the box extend to the group minimum and maximum values. For IE-1 level in patients with CS-CMVi, its median was equal to its 25th percentile and its minimum value.
Figure 3.
Correlation curve with intermediate-early 1 (IE-1) spot counts on the y-axis and phosphoprotein 65 (pp65) spot counts on the x-axis. Shown are values before the occurrence of clinically significant cytomegalovirus (CMV) infection (CS-CMVi) for 70 patients and maximum values for 171 patients who did not experience CS-CMVi.
Figure 4.
Cumulative incidence curves of clinically significant cytomegalovirus (CMV) infection (CS-CMVi) in patients with different CMV cell-mediated immunity (CMV-CMI) cutoffs. The curves show the likelihood of developing CS-CMVi in a particular patient at each time point. A, Cutoff 1 (intermediate-early 1 spot counts >100 as high CMV-CMI; P < .049). B, Cutoff 2 (IE-1 SPCs >100 and phosphoprotein 65 SPCs >100 as high CMV-CMI; P < .039). CMV-CMI (low/high) was a time-dependent variable in the cumulative incidence curves as it changed over time during the study period.
Figure 5.
Cumulative survival curves of patients with or without clinically significant cytomegalovirus infection (CS-CMVi).
Patient Outcomes and ELISPOT CMV Assay
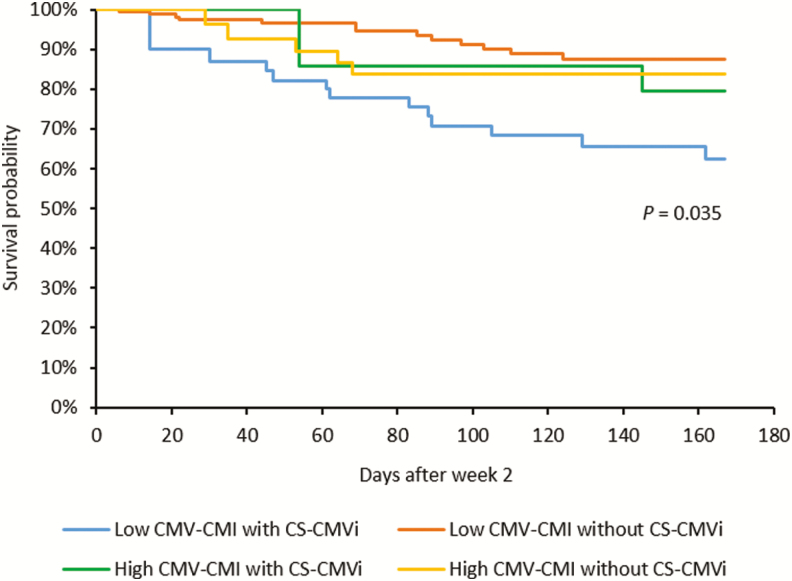
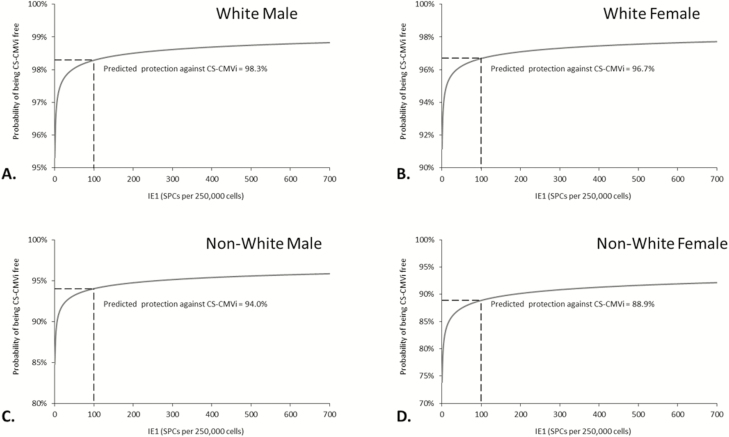
Cox regression analyses identified independent predictors of CS-CMVi and all-cause mortality. In a univariate analysis, patients’ sex (P = .026), race (P = .01), type of transplant (P = .022), ATG use (P = .008), steroid use (P = .002), and having acute GVHD (P = .039) were risk factors for CS-CMVi. In addition, low CMV-CMI was also a risk factor for CS-CMVi when cutoff 1 was used (P = .049; Table 2). In the multivariate analysis, sex (P = .023), race (P < .001), ATG use (P = .015), steroid use (P = .003), and low CMV-CMI (P = .049) remained as independent predictors of CS-CMVi (Table 2). Similar results were observed when cutoff 2 was used (Supplementary Table 1). Additionally, in the multivariate analysis, the time to engraftment was the only predictor of all-cause mortality (P = .033), but a trend was observed for patients with acute GVHD (P = .068) (Table 3 and Supplementary Table 2). Interestingly, patients with low CMV-CMI who experienced CS-CMVi had a higher mortality rate than patients with low CMV-CMI but without CS-CMVi or patients with high CMV-CMI with or without CS-CMVi (P = .035; Figure 6). In particular, there was a significant difference in mortality between patients with low CMV-CMI who experienced CS-CMVi (37%) vs those who did not (12%) using cutoff 1 (P = .003; Table 1 and Figure 6). The same results were observed when using cutoff 2 (P = .019). In addition, we constructed probability curves for predicted protection against CS-CMVi based on IE-1 levels (Figure 7A–D).
Table 2.
Impact of Cytomegalovirus (CMV) Cell-Mediated Immunity (CMI) on Developing Clinically Significant CMV Infection by Cox Regression Analysisa Using Cutoff of Immediate-Early 1 Antigen Spot Counts >100 as High CMV-CMI
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Crude HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| Age | 0.99 (.97–1.01) | .43 | … | |
| Sex | .026 | .015 | ||
| Male | Reference | Reference | ||
| Female | 1.82 (1.08–3.09) | 1.99 (1.15–3.46) | ||
| Race | .01 | < .001 | ||
| White | Reference | Reference | ||
| African American | 1.81 (.71–4.62) | 2.15 (.82–5.63) | ||
| Asian | 1.52 (.60–3.86) | 1.84 (.70–4.81) | ||
| Other | 3.85 (1.71–8.64) | 7.22 (3.04–17.17) | ||
| Type of transplantation | .022 | b | ||
| Match-related donor | Reference | … | ||
| Matched/mismatched unrelated | 2.65 (1.42–4.94) | … | ||
| Cord blood | c | … | ||
| Haploidentical | 1.61 (.65–3.98) | … | ||
| HCT donor status | .29 | |||
| CMV seronegative | Reference | … | ||
| CMV seropositive | 0.75 (.44–1.28) | … | ||
| Conditioning regimen | .78 | |||
| Myeloablative | 1.08 (.63–1.83) | … | ||
| Nonmyeloablative | Reference | … | ||
| Time from HCT to engraftment, d | 1.00 (.96–1.04) | .93 | … | |
| Steroid use | 9.02 (2.20–36.99) | .002 | 17.72 (2.43–129.13) | .005 |
| Acute GVHD | 1.77 (1.03–3.04) | .039 | … | b |
| Antithymocyte globulin | 2.16 (1.22–3.81) | .008 | 2.16 (1.16–4.02) | .015 |
| Cyclophosphamide | 1.18 (.68–2.04) | .55 | … | |
| Mycophenolate | 0.82 (.42–1.63) | .58 | … | |
| CMV-CMId | .049 | .043 | ||
| High (IE-1 >100) | Reference | Reference | ||
| Low (IE-1 ≤100) | 3.24 (1.01–10.46) | 3.37 (1.04–10.93) |
Abbreviations: CI, confidence interval; CMI, cell-mediated immunity; CMV, cytomegalovirus; GVHD, graft-vs-host disease; HCT, hematopoietic cell transplant; HR, hazard ratio; IE-1, intermediate-early 1 antigen.
aWeek 2 in the study was chosen as time 0 in the analysis.
bVariable was entered into the initial multivariate Cox regression model based on the P value of its univariate analysis (≤.20) and later removed from the final Cox regression model through the backward elimination procedure.
cHR and 95% CI failed to be estimated as there was only 1 patient with cord blood transplant in the analysis.
dCMV-CMI was treated as a time-dependent variable in the analysis.
Table 3.
Impact of Cytomegalovirus (CMV) Cell-Mediated Immunity (CMI) on All-Cause Mortality by Cox Regression Analysisa Using Cutoff of Intermediate-Early 1 Antigen Spot Counts >100 as High CMV-CMI
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Crude HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| Age | 1.01 (.99–1.04) | .34 | … | |
| Sex | .52 | |||
| Male | Reference | … | ||
| Female | 0.79 (.39–1.62) | … | ||
| Race | .75 | |||
| White | Reference | … | ||
| African American | 1.26 (.38–4.15) | … | ||
| Asian | 0.36 (.05–2.64) | … | ||
| Other | 1.07 (.25–4.51) | … | ||
| Type of transplantation | .013 | b | ||
| Match related donor | Reference | … | ||
| Matched/mismatched unrelated | 2.25 (.93–5.47) | … | ||
| Cord blood | 18.9 (2.28–157.3) | … | ||
| Haploidentical | 3.60 (1.31–9.94) | … | ||
| HCT donor status | .28 | |||
| CMV seronegative | Reference | … | ||
| CMV seropositive | 0.68 (.34–1.37) | … | ||
| Conditioning regimen | .51 | |||
| Myeloablative | 0.79 (.39–1.60) | … | ||
| Nonmyeloablative | Reference | … | ||
| Time from HCT to engraftment (every 10 days’ increase) | 1.63 (1.04–2.55) | .033 | 1.63 (1.04–2.56) | .033 |
| Steroid use | 1.50 (.58–3.88) | .41 | … | |
| Acute GVHDc | 1.62 (.79–3.32) | .19 | … | b |
| Antithymocyte globulin | 1.46 (.66–3.25) | .35 | … | |
| Cyclophosphamide | 1.06 (.51–2.19) | .88 | … | |
| Mycophenolate | 1.78 (.84–3.76) | .13 | … | b |
| CMV-CMIc | .79 | .93 | ||
| High (IE-1 >100) | Reference | Reference | ||
| Low (IE-1 ≤100) | 0.89 (.39–2.02) | 0.96 (.40–2.29) |
Abbreviations: CI, confidence interval; CMI, cell-mediated immunity; CMV, cytomegalovirus; GVHD, graft-vs-host disease; HCT, hematopoietic cell transplant; HR, hazard ratio; IE-1, intermediate-early 1 antigen.
aWeek 2 in the study was chosen as time 0 in the analysis.
bVariable was entered into the initial multivariate Cox regression model based on the P value of its univariate analysis (≤.20) and later removed from the final Cox regression model through the backward elimination procedure.
cGVHD and CMV-CMI were treated as time-dependent variables in the analysis.
Figure 6.
Cumulative survival curves of all patients using intermediate-early 1 (IE-1) >100 as high cytomegalovirus cell-mediated immunity (CMV-CMI) (cutoff 1). CMV-CMI level (high/low) and clinically significant cytomegalovirus infection (CS-CMVi) were time-dependent variables in the cumulative survival curves. There was only 1 significant difference among the 4 groups of patients: low CMV-CMI with CS-CMVi vs low CMV-CMI without CS-CMVi (P = .003).
Figure 7.
Probability curves for predicted protection against clinically significant cytomegalovirus infection of 4 typical cases of patients who received steroids during the study period: white male (A), white female (B), nonwhite male (C), and nonwhite female (D). Abbreviations: CS-CMVi, clinically significant cytomegalovirus infection; IE-1, intermediate-early 1 antigen; SPC, spot count.
Diagnostic Accuracy
The sensitivity of the ELISPOT CMV assay as a predictor of CS-CMVi was 94% when cutoff 1 was used and 96% when cutoff 2 was used. The negative predictive values, indicating protection against CS-CMVi in cases of high CMV-CMI, were 93% and 95% for cutoffs 1 and 2, respectively.
DISCUSSION
This international multicenter observational study enrolled a large cohort of allo-HCT recipients at risk for CMV infection from 13 centers to evaluate the accuracy of an ELISPOT CMV assay, by measuring CMV-CMI, as a predictor of clinically significant CMV infection. This study showed that CMV-CMI was a significant and independent predictor of CS-CMVi in addition to sex, race, and steroid use. Only time to engraftment independently predicted all-cause mortality. Interestingly, patients with CS-CMVi had higher all-cause mortality regardless of their CMV-CMI over time, and patients with low CMV-CMI and CS-CMVi (time-dependent variables) had the highest all-cause mortality (37%) within 6 months from transplant.
Our findings in this multicenter study substantiated previous single-center studies that supported the utility of CMV-CMI, as measured by the CMV-specific ELISPOT assay, to predict CS-CMVi [7, 17, 22–24]. Monitoring CMV-CMI in allo-HCT recipients may provide a more targeted approach when managing CMV infections after transplantation. Knowing the patient’s CMV specific immunity at a critical time point may lead to a reduction in the duration and intensity of CMV monitoring as well as the duration of antiviral therapy or of prophylaxis (either primary or secondary). Our findings were similar to those of a recently published prospective multicenter study evaluating the ELISPOT CMV assay in adult kidney transplant recipients [16].
CMV-CMI can be monitored using different IFN-γ release assays. The 2 commonly used tests in published studies are the CMV-specific ELISPOT and the enzyme-linked immunosorbent assay (ELISA). A head-to-head comparison of both assays in 124 kidney transplant recipients at risk for CMV infections showed that posttransplant CMV pp65 or IE-1 response, and not QuantiFERON-CMV response, was significantly associated with CMV DNAemia [16, 24]. However, data comparing both assays in allo-HCT recipients are lacking. Many studies evaluated either assay individually in SOT [10, 11, 14–16, 23, 24] or allo-HCT recipients [7, 9, 12, 17, 25–28]. Overall, the CMV-specific ELISPOT assay appears to be more sensitive when detecting patients at risk for CMV reactivation, especially those with low-level responses, with greater negative predictive value than the CMV-specific ELISA assay. Furthermore, the use of the ELISA-CMV assay often resulted in indeterminate results [25–28].
In addition to CMV-CMI, patients’ sex, race, ATG use, and steroid use were risk factors for CS-CMVi on multivariate analysis. Steroid use and ATG use, as predictors of CS-CMVi, are in accordance with other studies [5, 7, 29–35]. The use of high-dose steroids in particular, constitute a key risk factor for CMV reactivation and disease in HCT recipients [7, 29] decreasing the functional fractions of CMV-specific CD8+ and CD4+ T cells in HCT recipients [30, 33]. The impact of steroid use on CMV-CMI as measured by ELISPOT assays is not well understood and needs to be determined in future trials.
Time from transplant to engraftment was the only significant risk factor for all-cause mortality in our study, after we controlled for other variables and irrespective of GVHD prophylaxis. A recent study showed that neutrophil engraftment was protective and an absolute monocyte count of <300 cells/μL increased the risk of overall mortality [36]. On the other hand, higher all-cause mortality was observed in our cohort of patients who developed CS-CMVi, as seen in other larger studies [5, 37]. Recent cohort studies showed a strong association between CMV reactivation and poor posttransplant outcomes including nonrelapse mortality, independent of the use of preemptive therapy [5, 37]. Interestingly, patients who had low CMV-CMI and CS-CMVi in our cohort were more likely to die than other groups of patients. Whether the combination of low CMV-CMI and CS-CMVi constitutes a stronger predictor of mortality than each one alone needs to be determined in future prospective studies.
Our study has a few limitations. As a multicenter study, some data were missing or not collected for analysis, such as the cause of death, with nonrelapse mortality in particular. However, the available data yielded numerous informative results. Additionally, the study was only observational, and CMV management of enrolled patients was at the discretion of clinical providers and not based on real-time CMV-CMI assays’ results. The utility of real-time monitoring of CMV-CMI in allo-HCT recipients for CMV management and for more personalized preventive or treatment strategies should be determined in future interventional trials.
In conclusion, this multicenter observational study showed a strong correlation between CMV-CMI and the occurrence of CS-CMVi. Patients with low CMV-CMI were more likely to have CS-CMVi and to require anti-CMV therapy, whereas those with high CMV-CMI were protected. Thus, monitoring CMV-CMI by CMV ELISPOT assay of allo-HCT recipients at risk for CMV infection may reduce the unnecessary or prolonged use of antiviral therapy for patients with high CMV-CMI and may guide implementation of early preventive (ie, the newly US Food and Drug Administration–approved anti-CMV drug letermovir) or preemptive strategies for those at risk of developing CS-CMVi.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. E. A.-H. and R. F. C. designed the study. D. P. S. helped with the design of the study and some of the statistical analysis. Oxford Immunotec, Inc, performed the enzyme-linked immunospot (ELISPOT) assays and provided T-SPOT.CMV results and assistance with study design. E. A.-H. supervised the study. L. E. H. managed patients and samples at the University of Texas MD Anderson Cancer Center. R. F. C. and L. E. H. wrote the manuscript. All authors managed the study at their respective institutions, entered data, and edited the manuscript.
Acknowledgments. The authors thank Jodi Govan and Chris Ball from Oxford Immunotec, Inc, for providing ELISPOT cytomegalovirus results, and Bryan Tutt of Scientific Publications at the University of Texas MD Anderson Cancer Center, for copyediting the manuscript.
Disclaimer. The sponsor did not participate in the analysis and/or the interpretation of the data.
Financial support. This work was supported in part by research funding from Oxford Immunotec USA, Inc. The work was also supported by the National Cancer Institute of the National Institutes of Health under award number P30CA016672 (Clinical Trials Support Resource).
Potential conflicts of interest. R. F. C. has served as a consultant to Oxford Immunotec, Merck, Chimerix, Shire/Takeda, Astellas, and Clinigen, and has received research funding paid to his institution from Oxford Immunotec, AiCuris, Viracor, Merck, Shire/Takeda, Chimerix, and Novartis. D. J. W. has received research funding from Oxford Immunotec, Merck, Chimerix, Shire/Takeda, and Gilead, and has served as a consultant for Merck. S. D. R. has served as a consultant for Fate Therapeutics and Mesoblast and owns stocks in Allergan. K. M. M. has received research funding from Oxford Immunotec, Merck, Chimerix, Shire/Takeda, and Gilead, and has served as a consultant for Merck, GlaxoSmithKline, and ADMA Biologics. P. C. has received research funding paid to his institution from Oxford Immunotec, Inc. R. K. A. has received research funding paid to her institution from Oxford Immunotec, Shire/Takeda, Merck, Astellas, Chimerix, AiCuris, and Qiagen. P. C. has received grants from Oxford Immunotec, Inc. P. H. has received research funding and consulting fees from Celgene, Takeda, Bristol-Myers Squibb, Janssen, Sanofi, and Spectrum, and consulting fees from Gilead, Incyte, Kite, Karyopharm, Juno, and AbbVie. D. K. has received research funding paid to her institution from Oxford Immunotec, Shire, Merck, Qiagen, and Roche; consulting fees from Oxford Immunotec and Qiagen; and honoraria from Merck, Astellas, and Shire/Takeda. R. N. has served as a consultant to Oxford Immunotec, Inc. P. L. has received personal fees from AiCuris, and research funding from Merck, Shire/Takeda, Oxford Immunotec, Inc, and Astellas. S. B. M. has received research funding paid to his institution from Ansun Biopharma, Vistera, GlaxoSmithKline, and Merck, and personal fees from Clinigen. S. S. D. has served as a consultant to Merck, Clinigen, and Janssen, and has received research funding paid to his institution from Merck, Shire/Takeda, Gilead, Ansun Biopharma, and Oxford Immunotec. T. B. is an employee of Oxford Immunotec. E. A.-H. has received research funding paid to her institution from Oxford Immunotec and grants from Merck. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis 2002; 185:273–82. [DOI] [PubMed] [Google Scholar]
- 2. Yong MK, Ananda-Rajah M, Cameron PU, et al. Cytomegalovirus reactivation is associated with increased risk of late-onset invasive fungal disease after allogeneic hematopoietic stem cell transplantation: a multicenter study in the current era of viral load monitoring. Biol Blood Marrow Transplant 2017; 23:1961–7. [DOI] [PubMed] [Google Scholar]
- 3. Ariza-Heredia EJ, Nesher L, Chemaly RF. Cytomegalovirus diseases after hematopoietic stem cell transplantation: a mini-review. Cancer Lett 2014; 342:1–8. [DOI] [PubMed] [Google Scholar]
- 4. Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18:1687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 2016; 127:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am 2011; 25:151–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El Haddad L, Ariza-Heredia E, Shah DP, et al. The ability of a cytomegalovirus ELISPOT assay to predict outcome of low-level CMV reactivation in hematopoietic cell transplant recipients. J Infect Dis 2019; 219:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avetisyan G, Aschan J, Hägglund H, Ringdén O, Ljungman P. Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT. Bone Marrow Transplant 2007; 40:865–9. [DOI] [PubMed] [Google Scholar]
- 9. Avetisyan G, Larsson K, Aschan J, Nilsson C, Hassan M, Ljungman P. Impact on the cytomegalovirus (CMV) viral load by CMV-specific T-cell immunity in recipients of allogeneic stem cell transplantation. Bone Marrow Transplant 2006; 38:687–92. [DOI] [PubMed] [Google Scholar]
- 10. Gerna G, Lilleri D, Fornara C, et al. Monitoring of human cytomegalovirus-specific CD4 and CD8 T-cell immunity in patients receiving solid organ transplantation. Am J Transplant 2006; 6:2356–64. [DOI] [PubMed] [Google Scholar]
- 11. Kumar D, Chernenko S, Moussa G, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant 2009; 9:1214–22. [DOI] [PubMed] [Google Scholar]
- 12. Tormo N, Solano C, Benet I, et al. Reconstitution of CMV pp65 and IE-1-specific IFN-γ CD8(+) and CD4(+) T-cell responses affording protection from CMV DNAemia following allogeneic hematopoietic SCT. Bone Marrow Transplant 2011; 46:1437–43. [DOI] [PubMed] [Google Scholar]
- 13. Dinavahi R, Heeger PS. T-cell immune monitoring in organ transplantation. Curr Opin Organ Transplant 2008; 13:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poggio ED, Heeger PS. Use of cytokine enzyme-linked immunosorbent spot (ELISPOT) assay as an immune monitoring tool in solid organ transplantation. Transplant Rev 2004; 18:183–91. [Google Scholar]
- 15. Rittà M, Costa C, Sidoti F, et al. Pre-transplant assessment of CMV-specific immune response by ELISPOT assay in kidney transplant recipients. New Microbiol 2015; 38:329–35. [PubMed] [Google Scholar]
- 16. Kumar D, Chin-Hong P, Kayler L, et al. A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients. Am J Transplant 2019; 19:2505–16. [DOI] [PubMed] [Google Scholar]
- 17. Nesher L, Shah DP, Ariza-Heredia EJ, et al. Utility of the enzyme-linked immunospot interferon-γ-release assay to predict the risk of cytomegalovirus infection in hematopoietic cell transplant recipients. J Infect Dis 2016; 213:1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ljungman P, Boeckh M, Hirsch HH, et al. Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 19. Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 2017; 377:2433–44. [DOI] [PubMed] [Google Scholar]
- 20. Li J, Liu Y, Ma L, et al. The performance of T-cell Xtend reagent in increasing blood storage times for interferon gamma release assays. J Clin Lab Anal 2018; 32. doi:10.1002/jcla.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schultz LR, Peterson EL, Breslau N. Graphing survival curve estimates for time-dependent covariates. Int J Methods Psychiatr Res 2002; 11:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barabas S, Spindler T, Kiener R, et al. An optimized IFN-γ ELISpot assay for the sensitive and standardized monitoring of CMV protein-reactive effector cells of cell-mediated immunity. BMC Immunol 2017; 18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chanouzas D, Small A, Borrows R, Ball S. Assessment of the T-SPOT.CMV interferon-γ release assay in renal transplant recipients: a single center cohort study. PLoS One 2018; 13:e0193968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee H, Park KH, Ryu JH, et al. Cytomegalovirus (CMV) immune monitoring with ELISPOT and QuantiFERON-CMV assay in seropositive kidney transplant recipients. PLoS One 2017; 12:e0189488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clari MÁ, Muñoz-Cobo B, Solano C, et al. Performance of the QuantiFERON-cytomegalovirus (CMV) assay for detection and estimation of the magnitude and functionality of the CMV-specific gamma interferon-producing CD8(+) T-cell response in allogeneic stem cell transplant recipients. Clin Vaccine Immunol 2012; 19:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fleming T, Dunne J, Crowley B. Ex vivo monitoring of human cytomegalovirus-specific CD8(+) T-cell responses using the QuantiFERON-CMV assay in allogeneic hematopoietic stem cell transplant recipients attending an Irish hospital. J Med Virol 2010; 82:433–40. [DOI] [PubMed] [Google Scholar]
- 27. Morello E, Pagani E, Monsurrò V, et al. Quantiferon-CMV for immune-monitoring in patients undergoing allogeneic hematopoietic stem cell transplantation. Blood 2008;112:4369.19029454 [Google Scholar]
- 28. Tey SK, Kennedy GA, Cromer D, et al. Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PLoS One 2013; 8:e74744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Camargo JF, Komanduri KV. Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther 2017; 10:233–8. [DOI] [PubMed] [Google Scholar]
- 30. Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood 2003; 102:3060–7. [DOI] [PubMed] [Google Scholar]
- 31. Hamadani M, Blum W, Phillips G, et al. Improved nonrelapse mortality and infection rate with lower dose of antithymocyte globulin in patients undergoing reduced-intensity conditioning allogeneic transplantation for hematologic malignancies. Biol Blood Marrow Transplant 2009; 15:1422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kennedy L, Cavalier M, Howard D. Relationship of CMV reactivation and rabbit antithymocyte globulin administration in allogeneic stem cell transplant patients. Biol Blood Marrow Transplant 2017; 23:S282. [Google Scholar]
- 33. Ozdemir E, St John LS, Gillespie G, et al. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood 2002; 100:3690–7. [DOI] [PubMed] [Google Scholar]
- 34. Reusing JO Jr, Feitosa EB, Agena F, et al. Cytomegalovirus prophylaxis in seropositive renal transplant recipients receiving thymoglobulin induction therapy: outcome and risk factors for late CMV disease. Transpl Infect Dis 2018; 20:e12929. [DOI] [PubMed] [Google Scholar]
- 35. Wadhawan M, Gupta S, Goyal N, et al. Cytomegalovirus infection: its incidence and management in cytomegalovirus-seropositive living related liver transplant recipients: a single-center experience. Liver Transpl 2012; 18:1448–55. [DOI] [PubMed] [Google Scholar]
- 36. Hill JA, Mayer BT, Xie H, et al. The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood 2017; 129:2316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 2016; 3:e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.